Abstract
Definitions of what constitutes the ‘signal of interest’ in neuroscience can be controversial, due in part to continuously evolving notions regarding the significance of spontaneous neural activity. This review highlights how the challenge of separating brain signal from noise has led to new conceptualizations of brain functional organization at both micro and macroscopic levels. Recent debates in the functional neuroimaging community surrounding artifact removal processes have revived earlier discussions surrounding how to appropriately isolate and measure neuronal signals against a background of noise from various sources. Insights from electrophysiological studies and computational modeling can inform current theory and data analytic practices in human functional neuroimaging, given that signal and noise may be inextricably linked in the brain.
Keywords: artifact removal, brain signal variability, dynamical system, global signal regression, resting state fMRI, spontaneous neural activity
“Distinguishing the signal from the noise requires both scientific knowledge and self-knowledge: the serenity to accept the things we cannot predict, the courage to predict the things we can, and the wisdom to know the difference.”
-Nate Silver
What was once ‘noise’ is now ‘signal’
A primary problem with all measurement in science is proper modeling of signal and noise. While separating signal from noise appears at first glance to be an ontological issue, it is in fact epistemological in that it is influenced by our existing biases, values, and assumptions surrounding what is important or significant. As such, understanding and acknowledging our own biases, or self-knowledge, is an important first step in the endeavor to separate signal from noise. In the lab we often hear colleagues say, “These data are really noisy” or “I’m going to run this procedure to denoise the data”, but we seldom stop to consider the assumptions surrounding these judgements and decisions.
Signal-to-noise ratio (SNR) is a measure used in the sciences to quantify the level of a signal of interest with respect to the level of background noise, where noise can be considered random or irregular disturbances that interfere with or obscure the transfer of information. Although in some fields SNR has a precise definition, the phrase is often used more colloquially to refer to the ratio of meaningful or relevant information to background noise or unwanted signal. Distinguishing signal from noise can be relatively straightforward in fields with concrete, quantifiable variables, whereas it can present more of a challenge in areas where universally accepted taxonomies are still developing, like neuroscience.
As fields of science naturally progress, it is sometimes the case that what was previously considered ‘noise’ moves into the realm of ’signal’ as new empirical results lead to paradigm shifts requiring updates of existing theoretical frameworks. Here we will consider select examples from micro and macroscale studies in neuroscience ranging from the cellular level (optical imaging and single unit recording) to non-invasive whole-brain imaging (magnetic resonance imaging (MRI) and electroencephalography (EEG)) to illustrate the difficulty of separating signal from noise at multiple spatial and temporal scales, and highlight relevant considerations when attempting to tease these two apart. As noted in Table 1, non-neuronal physiological fluctuations, spontaneous neural activity, and variability of neuronal responses have all been referred to as ‘noise’ in the literature [1,2]. Here we will consider the continuously evolving notions of these and other putative noise sources.
Table 1.
Taxonomy of noise in neuroscience
| Category of putative ‘noise’ source |
Examples of‘noise’ | Consensus regarding classification as ‘noise’ |
Management and mitigation strategies |
|---|---|---|---|
| Measurement and hardware limitations | -thermal noise in extracellular recordings -MRI scanner artifacts (spikes, ghosting, signal dropout) |
Yes | -estimate based on known relationships [113] -quality control procedures (distortion correction, field mapping [114]) |
| Human physiological signals and behavior | -cardiac pulsatility -respiratory cycles -head motion |
Yes | -image-based correction methods [115] -denoising using independent component analysis [79] -global signal regression [64] |
| Spontaneous (non-evoked) neural activity | -random spiking of individual neurons -low-frequency fluctuations in BOLD signal |
No | -consider temporal context [89] -characterize as functional connectivity [116] |
| Variability | -synaptic noise due to variable transmission -BOLD signal variability -trial-to-trial differences in behavioral responses |
No | -relate to neural circuit plasticity [18] -characterize using MSSD, SD, MSE measures [117] -dense sampling of individual subjects [118] |
Methodological and theoretical considerations
In neuroscience, there are at least two factors that contribute to difficulties in separating signal from noise. The first is methodological; methods for detecting neural activity in vivo are often coarse, leading to measurement that can be noisy. In human neuroimaging in general, and functional MRI in particular, even at high resolution the basic unit of measurement (the voxel) can contain signals arising from averaging the activity over several thousand individual neurons. Multiple known issues complicate fMRI measurements of brain activity, not the least of which is the fact that the blood oxygen level dependent (BOLD) signal is a delayed vascular response to neuronal activity, rather than a direct measurement of that activity. The fact that blood flow and volume changes, while tightly coupled to neural activity (in particular the local field potential (LFP) [3], see Glossary), are secondary and slow responses to changes in neural activity adds to the difficulty in interpreting BOLD signals. Other issues that must be dealt with in fMRI data analysis come from known sources of noise such as non-neuronal fluctuations due to cardiac and respiratory processes [4]. These methodological issues can in principle be addressed with advances in data acquisition and processing that have been demonstrated to successfully reduce the impact of non-neuronal factors on fMRI measurements [5,6]. Still, much of the noise in fMRI data is so overlapping in characteristics with the signal that it can be difficult to separate the two [7,8].
The other major factor affecting our ability to separate brain signal from noise is theoretical, and more difficult to address. The issue is that in the past we have often been mistaken in our assessment of what aspects of the measured signal constitute ‘signal of interest’, from the standpoint of brain function [9]. That is, without an a priori understanding of how information is coded in the brain at multiple spatial and temporal scales, scientists have often made the simplifying assumption that spontaneous (e.g. intrinsic, or non-evoked) activity recorded in the absence of external stimulation is not functionally relevant and can safely be ignored. This assumption, however, has been challenged again and again by electrophysiology, computational modeling, and more recently functional neuroimaging studies demonstrating that spontaneous neural activity is structured across multiple spatiotemporal scales [10,11] and influences evoked responses [12-14].
In functional neuroimaging in particular, the data processing steps undertaken to mitigate the effects of known sources of noise on the measured BOLD signal can inadvertently remove signal of interest. This point is illustrated by recent work exploring the functional significance of the global signal, which is often treated as a source of noise in resting state functional MRI data analysis [15]. When the global signal is probed from the standpoint of treating it as ‘signal’ rather than ‘noise’, it becomes evident that it contains relevant information with respect to individual differences in behavior [16] and psychopathology [17].
Sources of noise in the nervous system at the cellular level such as electrical noise due to channel noise (random opening and closing of ion channels) and synaptic noise due to trial-to-trial variability in postsynaptic responses have been reviewed in other work [1,18]. Here we explore the phenomenon of spontaneous neural activity observed at the micro- and macro-scales to inform the question of whether to categorize such activity as signal or noise. We then leverage these insights to suggest ways in which contemporary debates in human functional neuroimaging can benefit from theoretical frameworks that shift the focus away from evoked/intrinsic neural activity dichotomies [19]. We suggest that several promising alternative approaches for neuroimaging data analysis already exist that require fewer assumptions regarding the separability of signal and noise. In addition, we highlight how computational models conceptualizing the brain as a complex nonlinear dynamical system [20] can provide formal descriptions of noise (Box 1).
Box 1. Dynamic systems accounts of noise in neuroscience.
In computational approaches to brain function, dynamic models are used to understand large-scale neural systems by describing how they evolve in time from a given state to a future state. A neuronal system with N state variables (where variables could be membrane potentials of neurons, neuronal firing rates, etc.) can be represented as a vector (X(t)={x1(t),x2(t),… xn(t)}) of length N. In a noise-free system, these variables would evolve according to an ordinary differential equation with a set of parameters, such as the concentration of acetylcholine, that tune the system. Random fluctuations at the level of the system (“state noise”) can be introduced by the addition of a scaled random (stochastic) term, which turns the equations into a stochastic differential equation. The state noise term can be purely additive, or it might be multiplied by the vector X (i.e. state-dependent noise). If the model describes the activity of a large neural population, this noise term could capture the random chatter of individual neurons. The system can also be autonomous or subject to structured external inputs, such as sensory perturbations.
Neural states X are not directly observable, but only apparent through an observation or measurement function, such as the hemodynamic response function in fMRI. These map the states of the vector X into a scalar measured signal S in the j-th observed voxel. We never have a pure measurement, but only one that is corrupted by the noise of the measurement device. ′ denotes that this is the corrupted signal which, or the composite of the "true" signal S and the measurement noise [106,107]. In fMRI, measurement is corrupted by at least two forms of noise: the non-neuronal contributions to the measured signal (such as respiration) and head motion (Table 1). As a complicating factor, the noise in the measurement channel might be correlated with the underlying neural states, either as a causal outcome (such as reflecting respiratory drive), or a cause (such as physiological monitoring of blood oxygenation in homeostatic brain centers). In this case, simple linear regression of measurement noise terms can diminish or confound estimation of the true neuronal state signal.
In sum, formal computational treatments dissect "noise" into quite distinct causal effects, and can potentially disambiguate various contributions of system effects, stochastic fluctuations intrinsic to the system, non-neuronal contributions, and extraneous artifacts and measurement noise [105,108,109].
Spontaneous single-unit and population activity influence function
Spontaneous activity refers to the firing of neurons in the absence of sensory input. This non-evoked or stimulus-independent activity represents a fundamental property of nervous systems. Electrophysiological studies of the intrinsic properties of single neurons and their relation to global function indicate that spontaneous firing of neurons underlies network oscillations [21]. Spontaneous cortical activity patterns can consist of “UP” states of persistent, organized network activity alternating with “DOWN” states of generalized neural silence [22]. To answer the question of what gives rise to this spontaneous activity, intrinsic oscillatory activity of mammalian neurons has been examined in vitro using brain slices. From observations of the spontaneous firing of inferior olive cells, researchers found that these neurons have ionic conductances which endow them with electrical autorhythmicity capable of generating synchronous firing in large populations of cells. This foundational work illustrates how the intrinsic activity of neurons can produce oscillations that may provide internal context to sensory input, and affect global functional states such as attention and consciousness [23].
Simultaneous recording of single-unit neuronal activity and real-time optical imaging in anesthetized cats permits the examination of the effects of spontaneous single neuron activity on the network in which it is embedded. Such studies reveal that the firing rate of a single spontaneously active neuron depends on the spatial patterns of ongoing population activity in a surrounding cortical area [24]. The fact that the spontaneous firing of single neurons is tightly linked to the cortical networks in which they are embedded highlights how stochastic activity of neighboring neurons is an important feature to consider when interpreting single-unit recording data.
Several lines of evidence suggest that spontaneous activity in cortical populations is highly structured in space and time, the spatio-temporal structure of spontaneous activity is linked to the underlying connectivity of the cortical network, and spontaneous cortical activity interacts with external stimulation to generate responses to individual presentations of a stimulus [25]. Furthermore, multiple investigations provide evidence that cortical responses evoked by natural stimulation are similar to those observed spontaneously [26,27]. Recordings from Purkinje neurons, vestibular nucleus neurons, and neurons in the suprachiasmatic nucleus demonstrate how spontaneous firing plays a central role in transforming synaptic input into spike output and encoding plasticity in neural circuits [28]. Taken together, these observations lead to the conclusion that far from being uninteresting, ongoing spontaneous cortical activity may represent a continuous prediction signal that interacts with incoming input to generate updated representations of the world [25].
Spontaneous large-scale network activity influences function
The phenomenon of spontaneous or intrinsic brain activity is ubiquitous in neuroscience, detectable at the level of single cells probed with electrophysiological techniques, to large-scale neural networks identifiable with EEG and fMRI (Figure 1). Indeed, the vast majority of the brain’s metabolic resources are devoted to maintaining spontaneous activity, rather than supporting evoked responses [29]. Resting state fluctuations seen in both EEG and fMRI signals were historically treated as noise in some instances. The alpha rhythm (8-13 Hz) was originally described as a “background” rhythm, as it was observed to be strongest in the absence of visual stimulation and attenuated during visual attention tasks. However, findings showing that alpha activity increases systematically with working memory load [30,31] demonstrate the significance of alpha activity for cognitive function.
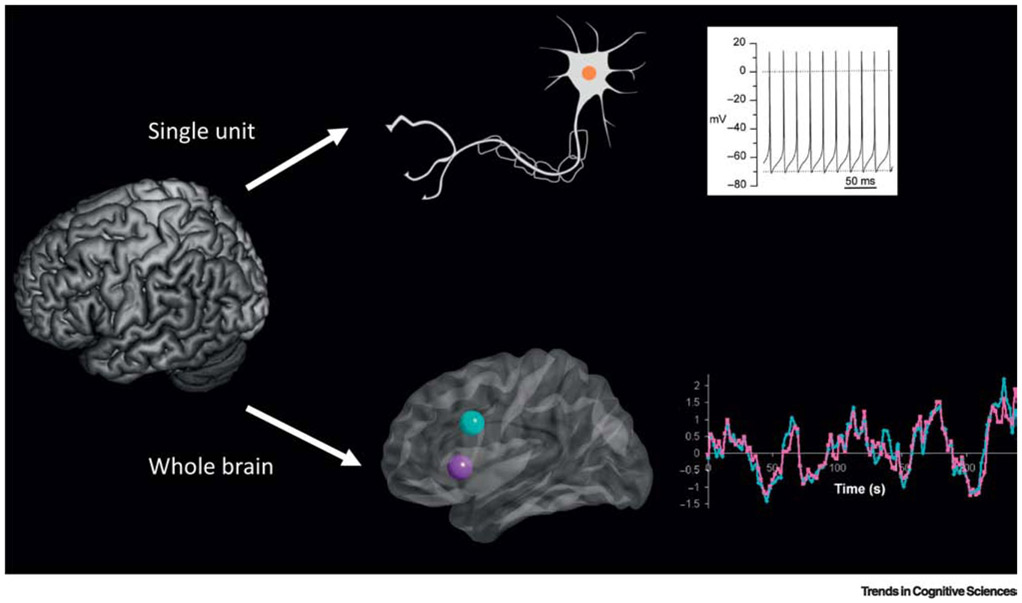
Figure 1. Spontaneous (intrinsic, non-evoked) activity is observed in the brain at multiple spatio-temporal scales.
Top: Spontaneous action potentials recorded from a neuron bathed in physiological saline (from [110]). Bottom: Functional connectivity measured using resting state fMRI (from [111]). Anatomically separate brain regions [insula (purple), and anterior cingulate cortex, light blue] that form known functional networks [salience/mid-cingulo-insular network [44,112]] exhibit spontaneous, low-frequency correlations even in the absence of cognitive task performance.
Spontaneous low-frequency oscillations in fMRI BOLD signal were also once considered less interesting than evoked responses. The common practice of averaging multiple trials during task performance and subtracting ‘active’ from ‘control’ cognitive states rests on the assumption that such averaging reduces the impact of unwanted background fluctuations on the signal of interest - in this case stimulus-evoked brain activation. The idea that fluctuations in the resting brain may be meaningfully structured was first demonstrated in an fMRI study of functional connectivity in the absence of task performance within the motor system [32]. This finding has since been shown to extend to multiple large-scale brain networks including the default mode network [33,34], attention networks [35], and many others [36]. Since the seminal work published in 1995, the field of resting state fMRI has exploded, with upwards of 12,000 publications as of 2020. Despite initial skepticism towards the idea that spontaneous, intrinsic fluctuations delineate functionally coherent systems, subsequent work has indicated that these functional connectomes can serve as fingerprints to identify individuals [37], closely resemble task-activation networks [38,39], and can be used to predict clinical outcomes [40]. Most researchers now agree that so-called resting state networks (RSNs) [41] or intrinsic connectivity networks (ICNs) [42], rather than representing uncorrelated noise, reflect the dynamic capacity of the brain to organize into multiple known functional network configurations [43,44]. It should be noted that despite increasing recognition of the functional significance of ICNs, in some contexts (depending on the paradigm), it may still be desirable to minimize the influence of low-frequency fluctuations.
Evidence consistent with a neuronal origin for spontaneous large-scale brain network fluctuations comes from electrophysiology. Examination of fluctuations in band-limited power of LFP signals recorded from multiple electrodes in the visual cortex of awake monkeys provides evidence for widespread coherent activity fluctuations (large amplitude in low frequencies < 0.1hz), lending further credence to the claim that such signals are of neuronal origin and do not simply constitute artifacts [45]. While there is now widespread agreement within the neuroimaging community that resting state functional connectivity can characterize meaningful aspects of the human connectome [46], it is important to remember that not long ago, these spontaneous fluctuations were often treated as merely sources of noise.
Variability of neuronal spiking and BOLD signal variability influence function
Yet another example of a scenario where what was once considered noise is now being reconsidered comes from the literature examining variability of neuronal responses. It has long been noted that cortical neurons show variability in the number and temporal distribution of spikes. It is thought that irregular interspike intervals allow neurons to achieve a dynamic range of responses. Irregularity of spike discharge solves the problem of synaptic integration in the high-input regime, where multiple excitatory inputs arrive for every spike that is output. Thus the “cost” of achieving a dynamic range of responses is noise, or variability [47]. Variability in interspike intervals can enhance sensitivity to weak signals and increase the fidelity of transmission for high-frequency signals [48]. The phenomenon that a subthreshold input has a better chance of being detected when noise is added is known as stochastic resonance [49]. The stochastic resonance phenomenon demonstrates how noise can be helpful in nonlinear systems [18], and strengthens the case for considering the brain from a dynamical systems perspective.
While spike threshold and pattern variability were traditionally viewed as noise, the alternative hypothesis that has come to dominate is that the ‘noise’ - the mismatch between the physical input and the neuronal response - reflects self-organized patterns in the brain [50]. Some have suggested that aspects of neuronal responses that appear to be noise, such as variability, may in fact be fundamental to information propagation or representation [1,51]. Biophysical models of neocortical microcircuitry suggest that stochastic neurotransmitter release can generate this variability [52].
Signal variability can also be observed at the level of BOLD fMRI. Measures of BOLD signal variability including standard deviation, mean square successive difference (MSSD), and multiscale entropy (MSE) [53] have been shown to capture important age-related effects [54,55]. There is now a burgeoning literature linking patterns of brain signal variability to behavioral performance in neurotypical populations [56,57], as well as clinical symptom severity in neurodevelopmental disorders [58,59]. These findings are linked to observations from computational models suggesting that variability enables the exploration of, and reflects, the brain’s dynamic repertoire. A dynamic system computationally solved in the presence of noise explores a greater range of network configurations [60]. This variability would allow the brain to generate predictions about the likely network configuration that would be optimal for a given input [61]. Taken together, these studies highlight how once again, an aspect of neural activity that was once believed to be of little consequence for function reveals its significance upon closer examination.
Global signal reflects function
In the early days of resting state fMRI research, the global signal was considered to mainly contain physiological noise, and was consequently removed from fMRI data via regression prior to analyses [33]. The rationale for removing the global signal is that it is thought to be dominated by fluctuations of non-neuronal origin, including physiological, hardware, and motion artifacts [15]. Although the consequences of global signal removal are more readily apparent in resting state fMRI data analysis [62], the discussion surrounding whether variations that are present in much of the brain should be considered nuisance effects to be eliminated predates resting state fMRI. In early task-based fMRI work from the 1990s, discussions of whether or not to include global signal covariates in data analysis pipelines highlighted the important point that the global signal could be significantly correlated with a given experimental paradigm [63].
Removal of the global signal as a fMRI preprocessing step significantly mitigates artifacts from a variety of sources [15,64]. However, the wisdom of this practice has been called into question [65,66] in light of evidence from electrophysiological recordings in macaques [67] and magnetic resonance spectroscopy studies in rodents [68] that the global signal also includes neural signals. The global signal was further demonstrated to have a direct neuronal source by pharmacological studies in macaques [69]. Using simultaneous resting state fMRI and measurements of calcium activity in awake rats, significant correspondence was recently demonstrated between the global signal measured with non-invasive neuroimaging and neural spiking activity [70], further bolstering the claim that the global signal contains significant neural components and not just noise [71].
Studies in humans using EEG and fMRI demonstrate that the global signal is related to vigilance [72] and arousal [73]. The global signal appears to exhibit a distinct topographic organization that can vary across individuals [74] and even time of day [75]. A recent study found evidence for behavioral relevance of the global signal in a sample of over 1000 healthy young adults. The first principal component of the global signal resembled the canonical lateral frontoparietal control network [44], and individual differences in global signal topography were significantly related to positive and negative life outcomes and psychological function [16]. These findings again illustrate how an aspect of measured brain activity that was once considered ‘noise’ is being reconsidered in light of its functional relevance.
The question of whether removal of the global signal amounts to throwing out the proverbial baby with the bathwater is difficult to resolve using fMRI, where the sources of the measured signal are mixed and potentially ambiguous [66]. A recent study conducted using an information-theoretic framework combined computational modeling (simulated resting state fMRI data) and calcium and hemodynamic recording in mice to address the question of what information is represented in the global signal. Using partial information decomposition, the authors disambiguate the unique and joint dynamics of the global signal and vessel BOLD signal, which represents non-neuronal physiological oscillations considered to be artifactual. Simulations demonstrated that differences in blood arrival time can explain how global signal and vessel BOLD signal affect dynamics of cortical areas, and comparison with results from calcium recordings confirmed the presence of vascular effects in the hemodynamic measurements [76]. This study illustrates the possibility of alternatives to removal of the global signal, which are indeed necessary in light of evidence of its partial neuronal origin. Several additional methods are available as alternatives to global signal regression, including spatial and temporal independent component analysis (ICA), modeling and removing non-neuronal physiological fluctuations, and others [77-80].
Quasi-periodic patterns (QPPs) represent reproducible patterns of large-scale brain dynamics that are thought to share a common mechanism with infraslow LFPs [81]. QPPs are repeated spatiotemporal patterns, exhibiting alternating high and low activity in particular brain areas and propagating along the cortex, that strongly coincide with specific phases of the global signal and likely contribute to it [82]. Interestingly, participant motion is not a major contributor to QPPs, and while they are influenced by physiological noise, this type of noise is not the primary source of QPPs [83]. While the functional significance of these dynamic patterns is currently unknown, QPP-derived metrics have been shown to improve classification of Alzheimer’s disease in a mouse model compared with conventional functional connectivity measures [84]. Altered infraslow network dynamics are also observed in mice with mutations that recapitulate a major genetic risk factor for autism spectrum disorder (ASD) [82]. Some have speculated that QPPs reflect spontaneous haemodynamic events [85], and have posited a role for them in attention and vigilance [83]. The QPP phenomenon is another illustration of the need for caution when making analytic decisions regarding what aspects of the neural signal to interpret and what aspects to discard.
Concluding remarks and future perspectives
The historical perspective outlined here highlights how the facets of neural activity we consider to be contributing to ‘noise’ today might in fact turn out to be signals of interest as methodological and theoretical innovations permit novel conceptualizations of brain function. This view has several implications for functional neuroimaging. In particular, consideration of the inseparability of signal and noise in the brain suggests that one potential way to move forward is to adopt approaches that consider whole brain “contexts” [86] and do not explicitly impose distinctions or separations between evoked and spontaneous or intrinsic brain activity.
We have previously argued for an alternative to consideration of separable evoked and intrinsic components of neural responses, as an evoked/intrinsic dichotomy necessarily imposes the need for unambiguous separation of signal and noise that is not feasible for the reasons outlined thus far. Instead, one might view the brain’s spatiotemporal dynamics as reflecting the enaction of a system of mutual constraints to move in and out of appropriate functional configurations in the face of task demands [19]. This view rests on the notion of neural synergies, or functional groupings of neural regions that are temporarily constrained to act as a coherent unit [87]. Studying the spatiotemporal patterns of neural activity across spontaneous (‘noisy’) and task-evoked states can provide a more comprehensive picture of brain function. This view is consistent with a larger paradigm shift in neuroscience ushered in by the introduction of dynamical systems theory [88], an area of mathematics in which the behavior of dynamical systems is described using differential equations. Viewed as a nonlinear dynamical system, brain activity is modulated in a manner that is determined by factors such as previous neural activity [89]. That is, rather than conceiving of brain activity as composed of distinct intrinsic and evoked processes, one can conceptualize brain activity in terms of its trajectory in multidimensional space before and after stimulus processing. Analysis of fMRI data within this framework has led to the surprising finding that spontaneous and evoked brain activity negatively interact, and the space occupied by the set of potential cortical activity trajectories shrinks following stimulus onset [90]. The finding that prestimulus and poststimulus activity interact in counterintuitive ways is not consistent with linear superposition, and further challenges the notion that one can readily separate brain signal from noise.
The challenges associated with separating signal from noise are formidable, particularly in fMRI where there is significant overlap between these components in the observed data. However, this issue can potentially be side-stepped by some model-free approaches to data analysis that do not rely on complex preprocessing pipelines and the assumptions that accompany them [91]. One methodological approach for circumventing the issue of separating signal from noise is inter-subject correlation (ISC) and its variants, including inter-subject phase synchronization (ISPC) [92] (Figure 2). First applied to fMRI data acquired during movie viewing [93], this approach has since been extended to other experimental paradigms in cognitive [94] and network [95,96] neuroscience. The inter-subject synchronization approach permits the assessment of dynamic task-related brain networks without the need for assumptions regarding the nature and form of brain responses or the specification of a ‘baseline’ condition. The only assumption this approach relies on is that brain regions exposed to the same stimulus will produce similar or conserved temporal dynamics across subjects. This exploratory approach is particularly effective for moving beyond the cognitive subtraction methodology in experimental paradigms for which assumptions of the conventional general linear model may be violated [97]. It also appears to be sensitive to tracking trait-level phenomenon, rendering it a promising means for probing individual differences [98]. There has been growing advocacy for going beyond trial-based paradigms and towards more ecologically valid approaches including ISC and ISPC. In particular, measurements of continuous brain activity and behavioral outputs have the potential to capture elements typically left out of conventional approaches as unexplained variance or noise [99] (see Outstanding Questions).
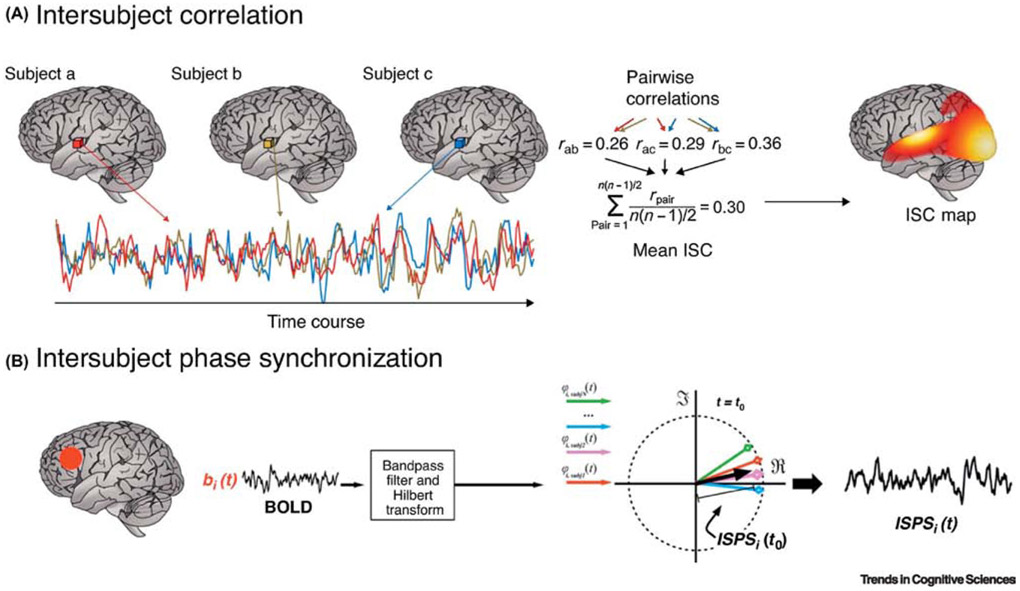
Figure 2. Analysis of inter-subject correlation of brain activity does not require a prior separation of signal and noise.
A. Inter-subject correlation (ISC) is a model-free analysis technique that requires the assumption that task-responsive brain regions will exhibit common patterns of responses (eg. changes in signal amplitude) across participants. In ISC, voxelwise time courses are correlated across all subjects, and averaged voxelwise correlations are used to create a map that represents brain areas maximally synchronized over time. B. In inter-subject phase synchronization (ISPS), a phase representation of each subject’s timeseries is created [94]. Mean voxelwise phase-based similarities are then computed across subjects and used to create an ISPS map (from [92]).
Outstanding Questions.
How can computational models and simulated data be leveraged to better understand the relative contributions of neural activity and artifacts to the global signal in fMRI?
How can multimodal neuroimaging potentially contribute to the question of what should be considered noise?
What are the tradeoffs to consider when denoising data? Are there specific circumstances in which aggressive denoising should be encouraged or discouraged?
How can novel data analytic approaches that are more agnostic to the question of separating signal and noise be developed?
Are there better ways of conceptualizing noise in neuroscience? For example, instead of the term ‘noise’ can we use the terms ‘explained’ and ‘unexplained’ sources of signal?
Large-scale computational models of the brain have the potential to provide mechanistic explanations of neural processes, while also considering intrinsic noise. One such model exploring synaptic activity of single nodes and simulated BOLD dynamics was used to demonstrate that the entropy of stimulus-evoked activity is lower than the entropy during spontaneous activity (Figure 3). The authors propose that higher entropy at rest represents a larger repertoire of potential brain states, whereas lower entropy in a stimulus-evoked brain state permits greater fidelity of information transmission [100].
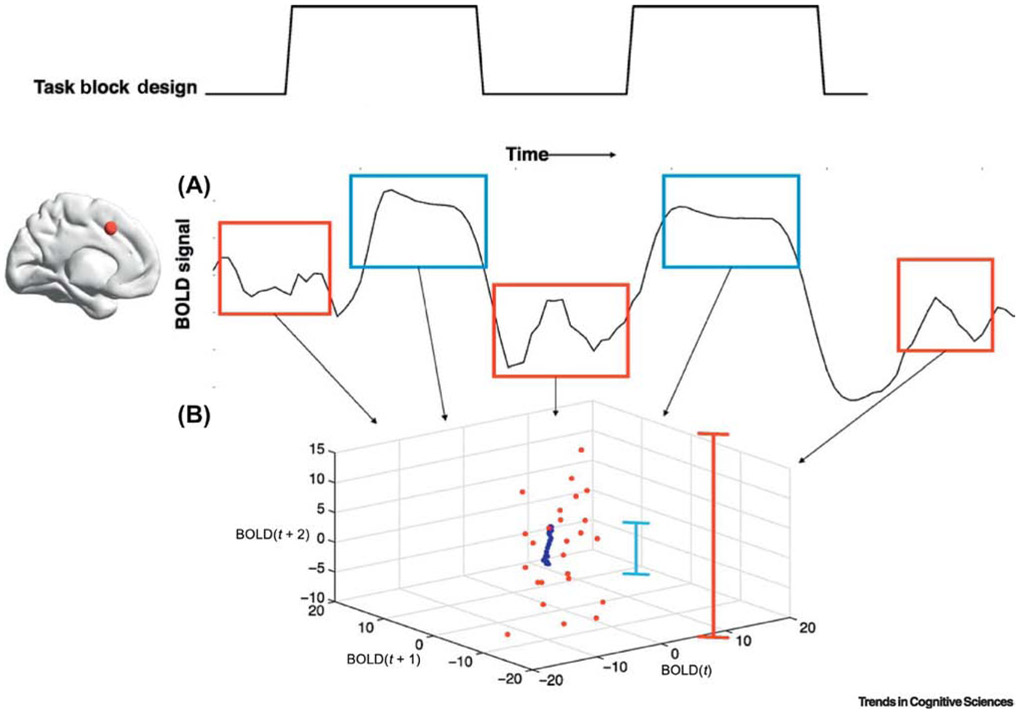
Figure 3. Modeling of cortical activity space in rest and task conditions.
A. The BOLD timeseries of a brain region exhibits increases in signal amplitude in response to a stimulus condition during task performance (blue) compared with a resting condition (red). B. BOLD signal amplitude is plotted at three time points (BOLD(t), BOLD(t+1), and BOLD (t+2). The trajectory of the brain region tightens during task performance periods and expands during resting conditions (indicated by length of the blue and red lines, respectively) [19]. This illustration of how one might conceptualize BOLD activity in terms of its trajectory in multidimensional space before and after stimulus processing is similar to computational approaches using simulated synaptic activity of brain regions to illustrate that in resting conditions, the network explores a larger volume of state space than it does in task conditions [100] (from [19], reprinted courtesy of The MIT Press).
Other methods that provide promising avenues for describing task-related brain activity in ways that make fewer assumptions about noise include approaches that characterize whole-brain states and network functional connectivity trajectories [101]. Approaches that utilize state-space and hidden Markov models [102], and applications of dynamical systems analysis [103,104] are other examples that have the potential to be used in an agnostic manner with respect to evoked/intrinsic divides.
If we are to accept the premise that the brain is best conceptualized as a nonlinear dynamical system, then increasing the sophistication of methods for modelling these dynamics will be the goal for future work. Spontaneous neural activity, from the micro- to macro-scale, is one aspect of brain function that is increasingly moving from the realm of’noise’ to signal. The challenge going forward will be clearly modeling and removing true noise and artifacts, while giving spontaneous neural activity due consideration. The most promising means for striking a balance between information extraction and over interpretation in the study of neural activity will involve integration using multiscale computational models [105]. As we continue to embrace the idea that there may be untapped information in spontaneous neural activity, perhaps we should not be so hasty in future efforts to separate brain signal from noise.
Highlights.
Separating neural signal from noise is methodologically and theoretically challenging.
Spontaneous activity can be observed at the level of individual neurons and at the level of large-scale brain networks.
Spontaneous activity and signal variability used to be considered noise, but both are now known to critically contribute to brain function.
Theoretical frameworks and data analytic approaches that focus on trajectories of whole-brain activity and do not rely on evoked/intrinsic activity dichotomies are promising.
Acknowledgments:
This work was supported by grants from the Canadian Institute for Advanced Research, a Gabelli Senior Scholar Award from the University of Miami, and R01MH107549 from the National Institute of Mental Health to LQU. The author would like to thank Michael Breakspear for thoughtful feedback on the topic of computational treatments of noise in neuroscience.
Glossary
- Global signal:
In fMRI data, the average value of all whole brain (or gray matter) signals. Global signal is sometimes removed from fMRI data prior to functional connectivity analysis in order to remove noise.
- Inter-subject correlation:
Inter-subject correlation (ISC) is a model-free approach for examining task-driven brain responses. This type of analysis assumes that a brain region entrains to a stimulus in a common way across participants. Inter-subject correlation amounts to conducting a functional connectivity analysis across multiple brains, rather than within one brain. Inter-subject phase synchronization (ISPC) is a variant of ISC in which phase representations of individual subject’s timeseries are used to identify groupwise synchronization estimates.
- Linear superposition:
The linear superposition assumption posits that spontaneous and evoked brain activity sum linearly. This assumption is challenged by studies demonstrating unexpected interactions between intrinsic and evoked brain activity.
- Local field potential:
The local field potential (LFP) represents the sum of excitatory and inhibitory dendritic potential recorded from a population of neurons.
- Mean squared successive difference:
Mean squared successive difference (MSSD) is used as an estimate of variability that is insensitive to gradual shifts in the mean. MSSD is calculated by taking the sum of the differences between consecutive observations squared, taking the mean of that sum, and dividing by the sample size minus one.
- Multiscale entropy:
Multiscale entropy (MSE) is a measure of the complexity of signals. It quantifies temporal predictability, and is often used as an index of variability of neural time series data.
- Nonlinear dynamical system:
A dynamical system evolves with time over a state space (which is the set of all possible states) according to a fixed rule. In a nonlinear system, the change of the output is not proportional to the change of the input, giving rise to behavior that can be unpredictable.
- Partial information decomposition:
Partial information decomposition (PID) permits the investigation of the joint effect of several driver variables over one or more targets. PID computes terms of information transfer at different time scales, and can decompose the joint and unique information being shared between drivers and target processes.
- Real-time optical imaging:
Real-time optical imaging uses voltage-sensitive dyes to image membrane potential changes of neuronal populations, providing a real-time view of neuronal activity spread.
- Resting state networks and intrinsic connectivity networks:
Resting state networks (RSNs) or intrinsic connectivity networks (ICNs) can be delineated using resting state fMRI data. RSNs/ICNs are spontaneous, coherent, low-frequency fluctuations observed across the entire brain, and bear resemblance to known functional large-scale brain networks.
- Stochastic resonance:
In non-linear systems, noise can be helpful for increasing the probability that the threshold for signal transmission is reached. Stochastic resonance is a phenomenon by which a signal that is initially too weak to be detected can be enhanced by a certain level of noise.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Faisal AA et al. (2008) Noise in the nervous system. Nat. Rev. Neurosci 9, 292–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones TB et al. (2008) Integration of motion correction and physiological noise regression in fMRI. Neuroimage 42, 582–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Logothetis NK et al. (2001) Neurophysiological investigation of the basis of the fMRI signal. Nature 412, 150–157 [DOI] [PubMed] [Google Scholar]
- 4.Murphy K et al. (2013) Resting-state fMRI confounds and cleanup. Neuroimage 80, 349–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Behzadi Y et al. (2007) A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang C and Glover GH (2009) Effects of model-based physiological noise correction on default mode network anti-correlations and correlations. Neuroimage 47, 1448–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bianciardi M et al. (2009) Sources of functional magnetic resonance imaging signal fluctuations in the human brain at rest: a 7 T study. Magn. Reson. Imaging 27, 1019–1029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mateo C et al. Entrainment of Arteriole Vasomotor Fluctuations by Neural Activity Is a Basis of Blood-Oxygenation-Level-Dependent “Resting-State” Connectivity. , Neuron, 96 (2017), 936–948.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huk AC and Hart E Parsing signal and noise in the brain. , Science, 364 19-April-(2019), 236–237 [DOI] [PubMed] [Google Scholar]
- 10.Fox MD and Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 8, 700–711 [DOI] [PubMed] [Google Scholar]
- 11.Foster BL et al. Spontaneous Neural Dynamics and Multi-scale Network Organization. , Frontiers in Systems Neuroscience, 10 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nierhaus T et al. (2009) Background and evoked activity and their interaction in the human brain. Magn. Reson. Imaging 27, 1140–1150 [DOI] [PubMed] [Google Scholar]
- 13.Sadaghiani S et al. (2015) Ongoing dynamics in large-scale functional connectivity predict perception. Proc. Natl Acad Sci. U. S. A 112, 8463–8468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Podvalny E et al. A dual role of prestimulus spontaneous neural activity in visual object recognition. , Nature Communications, 10 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Power JD et al. (2017) Sources and implications of whole-brain fMRI signals in humans. Neuroimage 146, 609–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J et al. (2019) Topography and behavioral relevance of the global signal in the human brain. Sci. Rep 9, 14286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang GJ et al. (2017) Altered Global Signal Topography in Schizophrenia. Cereb. Cortex 27, 5156–5169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rusakov DA et al. (2020) Noisy Synaptic Conductance: Bug or a Feature? Trends Neurosci. DOI: 10.1016/j.tins.2020.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bolt T et al. (2017) Beyond the evoked/intrinsic neural process dichotomy. Network Neuroscience In Press, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Breakspear M (2017) Dynamic models of large-scale brain activity. Nat. Neurosci 20, 340–352 [DOI] [PubMed] [Google Scholar]
- 21.Steriade M and Llinás RR (1988) The functional states of the thalamus and the associated neuronal interplay. Physiol Rev. 68, 649–742 [DOI] [PubMed] [Google Scholar]
- 22.Luczak A et al. Sequential structure of neocortical spontaneous activity in vivo. Proceedings of the National Academy of Sciences, 104 (2007), 347–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Llinas RR (1988) The intrinsic electrophysiological properties of mammalian neurons: insights into central nervous system function. Science 242, 1654–1664 [DOI] [PubMed] [Google Scholar]
- 24.Tsodyks M et al. (1999) Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science 286, 1943–1946 [DOI] [PubMed] [Google Scholar]
- 25.Ringach DL (2009) Spontaneous and driven cortical activity: implications for computation. Carr. Opin. Neurobiol. 19, 439–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luczak A et al. (2009) Spontaneous events outline the realm of possible sensory responses in neocortical populations. Neuron 62, 413–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh G et al. Topological analysis of population activity in visual cortex. , Journal of Vision, 8 (2008), 11–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Häusser M et al. (2004) The beat goes on: spontaneous firing in mammalian neuronal microcircuits. J. Neurosci 24, 9215–9219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raichle ME (2009) A paradigm shift in functional brain imaging. J. Neurosci 29, 12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen O et al. (2002) Oscillations in the Alpha Band (9–12 Hz) Increase with Memory Load during Retention in a Short-term Memory Task. Cereb. Cortex 12, 877–882 [DOI] [PubMed] [Google Scholar]
- 31.Tuladhar AM et al. Parieto-occipital sources account for the increase in alpha activity with working memory load. , Human Brain Mapping, 28 (2007), 785–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biswal B et al. (1995) Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med 34, 537–541 [DOI] [PubMed] [Google Scholar]
- 33.Fox MD et al. (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U. S. A 102, 9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greicius MD et al. (2003) Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A 100, 253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fox MD et al. (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A 103, 10046–10051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Damoiseaux JS et al. (2006) Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A 103, 13848–13853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finn ES et al. (2015) Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 18, 1664–1671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cole MW et al. (2014) Intrinsic and task-evoked network architectures of the human brain. Neuron 83, 238–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolt T et al. (2017) Correspondence between evoked and intrinsic functional brain network configurations. Hum. Brain Mapp 38, 1992–2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saggar M and Uddin LQ (2019) Pushing the Boundaries of Psychiatric Neuroimaging to Ground Diagnosis in Biology. eNeuro 6, [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luca MD et al. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. , NeuroImage, 29 (2006), 1359–1367 [DOI] [PubMed] [Google Scholar]
- 42.Seeley WW et al. (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci 27, 2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deco G et al. Resting brains never rest: computational insights into potential cognitive architectures. , Trends in Neurosciences, 36 (2013), 268–274 [DOI] [PubMed] [Google Scholar]
- 44.Uddin LQ et al. Towards a Universal Taxonomy of Macro-scale Functional Human Brain Networks. , Brain Topography, 32 (2019), 926–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leopold DA et al. (2003) Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb. Cortex 13, 422–433 [DOI] [PubMed] [Google Scholar]
- 46.Biswal BB et al. (2010) Toward discovery science of human brain function. Proc. Natl. Acad. Sci. U. S. A 107, 4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shadlen MN and Newsome WT (1998) The variable discharge of cortical neurons: implications for connectivity, computation, and information coding. J. Neurosci 18, 3870–3896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stein RB et al. (2005) Neuronal variability: noise or part of the signal? Nat. Rev. Neurosci 6, 389–397 [DOI] [PubMed] [Google Scholar]
- 49.Lu L et al. (2019) Effects of noise and synaptic weight on propagation of subthreshold excitatory postsynaptic current signal in a feed-forward neural network. Nonlinear Dyn. 95, 1673–1686 [Google Scholar]
- 50.Buzsáki G (2004) Large-scale recording of neuronal ensembles. Nat. Neurosci 7, 446–451 [DOI] [PubMed] [Google Scholar]
- 51.Ermentrout GB et al. (2008) Reliability, synchrony and noise. Trends Neurosci 31, 428–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nolte M et al. (2019) Cortical reliability amid noise and chaos. Nat. Conmrun 10, 3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Z et al. (2014) Brain entropy mapping using fMRI. PLoS One 9, e89948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Garrett DD et al. (2010) Blood oxygen level-dependent signal variability is more than just noise. J. Neurosci 30, 4914–4921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nomi JS et al. (2017) Moment-to-Moment BOLD Signal Variability Reflects Regional Changes in Neural Flexibility across the Lifespan. J. Neurosci 37, 5539–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garrett DD et al. (2011) The importance of being variable. J. Neurosci 31, 4496–4503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Armbruster-Genç DJN et al. (2016) Brain Signal Variability Differentially Affects Cognitive Flexibility and Cognitive Stability. J. Neurosci 36, 3978–3987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nomi JS et al. (2018) Resting-State Brain Signal Variability in Prefrontal Cortex Is Associated With ADHD Symptom Severity in Children. Front. Hum. Neurosci 12, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Easson AK and McIntosh AR BOLD signal variability and complexity in children and adolescents with and without autism spectrum disorder. , Developmental Cognitive Neuroscience, 36 (2019), 100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Deco G et al. (2011) Emerging concepts for the dynamical organization of resting-state activity in the brain. Nat. Rev. Neurosci 12, 43–56 [DOI] [PubMed] [Google Scholar]
- 61.McIntosh AR et al. (2010) The development of a noisy brain. Arch. Ital. Biol 148, 323–337 [PubMed] [Google Scholar]
- 62.Murphy K et al. (2009) The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage 44, 893–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Aguirre GK et al. (1998) The inferential impact of global signal covariates in functional neuroimaging analyses. Neuroimage 8, 302–306 [DOI] [PubMed] [Google Scholar]
- 64.Ciric R et al. (2018) Mitigating head motion artifact in functional connectivity MRI. Nat. Protoc 13, 2801–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Liu TT et al. (2017) The global signal in fMRI: Nuisance or Information? Neuroimage 150, 213–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Uddin LQ (2017) Mixed Signals: On Separating Brain Signal from Noise. Trends Cogti. Sci 21, 405–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scholvinck ML et al. (2010) Neural basis of global resting-state fMRI activity. Proc. Natl. Acad. Sci. U. S. A 107, 10238–10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hyder F and Rothman DL Neuronal correlate of BOLD signal fluctuations at rest: err on the side of the baseline. , Proceedings of the National Academy of Sciences of the United States of America, 107 15-June-(2010), 10773–10774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Turchi J et al. (2018) The Basal Forebrain Regulates Global Resting-State fMRI Fluctuations. Neuron 97, 940–952.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Y et al. (2020) Global brain signal in awake rats. Brain Struct. Funnct 225, 227–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murphy K and Fox MD (2017) Towards a consensus regarding global signal regression for resting state functional connectivity MRI. Neuroimage 154, 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wong CW et al. (2013) The amplitude of the resting-state fMRI global signal is related to EEG vigilance measures. Neuroimage 83, 983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu X et al. (2018) Subcortical evidence for a contribution of arousal to fMRI studies of brain activity. Nat. Commun 9, 395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Billings J and Keilholz S (2018) The Not-So-Global Blood Oxygen Level-Dependent Signal. Brain Connect. 8, 121–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Orban C et al. (2020) Time of day is associated with paradoxical reductions in global signal fluctuation and functional connectivity. PLoS Biol. 18, e3 000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Colenbier N et al. 12-April-(2019), Disambiguating the role of blood flow and global signal with Partial Information Decomposition. , bioRxiv, 596247. [DOI] [PubMed] [Google Scholar]
- 77.He H and Liu TT (2012) A geometric view of global signal confounds in resting-state functional MRI. Neuroimage 59, 2339–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glasser MF et al. (2018) Using temporal ICA to selectively remove global noise while preserving global signal in functional MRI data. Neuroimage 181, 692–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pruim RHR et al. (2015) ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage 112, 267–277 [DOI] [PubMed] [Google Scholar]
- 80.Aquino KM et al. (2020) Identifying and removing widespread signal deflections from fMRI data: Rethinking the global signal regression problem. Neuroimage 212, 116614. [DOI] [PubMed] [Google Scholar]
- 81.Thompson GJ et al. (2014) Quasi-periodic patterns (QPP): large-scale dynamics in resting state fMRI that correlate with local infraslow electrical activity. Neuroimage 84, 1018–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gutierrez-Barragan D et al. (2019) Infraslow State Fluctuations Govern Spontaneous fMRI Network Dynamics. Carr. Biol 29, 2295–2306.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yousefi B et al. (2018) Quasi-periodic patterns of intrinsic brain activity in individuals and their relationship to global signal. Neuroimage 167, 297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Belloy ME et al. Quasi-Periodic Patterns of Neural Activity improve Classification of Alzheimer’s Disease in Mice. , Scientific Reports, 8 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Belloy ME et al. (2018) Dynamic resting state fMRI analysis in mice reveals a set of Quasi-Periodic Patterns and illustrates their relationship with the global signal. Neuroimage 180, 463–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McIntosh AR (2004) Contexts and catalysts: a resolution of the localization and integration of function in the brain. Neuroinformatics 2, 175–182 [DOI] [PubMed] [Google Scholar]
- 87.Kelso JAS (2009) Synergies: atoms of brain and behavior. Adv. Exp. Med. Biol 629, 83–91 [DOI] [PubMed] [Google Scholar]
- 88.McKenna TM et al. (1994) The brain as a dynamic physical system. Neuroscience 60, 587–605 [DOI] [PubMed] [Google Scholar]
- 89.Buonomano DV and Maass W (2009) State-dependent computations: spatiotemporal processing in cortical networks. Nat. Rev. Neurosci 10, 113–125 [DOI] [PubMed] [Google Scholar]
- 90.He BJ (2013) Spontaneous and task-evoked brain activity negatively interact. J. Neurosci 33, 4672–4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nastase SA et al. (2019) Measuring shared responses across subjects using intersubject correlation. Soc. Cogn. Affect. Neurosci 14, 667–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nummenmaa L et al. Sharing the social world via intersubject neural synchronisation. , Current Opinion in Psychology, 24 (2018) ,7–14 [DOI] [PubMed] [Google Scholar]
- 93.Hasson U et al. (2004) Intersubject synchronization of cortical activity during natural vision. Science 303, 1634–1640 [DOI] [PubMed] [Google Scholar]
- 94.Bolt T et al. (2018) Inter-subject phase synchronization for exploratory analysis of task-fMRI. Neuroimage 176, 477–488 [DOI] [PubMed] [Google Scholar]
- 95.Betzel RF et al. (2020) Temporal fluctuations in the brain’s modular architecture during movie-watching. Neuroimage 213, 116687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bolton TAW et al. (2019) Dynamic Inter-subject Functional Connectivity Reveals Moment-to-Moment Brain Network Configurations Driven by Continuous or Communication Paradigms. J. Vis. Exp DOI: 10.3791/59083 [DOI] [PubMed] [Google Scholar]
- 97.Li J et al. (2019) Inter-subject phase synchronization differentiates neural networks underlying physical pain empathy. bioRxiv at <https://www.biorxiv.org/content/10.1101/841197v1.abstract> [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Finn ES et al. Trait paranoia shapes inter-subject synchrony in brain activity during an ambiguous social narrative. , Nature Communications, 9 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huk A et al. (2018) Beyond Trial-Based Paradigms: Continuous Behavior, Ongoing Neural Activity, and Natural Stimuli. J. Neurosci 38, 7551–7558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ponce-Alvarez A et al. (2015) Task-Driven Activity Reduces the Cortical Activity Space of the Brain: Experiment and Whole-Brain Modeling. PLoS Comput. Biol 11, el 004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ciric R et al. (2017) Contextual connectivity: A framework for understanding the intrinsic dynamic architecture of large-scale functional brain networks. Sci. Rep 7, 6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eavani H et al. (2013) Unsupervised learning of functional network dynamics in resting state fMRI. Inf. Process. Med. Imaging 23, 426–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fagerholm ED et al. (2015) Cascades and cognitive state: focused attention incurs subcritical dynamics. J. Neurosci 35, 4626–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tognoli E and Kelso JA (2014) The metastable brain. Neuron 81, 35–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deco G et al. (2008) The dynamic brain: from spiking neurons to neural masses and cortical fields. PLoS Comput. Biol 4, el000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roberts JA et al. (2017) Clinical Applications of Stochastic Dynamic Models of the Brain, Part I: A Primer. Biol Psychiatry Cogn Neurosci Neuroimaging 2, 216–224 [DOI] [PubMed] [Google Scholar]
- 107.Roberts JA et al. Clinical Applications of Stochastic Dynamic Models of the Brain, Part II: A Review. , Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 2 (2017), 225–234 [DOI] [PubMed] [Google Scholar]
- 108.Breakspear M and Siam CJ (2005) Dynamics of a neural system with a multiscale architecture. Philos. Trans. R. Soc. Lond. B Biol. Sci 360, 1051–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rolls ET and Deco G (2010) The noisy brain: stochastic dynamics as a principle of brain function, 34 Oxford university press; Oxford. [DOI] [PubMed] [Google Scholar]
- 110.Raman IM and Bean BP (1999) Ionic currents underlying spontaneous action potentials in isolated cerebellar Purkinje neurons. J. Neurosci 19, 1663–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fox MD et al. (2007) Intrinsic Fluctuations within Cortical Systems Account for Intertrial Variability in Human Behavior. Neuron 56, 171–184 [DOI] [PubMed] [Google Scholar]
- 112.Uddin LQ (2015) Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci 16, 55–61 [DOI] [PubMed] [Google Scholar]
- 113.Johnson JB (1928) Thermal Agitation of Electricity in Conductors. Phys. Rev 32, 97–109 [Google Scholar]
- 114.Poldrack RA et al. (2011) Handbook of Functional MRI Data Analysisis, Cambridge University Press. [Google Scholar]
- 115.Glover GH et al. (2000) Image-based method for retrospective correction of physiological motion effects in fiVIRI: RETROICOR. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 44, 162–167 [DOI] [PubMed] [Google Scholar]
- 116.Chen K et al. (2020) Resting-State Functional Connectivity: Signal Origins and Analytic Methods. Neuroimaging Clin. N. Am 30, 15–23 [DOI] [PubMed] [Google Scholar]
- 117.Garrett DD et al. (2013) Moment-to-moment brain signal variability: a next frontier in human brain mapping? Neurosci. Biobehav. Rev 37, 610–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gonzalez-Castillo J et al. (2012) Whole-brain, time-locked activation with simple tasks revealed using massive averaging and model-free analysis. Proc. Natl. Acad. Sci. U. S. A 109, 5487–5492 [DOI] [PMC free article] [PubMed] [Google Scholar]