Abstract
Genomic studies have revealed molecular mechanisms involved in the pathogenesis of Burkitt’s lymphoma, including the ID3/TCF3-dependent centroblast gene expression program, tonic PI3K-AKT-mTOR signaling, and deregulation of cell cycle and apoptosis through mutations in cyclin D3, CDKN2A, or TP53. Unfortunately, these advances have not been translated into treatment, which relies on dose-intense cytotoxic chemotherapy. While most patients achieve long-term survival, options for relapsed/refractory disease are lacking, as Burkitt lymphoma is often excluded from clinical trials of novel approaches. The lower-intensity, dose-adjusted EPOCH plus rituximab (DA-EPOCH-R) regimen constitutes a major advance allowing for treatment of older and HIV-positive patients but needs augmentation to better address the central nervous system involvement. Furthermore, DA-EPOCH-R provides a platform for the study of targeted or immunotherapeutic approaches while de-escalating cytotoxic agents and their associated adverse effects. In this review we discuss the epidemiology and molecular genetics of BL, first-line treatment considerations, and potential novel treatment strategies.
Keywords: Burkitt lymphoma, chemotherapy, immunotherapy, molecular cytogenetics, genomics
Introduction
Burkitt lymphoma (BL) is an aggressive mature B-cell lymphoma that occurs in adults and children. Multi-agent chemoimmunotherapy regimens adapted from B-cell acute lymphoblastic leukemia (B-ALL) result in long-term progression-free survival (PFS) for most patients.[1, 2] Nevertheless, treatment-related mortality (TRM) has limited application of high-intensity regimens among older patients, HIV-positive individuals, or in low-resource settings where endemic BL predominates. Recently, approaches aiming at decreasing toxicity while retaining efficacy have gained prominence to overcome these limitations and to reduce chemotherapy exposure in lower-risk disease. Still, about 20–40% of adult patients experience recurrent or refractory BL and lack efficacious salvage options. The past decade brought significant progress in characterizing molecular drivers of BL, yet this knowledge has not been translated into improved therapy. Better understanding of biology in combination with de-escalated chemotherapy provides a platform to develop novel treatments that could further limit reliance on high-intensity cytotoxic agents.
In this paper, we review advances in the epidemiology, molecular genetics, and therapy for sporadic BL. We will discuss currently available first-line regimens with a focus on toxicity and applicability in the context of central nervous system (CNS) involvement. We will then delineate future directions of clinical research.
Epidemiology
BL is categorized into 3 subtypes: endemic, sporadic, and immunodeficiency-associated, which have unique clinical and pathologic characteristics that impact treatment and outcomes. BL was first reported in 1958 by Dennis Burkitt as a “sarcoma of the jaw” among children in Uganda.[3] This endemic BL occurs at median age of 6 years, constituting half of childhood cancers and 90% of lymphomas in sub-Saharan Africa and Papua New Guinea.[2] Endemic BL commonly presents as an abdominal mass with involvement of the jaw, adrenals, and kidneys.[4, 5] It is unvaryingly associated with Epstein-Barr virus (EBV) infection and often with exposure to malaria.[6]
Sporadic BL is most commonly encountered by oncologists in developed countries. In the United States (US), sporadic BL represents ~1% of adult and ~30% of pediatric lymphomas.[7, 8] While the incidence of BL has slightly decreased between 2005 and 2016 (from 0.40 to 0.37 per 100,000 person-years), about 1,200 cases are diagnosed each year, at median age 42, and 2.6 times higher incidence among men than women.[9] Sporadic BL frequently involves extranodal sites (particularly gastrointestinal tract, bone marrow, and CNS), or presents as a mature B-ALL. In contrast to endemic BL, EBV infection is evident in only 20% of cases.
Immunodeficiency-associated BL was described in the 1980s in the context of HIV infection, with incidence 261 times higher than in the HIV-uninfected population.[10] The risk of BL is >3x higher when CD4+ lymphocyte count exceeds 250 cells/mL compared with <50 CD4 cells/mL, suggesting involvement of functional T-cells in the development of BL.[11] EBV is detected in ~30% of cases.[12] As a consequence of better HIV control the incidence of immunodeficiency-associated BL has declined, while its proportional share of HIV-associated lymphomas is increasing.[8] Accounting for prognostic factors, a similar proportion of HIV-negative and HIV-positive patients receiving active chemotherapy (89% vs. 90%, respectively), and their outcomes appear equivalent.[8]
Pathobiology of BL
BL is composed of small, dark, monomorphic B-cells with compact nuclei, scant cytoplasm, and numerous mitoses with a “starry sky” appearance. BL originates from a germinal center B-cell expressing BCL6, CD10, CD20, CD45, and CD79a, but not BCL2 or TdT. High proliferation rate is evidenced by Ki67 expression of ~100%. The characteristic morphology and immunophenotype often allow rapid diagnosis, but testing for genomic lesions is needed to differentiate BL from other high-grade pathologies: Burkitt-like lymphoma with 11q aberration, high-grade B-cell lymphoma (HGBL) with concurrent MYC, BCL2 and/or BCL6 rearrangements, HGBL, not otherwise specified (NOS), and some diffuse large B-cell lymphomas (DLBCL) with MYC rearrangement.[13]
The oncogenic role of MYC
The most important genomic feature of BL is the presence of MYC translocation, first discovered cytogenetically in 1976, with subsequent identification of MYC at a breakpoint alongside IGH in 1982.[14–16] The typical t(8;14)(q24;q32) rearrangement (MYC-IGH) occurs in 80%, with less frequent rearrangements to the light chain loci IGL t(2,8) or IGK t(8;22).[16] Rarely, MYC translocation may be absent in morphologically evident BL, usually resulting from cryptic rearrangements.[17, 18] MYC is an oncogenic transcription factor that promotes growth- and proliferation-oriented metabolism through many effector genes (>10% of all genes).[19] By amplifying the cell’s transcriptional program, MYC prepares DNA replication and division through biomass accumulation, energy production, and progression from G0/G1 to S phases of the cell cycle.[20, 21] In the germinal center, MYC is transiently expressed within the light zone cells destined to move to the highly proliferative centroblast phase (dark zone), where MYC is rapidly repressed by BCL6.[22–24] The MYC-IGH rearrangement breaks away BCL6 binding sites, preventing repression and leading to continued overexpression under IGH enhancers. However, MYC translocation alone is not sufficient for BL oncogenesis, as it occurs also in DLBCL, plasmablastic, transformed, and other non-Hodgkin lymphomas (NHL).[25, 26] Because MYC simultaneously sensitizes cells to apoptosis, additional protective mechanisms must operate to effect the uncontrolled malignant proliferation characteristic of high-grade lymphomas.[26, 27] For example, in “double-hit” lymphomas, MYC rearrangement co-occurs with BCL2-IGH translocation, leading to overexpression of the anti-apoptotic BCL2 protein, appearance of BL-like morphology, and an aggressive clinical course.[28]
Gene expression and mutational profile of BL
Over a decade ago, seminal studies delineated the BL gene expression profile and uncovered a BL-like signature in some DLBCLs (“molecular BL”).[29, 30] BL is characterized by overexpression of MYC, MYC targets, BCL6, a subset of GC B-cell genes, and distinctively low expression of NF-κB targets or MHC class I genes. Uniquely among B-cell lymphomas, the BL transcriptional profile closely resembles that of dark zone centroblast, even though MYC expression is typically restricted to the light zone of the germinal center.[31]
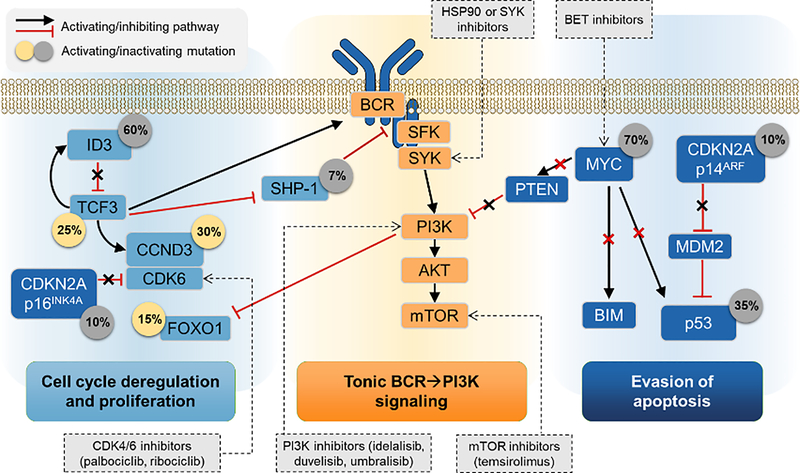
In 2012, three separate studies identified frequent mutations in the ID3 gene, which inhibits the transcription factor TCF3, also recurrently mutated in BL (Fig. 1).[32–34] TCF3 is the main regulator of the centroblast program through enhancement of proliferation and tonic BCR-PI3K signaling. Somatic mutations in the ID3 gene (which are inactivating and usually biallelic), or in the TCF3 gene (which are, conversely, activating) occur in 70–90% of sporadic, 67% of immunodeficiency-associated, and 40% of endemic BL.[32, 34–36] They are characteristically absent in DLBCL. Additionally, up to 30% of BL have mutations in CCND3 that increase intracellular accumulation of cyclin D3 and contribute to cell cycle deregulation.[32, 36] CCND3 is upregulated by TCF3, and acts in a complex with CDK6, which can be further disinhibited by recurrent deletions of p16INK4A/ CDKN2A.[37, 38] Whole genome sequencing confirmed prevalence of silencing ID3 mutations concentrated in its TCF3-binding domain, and ID3 knockout potentiates the proliferative effects of MYC.[39]
Fig. 1.
Molecular mechanisms in Burkitt lymphoma and potential targets for therapy. Prevalence of mutations in specific genes is indicated in circles.
Cell line and murine models demonstrate tonic activity of the PI3K-AKT-mTOR signaling in BL.[32, 40, 41] TCF3 upregulates expression of the B-cell receptor (BCR) and activates the PI3K signaling through downregulation of SYK inhibitor SHP-1 (PTPN6). Tonic BCR signaling differs from chronic signaling which depends on the activity of Bruton’s tyrosine kinase and NF-κB.[42, 43] In BL, tonic BCR signaling contributes to sustained cell survival, and its interruption is an attractive pharmacotherapeutic target, as multiple PI3K, mTOR, SYK, and HSP90 inhibitors are available.[44]
MYC itself is also the gene most frequently (70%) mutated in BL.[17, 32, 35, 45] MYC mutations often occur in positions that alter either MYC degradation or MYC-dependent transactivation of pro-apoptotic proteins BIM and p53.[46–48] TP53 is directly mutated in 35% of BL, and CDKN2A/p14ARF deletions further contribute to its decreased function, uncoupling the MYC-induced hyper-proliferative metabolism from sensitivity to apoptosis.[17, 32, 35]
These advances in the understanding of BL pathogenesis (cell cycle deregulation, tonic BCR-PI3K signaling, and evasion of apoptosis) have not yet been translated into therapy, which continues to rely on cytotoxic agents. However, they provide rationale for clinical trials, suggesting that multiple pathways need to be disrupted to overcome pathogenic mechanisms in BL.[44, 45, 49] These mechanisms are shared by sporadic and endemic BL, despite identified molecular differences. The EBV infection in endemic BL appears to functionally recapitulate some of the oncogenic events in sporadic BL. Endemic BL shows higher mutational load, but also frequent ID3/TCF3 axis mutations.[17, 39]
First-line therapy for BL
The rapid cellular growth and division in BL lays ground for the success of short-cycle, intensive chemotherapy modeled on B-ALL regimens (Table 1).[50] In contrast, treating BL with DLBCL strategies like R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) often leads to chemotherapy refractoriness and poor survival.[51] Phase 3 trials are difficult to conduct in BL given its rarity and aggressiveness,[52] but one pivotal multicenter trial showed improved event-free survival (EFS) with adding rituximab to first-line chemotherapy.[53] As a proof of concept, the lower-intensity, infusional regimen DA-EPOCH (dose-adjusted etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and rituximab) has exchanged the dose intensity for pharmacokinetically motivated, sustained drug exposure. This approach ameliorates toxicities of traditional high-intensity regimens, facilitating therapy for patients compromised by age, organ impairment, or HIV.
Table 1.
Outcomes of prospective clinical trials of first-line treatment regimens in BL.
| Regimen | Histology | N | Age, median (range) | Stage III/IV (or high risk) | CR | EFS/PFS (years) | OS (years) | TRM |
|---|---|---|---|---|---|---|---|---|
| CODOX-M/IVAC[113] | BL | 41 | 17 (3–59) | 80% | 95% | 92% EFS (2) | NR | 3% |
| CODOX-M/IVAC[114] | BL | 52 | 35 (15–60) | 61% | 77% | 65% EFS (2) | 73% (2) | 8% |
| AMC-048[64] a | HIV+ BL | 34 | 42 (19–55) | 74% | NR | 69% PFS (1) | 69% (2) | 3% |
| CODOX-M/IVAC[58] | BL/BL-like | 14 | 47 (18–65) | 64% | 90% | 64% PFS (2) | 71% (2) | 0% |
| R-CODOX-M/IVAC[115] a | BL | 25 | 44 (23–70) | (80%) | 92% | 80% PFS (2) | 84% (2) | 0% |
| Hyper-CVAD[56] | B-ALL | 26 | 58 (17–79) | 100% | 81% | 61% DOR (3) | 49% (3) | 19% |
| R-Hyper-CVAD[57] | BL B-ALL |
31 | 46 (17–77) | NR | 86% | 80% EFS (3) | 89% (3) | 0% |
| LMB89[68] b | BL B-ALL |
561 | 8 (0–18) | 79% | 97% | 91% EFS (5) | 92% (5) | 1% |
| LMB95[69] b | BL | 72 | 33 (18–76) | 67% | 72% | 65% EFS (2) | 70% (2) | 4% |
| LMB±R[53] b | BL | 257 | 47 (IQR 31–59) | 62% | NR | R 75% EFS (3) No R 62% EFS |
R 83% (3) No R 70% |
5% |
| BFM 90[70] b | BL B-ALL |
322 | 9 (1–18) | 60% | NR | 89% EFS (6) | NR | 3% |
| GMALL-B-ALL/NHL 2002[54] b | BL B-ALL |
363 | 42 (16–85) | 71% | 88% | 75% PFS (5) | 80% (5) | 4% |
| Alliance[72] b | BL B-ALL |
105 | 43 (19–79) | 49% | 83% | 78% EFS (2) | 80% (2) | 7% |
| SC-EPOCH-RR[61] | HIV+ BL | 11 | 44 (24–60) | 82% | 91% | 100% FFP (6) | 90% (6) | 0 |
| DA-EPOCH-R[61] | BL | 19 | 25 (15–88) | 58% | 100% | 95% FFP (7) | 100% (7) | 0 |
| DA-EPOCH-R[75] | BL HIV+ BL |
110 29 HIV+ |
49 (18–86) | 69% | NR | 86% PFS (3) | 86% (3) | 5% |
includes an optional prephase (corticosteroids +/− cyclophosphamide)
includes a mandatory prephase (corticosteroids +/− cyclophosphamide +/− vincristine)
B-ALL: B-cell acute lymphoblastic leukemia; BL: Burkitt lymphoma; DFS: disease-free survival; DOR: duration of remission; EFS: event-free survival; FFP: freedom from progression; IQR: interquartile range; NR: not reported; PFS: progression-free survival; R: rituximab; TRM: treatment-related mortality
An important aspect of the initial BL management is cytoreductive pre-phase that can ameliorate organ dysfunction and minimize tumor lysis. Administering 5 days of intravenous cyclophosphamide 200mg/m2 and oral prednisone 60 mg/m2 is built in as a mandatory pre-phase in the GMALL-B-ALL/NHL 2002 protocol.[54] Other high-intensity regimens include similar strategies. A pre-phase should be considered for all high-risk patients presenting with a large disease burden, organ dysfunction (e.g. hyperbilirubinemia), or tumor lysis.
The National Comprehensive Cancer Network guidelines recommend one of 3 chemotherapy regimens for first-line use in BL:[55]
R-hyperCVAD (rituximab, cyclophosphamide, vincristine, doxorubicin, and dexamethasone alternating with high-dose methotrexate and cytarabine),[56, 57]
CODOX-M/IVAC (cyclophosphamide, doxorubicin, vincristine, and methotrexate, alternating with ifosfamide, cytarabine, and etoposide) with or without rituximab,[58–60] or
In immunodeficiency-associated BL, modified CODOX-M/IVAC+/−R, and infusional DA-EPOCH-R (or its version SC-EPOCH-RR: a short-course etoposide, prednisone, vincristine, cyclophosphamide, doxorubicin, and two doses of rituximab) are preferred.[61–65] All regimens include intrathecal (IT) CNS prophylaxis with modifications in case of overt CNS involvement.
R-hyperCVAD
In 1999, Thomas et al. used hyperCVAD in 26 adults with B-ALL, of whom 81% achieved complete remission (CR) and 3-year overall survival (OS) of 49%.[56] CR was maintained in 57% of patients through >3 years of follow-up.[56] Addition of rituximab further increased CR to 86%, and 3-year OS to 89%.[57] Patients received 16 doses of IT prophylaxis (alternating methotrexate and cytarabine on days 2 and 7 of each cycle), with additional IT therapy administered twice weekly until CSF clearance for patients with CNS disease. Of note, the dose of IT methotrexate was 6 mg (rather than 12mg) when administered into Ommaya reservoirs. R-hyperCVAD has been subsequently applied in HIV+ BL.[63] However, it has proven difficult to deliver outside of centers of expertise, requiring over 6 months of high-intensity chemotherapy with severe myelosuppression and TRM in 12% of patients.[18, 66] Results with other regimens indicate that much shorter chemotherapy is sufficient to eradicate BL.
CODOX-M/IVAC ± rituximab and modified-CODOX-M/IVAC ± rituximab
In 1984, Magrath et al., published outcomes of CODOX-M in 34 adults with lymphoblastic lymphomas, who achieved 82% rate of CR and 60% OS at 3 years.[59] Mead et al. studied this regimen in an international cohort, alternating 2 cycles each of CODOX-M and IVAC for high-risk BL, whereas patients with low risk (defined as non-bulky stage I/II disease, normal lactate dehydrogenase (LDH), performance status ≤1, and ≤1 extranodal site) received 3 cycles of CODOX-M.[60] In high-risk BL, 2-year OS of 70% and EFS of 60% were achieved, but with high rates of severe mucositis and myelosuppression. Lacasce, et al. in a phase 2 study described a ‘modified Magrath’ regimen using altered doses and timing of agents, with 90% CR and 64% disease-free survival (DFS) at 29 months.[1, 58] Additionally, there was no grade 3/4 neuropathy and only one patient had grade 3/4 mucositis, pointing to excellent efficacy and tolerance. The definition of “low risk BL” was limited to a single site of lymphoma with normal LDH and tumor <10 cm (or completely resected ileocecal mass). Rituximab was added to this regimen in a retrospective study, demonstrating fewer relapses (7% vs. 33%), better 3-year PFS (74% vs. 61%), and OS (77% vs. 66%).[67]
In the AIDS Malignancy Consortium AMC-048 phase 2 trial, the CODOX-M/IVAC regimen was modified for HIV-positive patients by adding rituximab, capping the dose of vincristine, and altering the dosing of cyclophosphamide and methotrexate.[64] Sixty-eight percent of patients completed treatment with 2-year OS of 69%, and lower rates of grade 3/4 toxicities (79%) compared with the original regimen. Modified CODOX-M/IVAC+R is thus an appropriate regimen for immunodeficiency-related BL, with low TRM when delivered in academic setting.
Other intensive regimens
The Lymphome Malins de Burkitt (LMB) protocols are intensive regimens successively developed in France for mature B-ALL and BL. The pediatric LMB89 protocol saw promising CR rate of 97%, 5-year EFS and OS of 92% with minimal TRM (1%).[68] However, when adapted for adults (LMB95), the CR rate dropped to 72%, 2-year EFS to 65%, and TRM was 4%.[69] A subsequent phase 3 study demonstrated improved EFS (HR, 0.59; 95%CI, 0.38–0.94) and OS (HR, 0.51; 95%CI, 0.30–0.86) with addition of rituximab, which did not affect TRM (5%).[53]
The Berlin-Frankfurt-Münster (BFM) group studied intensive short-duration chemotherapy (NHL BFM-90), with 6-year EFS of 89% and TRM of 3%.[70] A similar short-duration regimen with rituximab was evaluated in the phase 2 German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia (GMALL) B-ALL/NHL2002 study which included BL-ALL, BL, atypical BL, and Burkitt-like lymphoma.[54] Patients age <55 experienced better CR (89% vs. 82%), 5-year PFS (82% vs. 60%), OS (86% vs. 62%), and lower TRM (2% vs. 11%).[54] This therapy showed significant toxicity when adopted for HIV+ patients.[71] Other intensive regimens were studied by the US Alliance for Clinical Trials in Oncology group with 2-year EFS of 78%, and by the Spanish PETHEMA group (Burkimab) with 4-year disease-free survival of 80%.[72, 73]
DA-EPOCH-R and SC-EPOCH-RR
Dunleavy et al. first reported the use of DA-EPOCH-R as treatment for BL in 2006, with subsequent 2013 publication of the phase 2 trial, reporting >90% rates of CR and PFS with no TRM.[61, 74] The SC-EPOCH-RR version for HIV+ patients included double-dosing of rituximab per cycle, no dose escalation, and response-guided treatment duration (3 to 6 cycles). At median follow-up of >5 years, rates of freedom from progression (FFP) and OS were 95% and 100%, respectively, after DA-EPOCH-R, and 100% and 90%, respectively, after SC-EPOCH-RR. [61] Febrile neutropenia occurred in 19% of treatment cycles with low rates of mucositis (5%), constipation or ileus (1%), but peripheral sensory neuropathy developed in 17%. The subsequent phase 2, multicenter study (NCI-9177) evaluated risk-adapted therapy with DA-EPOCH-R in 113 patients with BL, reporting 86% PFS at 3 years, unaffected by the HIV status or age (up to 86 years).[75] Most patients (88%) were considered high risk, and they had OS of 86% at 3 years after 6 cycles of DA-EPOCH-R. Notably, no deaths or progressions were recorded among 14 patients with low-risk BL treated with only 3 cycles of DA-EPOCH-R and no CNS prophylaxis. Similar to R-hyperCVAD protocols, patients with CNS involvement underwent intensive IT therapy (starting with twice-weekly administration) of methotrexate 12mg (6mg via Ommaya reservoir).
Choosing the treatment regimen in the context of CNS involvement
Although no randomized trials have compared various high-intensity regimens, retrospective data suggest similar outcomes, and the choice is often determined by institutional expertise.[76] The lower-intensity DA-EPOCH-R regimen is an important conceptual advancement, particularly for patients who are older or HIV+ positive, who historically had poor tolerance and worse outcomes with dose-intensive chemotherapy.[8, 77] Until the formal phase 3 trial (Eudara CT 2013–004394-27) will compare DA-EPOCH-R with R-CODOX-M/IVAC, the available observational data support DA-EPOCH-R as an option with outcomes similar to higher-intensity regimens. A recent retrospective of 557 adult BL patients treated in the US suggested no difference in PFS or OS among patients receiving hyperCVAD, CODOX-M/IVAC, or DA-EPOCH (P=.42) accounting for baseline prognostic factors (age, LDH, performance status, and CNS involvement).[18] Another international retrospective demonstrated 2-year EFS of 80% for patients treated with intensive immunochemotherapy, and a very low risk of recurrence among those maintaining CR for >12 months.[78]
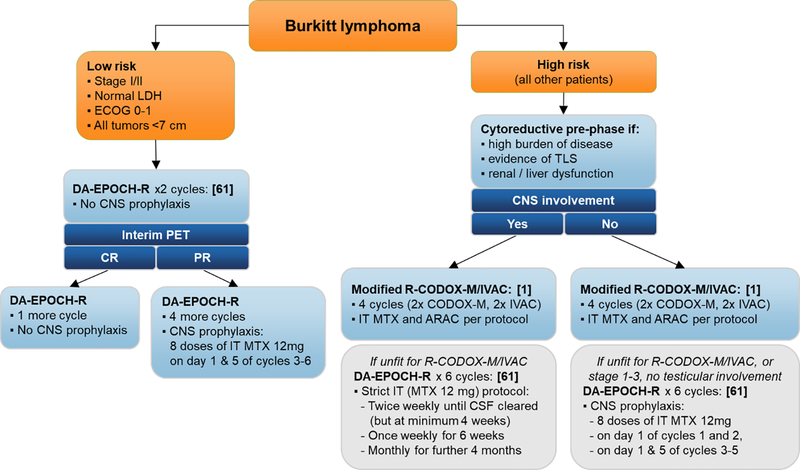
One concern with DA-EPOCH-R is increased risk of recurrence among patients with CNS or bone marrow involvement.[62] Rates of CNS involvement in BL are 14–41% at presentation and 6–42% at the time of relapse.[1, 2, 79–81] BL, similar to B-ALL, is prone to leptomeningeal spread, and in historical data most cases of CNS involvement were leptomeningeal, warranting a focus on IT therapy.[80] However, recent data suggest that while CNS recurrences still involve the leptomeningeal compartment in 76%, rates of intraparenchymal CNS involvement may be as high as 38%, similar to observations in DLBCL from the rituximab era, and suggesting an increasing role for systemic prophylaxis.[82, 83] While aggressive CNS-directed therapy can eradicate BL from the CNS compartment, median survival after relapse is <3 months.[66, 82] Consequently, all BL regimens include dedicated CNS prophylaxis (Table 2). DA-EPOCH-R has the same or higher intensity of IT injections as hyperCVAD and CODOX-M/IVAC, but it lacks classic agents (methotrexate, cytarabine) used for prophylaxis against parenchymal CNS disease. Only 1 patient with CNS involvement was included in the initial DA-EPOCH-R study. Ten patients with CNS or bone marrow involvement enrolled in the NCI 9177 trial showed significantly worse outcomes (PFS of 63% vs. 95%, P<.0001).[75] A single-institution series of 64 patients with BL or HGBL found that those selected for DA-EPOCH-R were significantly older and had other unfavorable factors, resulting in worse survival.[84] All CNS recurrences occurred within the first year of follow up and were among patients receiving DA-EPOCH-R, with a cumulative incidence of 36% (vs. 0% for high-intensity regimens, P=.0004). Similar conclusions emerged from the multi-institutional retrospective, where CNS recurrences were more frequent after DA-EPOCH (12%) than hyperCVAD or CODOX-M/IVAC (3–4%).[82] Risk factors for CNS recurrence included baseline CNS involvement and stage 4 disease. As a result, the adoption of DA-EPOCH-R as first-line therapy has been limited, and many centers favor high-intensity regimens for patients with CNS risk factors.[85] We favor R-CODOX-M/IVAC due to its shorter duration, and early IT therapy when DA-EPOCH-R is used (Fig. 2).
Table 2.
First-line treatment regimens and CNS outcomes
| Regimen | CNS involvement N (%) | CNS prophylaxis (N of doses) | CNS recurrence (%) |
|---|---|---|---|
| CODOX-M/IVAC[113] | 5 (12%) | HDMTX (2); HiDAC (8); IT MTX (4); IT Ara-C (4) CNS involvement: HDMTX (2); HiDAC (8); IT MTX (5); IT Ara-C (7) |
NR |
| CODOX-M/IVAC[114] | 6 (11%) | HDMTX (2); HiDAC (8); IT MTX (4); IT Ara-C (4) CNS involvement: HDMTX (2); HiDAC (8); IT MTX (6); IT Ara-C (10) |
NR |
| AMC-048[64] | 4 (12%) | HDMTX (2); HiDAC (8); IT MTX (4), IT Ara-C (2) CNS involvement: HDMTX (2); HiDAC (8); IT Liposomal Ara-C (2); IT MTX (4) |
1 (3%) |
| R-CODOX-M/IVAC[115] | 3 (12%) | HDMTX (2); HiDAC (8); IT MTX (4); IT Ara-C (4) CNS involvement: HDMTX (2); HiDAC (8); IT MTX (5); IT Ara-C (7) |
NR |
| Hyper-CVAD[56] | 11 (42%) | HDMTX (4); HiDAC (16); IT MTX (8); IT Ara-C (8) CNS involvement: HDMTX (4); HiDAC (16); Twice weekly IT until CSF cleared, then return to prophylactic regimen |
NR, but no isolated CNS recurrence |
| R-Hyper-CVAD[57] | 2 (6%) | HDMTX (4); HiDAC (16); IT MTX (8); IT Ara-C (8) CNS involvement: HDMTX (4); HiDAC (16); Twice weekly IT until CSF cleared, then weekly IT for 4 weeks (alt MTX & Ara-C), the return to prophylactic regimen |
0 |
| SC-EPOCH-RR[61] | 0 | IT MTX (6) CNS involvement: IT MTX (18) |
0 |
| DA-EPOCH-R[61] | 1 (5%) | IT MTX (8) CNS involvement: IT MTX (18) |
0 |
| DA-EPOCH-R[75] | 11 (10%) | IT MTX (8) (high risk only) CNS involvement: IT MTX twice weekly for 2 weeks after negative CSF, then weekly for 6 doses, then monthly for 6 doses (min. 16) |
3% (parenchymal) |
| LMB89[68] | 67 (12%) | HDMTX 3g/m2 (5); IT MTX (8); IT Ara-C (2) CNS involvement: HDMTX 8g/m2 (3); HiDAC (2); Cranial RT 24Gy; IT MTX + Ara-C (6) |
6 (1%) |
| LMB95[69] | 11 (15%) | HDMTX 3g/m2 (5); IT MTX (8); IT Ara-C (2) CNS involvement: HDMTX 8g/m2 (3); HiDAC (2); Cranial RT 24Gy; IT MTX + Ara-C (10) |
1 (1%) |
| LMB ± R[53] | 65 (12%) | HDMTX 3g/m2 (5); IT MTX + HC (8); IT Ara-C + HC (2) CNS involvement: HDMTX 8g/m2 (3); HiDAC (2); Cranial RT 18Gy; IT MTX + HC + Ara-C (10) |
NR |
| BFM 90[70] | 26 (6%) | HDMTX 5g/m2 (4); IT MTX + Ara-C + Pred (9) CNS involvement: HDMTX 5g/m2 (6); IT MTX + Ara-C + Pred (13) |
2 (1%) |
| GMALL-B-ALL/NHL 2002[54] | 35 (10%) |
</=55 yrs: HDMTX 1.5 g/m2 (6); HiDAC (4); IT MTX (1); IT MTX + Ara-C (4 or 8) >55 yrs: HDMTX 1.5 g/m2 (3) & 0.5 g/m2 (3); IT MTX (1); IT MTX + Ara-C (6 or 12) CNS involvement </=55 yrs: HDMTX 1.5 g/m2 (6); HiDAC (4); twice weekly IT until CSF cleared, then IT MTX (1); IT MTX + Ara-C (8) CNS involvement >55 yrs: HDMTX 1.5 g/m2 (3) + 0.5 g/m2 (3); twice weekly IT until CSF cleared, then IT MTX (1); IT MTX + Ara-C (12) |
12 (3%) after cR |
| Alliance[72] | 14 (14%) | HDMTX (6); HiDAC (6); IT MTX (6); IT Ara-C (6) CNS involvement: additional twice-weekly IT therapy until CSF clearance, then weekly x4, then cranial RT 24 Gy |
4 (4%) |
Ara-C: cytarabine; CNS: central nervous system; CSF: cerebrospinal fluid; HDMTX: high-dose methotrexate; HiDAC: high-dose cytarabine (≥1 g/m2); IT: intrathecal; MTX: methotrexate; NR: not reported; RT: radiation therapy
Fig. 2.
The authors’ preferred treatment strategy for Burkitt lymphoma according to risk group and baseline CNS involvement status.
ARAC: cytarabine; CNS: central nervous system; IT: intrathecal; LDH: lactate dehydrogenase; MTX: methotrexate; PET: position emission tomography; TLS: tumor lysis syndrome
Relapsed and refractory BL
While standard first-line regimens show robust CR and PFS rates, 20–40% of adult patients experience primary refractory or recurrent BL. In that setting, high-level evidence for best therapy is lacking. Patients with relapses >6 months from initial therapy can achieve a second remission with further combinations like ICE (ifosfamide, carboplatin, and etoposide), DA-EPOCH-R, IVAC, GDP (gemcitabine, dexamethasone & cisplatin), or high-dose cytarabine. These are often delivered with rituximab, and remissions consolidated with autologous or allogeneic stem cell transplantation (SCT).[86] While SCT in second CR resulted in OS of up to 70% in the pre-rituximab era,[86] most patients with BL experience early, chemotherapy-resistant relapse, and <10% survive a relapse following a high-intensity induction.[66] Recent data indicate that patients in second CR attain 44% long-term PFS after autologous, and 27% after allogeneic SCT, whereas with partial response 5-year PFS is only 19% and 11%, respectively.[87] The number of SCT procedures for BL in the US has declined from 48 in 2013 to 23 in 2017.[88]
Given the need for efficacious second-line therapy, clinical trials are evaluating novel approaches, yet BL-specific data remain extremely limited. The most promising strategies involve immunotherapy (including novel cellular therapies), or rationally selected targeted therapies.
Targeted therapies
Based on the molecular pathobiology of BL, targeted therapeutic approaches may include inhibition of the B-cell receptor (BCR), PI3K or SYK (directly or indirectly through the chaperone heat-shock protein 90 [HSP90]), disruption of MYC function using inhibitors of proteins with bromodomain and extra-terminal motifs (BET/BRD), deregulation of metabolism (devimistat), or disturbing the proliferation/apoptosis balance by inhibiting cyclin-dependent kinases CDK4/6 or apoptosis regulators like MCL-1. Unfortunately, despite encouraging pre-clinical data, these approaches are only starting to be clinically explored.
Abemaciclib and palbociclib, two structurally different CDK4/6 inhibitors, suppress proliferation and induce apoptosis in aggressive NHL cell lines, although the effects of abemaciclib appeared to be greater and more dependent on cyclin D3 expression.[89] JQ1, a small molecule BRD inhibitor, exerted in vitro activity against BL cell lines, and addition of a PI3K/mTOR inhibitor omipalisib augmented this response.[90] Other preclinical observations suggest activity of PI3K/mTOR inhibitors omipalisib and idelalisib, alone or in combination with chemotherapy.[91, 92] Molibresib, a selective BRD/BET inhibitor, showed modest clinical activity in a phase 1 study in relapsed/refractory NHL, but actually not among subjects with BL.[93] The MCL-1 inhibitor, AMG-176, showed so far pre-clinical activity against BL cell lines.[94] One novel targeted approach already translated into a clinical study involves disrupting the MYC-driven glycolytic metabolism that relies on the tricarboxylic acid cycle using the novel lipoic acid analogue devimistat (CPI-613). Devimistat is being investigated in a phase 2 study following a sustained response observed in a patient with BL.[95, 96].
Immunotherapy
Novel immunotherapies are among the most successful approaches in aggressive B-cell lymphomas. Unfortunately, specific experience with these treatments in BL is lacking, although strategies summarized below are often described as reasonable salvage approaches in BL through data extrapolation from both B-ALL and DLBCL.[97]
Chimeric antigen receptor (CAR) T-cell therapy
CD19-directed CAR T-cells are a novel form of cellular therapy, inducing 50–90% response rates and durable remissions in 30–40% of patients with relapsed/refractory precursor B-ALL or DLBCL.[98–100] Unfortunately, BL was excluded from pivotal trials of CAR T-cell therapies. Their use for BL remains experimental, with only case reports of anecdotal efficacy and no reported experience in real-world data on commercial products (axicabtagene ciloleucel and tisagenlecleucel).[101, 102] The applicability of CAR T-cells is compromised by the rapid pace of clinical progression in BL and time required to produce CAR T-cells. However, new trials suggest feasibility of this strategy in BL. One ongoing study evaluates sequential CD19, CD20, and CD22-directed CAR T-cells in relapsed/refractory pediatric leukemias and lymphomas, predominantly in BL (13 of 17 subjects). The researchers reported a preliminary 42% response rate to the first CD19-directed infusion, and overall 94% response rate with 71% CRs.[103] One of 2 BL patients who enrolled in a trial of anti-CD19/anti-CD22 CAR T-cell “cocktail” obtained a remission.[104] Additional targets for CAR T-cell therapy are under investigation, including a modular anti-CD10 CAR T-cell system, and a multiantigen-targeting CAR NK-cell product.[105, 106]. The CARPALL study of a novel ‘fast off-rate’ anti-CD19 CAR T-cell therapy is recruiting pediatric patients with Burkitt lymphoma (NCT02443831).
Bispecific T-cell engagers (BiTEs)
BiTEs, which are complete or truncated monoclonal antibodies that simultaneously target B-cell and T-cell antigens (CD19/20 and CD3, respectively), can induce immune-mediated killing of malignant B-cells with a ready-to-use product, obviating the delay needed to generate CAR T-cells. Blinatumomab, a CD19/CD3 BiTE, is approved for treatment of relapsed or refractory precursor B-ALL,[107] but its efficacy in BL is limited to case reports, some disappointingly reporting progressive disease.[108, 109] An ongoing phase I study is evaluating a combination of blinatumomab with the immunomodulatory drug lenalidomide in relapsed/refractory NHL, including BL.[110] Clinical trials of other BiTEs like mosunetuzumab, REGN1979, or GEN3013 have shown promising responses in relapsed/refractory aggressive NHL, although specific experience is BL is currently lacking.[111, 112]
Antibody-drug conjugates
BL may be amenable to therapy with novel antibody-drug conjugates like inotuzumab ozogamicin (targeting CD22) or polatuzumab vedotin (targeting CD79b), but so far clinical data are lacking pending results of early-phase clinical trials (e.g. S1312/NCT01925131).
Conclusions
BL is characterized by high initial response rates to combination cytotoxic chemotherapy, but traditional regimens are quite toxic and standard options have very limited efficacy in relapsed/refractory disease. While DA-EPOCH-R is a major advance in the treatment of BL, demonstrating that it can be cured with lower-intensity chemotherapy, the regimen needs augmentation to address the risk of CNS involvement. DA-EPOCH-R provides an excellent platform to implement targeted or immune-directed therapies that could meaningfully improve outcomes. Research opportunities include short debulking chemotherapy with consolidative immunotherapy to eradicate minimal residual disease, or concurrent addition of targeted agents to first-line regimen. Trials are needed to translate the accumulating molecular findings into the clinical setting, potentially using combinations of agents for optimal efficacy.
Acknowledgments
Funding: This work was supported by the National Institute of General Medical Sciences at National Institutes of Health under Grant U54GM115677.
Footnotes
Conflict of Interest: AZ declares no conflict of interest. AJO reports research grants (to the institution) by Genentech/Roche, Spectrum Pharmaceuticals, and TG Therapeutics.
References
- 1.Jacobson C, LaCasce A. How I treat Burkitt lymphoma in adults. Blood 2014; 124: 2913–2920. [DOI] [PubMed] [Google Scholar]
- 2.Molyneux EM, Rochford R, Griffin B, et al. Burkitt’s lymphoma. Lancet 2012; 379: 1234–1244. [DOI] [PubMed] [Google Scholar]
- 3.Burkitt D A sarcoma involving the jaws in african children. BJS 1958; 46: 218–223. [DOI] [PubMed] [Google Scholar]
- 4.Bouda GC, Traore F, Couitchere L, et al. Advanced Burkitt Lymphoma in Sub-Saharan Africa Pediatric Units: Results of the Third Prospective Multicenter Study of the Groupe Franco-Africain d’Oncologie Pediatrique. J Glob Oncol 2019; 5: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Painschab MS, Westmoreland KD, Kasonkanji E, et al. Prospective study of Burkitt lymphoma treatment in adolescents and adults in Malawi. Blood Adv 2019; 3: 612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopal S, Gross TG. How I treat Burkitt lymphoma in children, adolescents, and young adults in sub-Saharan Africa. Blood 2018; 132: 254–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morton LM, Wang SS, Devesa SS, Hartge P, Weisenburger DD, Linet MS. Lymphoma incidence patterns by WHO subtype in the United States, 1992–2001. Blood 2006; 107: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olszewski AJ, Fallah J, Castillo JJ. Human immunodeficiency virus-associated lymphomas in the antiretroviral therapy era: Analysis of the National Cancer Data Base. Cancer 2016; 122: 2689–2697. [DOI] [PubMed] [Google Scholar]
- 9.NPCR and SEER Incidence - U.S. Cancer Statistics Public Use Database with Puerto Rico, Nov 2018 submission (2005–2016). United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2019, based on the November 2018 submission. Accessed at www.cdc.gov/cancer/npcr/public-use.
- 10.Cote TR, Biggar RJ, Rosenberg PS, et al. Non-Hodgkin’s lymphoma among people with AIDS: incidence, presentation and public health burden. AIDS/Cancer Study Group. Int J Cancer 1997; 73: 645–650. [DOI] [PubMed] [Google Scholar]
- 11.Guech-Ongey M, Simard EP, Anderson WF, et al. AIDS-related Burkitt lymphoma in the United States: what do age and CD4 lymphocyte patterns tell us about etiology and/or biology? Blood 2010; 116: 5600–5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hamilton-Dutoit SJ, Raphael M, Audouin J, et al. In situ demonstration of Epstein-Barr virus small RNAs (EBER 1) in acquired immunodeficiency syndrome-related lymphomas: correlation with tumor morphology and primary site. Blood 1993; 82: 619–624. [PubMed] [Google Scholar]
- 13.Swerdlow SH, World Health Organization, International Agency for Research on Cancer. WHO classification of tumours of haematopoietic and lymphoid tissues (ed Revised 4th edition.). Lyon: International Agency for Research on Cancer; 2017. [Google Scholar]
- 14.Zech L, Haglund U, Nilsson K, Klein G. Characteristic chromosomal abnormalities in biopsies and lymphoid-cell lines from patients with Burkitt and non-Burkitt lymphomas. Int J Cancer 1976; 17: 47–56. [DOI] [PubMed] [Google Scholar]
- 15.Taub R, Kirsch I, Morton C, et al. Translocation of the c-myc gene into the immunoglobulin heavy chain locus in human Burkitt lymphoma and murine plasmacytoma cells. Proc Natl Acad Sci U S A 1982; 79: 7837–7841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A 1982; 79: 7824–7827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grande BM, Gerhard DS, Jiang A, et al. Genome-wide discovery of somatic coding and noncoding mutations in pediatric endemic and sporadic Burkitt lymphoma. Blood 2019; 133: 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alderuccio JP, Olszewski AJ, Portell CA, et al. The Evaluation and Treatment (Tx) of Burkitt Lymphoma (BL) in the Modern Era: Real World (RW) Outcomes and Prognostication across 26 US Cancer Centers (CC). Blood 2019; 134: 397. [Google Scholar]
- 19.Boxer LM, Dang CV. Translocations involving c-myc and c-myc function. Oncogene 2001; 20: 5595–5610. [DOI] [PubMed] [Google Scholar]
- 20.Lin CY, Loven J, Rahl PB, et al. Transcriptional amplification in tumor cells with elevated c-Myc. Cell 2012; 151: 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dang CV. MYC on the path to cancer. Cell 2012; 149: 22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calado DP, Sasaki Y, Godinho SA, et al. The cell-cycle regulator c-Myc is essential for the formation and maintenance of germinal centers. Nat Immunol 2012; 13: 1092–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dominguez-Sola D, Victora GD, Ying CY, et al. The proto-oncogene MYC is required for selection in the germinal center and cyclic reentry. Nat Immunol 2012; 13: 1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basso K, Dalla-Favera R. Germinal centres and B cell lymphomagenesis. Nat Rev Immunol 2015; 15: 172–184. [DOI] [PubMed] [Google Scholar]
- 25.Castillo JJ, Bibas M, Miranda RN. The biology and treatment of plasmablastic lymphoma. Blood 2015; 125: 2323–2330. [DOI] [PubMed] [Google Scholar]
- 26.Ott G, Rosenwald A, Campo E. Understanding MYC-driven aggressive B-cell lymphomas: pathogenesis and classification. Blood 2013; 122: 3884–3891. [DOI] [PubMed] [Google Scholar]
- 27.Hoffman B, Liebermann DA. Apoptotic signaling by c-MYC. Oncogene 2008; 27: 6462–6472. [DOI] [PubMed] [Google Scholar]
- 28.Petrich AM, Nabhan C, Smith SM. MYC-associated and double-hit lymphomas: a review of pathobiology, prognosis, and therapeutic approaches. Cancer 2014; 120: 3884–3895. [DOI] [PubMed] [Google Scholar]
- 29.Dave SS, Fu K, Wright GW, et al. Molecular diagnosis of Burkitt’s lymphoma. N Engl J Med 2006; 354: 2431–2442. [DOI] [PubMed] [Google Scholar]
- 30.Hummel M, Bentink S, Berger H, et al. A biologic definition of Burkitt’s lymphoma from transcriptional and genomic profiling. N Engl J Med 2006; 354: 2419–2430. [DOI] [PubMed] [Google Scholar]
- 31.Victora GD, Dominguez-Sola D, Holmes AB, Deroubaix S, Dalla-Favera R, Nussenzweig MC. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas. Blood 2012; 120: 2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz R, Young RM, Ceribelli M, et al. Burkitt lymphoma pathogenesis and therapeutic targets from structural and functional genomics. Nature 2012; 490: 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richter J, Schlesner M, Hoffmann S, et al. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet 2012; 44: 1316–1320. [DOI] [PubMed] [Google Scholar]
- 34.Love C, Sun Z, Jima D, et al. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet 2012; 44: 1321–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Richter J, Schlesner M, Hoffmann S, et al. Recurrent mutation of the ID3 gene in Burkitt lymphoma identified by integrated genome, exome and transcriptome sequencing. Nat Genet 2012; 44: 1316–1320. [DOI] [PubMed] [Google Scholar]
- 36.Rohde M, Bonn BR, Zimmermann M, et al. Relevance of ID3-TCF3-CCND3 pathway mutations in pediatric aggressive B-cell lymphoma treated according to the non-Hodgkin Lymphoma Berlin-Frankfurt-Munster protocols. Haematologica 2017; 102: 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Havelange V, Pepermans X, Ameye G, et al. Genetic differences between paediatric and adult Burkitt lymphomas. Br J Haematol 2016; 173: 137–144. [DOI] [PubMed] [Google Scholar]
- 38.Bouska A, Bi C, Lone W, et al. Adult high-grade B-cell lymphoma with Burkitt lymphoma signature: genomic features and potential therapeutic targets. Blood 2017; 130: 1819–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Panea RI, Love CL, Shingleton JR, et al. The whole-genome landscape of Burkitt lymphoma subtypes. Blood 2019; 134: 1598–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sander S, Calado DP, Srinivasan L, et al. Synergy between PI3K signaling and MYC in Burkitt lymphomagenesis. Cancer Cell 2012; 22: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walter R, Pan KT, Doebele C, et al. HSP90 promotes Burkitt lymphoma cell survival by maintaining tonic B-cell receptor signaling. Blood 2017; 129: 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov 2013; 12: 229–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Corso J, Pan KT, Walter R, et al. Elucidation of tonic and activated B-cell receptor signaling in Burkitt’s lymphoma provides insights into regulation of cell survival. Proc Natl Acad Sci U S A 2016; 113: 5688–5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomska K, Kurilov R, Lee KS, et al. Drug-based perturbation screen uncovers synergistic drug combinations in Burkitt lymphoma. Sci Rep 2018; 8: 12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz R, Ceribelli M, Pittaluga S, Wright G, Staudt LM. Oncogenic mechanisms in Burkitt lymphoma. Cold Spring Harb Perspect Med 2014; 4: doi: 10.1101/cshperspect.a014282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuttler F, Ame P, Clark H, et al. c-myc box II mutations in Burkitt’s lymphoma-derived alleles reduce cell-transformation activity and lower response to broad apoptotic stimuli. Oncogene 2001; 20: 6084–6094. [DOI] [PubMed] [Google Scholar]
- 47.Bahram F, von der Lehr N, Cetinkaya C, Larsson LG. c-Myc hot spot mutations in lymphomas result in inefficient ubiquitination and decreased proteasome-mediated turnover. Blood 2000; 95: 2104–2110. [PubMed] [Google Scholar]
- 48.Hemann MT, Bric A, Teruya-Feldstein J, et al. Evasion of the p53 tumour surveillance network by tumour-derived MYC mutants. Nature 2005; 436: 807–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Derenzini E, Mondello P, Erazo T, et al. BET Inhibition-Induced GSK3beta Feedback Enhances Lymphoma Vulnerability to PI3K Inhibitors. Cell Rep 2018; 24: 2155–2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blum KA, Lozanski G, Byrd JC. Adult Burkitt leukemia and lymphoma. Blood 2004; 104: 3009–3020. [DOI] [PubMed] [Google Scholar]
- 51.Wasterlid T, Brown PN, Hagberg O, et al. Impact of chemotherapy regimen and rituximab in adult Burkitt lymphoma: a retrospective population-based study from the Nordic Lymphoma Group. Ann Oncol 2013; 24: 1879–1886. [DOI] [PubMed] [Google Scholar]
- 52.Olszewski AJ, Ollila T, Reagan JL. Time to treatment is an independent prognostic factor in aggressive non-Hodgkin lymphomas. Br J Haematol 2018; 181: 495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ribrag V, Koscielny S, Bosq J, et al. Rituximab and dose-dense chemotherapy for adults with Burkitt’s lymphoma: a randomised, controlled, open-label, phase 3 trial. Lancet 2016; 387: 2402–2411. [DOI] [PubMed] [Google Scholar]
- 54.Hoelzer D, Walewski J, Dohner H, et al. Improved outcome of adult Burkitt lymphoma/leukemia with rituximab and chemotherapy: report of a large prospective multicenter trial. Blood 2014; 124: 3870–3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.B-cell lymphomas. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) version 6.2019, November 26, 2019; available from https://www.nccn.org/professionals/physician_gls/.
- 56.Thomas DA, Cortes J, O’Brien S, et al. Hyper-CVAD program in Burkitt’s-type adult acute lymphoblastic leukemia. J Clin Oncol 1999; 17: 2461–2470. [DOI] [PubMed] [Google Scholar]
- 57.Thomas DA, Faderl S, O’Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer 2006; 106: 1569–1580. [DOI] [PubMed] [Google Scholar]
- 58.Lacasce A, Howard O, Lib S, et al. Modified magrath regimens for adults with Burkitt and Burkitt-like lymphomas: preserved efficacy with decreased toxicity. Leuk Lymphoma 2004; 45: 761–767. [DOI] [PubMed] [Google Scholar]
- 59.Magrath IT, Janus C, Edwards BK, et al. An effective therapy for both undifferentiated (including Burkitt’s) lymphomas and lymphoblastic lymphomas in children and young adults. Blood 1984; 63: 1102–1111. [PubMed] [Google Scholar]
- 60.Mead GM, Sydes MR, Walewski J, et al. An international evaluation of CODOX-M and CODOX-M alternating with IVAC in adult Burkitt’s lymphoma: results of United Kingdom Lymphoma Group LY06 study. Ann Oncol 2002; 13: 1264–1274. [DOI] [PubMed] [Google Scholar]
- 61.Dunleavy K, Pittaluga S, Shovlin M, et al. Low-intensity therapy in adults with Burkitt’s lymphoma. N Engl J Med 2013; 369: 1915–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roschewski M, Dunleavy K, Abramson JS, et al. Risk-Adapted Therapy in Adults with Burkitt Lymphoma: Results of NCI 9177, a Multicenter Prospective Phase II Study of DA-EPOCH-R. Blood 2017; 130: 188. [Google Scholar]
- 63.Cortes J, Thomas D, Rios A, et al. Hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone and highly active antiretroviral therapy for patients with acquired immunodeficiency syndrome-related Burkitt lymphoma/leukemia. Cancer 2002; 94: 1492–1499. [DOI] [PubMed] [Google Scholar]
- 64.Noy A, Lee JY, Cesarman E, et al. AMC 048: modified CODOX-M/IVAC-rituximab is safe and effective for HIV-associated Burkitt lymphoma. Blood 2015; 126: 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodrigo JA, Hicks LK, Cheung MC, et al. HIV-Associated Burkitt Lymphoma: Good Efficacy and Tolerance of Intensive Chemotherapy Including CODOX-M/IVAC with or without Rituximab in the HAART Era. Adv Hematol 2012; 2012: 735392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Short NJ, Kantarjian HM, Ko H, et al. Outcomes of adults with relapsed or refractory Burkitt and high-grade B-cell leukemia/lymphoma. Am J Hematol 2017; 92: E114–E117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barnes JA, Lacasce AS, Feng Y, et al. Evaluation of the addition of rituximab to CODOX-M/IVAC for Burkitt’s lymphoma: a retrospective analysis. Ann Oncol 2011; 22: 1859–1864. [DOI] [PubMed] [Google Scholar]
- 68.Patte C, Auperin A, Michon J, et al. The Societe Francaise d’Oncologie Pediatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood 2001; 97: 3370–3379. [DOI] [PubMed] [Google Scholar]
- 69.Divine M, Casassus P, Koscielny S, et al. Burkitt lymphoma in adults: a prospective study of 72 patients treated with an adapted pediatric LMB protocol. Ann Oncol 2005; 16: 1928–1935. [DOI] [PubMed] [Google Scholar]
- 70.Reiter A, Schrappe M, Tiemann M, et al. Improved treatment results in childhood B-cell neoplasms with tailored intensification of therapy: A report of the Berlin-Frankfurt-Munster Group Trial NHL-BFM 90. Blood 1999; 94: 3294–3306. [PubMed] [Google Scholar]
- 71.Oriol A, Ribera JM, Bergua J, et al. High-dose chemotherapy and immunotherapy in adult Burkitt lymphoma: comparison of results in human immunodeficiency virus-infected and noninfected patients. Cancer 2008; 113: 117–125. [DOI] [PubMed] [Google Scholar]
- 72.Rizzieri DA, Johnson JL, Byrd JC, et al. Improved efficacy using rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or aggressive lymphomas: cancer and Leukemia Group B study 10 002. Br J Haematol 2014; 165: 102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ribera JM, Garcia O, Grande C, et al. Dose-intensive chemotherapy including rituximab in Burkitt’s leukemia or lymphoma regardless of human immunodeficiency virus infection status: final results of a phase 2 study (Burkimab). Cancer 2013; 119: 1660–1668. [DOI] [PubMed] [Google Scholar]
- 74.Dunleavy K, Pittaluga S, Janik J, et al. Novel Treatment of Burkitt Lymphoma with Dose-Adjusted EPOCH-Rituximab: Preliminary Results Showing Excellent Outcome. 2006; 108: 2736–2736. [Google Scholar]
- 75.Roschewski M, Dunleavy K, Abramson JS, et al. Risk-Adapted Therapy in Adults with Burkitt Lymphoma: Results of NCI 9177, a Multicenter Prospective Phase II Study of DA-EPOCH-R. 2017; 130: 188–188. [Google Scholar]
- 76.Oosten LEM, Chamuleau MED, Thielen FW, et al. Treatment of sporadic Burkitt lymphoma in adults, a retrospective comparison of four treatment regimens. Ann Hematol 2018; 97: 255–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castillo JJ, Winer ES, Olszewski AJ. Population-based prognostic factors for survival in patients with Burkitt lymphoma: an analysis from the Surveillance, Epidemiology, and End Results database. Cancer 2013; 119: 3672–3679. [DOI] [PubMed] [Google Scholar]
- 78.Jakobsen LH, Ellin F, Smeland KB, et al. Minimal relapse risk and early normalization of survival for patients with Burkitt lymphoma treated with intensive immunochemotherapy: an international study of 264 real-world patients. Br J Haematol 2020: doi: 10.1111/bjh.16425 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 79.Nkrumah FK, Perkins IV. Relapse in Burkitt’s lymphoma. Int J Cancer 1976; 17: 455–460. [DOI] [PubMed] [Google Scholar]
- 80.Sariban E, Edwards B, Janus C, Magrath I. Central nervous system involvement in American Burkitt’s lymphoma. J Clin Oncol 1983; 1: 677–681. [DOI] [PubMed] [Google Scholar]
- 81.Boehme V, Zeynalova S, Kloess M, et al. Incidence and risk factors of central nervous system recurrence in aggressive lymphoma--a survey of 1693 patients treated in protocols of the German High-Grade Non-Hodgkin’s Lymphoma Study Group (DSHNHL). Ann Oncol 2007; 18: 149–157. [DOI] [PubMed] [Google Scholar]
- 82.Zayac A, Evens AM, Stadnik A, et al. Outcomes of Patients with Newly-Diagnosed Burkitt Lymphoma (BL) and Central Nervous System (CNS) Involvement Treated in the Modern Era: A Multi-Institutional Real-World Analysis. Blood 2019; 134: 402. [Google Scholar]
- 83.Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol 2018; 19: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Decker DP, Egan PC, Zayac AS, Treaba DO, Olszewski AJ. Treatment strategies and risk of central nervous system recurrence in high-grade B-cell and Burkitt lymphoma. Leuk Lymphoma 2020; 61: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Alderuccio JP, Lossos IS. DA-EPOCH-R for Adult Burkitt’s Lymphoma: Pros and Cons. J Oncol Pract 2018; 14: 676–678. [DOI] [PubMed] [Google Scholar]
- 86.Sweetenham JW, Pearce R, Taghipour G, Blaise D, Gisselbrecht C, Goldstone AH. Adult Burkitt’s and Burkitt-like non-Hodgkin’s lymphoma--outcome for patients treated with high-dose therapy and autologous stem-cell transplantation in first remission or at relapse: results from the European Group for Blood and Marrow Transplantation. J Clin Oncol 1996; 14: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 87.Maramattom LV, Hari PN, Burns LJ, et al. Autologous and allogeneic transplantation for burkitt lymphoma outcomes and changes in utilization: a report from the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2013; 19: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Center for International Blood & Marrow Transplant Research. Transplant Activity Report. Accessed on 12 January, 2020 at https://bloodstemcell.hrsa.gov/data/donation-and-transplantation-statistics/transplant-activity-report.2020.
- 89.Tanaka Y, Momose S, Tabayashi T, et al. Abemaciclib, a CDK4/6 inhibitor, exerts preclinical activity against aggressive germinal center-derived B-cell lymphomas. Cancer Sci 2020; 111: 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ippolito T, Gu JJ, Tang G, et al. Targeting BET Bromodomains in Pre-Clinical Models of Burkitt Lymphoma. Blood 2016; 128: 5381–5381. [Google Scholar]
- 91.Ippolito T, Mavis C, Gu J, Hernandez-Ilizaliturri FJ, Barth MJ. Omipalisib (GSK458), a Dual an-PI3K/mTOR Inhibitor, Exhibits in Vitro and In Vivo activity in Chemotherapy-Sensitive and -Resistant Models of Burkitt Lymphoma. Blood 2018; 132: 2951–2951. [Google Scholar]
- 92.Bhatti M, Ippolito T, Mavis C, et al. Pre-clinical activity of targeting the PI3K/Akt/mTOR pathway in Burkitt lymphoma. Oncotarget 2018; 9: 21820–21830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dickinson M, Kamdar M, Huntly BJP, et al. A Phase I Study of Molibresib (GSK525762), a Selective Bromodomain (BRD) and Extra Terminal Protein (BET) Inhibitor: Results from Part 1 of a Phase I/II Open Label Single Agent Study in Subjects with Non-Hodgkin’s Lymphoma (NHL). Blood 2018; 132: 1682–1682. [Google Scholar]
- 94.Daly T, Ippolito T, Gu JJ, et al. MCL-1 Inhibition By the Selective MCL-1 Inhibitor AMG-176 Induces in Vitro Activity Against Burkitt Lymphoma Cell Lines and Synergistically Enhances the Cytotoxic Effect of Chemotherapy and BH3 Mimetics. Blood 2019; 134: 5303–5303. [Google Scholar]
- 95.Pardee TS, Lee K, Luddy J, et al. A phase I study of the first-in-class antimitochondrial metabolism agent, CPI-613, in patients with advanced hematologic malignancies. Clin Cancer Res 2014; 20: 5255–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Noy A, Pardee TS, Nikolaenko L, et al. A Phase II Clinical Trial of Cpi-613 (devimistat) in Patients with Relapsed or Refractory Burkitt Lymphoma/Leukemia or High-Grade B-Cell Lymphoma with Rearrangements of MYC and BCL2and/or BCL6. Blood 2019; 134: 4087. [Google Scholar]
- 97.Dunleavy K Approach to the Diagnosis and Treatment of Adult Burkitt’s Lymphoma. J Oncol Pract 2018; 14: 665–671. [DOI] [PubMed] [Google Scholar]
- 98.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N Engl J Med 2017; 377: 2531–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N Engl J Med 2019; 380: 45–56. [DOI] [PubMed] [Google Scholar]
- 100.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014; 371: 1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Avigdor A, Shouval R, Jacoby E, et al. CAR T cells induce a complete response in refractory Burkitt Lymphoma. Bone Marrow Transplant 2018; 53: 1583–1585. [DOI] [PubMed] [Google Scholar]
- 102.Riedell PA, Walling C, Nastoupil LJ, et al. A Multicenter Retrospective Analysis of Clinical Outcomes, Toxicities, and Patterns of Use in Institutions Utilizing Commercial Axicabtagene Ciloleucel and Tisagenlecleucel for Relapsed/Refractory Aggressive B-Cell Lymphomas. Blood 2019; 134: 1599. [Google Scholar]
- 103.Zhang W, Hu B, Jing L, et al. Early Response Observed in Pediatric Patients with Refractory/Relapsed B-Cell Non-Hodgkin Lymphoma Treated with Sequential Chimeric Antigen Receptor T Cells. Blood 2019; 134: 1945–1945. [Google Scholar]
- 104.Wang N, Hu X, Cao W, et al. Efficacy and safety of CAR19/22 T-cell cocktail therapy in patients with refractory/relapsed B-cell malignancies. Blood 2020; 135: 17–27. [DOI] [PubMed] [Google Scholar]
- 105.Feldmann A, Koristka S, Arndt C, et al. Development of Novel Anti-CD10 Target Modules for Redirection of Universal CAR T Cells Against CD10-Positive Malignancies. Blood 2019; 134: 5612–5612. [Google Scholar]
- 106.Goodridge JP, Mahmood S, Zhu H, et al. FT596: Translation of First-of-Kind Multi-Antigen Targeted Off-the-Shelf CAR-NK Cell with Engineered Persistence for the Treatment of B Cell Malignancies. Blood 2019; 134: 301–301. [Google Scholar]
- 107.Kantarjian H, Stein A, Gokbuget N, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med 2017; 376: 836–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Duell J, Zugmaier G, Eisele F, et al. Treatment of R/R Burkitt Lymphoma with Blinatumomab Is Feasible and Induced a Long Lasting Complete Remission. HemaSphere 2019; 3: 816–817. [Google Scholar]
- 109.Sokol KA, Barlogie B, Steinberg A. Blinatumumab as a Bridge to Transplant in ALL and the First Reported Use in Myeloma: Successes in Reaching Transplant but with Rapid Relapse. the Mount Sinai Hospital’s Institutional Experience. Biol Blood Marrow Transplant 2017; 23: S164. [Google Scholar]
- 110.Poh C, Frankel P, Ruel C, et al. Blinatumomab/Lenalidomide in Relapsed/Refractory Non-Hodgkin’s Lymphoma: A Phase I California Cancer Consortium Study of Safety, Efficacy and Immune Correlative Analysis. Blood 2019; 134: 760–760. [Google Scholar]
- 111.Budde LE, O’Hear C, Li C-C, et al. Mosunetuzumab Induces Complete Remissions in Poor Prognosis Non-Hodgkin Lymphoma Patients, Including Those Who Are Resistant to or Relapsing After Chimeric Antigen Receptor T-Cell (CAR-T) Therapies, and Is Active in Treatment through Multiple Lines. Blood 2019; 134: 6.31273004 [Google Scholar]
- 112.Hutchings M, Ahmadi T, Gupta M, et al. First-in-Human, Phase 1/2 Trial to Assess the Safety and Clinical Activity of Subcutaneous GEN3013 (DuoBody®-CD3×CD20) in B-Cell Non-Hodgkin Lymphomas. Blood 2019; 134: 758. [Google Scholar]
- 113.Magrath I, Adde M, Shad A, et al. Adults and children with small non-cleaved-cell lymphoma have a similar excellent outcome when treated with the same chemotherapy regimen. J Clin Oncol 1996; 14: 925–934. [DOI] [PubMed] [Google Scholar]
- 114.Mead GM, Barrans SL, Qian W, et al. A prospective clinicopathologic study of dose-modified CODOX-M/IVAC in patients with sporadic Burkitt lymphoma defined using cytogenetic and immunophenotypic criteria (MRC/NCRI LY10 trial). Blood 2008; 112: 2248–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Evens AM, Carson KR, Kolesar J, et al. A multicenter phase II study incorporating high-dose rituximab and liposomal doxorubicin into the CODOX-M/IVAC regimen for untreated Burkitt’s lymphoma. Ann Oncol 2013; 24: 3076–3081. [DOI] [PMC free article] [PubMed] [Google Scholar]