Abstract
Hypoxia is a common feature in tumors, driving pathways that promote epithelial-to-mesenchymal transition, invasion, and metastasis. Clinically, high levels of hypoxia-inducible factor (HIF) expression and stabilization at the primary site in many cancer types is associated with poor patient outcomes. Experimental evidence suggests that HIF signaling in the primary tumor promotes their dissemination to the bone, as well as the release of factors such as LOX that act distantly on the bone to stimulate osteolysis and form a pre-metastatic niche. Additionally, the bone itself is a generally hypoxic organ, fueling the activation of HIF signaling in bone resident cells, promoting tumor cell homing to the bone as well as osteoclastogenesis. The hypoxic microenvironment of the bone also stimulates the vicious cycle of tumor-induced bone destruction, further fueling tumor cell growth and osteolysis. Furthermore, hypoxia appears to regulate key tumor dormancy factors. Thus, hypoxia acts both on the tumor cells as well as the metastatic site itself to promote tumor cell metastasis.
Keywords: Cancer, HIF, Oxygen, Dormancy, Vasculature
1. Introduction
Hypoxia (low oxygen tensions) is a common feature in solid tumors, since the existing vasculature cannot support the high nutrient and oxygen demands of the dense mass of rapidly proliferating malignant cells. This acts as a stimulus to trigger angiogenesis and other cellular changes to help the tumor cells adapt to survive in this hostile environment. However, tumors will often still have regions of hypoxia even after the establishment of new blood vessels, since these new blood vessels are poorly organized and structurally abnormal, causing them to be leaky and leading to inefficient perfusion of the tumor [1]. For example, the median oxygen tension in breast tumors has been measured at approximately 28 mmHg (3.7%), whereas normal healthy breast tissue’s oxygen levels were around 65 mmHg (8.6%) [2]. Similarly, squamous cell carcinoma of the uterine cervix had a median pO2 of 9 mmHg (1.2%) while normal cervical tissue was around 42 mmHg (5.5%).
Cells sense and respond to hypoxia through the hypoxia-inducible factor (HIF) signaling pathway. The functional HIF transcription factor heterodimer forms when an oxygen-sensitive α subunit (HIF1α or HIF2α) binds to the constitutively expressed β subunit (HIF1β, encoded by the ARNT gene) [3-6]. While a third α subunit variant, HIF3α, has been identified, many splice variants of HIF3α are not oxygen sensitive, cannot dimerize with HIF1β, and do not have transcriptional regulatory functions [7-10]. Furthermore, the most common isoform of HIF3α shares only 74% identity with HIF1α and 52-58% identity with HIF2α [11, 12]. Therefore, HIF3α will not be discussed further in the context of this review, since HIF1α and HIF2α are the main drivers of hypoxia responsive pathways.
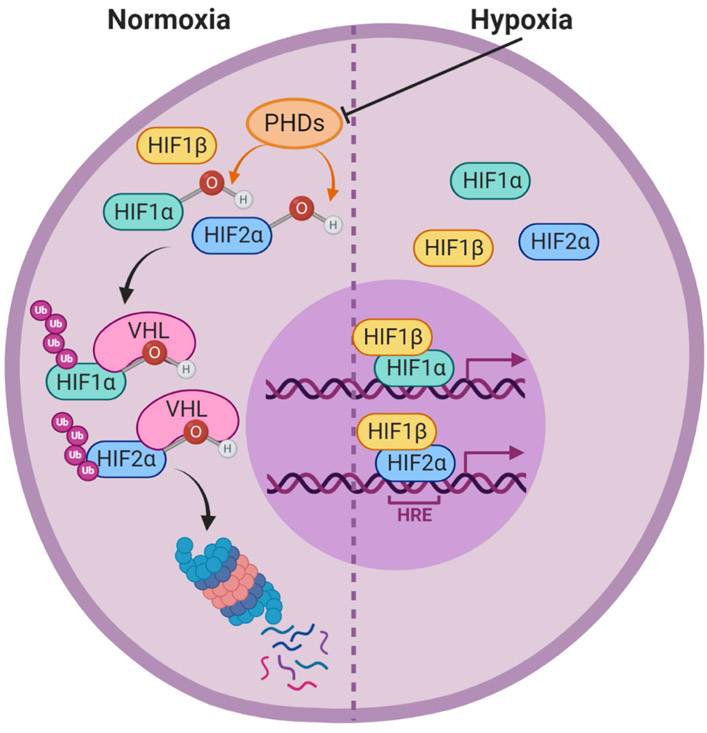
Under normoxia, α subunits are hydroxylated on certain prolyl residues by prolyl hydroxylase domain-containing enzymes (PHD1-4). These hydroxylations allow the von Hippel Lindau (VHL) E3 ubiquitin ligase to bind the α subunits and polyubiquitinate them, marking them for degradation by the proteasome [13-15]. PHD enzymes require molecular oxygen, α-ketoglutarate, ascorbate, and iron in order to be functional [16, 17]. Thus, under hypoxic conditions the PHD enzymes are non-functional and the α subunits are not hydroxylated and degraded. This allows the dimerization and nuclear translocation of the α and β subunit complex, which functions as a transcription factor for target genes by binding to hypoxia response elements (HREs) in the promoter region [18-20] (Figure 1). HIF signaling regulates the expression of many genes involved in angiogenesis and metabolism, allowing cells to survive in low oxygen while they recruit new blood vessels to restore ideal oxygen tensions [21-23]. One of the key genes that is upregulated in response to HIF signaling is vascular endothelial growth factor (VEGF), which stimulates angiogenesis by binding to VEGF receptor (VEGFR) on the surface of endothelial cells and causing them to migrate and assemble into new blood vessels [24, 25]. Increased microvessel density improves the delivery of oxygen and nutrients to the hypoxic cells, supporting further cell proliferation. HIF signaling also increases glycolysis by stimulating the expression of glucose transporters, like GLUT1, and glycolytic enzymes, like lactate dehydrogenase A [26-29]. These transcriptional changes allow cells to take up more glucose from their environment and derive sufficient energy from glycolysis alone during the time that the oxygen dependent electron transport chain is inactive, thus enabling cells to survive in oxygen-poor microenvironments.
Figure 1. Hypoxia-inducible factor signaling pathway.
Under normoxic conditions, prolyl hydroxylase domain containing enzymes (PHDs) hydroxylate the hypoxia inducible factor alpha subunits (HIF1α and HIF2α). These hydroxylations allow the E3 ubituitin ligase von Hippen Lindau (VHL) to ubiquitinate HIF1α and HIF2α, leading to their proteasomal degradation. Under hypoxic conditions, the PHD enzymes are inactive, allowing HIF1α and HIF2α to accumulate in the cytoplasm. The alpha subunits will then dimerize with HIF1β, enter the nucleus, and bind to hypoxia response elements (HREs) in the DNA to act as transcription factors and drive the expression of hypoxia responsive genes.
In the context of tumor cells, HIF signaling also regulates genes involved in epithelial-to-mesenchymal transition, invasion, and the regulation of matrix composition, which promotes the metastasis of malignant cells [30-32]. This is supported by clinical data from breast cancer patients, which found that more intense HIF1α staining in the primary tumor was associated with poor outcomes such as reduced overall survival, therapy resistance, and early relapse [33-36]. HIF1α overexpression was also found to be associated with shorter overall patient survival as and recurrence-free survival in lung cancer [37], and increased intratumoral HIF1α staining predicts poor outcome and response to therapy in many tumor types, including oropharyngeal cancer [38], oral squamous cell carcinoma [39], oligodendroglioma [40], epithelial ovarian cancer [41], and cervical cancer [42]. A meta-analysis of HIF2α as a prognostic marker similarly showed that high HIF2α expression was associated with decreased overall survival, disease-specific survival, disease-free survival, metastasis-free survival, and progression-free survival in patients suffering from various types of solid tumors [43]. While hypoxia has classically been considered a feature of solid tumors rather than hematological malignancies, the increased hypoxia in myelomatous and leukemic bone marrow is now well-established [44-47]. Furthermore, in acute myeloid leukemia, hypoxia promotes chemoresistance [48] and increased HIF1α expression is associated with decreased overall survival and event-free survival [49].
Hypoxia and HIF signaling also play critical roles in skeletal development and osteogenesis. For example, HIF1α and VHL expression in osteoblast-lineage cells, defined by Osterix (OSX) expression, impacts bone mass in mouse models, with Hif1α deletion resulting in reduced bone mass and Vhl deletion resulting in dramatically increased bone volume [50]. Similarly, combined deletion of PHD1, PHD2, and PHD3, which stabilizes HIF, in OSX-lineage cells causes excessive trabecular bone growth due to overly active HIF signaling [51]. While the actions of HIF signaling in endochondral ossification and growth plate development, for example, are well described in the literature [52], this review will focus on the role of hypoxia in metastasis, tumor growth in the bone, and osteolysis.
2. Blood flow and hypoxia in bone
The bone marrow is the site of hematopoiesis and thus houses a vast array of cell types including hematopoietic stem cells, various progenitor cells, mature immune cells, megakaryocytes, as well as osteoblasts and osteoclasts that control bone turnover and homeostasis. Thus, the bone marrow is highly vascularized to support the metabolic needs of all of these cells. The vascular structure of the bone was first described in the 1950s and 1960s, where it was found to possess a similar hierarchical organization of blood flow as many other organs [53-56]. The bone has large arterial vessels that feed into a dense capillary network before eventually draining into a large central vein, which in the case of long bones runs along the center of the diaphysis, the main shaft that contains the marrow [55, 57, 58]. As very dense cortical bone encases the bone marrow, blood vessels can only enter and exit the bone at specific points. High-resolution microscopy of murine long bones has revealed that they are supplied by approximately 16 nutrient arteries that feed into the endosteal capillary network [59]. These arterial capillaries eventually connect to the venous tree, where blood drains into the large central sinus and then exits the bone through one of two exit sites. In addition, hundreds of transcortical capillaries were discovered that traverse perpendicularly through the cortical bone along the entire bone shaft, forming a direct connection between the endosteal and periosteal circulations. Surprisingly, these transcortical capillaries are responsible for roughly 80% of arterial, and 59% of venous blood flow, indicating that these small vessels are responsible for the majority of blood supply to the bone rather than the larger nutrient arteries.
Another challenge that is presented by the dense cortical bone is that it has been extremely challenging to measure the oxygen tensions within the bone marrow. Mathematical models have attempted to estimate the oxygen levels in bone marrow, but the complicated cellular composition and structural intricacies of the bone limit the accuracy of the estimates [60-62]. For example, the various bone marrow resident cell types have different oxygen consumption rates, the distribution of different cell types throughout bone marrow niches is not uniform or well defined, and the structure of the trabecular bone and capillaries beds are extremely complex. These models estimate that the oxygen tensions at the inner layer of trabecular bone range from 2.5 to 35.2 mmHg (0.33-4.63% pO2), depending on the thickness of the trabeculum and the distance from a capillary [62].
While the oxygen levels in long bones have not yet been measured, two-photon phosphorescence lifetime microscopy has allowed for the direct measurement of calvarial bone marrow oxygen tensions in live mice [63]. This revealed that while the bone marrow is a highly vascularized tissue, the bone is a generally hypoxic organ that contains niches of severe hypoxia. Using two-photon in vivo imaging, Spencer et al. followed individual blood vessels as they ran deeper into the bone marrow. From this longitudinal analysis, they observed rapid oxygen depletion as the blood traveled from the cortical bone, a region of low cellularity, to the bone marrow, a densely cellular tissue. Endosteal regions were found to be better oxygenated, with a mean intravascular pO2 of 21.9 mmHg (2.9%) and extravascular pO2 of 13.5 mmHg (1.8%), compared to deeper sinusoidal regions that had a mean intravascular pO2 of 17.7 mmHg (2.4%) and extravascular pO2 of 9.9 mmHg (1.3%). These measurements also show that in addition to oxygen tensions decreasing along the length of the blood vessel, they drop sharply between intravascular and extravascular readings. Thus, even bone marrow that is close to blood vessels experience low oxygen levels, with extravascular oxygen tension measurements ranging from 4.8 to 21.1 mmHg (0.6-2.8%). This rapid oxygen depletion, both longitudinally and laterally, is likely due to the high metabolic demands of the bone marrow, in accordance with its highly cellular nature and actively dividing cell populations. It is important to note that these measurements are from the calvaria of mice, which is structurally distinct from other bones such as long bones or vertebrae that are also common anatomical sites of metastasis. However, bone marrow in these other sites would likely be subject to oxygen tensions as low as what was measured here, or possibly even lower. As will be discussed below, the hypoxic conditions that bone metastatic cells and bone resident cells experience have important implications for tumor growth in the bone and osteolysis.
3. Hypoxia in Bone Metastasis
While hypoxia and active HIF signaling in primary tumors is predicted to drive distant metastasis, a direct relationship between tumor hypoxia and bone metastasis has been difficult to establish. Clinical evidence supports this theory, as increased HIF1α expression, as well as decreased VHL expression, has been observed in primary breast tumor samples from patients with bone marrow metastasis compared to those with no tumor cells detectable in their bone marrow [64].
Lu et al. attempted to address this question of whether HIF signaling in the primary tumor drives bone metastasis. They found that MDA-MB-231 variant cells expressing DN-HIF1α formed significantly fewer bone metastases than control cells, following mammary fat pad injection [65]. Liao et al. performed a similar study using a genetically modified mouse model that had mammary specific deletion of Hif1α and expression of the polyoma middle T antigen (PyMT) that drives spontaneous mammary tumorigenesis [66]. These mice had decreased pulmonary metastasis compared to control mice, but tumor dissemination to bone was not evaluated in this study. Similarly, a recent study found that a HIF2α-driven long non-coding RNA, RAB11B-AS1, increases dissemination to, and colonization of, the lung and liver following orthotopic injection of human breast cancer MDA-MB-231 cells [67]. This study found that RAB11B-AS1 overexpression in MDA-MB-231 cells increased their migration and invasion and upregulated the expression of angiogenesis genes like VEGFA and ANGPTL4, thereby promoting distant metastasis. While these studies establish that active HIF signaling in the primary tumor is capable of driving dissemination to distant sites, only the study by Lu et al. evaluated dissemination to the bone. No studies using a spontaneous tumor model have yet validated that HIF signaling factors drive bone metastasis.
Whether dissemination from the primary site to the bone is HIF dependent remains unclear, but the role of HIF signaling in tumor growth and osteolysis once tumor cells reach the bone marrow has been investigated more directly. Hiraga et al. generated MDA-MB-231 cells that expressed a dominant negative (DN) form of HIF1α or a constitutively active (CA) HIF1α and compared tibial tumor volume following intracardiac inoculation [68]. The mice injected with DN-HIF1α cells had significantly reduced tumor burden in the tibia compared to empty vector control cells, while CA-HIF1α cells grew more aggressively in the tibia and induced greater osteolysis. CA-HIF1α cells expressed significantly higher levels of VEGF in vitro, and hypoxia inhibited the differentiation of mesenchymal cells (C3H10T1/2) to alkaline phosphatase-positive osteoblast-like cells in response to BMP2 stimulation. Hypoxia also increased TRAP-positive osteoclast-like cell formation from spleen cell cultures treated with soluble RANKL and M-CSF. Taken together, this indicates that hypoxia causes tumor cells to recruit additional vasculature to support their outgrowth in the bone, while simultaneously shifting the activity of bone resident cells to promote osteolysis. In a separate study, HIF1α knockdown in MDA-MB-231 cells decreased bone colonization and increased survival in nude mice inoculated with the tumor cells via intracardiac injection [69]. HIF1α knockdown also decreased the microvessel density within the bone metastatic lesions they observed in vivo, and culturing MDA-MB-231 cells in 1% O2 caused the cells to upregulate VEGF and CXCR4 transcription in a HIF1α-dependent manner.
When discussing bone metastasis, it is important to recognize that the immune system also impacts primary tumor growth, metastasis, and growth of disseminated cells [70]. Tumor associated macrophages have been recognized for their importance in driving angiogenesis, tumor growth, invasion, and metastasis [71-73]. In the primary tumor, the presence of regulatory T cells (Tregs) has been associated with poor prognosis [74], and Tregs are increased in the bone marrow of prostate cancer patients with bone marrow metastases [75]. In addition, Tregs have been found to produce RANKL [76], suggesting they may contribute to the cycle of bone destruction. Cytotoxic T cells are one of the main cell types responsible for the destruction of tumor cells, but their activity can be inhibited by TGF-β released from osteoclastic resorption of bone [77, 78]. Along with cytotoxic T cells, natural killer (NK) cells are important in tumor cell destruction and are often responsible for killing metastatic cells while they are in circulation, preventing metastatic seeding [79]. Furthermore, depletion of NK cells and cytotoxic T cells has been shown to drive metastasis to bone in a 4T1 cell model of breast cancer [80].
It is important to note that many of the studies investigating the role of hypoxia in bone metastasis and tumor growth in bone have used MDA-MB-231 human breast cancer cells to model tumor growth in mice. While MDA-MB-231 cells grow aggressively and reliably colonize the bone without the need for exogenous estradiol supplementation, allowing researchers to study mechanisms involved in dissemination and osteolysis with phenotypically normal bone, they require the use of immunocompromised mice. Athymic nude mice are most frequently used, meaning the impact of T cells on the processes under investigation cannot be modeled [81]. Studies employing NSG mice would be further limited in their ability to model physiological disease processes, as they lack functional T and B cells, NK cells, macrophages, and dendritic cells (REF). Hypoxia is known to modulate the function of various immune cell populations, and could thus affect the frequency of metastasis from the primary tumor, or the ability of tumor cells to colonize the bone marrow. Culturing T-cells in 1% O2 was found to induce the expression of FOXP3, a key regulator of Treg cell differentiation, in a HIF1α dependent manner [82]. Thus, extremely hypoxic regions of the bone microenvironment may increase Treg cell differentiation and promote bone metastasis or osteolysis [74-76]. Additionally, HIF1α, but not HIF2α, was shown to drive the expression of programmed death ligand 1 (PD-L1), an immune checkpoint regulator, by binding to an HRE in the PD-L1 proximal promoter [83]. Hypoxia was found to drive PD-L1 expression in myeloid derived suppressor cells, macrophages, dendritic cells, as well tumor cells (B16-F10, LLC, CT26, 4T1). Increased interactions of PD-L1 and PD-1 on T cells leads to the inactivation of T cells, creating a permissive environment for tumor cell growth. This could occur in the primary tumor or in metastatic lesions. Furthermore, microenvironmental acidification, a common feature among hypoxic solid tumors, can cause T cells to become anergic [84], similarly leading to a growth permissive microenvironment. Taken together, hypoxia drives the generation of a microenvironment in which T cell functions are inhibited, or are skewed toward an immunosuppressive phenotype. Interestingly, HIF signaling also plays a role in macrophage polarization, where HIF1α is induced by Th1 cytokine during M1 polarization, while HIF2α is induced in response to Th2 cytokines during M2 polarization [85]. HIF1α then drives the expression of pro-inflammatory M1 macrophage effector genes like nitric oxide synthase (NOS), whereas HIF2α regulates effectors in the anti-inflammatory, pro-repair, or tumor-associated M2 macrophages like arginase I (Arg-I). In vitro studies have shown that intermittent hypoxia promotes M1 polarization [86, 87]. Since primary tumors often have fluctuating oxygen levels due to inefficient and disorganized vasculature [88], these conditions could promote pro-inflammatory M1 polarization. However, with the other immune dampening effect of hypoxia, it is difficult to predict whether these M1 macrophages could still exert their typical anti-tumor effects. As a whole, hypoxia appears to dampen the anti-tumor immune response, thereby further tipping the scale in favor of tumor growth and metastasis.
In addition to hypoxia playing a role in tumor growth and metastasis to the bone, hypoxia also promotes the proliferation of osteosarcoma cells, a primary malignant bone tumor. Zhang et al. [89] showed that osteosarcomas are often hypoxic and that hypoxia is capable of upregulating platelet-derived growth factor (PDGF) and platelet-derived growth factor receptor (PDGFR) in osteosarcoma cell in vitro, which has previously been shown to be associated with poor patient outcomes [90-93]. Specifically, they showed that hypoxia increases PDGF-BB and PDGFR-β, and that this expression was HIF1α dependent. PDGF-BB was found to promote osteosarcoma cell proliferation and migration by activating AKT, ERK1/2, and STAT3 signaling.
As discussed above, due to the shifting metabolic needs of tumors and the abnormal vasculature that is formed, hypoxia within tumors is highly variable, heterogeneous, and transient. Thus, not all tumor cells within a tumor experience hypoxia. Harrison et al. modeled this heterogeneity by creating an inducible HIF1α construct [94]. They fused a mutant form of HIF1α that is stable under normoxia to a destabilization domain, creating an inducible HIF1α that is only stable in the presence of the inducer molecule trimethoprim. MCF7 cells expressing this construct were co-cultured with wild type MCF7 cells, creating a mixture of cells mimicking normoxic and hypoxic cell signaling. Increased hypoxia response gene expression was observed in wild type MCF7 cells following co-culture, as indicated by elevated VEGF, erythropoietin (EPO), and carbonic anhydrase IX (CAIX) mRNA. This suggests that cells with active HIF signaling are capable of inducing a hypoxic-like state in neighboring cells, even when these neighboring cells do not have direct hypoxia-triggered HIF activation. While the mechanism by which this occurs is unclear, these findings suggest that tumoral hypoxia in one region of the tumor could promote the invasive and metastatic capabilities of neighboring tumor cells, contributing to tumor invasion, metastasis, and poor patient outcomes.
Supporting this idea, tumor cells experiencing hypoxia have also been shown to exert effects on surrounding tumor cells in their microenvironment that promote tumor cell survival, invasion, and metastasis. Hypoxia was found to increase extracellular vesicle release from prostate cancer cell lines (MiaPaCa and AsPC-1) in a HIF1α dependent manner, and change the size distribution of the extracellular vesicles that were released, favoring smaller vesicles [95]. Furthermore, treating these prostate cancer cells with the small extracellular vesicles released from hypoxic cells conferred a growth advantage under hypoxic conditions, compared to treatment by small extracellular vesicles collected from normoxic cells. This suggests that hypoxia not only alters the number and size of the extracellular vesicles, but also the composition of the extracellular vesicles to increase tumor cell survival in these growth-limiting conditions. Another study showed that the expression of RAB22A, a small GTPase that localizes at the plasma membrane with budding microvesicles, has been shown to be hypoxia dependent [96]. Culturing breast cancer cells (MDA-MB-231, MDA-MB-453, or MCF7) in hypoxia increased the number of microvesicles formed as well as RAB22A mRNA levels, and incubating breast cancer cells with these microvesicles induces focal adhesion formation and invasion, in vitro. Furthermore, RAB22A knockdown in MDA-MB-231 cells reduced pulmonary metastasis following orthotopic implantation. Clinically, RAB22A mRNA overexpression in primary tumors was found to be associated with decreased overall survival and metastasis-free survival. The implications of RAB22A induced tumor microvesicles on bone metastasis, however, was not investigated. It is unclear whether these microvesicles could localize to the bone marrow and contribute to the establishment of a pre-metastatic niche by altering the bone marrow to make an environment that is more permissive to the survival and outgrowth of metastatic cells. Other studies have shown that tumor-derived exosomes, another type of extracellular vesicle, are capable of localizing to the bone marrow after intravenous injection, supporting the possibility that hypoxia stimulated extracellular vesicles may similarly be able to home to the bone [97]. Furthermore, tumor-derived extracellular vesicles have been shown to alter the activity of bone-resident cells in multiple tumor types [98]. For example, amphiregulin-containing exosomes released from non-small cell lung cancer cells have been found to induce EGFR signaling in preosteoclasts and promote osteoclastogenesis in vitro [99], and multiple myeloma exosomes simultaneously enhanced osteoclast activity and inhibited osteoblast differentiation and activity [100]. Additionally, exosome-mediated transfer of pyruvate kinase M2 (PKM2) from primary prostate cancer cells to bone marrow stromal cells was shown to increase CXCL12 expression in the bone marrow stromal cells in a HIF1α-dependent manner, enhancing seeding and growth of metastatic cells in the bone marrow [101]. These examples illustrate how hypoxic tumor cell-derived extracellular vesicles may be capable of altering the bone metastatic niche, an already fertile soil for tumor cells, and make it even more amenable to metastatic seeding and outgrowth. The establishment of such pre-metastatic niches has mainly been reported in the lungs, where primary breast tumor secreted factors and extracellular vesicles have been shown to induce vascular leakiness, alter the behavior of stromal cells, remodel the extracellular matrix (ECM), trigger the recruitment of bone marrow derived cells (BMDCs) to the lungs [102-105]. These BMDCs then secrete inflammatory cytokines, growth factors, proangiogenic factors, and ECM altering factors such as matrix metalloproteinase 2 (MMP2), preparing the site for the arrival of metastatic cells [102, 103]. Tumor derived factors have also been shown to modulate the behavior of perivascular cells, inducing a phenotypic switch to a less differentiated state, marked by expression of the pluripotency gene KLF4 [106]. These perivascular cells have enhanced ECM deposition, which in turn supports metastasis. Importantly, recent research supports the notion that pre-metastatic niches can form in the bone marrow as well [101]. This new paradigm may further deepen our understanding of why certain cancer types preferentially metastasize to the bone, or why certain patients develop bone metastases while other do not.
Apart from the role of HIF signaling in the tumor cells, active HIF signaling in bone resident cells also controls metastasis to the bone. Devignes et al. established that HIF1α and VHL expression in osteoblast-lineage cells could influence the ability of injected tumor cells to colonize the bone [107]. When mice lacking Hif1a expression specifically in osteoblast-lineage cells (ΔHif1αOSX mice) were inoculated with a PyMT-derived cell line via intracardiac or orthotopic injection, ΔHif1αOSX mice developed bone metastases less frequently than control mice. Interestingly, the primary tumors that grew after orthotopic injection were also significantly smaller in ΔHif1αOSX mice. Conversely, ΔVhlOSX mice developed bone metastases more frequently and more rapidly than control mice after intracardiac or orthotopic injection, and grew larger, more proliferative primary tumors. This indicates that signals from bone resident cells are capable of controlling distant tumor proliferation. This bone-imposed control of tumor growth was found to be CXCL12 mediated, where ΔHif1αOSX mice had decreased numbers of CXCL12+ osteoprogenitor cells, whereas ΔVhlOSX mice had significantly more CXCL12+ osteoprogenitor cells and also had higher circulating plasma CXCL12 levels. CXCL12 expression can be induced by hypoxia [108-111] and is known to promote mammary tumor cell growth and dissemination by signaling through CXCR4 on breast cancer cells [112]. These findings underscore the fact that the effects of hypoxia and HIF signaling do not only have local effects, but can exert control on distant seemingly unrelated organs to impact tumor growth.
4. Hypoxia’s Role in Tumor-Induced Osteolysis
Hypoxic signaling in the bone can exert distant effects on tumor cells to influence their growth and metastasis, but HIF signaling in tumor cells can also have distant effects on the bone to promote osteolysis and prepare the bone for colonization (Figure 2). One such hypoxia-induced tumor-secreted factor is lysyl oxidase (LOX). The LOX family of secreted copper-dependent amine oxidases covalently crosslink collagen and elastin to increase ECM, which has been shown to increase tumor cell invasion in breast and colorectal cancer [113-115]. LOX expression can be HIF1α induced, and increased expression of some LOX family members has been reported in colorectal, breast, prostate, lung, and bladder cancer [113, 114, 116-118]. Reynaud et al. identified that tumor-secreted LOX in colorectal cancer generates osteolytic lesions in mice following intra-arterial inoculation of LOX overexpression Hct116 human colorectal cancer cells [119]. LOX overexpression led to greater total bone lysis area, increased bone tumor burden, and increased numbers of osteoclasts in the bone. Furthermore, treating LOX overexpressing Hct116 tumor bearing mice with a LOX inhibitor prolonged metastasis-free survival to control levels, confirming that LOX is capable of driving this increased metastasis and osteolysis. Similar findings are reported from Cox et al. in the context of breast cancer showing that LOX drives osteoclastogenesis [120]. They found that injecting mice with 4T1 mouse mammary carcinoma cells (expressing high levels of LOX) resulted in osteolytic lesion formation, which could be mitigated by genetic silencing or antibody-based inhibition of LOX. Additionally, they found that LOX acts as a potent activator of osteoclastogenesis by triggering the nuclear translocation of NFATc1, the master regulator of osteoclastogenesis. Pre-conditioning mice with conditioned media from 4T1 cells increased tumor burden following intracardiac injection of 4T1 cells, suggesting that LOX secreted by tumors induces osteoclastogenesis to create a pre-metastatic niche that is more favorable for tumor growth.
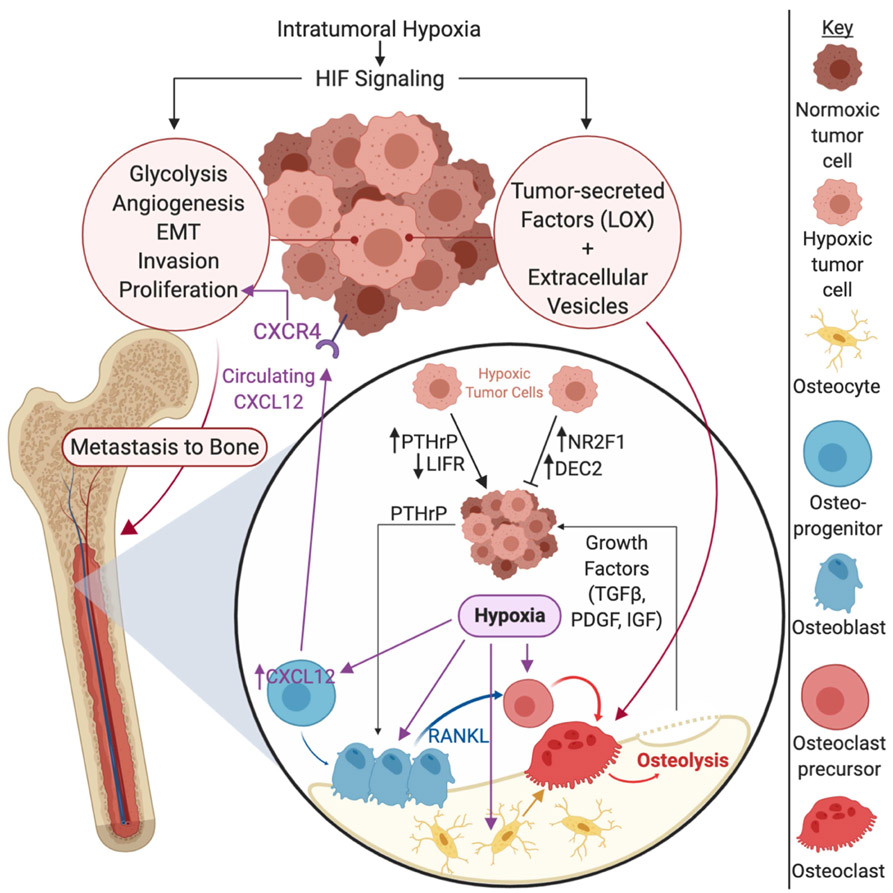
Figure 2. Hypoxia driven factors in the primary tumor and bone metastatic site.
Intratumoral hypoxia in the tumor drives hypoxia inducible factor (HIF) signaling within tumor cells, which drives pro-survival and pro-metastatic processes such as glycolysis, angiogenesis, epithelial-to-mesenchymal transition (EMT), invasion, and tumor cell proliferation. HIF signaling also causes tumor cells to secrete factors such as lysyl oxidase (LOX) and to alter extracellular vesicle production, promoting osteolysis. Once tumor cells reach the bone, a physiologically hypoxic microenvironment, hypoxic tumor cells can be maintained in a dormant state, marked by increased expression of nuclear receptor subfamily 2 group F member 1 (NR2F1) and differentially expressed in chondrocytes 2 (DEC2). On the other hand, hypoxia may drive tumor cells out of dormancy by inducing parathyroid hormone related protein (PTHrP) expression and down regulating leukemia inhibitory factor receptor (LIFR), promoting the establishment of clinically significant metastatic lesions. Hypoxia also promotes the expression of PTHrP, driving the vicious cycle of bone destruction. Hypoxia in the bone also promotes osteolysis by direct stimulation of osteoclastogenic factors by osteoblasts, bone marrow stromal cells, and osteocytes. Intermittent hypoxia also stimulates osteoclastogenesis and osteoclast function. Additionally, hypoxia stimulates CXCL12 expression on osteoblast progenitors and increases circulating CXCL12 levels, which can stimulate the growth and metastasis of primary breast cancer cells through CXCR4, indicating that hypoxia in the bone can exert effects on the primary tumor site as well. Additional abbreviations: TGFβ = transforming growth factor receptor beta; PDGF = platelet derived growth factor; IGF = insulin-like growth factor.
These osteolytic lesions often result from a process called the vicious cycle of bone destruction [121], which is observed in several cancer types but has been mainly characterized in breast cancer. In this cycle, tumor cells growing in the bone marrow produce parathyroid hormone related protein (PTHrP) [122-127], which functions in a similar manner to parathyroid hormone (PTH), which stimulates calcium release from bone [128]. PTHrP binds to the parathyroid hormone 1 receptor (PTH1R) on the surface of osteoblasts [129-131], which will in turn stimulate the production of receptor activator of nuclear factor kappa-B ligand (RANKL) and inhibit osteoprotegrin (OPG) production from osteoblasts [132]. RANKL can then bind RANK on the surface of osteoclast precursors to stimulate their maturation [133-135]. OPG is a soluble decoy receptor for RANKL [136], so an increase in RANKL and a decrease in OPG will collectively increase osteoclastogenesis and bone resorption, causing the release of growth factors such as transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF) and insulin-like growth factors (IGFs) that are stored in the bone matrix [137-141]. These growth factors will then stimulate the growth of the tumor cells to drive the cycle forward and cause further osteoclastogenesis and osteolysis [121, 142]. HIF signaling is known to partially drive this process, as HIF2α, but not HIF1α, stimulates the expression of PTHrP [143]. Thus, the hypoxic conditions that metastatic tumor cells experience in the bone, a generally hypoxic organ, could promote PTHrP production from tumor cells. Recent studies have also shown that PTHrP expression is hypoxia inducible in chondrocytes, confirming that this regulation is not specific to breast cancer cells alone [144, 145]. PTHrP transcription in chondrocytes is responsive to both HIF1α and HIF2α, though, indicating that the exact mechanism of regulation may be subtly different between cell types [145]. Hypoxia has also been shown to drive RANK and RANKL expression in breast cancer cells [146], potentially driving osteolysis through a more direct tumor-osteoclast interaction.
Hypoxia can also cause other cell types in the bone to produce osteoclastogenic factors. Hypoxia causes osteoblastic cells to upregulate VEGF [147, 148] production, bone marrow stromal cells to increase IGF2 production [149], and osteocytes to upregulate the production of growth differentiation factor 15 (GDF15) [150], all of which promotes osteoclastic differentiation. Interestingly, HIF-signaling in osteoblasts has also been shown to drive OPG production, thus inhibiting osteoclastogenesis [51, 151]. There are some conflicting reports, however, on whether the regulation of RANKL, a key osteoclast stimulatory factor, is HIF dependent. Lee et al. recently reported that genetic deletion of HIF2α in osteoblasts (using Col1a1-Cre) resulted in mice with increased bone mass, and that RANKL is regulated in a HIF2α-dependent manner [152]. Wu et al. had previously reported that increased HIF signaling resulting from genetic deletion of PHD2 and PHD3 in osteoprogenitor cells (using Osx-Cre) caused increased bone mass through the induction of OPG, but did not alter RANKL production of osteoblasts [51]. Additionally, Wu et al. created variants of the PHD2/3 knockout mice expressing constitutively active forms of HIF1α or HIF2α in their osteoblasts and confirmed that RANKL expression was not significantly increased in either case. These findings are consistent with a report by Shao et al. in which they found that in vitro deletion of VHL in primary calvarial osteoblasts, and thus upregulation of HIF1α (HIF2α was not evaluated), increased OPG expression but not RANKL expression [151]. Furthermore, these results are supported by earlier reports that VHL deletion in osteoblasts causes high bone density [153]. The reason for the differing results pertaining to the role of HIF2α in RANKL induction is unclear, but could be due to differences in the mouse strains or genetic markers used for osteoblast-specific deletion of targets of interest. Taken together, it is clear that HIF signaling is capable of modulating the activity of many bone resident cells and plays a critical role in the regulation of bone turnover and the development of osteolytic lesions.
Hypoxia has also been shown to stimulate osteoclastogenesis more directly. Arnett et al. found that culturing mouse marrow cells with M-CSF and RANKL on dentine in 2% oxygen in vitro increased resorption area by 9.5-fold and osteoclast number by 3.5-fold over a 13 day period, compared to atmospheric oxygen tensions [154]. Interestingly, culturing mature rat osteoclasts on dentine in low oxygen tensions reduced the number of osteoclasts and the resorption area, suggesting that the hypoxic stimulation of osteolysis is likely acting on preosteoclasts. These experiments were performed in sealed flasks that were filled with gas mixtures containing specific percentages of oxygen, and re-gassed daily. This means that the exact oxygen tension in the flask would fluctuate as the oxygen is depleted and then replenished, causing the cells to undergo cycles of reoxygenation. A later study using a gloved hypoxia chamber confirmed that short-term hypoxia (24 hours) stimulates osteoclast activity but also initiates osteoclast apoptosis [155]. Furthermore, this study confirmed that reoxygenation following short-term hypoxia is able to rescue osteoclasts from apoptosis, indicating that short-term hypoxia may promote osteoclastogenesis, while osteoclasts are unable to survive in extended periods of hypoxia. A recent study confirmed that constant hypoxia inhibits osteoclast differentiation, and thus bone resorption, and found that this was mediated by decreasing the phosphorylation of c-Jun N-terminal kinase (JNK) and nuclear factor κBα (IκBα) [156]. This study also found that hypoxia abrogated NFATc1 transcriptional upregulation in response to M-CSF and soluble RANKL treatment in RAW264.7 macrophage cells or bone marrow derived monocytes. Several studies also show that HIF1α induction inhibits osteoclastogenesis [157-159], but some of the findings indicate that osteoclast differentiation in hypoxia enhances the resorptive capabilities of the cells once they mature [158]. Thus, the regulation of osteoclastogenesis and osteoclast activity appears to be very sensitive to the severity and duration of hypoxia, as well as the timing of when the differentiating cells experience hypoxia.
A recent study proposed a promising new hypoxia modulating therapeutic avenue to treat bone metastases and decrease osteolytic bone destructions [160]. Transcutaneous CO2 administration increases the CO2 concentration in the treated tissues, causing increased oxygen unloading from hemoglobin in red blood cells. Following intratibial inoculation of mice with MDA-MB-231 cells, transcutaneous CO2 administration decreased tumor growth by 2-fold, inhibited osteolysis, and significantly decreased HIF1α stabilization. The number of osteoclasts was also significantly decreased and RANKL expression in the bone was similarly inhibited. Notably, transcutaneous CO2 treatment was initiated 4 weeks post tumor cell inoculation, and was only performed for 10 minutes, twice a week, for 2 weeks. The ability of this minimally invasive treatment to stall the growth of established tumors makes it a clinically attractive avenue that warrants further investigation.
Since high intratumoral HIF1α and HIF2α levels are known to correlate with poor patient outcomes in many cancer types, as discussed previously, several HIF1α and HIF2α targeting agents have been tested clinically. PX-478, a selective HIF1α inhibitor (NCT00522652), EZN-2968, an antisense oligonucleotide inhibitor of HIF1α (NCT00466583 and NCT01120288), and RO7070179, a HIF1α mRNA antagonist (NCT02564614), have all completed phase 1 clinical trials for patient with advanced solid tumors. One of the EZN-2968 trials (NCT01120288) exhibited some preliminary evidence that this agent was capable of decreasing HIF1α mRNA and proteins levels in solid tumors, but had to be closed prematurely when further development was suspended [161]. Several trials investigating HIF2α inhibitors are also currently underway, most of which focus on clear cell renal cell carcinoma: PT2977 (Phase 1 NCT02974738 and Phase 2 NCT03634540), MK-6482 (Phase 3 NCT04195750), PT2385 (Phase 1 NCT02293980 and Phase 2 NCT03108066, NCT03216499). An RNA interference therapeutic (ARO-HIF2) is also currently under investigation in a phase 1 trial (NCT04169711). In addition to these drugs that directly target HIF subunits, some drugs are being developed that are only activated in hypoxia in hopes of improving tumor-specific drug activity. For example, TH-302, a hypoxia-activated prodrug has completed phase 1 testing for use in treating patients with advanced kidney cancer or liver cancer that cannot be removed by surgery (NCT01497444). Thus, while hypoxia targeting therapies are not currently in routine clinical use, this therapeutic direction is actively under investigation.
5. Hypoxia’s Role in Tumor Dormancy
Upon tumor dissemination to bone, the cells may begin to grow and form clinically detectable macrometastatic lesions that degrade the bone and cause severe bone pain, fractures, and hypercalcemia [162, 163]. Alternatively, tumor cells may enter a dormant state after arrival in the bone, where they can remain for a prolonged period before eventually growing into a macrometastatic lesion. The theory of tumor dormancy is supported by clinical observations that some cancer patients relapse many years after initial treatment and declaration of “cancer free” status. In breast cancer, estrogen receptor (ER) positive disease is particularly notable for its long window between initial treatment and relapse [164]. Additionally, the bone is the most common site of metastasis for breast cancer [165]; thus tumor dormancy in bone is frequently studied in the context of this disease.
Tumor dormancy remains a poorly defined process, and multiple types of dormancy have been described and hypothesized [164]. The most commonly accepted definition of dormancy is a non-proliferative quiescent tumor cell that is arrested in G0-G1 phase of the cell cycle, as indicated by negative staining for Ki-67 and/or PCNA [166, 167]. In this “cellular dormancy” model, some reactivation stimulus would cause this dormant disseminated tumor cell to reenter the cell cycle, proliferate, and grow into a detectable metastasis. In the “micrometastatic dormancy” model, the disseminated tumor cell exists in the context of a cluster of cells as a clinically undetectable micrometastasis. These micrometastases are hypothesized to have balanced rates of proliferation and cell death, leading to a stable micrometastasis. Another mechanism that has been proposed in the micrometastatic dormancy paradigm is the possibility that micrometastases may be composed of slow cycling cells that take an extended period to grow into a lesion that is large enough to be clinically detected. In either case, this model posits that the disseminated tumor burden remains static until the tumor cells are reactivated by exogenous stimuli. It is important to note that these models are not mutually exclusive. Individual quiescent cells may exist in the context of a micrometastasis, and a cell could first escape cellular dormancy, grow into a micrometastasis, where it again stalls for a period, before eventually developing into an overt metastasis.
Hypoxia and angiogenesis are proposed to play key roles in dormancy by limiting the availability of nutrients that tumor cells need in order to proliferate. This “angiogenic dormancy” model proposes that the inability of the disseminated tumor cells to induce angiogenesis is what keeps them what growing past approximately 1-2 mm in diameter [168]. High expression of anti-angiogenic factors such as thrombospondin 1 (TSP1 or THBS1) in the microenvironment [169] or low expression of angiogenic signals such as VEGF and basic fibroblast growth factor (bFGF) in the tumor cells may cause the micrometastasis to remain static in size [168].
Hypoxia also alters the expression of genes in the tumor cells that are involved in dormancy maintenance, such as leukemia inhibitory factor receptor (LIFR), which acts as a breast cancer tumor suppressor in MCF7 (ER-positive human breast cancer) cells [170, 171] and confers a dormant phenotype in bone-disseminated MCF7 breast cancer cells [172]. Hypoxia decreases the expression of LIFR in both MCF7 and SUM159 (ER-negative human breast cancer) cells in vitro and is negatively correlated with LIFR mRNA levels in patient samples [172]. LIFR regulation was, however, found to be HIF-independent, suggesting that HIF is not required for hypoxia to influence dormancy in the bone marrow. LIFR downregulation was observed when breast cancer cells were cultured at 0.5% pO2, suggesting that regions of extreme hypoxia in the bone marrow may promote tumor cell exit from dormancy. Furthermore, as tumors grow in the bone marrow they will become increasingly hypoxic, which may lead to prolonged repression of LIFR. Additionally, PTHrP has been shown to negatively regulate the expression of LIFR [172] and other dormancy genes in MCF7 breast cancer cells in vitro [173]. Since PTHrP is hypoxia responsive [143], this demonstrates another mechanism by which hypoxia may push tumor cells out of dormancy.
There is also evidence that suggests hypoxia may promote dormancy. The dormancy markers NR2F1 (Nuclear Receptor Subfamily 2 Group F Member 1) [174, 175] and DEC2 (Differentially Expressed In Chondrocytes 2, also known as SHARP1) [176] have been reported to be co-expressed with HIF1α and GLUT1, hypoxia markers, in MDA-MB-231 cells in primary tumors following mammary fat pad injection [177]. Analysis of human head and neck squamous cell carcinoma (HNSCC) patient samples similarly showed that hypoxic GLUT1-high portions of tumors frequently had upregulated NR2F1. Additionally, growing HEp3 HNSCC cells using the chick chorioallantoic membrane (CAM) model, non-cycling cells were shown to exhibit more intense pimonidozole staining, which is a marker of hypoxia. Similarly, treating CAM-implanted tumors with desferrioxamine (DFOM, a hypoxia mimicking drug that causes the accumulation of HIF1α) showed that DFOM treated tumors had significantly more quiescent cells. Collectively, these findings suggest that hypoxia may also promote quiescence in the primary tumor.
Interestingly, NR2F1 appears to function differently as a dormancy regulator depending on the site of tumor growth. Following subcutaneous implantation, growth, and resection of HEp3 cells in mice, NR2F1 was knocked down using a tetracycline-inducible method in order to study the effect of NR2F1 expression on the growth of disseminated tumor cells (DTCs) in the lung and bone marrow [174]. While NR2F1 knockdown allowed the disseminated HNSCC cells in the lung to grow more aggressively, NR2F1 knockdown had the opposite effect on bone-disseminated cells, suggesting that NR2F1 expression promotes tumor cell growth or survival in bone. Thus, some dormancy maintenance factors may act in a tumor-type or tissue-type specific manner. It is also important to note that NR2F1 has not been shown to be directly induced by HIF.
These seemingly opposing findings for the role of hypoxia in dormancy maintenance or escape may be explained by the degree of hypoxia that the cells experience or the size and location of the bone metastatic lesion. A better understanding of the cellular and molecular makeup of heterogeneous lesions in the bone metastatic niche is needed to understand the impact of hypoxia on tumor dormancy in bone. Pro-dormancy effects of hypoxia have also not been observed in the context of the bone, suggesting that other signals from the microenvironment may alter how cells respond to hypoxia.
Another avenue by which hypoxia may influence dormancy or the survival of DTCs, is through promoting the stem-like status of cancer cells. It has been proposed that disseminated tumors cells may adopt a cancer stem cell phenotype, thereby allowing the cell to survive in circulation and grow from a single disseminated tumor cell into a metastasis in the secondary site [178]. Indeed, dormant DTCs and pluripotent stem cells share some prominent characteristics, such as self-renewal and differentiation, quiescence, and chemotherapeutic resistance [178]. It is therefore theorized that the DTCs that survive, escape dormancy, and grow into clinically detectable metastases may be cancer stem cells (CSCs). Hypoxia has been shown in multiple cancer types to promote a CSC phenotype. In breast cancer, hypoxia-induced HIF1α expression was found to increase expression of adenosine receptor 2B (A2BR) in multiple breast cancer cell lines, driving CSC enrichment in vitro and lung metastasis in vivo [179]. CSC enrichment was measured by mammosphere formation and aldehyde dehydrogenase expression in vitro, and A2BR signaling was found to drive this enrichment through activation of protein kinase C-δ (PKCδ). PKCδ in turn activated STAT3, driving the expression of interleukin 6 and NANOG, which are two key mediators of the CSC phenotype. In glioblastoma, chemotherapy-induced HIF1α and HIF2α was found to mediate the dedifferentiation of non-stem glioblastoma cells to CSCs, as marked by the expression of CD133, CD15, or SOX2 [180]. In bladder cancer, HIF1α has been shown to drive CD24 expression, which is a CSC marker, by binding to a functional HRE in the CD24 promoter [181]. These examples illustrate how hypoxia can drive CSC enrichment, thus potentially increasing the number of DTCs that metastasize and survive at distant sites, eventually growing into clinically significant lesions.
5. Conclusion
Hypoxia is a common pathological feature in tumors and a physiological feature of the bone. HIF signaling causes a host of adaptive changes in the primary tumor that drive invasion and metastasis to distant organs, including the bone. Hypoxia in the primary tumor can also induce the expression of factors like LOX that act distantly on the bone to induce osteolysis and prepare the bone for tumor cell seeding. Reciprocally, hypoxia in the bone can alter the cytokine expression profile of osteoprogenitor cells to fuel primary tumor growth, proliferation, and metastasis. Hypoxia in the bone itself is also an important factor controlling osteoclastogenesis, which is a key part of the vicious cycle of bone destruction. This could play an increasingly important role as patients age, since bone vascularization decreases with age [182, 183]. While the understanding of tumor dormancy is still evolving, it is clear that hypoxia plays an important role in this process that has significant implications for the long-term care of cancer survivors.
Acknowledgements
Rachelle Johnson and Vera Todd are supported in part by NIH R00CA194198 (RWJ) and DoD CDMRP Breakthrough Award W81XWH-18-1-0029 (RWJ).
Footnotes
Conflict of interest statement
The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azzi S, Hebda JK, and Gavard J, Vascular permeability and drug delivery in cancers. Front Oncol, 2013. 3: p. 211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaupel P, Hockel M, and Mayer A, Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal, 2007. 9(8): p. 1221–35. [DOI] [PubMed] [Google Scholar]
- 3.Semenza GL and Wang GL, A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol, 1992. 12(12): p. 5447–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GL, et al. , Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A, 1995. 92(12): p. 5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tian H, McKnight SL, and Russell DW, Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev, 1997. 11(1): p. 72–82. [DOI] [PubMed] [Google Scholar]
- 6.Gu YZ, et al. , Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr, 1998. 7(3): p. 205–13. [PMC free article] [PubMed] [Google Scholar]
- 7.Makino Y, et al. , Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem, 2002. 277(36): p. 32405–8. [DOI] [PubMed] [Google Scholar]
- 8.Maynard MA, et al. , Multiple splice variants of the human HIF-3 alpha locus are targets of the von Hippel-Lindau E3 ubiquitin ligase complex. J Biol Chem, 2003. 278(13): p. 11032–40. [DOI] [PubMed] [Google Scholar]
- 9.Pasanen A, et al. , Hypoxia-inducible factor (HIF)-3alpha is subject to extensive alternative splicing in human tissues and cancer cells and is regulated by HIF-1 but not HIF-2. Int J Biochem Cell Biol, 2010. 42(7): p. 1189–200. [DOI] [PubMed] [Google Scholar]
- 10.Heikkila M, et al. , Roles of the human hypoxia-inducible factor (HIF)-3alpha variants in the hypoxia response. Cell Mol Life Sci, 2011. 68(23): p. 3885–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hara S, et al. , Expression and characterization of hypoxia-inducible factor (HIF)-3alpha in human kidney: suppression of HIF-mediated gene expression by HIF-3alpha. Biochem Biophys Res Commun, 2001. 287(4): p. 808–13. [DOI] [PubMed] [Google Scholar]
- 12.Dengler VL, Galbraith M, and Espinosa JM, Transcriptional regulation by hypoxia inducible factors. Crit Rev Biochem Mol Biol, 2014. 49(1): p. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang LE, et al. , Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci U S A, 1998. 95(14): p. 7987–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohh M, et al. , Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol, 2000. 2(7): p. 423–7. [DOI] [PubMed] [Google Scholar]
- 15.Tanimoto K, et al. , Mechanism of regulation of the hypoxia-inducible factor-1 alpha by the von Hippel-Lindau tumor suppressor protein. EMBO J, 2000. 19(16): p. 4298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ivan M, et al. , HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science, 2001. 292(5516): p. 464–8. [DOI] [PubMed] [Google Scholar]
- 17.Jaakkola P, et al. , Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science, 2001. 292(5516): p. 468–72. [DOI] [PubMed] [Google Scholar]
- 18.Schodel J, et al. , High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood, 2011. 117(23): p. e207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mole DR, et al. , Genome-wide association of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha DNA binding with expression profiling of hypoxia-inducible transcripts. J Biol Chem, 2009. 284(25): p. 16767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xia X and Kung AL, Preferential binding of HIF-1 to transcriptionally active loci determines cell-type specific response to hypoxia. Genome Biol, 2009. 10(10): p. R113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takeda N, et al. , Endothelial PAS domain protein 1 gene promotes angiogenesis through the transactivation of both vascular endothelial growth factor and its receptor, Flt-1. Circ Res, 2004. 95(2): p. 146–53. [DOI] [PubMed] [Google Scholar]
- 22.Benita Y, et al. , An integrative genomics approach identifies Hypoxia Inducible Factor-1 (HIF-1)-target genes that form the core response to hypoxia. Nucleic Acids Res, 2009. 37(14): p. 4587–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Semenza GL, Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci, 2012. 33(4): p. 207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shweiki D, et al. , Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature, 1992. 359(6398): p. 843–5. [DOI] [PubMed] [Google Scholar]
- 25.Tsuzuki Y, et al. , Vascular endothelial growth factor (VEGF) modulation by targeting hypoxia-inducible factor-1alpha--> hypoxia response element--> VEGF cascade differentially regulates vascular response and growth rate in tumors. Cancer Res, 2000. 60(22): p. 6248–52. [PubMed] [Google Scholar]
- 26.Firth JD, Ebert BL, and Ratcliffe PJ, Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements. J Biol Chem, 1995. 270(36): p. 21021–7. [DOI] [PubMed] [Google Scholar]
- 27.Gleadle JM and Ratcliffe PJ, Induction of hypoxia-inducible factor-1, erythropoietin, vascular endothelial growth factor, and glucose transporter-1 by hypoxia: evidence against a regulatory role for Src kinase. Blood, 1997. 89(2): p. 503–9. [PubMed] [Google Scholar]
- 28.Firth JD, et al. , Oxygen-regulated control elements in the phosphoglycerate kinase 1 and lactate dehydrogenase A genes: similarities with the erythropoietin 3' enhancer. Proc Natl Acad Sci U S A, 1994. 91(14): p. 6496–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ebert BL, Firth JD, and Ratcliffe PJ, Hypoxia and mitochondrial inhibitors regulate expression of glucose transporter-1 via distinct Cis-acting sequences. J Biol Chem, 1995. 270(49): p. 29083–9. [DOI] [PubMed] [Google Scholar]
- 30.Petrella BL, Lohi J, and Brinckerhoff CE, Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene, 2005. 24(6): p. 1043–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erler JT, et al. , Lysyl oxidase is essential for hypoxia-induced metastasis. Nature, 2006. 440(7088): p. 1222–6. [DOI] [PubMed] [Google Scholar]
- 32.Yang MH, et al. , Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nat Cell Biol, 2008. 10(3): p. 295–305. [DOI] [PubMed] [Google Scholar]
- 33.Schindl M, et al. , Overexpression of hypoxia-inducible factor 1alpha is associated with an unfavorable prognosis in lymph node-positive breast cancer. Clin Cancer Res, 2002. 8(6): p. 1831–7. [PubMed] [Google Scholar]
- 34.Dales JP, et al. , Overexpression of hypoxia-inducible factor HIF-1alpha predicts early relapse in breast cancer: retrospective study in a series of 745 patients. Int J Cancer, 2005. 116(5): p. 734–9. [DOI] [PubMed] [Google Scholar]
- 35.Bos R, et al. , Levels of hypoxia-inducible factor-1alpha independently predict prognosis in patients with lymph node negative breast carcinoma. Cancer, 2003. 97(6): p. 1573–81. [DOI] [PubMed] [Google Scholar]
- 36.Generali D, et al. , Hypoxia-inducible factor-1alpha expression predicts a poor response to primary chemoendocrine therapy and disease-free survival in primary human breast cancer. Clin Cancer Res, 2006. 12(15): p. 4562–8. [DOI] [PubMed] [Google Scholar]
- 37.Hung JJ, et al. , Prognostic significance of hypoxia-inducible factor-1alpha, TWIST1 and Snail expression in resectable non-small cell lung cancer. Thorax, 2009. 64(12): p. 1082–9. [DOI] [PubMed] [Google Scholar]
- 38.Aebersold DM, et al. , Expression of hypoxia-inducible factor-1alpha: a novel predictive and prognostic parameter in the radiotherapy of oropharyngeal cancer. Cancer Res, 2001. 61(7): p. 2911–6. [PubMed] [Google Scholar]
- 39.Zhou J, et al. , Clinical and prognostic significance of HIF-1alpha overexpression in oral squamous cell carcinoma: a meta-analysis. World J Surg Oncol, 2017. 15(1): p. 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Birner P, et al. , Expression of hypoxia-inducible factor-1 alpha in oligodendrogliomas: its impact on prognosis and on neoangiogenesis. Cancer, 2001. 92(1): p. 165–71. [DOI] [PubMed] [Google Scholar]
- 41.Birner P, et al. , Expression of hypoxia-inducible factor 1alpha in epithelial ovarian tumors: its impact on prognosis and on response to chemotherapy. Clin Cancer Res, 2001. 7(6): p. 1661–8. [PubMed] [Google Scholar]
- 42.Birner P, et al. , Overexpression of hypoxia-inducible factor 1alpha is a marker for an unfavorable prognosis in early-stage invasive cervical cancer. Cancer Res, 2000. 60(17): p. 4693–6. [PubMed] [Google Scholar]
- 43.Moreno Roig E, et al. , Prognostic Role of Hypoxia-Inducible Factor-2alpha Tumor Cell Expression in Cancer Patients: A Meta-Analysis. Front Oncol, 2018. 8: p. 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Asosingh K, et al. , Role of the hypoxic bone marrow microenvironment in 5T2MM murine myeloma tumor progression. Haematologica, 2005. 90(6): p. 810–7. [PubMed] [Google Scholar]
- 45.Jensen PO, et al. , Increased cellular hypoxia and reduced proliferation of both normal and leukaemic cells during progression of acute myeloid leukaemia in rats. Cell Prolif, 2000. 33(6): p. 381–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konopleva M, et al. , Phase I/II study of the hypoxia-activated prodrug PR104 in refractory/relapsed acute myeloid leukemia and acute lymphoblastic leukemia. Haematologica, 2015. 100(7): p. 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irigoyen M, Garcia-Ruiz JC, and Berra E, The hypoxia signalling pathway in haematological malignancies. Oncotarget, 2017. 8(22): p. 36832–36844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petit C, et al. , Hypoxia promotes chemoresistance in acute lymphoblastic leukemia cell lines by modulating death signaling pathways. BMC Cancer, 2016. 16(1): p. 746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deeb G, et al. , Hypoxia-inducible factor-1alpha protein expression is associated with poor survival in normal karyotype adult acute myeloid leukemia. Leuk Res, 2011. 35(5): p. 579–84. [DOI] [PubMed] [Google Scholar]
- 50.Rankin EB, et al. , The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell, 2012. 149(1): p. 63–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu C, et al. , Oxygen-sensing PHDs regulate bone homeostasis through the modulation of osteoprotegerin. Genes Dev, 2015. 29(8): p. 817–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson RW, Schipani E, and Giaccia AJ, HIF targets in bone remodeling and metastatic disease. Pharmacol Ther, 2015. 150: p. 169–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crock HV, A Revision of the Anatomy of the Arteries Supplying the Upper End of the Human Femur. J Anat, 1965. 99: p. 77–88. [PMC free article] [PubMed] [Google Scholar]
- 54.Trueta J and Harrison MH, The normal vascular anatomy of the femoral head in adult man. J Bone Joint Surg Br, 1953. 35-B(3): p. 442–61. [DOI] [PubMed] [Google Scholar]
- 55.Trueta J and Morgan JD, The vascular contribution to osteogenesis. I. Studies by the injection method. J Bone Joint Surg Br, 1960. 42-B: p. 97–109. [DOI] [PubMed] [Google Scholar]
- 56.Branemark P, Experimental Investigation of Microcirculation in Bone Marrow. Angiology, 1961. 12(7): p. 293-&. [Google Scholar]
- 57.Kusumbe AP, Ramasamy SK, and Adams RH, Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature, 2014. 507(7492): p. 323–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sivaraj KK and Adams RH, Blood vessel formation and function in bone. Development, 2016. 143(15): p. 2706–15. [DOI] [PubMed] [Google Scholar]
- 59.Gruneboom A, et al. , A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nat Metab, 2019. 1(2): p. 236–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chow DC, et al. , Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh's model. Biophys J, 2001. 81(2): p. 675–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chow DC, et al. , Modeling pO(2) distributions in the bone marrow hematopoietic compartment. II. Modified Kroghian models. Biophys J, 2001. 81(2): p. 685–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zahm AM, et al. , Numerical modeling of oxygen distributions in cortical and cancellous bone: oxygen availability governs osteonal and trabecular dimensions. Am J Physiol Cell Physiol, 2010. 299(5): p. C922–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spencer JA, et al. , Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature, 2014. 508(7495): p. 269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Woelfle U, et al. , Molecular signature associated with bone marrow micrometastasis in human breast cancer. Cancer Res, 2003. 63(18): p. 5679–84. [PubMed] [Google Scholar]
- 65.Lu X, et al. , In vivo dynamics and distinct functions of hypoxia in primary tumor growth and organotropic metastasis of breast cancer. Cancer Res, 2010. 70(10): p. 3905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liao D, et al. , Hypoxia-inducible factor-1alpha is a key regulator of metastasis in a transgenic model of cancer initiation and progression. Cancer Res, 2007. 67(2): p. 563–72. [DOI] [PubMed] [Google Scholar]
- 67.Niu Y, et al. , HIF-2-induced long non-coding RNA RAB11B-AS1 promotes hypoxia-mediated angiogenesis and breast cancer metastasis. Cancer Res, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hiraga T, et al. , Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res, 2007. 67(9): p. 4157–63. [DOI] [PubMed] [Google Scholar]
- 69.Dunn LK, et al. , Hypoxia and TGF-beta drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS One, 2009. 4(9): p. e6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiang L and Gilkes DM, The Contribution of the Immune System in Bone Metastasis Pathogenesis. Int J Mol Sci, 2019. 20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee HW, et al. , Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim Biophys Acta, 2013. 1835(2): p. 170–9. [DOI] [PubMed] [Google Scholar]
- 72.Karnevi E, Andersson R, and Rosendahl AH, Tumour-educated macrophages display a mixed polarisation and enhance pancreatic cancer cell invasion. Immunol Cell Biol, 2014. 92(6): p. 543–52. [DOI] [PubMed] [Google Scholar]
- 73.Sinder BP, Pettit AR, and McCauley LK, Macrophages: Their Emerging Roles in Bone. J Bone Miner Res, 2015. 30(12): p. 2140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bates GJ, et al. , Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol, 2006. 24(34): p. 5373–80. [DOI] [PubMed] [Google Scholar]
- 75.Zhao E, et al. , Regulatory T cells in the bone marrow microenvironment in patients with prostate cancer. Oncoimmunology, 2012. 1(2): p. 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan W, et al. , Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature, 2011. 470(7335): p. 548–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thomas DA and Massague J, TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell, 2005. 8(5): p. 369–80. [DOI] [PubMed] [Google Scholar]
- 78.Fournier PG, Chirgwin JM, and Guise TA, New insights into the role of T cells in the vicious cycle of bone metastases. Curr Opin Rheumatol, 2006. 18(4): p. 396–404. [DOI] [PubMed] [Google Scholar]
- 79.Palumbo JS, et al. , Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood, 2005. 105(1): p. 178–85. [DOI] [PubMed] [Google Scholar]
- 80.Bidwell BN, et al. , Silencing of Irf7 pathways in breast cancer cells promotes bone metastasis through immune escape. Nat Med, 2012. 18(8): p. 1224–31. [DOI] [PubMed] [Google Scholar]
- 81.Wright LE, et al. , Murine models of breast cancer bone metastasis. Bonekey Rep, 2016. 5: p. 804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clambey ET, et al. , Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A, 2012. 109(41): p. E2784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Noman MZ, et al. , PD-L1 is a novel direct target of HIF-1alpha, and its blockade under hypoxia enhanced MDSC-mediated T cell activation. J Exp Med, 2014. 211(5): p. 781–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calcinotto A, et al. , Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res, 2012. 72(11): p. 2746–56. [DOI] [PubMed] [Google Scholar]
- 85.Takeda N, et al. , Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev, 2010. 24(5): p. 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schaefer E, et al. , Intermittent hypoxia is a proinflammatory stimulus resulting in IL-6 expression and M1 macrophage polarization. Hepatol Commun, 2017. 1(4): p. 326–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou J, et al. , Intermittent Hypoxia Enhances THP-1 Monocyte Adhesion and Chemotaxis and Promotes M1 Macrophage Polarization via RAGE. Biomed Res Int, 2018. 2018: p. 1650456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Michiels C, Tellier C, and Feron O, Cycling hypoxia: A key feature of the tumor microenvironment. Biochim Biophys Acta, 2016. 1866(1): p. 76–86. [DOI] [PubMed] [Google Scholar]
- 89.Zhang D, et al. , Hypoxia promotes osteosarcoma cell proliferation and migration through enhancing platelet-derived growth factor-BB/platelet-derived growth factor receptor-beta axis. Biochem Biophys Res Commun, 2019. 512(2): p. 360–366. [DOI] [PubMed] [Google Scholar]
- 90.Betsholtz C, et al. , Coexpression of a PDGF-like growth factor and PDGF receptors in a human osteosarcoma cell line: implications for autocrine receptor activation. Cell, 1984. 39(3 Pt 2): p. 447–57. [DOI] [PubMed] [Google Scholar]
- 91.Heldin CH, et al. , A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature, 1986. 319(6053): p. 511–4. [DOI] [PubMed] [Google Scholar]
- 92.Sulzbacher I, et al. , Platelet-derived growth factor-AA and -alpha receptor expression suggests an autocrine and/or paracrine loop in osteosarcoma. Mod Pathol, 2000. 13(6): p. 632–7. [DOI] [PubMed] [Google Scholar]
- 93.Oda Y, et al. , Expression of growth factors and their receptors in human osteosarcomas. Immunohistochemical detection of epidermal growth factor, platelet-derived growth factor and their receptors: its correlation with proliferating activities and p53 expression. Gen Diagn Pathol, 1995. 141(2): p. 97–103. [PubMed] [Google Scholar]
- 94.Harrison H, et al. , HIF1-alpha expressing cells induce a hypoxic-like response in neighbouring cancer cells. BMC Cancer, 2018. 18(1): p. 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patton MC, et al. , Hypoxia alters the release and size distribution of extracellular vesicles in pancreatic cancer cells to support their adaptive survival. J Cell Biochem, 2020. 121(1): p. 828–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang T, et al. , Hypoxia-inducible factors and RAB22A mediate formation of microvesicles that stimulate breast cancer invasion and metastasis. Proc Natl Acad Sci U S A, 2014. 111(31): p. E3234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Peinado H, et al. , Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med, 2012. 18(6): p. 883–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rossi M, et al. , The Role of Extracellular Vesicles in Bone Metastasis. Int J Mol Sci, 2018. 19(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Taverna S, et al. , Amphiregulin contained in NSCLC-exosomes induces osteoclast differentiation through the activation of EGFR pathway. Sci Rep, 2017. 7(1): p. 3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Faict S, et al. , Exosomes play a role in multiple myeloma bone disease and tumor development by targeting osteoclasts and osteoblasts. Blood Cancer J, 2018. 8(11): p. 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dai J, et al. , Primary prostate cancer educates bone stroma through exosomal pyruvate kinase M2 to promote bone metastasis. J Exp Med, 2019. 216(12): p. 2883–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Erler JT, et al. , Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell, 2009. 15(1): p. 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Liu Y and Cao X, Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell, 2016. 30(5): p. 668–681. [DOI] [PubMed] [Google Scholar]
- 104.Peinado H, et al. , Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer, 2017. 17(5): p. 302–317. [DOI] [PubMed] [Google Scholar]
- 105.Kaplan RN, et al. , VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature, 2005. 438(7069): p. 820–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murgai M, et al. , KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med, 2017. 23(10): p. 1176–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Devignes CS, et al. , HIF signaling in osteoblast-lineage cells promotes systemic breast cancer growth and metastasis in mice. Proc Natl Acad Sci U S A, 2018. 115(5): p. E992–E1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ceradini DJ, et al. , Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med, 2004. 10(8): p. 858–64. [DOI] [PubMed] [Google Scholar]
- 109.Du R, et al. , HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell, 2008. 13(3): p. 206–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hitchon C, et al. , Hypoxia-induced production of stromal cell-derived factor 1 (CXCL12) and vascular endothelial growth factor by synovial fibroblasts. Arthritis Rheum, 2002. 46(10): p. 2587–97. [DOI] [PubMed] [Google Scholar]
- 111.Zou D, et al. , Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1alpha mediated BMSCs. Biomaterials, 2012. 33(7): p. 2097–108. [DOI] [PubMed] [Google Scholar]
- 112.Guo F, et al. , CXCL12/CXCR4: a symbiotic bridge linking cancer cells and their stromal neighbors in oncogenic communication networks. Oncogene, 2016. 35(7): p. 816–26. [DOI] [PubMed] [Google Scholar]
- 113.Barker HE, et al. , LOXL2-mediated matrix remodeling in metastasis and mammary gland involution. Cancer Res, 2011. 71(5): p. 1561–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Barker HE, Cox TR, and Erler JT, The rationale for targeting the LOX family in cancer. Nat Rev Cancer, 2012. 12(8): p. 540–52. [DOI] [PubMed] [Google Scholar]
- 115.Levental KR, et al. , Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell, 2009. 139(5): p. 891–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pez F, et al. , The HIF-1-inducible lysyl oxidase activates HIF-1 via the Akt pathway in a positive regulation loop and synergizes with HIF-1 in promoting tumor cell growth. Cancer Res, 2011. 71(5): p. 1647–57. [DOI] [PubMed] [Google Scholar]
- 117.Baker AM, et al. , Lysyl oxidase enzymatic function increases stiffness to drive colorectal cancer progression through FAK. Oncogene, 2013. 32(14): p. 1863–8. [DOI] [PubMed] [Google Scholar]
- 118.Cox TR, et al. , LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res, 2013. 73(6): p. 1721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reynaud C, et al. , Lysyl Oxidase Is a Strong Determinant of Tumor Cell Colonization in Bone. Cancer Res, 2017. 77(2): p. 268–278. [DOI] [PubMed] [Google Scholar]
- 120.Cox TR, et al. , The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature, 2015. 522(7554): p. 106–110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 121.Mundy GR, Mechanisms of bone metastasis. Cancer, 1997. 80(8 Suppl): p. 1546–56. [DOI] [PubMed] [Google Scholar]
- 122.Kohart NA, et al. , Parathyroid hormone-related protein promotes bone loss in T-cell leukemia as well as in solid tumors. Leuk Lymphoma, 2020. 61(2): p. 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Powell GJ, et al. , Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res, 1991. 51(11): p. 3059–61. [PubMed] [Google Scholar]
- 124.Vargas SJ, et al. , Localization of parathyroid hormone-related protein mRNA expression in breast cancer and metastatic lesions by in situ hybridization. J Bone Miner Res, 1992. 7(8): p. 971–9. [DOI] [PubMed] [Google Scholar]
- 125.Kohno N, et al. , Parathyroid Hormone-related Protein in Breast Cancer Tissues: Relationship between Primary and Metastatic Sites. Breast Cancer, 1994. 1(1): p. 43–49. [DOI] [PubMed] [Google Scholar]
- 126.Johnson RW, et al. , Wnt signaling induces gene expression of factors associated with bone destruction in lung and breast cancer. Clin Exp Metastasis, 2014. 31(8): p. 945–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kuo PL, et al. , MicroRNA-33a functions as a bone metastasis suppressor in lung cancer by targeting parathyroid hormone related protein. Biochim Biophys Acta, 2013. 1830(6): p. 3756–66. [DOI] [PubMed] [Google Scholar]
- 128.Poole KE and Reeve J, Parathyroid hormone - a bone anabolic and catabolic agent. Curr Opin Pharmacol, 2005. 5(6): p. 612–7. [DOI] [PubMed] [Google Scholar]
- 129.Abou-Samra AB, et al. , Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A, 1992. 89(7): p. 2732–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Juppner H, et al. , A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science, 1991. 254(5034): p. 1024–6. [DOI] [PubMed] [Google Scholar]
- 131.Pioszak AA, et al. , Structural basis for parathyroid hormone-related protein binding to the parathyroid hormone receptor and design of conformation-selective peptides. J Biol Chem, 2009. 284(41): p. 28382–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Halladay DL, et al. , Identification of signal transduction pathways and promoter sequences that mediate parathyroid hormone 1-38 inhibition of osteoprotegerin gene expression. J Cell Biochem, 2001. 84(1): p. 1–11. [DOI] [PubMed] [Google Scholar]
- 133.Udagawa N, et al. , Osteoblasts/stromal cells stimulate osteoclast activation through expression of osteoclast differentiation factor/RANKL but not macrophage colony-stimulating factor: receptor activator of NF-kappa B ligand. Bone, 1999. 25(5): p. 517–23. [DOI] [PubMed] [Google Scholar]
- 134.Hsu H, et al. , Tumor necrosis factor receptor family member RANK mediates osteoclast differentiation and activation induced by osteoprotegerin ligand. Proc Natl Acad Sci U S A, 1999. 96(7): p. 3540–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jansen ID, et al. , Osteoclast fusion and fission. Calcif Tissue Int, 2012. 90(6): p. 515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lacey DL, et al. , Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell, 1998. 93(2): p. 165–76. [DOI] [PubMed] [Google Scholar]
- 137.Hauschka PV, et al. , Growth factors in bone matrix. Isolation of multiple types by affinity chromatography on heparin-Sepharose. J Biol Chem, 1986. 261(27): p. 12665–74. [PubMed] [Google Scholar]
- 138.Hiraga T, et al. , Bone-derived IGF mediates crosstalk between bone and breast cancer cells in bony metastases. Cancer Res, 2012. 72(16): p. 4238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Frolik CA, Ellis LF, and Williams DC, Isolation and characterization of insulin-like growth factor-II from human bone. Biochem Biophys Res Commun, 1988. 151(3): p. 1011–8. [DOI] [PubMed] [Google Scholar]
- 140.Seyedin SM, et al. , Cartilage-inducing factor-A. Apparent identity to transforming growth factor-beta. J Biol Chem, 1986. 261(13): p. 5693–5. [PubMed] [Google Scholar]
- 141.Bonewald LF and Mundy GR, Role of transforming growth factor-beta in bone remodeling. Clin Orthop Relat Res, 1990(250): p. 261–76. [PubMed] [Google Scholar]
- 142.Sterling JA, et al. , Advances in the biology of bone metastasis: how the skeleton affects tumor behavior. Bone, 2011. 48(1): p. 6–15. [DOI] [PubMed] [Google Scholar]
- 143.Manisterski M, et al. , Hypoxia induces PTHrP gene transcription in human cancer cells through the HIF-2alpha. Cell Cycle, 2010. 9(18): p. 3723–9. [PubMed] [Google Scholar]
- 144.Browe DC, et al. , Hypoxia Activates the PTHrP -MEF2C Pathway to Attenuate Hypertrophy in Mesenchymal Stem Cell Derived Cartilage. Sci Rep, 2019. 9(1): p. 13274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Pelosi M, et al. , Parathyroid hormone-related protein is induced by hypoxia and promotes expression of the differentiated phenotype of human articular chondrocytes. Clin Sci (Lond), 2013. 125(10): p. 461–70. [DOI] [PubMed] [Google Scholar]
- 146.Tang ZN, et al. , Hypoxia induces RANK and RANKL expression by activating HIF-1alpha in breast cancer cells. Biochem Biophys Res Commun, 2011. 408(3): p. 411–6. [DOI] [PubMed] [Google Scholar]
- 147.Dandajena TC, et al. , Hypoxia triggers a HIF-mediated differentiation of peripheral blood mononuclear cells into osteoclasts. Orthod Craniofac Res, 2012. 15(1): p. 1–9. [DOI] [PubMed] [Google Scholar]
- 148.Knowles HJ and Athanasou NA, Hypoxia-inducible factor is expressed in giant cell tumour of bone and mediates paracrine effects of hypoxia on monocyte-osteoclast differentiation via induction of VEGF. J Pathol, 2008. 215(1): p. 56–66. [DOI] [PubMed] [Google Scholar]
- 149.Fukuoka H, et al. , Hypoxic stress enhances osteoclast differentiation via increasing IGF2 production by non-osteoclastic cells. Biochem Biophys Res Commun, 2005. 328(4): p. 885–94. [DOI] [PubMed] [Google Scholar]
- 150.Hinoi E, et al. , Positive regulation of osteoclastic differentiation by growth differentiation factor 15 upregulated in osteocytic cells under hypoxia. J Bone Miner Res, 2012. 27(4): p. 938–49. [DOI] [PubMed] [Google Scholar]
- 151.Shao J, et al. , HIF-1alpha disturbs osteoblasts and osteoclasts coupling in bone remodeling by up-regulating OPG expression. In Vitro Cell Dev Biol Anim, 2015. 51(8): p. 808–14. [DOI] [PubMed] [Google Scholar]
- 152.Lee SY, et al. , Controlling hypoxia-inducible factor-2alpha is critical for maintaining bone homeostasis in mice. Bone Res, 2019. 7: p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang Y, et al. , The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest, 2007. 117(6): p. 1616–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Arnett TR, et al. , Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol, 2003. 196(1): p. 2–8. [DOI] [PubMed] [Google Scholar]
- 155.Knowles HJ and Athanasou NA, Acute hypoxia and osteoclast activity: a balance between enhanced resorption and increased apoptosis. J Pathol, 2009. 218(2): p. 256–64. [DOI] [PubMed] [Google Scholar]
- 156.Ma Z, et al. , Constant hypoxia inhibits osteoclast differentiation and bone resorption by regulating phosphorylation of JNK and IkappaBalpha. Inflamm Res, 2019. 68(2): p. 157–166. [DOI] [PubMed] [Google Scholar]
- 157.Leger AJ, et al. , Inhibition of osteoclastogenesis by prolyl hydroxylase inhibitor dimethyloxallyl glycine. J Bone Miner Metab, 2010. 28(5): p. 510–9. [DOI] [PubMed] [Google Scholar]
- 158.Hulley PA, et al. , Hypoxia-inducible factor 1-alpha does not regulate osteoclastogenesis but enhances bone resorption activity via prolyl-4-hydroxylase 2. J Pathol, 2017. 242(3): p. 322–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kang H, et al. , Osteoblast Hypoxia-Inducible Factor-1alpha Pathway Activation Restrains Osteoclastogenesis via the Interleukin-33-MicroRNA-34a-Notch1 Pathway. Front Immunol, 2017. 8: p. 1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Takemori T, et al. , Transcutaneous carbon dioxide application suppresses bone destruction caused by breast cancer metastasis. Oncol Rep, 2018. 40(4): p. 2079–2087. [DOI] [PubMed] [Google Scholar]
- 161.Jeong W, et al. , Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1alpha), in patients with refractory solid tumors. Cancer Chemother Pharmacol, 2014. 73(2): p. 343–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Johnson RW and Suva LJ, Hallmarks of Bone Metastasis. Calcif Tissue Int, 2018. 102(2): p. 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.David Roodman G and Silbermann R, Mechanisms of osteolytic and osteoblastic skeletal lesions. Bonekey Rep, 2015. 4: p. 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gomis RR and Gawrzak S, Tumor cell dormancy. Mol Oncol, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wu Q, et al. , Breast cancer subtypes predict the preferential site of distant metastases: a SEER based study. Oncotarget, 2017. 8(17): p. 27990–27996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Naumov GN, et al. , Persistence of solitary mammary carcinoma cells in a secondary site: A possible contributor to dormancy. Cancer Research, 2002. 62(7): p. 2162–2168. [PubMed] [Google Scholar]
- 167.Ghiso JAA, Kovalski K, and Ossowski L, Tumor dormancy induced by downregulation of urokinase receptor in human carcinoma involves integrin and MAPK signaling. Journal of Cell Biology, 1999. 147(1): p. 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Naumov GN, Akslen LA, and Folkman J, Role of angiogenesis in human tumor dormancy: animal models of the angiogenic switch. Cell Cycle, 2006. 5(16): p. 1779–87. [DOI] [PubMed] [Google Scholar]
- 169.Ghajar CM, et al. , The perivascular niche regulates breast tumour dormancy. Nat Cell Biol, 2013. 15(7): p. 807–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Iorns E, et al. , Whole genome in vivo RNAi screening identifies the leukemia inhibitory factor receptor as a novel breast tumor suppressor. Breast Cancer Res Treat, 2012. 135(1): p. 79–91. [DOI] [PubMed] [Google Scholar]
- 171.Chen D, et al. , LIFR is a breast cancer metastasis suppressor upstream of the Hippo-YAP pathway and a prognostic marker. Nat Med, 2012. 18(10): p. 1511–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johnson RW, et al. , Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol, 2016. 18(10): p. 1078–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Johnson RW, et al. , Parathyroid Hormone-Related Protein Negatively Regulates Tumor Cell Dormancy Genes in a PTHR1/Cyclic AMP-Independent Manner. Front Endocrinol (Lausanne), 2018. 9: p. 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sosa MS, et al. , NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat Commun, 2015. 6: p. 6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Thompson VC, et al. , A gene signature identified using a mouse model of androgen receptor-dependent prostate cancer predicts biochemical relapse in human disease. Int J Cancer, 2012. 131(3): p. 662–72. [DOI] [PubMed] [Google Scholar]
- 176.Montagner M, et al. , SHARP1 suppresses breast cancer metastasis by promoting degradation of hypoxia-inducible factors. Nature, 2012. 487(7407): p. 380–4. [DOI] [PubMed] [Google Scholar]
- 177.Fluegen G, et al. , Phenotypic heterogeneity of disseminated tumour cells is preset by primary tumour hypoxic microenvironments. Nature Cell Biology, 2017. 19(2): p. 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Aguirre-Ghiso JA, Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer, 2007. 7(11): p. 834–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Lan J, et al. , Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proc Natl Acad Sci U S A, 2018. 115(41): p. E9640–E9648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lee G, et al. , Dedifferentiation of Glioma Cells to Glioma Stem-like Cells By Therapeutic Stress-induced HIF Signaling in the Recurrent GBM Model. Mol Cancer Ther, 2016. 15(12): p. 3064–3076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Thomas S, et al. , CD24 is an effector of HIF-1-driven primary tumor growth and metastasis. Cancer Res, 2012. 72(21): p. 5600–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lahtinen T, et al. , The effect of age on blood flow in the proximal femur in man. J Nucl Med, 1981. 22(11): p. 966–72. [PubMed] [Google Scholar]
- 183.Prisby RD, et al. , Aging reduces skeletal blood flow, endothelium-dependent vasodilation, and NO bioavailability in rats. J Bone Miner Res, 2007. 22(8): p. 1280–8. [DOI] [PubMed] [Google Scholar]