Abstract
The nitrate-nitrite-nitric oxide pathway regulates NO synthase-independent vasodilation and NO signaling. Ingestion of inorganic nitrite has vasodilatory and blood pressure lowering effects. Pre-clinical studies in rodent models suggest there may be a benefit of nitrite in lowering serum triglyceride levels and improving the metabolic syndrome. In a phase 2 study we evaluated the safety and efficacy of chronic oral nitrite therapy in patients with hypertension and the metabolic syndrome.
Twenty adult subjects with stage 1 or 2 hypertension and the metabolic syndrome were enrolled in an open-label safety and efficacy study. The primary efficacy endpoint was blood pressure reduction; secondary endpoints included insulin-dependent glucose disposal and endothelial function measured by flow-mediated vasodilation of the brachial artery and intima-media diameter of the carotid artery.
Chronic oral nitrite therapy (40mg/three times daily) was well tolerated. Oral nitrite significantly lowered systolic, diastolic, and mean arterial pressures, but tolerance was observed after 10-12 weeks of therapy. There was significant improvement in the intima-media thickness of the carotid artery and trends toward improvements in flow-mediated vasodilation of the brachial artery and insulin sensitivity.
Chronic oral nitrite therapy is safe in patients with hypertension and the metabolic syndrome. Despite an apparent lack of enzymatic tolerance to nitrite, we observed tolerance after 10 weeks of chronic therapy, which requires additional mechanistic studies and possible therapeutic dose titration in clinical trials. Nitrite may be a safe therapy to concominantly improve multiple features of the metabolic syndrome including hypertension, insulin resistance, and endothelial dysfunction.
Clinical Trial Registration:
URL: https://clinicaltrials.gov Unique Identifier: NCT01681810
Keywords: metabolic syndrome, nitrite, clinical trial, hypertension, blood pressure
Graphical Abstract
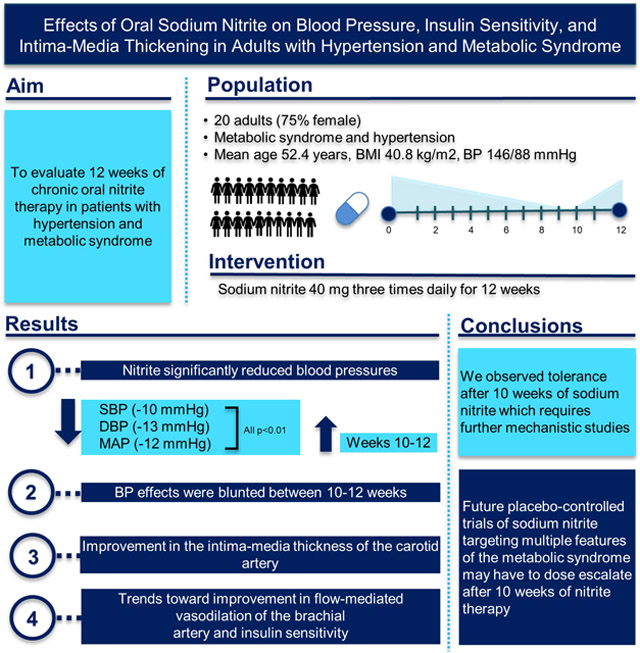
Summary
Oral nitrite significantly lowered systolic, diastolic, and mean arterial pressures, but tolerance was observed after 10-12 weeks of therapy. There was a significant improvement in the intima-media thickness of the carotid artery and trends toward improvements in flow-mediated vasodilation of the brachial artery and insulin sensitivity.
INTRODUCTION
NO is produced by the vascular endothelium and serves a critical function as a vasodilator, anti-inflammatory, and anti-thrombotic signaling molecule.1 Reduced NO signaling contributes to endothelial dysfunction, inflammation, platelet activation, and smooth muscle proliferation and migration.2 Recent evidence suggests that the alternate NO-signaling pathway, involving nitrate (NO3−) and nitrite (NO2−) reduction, also regulates blood pressure and improves the metabolic syndrome through increased NO bioavailability. Nitrate, present in leafy green vegetables, roots (beets for example), and fruit,3 is reduced to nitrite by oral bacterial flora in the saliva.4 Nitrite, either formed from oral bacterial nitrate reductase activity, nitric oxide (NO; from NO synthases) oxidation, or delivered as a therapeutic infusion or oral formulation, is further reduced to nitric oxide (NO). The reduction of nitrite to NO occurs by concerted electron-proton transfer reactions from hemoglobin, myoglobin, neuroglobin, cytoglobin, globin X, and members of the molybdopterin family of enzymes, which include xanthine and aldehyde oxidoreductases, the mitochondrial amidoximine complex, and sulfite oxidase.5-9 Nitric oxide can then directly bind and activate soluble guanylyl cyclase (sGC), producing cGMP which further results in vascular smooth muscle relaxation.1,10-12
Human studies and animal models have demonstrated the canonical vasodilatory and blood pressure (BP) lowering effects and prevention of endothelial dysfunction with exposure to inorganic and dietary nitrate and nitrite.13, 14 It has been proposed that nitrite reduction to NO is responsible for vasodilation after administering low dose IV nitrite infusions into forearm brachial arteries in healthy adults.15,16 In these studies, forearm blood flow increased with a simultaneous rise in NO formation. Several human studies have also demonstrated a reduction in BP in healthy adults following a single ingestion of nitrate,17,18 for up to 15 days of treatment with nitrate-rich beetroot juice,19-21 and eating a traditional Japanese diet, known to be a rich source of dietary nitrates.22 Multiple clinical trials confirm that various dosages of inorganic potassium nitrate,18 sodium nitrate,23 and single doses of oral and intravenous sodium nitrite solutions24 significantly reduce BP in healthy adults.18, 23 We recently demonstrated that in 10 healthy adults, a single oral dose of sodium nitrite 20mg increases plasma nitrite, which is then reduced directly to NO, as detected by the formation of iron-nitrosyl-hemoglobin.25 Significant acute vasodilatory effects of oral nitrite were observed, with decreases in systolic, diastolic, and mean arterial pressure (MAP). In humans, endothelial dysfunction develops in patients with hypertension and in the metabolic syndrome,26-29 and plasma nitrite levels are lower and flow-mediated dilation (FMD) is worse in individuals with endothelial dysfunction.30 A recent study in older adults showed that 4 weeks of sodium nitrate supplementation improved endothelial function assessed by FMD.31 It should be noted that the half-life of orally dosed nitrate is approximately 6 hours and results in sustained production of nitrite for 24 hours, while nitrite has a shorter half-life of less than one hour.25 Nitrite is rapidly reduced to NO and vasodilates acutely with an effect that peaks in 20-minutes and lasts only 4-6 hours.25 Both nitrate and nitrite are inorganic salts, while nitroglycerin is an organic nitrate that is rapidly metabolized to form NO in minutes.1,10 In aggregate, these clinical studies suggest that nitrate and nitrite may improve NO bioavailability, endothelial function, and blood pressure.
Accumulating evidence also suggests that inorganic nitrate and nitrite therapy may ameliorate key features of cardiovascular disease, type 2 diabetes, and the metabolic syndrome (a clustering of risk factors that include abdominal obesity, hypertension, dyslipidemia, and impaired blood glucose regulation). For example, eNOS knock-out mice are unable to generate endothelial NO and are obese, hypertensive, insulin resistant, and hyperlipidemic, similar to humans with metabolic syndrome.32 In these mice, 10 weeks of dietary nitrate treatment reduced BP, fasting blood sugar, triglycerides, body weight, and visceral fat. Similarly, collaborative studies within our research group demonstrate that in obese mice, seven days of intravenous nitrite reduces BP and increases glucose disposal, without differences in body weight as seen with rosiglitazone.33 Taken together, these murine studies suggest that inorganic nitrite therapy can impact glucose regulation and other aspects of the metabolic syndrome.
Collectively, evidence from animal models and early human studies suggest a potential therapeutic role of supplementation with nitrite in hypertension and metabolic syndrome to enhance NO bioavailability, relax vascular smooth muscle to lower blood pressure, and to preserve endothelial function. The current study evaluates the safety and efficacy of a GMP drug formulation of oral NO2− capsules to target multiple metabolic pathways that currently require polypharmacy to effectively treat, including blood pressure, endothelial function, vascular smooth muscle and intima proliferation, and insulin sensitivity.
METHODS
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study overview.
This single-arm, open label phase 2 proof of concept investigational drug trial (NCT01681810) was designed to examine the effects of 12 weeks of oral sodium nitrite at a dose of 40mg three times daily on blood pressure, insulin sensitivity, body composition and vascular structure (intima-medial thickness), and endothelial function (flow mediated vasodilation) in overweight/obese adults with metabolic syndrome and uncontrolled hypertension. Insulin stimulated glucose disposal was pre-defined as the primary endpoint.
Study population.
The study was approved by the University of Pittsburgh Institutional Review Board and the US Food and Drug Administration for use of the investigational new drug. 387 subjects underwent phone screening and 344 were ineligible based on stringent inclusion and exclusion criteria summarized below (and shown in Supplemental Figure S1). 43 subjects aged 18-60 years with metabolic syndrome (according to NCEP ATP III definition34) and hypertension were recruited to the University of Pittsburgh Montefiore Hospital Clinical and Translational Research Center for a screening visit. Before performing any of the study procedures or interventions, subjects provided written informed consent and procedures were followed in accordance with institutional guidelines. 34 subjects consented, and 20 were eligible for study participation as they met hypertension criteria: systolic blood pressure ≥130 and/or diastolic blood pressure ≥85 mmHg at the visit, had BMI ≥30 kg/m2 and waist circumference >102 cm in men or >88 cm in women. Additional metabolic syndrome parameters assessed included fasting glucose, HDL, and triglycerides. TSH and hemoglobin were measured and subjects were excluded if TSH was >8 mIU/mL or they were anemic (hemoglobin <11 g/dL).
Subjects were also excluded if currently receiving ≥3 anti-hypertensive agents, regardless of blood pressure control, or if they were normotensive on a single or double agent, used PDE5 inhibitors, organic nitrates, and medications affecting glucose metabolism including oral hypoglycemics, insulin, and antipsychotics. Subjects were also excluded due to the recent addition or change in dosing of hormonal contraception medications (oral contraceptive pills and long-acting reversible contraceptives including intrauterine devices, DepoProvera injections, and subdermal contraceptive implants) or if they were not planning to remain on their current dose of medications. Exclusion criteria also included current smoking, chronic psychiatric or medical conditions including diabetes, liver or kidney disease, or positive urine pregnancy test or breastfeeding.
Drug formulation and dose.
The sodium nitrite drug formulation was initially developed at the Pharmacy Development Service of the NIH Clinical Center and prepared for this study at the Investigational Drug Pharmacy Service at the Triangle Compounding Pharmacy (Cary, NC). The University of Pittsburgh Medical Center Investigational Drug Pharmacy Service (UPMC-IDS) dispensed the study drug. Our study team identified a safe dosing strategy over 12 weeks of sodium nitrite 40mg taken three times daily, based on the observed half-life of nitrite of ~40 minutes and safety results from our phase 1 study.25 We considered the Joint Food and Agriculture Organization/World Health Organization Expert Committee on Food Additives ADI for sodium nitrite35, modeling of the fruit and vegetable consumption patterns of the Dietary Approaches to Stop Hypertension (DASH) diet,3 and observed human24 and rodent effects of nitrite on BP and glycemia.14,36
Study endpoints.
Following the screening visit, subjects returned for three baseline study visits (Supplemental Figure S2) which were repeated at the end of 12 weeks. At the initial baseline drug dose visit and in the week 12 drug dose and 2 hour vital sign visit, subjects fasted for 8 hours. Blood pressures were obtained with a Dinamap DPC200X (GE Medical Systems Information Technology, Milwaukee, WI) using an appropriately sized cuff with the subject in the supine position with legs uncrossed and sitting upright in a hospital bed in a quiet room throughout the study visits; for each time point three measurements were obtained and averaged. Subjects were blinded to the values of the BP readings. Pill counts were assessed throughout the trial and daily diary dosing cards were reviewed at bi-weekly visits and by phone contact on interim weeks.
Methemoglobin (MetHb) was assessed using non-invasive co-oximetry (Masimo Corp, Irvine, CA) at times 0 (baseline or trough before study drug), 0.5, 1, and 2 hours post-drug administration.
Four-hour hyperinsulinemic-euglycemic clamps were performed at baseline and at 12 weeks to evaluate in vivo insulin action in stimulating glucose disposal and inhibiting endogenous glucose production.37 HumuLIN® R regular insulin was infused at 40 mU/m2/min. Plasma glucose was clamped between 85-95 mg/dL with a variable infusion rate of 20% dextrose in water. The rate of glucose infusion was adjusted based on arterialized plasma glucose measurements obtained every 5 minutes. The rates of basal and insulin-induced glucose metabolism were determined using a non-radioactive glucose isotope dilution method38 based on a primed, then constant infusion of the 98+% enriched stable isotope of D-glucose [6,6-D2] (0.22 μmol•kg-1, 17.6 μmol•kg-1 prime) and expressed per kg of lean body mass. This was continued during the insulin infusion to determine rates of systemic glucose utilization (peripheral insulin resistance) and to determine insulin suppression of hepatic glucose production (hepatic insulin resistance). The deuterium labeled glucose isotope chemical purity was provided by Isotec, Inc. and was tested for sterility and pyrogenicity by the manufacturer before being shipped.
Pulse wave velocity (PWV) was performed in duplicate at the carotid and femoral arteries using the SphygmoCor machine (AtCor Medical, Sydney, Australia. Model MM3. Software version 7.1) equipped with a single tonometric transducer SPT-304.
A GE VIVID-7 ultrasound machine (GE Healthcare, Milwaukee, WI), equipped with a high resolution 9L linear transducer was used for measurement of the intima media thickness (IMT) of the carotid artery. Bilateral assessment of the distal common carotid artery, approximately 2 cm proximal to bifurcation at the posterior (far) vessel wall, was performed using longitudinal B-mode ultrasound cine-loop (5 beats). Semi-automated edge detection software, which traces the luminal intima and hypoechoic boundary between media and adventitia, was used to calculate the IMT.
Endothelial-dependent flow-mediated vasodilation (FMD) of the brachial artery was obtained at baseline and the end of 12 weeks to evaluate endothelial function, at a separate visit from the clamp. A 5 cm wide occlusive cuff (Hokanson SC 5) with rapid release sphygmomanometer (Hokanson DS400) was attached to the upper forearm approximately 5 cm below the elbow on the arm opposite the control arm. Brachial artery diameter and flow velocities were examined by high resolution GE VIVID-7 Duplex ultrasound (GE Healthcare, Milwaukee, WI), equipped with a 9L high-resolution linear array transducer preset on a specifically dedicated scanning protocol. The occlusion cuff was manually inflated to 200 mmHg (or 50 mmHg above SBP) for 5 minutes. Images were captured at baseline and for 3 minutes after deflation during the hyperemic period at the highest optimal resolution with spectral velocity Doppler imaging.
Whole body dual-energy x-ray absorptiometry (Lunar iDXA, GE Healthcare) was completed at baseline and at 12 weeks to assess body composition.
Statistical analysis.
Analyses were performed using GraphPad Prism v6.0e statistical software (GraphPad Software, San Diego, CA). Paired t-tests were used to evaluate the primary endpoint measure of insulin sensitivity, in addition to vascular studies, laboratory tests, and body composition measures. Repeated measures ANOVA with visit (pre-, post-treatment) as the within-subject effect were used to evaluate response to treatment for the secondary endpoint measures of BP, heart rate, MetHb levels, and drug compliance. Data are presented as mean ± standard error of the mean (SEM).
RESULTS
Human subjects.
Twenty subjects with hypertension (25% with stage 1 and 75% with stage 2) and metabolic syndrome were recruited and eligible for intervention (Supplemental Figure S1) with mean age 52.4 years, BMI 40.8 kg/m2, and BP 146/87.8 mmHg, 75% were female and 60% were Caucasian (Table 1). All subjects were obese (BMI >30 kg/m2) and hypertensive and all men had waist circumference >102 cm and all women had waist circumference >88 cm. 12/20 (60%) had fasting glucose ≥100 mg/dL, 9/20 (45%) had HDL <50 mg/dL and 6/20 (30%) had fasting TG >150 mg/dL. Subjects had normal laboratory values for kidney, liver, and thyroid function and were not diabetic.
Table 1.
Baseline Characteristics (Data are presented as mean ± standard error of the mean (SEM).
| Number of subjects | 20 |
| Age (years) | 52.4 ± 1.3 |
| Sex | 15 female, 5 male |
| Race | |
| Caucasian | 12 |
| African American | 6 |
| Native American | 2 |
| BMI (kg/m2) | 40.8 ± 1.5 |
| Waist circumference (cm) | |
| Males | 133 ± 5 |
| Females | 117 ± 3 |
| Creatinine (mg/dL) | 0.8 ± 0.04 |
| AST (IU/L) | 21 ± 2 |
| ALT (IU/L) | 23 ± 3 |
| TSH (mIU/mL) | 1.93 ± 0.3 |
| Hemoglobin A1c (%) | 5.7 ± 0.1 (range 4.9 - 6.3) |
| MetHb (%) | 1.1 ± 0.1 |
| Heart rate (bpm) | 66 ± 1.6 |
Drug Dosing and Adverse Events.
Biweekly drug compliance by pill counts was excellent and did not differ over time (Fig. 1F), with mean compliance ranging from 92.7-96.3% at each visit. Study and drug AEs are summarized in Supplemental Table S1. The most frequently reported AEs (% of individuals experiencing an event are reported in parentheses) were headache (75%, average of 3 headaches per subject across the study period), nausea (45%), dry mouth (30%), flushing (25%), dizziness (25%), vomiting (20%), and abdominal pain (20%). All events except dry mouth were pre-specified as anticipated and possibly or probably related to study drug. All other AEs were mild to moderate in severity. None of the AEs required drug discontinuation or dose reduction.
Figure 1.
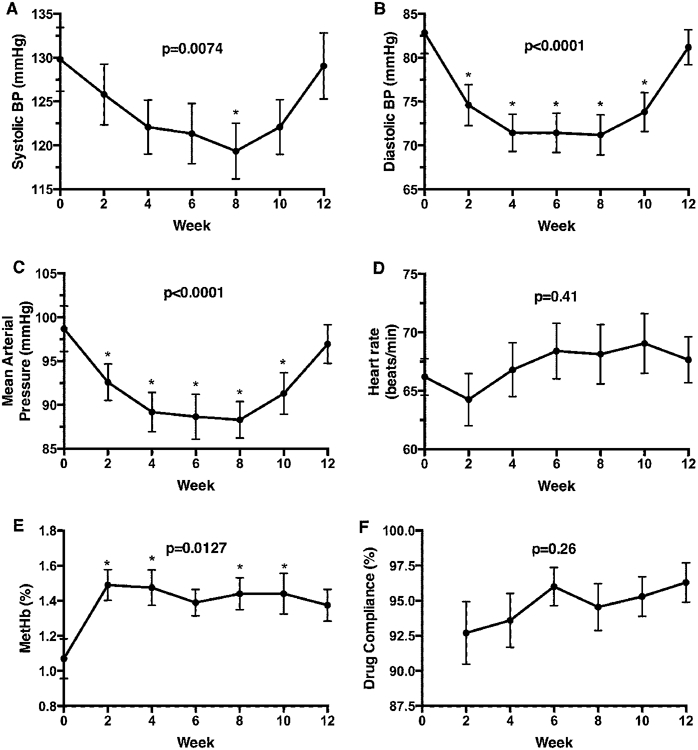
Hemodynamic responses and methemoglobin formation over 12 weeks of nitrite treatment. Compared to baseline, systolic (−10 mmHg, A), diastolic (−13 mmHg, B) and mean arterial pressures (−12 mmHg, C) were reduced significantly during the oral nitrite treatment period (all p<0.01). There was no observed rebound tachycardia with changes in blood pressures (D). The % blood methemoglobin increased significantly compared to baseline, but remained within the normal range and ≤3.1% in all subjects (E). Biweekly drug compliance by pill counts did not differ over time (F). Data are presented as mean ± standard error of the mean (SEM).
Hemodynamic Analysis and Methemoglobin Formation.
Compared to baseline, systolic (−10 mmHg), diastolic (−13 mmHg), and mean arterial pressures (−12 mmHg) were significantly reduced during the treatment period (all p<0.01, Fig. 1A-C) without differences in pulse pressure (Supplemental Table S2). During weeks 10-12 of the study, the blood pressure reduction effect was blunted with a diminution in Met-Hb levels, despite directly observed nitrite dosing and blinded automatic blood pressures obtained by subjects during outpatient bi-weekly safety visits. There was no observed rebound tachycardia with changes in blood pressures (Fig. 1D).
Nitrite reacts with both oxy- and deoxy-hemoglobin to form methemoglobin, which was carefully monitored for safety. The % blood methemoglobin increased significantly compared to baseline, but remained within the normal range and ≤3.1% in all subjects. The levels of MetHb were also well below the 5% threshold which would have required dose reduction of nitrite per protocol (Fig. 1E).
Endothelial function and vascular remodeling.
Carotid IMT decreased from 0.773±0.02 at baseline to 0.730±0.02 mm after 12 weeks of nitrite treatment (p<0.01, Fig. 2A). IMT was reduced 0.04 mm over 12 weeks, whereas typical age-related progression of CCA IMT is to increase 0.0015-0.0022 mm every 12 weeks.39 Although not statistically significant, there were concordant trends towards a reduction in arterial stiffness as measured by PWV (Fig. 2B) and improvements in endothelial function as measured by FMD of the brachial artery (Fig. 2C). The baseline vascular diameter also increased significantly after 12 weeks of nitrite therapy (from 0.36±0.02 mm to 0.40±0.02 mm; p=0.0009; Supplemental Table S2).
Figure 2.
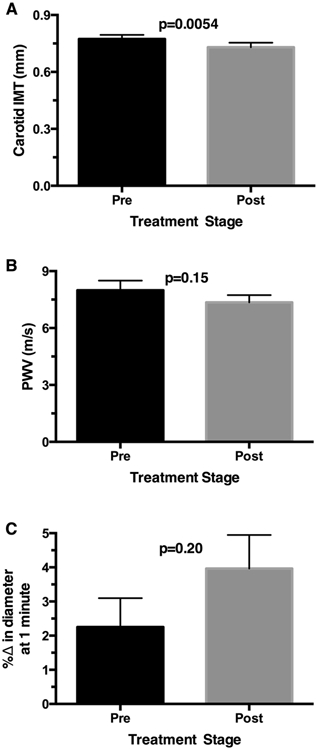
Endothelial function and vascular remodeling formation over 12 weeks of nitrite treatment. Carotid IMT decreased from baseline after 12 weeks of nitrite treatment (p<0.01, A). There were concordant trends towards a reduction in arterial stiffness as measured by PWV (B) and improvements in endothelial function as measured by FMD of the brachial artery (C). Data are presented as mean ± standard error of the mean (SEM).
Compared to baseline values, there were no changes in body weight, BMI, DEXA measures of lean mass, fat-free mass, bone mineral density or content, or glycemic measures including fasting glucose, HbA1c, and basal endogenous glucose production or fasting lipids with chronic nitrite treatment (Table 2). There was a 0.5% increase in % body fat (Table 2). This last finding was unexpected as prior studies in rodent models have suggested that nitrate treatment in the diet could reduce body fat and triglycerides.32 However, we recognize the caveat that this was one of many exploratory secondary endpoints.
Table 2.
Metabolic Treatment Characteristics. Data are presented as mean ± standard error of the mean (SEM).
| Variables | Pre-drug | 12-week Post-drug | P value |
|---|---|---|---|
| Body weight (kg) | 114.2 ± 5.4 | 114.2 ± 5.5 | 0.99 |
| BMI (kg/m2) | 40.8 ± 1.5 | 40.8 ± 1.5 | 0.91 |
| Lean mass (kg) | 55.9 ± 2.9 | 55.2 ± 2.9 | 0.10 |
| Fat free mass (kg) | 58.8 ± 3.0 | 58.1 ± 3.1 | 0.10 |
| Body fat (%) | 47.7 ± 1.1 | 48.3 ± 1.1 | 0.04 |
| Bone mineral density (grams/cm2) | 1.28 ± 0.0 | 1.28 ± 0.0 | 0.88 |
| Bone mineral content (grams) | 2878 ± 150 | 2873 ± 144 | 0.79 |
| Fasting glucose (mg/dL) | 102.2 ± 2.5 | 102.6 ± 3.1 | 0.87 |
| Hemoglobin A1c (%) | 5.7 ± 0.1 | 5.7 ± 0.1 | 0.87 |
| Basal endogenous glucose production (mg/kg/min) | 4.0 ± 0.2 | 4.1 ± 0.2 | 0.81 |
| Total cholesterol (mg/dL) | 187.0 ± 7.3 | 188.5 ± 6.5 | 0.82 |
| LDL cholesterol (mg/dL) | 112.1 ± 6.6 | 117.3 ± 5.6 | 0.33 |
| HDL cholesterol (mg/dL) | 50.2 ± 2.9 | 48.7 ± 3.6 | 0.45 |
| Triglycerides (mg/dL) | 122.9 ± 15.6 | 114.6 ± 9.6 | 0.61 |
Insulin sensitivity.
Among the 19 of 20 subjects with complete isotope analyses, following oral nitrite treatment for 12 weeks, there was a trend towards improvement in suppression of endogenous glucose production (EGP) in the final hour of the clamp (p=0.078; Fig. 3A). Overall there was no statistically significant increase in the rate of glucose infusion (GIR) required to maintain euglycemia (Fig. 3B) and no statistically significant increase in insulin stimulated glucose disposal (Rd) using 6,6,2 stable glucose isotope analyses during the hyperinsulinemic euglycemic clamp (+10%; 7.3±0.7 at baseline to 8.0±0.8 mg/kg/min post, p=0.21, Fig. 3C).
Figure 3.
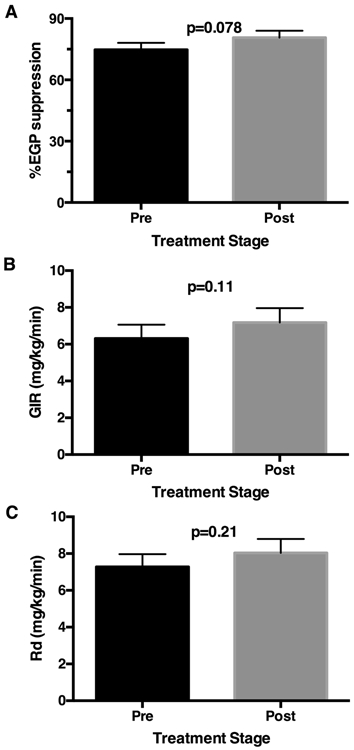
Insulin sensitivity over 12 weeks of nitrite treatment. Following 12 weeks of oral nitrite treatment, there was a trend towards improvement in suppression of endogenous glucose production in the final hour of the clamp (p=0.078; A). Overall there was no statistically significant increase in the rate of glucose infusion (GIR) required to maintain euglycemia (p=0.11; B) and no statistically significant increase in insulin-stimulated glucose disposal (Rd) using 6,6,2 stable glucose isotope analyses during the hyperinsulinemic euglycemic clamp (p=0.21; C). Data are presented as mean ± standard error of the mean (SEM).
DISCUSSION
In mammals, NO2− is generated via two primary mechanisms. In the endogenous pathway, the enzyme nitric oxide synthase (NOS) oxidizes L-Arginine to produce the free radical NO, which is further oxidized in the blood and the tissue to form NO2−.1,40 A complementary pathway of NO generation is the nitrate-nitrite-nitric oxide pathway. Dietary NO3− is consumed predominantly via leafy vegetables and beets.1,40 However, mammals lack the enzymes necessary to reduce exogenous nitrate, but bacteria present in the oral microbiome reduce NO3− into NO2−. In the acidic milleiu of the stomach, some of the NO2− is further reduced to NO. The remaining NO2− is subsequently absorbed into the gastrointestinal tract where it is ultimately further reduced to NO by the nitrite reductases hemoglobin, myoglobin, ascorbate, polyphenols, protons, and xanthine oxidoreductase along a pH and oxygen gradient.13, 40 Nitrite, generated both through the endogenous and exogenous pathways, therefore, serves as a circulating vascular reservoir for the potent vasodilator NO. Previous work performed by our group demonstrated an acute reduction in systolic blood pressure (−12.1 mmHg, p<0.001), diastolic blood pressure (−8.1 mmHg, p=0.002) and mean arterial pressure (−10.4 mmHg, p=0.001) after the administration of a single dose of oral sodium nitrite in healthy adults.25 Furthermore, 6 hours after acute dosing, inhibition of platelet activation was observed. The effect of nitrite on both blood pressure and platelets was eliminated by the co-administration of conjugated linoleic acid.25
The findings from this current study evaluating 12 weeks of therapy indicate that chronic oral nitrite administration to patients with hypertension and the metabolic syndrome results in the generation of NO, as evident by a rise in methemoglobin production (which can also form from direct reaction of nitrite with oxy- and deoxy-hemoglobin), and yields a statistically significant reduction in systolic blood pressure (−10 mmHg, p=0.0074), diastolic blood pressure (−13 mmHg, p<0.0001), and mean arterial pressure (−12 mmHg, p<0.0001). This blood pressure lowering effect was blunted at the 10-week mark with a resultant increase in blood pressure between weeks 10-12. This effect is not attributed to adherence to the study nitrite, as measurements were performed after directly observing the morning dose of nitrite. This finding was unexpected and may suggest subjects developed tolerance to the blood pressure reducing effects of sodium nitrite, but this requires further exploration. In prior studies in primates, tolerance was not observed over 2 weeks of therapy.16
Beyond the antihypertensive properties of oral nitrite, this clinical trial demonstrated a significant reduction in carotid IMT over the 12-week period. Carotid IMT is a measure of carotid atherosclerosis which is linked to a dysfunction in endothelial NO production.14 Improved endothelial function with chronic oral nitrite is further reflected in a trend towards improved FMD. Other studies have shown that therapy with nitrate, which is metabolized by the oral microbiome to form nitrite,41 can also reduce IMT and FMD.42-46 Higher vegetable nitrate intake has been significantly and independently associated with lower mean and maximal carotid IMT, and was associated with lower 15 year risk of cerebrovascular disease events.47 There was no significant change in patient weight, BMI, or DEXA measures of lean mass, fat-free mass, bone mineral density or content after chronic nitrite therapy. These findings suggest that oral nitrite may have activity for vascular disease and atherosclerosis, but additional larger and placebo-controlled studies are required for confirmation.
Data from the hyperinsulinemic-euglycemic clamp revealed a trend towards a decrease in production of endogenous glucose, denoting improved hepatic insulin sensitivity, and a trend towards improved peripheral insulin sensitivity by 10%. While less significant, this was in line with the increase in insulin sensitivity by 31.5% observed in prior work conducted in obese Lepob mice administered nitrite and rosiglitazone (6mg/kg/day s.q.; an insulin sensitizing drug positive control group) compared to similarly obese vehicle controls (rosiglitazone 8.0 ± 0.3 ml/kg/min and nitrite 8.6 ± 0.7 ml/kg/min vs. saline 6.6 ± 0.5 mg/kg/min).33 Clearly, our sample size was not sufficiently powered to detect this 10% improvement in insulin sensitivity. We excluded patients with type 2 diabetes, who are characterized by their marked insulin resistance, and insulin sensitivity values in our study subjects at baseline were relatively normal. It is plausible that oral nitrite therapy would be more efficacious in patients with type 2 diabetes or in those who are more insulin resistant, however a placebo-controlled human study of oral beetroot juice in diabetic patients, most receiving background metformin therapy, failed to show significant effects on insulin sensitivity.48,49 It also remains possible that the reduced efficacy on blood pressure reduction observed at 10-12 weeks may similarly have limited effects on insulin sensitivity. Based on the findings from this study, chronic inorganic nitrite supplementation offers promise as an effective antihypertensive agent and may improve vascular remodeling and insulin sensitivity. Confirmation of these findings will require larger and blinded placebo-controlled studies with consideration to measure vascular and insulin sensitivity endpoints at the midpoint of chronic nitrite therapy.
The blood pressure lowering effect of chronic oral nitrite administration was unequivocal in this clinical trial. However, this effect was abrogated after week 10 with a gradual normalization of blood pressure during weeks 10-12. This may be a manifestation of tolerance, cross-tolerance or pseudotolerance, phenomena initially described in 1888 secondary to chronic administration of the organic nitrate nitroglycerin. With chronic nitroglycerin treatment, patients have developed acetylcholine-induced paradoxical vasoconstriction with a suspected reduction in vascular NO bioavailability and NOS dysfunction.50 Plasma expansion has been documented with the use of sodium nitrite in both animal studies and clinical trials.51-53 Physiologically, sodium nitrite results in a reduction in adrenal and gonadal steroidogenesis which impairs water excretion and results in volume expansion. Aldosterone deficiency yields sodium loss and increased renin which results in volume expansion absent sodium retention.51, 53, 54 The trend towards an improvement in brachial FMD over the study period argues against increased sensitivity to receptor-dependent vasoconstrictors, or paradoxical vasoconstriction, reduced vascular NO bioavailability, and NOS dysfunction. Prior pre-clinical rodent studies have reported a decrease in eNOS expression with chronic nitrate treatment.55 However, our patients did demonstrate a reduction in serum hemoglobin from the screening visit to week 10 (14.07 g/dL +/− 0.28 to 13.11 g/dL +/− 0.30, p <0.0001), which suggests possible plasma expansion from chronic nitrite administration, consistent with pseudotolerance. It is also notable that the increase in MAP at week 12 is also associated with a decrease in Met-Hb levels, suggesting that the formation of NO from nitrite may be limited starting at 10-12 weeks (Fig 1). The gradual blunting of the blood pressure lowering effect of oral nitrite suggests, as is the case with many other oral anti-hypertensives, that drug dosing should be increased after the initial dose and titrated to adequate blood pressure control. Alternatively, the drug can be combined with a renin-angiotension-aldosterone inhibitor or a diuretic.
To date, nitrite has been evaluated in several disease states. Ischemia reperfusion is a state characterized by low oxygen and a deficiency in bioavailable NO. In pre-clinical models, nitrite therapy has demonstrated a protective effect at the level of the brain, heart, kidneys, liver, and lungs if administered during, or shortly thereafter, the ischemia phase.1,56 The findings have been more heterogenous in clinical trials. Work by Jones et al. failed to demonstrate a reduction in infarct size in a cohort of patients suffering an myocardial infarction who were treated with intracoronary nitrite.57 In a sub-group analysis in this trial, however, intracoronary nitrite did reduce infarct volume in patients with a significantly reduced coronary perfusion (as measured by TIMI flow score) at the time of infarct. Finally, a follow-up analysis of major adverse cardiac event rates showed 5.2% in the nitrite treatment group versus 25.0% with placebo at 3 years of follow up (p= 0.013), and a reduction in non-stustained ventricular tachycardia with nitrite therapy.58,59 A second trial of systemic intravenous nitrite infusion for ST elevation myocardial infarction (STEMI) given prior to reperfusion failed to show a significant reduction in infarct size.60
A number of studies have evaluated the effects of inhaled nitrite on hemodynamic responses at baseline and to exercise in patients with HFpEF (with and without high transpulmonary gradients) and reported decreases in left ventricular end-diastolic, pulmonary artery, and central venous pressures with therapy.61, 62 However, a randomized, placebo-controlled trial of inhaled nitrite failed to improve exercise performance in HFpEF patients.63 Inhaled nitrite has recently been explored as treatment for pulmonary hypertension in patients with β-thalassemia. Acute inhalation resulted in a 13.5% reduction in mean pulmonary artery pressure which was transient, as the effect was lost 15 minutes after the drug was administered.64 Ongoing studies continue to investigate the effects of oral nitrite in patients with chronic disease states such as pulmonary hypertension secondary to heart failure with preserved ejection fraction (PH-HFpEF) (NCT03015402).
In conclusion, this phase 2 proof of concept investigational drug trial enrolled 20 patients to study the effect of chronic oral nitrite administration in patients with hypertension and the metabolic syndrome. Oral nitrite lowered blood pressure and improved markers of endothelial dysfunction (IMT with trends toward improved FMD) without a significant effect on insulin resistance. While many of these measurements are objective, the unblinded nature of this trial represents an intrinsic weakness. It is notable that baseline brachial artery vascular diameter was increased significantly after 12 weeks of nitrite therapy (from 0.36±0.02 mm to 0.40±0.02 mm; p=0.0009; Supplemental Table S2), and this change could affect measurements of IMT and FMD. Additional larger randomized and placebo-controlled clinical trials are needed to further explore these findings.
Perspectives
This is the first study to evaluate the effects of an oral formulation of sodium nitrite over a 12-week time period on multiple measures of the metabolic syndrome and endothelial function. Oral nitrite significantly lowered systolic, diastolic, and mean arterial pressures, but tolerance was observed after 10-12 weeks of therapy. There was a significant improvement in the intima-media thickness of the carotid artery and trends toward improvements in flow-mediated vasodilation of the brachial artery and insulin sensitivity. This safety and efficacy study suggests that future placebo-controlled trials of sodium nitrite targeting multiple features of the metabolic syndrome including hypertension, insulin resistance, and endothelial dysfunction may have to plan dose escalation approaches after 10 weeks of therapy.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first study to evaluate the effects of an oral formulation of sodium nitrite over a 12-week time period on multiple measures of the metabolic syndrome and endothelial function, including blood pressure responses over 12 weeks, insulin sensitivity, flow mediated vasodilation, intimal media thickness and pulse wave velocity. The study is also the first to show tolerance between 10 and 12 weeks of nitrite therapy on blood pressure responses, suggesting that future placebo-controlled trials may have to plan dose escalation approaches after 10 weeks of therapy.
What Is Relevant?
This safety and efficacy study will inform future placebo-controlled trials of sodium nitrite for hypertension, insulin resistance, and endothelial dysfunction.
Acknowledgements
Dr. Gladwin receives research support from NIH grants 5R01HL098032, 2R01HL125886, UG3 HL143192, and 5P01HL103455, 5T32HL110849, the Burroughs Wellcome Foundation, the Institute for Transfusion Medicine, and the Hemophilia Center of Western Pennsylvania.
Sources of funding This study was supported by K12HD052892 (K.S.H.), K23HL124051 (K.S.H.), Cochrane-Weber Endowed Fund in Diabetes Research grant (K.S.H.), Pilot Project Program in Hemostasis and Vascular Biology grant (K.S.H.) and a generous monetary gift from McKamish, Inc. (K.S.H., M.T.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosures Dr. Gladwin is a co-inventor of pending patent applications and planned patents directed to the use of recombinant neuroglobin and heme-based molecules as antidotes for CO poisoning, which have been licensed by Globin Solutions, Inc. Globin Solutions, Inc. also has an option to a potential therapeutic for CO poisoning from VCU, hydroxycobalamin. Dr. Gladwin is a shareholder, advisor, and director in Globin Solutions, Inc. Dr. Gladwin is also co-inventor on patents directed to the use of nitrite salts in cardiovascular diseases, which were previously licensed to United Therapeutics and Hope Pharmaceuticals, and is now licensed to Globin Solutions. Additionally, Dr. Gladwin is a co-investigator in a research collaboration with Bayer Pharmaceuticals to evaluate riociguate as a treatment for patients with SCD.
REFERENCES
- 1.Lundberg JO, Gladwin MT and Weitzberg E. Strategies to increase nitric oxide signalling in cardiovascular disease. Nature reviews Drug discovery. 2015;14:623–41. [DOI] [PubMed] [Google Scholar]
- 2.Napoli C and Ignarro LJ. Nitric oxide and pathogenic mechanisms involved in the development of vascular diseases. Archives of pharmacal research. 2009;32:1103–8. [DOI] [PubMed] [Google Scholar]
- 3.Hord NG, Tang Y and Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. The American journal of clinical nutrition. 2009;90:1–10. [DOI] [PubMed] [Google Scholar]
- 4.Doel JJ, Benjamin N, Hector MP, Rogers M and Allaker RP. Evaluation of bacterial nitrate reduction in the human oral cavity. European journal of oral sciences. 2005;113:14–9. [DOI] [PubMed] [Google Scholar]
- 5.Liu C, Wajih N, Liu X, Basu S, Janes J, Marvel M, Keggi C, Helms CC, Lee AN, Belanger AM, Diz DI, Laurienti PJ, Caudell DL, Wang J, Gladwin MT and Kim-Shapiro DB. Mechanisms of human erythrocytic bioactivation of nitrite. The Journal of biological chemistry. 2015;290:1281–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tejero J, Sparacino-Watkins CE, Ragireddy V, Frizzell S and Gladwin MT. Exploring the mechanisms of the reductase activity of neuroglobin by site-directed mutagenesis of the heme distal pocket. Biochemistry. 2015;54:722–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang J, Krizowski S, Fischer-Schrader K, Niks D, Tejero J, Sparacino-Watkins C, Wang L, Ragireddy V, Frizzell S, Kelley EE, Zhang Y, Basu P, Hille R, Schwarz G and Gladwin MT. Sulfite Oxidase Catalyzes Single-Electron Transfer at Molybdenum Domain to Reduce Nitrite to Nitric Oxide. Antioxidants & redox signaling. 2015;23:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sparacino-Watkins CE, Tejero J, Sun B, Gauthier MC, Thomas J, Ragireddy V, Merchant BA, Wang J, Azarov I, Basu P and Gladwin MT. Nitrite reductase and nitric-oxide synthase activity of the mitochondrial molybdopterin enzymes mARC1 and mARC2. The Journal of biological chemistry. 2014;289:10345–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khambata RS, Ghosh SM and Ahluwalia A. "Repurposing" of Xanthine Oxidoreductase as a Nitrite Reductase: A New Paradigm for Therapeutic Targeting in Hypertension. Antioxidants & redox signaling. 2015;23:340–53. [DOI] [PubMed] [Google Scholar]
- 10.Tejero J, Shiva S and Gladwin MT. Sources of Vascular Nitric Oxide and Reactive Oxygen Species and Their Regulation. Physiological reviews. 2019;99:311–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amdahl MB, Sparacino-Watkins CE, Corti P, Gladwin MT and Tejero J. Efficient Reduction of Vertebrate Cytoglobins by the Cytochrome b5/Cytochrome b5 Reductase/NADH System. Biochemistry. 2017;56:3993–4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corti P, Xue J, Tejero J, Wajih N, Sun M, Stolz DB, Tsang M, Kim-Shapiro DB and Gladwin MT. Globin X is a six-coordinate globin that reduces nitrite to nitric oxide in fish red blood cells. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:8538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lundberg JO, Carlstrom M, Larsen FJ and Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovascular research. 2011;89:525–32. [DOI] [PubMed] [Google Scholar]
- 14.Stokes KY, Dugas TR, Tang Y, Garg H, Guidry E and Bryan NS. Dietary Nitrite Prevents Hypercholesterolemic Microvascular Inflammation and Reverses Endothelial Dysfunction. American journal of physiology Heart and circulatory physiology. 2009:012912008. [DOI] [PubMed] [Google Scholar]
- 15.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO 3rd and Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature medicine. 2003;9:1498–505. [DOI] [PubMed] [Google Scholar]
- 16.Dejam A, Hunter CJ, Tremonti C, Pluta RM, Hon YY, Grimes G, Partovi K, Pelletier MM, Oldfield EH, Cannon RO 3rd, Schechter AN and Gladwin MT. Nitrite infusion in humans and nonhuman primates: endocrine effects, pharmacokinetics, and tolerance formation. Circulation. 2007;116:1821–31. [DOI] [PubMed] [Google Scholar]
- 17.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ and Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, Arghandawi S, Pearl V, Benjamin N, Loukogeorgakis S, Macallister R, Hobbs AJ, Webb AJ and Ahluwalia A. Inorganic nitrate supplementation lowers blood pressure in humans: role for nitrite-derived NO. Hypertension. 2010;56:274–81. [DOI] [PubMed] [Google Scholar]
- 19.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N and Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. Journal of applied physiology. 2010;109:135–48. [DOI] [PubMed] [Google Scholar]
- 20.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N and Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. Journal of applied physiology. 2009;107:1144–55. [DOI] [PubMed] [Google Scholar]
- 21.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG and Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. American journal of physiology Regulatory, integrative and comparative physiology. 2010;299:R1121–31. [DOI] [PubMed] [Google Scholar]
- 22.Sobko T, Marcus C, Govoni M and Kamiya S. Dietary nitrate in Japanese traditional foods lowers diastolic blood pressure in healthy volunteers. Nitric oxide : biology and chemistry. 2010;22:136–40. [DOI] [PubMed] [Google Scholar]
- 23.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO and Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. The New England journal of medicine. 2006;355:2792–3. [DOI] [PubMed] [Google Scholar]
- 24.Hunault CC, van Velzen AG, Sips AJ, Schothorst RC and Meulenbelt J. Bioavailability of sodium nitrite from an aqueous solution in healthy adults. Toxicology letters. 2009;190:48–53. [DOI] [PubMed] [Google Scholar]
- 25.Hughan KS, Wendell SG, Delmastro-Greenwood M, Helbling N, Corey C, Bellavia L, Potti G, Grimes G, Goodpaster B, Kim-Shapiro DB, Shiva S, Freeman BA and Gladwin MT. Conjugated Linoleic Acid Modulates Clinical Responses to Oral Nitrite and Nitrate. Hypertension. 2017;70:634–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaidya D, Yanek LR, Faraday N, Moy TF, Becker LC and Becker DM. Native platelet aggregation and response to aspirin in persons with the metabolic syndrome and its components. Metabolic syndrome and related disorders. 2009;7:289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Serebruany VL, Malinin A, Ong S and Atar D. Patients with metabolic syndrome exhibit higher platelet activity than those with conventional risk factors for vascular disease. Journal of thrombosis and thrombolysis. 2008;25:207–13. [DOI] [PubMed] [Google Scholar]
- 28.Minuz P, Patrignani P, Gaino S, Seta F, Capone ML, Tacconelli S, Degan M, Faccini G, Fornasiero A, Talamini G, Tommasoli R, Arosio E, Santonastaso CL, Lechi A and Patrono C. Determinants of platelet activation in human essential hypertension. Hypertension. 2004;43:64–70. [DOI] [PubMed] [Google Scholar]
- 29.Moss MB, Siqueira MA, Mann GE, Brunini TM and Mendes-Ribeiro AC. Platelet aggregation in arterial hypertension: is there a nitric oxide-urea connection? Clinical and experimental pharmacology & physiology. 2010;37:167–72. [DOI] [PubMed] [Google Scholar]
- 30.Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M and Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free radical biology & medicine. 2006;40:295–302. [DOI] [PubMed] [Google Scholar]
- 31.Rammos C, Hendgen-Cotta UB, Sobierajski J, Bernard A, Kelm M and Rassaf T. Dietary nitrate reverses vascular dysfunction in older adults with moderately increased cardiovascular risk. Journal of the American College of Cardiology. 2014;63:1584–5. [DOI] [PubMed] [Google Scholar]
- 32.Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E and Lundberg JO. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17716–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singamsetty S, Watanabe Y, Guo L, Corey C, Wang Y, Tejero J, McVerry BJ, Gladwin MT, Shiva S and O'Donnell CP. Inorganic nitrite improves components of the metabolic syndrome independent of weight change in a murine model of obesity and insulin resistance. The Journal of physiology. 2015;593:3135–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr., Spertus JA, Costa F, American Heart A, National Heart L and Blood I. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. [DOI] [PubMed] [Google Scholar]
- 35.EFS A. Nitrate in vegetables: scientific opinion of the panel on contaminants in the food chain. The EFSA Journal. 2008:1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schopfer FJ, Cole MP, Groeger AL, Chen CS, Khoo NK, Woodcock SR, Golin-Bisello F, Motanya UN, Li Y, Zhang J, Garcia-Barrio MT, Rudolph TK, Rudolph V, Bonacci G, Baker PR, Xu HE, Batthyany CI, Chen YE, Hallis TM and Freeman BA. Covalent peroxisome proliferator-activated receptor gamma adduction by nitro-fatty acids: selective ligand activity and anti-diabetic signaling actions. The Journal of biological chemistry. 2010;285:12321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodpaster BH, Kelley DE, Wing RR, Meier A and Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–47. [DOI] [PubMed] [Google Scholar]
- 38.Steele R Influences of glucose loading and of injected insulin on hepatic glucose output. Annals of the New York Academy of Sciences. 1959;82:420–30. [DOI] [PubMed] [Google Scholar]
- 39.Tzou WS, Douglas PS, Srinivasan SR, Bond MG, Tang R, Li S, Chen W, Berenson GS and Stein JH. Distribution and predictors of carotid intima-media thickness in young adults. Preventive cardiology. 2007;10:181–9. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg JO, Weitzberg E and Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature reviews Drug discovery. 2008;7:156–67. [DOI] [PubMed] [Google Scholar]
- 41.Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E and Morris A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free radical biology & medicine. 2017;105:48–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shepherd AI, Costello JT, Bailey SJ, Bishop N, Wadley AJ, Young-Min S, Gilchrist M, Mayes H, White D, Gorczynski P, Saynor ZL, Massey H and Eglin CM. "Beet" the cold: beetroot juice supplementation improves peripheral blood flow, endothelial function, and anti-inflammatory status in individuals with Raynaud's phenomenon. Journal of applied physiology. 2019;127:1478–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Walker MA, Bailey TG, McIlvenna L, Allen JD, Green DJ and Askew CD. Acute Dietary Nitrate Supplementation Improves Flow Mediated Dilatation of the Superficial Femoral Artery in Healthy Older Males. Nutrients. 2019;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee JS, Stebbins CL, Jung E, Nho H, Kim JK, Chang MJ and Choi HM. Effects of chronic dietary nitrate supplementation on the hemodynamic response to dynamic exercise. American journal of physiology Regulatory, integrative and comparative physiology. 2015;309:R459–66. [DOI] [PubMed] [Google Scholar]
- 45.Joris PJ and Mensink RP. Beetroot juice improves in overweight and slightly obese men postprandial endothelial function after consumption of a mixed meal. Atherosclerosis. 2013;231:78–83. [DOI] [PubMed] [Google Scholar]
- 46.Velmurugan S, Gan JM, Rathod KS, Khambata RS, Ghosh SM, Hartley A, Van Eijl S, Sagi-Kiss V, Chowdhury TA, Curtis M, Kuhnle GG, Wade WG and Ahluwalia A. Dietary nitrate improves vascular function in patients with hypercholesterolemia: a randomized, double-blind, placebo-controlled study. The American journal of clinical nutrition. 2016;103:25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bondonno CP, Blekkenhorst LC, Prince RL, Ivey KL, Lewis JR, Devine A, Woodman RJ, Lundberg JO, Croft KD, Thompson PL and Hodgson JM. Association of Vegetable Nitrate Intake With Carotid Atherosclerosis and Ischemic Cerebrovascular Disease in Older Women. Stroke. 2017;48:1724–1729. [DOI] [PubMed] [Google Scholar]
- 48.Shepherd AI, Gilchrist M, Winyard PG, Jones AM, Hallmann E, Kazimierczak R, Rembialkowska E, Benjamin N, Shore AC and Wilkerson DP. Effects of dietary nitrate supplementation on the oxygen cost of exercise and walking performance in individuals with type 2 diabetes: a randomized, double-blind, placebo-controlled crossover trial. Free radical biology & medicine. 2015;86:200–8. [DOI] [PubMed] [Google Scholar]
- 49.Gilchrist M, Winyard PG, Aizawa K, Anning C, Shore A and Benjamin N. Effect of dietary nitrate on blood pressure, endothelial function, and insulin sensitivity in type 2 diabetes. Free radical biology & medicine. 2013;60:89–97. [DOI] [PubMed] [Google Scholar]
- 50.Munzel T, Daiber A and Mulsch A. Explaining the phenomenon of nitrate tolerance. Circulation research. 2005;97:618–28. [DOI] [PubMed] [Google Scholar]
- 51.Munzel T, Heitzer T, Kurz S, Harrison DG, Luhman C, Pape L, Olschewski M and Just H. Dissociation of coronary vascular tolerance and neurohormonal adjustments during long-term nitroglycerin therapy in patients with stable coronary artery disease. Journal of the American College of Cardiology. 1996;27:297–303. [DOI] [PubMed] [Google Scholar]
- 52.Munzel T, Daiber A and Gori T. Nitrate therapy: new aspects concerning molecular action and tolerance. Circulation. 2011;123:2132–44. [DOI] [PubMed] [Google Scholar]
- 53.Parker JD, Farrell B, Fenton T, Cohanim M and Parker JO. Counter-regulatory responses to continuous and intermittent therapy with nitroglycerin. Circulation. 1991;84:2336–45. [DOI] [PubMed] [Google Scholar]
- 54.Panesar NS and Chan KW. Decreased steroid hormone synthesis from inorganic nitrite and nitrate: studies in vitro and in vivo. Toxicology and applied pharmacology. 2000;169:222–30. [DOI] [PubMed] [Google Scholar]
- 55.Carlstrom M, Liu M, Yang T, Zollbrecht C, Huang L, Peleli M, Borniquel S, Kishikawa H, Hezel M, Persson AE, Weitzberg E and Lundberg JO. Cross-talk Between Nitrate-Nitrite-NO and NO Synthase Pathways in Control of Vascular NO Homeostasis. Antioxidants & redox signaling. 2015;23:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lundberg JO, Gladwin MT, Ahluwalia A, Benjamin N, Bryan NS, Butler A, Cabrales P, Fago A, Feelisch M, Ford PC, Freeman BA, Frenneaux M, Friedman J, Kelm M, Kevil CG, Kim-Shapiro DB, Kozlov AV, Lancaster JR Jr., Lefer DJ, McColl K, McCurry K, Patel RP, Petersson J, Rassaf T, Reutov VP, Richter-Addo GB, Schechter A, Shiva S, Tsuchiya K, van Faassen EE, Webb AJ, Zuckerbraun BS, Zweier JL and Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nature chemical biology. 2009;5:865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jones DA, Pellaton C, Velmurugan S, Rathod KS, Andiapen M, Antoniou S, van Eijl S, Webb AJ, Westwood MA, Parmar MK, Mathur A and Ahluwalia A. Randomized phase 2 trial of intracoronary nitrite during acute myocardial infarction. Circulation research. 2015;116:437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jones DA, Whittaker P, Rathod KS, Richards AJ, Andiapen M, Antoniou S, Mathur A and Ahluwalia A. Sodium Nitrite-Mediated Cardioprotection in Primary Percutaneous Coronary Intervention for ST-Segment Elevation Myocardial Infarction: A Cost-Effectiveness Analysis. J Cardiovasc Pharmacol Ther. 2019;24:113–119. [DOI] [PubMed] [Google Scholar]
- 59.Jones DA, Rathod KS, Williamson A, Harrington D, Andiapen M, van Eijl S, Westwood M, Antoniou S, Schilling RJ, Ahluwalia A and Mathur A. The effect of intracoronary sodium nitrite on the burden of ventricular arrhythmias following primary percutaneous coronary intervention for acute myocardial infarction. Int J Cardiol. 2018;266:1–6. [DOI] [PubMed] [Google Scholar]
- 60.Siddiqi N, Neil C, Bruce M, MacLennan G, Cotton S, Papadopoulou S, Feelisch M, Bunce N, Lim PO, Hildick-Smith D, Horowitz J, Madhani M, Boon N, Dawson D, Kaski JC, Frenneaux M and investigators N. Intravenous sodium nitrite in acute ST-elevation myocardial infarction: a randomized controlled trial (NIAMI). European heart journal. 2014;35:1255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Simon MA, Vanderpool RR, Nouraie M, Bachman TN, White PM, Sugahara M, Gorcsan J 3rd, Parsley EL and Gladwin MT. Acute hemodynamic effects of inhaled sodium nitrite in pulmonary hypertension associated with heart failure with preserved ejection fraction. JCI insight. 2016;1:e89620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Borlaug BA, Melenovsky V and Koepp KE. Inhaled Sodium Nitrite Improves Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. Circulation research. 2016;119:880–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Borlaug BA, Anstrom KJ, Lewis GD, Shah SJ, Levine JA, Koepp GA, Givertz MM, Felker GM, LeWinter MM, Mann DL, Margulies KB, Smith AL, Tang WHW, Whellan DJ, Chen HH, Davila-Roman VG, McNulty S, Desvigne-Nickens P, Hernandez AF, Braunwald E, Redfield MM, National Heart L and Blood Institute Heart Failure Clinical Research N. Effect of Inorganic Nitrite vs Placebo on Exercise Capacity Among Patients With Heart Failure With Preserved Ejection Fraction: The INDIE-HFpEF Randomized Clinical Trial. Jama. 2018;320:1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yingchoncharoen T, Rakyhao T, Chuncharunee S, Sritara P, Pienvichit P, Paiboonsukwong K, Sathavorasmith P, Sirirat K, Sriwantana T, Srihirun S and Sibmooh N. Inhaled nebulized sodium nitrite decreases pulmonary artery pressure in beta-thalassemia patients with pulmonary hypertension. Nitric oxide : biology and chemistry. 2018;76:174–178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


