Abstract
Objective:
Cognitive training is an effective means of improving performance in a range of populations. Whether it may serve to facilitate cognitive recovery and longer-term outcomes in persons with alcohol use disorders (AUDs) is unclear. Here, we review historical and current literature and offer perspectives for model development and potential implementation.
Method:
We considered a large literature regarding the nature of alcohol-related compromise, early efforts to clarify the nature of recovery and current models and methods underlying cognitive training paradigms. We then constructed a narrative review demonstrating evolving frameworks and empirical data informing the critical review of cognitive training methods as a means of mitigating compromise and facilitating functional outcomes.
Results:
Cognitive improvement with abstinence is generally noted, but training protocols may enhance performance and generalize benefit to untrained, but highly similar, tasks. Transfer of training to dissimilar tasks and functional outcomes is uncommonly reported. It is noteworthy that some work suggests that clinician ratings for participants are improved. Inconsistency in sample characteristics, training protocols, and outcome measures constrain general conclusions while suggesting opportunities for study and development.
Conclusions:
Cognitive training protocols have shown benefit in a variety of populations but have been examined infrequently in persons with AUDs. This overview indicates significant opportunity for cognitive improvement and recovery and thus a strong potential role for training protocols. However, supportive data are not robustly obtained. We suggest that one step in bridging this gap is the implementation of a conceptual framework incorporating contextual, behavioral, and neurobiological variables.
Keywords: cognitive training, alcoholism, alcohol use disorder, cognition
General Scientific Summary
Alcohol use disorders are associated with a range of cognitive deficits. Cognitive interventions have been proposed to facilitate cognitive recovery and improve long-term drinking outcomes and psychosocial adaptation. This review summarizes current literature and offers direction for future research and implementation.
Decades of research demonstrate alcohol-related neurocognitive compromise persisting after detoxification among persons with alcohol use disorders (AUDs) but who do not suffer from Korsakoff’s syndrome or alcoholic dementia (Parsons, 1987a; Oscar-Berman et al., 2014). Although the specific number of those affected is not known, it is estimated that 30% to 40% of those with AUD exhibit clinically relevant levels of neurocognitive compromise throughout the first 2 months of abstinence (Diagnostic & Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association, 2013). Estimates derived from research samples suggest that 50% to 80% of detoxified treatment seekers perform significantly worse than healthy age and education equivalent peers with a portion of these participants demonstrating clear clinical impairment (Bates, Bowden, & Barry, 2002; Fals-Stewart & Lam, 2010; Parsons, 1986; Parsons & Nixon, 1993; Stavro, Pelletier, & Potvin, 2013; Sullivan, Fama, Rosenbloom, & Pfefferbaum, 2002). Although verbal functions are relatively spared, alcohol-related deficits are observed across diverse cognitive abilities (Oscar-Berman et al., 2014; Parsons & Nixon, 1993). Consistent with variability in the estimates of those affected, there is notable heterogeneity in the level of severity across tests within individuals as well as across studies (Goldman, 1983; Le Berre, Fama, & Sullivan, 2017; Pitel, Beaunieux, et al., 2007, Pitel, Witkowski, et al. 2007b). That said, deficits in abilities strongly dependent on the integrity of the prefrontal cortex (PFC) and its diverse networks such as attention, inhibitory control, behavioral regulation, and decision-making, that is, those commonly referenced as components of executive function (EF), are most vulnerable (Oscar-Berman et al., 2014; Sullivan et al., 2002; Tivis, Beatty, Nixon, & Parsons, 1995).
An expansive literature suggests a relationship between alcohol-related neurobehavioral or cognitive deficits and poorer treatment outcomes as reflected in measures of treatment engagement, treatment completion, and posttreatment abstinence (Buckman, Bates, & Morgenstern, 2008; Domínguez-Salas, Díaz-Batanero, Lozano-Rojas, & Verdejo-García, 2016; Fals-Stewart & Lam, 2010; Fals-Stewart, Schafer, Lucente, Rustine, & Brown, 1994; Glenn, Sinha, & Parsons, 1993; Guthrie & Elliott, 1980; Leber, Parsons, & Nichols, 1985; McCrady & Smith, 1986; Teichner, Horner, Harvey, & Johnson, 2001; Walker, Donovan, Kivlahan, & O’Leary, 1983). Thus, not surprisingly, there is long-standing interest in exploring whether efforts to enhance cognition during treatment might benefit therapeutic process and posttreatment outcomes (Bates, Buckman, & Nguyen, 2013; Sofuoglu, DeVito, Waters, & Carroll, 2013; Walker et al., 1983). In response to early interest, a number of studies focusing on facilitating cognitive improvement were conducted in the late 20th century (see Goldman, 1986, 1987, 1995; Parsons, 1987b). Perhaps challenged by the inherent variability in alcohol-related deficits, inconsistent effect sizes, and/or conflicting resource demands, promising interventions were rarely implemented and programmatic study declined steeply with little reported advance across ~15–20 years. That said, fueled by developments in addiction and brain science, the field is enjoying a resurgence that is giving rise to new approaches offering promising outcomes (e.g., Rupp, Kemmler, Kurz, Hinterhuber, & Fleishhacker, 2012; for reviews, see Bates et al., 2013; Bickel, Quisenberry, Moody, & Wilson, 2015).
Although we focus on cognitive and behavioral outcomes, we recognize that substantive cognitive change is accompanied by neuroadaptive accommodation (Buschkuehl, Jaeggi, & Jonides, 2012; Klingberg, 2010; Strenziok et al., 2014). This conclusion is supported by a substantial body of work demonstrating neuroadaptations associated with recovery/abstinence (Chanraud & Sullivan, 2014; Fein & Chang, 2006; Fein, Torres, Price, & Di Sclafani, 2006; Naqvi & Morgenstern, 2015; Pfefferbaum et al., 2001; Prochaska & DiClemente, 1992; Rangaswamy & Porjesz, 2014; Seo & Sinha, 2015). Relatedly, there is also a developing literature suggesting treatment-specific effects on neural structure/function (Feldstein Ewing, Filbey, Sabbineni, Chandler, & Hutchinson, 2011; Vollstädt-Klein et al., 2011). However, as noted by Naqvi and Morgenstern (2015), systematic investigations exploring to what extent cognitive interventions might alter these trajectories are largely lacking.
As evidenced in later sections, we agree with investigators and clinicians who emphasize that clinically relevant intervention programs must ultimately benefit functional outcomes, that is, demonstrate real-world relevance (Allen, Goldstein, & Seaton, 1997; Bates et al., 2013). The definition of functional outcomes varies by disorder. Among persons with AUDs or other substance use disorders (SUDs), those of particular import include an improved capacity to mitigate/avoid harmful use and enhanced competency in managing personal, professional, and community relationships (Bates et al., 2013; Khemiri, Brynte, Stunkel, Klingberg, & Jayaram-Lindstrom, 2018; Kornreich et al., 2002; Oscar-Berman et al., 2014; Rupp, Derntl, Osthaus, Kemmler, & Fleischhacker, 2017). Engagement in treatment, self-efficacy, and willingness to change also constitute relevant outcomes and are impacted by cognitive impairment (e.g., Bates, Pawlak, Tonigan, & Buckman, 2006; Le Berre et al., 2012). Yet, they remain underappreciated as outcome measures in cognitive interventions (e.g., see Table 1).
Table 1.
Recent Works
| Reference | Diagnosis & details | Setting | M age | N | Training details | Control group | Sessions (min/per) | Transfer domain | Transfer measure/instrument | Significance | Effect size/95% Cl |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fals-Stewart & Lam, 2010 | SUD | Inpatient | ~33 | 160 | Multidomain | Active | 24 (50) | Cognitive | Neuropsych Assessment Battery (NAB-SM) | z = 2.35, p < .05 | d = .37 |
| Typing tutorial | Functional | Working Alliance Inventory (WAI-S) | z = 2.68, p < .01 | d = .43 | |||||||
| Staff Rating Scale (SRS) | z = 3.29, p < .01 | d = .57 | |||||||||
| Client Assessment Scale (CAS) | z = 2.78, p < .01 | d = .94 | |||||||||
| Addiction Severity Index (ASI)—Alcohol 12 mo. | p < .01 | d = .61 | |||||||||
| (ASI) Drug 12 mo. | p < .01 | d = .99 | |||||||||
| (ASI) Employement 12 mo. | ns | — | |||||||||
| (ASI) Medical 12 mo. | ns | — | |||||||||
| (ASI) Psychiatric 12 mo. | ns | — | |||||||||
| (ASI) Psychiatric 12 mo. | p < .01 | d = .47 | |||||||||
| (ASI) Legal 12 mo. | p < .01 | d = .44 | |||||||||
| Drinking/use | Timeline Followback (TLFB) % Days Abstinent | p < .01 | d = .65 | ||||||||
| Houben, Wiers, and Jansen (2011) | No diagnosis Heavy drinkers | Community | ~44 | 48 | WM Online delivery | Active nonadaptive | 25 (30) | Cognitive | None | — | — |
| Functional | None | — | — | ||||||||
| Drinking/use | Alcohol Timeline Followback(TLFB) | F = 6.60, p = .01 | pEta2 = .160 | ||||||||
| Rupp, Kemmler, Kurz, Hinterhuber, & Fleishhacker, 2012 | AUD | Inpatient | ~45 | 41 | Multidomain | Treatment as usual | 12 (45–60) | Cognitive | Alertness | F = 4.13, p = .05 | pEta2 = .105 |
| Divided Attention | F = 4.21,p = .05 | pEta2 = .107 | |||||||||
| Mild cognitive impairments (>1 SD below norms) | Digit Span (DS) - backward | F = 4.68, p = .04 | pEta2 = .118 | ||||||||
| 2-back working memory task | F = 4.34, p = .04 | pEta2 = .110 | |||||||||
| Incompatibility | ns | — | |||||||||
| Verbal Fluency | ns | — | |||||||||
| Trail Making Test A (TMT-A) | ns | — | |||||||||
| Trail Making Test B (TMT-B) | ns | — | |||||||||
| Digit Span (DS)—forward | ns | — | |||||||||
| Munich Verbal Learning (MVG)—Total | ns | — | |||||||||
| Munich Verbal Learning (MVG)—Recall | F = 4.71, p = .04 | pEta2 = .119 | |||||||||
| Munich Verbal Learning (MVG) - Recognition | ns | — | |||||||||
| Complex Figure Test (CFT) recall-long delay | F = 3.32, p = .08 | pEta2 = .087 | |||||||||
| Complex Figure Test (CFT) copy | F = 4.43, p = .04 | pEta2 = .112 | |||||||||
| Block Design | ns | ||||||||||
| Mini-Mental State Examination (MMSE) | F = 5.77, p = .02 | pEta2 = .142 | |||||||||
| Beck Depression Index (BDI) | ns | — | |||||||||
| Symptoms Checklist (SCL-90-R)—Distress | F = 6.23, p = .02 | pEta2 = .151 | |||||||||
| SCL-90-R–Symptom Intensity | ns | — | |||||||||
| SCL-90-R-Number of Symptoms | F = 4.56, p = .04 | pEta2 = .115 | |||||||||
| Obsessive Drinking Scale (OCDS) | ns | — | |||||||||
| Compulsive Drinking Scale (OCDS) | F = 4.13, p = .05 | pEta2 = .105 | |||||||||
| Drinking/Use | None | — | — | ||||||||
| Gamito et al. (2014) | AUD | Inpatient | ~45 | 54 | Multidomain | Treatment as usual | 10 (60) | Cognitive | Wisconsin Card Sort (WCST)—Correct | ns | — |
| Frontal Assessment Battery (FAB) | F = 8.00, p = .01 | Eta2 = .16 | |||||||||
| Tablet delivery | Color Trail Task 1 (CTT1) | ns | — | ||||||||
| Color Trail Task 2 (CTT2) | ns | — | |||||||||
| Functional | Mini-Mental State Examination (MMSE) | ns | — | ||||||||
| Drinking/use | none | — | — | ||||||||
| Bell, Vissicchio, and Weinstein (2016) | AUD | Inpatient (first 30 days) | ~55 | 31 | Multidomain | Work therapy | 65 (60) | Cognitive | Hopkin’s Verbal Learning (HVLT-R) - memory | F = 7.98, p < .01 | d = 1.01 |
| Veterans; men only | Outpatient (last 60 days) | Hopkin’s Verbal Learning (HVLT-R)—learning | F = 10.73, p < .01 | d = 1.09 | |||||||
| Functional | None | — | |||||||||
| Drinking/use | None | — | |||||||||
| Khemiri, Brynte, Stunkel, Klingberg, & Jayaram-Lindstrom, 2018 | AUD | Outpatient | ~50 | 50 | WM | Active nonadaptive | 20 (30–45) | Cognitive | Digit Span (DS)—Total | F = 6.12, p = .02 | pEta2 = .142 |
| Digit Span (DS)—Backward | F = 6.14, p = .02 | pEta2 = .142 | |||||||||
| Online delivery | Digit Span (DS)–Forward | ns | — | ||||||||
| Spatial Working Memory (SWM) | ns | — | |||||||||
| Stop Signal Task (SST) | ns | — | |||||||||
| Rapid Visual Processing (RVP) | ns | — | |||||||||
| Towers of London (ToL) | ns | — | |||||||||
| Functional | Cambridge Gambling Task (CGT) | ns | — | ||||||||
| Delay Discounting (DD) | ns | — | |||||||||
| Obsessive Compulsive Drinking Scale (OCDS) | ns | — | |||||||||
| Desires for Alcohol Questionnaire (DAQ) | ns | — | |||||||||
| Montgomery-Asperg Depression (MADRS) | ns | — | |||||||||
| Drinking/use | (Alc-TLFB) % Heavy Drinking Days | ns | — | ||||||||
| (Alc-TLFB) Drinks/Drinking Day | F = 3.48, p = .07 | pEta2 = .086 | |||||||||
| (Alc-TLFB) % Drinking Days | ns | — | |||||||||
| (Alc-TLFB) Drinks/Day | ns | — | |||||||||
| Snider et al. (2018) | AUD | Community | ~42 | 50 | WM | Active nonadaptive | 20 | Cognitive | Functional “Instructions” Working Memory Task | F = 2.65, p = .01 | d = .76 |
| Functional | Delay Discounting (DD) | ns | — | ||||||||
| DD + Episode Future Thinking (EFT DD) | ns | — | |||||||||
| Alcohol Use Disorder Identification Test (AUDIT) | ns | — | |||||||||
| Drinking/use | None | — | — | ||||||||
| Gunn, Gerst, Wiemers, Redick, and Finn (2018) | AUD | Community | ~22 | 145 | WM | Active/Adaptive | 15 | Cognitive | Rotation Span Task (RTS) | B = −7.79, p < .01 | (−12.37, −3.20) |
| Reading Span (RDS) | ns | — | |||||||||
| Primarily university students | Auditory Consonant Trigram (ACT) | B = −2.85, p < .05 | (−5.33, −.37) | ||||||||
| Visual search task | Running Letter Span (RLS) | ns | — | ||||||||
| Running Spatial Span (RSS) | B = −4.47, p < .05 | (−8.54, −.40) | |||||||||
| Keep Track task (KT) | ns | — | |||||||||
| Functional | None | — | — | ||||||||
| Drinking/use | None | — | — | ||||||||
| Hendershot et al. (2018) | SUD | Inpatient | ~40 | 110 | WM | Active nonadaptive | 25 (45) | Cognitive | Digit Span (DS) backward | B = .78, p = .05 | (.02, 1.54) |
| Letter-number sequencing (L-NS) | ns | — | |||||||||
| Inpatient + online | Spatial Span backward | ns | — | ||||||||
| Delivery | Symbol Span | ns | — | ||||||||
| Functional | Delay Discounting (DD) | ns | — | ||||||||
| Drinking/use | Multisubstance TLFB | ns | — | ||||||||
| Jones et al. (2018) | No diagnosis | Community | ~41 | 205 | Inhibitory control | Active | 14 | Cognitive | Stop-Signal Response Task (SSRT) | ns | — |
| Heavy drinkers w/desire to reduce | Online delivery | Category task | Functional | Alcohol SSRT | ns | — | |||||
| Alcohol Implicit Association Task (IAT) | ns | — | |||||||||
| Drinking/use | Alcohol volume consumed during training | ns | — | ||||||||
| Number of heavy drinking days | ns | — | |||||||||
Note. SUD = substance use disorder; AUD = alcohol use disorder; WM = working memory.
Finally, even a casual review of extant literature reveals multiple labels for methods directed to improving cognition (e.g., cognitive rehabilitation, cognitive remediation, and cognitive retraining). The terms are often used interchangeably. But, at least partially, they reflect differences in the presumed etiology of cognitive improvement, specifically whether improvement occurs through reestablishing knowledge/skills or via developing alternate or compensatory means of accomplishing a task (Allen et al., 1997; Harvey, McGurk, Mahncke, & Wykes, 2018). Here, we use the term cognitive training in reference to programmatic efforts to improve cognitive performance in and across cognitive abilities without consideration of the presumed mechanism of change.
Below, we present a brief summary of general issues confronting the study of alcohol-related cognitive deficits and their recovery. Then, because scientific progress is contingent on appreciation of past work, we provide an overview of early studies addressing cognitive improvement in AUD populations. In the third section, we discuss investigations using current and emerging research strategies. Finally, in our concluding sections, we provide a working model illustrating core constructs underlying cognitive training and suggest areas for further development.
General Issues/Challenges
Severity of Impairment
An important, but often underappreciated, factor is that training benefits are necessarily constrained by the capacity for improvement. Those abilities that are initially compromised would be expected to demonstrate greater benefit with training than would those unaffected at baseline. Thus, when evaluating the relative effectiveness of training protocols, a complete understanding of baseline performance across relevant cognitive abilities or domains is imperative. In short, null outcomes may be related to the absence of impairment, rather than an overarching failure in the training method. (e.g., Jaeggi, Buschkuehl, Shah, & Jonides, 2014). This observation is of particular import when seeking to develop cognitive training programs for use in populations, such as AUD, characterized by highly variable and generally mild cognitive deficits.
Distinguishing Sources of Improvement
Substantial, but incomplete, improvement in cognition and brain dysregulation is observed in the first weeks/months of abstinence (Fernandez-Serrano, Perez-Garcia, & Verdejo-Garcia, 2011; Page & Schaub, 1977; Pitel et al., 2009; Stavro et al., 2013). This progress, achieved without directed cognitive intervention, has been called time-dependent or spontaneous recovery and is generally assumed to accompany continuing abstinence (see Goldman, 1987, 1995). It should be noted, however, even in the absence of programmatic cognitive interventions, participants are cognitively and emotionally challenged throughout the course of treatment (Goldman, 1995). Therefore, one must be cautious in concluding that improvement achieved during treatment is due solely to abstinence. Also, when designs include non-AUD comparison groups, AUD and non-AUD groups often demonstrate equivalent magnitudes of change (i.e., equivalent slopes; for review see Parsons, 1987b). This pattern suggests that both groups benefit from practice. Recovery, on the other hand, would be demonstrated if the AUD group showed differential improvement. Importantly, recovery is not dependent on AUD groups achieving performance levels equivalent to that of the comparison group. Rather, it is contingent on the AUD group producing a steeper change slope than does the comparison group. (Parsons, 1987b).
Disentangling sources of improvement is also impacted by training methods and context. For example, training frequency/duration, task characteristics, nonspecific context variables, and a host of individual variables must be defined and evaluated (Goldman, 1986; Jaeggi et al., 2014; Khemiri et al., 2018; Parsons, 1986). Otherwise, the differential contributions of practice, training, and transfer may be confounded. Relatedly, when assessing improvement across time, longitudinal designs offer obvious advantages. However, they also introduce the opportunity for differential attrition and potential shifts in the fidelity of the training protocol/intervention. Similarly, fully balanced designs that provide direct comparisons of multiple sources of improvement are desired but increase complexity and threaten feasibility. Taken together, it is clear that identifying the underlying source(s) of improvement across time requires thoughtful design, sufficient sample sizes to address heterogeneity, and appropriate statistical methods. In the conduct of clinical research, there are inevitable limitations. Thus, we remain mindful that imperfect studies may yield relevant data.
Distinguishing Training Gains from Transfer of Training
Cognitive improvements occurring within and across training sessions are referred to as training gains. Training gains are most readily achieved when the training protocol is responsive to initial and changing performance levels, that is, it is dynamic (e.g., Holmes, Gathercole, & Dunning, 2009; Lilienthal, Tamez, Shelton, Myerson, & Hale, 2013). Performance during training is negatively associated with subjective ratings of task “difficulty” and “effortfulness,” but positively associated with ratings of “challenging” and “not overwhelming” (Jaeggi, Buschkuehl, Jonides, & Shah, 2011). These observations are consistent with fundamental principles of learning and motivation and reflect the fact that optimal improvement requires a balance between challenge and signals of increasing competency (Jaeggi et al., 2014).
The ultimate test for training protocols is the degree to which improvement achieved during training (i.e., training gains) subsequently benefits performance on untrained tests (i.e., transfer of training). Training transfer is commonly classified as either proximal or distal. Proximal transfer references benefit from the training task to an untrained test task that is presumed to demand highly similar cognitive abilities. Distal transfer refers to transfer from a training task to a dissimilar task. Although there are conflicting views regarding the degree to which transfer of training is possible (Owen et al., 2010; Sala & Gobet, 2017; Salminen, Strobach, & Schubert, 2012; Westerberg et al., 2007), it is clear that transfer is facilitated if training and transfer tasks engage common underlying neurobehavioral processes and neural networks (Constantinidis & Klingberg, 2016; Forsberg & Goldman, 1987; Klingberg, 2010). We return to discussion of levels of transfer in the later section describing a conceptual framework.
Individual Differences
Space prohibits a full discussion of relevant individual differences. Here, we introduce select variables relevant to alcohol and cognitive training studies.
AUD chronicity/exposure levels.
Increased AUD chronicity and recent/pretreatment levels of drinking are modestly associated with cognitive impairment (Beatty, Tivis, Stott, Nixon, & Parsons, 2000; Eckardt et al., 1998; Parsons, 1994; but see Ruiz et al., 2013). This finding is consistent with the previously noted heterogeneity within the AUD population. In the current cognitive training literature, these factors have received minimal attention. However, as programs evolve and expand, it will be pertinent that we determine whether they directly impact response to training protocols.
Age.
Although individual cognitive abilities are not equally impacted, it is well-established that increasing age is associated with cognitive change (e.g., Salthouse, 2010). Importantly, older adults with AUD commonly exhibit greater degrees of cognitive compromise relative to their non-AUD age cohorts than do younger adults with AUD (Leber & Parsons, 1982; Oscar-Berman & Marinkovic, 2007; Pfefferbaum, Adalsteinsson, & Sullivan, 2006; Pitel, Eustache, & Beaunieux, 2014; Rourke & Grant, 1999; Sullivan & Pfefferbaum, 2003). Furthermore, investigations (discussed below) reveal that older persons with AUD experience less cognitive recovery during early abstinence than do younger persons with AUD. We note that study of age effects among treatment-seekers has been constrained by the age ranges of those typically available for study, which seldom exceed the early 60s. In addition, because excessive drinking is associated with early death, persons with AUD surviving into older ages are unlikely representatives of typical older problem-drinkers (Fein & McGillivray, 2007). Given expected increases in the numbers of adults over the age of 65 and current reports of increased drinking in older ages (Breslow, Castle, Chen, & Graubard, 2017), developing work should be highly cognizant of age as both a grouping and a correlative variable.
Sex.
Complex and often inconsistent literatures surround the study of sex differences in alcohol-related cognitive outcomes. Although some studies find that women are differentially sensitive to alcohol, others find no sex differences (Bobzean, DeNobrega, & Perrotti, 2014; Fabian, Parsons, & Sheldon, 1984; Nixon, 1994; Nixon, Prather, & Lewis, 2014). An understudied aspect of these comparisons is that although sex differences in chronicity or typical drinks may be reported and analyzed, estimates of typical or chronic blood alcohol concentrations (BACs) are largely unreported. Women typically achieve higher BACs than men when consuming equivalent doses and therefore experience greater exposure to alcohol’s neurotoxic effects (Marshall, Kingstone, Boss, & Morgan, 1983). Hence, it could be argued that greater compromise in women versus men with AUD may not reflect an inherent differential vulnerability but arise from greater CNS exposure among heavy drinking women. Given the inconclusive nature of current research, sex differences merit directed attention in cognitive training studies.
Comorbid conditions.
Comorbid conditions are common in AUDs and may confound study of cognition. Even when individuals with significant comorbid clinical disorders such as the psychotic disorders, PTSD, and current major depression are excluded from study, treatment-seekers often endorse more psychiatric symptoms, particularly levels of negative affect. (Bates et al., 2004; Boissoneault, Lewis, & Nixon, 2019; Domenico, Lewis, Hazarika, & Nixon, 2018; Fein, 2015). In well-designed studies, the impact of these factors is mitigated by selection criteria and statistical control/methods. Perhaps more problematic in clarifying alcohol’s effects is the fact that polysubstance use/misuse in AUD is the dominant use pattern (e.g., Staines, Magura, Foote, Deluca, & Kosanke, 2001). To address this issue, participants may be subgrouped on the basis of their patterns of polysubstance use, and subgroups directly compared (e.g., Schrimsher, O’Bryant, Parker, & Burke, 2005; Beatty, Blanco, Hames, & Nixon, 1997; Gilbertson, Boissoneault, Prather, & Nixon, 2011; Lawton-Craddock, Nixon, & Tivis, 2003; Nixon, 1999). In other settings, regression methods may be applied (Verdejo-García, López-Torrecillas, Aguilar de Arcos, & Pérez-García, 2005). Neither approach is fully satisfying although both improve interpretation. Attention to specific drug effects among polysubstance abusers is uncommon in studies of cognitive training in SUD/AUD populations. Consequently, we encourage their more deliberate consideration in future research.
Early Studies of Cognitive Improvement in AUD
Building on available work (e.g., Allen, Faillace, & Reynolds, 1971; Page & Linden, 1974; Long & McLachlan, 1974; Tarter & Jones, 1971), Goldman conducted a series of seminal studies in the late 1970s and 1980s (e.g., Goldman, 1983, 1986, 1995; Goldman & Goldman, 1988; many reviewed in Goldman, 1987). In these investigations, Goldman and colleagues systematically examined spontaneous/time-dependent and practice dependent improvement as well as training-related recovery. Initial studies showed significant, but uneven, improvement in the early weeks after detoxification. Verbal skills improved to estimated premorbid levels within weeks of initiating treatment (Sharp, Rosenbaum, Goldman, & Whitman, 1977), whereas others remained compromised (Ellenberg, Rosenbaum, Goldman, & Whitman, 1980). Critically, differences in task difficulty could not account for this discrepancy. Continuing research reveals similar outcomes with verbal skills being unimpaired or improving early in abstinence whereas other cognitive abilities, particularly those heavily reliant on PFC integrity, demonstrate not only greater initial impairment but also less initial improvement (e.g., Le Berre et al., 2017; Long & McLachlan, 1974; Brandt, Butters, Ryan, & Bayog, 1983; Oscar-Berman & Ellis, 1987; Parsons & Leber, 1981; Fabian & Parsons, 1983).
Goldman’s work also addressed age-related differences. Deconstruction of their earlier data showing minimal or no improvement (Ellenberg et al., 1980) revealed that performance stability/improvement was not uniform within the AUD group. They found that the observed lack of improvement was driven by older alcoholics with longer drinking histories. Further analyses indicated that age was the more robust correlate (Goldman, 1987). This finding is consistent with a large literature demonstrating age-related vulnerability to both acute (e.g., Lewis, Boissoneault, Gilbertson, Prather, & Nixon, 2013; Price, Lewis, Boissoneault, Frazier, & Nixon, 2018) and chronic alcohol effects (e.g., Brandt et al., 1983; Oscar-Berman & Marinkovic, 2007; Pfefferbaum et al., 2006; Sullivan & Pfefferbaum, 2003). Given current epidemiological trends (Breslow et al., 2017), it is noteworthy that within this early literature the label of “older” applied to individuals as young as 35 (Ellenberg et al., 1980).
The large majority of participants in early studies were men. In an exception, Fabian and Parsons (1983), used a comprehensive battery to assess performance in three groups of women; short-term abstinent (1 month), longer-term abstinent (4 years), and controls. The three groups were equivalent on tests of verbal ability. However, on tests dependent on PFC integrity (i.e., EFs), AUD groups, regardless of length of abstinence, performed more poorly than controls. In a related experiment, AUD women and controls were tested at baseline and again ~2 years later. Although both groups improved, the AUD group did not show the differential improvement expected to accompany recovery. An underappreciated caveat in this study is the fact that over the 2-year study period, over 33% of the AUD group resumed drinking, although at levels below pretreatment levels. When analysis by resumer status (i.e., resumer vs. abstainer) was conducted, those who resumed performed more poorly at both initial testing and retest, suggesting a link between poorer cognitive performance and poorer outcomes. Later research by this and other groups reinforces the importance of abstinence for sustained cognitive and neurobiological benefit (see Bartels et al., 2006; Parsons, 1993; Pitel et al., 2009; Rosenbloom, Rohlfing, O’Reilly, Sassoon, Pfefferbaum, & Sullivan, 2007; Sullivan, Harris, & Pfefferbaum, 2010).
Goldman’s later investigations implemented modifications to better account for practice effects, determine differential improvement accompanying recovery, and explore transfer of training. For example, Forsberg and Goldman (1985) assessed performance across four practice sessions of both verbal and visual-spatial tests in AUD and control groups. At the end of practice, groups were tested on untrained tests of both types. Consistent with other reports, verbal performance across tests/time was unaffected by AUD, whereas all groups showed training gains on the visual-spatial test. Furthermore, groups also showed transfer of training to the untrained visual-spatial task. Importantly, AUD performance at the conclusion of training approached performance levels achieved by the control group on their first session. Together, the outcomes reflect transfer of training for both groups and recovery of function for the AUD group. One of the limitations in generalizing from these findings is the fact that although untrained, the transfer task was an alternate form of the training tasks and thus did not provide a rigorous test of transfer. A later study (Forsberg & Goldman, 1987) demonstrated transfer to tests with greater dissimilarity, but only if training and transfer tasks engaged the same sensory modality (i.e., auditory or visual).
In further efforts, Goldman and colleagues used a complex experimental design to extract practice effects and examine nonspecific contextual variables (Stringer, 1984; detailed in Goldman, 1987). They found that training on task-relevant strategies as well as repeated practice on a similar task differentially improved test performance on the Wechsler Adult Intelligence Scale Block Design (Wechsler & De Lemos, 1981) relative to conditions controlling for previous test exposure (pre–post test) or spontaneous recovery. Reflecting the complexity of AUD research, although the study showed training benefits, it failed to evidence expected spontaneous recovery or practice effects.
In this same period, Yohman, Schaeffer, and Parsons (1988) conducted a transfer of training study in which men receiving treatment for AUD were assigned to either no cognitive training, an intervention focused on developing strategies for more effective memory, or one focused on developing strategies for more effective problem-solving. Training occurring over a 2-week period with a total duration of 12 hr. Prior to and after the intervention, groups completed a neuropsychological battery assessing the targeted abilities and perceptual-motor skills. Analyses revealed that problem-solving training differentially improved problem-solving performance but did not impact improvement in memory. Memory training was not effective in differentially improving either of the targeted abilities. On the untrained domain, perceptual-motor function, the groups showed equivalent improvement. Overall, training was relatively ineffective. Subsequent post hoc analyses suggested that lower levels of initial impairment and younger age were associated with greater, but task-specific, improvement.
Over the next decade, Fals-Stewart and Lucente (1994) published one of the few studies of cognitive training in SUDs. In an ambitious study of primarily male participants (74%), they examined cognitive change and clinician ratings across 6 months in court-mandated treatment-seekers with diverse substance use histories. In contrast to earlier studies, only individuals with demonstrable cognitive compromise were recruited. Participants were assigned to either a computer-assisted cognitive training (CACT) group, or one of three control conditions. Neuropsychological testing was administered at admission and monthly thereafter. The CACT group experienced significantly greater cognitive benefit than other groups until the 5th month assessment. The three groups that did not receive CACT demonstrated steeper gains in Months 3, 4, and 5, resulting in equivalent performance across the groups in Months 5 and 6. Clinician ratings of treatment engagement were higher for the CACT group throughout the assessment period. Postrelease substance use variables were not obtained. Of particular relevance to this review, later work by the group (Grohman & Fals-Stewart, 2003) examined CACT in a sample without notable cognitive deficits and found that it was associated with improved commitment to treatment, successful treatment completion, and reduced drinking days at 6 months after discharge.
Taken together, this early body of work significantly influenced understanding of cognitive improvement during early abstinence, reinforced the differential susceptibility of specific cognitive abilities or domains, operationally distinguished practice from recovery outcomes, and initiated examination of transfer effects. Most, but not all, of these studies used components of standard neuropsychological batteries and intellectual assessments. With some exceptions, studies were limited by relatively small sample sizes, constrained training and assessment periods, and primary inclusion of male participants. Although not detailed here, in contrast to current trends and with the exception of nicotine, few participants reported significant polysubstance use and most were recruited from residential/inpatient treatment facilities. In addition, training task difficulty was largely standardized rather than being responsive to individual training performance, that is, it was not adaptive. Finally, although investigators explicitly recognized the import of functional outcomes, few studies obtained these data.
In the following 10–15 years, advances in theory and methods significantly expanded understanding of alcohol’s effects across neurobehavioral systems (Hunt, Nixon, & National Institute on Alcohol Abuse and Alcoholism 1993; Naqvi & Morgenstern, 2015; Oscar-Berman & Marinkovic, 2007; Pfefferbaum, Rosenbloom, Fama, Sassoon, & Sullivan, 2010). Congruent with earlier research, this work reinforced conclusions of a prolonged period of cognitive impairment with differential trajectories for specific abilities or behavioral domains (e.g., Bartels et al., 2006; Bates et al., 2006; Fein et al., 2006; Sullivan, Rosenbloom, & Pfefferbaum, 2000; Sullivan et al., 2002). However, the potential role of cognitive training in facilitating more effective outcomes in AUD/SUD populations received relatively little attention (Bates et al., 2013). Recently, investigations of cognitive training have been reported using diverse clinical samples including persons with schizophrenia, traumatic brain injury, neurodegenerative disorders, and psychiatric disorders (for recent review, see Kim, Bahk, Lee, Lee, & Choi, 2018). These studies, combined with developing technologies and statistical methods, have revived interest in using cognitive training as a component of SUD/AUD treatment (see Bates et al., 2013; Bickel, Moody, & Quisenberry, 2014; Bickel et al., 2015; Sofuoglu et al., 2013; Verdejo-Garcia, 2016).
Current Research and Frameworks
Commercial, clinical, and academic interest in cognitive training has risen rapidly. As described by Harvey et al. (2018), over half of the approximately 4,200 cognitive training studies published in the last two decades were produced in the last 5 years. Using advances in the cognitive and behavioral neurosciences, these studies leverage the interdependence of neurobehavioral structures and processes. Their fundamental assumption is that targeting training to cognitive abilities that are known to engage diversely integrated neural networks should enhance the probability that training gains will show transfer benefit to untrained tests impacted by common neural networks (Constantinidis & Klingberg, 2016). Given their relevance to adaptive behavior and the diversity of neural networks with which they interact, EFs provide a likely target. Among these, working memory (WM) is frequently, but not exclusively, targeted. It should be noted that this approach may be particularly appropriate when directed to populations characterized by diffuse, relatively subtle cognitive compromise, such as those with AUDs (Kaplan, 1988; Sternberg, 1984; Nixon, 1993).
Recent Work
In the following section, we summarize recent work exploring cognitive training interventions in AUD populations and where appropriate refer to findings in the older literature. Among current studies of cognitive training in SUD, most have not distinguished performance on the basis of the specific SUD. Therefore, it is often difficult to distinguish the effects of alcohol from those of other drugs. To minimize this potential confound, we include studies with diverse substance use histories if samples included substantive proportions (>70%) of problem drinkers. Also, several contemporary studies use commercially available computerized training software. We proffer no opinion regarding the relative strengths of these programs and thus omit notation of specific product/company names. In Table 1, we summarize alcohol-associated cognitive training studies conducted over the last decade, with interpretation and summary of critical findings, methodological considerations, and future directions included below.
Cognitive Outcomes
Consistent with the larger cognitive training literature, training-associated cognitive gains are observed in a number of alcohol-related investigations. Importantly, and consistent with historical reports, improvements are largely relegated to proximal transfer or general/nonspecific transfer; distal transfer remains difficult to characterize.
Encouragingly, several groups applying broad/multidomain training paradigms report training-contingent improvements across diverse measures. Fals-Stewart and Lam (2010) demonstrated transfer to an untrained neuropsychological assessment battery but failed to report domain-specific subscale scores. Rupp et al. (2012) examined a broad range of cognitive outcomes, including indices of “attention/executive function” (e.g., Trail-Making Tasks), “memory” (e.g., Munich Verbal Memory Test), and “miscellaneous measures.” Although significant improvements were observed in less than half of the individual measures, multivariate composite scores indicated substantial transfer across tasks. Gamito and colleagues (2014) used a web-based program to provide training on a battery of tests tasking attention, memory, decision-making, spatial vision, perception, reasoning, and processing speed. Four weeks of training differentially benefitted performance, but only on tasks within the Frontal Assessment Battery (Dubois, Slachevsky, Litvan, & Pillon, 2000). Both Gamito et al. (2014) and Rupp et al. (2012) assessed performance on the Mini-Mental State Examination, which includes short assessments of sentence construction/comprehension and orientation to time and place. Gamito and colleagues (2014) observed no training benefits. Rupp et al.’s (2012) positive findings suggest, not surprisingly given the nature of the Mini-Mental State Examination, that it may be more sensitive to training when used with individuals with more significant impairment. Finally, Bell, Vissicchio, and Weinstein (2016) used a training protocol directed to increasing memory capacity and observed training-associated transfer for untrained verbal learning and verbal memory measures. These outcomes offer promise. Yet, heterogeneity among training and transfer tasks challenges characterization of proximal versus distal transfer.
The remaining work reported in Table 1 used training paradigms focused on single domains/processes. Jones et al. (2018) investigated inhibitory control training. Despite use of a stop signal task as both a training and outcome measure, no training-associated improvements were observed. Excepting Jones et al. (2018), and consistent with the larger cognitive training literature, WM has been the predominant domain of focus. Gunn, Gerst, Wiemers, Redick, and Finn (2018) assessed transfer on six nontrained WM tasks. Transfer was observed on three of the six, with two evincing sustained improvement at 1-month follow-up. Khemiri and colleagues (2018) observed transfer in a verbal WM task, but not across a range of additional measures, including several alternate WM instruments. Similarly, Hendershot et al. (2018) observed training-associated improvement relative to active controls in a digit span task, but not in three other related WM measures.
Snider and colleagues (2018) assessed only a single cognitive outcome, employing a task in which participants listened to a series of sequential instructions and responded by manipulating objects on a virtual desk (e.g., “put the yellow crayon in the green box”). Following their WM training, Snider et al. (2018) observed proximal transfer using this measure, which they designated as a “functional” WM task. Although Houben, Wiers, and Jansen (2011) demonstrated training-associated WM performance enhancements at posttest and follow-up sessions, performance was measured using only the training tasks, precluding dissociation of transfer and practice effects.
Of the recent work reviewed in Table 1, Khemiri et al. (2018) provide the sole example of a single-process training experiment that included multidomain test assessment. Their group observed proximal transfer of WM training to several untrained WM tasks, but no transfer to other EF components or visual-spatial abilities. Thus, despite consideration of distal transfer as a “gold standard” measure in the larger literature, it remains both unobserved and largely uninvestigated in the alcohol-associated literature. Taken together, current evidence suggests training approaches targeting WM produce relatively effective proximal transfer, with some evidence for persistence at least one month following training. However, although observations of proximal transfer in these studies are encouraging, the lack of within-study consistency in results across similar measures and substantial between-study heterogeneity in selection of measures challenge interpretation.
Discounting of delayed rewards.
Although much of the training literature uses “classic” neuropsychological measures or variants thereof, several groups have used measures that may be reasonable considered as both cognitive and functional outcomes. An exemplar of these is the discounting of delayed rewards (for review see Odum, 2011), on which performance appears at least partially reliant on WM (Kurth-Nelson, Bickel, & Redish, 2012). In a promising demonstration of training transfer, Bickel and colleagues (2011) observed that WM training reduced discounting of delayed rewards in psychostimulant abusers. In their recent alcohol-focused work, this group (Snider et al., 2018) included two discounting measures. The first was a standard discounting task. The second, labeled an episodic future thinking delayed discounting task (EFT DD; Snider, LaConte, & Bickel, 2016) incorporated presentation of participant-generated cues of positive future events (gathered during baseline interview) during assessment. Although their analyses indicated the import of baseline function in training outcome (see related discussion below), they failed to reveal group-level differences in change on either the standard or EFT DD tasks. Consistent with these findings, Khemiri et al. (2018) and Hendershot et al. (2018) both observed null results in DD assessments following WM training. Excepting these studies, few indices spanning cognitive/functional boundaries have been used in recent alcohol-associated work, despite recognition of their import in recent cognitive training reviews (e.g., Harvey et al., 2018).
Capacity for improvement/personalized interventions.
As detailed previously, the relationship between individual differences in baseline cognitive function and training efficacy is an important consideration. Understanding their potential impact will facilitate greater personalization of training interventions. Rupp and colleagues (2012) provided crucial demonstration of a personalized approach. In their work, training was applied only to individuals with AUD meeting criteria for mild cognitive impairments (defined as ≥1 SD below normative scores on baseline assessments). Further, training tasks were individualized, with selection based on domain-specific impairments in baseline function. Notably, this approach evinced cognitive and functional transfer across numerous tasks.
Snider et al. (2018) and Gunn et al. (2018) also reinforced conclusions that training effects may vary as a function of baseline performance. Gunn and colleagues (2018) reported associations between baseline IQ and greater gains in the active training task, subsequently demonstrating that training performance predicted cognitive transfer. Both baseline IQ and WM function predicted transfer, however differential associations between control/active training groups were not analyzed. Snider and colleagues (2018) assessed individual differences in transfer effects using Oldham’s correlation between the difference in pre/posttest scores and mean of those scores. Using this approach, they observed significant correlations in the trained group for both cognitive and functional outcomes that were not apparent among controls. In contrast, Khemiri et al. (2018) calculated Oldham’s correlations across a variety of outcome measures but found no evidence of rate-dependent improvements in the trained group.
Functional Outcomes
The import of improved cognitive abilities is ultimately defined by the impact of this improvement on clinically relevant outcomes. As such, the paucity of emphasis on functional outcomes in alcohol-associated cognitive training studies is regrettable. As introduced earlier, functional outcomes represent a broad category of dependent measures, including direct drinking/use outcomes (e.g., days abstinent; drinks per day), indirect measures associated with use (e.g., craving; delay discounting), outcomes associated with treatment success (e.g., self-efficacy), and quality-of-life measures (e.g., social support). Below, we summarize the small literature informing this issue.
Treatment engagement/motivation.
Cognitive impairments are associated with poorer responses to SUD treatment, including decreased treatment engagement (Katz, Jaeggi, Buschkuehl, Stegman, & Shah, 2014), lower self-efficacy (Bates et al., 2006), and reduced insight (Shelton & Parsons, 1987). While training-associated improvements in these measures provide a hypothesized mechanism by which cognitive enhancement can impact treatment efficacy, they remain largely understudied. Fals-Stewart and Lam (2010) constituted an exception, observing that AUD patients who received cognitive training were more engaged and committed to treatment. Outcome measures included length of stay, ratings of therapeutic alliance, and ratings of treatment participation provided by treatment staff. Critically, treatment engagement appeared to mediate the effect of training on postdischarge abstinence (see Bates et al., 2013).
Drinking/use outcomes.
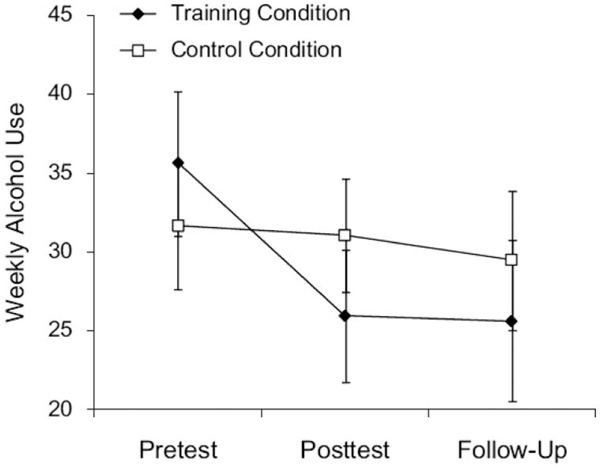
Half of the 10 studies reviewed in Table 1 include direct measures of substance use as intervention outcomes. Among these, the most striking results are reported by Houben and colleagues (2011) who observed a training-associated reduction of approximately ~8 drinks/week relative to controls (~28% overall reduction) with reductions persisting across 1 month in their sample of nontreatment seeking heavy drinkers (see Figure 1). Analyses revealed a conditional mediation effect involving implicit impulses to consume alcohol. Specifically, increases in performance across training sessions were more strongly associated with reductions in alcohol consumption among individuals with high positive implicit alcohol associations than those with low implicit associations. This pattern led the authors to assert that reduced drinking was mediated by increases in impulse control enabled by training-associated WM improvements.
Figure 1.
Reduction in weekly alcohol use during and following working memory training. Taken from Houben et al. (2011). Depicts estimated marginal means for alcohol consumption at pretest (initial session), posttest (1 week following training), and follow-up (30 days following posttest) periods. Consumption is represented as average alcoholic drinks per week. Errors bars represents standard errors of the mean. From “Getting a Grip on Drinking Behavior: Training Working Memory to Reduce Alcohol Abuse,” by K. Houben, R. W. Weirs, and A. Jansen, 2011, Psychological Science, p. 968–975. Copyright 2011 by SAGE Publications, Inc. Reprinted with permission.
Other WM approaches report inconsistent findings. Hendershot et al. (2018) observed no group differences in relapse rate at 1-month follow-up, although alcohol-specific rates/quantities were not reported in this SUD sample. In contrast, Khemiri et al. (2018) observed a trend toward reduction in drinks per drinking day, but no effect on several other measures (e.g., heavy drinking days).
Studies not specifically directed to WM training (including single-process training and more broad approaches incorporating WM components) also report mixed results. Jones et al. (2018) observed no alterations in drinking behavior, in contrast to the increased percentage of postdischarge days abstinent noted by Fals-Stewart and Lam (2010). Observations from the latter study are highly pertinent, as measurements were conducted up to one year after the training, and increased abstinence was accompanied by significant training-associated reductions in several addiction severity indices, specifically including alcohol. Among studies not applying direct drinking/use measures, only Rupp and colleagues (2012) employed craving indices. They observed no alteration in alcohol-associated obsessions, but a significant reduction in compulsions to drink.
Among training studies addressing functional outcomes, most focus on changes in drinking/use. Despite some strong indications of reduction in drinking/use, outcomes for both of these domains remain mixed. Unfortunately, with the exceptions of Fals-Stewart and Lam (2010) and Houben et al. (2011), empirical investigations of potential mediators through which training may exert alterations in drinking or other functional outcomes remain rare.
Absent are measures or consideration of social cognition, critical skills for sustained recovery and psychosocial adaptation (e.g., Kornreich et al., 2002; Maurage et al., 2011; Rupp et al., 2017). Though overlooked, these complex outcomes rely on integration of diverse neural networks and may benefit from appropriately designed training (Quaglino, De Wever, & Maurage, 2015; Valmas, Mosher Ruiz, Gansler, Sawyer, & Oscar-Berman, 2014). Also largely absent are “quality of life” measures, ranging from potential alterations in interpersonal problems, stress/stressful experiences, social support, and employment status. In another example of its breadth, Fals-Stewart and Lam (2010) observed improvements in legal issues and family/social relationships one year after training. Taken together, although findings from outcome measures discussed above provide cause for optimism regarding the functional efficacy of training, they remain both narrowly applied and inconsistently observed.
Environmental Context
The environmental context in which the studies presented in Table 1 were conducted varied substantially. Although numerous environmental factors bear consideration, the most salient difference is whether or not training occurred in the context of treatment.
Six studies were conducted as adjunctive interventions delivered during treatment. Among these, only four were conducted in residential/inpatient contexts. This distinction is critical, given the multitude of differences in novelty, social interaction, and density of abstinence-promoting activities present in inpatient versus outpatient settings. These differences were discussed in a recent review by Sampedro-Piquero, Alvarez-Suarez, and Begega (2018). In this article, the authors drew parallels between inpatient treatment environments and animal models of “environmental enrichment,” which are known to activate neuroplasticity in regions critical for reward processing, habit formation, emotion regulation, and executive functioning. If this analogy is accurate, the stimulating inpatient environment may enhance and thus heighten training benefits either directly or indirectly through enhanced self-efficacy, motivation, and so forth. On the other hand, the benefits derived from the stimulating environment mightrender specific training unnecessary and thereby ineffective, suggesting that training effects may be more easily observed in contexts in which fewer environmental factors may confound outcomes.
Heterogeneity among outpatient programs further complicates this distinction. For instance, Bell and colleagues (2016), who reported benefits to verbal learning and memory, applied training in the context of a work therapy program in which a 30-day residential program was followed by an outpatient day program that included structure and intensity comparable to some inpatient contexts. In contrast, few details were available regarding the outpatient treatment context in which Khemiri et al. (2018), who also observed transfer, delivered their training. Nonetheless, the potential impact of differences in therapeutic context between these studies is highlighted by methodological differences in training delivery. Participants in Bell et al. (2016) completed training as part of their therapy program, in lieu of additional work therapy activities. Participants in Khemiri et al. (2018) participated in training online, from their homes, thus training was not an integrated aspect of their recovery program. Given their disparate outcomes, these contrasts present an illustrative example of challenges in synthesizing results across this limited literature.
Similar complications arise in evaluating research conducted outside a treatment context. This distinction applies to four of the studies in Table 1. Two targeted clinical samples (i.e., nontreatment seeking individuals with AUD; Snider et al., 2018; Gunn et al., 2018), with both demonstrating proximal WM transfer (neither assessed drinking/use outcomes). Two targeted heavy drinkers defined on the basis of high Alcohol Use Disorders Identification Test scores or self-reported consumption levels. Among the heavy drinking samples, participants expressed desire to reduce their drinking in one study (Jones et al., 2018) but not the other (Houben et al., 2011). Thus, despite sound methodology in both experiments, comparison of efficacy between the utilized training approaches (i.e., WM vs. inhibitory control) remains problematic.
Experimental Design
Experimental control.
Although the selection of control and comparison groups in recent studies is more balanced than earlier work, some methodological issues persist. Nontreated comparison groups maintain some utility in recently abstinent AUD/SUD samples by facilitating estimation of time-dependent and/or treatment-dependent recovery. That said, nontreated comparison groups fail to control for a host of relevant confounds including potential impacts of placebo effects, training novelty/engagement, and interpersonal interaction with staff. Thus, active controls represent a critical component of cognitive training designs. Neither of the two recent investigations specific to residential treatment for AUD utilized active controls (Rupp et al., 2012; Gamito et al., 2014).
Active controls were used by the other eight studies, although with substantial variance in utilization. Consistent with the larger literature, several groups used identical training tasks in both experimental and control groups but maintained consistent low-difficulty training conditions among controls (designated “active nonadaptive” in Table 1; e.g., Houben et al., 2011). In contrast, alternative tasks/activities have been used, including a computerized typing tutorial (Fals-Stewart & Lam, 2010) and categorization task (Jones et al., 2018). The latter method was also used by Gunn et al. (2018) in an innovative design which used a visual search task programmed for adaptive difficulty. Although none of these active approaches is inappropriate, each controls for different aspects of training interventions. Thus, as this literature grows, heterogeneity in control methods may strengthen cross-study analysis of specific training components critical to improving alcohol-related outcomes. Unfortunately, at present this heterogeneity remains a challenge to cross-study synthesis.
Training intensity/dose.
In the SUD/AUD literature, total training time often falls in the range of 10–14 hr. This range is consistent with that reported in the larger cognitive training literature (e.g., Anguera et al., 2013; Klingberg, Forssberg, & Westerberg, 2002; Salminen et al., 2012). A dose–response analysis conducted by Stepankova and colleagues (2014) supports the utility of this common dose but demonstrated that although increased training sessions can enhance efficacy, the enhancement is subject to diminishing returns. Another source of variability is the duration of training. Although training sessions are commonly conducted over ~4 weeks, some studies have used more intensive protocols extending across several months. Finally, the number and spacing of individual sessions is also highly variable with the number of sessions ranging from 10 to 65 and their duration ranging from 30 to 60 min. Thus, although the current literature provides some guidance for future studies, optimal training doses remain uncharacterized. That said, a recent meta-analysis indicated little evidence for dose effects within the larger cognitive training literature (Soveri, Antfolk, Karlsson, Salo, & Laine, 2017). This latter report offers hope that less rigorous cognitive interventions may have utility. This is particularly important when training is embedded with ongoing treatment demands and must be confined to a few weeks.
Motivation and usability.
There is currently little evidence that training methods employed in the current alcohol literature are sufficiently motivating to provide a means of intervention that uncompensated individuals will initiate and use routinely. This is a substantial challenge to usability, but also an opportunity to enhance aspects of training (e.g., intrinsic reward) likely to facilitate both adherence and efficacy. Training performance and subsequent transfer are enhanced by participant motivation, when motivation is intrinsic to the training task. Thus, sustaining task engagement is critical. Engagement is a multidimensional phenomenon including persistent, positive involvement with the task. Computerized programs for training, education, and rehabilitation purposes, specifically engineered to maximize engagement are often referred to as “serious games” (e.g., Lau, Smit, Fleming, & Riper, 2017). Over the last decade, training studies have increasingly leveraged computer science practices to maximize engagement in gamified tasks (e.g., Anguera et al., 2013). To date, this aspect of training has received little attention in substance abuse studies (but see Gamito et al., 2014). SUD samples are characterized by deficits in attention and motivation, suggesting strategies to maximize engagement may be particularly beneficial. However, although training modifications directed to enhancing motivation can improve task engagement, performance, and transfer (e.g., Prins, Dovis, Ponsioen, ten Brink, & van der Oord, 2011), negative findings caution against potential introduction of distracting or frustrating elements (e.g., persistent display of score; Katz et al., 2014). Thus, future intervention development efforts should be directed to maximizing engagement but must be informed by contemporary theory and practice in computer science, human–computer interaction, and serious game design.
Targeted processes.
With some exception (e.g., Jones et al., 2018), recent training approaches targeting single domains/processes have consistently focused on WM over the past decade. In AUD/SUD contexts, these approaches appear informed at least in part by Bickel and colleagues’ (e.g., Bickel et al., 2011; Bickel et al., 2014) suggestion that WM processes provide an optimal target for improving functional outcomes. The literature cited here provides modest support for WM-specific approaches, but little evidence for preferential benefit relative to broad multidomain approaches targeting diverse processes. A multidomain approach was employed by two recent studies providing particularly strong evidence of training efficacy in AUD (Rupp et al., 2012; Fals-Stewart & Lam, 2010). In contrast, Houben et al. (2011) provide compelling data for the utility of WM-specific approaches in a subclinical sample. Complicating this distinction, many multidomain approaches heavily target WM processes but do not train them exclusively. In short, although both approaches appear to have potential, more thorough study of domain-specific versus generalized strategies is needed.
Source/number of training tasks.
The number of training tasks applied across these studies varies substantially. Some experiments (e.g., Houben et al., 2011; Gunn et al., 2018) have constrained training to ≤3 tasks, whereas several studies use large selections of tasks (with several failing to report the total number employed). For example, Rupp and colleagues (2012) administered up to 62 training tasks. Using diverse tasks within and across studies is useful in preliminary demonstrations of feasibility/efficacy and facilitates the process of defining initial empirical and conceptual boundaries. As reports accrue, however, the practice confounds cross-study comparison and obfuscates analyses directed to understanding which individual tasks may confer greater/lesser transfer to cognitive or functional outcomes.
Duration of effects.
A persistent issue in the larger cognitive training literature regards the duration of training effects. Unfortunately, few of the recent alcohol-associated cognitive training investigations characterized transfer effects in follow-up assessments, and among these training duration varied widely. Improvements in verbal memory and verbal learning reported by Bell et al. (2016) persisted 3 months after training, and remained strong effects (Cohen’s d ≥ 1.18). Of the three measures reflecting training-associated transfer at posttest, Gunn et al. (2018) noted sustained improvement at 1-month follow-up on rotation span and auditory consonant trigram tasks, but not a spatial span task. Although not examining transfer per se, Houben et al. (2011) noted that gains on the WM training task persisted at 1-month follow-up.
Study dropout is a relevant consideration for any interventional study, particularly those evaluating longevity of intervention effects. Among the aforementioned studies, retention-related issues appeared minimally confounding. All participants included in Houben et al. (2011) completed follow-up assessments, although the authors do not report dropout rates for individuals who may have been excluded. Gunn et al. (2018) included only individuals completing follow-ups in analyses but reported ~30% loss from the initially recruited sample. Bell et al. (2016) included individuals not completing follow-ups in analysis but reported only modest loss (~10%) relative to posttraining assessment. Among investigations of long-term drinking outcomes, Fals-Stewart and Lam (2010) reported missing data from only 8% of participants across multiple follow-up assessments over a 1-year period. In contrast, Hendershot et al. (2018) reported loss of approximately 41% of the sample between posttest and the 1-month follow-up. Given the aforementioned association between continued abstinence and cognitive recovery (e.g., Chanraud & Sullivan, 2014; Pfefferbaum et al., 2001), we anticipate that the persistence of training effects may be similarly dependent. To our knowledge, this pertinent issue has not yet been studied.
Although maintaining training-associated improvements is desirable, in the context of training as an adjunct to AUD treatment, lack of group differences in follow-up assessments must be carefully interpreted. At follow-up, failure to observe previously detected group differences may reflect early training-associated acceleration of cognitive recovery (as noted by Fals-Stewart & Lucente, 1994), rather than a persistent shift in magnitude of recovery. However, by advantaging cognitive function early in the treatment process, training effects on functional outcomes (via enhanced treatment efficacy) may persist even in the absence of long-term cognitive differences. Unfortunately, longitudinal examinations of functional outcomes remain rare, though exceptional examples of persistent effects are noted by Fals-Stewart and Lam (2010).
Summary of Recent Work
Our review reveals both potential and challenges facing the application of cognitive training interventions in persons with AUDs. There are promising examples of improvement in specific cognitive abilities, drinking outcomes and benefit to therapeutic processes. However, variability in methods, study sample, and training context, combined with the body of mixed results and the small number of published reports precludes meaningful conclusions of efficacy or estimates of effect size. Stated otherwise, there is a strong basis for the continued investigation of training intervention accompanied by only a weak basis to suggest that current training methods represent efficacious evidence-based interventions.
Although some criticisms of the recent studies are founded (e.g., lack of functional measures, longitudinal outcomes, appropriate controls), it bears noting that cognitive training interventions, particularly in the context of residential AUD/SUD treatment, are tremendously challenging and often incur practical limitations. Encouragingly, as awareness of relevant issues has grown, so too has its methodological sophistication (e.g., Khemiri et al., 2018). Thus, despite the necessity of discussing their constraints, we believe these recent reports reflect potential and provide guidance for future work. Many of the challenges in synthesizing findings across this emerging literature can be readily addressed through programmatic research. In the subsequent section, we outline a working framework that we hope will inform a path forward.
Visualizing a Framework
As evidenced throughout, the disappointing hallmark of cognitive training efforts in AUD is the paucity of robust transfer of training effects. As we were conducting this review, we were increasingly convinced of the inadequacy of the binary system for classifying transfer.
We recognize that Harvey and colleagues (2018) reached a similar conclusion. They suggested that training efficacy can be better evaluated at four levels; training engagement, benefit to cognitive performance, benefits to cognitive abilities with direct real-world relevance, and transfer to real-world environments. In the context of the more typical nomenclature, training engagement is roughly equivalent to training gains; benefit to cognition includes both proximal and distal transfer; benefit to cognitive abilities with real-world relevance would be typically referenced as distal transfer; and environmental transfer would align with a specific category of distal outcomes, functional outcomes. We appreciate the implications of their model and believe it will benefit the articulation of empirical questions and targeted outcomes.
Our conceptual framework approaches similar concerns but is not a competing model. We believe our framework encourages broader consideration of the potential strengths of cognitive training protocols, when core issues are addressed. We assert that the terms distal and proximal transfer occupy relative and dynamic positions, rather than discrete/distinct categories. The subsequent discussion suggests there are multiple pathways through which positive transfer may occur and notes conditions that will challenge any degree of transfer. Thus, we propose that (a) transfer may be facilitated through training on both the targeted cognitive ability as well as nontargeted abilities that inadvertently gain benefit through training, and (b) performance may be improved through indirect transfer. Although not illustrated, we anticipate that the degree of transfer enabled by nontargeted abilities or indirect transfer is likely, highly variable, and possibly relate to training duration.
To reflect these possibilities, we developed a Venn diagram illustrating how these factors might interface and impact transfer (see Figure 2). In developing the figure, we accept the premise that directed training across the full range of AUD deficits would require a broad battery of training and transfer tasks. As illustrated in previous sections, this challenge is not unique to AUDs and has been approached by using training tasks that engage diverse interrelated neural networks; the assumption being that training on these tasks will activate multiple circuits and thereby benefit performance on a wide array of cognitive abilities. As previously referenced, the PFC has extensive cortical networks and is the “hub” for cognitive abilities including attention, response inhibition, WM, error detection/response adaptation, and decision-making—abilities essential for purposeful, adaptive behavior. Thus, cognitive abilities mediated by the PFC are often targeted in training protocols. Among these, as illustrated in Table 1 and previous sections, WM has been most frequently, but not exclusively, examined.
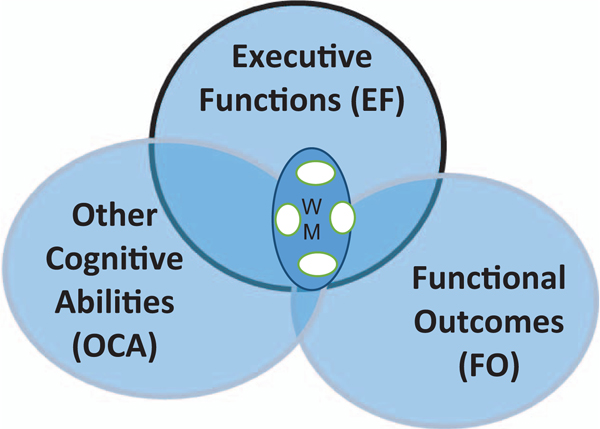
Figure 2.
Conceptual framework of cognitive training constructs. The three large circles represent areas of alcohol-related compromise. The frequent reliance on working memory training in cognitive training protocols is reflected in the circle labeled “WM.” The three large circles in Figure 2 represent three general cognitive/behavioral domains known to be sensitive to alcohol-related impairment. The center circle represents EFs; the one on the left, other non-EF cognitive abilities; and on the right, functional behaviors/outcomes. See the online article for the color version of this figure.
WM and EF
WM training tasks are commonly used in cognitive training, yet because WM is only one of several EF,s a small circle labeled WM is embedded in the EF circle. In a full illustration, other EFs and their overlap would be depicted. To avoid distraction from the primary intent of the discussion, such a figure was not included. To indicate variability in WM tasks across protocols, an arbitrary number of smaller circles are evident within the WM circle. Training on any one of the WM tasks depicted is expected to benefit performance on untrained WM tasks. If the premise that training benefit is rendered on the basis of activation in interrelated networks is correct, performance on other EFs such as attention, behavioral inhibition, or decision-making should also benefit from WM training, albeit to a lesser degree.
Other Cognitive Abilities
Tasks used to assess alcohol-related compromise in non-EFs are represented in other cognitive abilities (OCA). The overlap between OCA and WM indicates that WM training may enable transfer to these tasks, at least under certain task conditions. For example, WM training could be predicted to impart greater benefit to a list learning transfer task with embedded distractors than to tasks assessing visual-spatial or mental rotation abilities. Tests tasking these latter skills are posited to lie in OCA outside the area of overlap with EF. We hypothesize that if WM training enhances other EFs (e.g., selective attention, behavioral inhibition), and if these EFs, in turn, play a role in performance on non-EF tasks, positive transfer might be achieved, illustrating indirectly modulated (distal) transfer. Similarly, if training enhances general variables such as arousal or motivation, performance benefits may be observed, regardless of cognitive task.
Functional Outcomes
As previously detailed, investigators have considered a range of functional outcomes including those related to the therapeutic process, postdischarge drinking, affect/mood, and interpersonal well-being. Given the role of EFs in supporting relevant behaviors such as enacting refusal skills or inhibiting initial responses, our diagram shows FO overlapping with EF more fully than it does with OCA. There is also an area of overlap between OCA and FO not shared by EF. Although this overlap is not commonly considered, it represents the possibility that training on tasks focused on visual-spatial ability or spatial memory, for example, may provide transfer benefit to certain functional outcomes. For example, one possibility is that training on spatial memory might benefit avoidance of former oft-visited bars or drinking establishments by enhancing cognitive/spatial mapping skills.
The large area of FO that remains separate from the other circles is consistent with the limited reports of successful transfer. This area reflects the fact that functional outcomes are complex integrated behaviors, influenced by individual differences as well as psychosocial and cultural contexts and incompletely accounted for by traditionally assessed abilities. That said, some functional outcomes have shown sensitivity to cognitive training. Among those, the most robust findings are related to therapeutic processes assessed during treatment. This outcome may be an example of indirect benefit achieved through the training effects on motivation, attentional abilities, or self-efficacy experienced during treatment, or it may represent direct transfer from the training task(s) to treatment engagement or related variables. Studies to date cannot disentangle these possibilities. It should be noted that these therapeutic gains may not directly impact posttreatment adaptation but may reflect potent intermediary gains that differentially advantage AUD subgroups after treatment (e.g., Bates et al., 2013).
Alcohol-related compromise in interpersonal skills and emotion regulation have long been noted (Kornreich et al., 2002; Lewis, Price, Garcia, & Nixon, 2019). Given their relevance to effective psychosocial adaptation, they represent an important target for intervention. It is unclear where one might locate these abilities within FO. Indeed, improvement in EFs, particularly those related to behavioral inhibition and decision-making, may offer some benefit in these domains. However, given their multifactorial nature, we would not expect that specific skill training would fully remedy these deficits.
Expanding cognitive training or focusing on different EFs might provide added benefit. That said, initial studies using these approaches are not conclusive (Jones et al., 2018). Thus, taken together, existing data and the proposed framework reinforce the import of rethinking the structure and objective of CT protocols. More directly, protocols might be explicitly focused on behavioral components of functional outcomes and attempt to account for variables that would interfere with execution of desired responses. In partial response to this issue, we are conducting a pilot study using cognitive training methods in which we examine the role of emotion (words and faces) and sensory modality on WM learning and transfer of gains to other cognitive domains. Our objective is to examine the degree to which emotional stimuli impede WM learning and whether, if observed, this interference can be overcome with training. As the work matures, we will examine the association between patterns of training gains, posttreatment drinking, and interpersonal function. Regardless of the study design, we recognize that because functional outcomes are multiply determined and rely on the integration of separate, but interdependent neurobehavioral processes and cognitive abilities, transfer effects are unlikely to be large. However, if future refinements in training lead to reliable reproduction of even small effects, given the scalability and accessibility of cognitive training as an adjunctive intervention, the public health impact of these small effects would be substantial.
Concluding Comments
As we close this section, we reiterate that our propositions and assumptions do not meet standards for a theoretical model. The diagram provides visualization of core constructs in cognitive training paradigms and depicts how tasks within and across the three circles vary in their sensitivity to transfer effects. In considering the varying degrees of separation and overlap, the framework highlights the pertinence of an a priori definition of desired outcomes with training tasks being selected on this basis. The patterns of potential change and their interrelatedness reflect the need for statistical models appropriate for defining dynamic interactions. Finally, it is worth stating explicitly that in a translational context, the more meaningful outcomes may lie at the edges of our hypothetical circles and require integration of ostensibly disparate processes. Practical concerns related to matters such as training length, duration or the ability to sustain engagement are not addressed and contribute another layer of complexity. Furthermore, based on existing literature, we concede that training protocols will not benefit all participants, nor are they likely to mitigate compromise across the full range of deficits observed in AUD populations. These anticipated limitations may be leveraged to motivate and inform future work.
Future Directions
Our framework suggests that expanding the overlap across training processes, cognitive outcomes, and functional outcomes would improve transfer and, ultimately, outcomes. Although not depicted in the figure, the larger conceptual framework recognizes the possibility that identifying ways of optimizing generalization among untrained, but shared, processes might also enhance training efficiency. At this developmental phase, effective methods to achieve these ends remain undefined. Importantly, although the framework was derived from current work using directed cognitive training, it does not preclude the possibility that combining cognitive training with other promising interventions may provide added benefit.
One such approach is bias modification. Cognitive biases for alcohol refer to both the implicit direction of attention toward alcohol cues and the facilitation of alcohol-associated approach behaviors. These biases contribute to loss of behavioral control in AUD (for review, see Stacy & Wiers, 2010) and are targeted in bias modification interventions. Bias modification approaches weaken stimulus–response associations and reactivity to alcohol cues (e.g., Fadardi & Cox, 2009). Wiers and colleagues developed the alcohol Approach-Avoidance Task, a bias modification method using repeated sessions of directed avoidance responses to alcohol cues (Wiers, Rinck, Dictus, & van den Wildenberg, 2009). Approach–avoidance training is reported to reduce relapse rates (Wiers, Eberl, Rinck, Becker, & Lindenmeyer, 2011; Eberl et al., 2013; Manning et al., 2016), although recent meta-analysis suggests the strength of these effects is equivocal (Cristea, Kok, & Cuijpers, 2016). Mechanisms purported to underlie bias modification and cognitive training effects are distinct (i.e., weakening of “bottom–up” alcohol associations vs. strengthening of “top–down” control, respectively). However, combination of these mechanistically distinct approaches may offer the potential for additive effects. Although this possibility remains largely unexamined, two of the three active intervention groups used by Jones et al. (2018) shared this approach. Jones and colleagues used “associative” Go/No-Go and Stop Signal training tasks in which stimuli utilized at “No-Go” and “Stop” signals were alcohol related, thus training reflected attempts to both strengthen cognitive control and weaken positive alcohol associations. Although neither task appeared efficacious, this combined approach provides a model for future development of novel, innovative, cognitive interventions.
Another opportunity lies in considering the benefits of exercise. Substantive literatures demonstrate that even moderate levels of exercise are associated with cognitive benefits (van Praag, Kempermann, & Gage, 1999; other). Studies with problem-drinkers showed that exercise was associated with both cognitive and functional benefits (e.g., Brown et al., 2009). Finally, an investigation by Shors, Anderson, Curlik, and Nokia (2012) suggests effortful learning over extended periods promotes the survival of nascent neurons and facilitates integration into existing neural networks. Thus, it follows that, if combined, exercise and cognitive training may have synergistic potential. Interestingly, it is possible that exercise would result in generalized activation, serving to enhance the breadth of the cognitive transfer. Although attractive, to the best of our knowledge, this approach remains untested.
We briefly introduce these three because of their behavioral focus. We recognize there are almost endless opportunities including the combination of cognitive training with mindfulness training and/or modulation of autonomic systems (Eddie, Vaschillo, Vaschillo, & Lehrer, 2015; Roos, Bowen, Witkiewitz, 2017). That noted, the spectrum of possibilities also encompasses those combining cognitive training with interventions directly affecting neurobiology such as pharmacologic interventions (e.g., medications used to enhance cognition) and/or brain stimulation (e.g., transcranial magnetic stimulation, transcranial direct current stimulation).
Defining the overarching parameters of training interventions is one factor determining next steps. Another is the possibility that the current methods for delivering training and/or the specific training curricula may not be sufficiently engaging to sustain motivation in clinical populations who are not research participants. One option is to use computer programs specifically engineered to maximize engagement in training, education and rehabilitation contexts, so-called “serious games” (e.g., Lau et al., 2017). Over the last decade, cognitive training studies have increasingly leveraged computer science practices to maximize engagement in gamified tasks (e.g., Anguera et al., 2013). Importantly, although certain modifications may enhance performance (e.g., Prins et al., 2011), others may increase distraction or heighten frustration (e.g., persistent display of score; Katz et al., 2014). To date, despite their potential, these approaches have received little attention in substance abuse studies (but see Gamito et al., 2014).
Conclusion
In closing, data regarding a generalized benefit of cognitive training in the treatment of SUDs/AUDS are not robust. Although individuals with AUD often show improvement with cognitive training, the degree to which this training improves diverse aspects of cognitive performance and/or functional outcomes such as drinking and psychosocial adaptation is unclear. Furthermore, in current studies, the training and transfer tasks, functional outcomes, training protocols, and participant characteristics are highly diverse and constrain comparison and generalization. That said, although we have endeavored to remain conservative in our discussions of this literature, we maintain a continued scientific enthusiasm for the potential of cognitive training as a means of facilitating sustained recovery. When viewed as a whole, there is reason to be hopeful that these methods can be refined to meet the needs those with AUD. To meet this objective, we must encourage the conduct of additional programmatic research using carefully defined methods and guided by conceptual models and frameworks.
Acknowledgments
Article preparation was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Grant Numbers R03 AA 025430 (Sara Jo Nixon, PI) and K01 AA 026893 (Ben Lewis, PI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- Allen DN, Goldstein G, & Seaton BE (1997). Cognitive rehabilitation of chronic alcohol abusers. Neuropsychology Review, 7, 21–39. 10.1007/BF02876971 [DOI] [PubMed] [Google Scholar]
- Allen RP, Faillace LA, & Reynolds DM (1971). Recovery of memory functioning in alcoholics following prolonged alcohol intoxication. Journal of Nervous and Mental Disease, 153, 417–423. [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed). Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Anguera JA, Boccanfuso J, Rintoul JL, Al-Hashimi O, Faraji F, Janowich J, … Gazzaley A. (2013). Video game training enhances cognitive control in older adults. Nature, 501, 97–101. 10.1038/nature12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels C, Kunert HJ, Stawicki S, Kröner-Herwig B, Ehrenreich H, & Krampe H. (2006). Recovery of hippocampus-related functions in chronic alcoholics during monitored long-term abstinence. Alcohol and Alcoholism, 42, 92–102. 10.1093/alcalc/agl104 [DOI] [PubMed] [Google Scholar]
- Bates ME, Barry D, Labouvie EW, Fals-Stewart W, Voelbel G, & Buckman JF (2004). Risk factors and neuropsychological recovery in clients with alcohol use disorders who were exposed to different treatments. Journal of Consulting and Clinical Psychology, 72, 1073–1080. 10.1037/0022-006X.72.6.1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, & Barry D. (2002). Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Experimental and Clinical Psychopharmacology, 10, 193–212. 10.1037/1064-1297.10.3.193 [DOI] [PubMed] [Google Scholar]
- Bates ME, Buckman JF, & Nguyen TT (2013). A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychology Review, 23, 27–47. 10.1007/s11065-013-9228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates ME, Pawlak AP, Tonigan JS, & Buckman JF (2006). Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychology of Addictive Behaviors, 20, 241–253. 10.1037/0893-164X.20.3.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Blanco CR, Hames KA, & Nixon SJ (1997). Spatial cognition in alcoholics: Influence of concurrent abuse of other drugs. Drug and Alcohol Dependence, 44, 167–174. 10.1016/S0376-8716(97)01334-3 [DOI] [PubMed] [Google Scholar]
- Beatty WW, Tivis R, Stott HD, Nixon SJ, & Parsons OA (2000). Neuropsychological deficits in sober alcoholics: Influences of chronicity and recent alcohol consumption. Alcoholism: Clinical and Experimental Research, 24, 149–154. 10.1111/j.1530-0277.2000.tb04584.x [DOI] [PubMed] [Google Scholar]
- Bell MD, Vissicchio NA, & Weinstein AJ (2016). Cognitive training and work therapy for the treatment of verbal learning and memory deficits in veterans with alcohol use disorders. Journal of Dual Diagnosis, 12, 83–89. 10.1080/15504263.2016.1145779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Moody L, & Quisenberry A. (2014). Computerized working-memory training as a candidate adjunctive treatment for addiction. Alcohol Research: Current Reviews, 36, 123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Quisenberry AJ, Moody L, & Wilson AG (2015). Therapeutic opportunities for self-control repair in addiction and related disorders: Change and the limits of change in trans-disease processes. Clinical Psychological Science, 3, 140–153. 10.1177/2167702614541260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Yi R, Landes RD, Hill PF, & Baxter C. (2011). Remember the future: Working memory training decreases delay discounting among stimulant addicts. Biological Psychiatry, 69, 260–265. 10.1016/j.biopsych.2010.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobzean SA, DeNobrega AK, & Perrotti LI (2014). Sex differences in the neurobiology of drug addiction. Experimental Neurology, 259, 64–74. 10.1016/j.expneurol.2014.01.022 [DOI] [PubMed] [Google Scholar]
- Boissoneault J, Lewis B, & Nixon SJ (2019). Characterizing chronic pain and alcohol use trajectory among treatment-seeking alcoholics. Alcohol, 75, 47–54. 10.1016/j.alcohol.2018.05.009 [DOI] [PubMed] [Google Scholar]
- Brandt J, Butters N, Ryan C, & Bayog R. (1983). Cognitive loss and recovery in long-term alcohol abusers. Archives of General Psychiatry, 40, 435–442. 10.1001/archpsyc.1983.01790040089012 [DOI] [PubMed] [Google Scholar]
- Breslow RA, Castle IP, Chen CM, & Graubard BI (2017). Trends in alcohol consumption among older Americans: National Health Interview Surveys, 1997 to 2014. Alcoholism, Clinical and Experimental Research, 41, 976–986. 10.1111/acer.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Abrantes AM, Read JP, Marcus BH, Jakicic J, Strong DR, … Gordon AA (2009). Aerobic exercise for alcohol recovery: Rationale, program description, and preliminary findings. Behavior Modification, 33, 220–249. 10.1177/0145445508329112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckman JF, Bates ME, & Morgenstern J. (2008). Social support and cognitive impairment in clients receiving treatment for alcohol- and drug-use disorders: A replication study. Journal of Studies on Alcohol and Drugs, 69, 738–746. 10.15288/jsad.2008.69.738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschkuehl M, Jaeggi SM, & Jonides J. (2012). Neuronal effects following working memory training. Developmental Cognitive Neuroscience, 2 (Suppl. 1), S167–S179. 10.1016/j.dcn.2011.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, & Sullivan EV (2014). Compensatory recruitment of neural resources in chronic alcoholism. Handbook of Clinical Neurology, 125, 369–380. 10.1016/B978-0-444-62619-6.00022-7 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, & Klingberg T. (2016). The neuroscience of working memory capacity and training. Nature Reviews Neuroscience, 17, 438–449. 10.1038/nrn.2016.43 [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, & Cuijpers P. (2016). The effectiveness of cognitive bias modification interventions for substance addictions: A meta-analysis. PLoS ONE, 11(9), e0162226. 10.1371/journal.pone.0162226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domenico LH, Lewis B, Hazarika M, & Nixon SJ (2018). Characterizing anxiety among individuals receiving treatment for alcohol and substance use disorders. Journal of the American Psychiatric Nurses Association, 24, 343–351. 10.1177/1078390317739106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Salas S, Díaz-Batanero C, Lozano-Rojas OM, & Verdejo-García A (2016). Impact of general cognition and executive function deficits on addiction treatment outcomes: Systematic review and discussion of neurocognitive pathways. Neuroscience and Biobehavioral Reviews, 71, 772–801. 10.1016/j.neubiorev.2016.09.030 [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, & Pillon B. (2000). The FAB: A Frontal Assessment Battery at bedside. Neurology, 55, 1621–1626. 10.1212/WNL.55.11.1621 [DOI] [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, & Lindenmeyer J. (2013). Approach bias modification in alcohol dependence: Do clinical effects replicate and for whom does it work best? Developmental Cognitive Neuroscience, 4, 38–51. 10.1016/j.dcn.2012.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckardt MJ, File SE, Gessa GL, Grant KA, Guerri C, Hoffman PL, … Tabakoff B. (1998). Effects of moderate alcohol consumption on the central nervous system. Alcoholism, Clinical and Experimental Research, 22, 998–1040. 10.1111/j.1530-0277.1998.tb03695.x [DOI] [PubMed] [Google Scholar]
- Eddie D, Vaschillo E, Vaschillo B, & Lehrer P. (2015). Heart rate variability biofeedback: Theoretical basis, delivery, and its potential for the treatment of substance use disorders. Addiction Research & Theory, 23, 266–272. 10.3109/16066359.2015.1011625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberg L, Rosenbaum C, Goldman MS, & Whitman RD (1980). Recoverability of psychological functioning following alcohol abuse: Lateralization effects. Journal of Consulting and Clinical Psychology, 48, 503–510. 10.1037/0022-006X.48.4.503 [DOI] [PubMed] [Google Scholar]
- Fabian MS, & Parsons OA (1983). Differential improvement of cognitive functions in recovering alcoholic women. Journal of Abnormal Psychology, 92, 87–95. 10.1037/0021-843X.92.1.87 [DOI] [PubMed] [Google Scholar]
- Fabian MS, Parsons OA, & Sheldon MD (1984). Effects of gender and alcoholism on verbal and visual-spatial learning. Journal of Nervous and Mental Disease, 172, 16–20. 10.1097/00005053-198401000-00005 [DOI] [PubMed] [Google Scholar]
- Fadardi JS, & Cox WM (2009). Reversing the sequence: Reducing alcohol consumption by overcoming alcohol attentional bias. Drug and Alcohol Dependence, 101, 137–145. 10.1016/j.drugalcdep.2008.11.015 [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, & Lam WK (2010). Computer-assisted cognitive rehabilitation for the treatment of patients with substance use disorders: A randomized clinical trial. Experimental and Clinical Psychopharmacology, 18, 87–98. 10.1037/a0018058 [DOI] [PubMed] [Google Scholar]
- Fals-Stewart W, & Lucente S. (1994). The effect of cognitive rehabilitation on the neuropsychological status of patients in drug abuse treatment who display neurocognitive impairment. Rehabilitation Psychology, 39, 75–94. 10.1037/h0080316 [DOI] [Google Scholar]
- Fals-Stewart W, Schafer J, Lucente S, Rustine T, & Brown L. (1994). Neurobehavioral consequences of prolonged alcohol and substance abuse: A review of findings and treatment implications. Clinical Psychology Review, 14, 755–778. 10.1016/0272-7358(94)90041-8 [DOI] [Google Scholar]
- Fein G. (2015). Psychiatric comorbidity in alcohol dependence. Neuropsychology Review, 25, 456–475. 10.1007/s11065-015-9304-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, & Chang M. (2006). Visual P300s in long-term abstinent chronic alcoholics. Alcoholism, Clinical and Experimental Research, 30, 2000–2007. 10.1111/j.1530-0277.2006.00246.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, & McGillivray S. (2007). Cognitive performance in long-term abstinent elderly alcoholics. Alcoholism: Clinical and Experimental Research, 31, 1788–1799. 10.1111/j.1530-0277.2007.00481.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, & Di Sclafani V. (2006). Cognitive performance in long-term abstinent alcoholic individuals. Alcoholism: Clinical and Experimental Research, 30, 1538–1544. 10.1111/j.1530-0277.2006.00185.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Sabbineni A, Chandler LD, & Hutchison KE (2011). How psychosocial alcohol interventions work: A preliminary look at what FMRI can tell us. Alcoholism: Clinical and Experimental Research, 35, 643–651. 10.1111/j.1530-0277.2010.01382.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Pérez-García M, & Verdejo-García A. (2011). What are the specific vs. generalized effects of drugs of abuse on neuropsychological performance? Neuroscience and Biobehavioral Reviews, 35, 377–406. 10.1016/j.neubiorev.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Forsberg LK, & Goldman MS (1985). Experience-dependent recovery of visuospatial functioning in older alcoholic persons. Journal of Abnormal Psychology, 94, 519–529. 10.1037/0021-843X.94.4.519 [DOI] [PubMed] [Google Scholar]
- Forsberg LK, & Goldman MS (1987). Experience-dependent recovery of cognitive deficits in alcoholics: Extended transfer of training. Journal of Abnormal Psychology, 96, 345–353. 10.1037/0021-843X.96.4.345 [DOI] [PubMed] [Google Scholar]
- Gamito P, Oliveira J, Lopes P, Brito R, Morais D, Silva D, … Deus A. (2014). Executive functioning in alcoholics following an mHealth cognitive stimulation program: Randomized controlled trial. Journal of Medical Internet Research, 16(4), e102 10.2196/jmir.2923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson R, Boissoneault J, Prather R, & Nixon SJ (2011). Nicotine effects on immediate and delayed verbal memory after substance use detoxification. Journal of Clinical and Experimental Neuropsychology, 33, 609–618. 10.1080/13803395.2010.543887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn SW, Sinha R, & Parsons OA (1993). Electrophysiological indices predict resumption of drinking in sober alcoholics. Alcohol, 10, 89–95. 10.1016/0741-8329(93)90086-4 [DOI] [PubMed] [Google Scholar]
- Goldman MS (1983). Cognitive impairment in chronic alcoholics. Some cause for optimism. American Psychologist, 38, 1045–1054. 10.1037/0003-066X.38.10.1045 [DOI] [PubMed] [Google Scholar]
- Goldman MS (1986). Neuropsychological recovery in alcoholics: Endogenous and exogenous processes. Alcoholism: Clinical and Experimental Research, 10, 136–144. 10.1111/j.1530-0277.1986.tb05060.x [DOI] [PubMed] [Google Scholar]
- Goldman MS (1987). The role of time and practice in recovery of function in alcoholics In Parsons OA, Butters N, & Nathan PE (Eds.), Neuropsychology of alcoholism: Implications for diagnosis and treatment (pp. 291–321). New York, NY: Guilford Press. [Google Scholar]
- Goldman MS (1995). Recovery of cognitive functioning in alcoholics: The relationship to treatment. Alcohol Health and Research World, 19, 148–154. [PMC free article] [PubMed] [Google Scholar]
- Goldman RS, & Goldman MS (1988). Experience-dependent cognitive recovery in alcoholics: A task component strategy. Journal of Studies on Alcohol, 49, 142–148. 10.15288/jsa.1988.49.142 [DOI] [PubMed] [Google Scholar]
- Grohman K, & Fals-Stewart W. (2003). Computer-assisted cognitive rehabilitation with substance-abusing patients: Effects on treatment response. Journal of Cognitive Rehabilitation, 21, 10–17. [Google Scholar]
- Gunn RL, Gerst KR, Wiemers EA, Redick TS, & Finn PR (2018). Predictors of effective working memory training in individuals with alcohol use disorders. Alcoholism: Clinical and Experimental Research, 42, 2432–2441. 10.1111/acer.13892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie A, & Elliott WA (1980). The nature and reversibility of cerebral impairment in alcoholism; treatment implications. Journal of Studies on Alcohol, 41, 147–155. 10.15288/jsa.1980.41.147 [DOI] [PubMed] [Google Scholar]
- Harvey PD, McGurk SR, Mahncke H, & Wykes T. (2018). Controversies in computerized cognitive training. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 907–915. [DOI] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Vandervoort J, McPhee MD, Keough MT, & Quilty LC (2018). Randomized trial of working memory training as an adjunct to inpatient substance use disorder treatment. Psychology of Addictive Behaviors, 32, 861–872. 10.1037/adb0000415 [DOI] [PubMed] [Google Scholar]
- Holmes J, Gathercole SE, & Dunning DL (2009). Adaptive training leads to sustained enhancement of poor working memory in children. Developmental Science, 12(4), F9–F15. 10.1111/j.1467-7687.2009.00848.x [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW, & Jansen A. (2011). Getting a grip on drinking behavior: Training working memory to reduce alcohol abuse. Psychological Science, 22, 968–975. 10.1177/0956797611412392 [DOI] [PubMed] [Google Scholar]
- Hunt WA, Nixon SJ, & National Institute on Alcohol Abuse and Alcoholism. (1993). Alcohol-induced brain damage (Vol. 22). Rockville, MD: National Institutes of Health. [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, & Shah P. (2011). Short- and long-term benefits of cognitive training. PNAS Proceedings of the National Academy of Sciences of the United States of America, 108, 10081–10086. 10.1073/pnas.1103228108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Shah P, & Jonides J. (2014). The role of individual differences in cognitive training and transfer. Memory & Cognition, 42, 464–480. 10.3758/s13421-013-0364-z [DOI] [PubMed] [Google Scholar]
- Jones A, McGrath E, Robinson E, Houben K, Nederkoorn C, & Field M. (2018). A randomized controlled trial of inhibitory control training for the reduction of alcohol consumption in problem drinkers. Journal of Consulting and Clinical Psychology, 86, 991–1004. 10.1037/ccp0000312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E. (1988). A process approach to neuropsychological assessment In Boll T & Bryant BK (Eds.), Clinical neuropsychology and brain function: Research, measurement, and practice (pp. 127–167). Washington, DC: American Psychological Association; 10.1037/10063-004 [DOI] [Google Scholar]
- Katz B, Jaeggi S, Buschkuehl M, Stegman A, & Shah P. (2014). Differential effect of motivational features on training improvements in school-based cognitive training. Frontiers in Human Neuroscience, 8, 242 10.3389/fnhum.2014.00242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemiri L, Brynte C, Stunkel A, Klingberg T, & Jayaram-Lindstrom N. (2018). Working memory training in alcohol use disorder: A randomized controlled trial. Alcoholism, Clinical and Experimental Research, 43, 135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Bahk YC, OH H, Lee WH, Lee JS, & Choi KH (2018). Current status of cognitive remediation for psychiatric disorders: A review. Frontiers in Psychiatry, 9, 461 10.3389/fpsyt.2018.00461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingberg T. (2010). Training and plasticity of working memory. Trends in Cognitive Sciences, 14, 317–324. 10.1016/j.tics.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Klingberg T, Forssberg H, & Westerberg H. (2002). Training of working memory in children with ADHD. Journal of Clinical and Experimental Neuropsychology, 24, 781–791. 10.1076/jcen.24.6.781.8395 [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, … Verbanck P. (2002). Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol and Alcoholism, 37, 394–400. 10.1093/alcalc/37.4.394 [DOI] [PubMed] [Google Scholar]
- Kurth-Nelson Z, Bickel W, & Redish AD (2012). A theoretical account of cognitive effects in delay discounting. European Journal of Neuroscience, 35, 1052–1064. 10.1111/j.1460-9568.2012.08058.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau HM, Smit JH, Fleming TM, & Riper H. (2017). Serious games for mental health: Are they accessible, feasible, and effective? A systematic review and meta-analysis. Frontiers in Psychiatry, 7, 209 10.3389/fpsyt.2016.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawton-Craddock A, Nixon SJ, & Tivis R. (2003). Cognitive efficiency in stimulant abusers with and without alcohol dependence. Alcoholism: Clinical and Experimental Research, 27, 457–464. 10.1097/01.ALC.0000056620.98842.E6 [DOI] [PubMed] [Google Scholar]
- Leber WR, & Parsons OA (1982). Premature aging and alcoholism. The International Journal of the Addictions, 17, 61–88. 10.3109/10826088209054610 [DOI] [PubMed] [Google Scholar]
- Leber WR, Parsons OA, & Nichols N. (1985). Neuropsychological test results are related to ratings of men alcoholics’ therapeutic progress: A replicated study. Journal of Studies on Alcohol, 46, 116–121. 10.15288/jsa.1985.46.116 [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Fama R, & Sullivan EV (2017). Executive functions, memory, and social cognitive deficits and recovery in chronic alcoholism: A critical review to inform future research. Alcoholism: Clinical and Experimental Research, 41, 1432–1443. 10.1111/acer.13431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Vabret F, Cauvin C, Pinon K, Allain P, Pitel AL, … Beaunieux H. (2012). Cognitive barriers to readiness to change in alcohol-dependent patients. Alcoholism: Clinical and Experimental Research, 36, 1542–1549. 10.1111/j.1530-0277.2012.01760.x [DOI] [PubMed] [Google Scholar]
- Lewis B, Boissoneault J, Gilbertson R, Prather R, & Nixon SJ (2013). Neurophysiological correlates of moderate alcohol consumption in older and younger social drinkers. Alcoholism: Clinical and Experimental Research, 37, 941–951. 10.1111/acer.12055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Price JL, Garcia CC, & Nixon SJ (2019). Emotional face processing among treatment-seeking individuals with alcohol use disorders: Investigating sex differences and relationships with interpersonal functioning. Alcohol and Alcoholism. Advance online publication. 10.1093/alcalc/agz010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilienthal L, Tamez E, Shelton JT, Myerson J, & Hale S. (2013). Dual n-back training increases the capacity of the focus of attention. Psychonomic Bulletin & Review, 20, 135–141. 10.3758/s13423-012-0335-6 [DOI] [PubMed] [Google Scholar]
- Long JA, & McLachlan JF (1974). Abstract reasoning and perceptual-motor efficiency in alcoholics. Impairment and reversibility. Quarterly Journal of Studies on Alcohol, 35, 1220–1229. [PubMed] [Google Scholar]
- Manning V, Staiger PK, Hall K, Garfield JB, Flaks G, Leung D, … Verdejo-Garcia A. (2016). Cognitive bias modification training during inpatient alcohol detoxification reduces early relapse: A randomized controlled trial. Alcoholism: Clinical and Experimental Research, 40, 2011–2019. 10.1111/acer.13163 [DOI] [PubMed] [Google Scholar]
- Marshall AW, Kingstone D, Boss M, & Morgan MY (1983). Ethanol elimination in males and females: Relationship to menstrual cycle and body composition. Hepatology, 3, 701–706. 10.1002/hep.1840030513 [DOI] [PubMed] [Google Scholar]
- Maurage P, Grynberg D, Noël X, Joassin F, Philippot P, Hanak C, … Campanella S. (2011). Dissociation between affective and cognitive empathy in alcoholism: A specific deficit for the emotional dimension. Alcoholism: Clinical and Experimental Research, 35, 1662–1668. 10.1111/j.1530-0277.2011.01512.x [DOI] [PubMed] [Google Scholar]
- McCrady BS, & Smith DE (1986). Implications of cognitive impairment for the treatment of alcoholism. Alcoholism: Clinical and Experimental Research, 10, 145–149. 10.1111/j.1530-0277.1986.tb05061.x [DOI] [PubMed] [Google Scholar]
- Naqvi NH, & Morgenstern J. (2015). Cognitive neuroscience approaches to understanding behavior change in alcohol use disorder treatments. Alcohol Research: Current Reviews, 37, 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ (1993). Application of theoretical models to the study of alcohol-induced brain damage In Hunt WA & Nixon SJ (Eds.), Alcohol-induced brain damage (Vol. 22, pp. 213–230). Rockville, MD: National Institutes of Health. [Google Scholar]
- Nixon SJ (1994). Cognitive deficits in alcoholic women. Alcohol Health & Research World, 18, 228–232. [PMC free article] [PubMed] [Google Scholar]
- Nixon SJ (1999). Neurocognitive performance in alcoholics: Is polysubstance abuse important? Psychological Science, 10, 181–185. 10.1111/1467-9280.00130 [DOI] [Google Scholar]
- Nixon SJ, Prather R, & Lewis B. (2014). Sex differences in alcohol-related neurobehavioral consequences. Handbook of Clinical Neurology, 125, 253–272. 10.1016/B978-0-444-62619-6.00016-1 [DOI] [PubMed] [Google Scholar]
- Odum AL (2011). Delay discounting: I’m a k, you’re a k. Journal of the Experimental Analysis of Behavior, 96, 427–439. 10.1901/jeab.2011.96-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, & Ellis RJ (1987). Cognitive deficits related to memory impairments in alcoholism. Recent Developments in Alcoholism, 5, 59–80. 10.1007/978-1-4899-1684-6_3 [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, & Marinković K. (2007). Alcohol: Effects on neurobehavioral functions and the brain. Neuropsychology Review, 17, 239–257. 10.1007/s11065-007-9038-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, & Gravitz ZR (2014). Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handbook of Clinical Neurology, 125, 183–210. 10.1016/B978-0-444-62619-6.00012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen AM, Hampshire A, Grahn JA, Stenton R, Dajani S, Burns AS,... Ballard CG (2010). Putting brain training to the test. Nature, 465, 775–778. 10.1038/nature09042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page RD, & Linden JD (1974). “Reversible” organic brain syndrome in alcoholics. A psychometric evaluation. Quarterly Journal of Studies on Alcohol, 35, 98–107. [PubMed] [Google Scholar]
- Page RD, & Schaub LH (1977). Intellectual functioning in alcoholics during six months’ abstinence. Journal of Studies on Alcohol, 38, 1240–1246. 10.15288/jsa.1977.38.1240 [DOI] [PubMed] [Google Scholar]
- Parsons OA (1986). Alcoholics’ neuropsychological impairment: Current findings and conclusions. Annals of Behavioral Medicine, 8(2–3), 13–19. [DOI] [Google Scholar]
- Parsons OA (1987a). Neuropsychological consequences of alcohol abuse: Many questions - some answers In Parsons OA, Butters N, & Nathan PE (Eds.), Neuropsychology of alcoholism: Implications for diagnosis and treatment (pp. 153–175). New York, NY: Guilford Press. [Google Scholar]
- Parsons OA (1987b). Do neuropsychological deficits predict alcoholics’ treatment course and posttreatment recovery? In Parsons OA, Butters N, & Nathan PE (Eds.), Neuropsychology of alcoholism: Implications for diagnosis and treatment (pp. 273–290). New York, NY: Guilford Press. [Google Scholar]
- Parsons OA (1993). Impaired neuropsychological cognitive functioning in sober alcoholics In Hunt WA & Nixon SJ (Eds.), Alcohol-induced brain damage (Vol. 22, pp. 173–194). Rockville, MD: National Institutes of Health. [Google Scholar]
- Parsons OA (1994). Determinants of cognitive deficits in alcoholics The search continues. Clinical Neuropsychologist, 8, 39–58. 10.1080/13854049408401542 [DOI] [Google Scholar]
- Parsons OA, & Leber WR (1981). The relationship between cognitive dysfunction and brain damage in alcoholics: Causal, interactive, or epiphenomenal? Alcoholism, Clinical and Experimental Research, 5, 326–343. 10.1111/j.1530-0277.1981.tb04906.x [DOI] [PubMed] [Google Scholar]
- Parsons OA, & Nixon SJ (1993). Neurobehavioral sequelae of alcoholism. Neurologic Clinics, 11, 205–218. 10.1016/S0733-8619(18)30178-6 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, & Sullivan EV (2006). Dysmorphology and microstructural degradation of the corpus callosum: Interaction of age and alcoholism. Neurobiology of Aging, 27, 994–1009. 10.1016/j.neurobiolaging.2005.05.007 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, & Sullivan EV (2001). Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. Neuroimage, 14(1, Pt 1), 7–20. 10.1006/nimg.2001.0785 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Fama R, Sassoon SA, & Sullivan EV (2010). Transcallosal white matter degradation detected with quantitative fiber tracking in alcoholic men and women: Selective relations to dissociable functions. Alcoholism: Clinical and Experimental Research, 34, 1201–1211. 10.1111/j.1530-0277.2010.01197.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, Quinette P, … Eustache F. (2007). Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcoholism, Clinical and Experimental Research, 31, 1169–1178. 10.1111/j.1530-0277.2007.00418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Eustache F, & Beaunieux H. (2014). Component processes of memory in alcoholism: Pattern of compromise and neural substrates. Handbook of Clinical Neurology, 125, 211–225. 10.1016/B978-0-444-62619-6.00013-6 [DOI] [PubMed] [Google Scholar]
- Pitel AL, Rivier J, Beaunieux H, Vabret F, Desgranges B, & Eustache F. (2009). Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcoholism, Clinical and Experimental Research, 33, 490–498. 10.1111/j.1530-0277.2008.00859.x [DOI] [PubMed] [Google Scholar]
- Pitel AL, Witkowski T, Vabret F, Guillery-Girard B, Desgranges B, Eustache F, & Beaunieux H. (2007b). Effect of episodic and working memory impairments on semantic and cognitive procedural learning at alcohol treatment entry. Alcoholism, Clinical and Experimental Research, 31, 238–248. 10.1111/j.1530-0277.2006.00301.x [DOI] [PubMed] [Google Scholar]
- Price JL, Lewis B, Boissoneault J, Frazier IR, & Nixon SJ (2018). Effects of acute alcohol and driving complexity in older and younger adults. Psychopharmacology, 235, 887–896. 10.1007/s00213-017-4806-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prins PJ, Dovis S, Ponsioen A, ten Brink E, & van der Oord S. (2011). Does computerized working memory training with game elements enhance motivation and training efficacy in children with ADHD? Cyberpsychology, Behavior, and Social Networking, 14, 115–122. 10.1089/cyber.2009.0206 [DOI] [PubMed] [Google Scholar]
- Prochaska JO, & DiClemente CC (1992). Stages of change in the modification of problem behaviors. Progress in Behavior Modification, 28, 183–218. [PubMed] [Google Scholar]
- Quaglino V, De Wever E, & Maurage P. (2015). Relations between cognitive abilities, drinking characteristics, and emotional recognition in alcohol dependence: A preliminary exploration. Alcoholism: Clinical and Experimental Research, 39, 2032–2038. 10.1111/acer.12841 [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, & Porjesz B. (2014). Understanding alcohol use disorders with neuroelectrophysiology. Handbook of Clinical Neurology, 125, 383–414. 10.1016/B978-0-444-62619-6.00023-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos CR, Bowen S, & Witkiewitz K. (2017). Baseline patterns of substance use disorder severity and depression and anxiety symptoms moderate the efficacy of mindfulness-based relapse prevention. Journal of Consulting and Clinical Psychology, 85, 1041–1051. 10.1037/ccp0000249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O’Reilly AW, Sassoon SA, Pfefferbaum A, & Sullivan EV (2007). Improvement in memory and static balance with abstinence in alcoholic men and women: Selective relations with change in brain structure. Psychiatry Research: Neuroimaging, 155, 91–102. 10.1016/j.pscychresns.2006.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, & Grant I. (1999). The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: A 2-year follow-up study. Journal of the International Neuropsychological Society, 5, 234–246. 10.1017/S1355617799533067 [DOI] [PubMed] [Google Scholar]
- Ruiz SM, Oscar-Berman M, Sawyer KS, Valmas MM, Urban T, & Harris GJ (2013). Drinking history associations with regional white matter volumes in alcoholic men and women. Alcoholism: Clinical and Experimental Research, 37, 110–122. 10.1111/j.1530-0277.2012.01862.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp CI, Derntl B, Osthaus F, Kemmler G, & Fleischhacker WW (2017). Impact of social cognition on alcohol dependence treatment outcome: Poorer facial emotion recognition predicts relapse/dropout. Alcoholism, Clinical and Experimental Research, 41, 2197–2206. 10.1111/acer.13522 [DOI] [PubMed] [Google Scholar]
- Rupp CI, Kemmler G, Kurz M, Hinterhuber H, & Fleischhacker WW (2012). Cognitive remediation therapy during treatment for alcohol dependence. Journal of Studies on Alcohol and Drugs, 73, 625–634. 10.15288/jsad.2012.73.625 [DOI] [PubMed] [Google Scholar]
- Sala G, & Gobet F. (2017). Does far transfer exist? Negative evidence from chess, music, and working memory training. Current Directions in Psychological Science, 26, 515–520. 10.1177/0963721417712760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen T, Strobach T, & Schubert T. (2012). On the impacts of working memory training on executive functioning. Frontiers in Human Neuroscience, 6, 166 10.3389/fnhum.2012.00166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2010). Selective review of cognitive aging. Journal of the International Neuropsychological Society, 16, 754–760. 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampedro-Piquero P, Alvarez-Suarez P, & Begega A. (2018). Coping with stress during aging: The importance of a resilient brain. Current Neuropharmacology, 16, 284–296. 10.2174/1570159X15666170915141610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrimsher GW, O’Bryant SE, Parker JD, & Burke RS (2005). The relation between ethnicity and cognistat performance in males seeking substance use disorder treatment. Journal of Clinical and Experimental Neuropsychology, 27, 873–885. 10.1080/13803390490919173 [DOI] [PubMed] [Google Scholar]
- Seo D, & Sinha R. (2015). Neuroplasticity and predictors of alcohol recovery. Alcohol Research: Current Reviews, 37, 143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp JR, Rosenbaum G, Goldman MS, & Whitman RD (1977). Recoverability of psychological functioning following alcohol abuse: Acquisition of meaningful synonyms. Journal of Consulting and Clinical Psychology, 45, 1023–1028. 10.1037/0022-006X.45.6.1023 [DOI] [PubMed] [Google Scholar]
- Shelton MD, & Parsons OA (1987). Alcoholics’ self-assessment of their neuropsychological functioning in everyday life. Journal of Clinical Psychology, 43, 395–403. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Anderson ML, Curlik DM II, & Nokia MS (2012). Use it or lose it: How neurogenesis keeps the brain fit for learning. Behavioural Brain Research, 227, 450–458. 10.1016/j.bbr.2011.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, Deshpande HU, Lisinski JM, Koffarnus MN, LaConte SM, & Bickel WK (2018). Working memory training improves alcohol users’ episodic future thinking: A rate-dependent analysis. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3, 160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider SE, LaConte SM, & Bickel WK (2016). Episodic future thinking: Expansion of the temporal window in individuals with alcohol dependence. Alcoholism: Clinical and Experimental Research, 40, 1558–1566. 10.1111/acer.13112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, & Carroll KM (2013). Cognitive enhancement as a treatment for drug addictions. Neuropharmacology, 64, 452–463. 10.1016/j.neuropharm.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soveri A, Antfolk J, Karlsson L, Salo B, & Laine M. (2017). Working memory training revisited: A multi-level meta-analysis of n-back training studies. Psychonomic Bulletin & Review, 24, 1077–1096. 10.3758/s13423-016-1217-0 [DOI] [PubMed] [Google Scholar]
- Stacy AW, & Wiers RW (2010). Implicit cognition and addiction: A tool for explaining paradoxical behavior. Annual Review of Clinical Psychology, 6, 551–575. 10.1146/annurev.clinpsy.121208.131444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staines GL, Magura S, Foote J, Deluca A, & Kosanke N. (2001). Polysubstance use among alcoholics. Journal of Addictive Diseases, 20, 57–69. 10.1300/J069v20n04_06 [DOI] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, & Potvin S. (2013). Widespread and sustained cognitive deficits in alcoholism: A meta-analysis. Addiction Biology, 18, 203–213. 10.1111/j.1369-1600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- Stepankova H, Lukavsky J, Buschkuehl M, Kopecek M, Ripova D, & Jaeggi SM (2014). The malleability of working memory and visuospatial skills: A randomized controlled study in older adults. Developmental Psychology, 50, 1049–1059. 10.1037/a0034913 [DOI] [PubMed] [Google Scholar]
- Sternberg R. (1984). Toward a triarchic theory of human intelligence. Behavioral and Brain Sciences, 7, 269–287. 10.1017/S0140525X00044629 [DOI] [Google Scholar]
- Strenziok M, Parasuraman R, Clarke E, Cisler DS, Thompson JC, & Greenwood PM (2014). Neurocognitive enhancement in older adults: Comparison of three cognitive training tasks to test a hypothesis of training transfer in brain connectivity. NeuroImage, 85(Pt 3), 1027–1039. 10.1016/j.neuroimage.2013.07.069 [DOI] [PubMed] [Google Scholar]
- Stringer AYGMS (1984). Remediation of spatial and problem solving deficits in male alcoholics. Paper presented at the American Psychological Association, Toronto, Canada. [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, & Pfefferbaum A. (2002). A profile of neuropsychological deficits in alcoholic women. Neuropsychology, 16, 74–83. 10.1037/0894-4105.16.1.74 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Harris RA, & Pfefferbaum A. (2010). Alcohol’s effects on brain and behavior. Alcohol Research & Health, 33, 127–143. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A. (2003). Diffusion tensor imaging in normal aging and neuropsychiatric disorders. European Journal of Radiology, 45, 244–255. 10.1016/S0720-048X(02)00313-3 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, & Pfefferbaum A. (2000). Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcoholism: Clinical and Experimental Research, 24, 611–621. 10.1111/j.1530-0277.2000.tb02032.x [DOI] [PubMed] [Google Scholar]
- Tarter RE, & Jones BM (1971). Absence of intellectual deterioration in chronic alcoholics. Journal of Clinical Psychology, 27, 453–455. [PubMed] [Google Scholar]
- Teichner G, Horner MD, Harvey RT, & Johnson RH (2001). Neuropsychological predictors of the attainment of treatment objectives in substance abuse patients. International Journal of Neuroscience, 106, 253–263. 10.3109/00207450109149753 [DOI] [PubMed] [Google Scholar]
- Tivis R, Beatty WW, Nixon SJ, & Parsons OA (1995). Patterns of cognitive impairment among alcoholics: Are there subtypes? Alcoholism, Clinical and Experimental Research, 19, 496–500. 10.1111/j.1530-0277.1995.tb01537.x [DOI] [PubMed] [Google Scholar]
- Valmas MM, Mosher Ruiz S, Gansler DA, Sawyer KS, & Oscar-Berman M. (2014). Social cognition deficits and associations with drinking history in alcoholic men and women. Alcoholism: Clinical and Experimental Research, 38, 2998–3007. 10.1111/acer.12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, & Gage FH (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature Neuroscience, 2, 266–270. 10.1038/6368 [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A. (2016). Cognitive training for substance use disorders: Neuroscientific mechanisms. Neuroscience and Biobehavioral Reviews, 68, 270–281. 10.1016/j.neubiorev.2016.05.018 [DOI] [PubMed] [Google Scholar]
- Verdejo-García AJ, López-Torrecillas F, Aguilar de Arcos F, & Pérez-García M. (2005). Differential effects of MDMA, cocaine, and cannabis use severity on distinctive components of the executive functions in polysubstance users: A multiple regression analysis. Addictive Behaviors, 30, 89–101. 10.1016/j.addbeh.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Loeber S, Kirsch M, Bach P, Richter A, Bühler M, … Kiefer F. (2011). Effects of cue-exposure treatment on neural cue reactivity in alcohol dependence: A randomized trial. Biological Psychiatry, 69, 1060–1066. 10.1016/j.biopsych.2010.12.016 [DOI] [PubMed] [Google Scholar]
- Walker RD, Donovan DM, Kivlahan DR, & O’Leary MR (1983). Length of stay, neuropsychological performance, and aftercare: Influences on alcohol treatment outcome. Journal of Consulting and Clinical Psychology, 51, 900–911. 10.1037/0022-006X.51.6.900 [DOI] [PubMed] [Google Scholar]
- Wechsler D, & De Lemos MM (1981). Wechsler Adult Intelligence Scale—Revised. New York, NY: Harcourt Brace Jovanovich. [Google Scholar]
- Westerberg H, Jacobaeus H, Hirvikoski T, Clevberger P, Ostensson ML, Bartfai A, & Klingberg T. (2007). Computerized working memory training after stroke—A pilot study. Brain Injury, 21, 21–29. 10.1080/02699050601148726 [DOI] [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, & Lindenmeyer J. (2011). Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychological Science, 22, 490–497. 10.1177/0956797611400615 [DOI] [PubMed] [Google Scholar]
- Wiers RW, Rinck M, Dictus M, & van den Wildenberg E. (2009). Relatively strong automatic appetitive action-tendencies in male carriers of the OPRM1 G-allele. Genes, Brain & Behavior, 8, 101–106. 10.1111/j.1601-183X.2008.00454.x [DOI] [PubMed] [Google Scholar]
- Yohman JR, Schaeffer KW, & Parsons OA (1988). Cognitive training in alcoholic men. Journal of Consulting and Clinical Psychology, 56, 67–72. 10.1037/0022-006X.56.1.67 [DOI] [PubMed] [Google Scholar]