Abstract
Alcohol‐use disorders are chronically relapsing conditions characterized by cycles of use, abstinence and relapse. The ventral pallidum (VP) is a key node in the neural circuits controlling relapse to alcohol seeking and a key target of pharmacotherapies for relapse prevention. There has been a significant increase in our understanding of the molecular, anatomical, pharmacological and functional properties of the ventral pallidum, laying foundations for a new understanding of its role in relapse to alcohol seeking and motivation. Here we review these advances, placing special emphasis on how advances in understanding in the cellular and circuit architectures of ventral pallidum contributes to the relapse to alcohol seeking. We show how this knowledge improves mechanistic understanding of current relapse prevention pharmacotherapies, how it may be used to tailor these against different forms of relapse and how it may help provide insights into the mental health problems frequently co‐morbid with alcohol‐use disorders.
Abbreviations
- AcbC
nucleus accumbens core
- AcbSh
nucleus accumbens shell
- BLA
basolateral amygdala
- Drd1
D1 receptor gene
- Drd2
D2 receptor gene
- LH
lateral hypothalamus
- LHb
lateral habenula
- PL
prelimbic cortex
- PV
parvalbumin
- RMTg
rostromedial tegmentum
- SNc
substantia nigra pars compacta
- SNr
substantia nigra pars reticulata
- STN
subthalamic nucleus
- vGlut 2
vesicular glutamate transporter 2
- VP
ventral pallidum
- VTA
ventral tegmental area
1. INTRODUCTION
Alcohol‐use disorders impose significant burdens on individuals, their families and communities (Whiteford et al., 2013). They are among leading causes of preventable morbidity and mortality, worldwide. Alcohol‐use disorders are characterized by persistent and repeated alcohol use despite the adverse personal, health and financial consequences caused by this use. A major treatment goal is promoting abstinence from drinking. However, the chronic relapsing nature of alcohol‐use disorders remains a major impediment (Jonas et al., 2014). Relapse to drinking after a period of treatment and abstinence is common and it can be precipitated by a wide range of events, including stressors and negative emotions, positive emotions, exposure to alcohol‐associated cues as well as places and exposure to alcohol itself.
To this end, there are a handful of approved medications for alcohol‐use disorders (e.g. naltrexone and acamprosate) that can have strong efficacy in a subset of individual drinkers. When effective, these treatments can reduce heavy drinking and reduce the return to drinking after periods of abstinence (e.g. Anton et al., 2006). Encouragingly, other candidate medications (e.g. baclofen) provide additional treatment options (Agabio et al., 2018). Nonetheless, there remains considerable heterogeneity in both the clinical presentation (including continuum of severity of alcohol‐use disorder as well as the presence, extent and type of co‐morbidities) and treatment responsiveness among sufferers of alcohol‐use disorders. Heterogeneity in treatment responsiveness remains poorly understood, but there is widespread recognition that advances in successful, personalized addiction medicines will depend on understanding these (Litten, Ryan, Falk, Reilly, Fertig, & Koob, 2015).
Here, we review recent findings regarding the neuropharmacology, neuroanatomy and neurobiology of relapse to seeking alcohol and other drugs of abuse. Our focus is on the role of the ventral pallidum (VP) in relapse. In particular, we focus on findings from preclinical animal models showing how relapse to alcohol seeking is assembled from heterogeneous cell types and neural circuits involving the VP. There has been a significant increase in understanding in the cellular, circuit and functional heterogeneity of the VP that has led to new discoveries and previously unrecognized complexities in how the VP controls relapse. These include demonstrations that different forms of relapse (e.g. reacquisition and context‐induced reinstatement) depend on difference in the VP cellular and circuit architectures. Understanding and parsing this cellular and circuit heterogeneity in preclinical models of relapse provide a crucial evidence base for understanding when and why relapse prevention medications may be effective or ineffective. It therefore provides a mechanistic bridge to understanding heterogeneity in treatment responsiveness among individuals with alcohol‐use disorders.
2. VENTRAL PALLIDUM (VP) AND RELAPSE
The VP is located in the rostral, subcommissural portion of the substantia innominata and is a critical component of the ventral striatopallidal system. In animal models, cellular activity and manipulation studies (e.g. lesions, pharmacological, chemogenetic and optogenetic) consistently and robustly implicate the VP in a variety of forms of relapse to seeking a variety of drugs of abuse, including alcohol, cocaine, heroin, remifentanil and natural reinforcers such as sucrose and food (e.g. Mahler et al., 2014; Mohammadkhani, James, Pantazis, & Aston‐Jones, 2020; Perry & McNally, 2013; Rogers, Ghee, & See, 2008; Smith & Berridge, 2005; Tang, McFarland, Cagle, & Kalivas, 2005). Indeed, the role of the VP in different forms of relapse to seeking a variety of drugs of abuse is so robust that the VP was proposed as key component of a “final common pathway” for relapse (Kalivas & Volkow, 2005) (Figure 1a). Within this influential framework, different triggers for relapse to drug seeking (stressors, drug‐associated cues, contexts and drugs themselves) recruit distinct and non‐overlapping neural circuits that converge on the dorsomedial prefrontal cortex, which orchestrates relapse behaviour via glutamate projections to GABA neurons in the nucleus accumbens core (AcbC), which in turn send dense projections to the VP. The VP then serves as both a key output nucleus sending projections outside the basal ganglia (e.g. to mediodorsal thalamus) and as an indirect relay to midbrain dopamine neurons to determine propensity to relapse.
FIGURE 1.
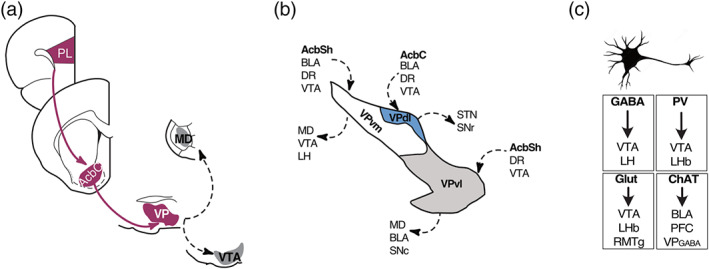
(a) Ventral pallidum as a key element in a final common pathway controlling relapse. (b) Anatomical subdivisions of the VP with partially overlapping and partially segregated afferents and efferents (modelled after Root et al., 2015). (c) Cell‐type and projection‐specific heterogeneity in the VP. AcbC, nucleus accumbens core; AcbSh, nucleus accumbens shell; BLA, basolateral amygdala; DR, dorsal raphé; Glut, glutamate; LH, lateral hypothalamus; LHb, lateral habenula; MD, mediodorsal thalamus; PFC, prefrontal cortex; PL, prelimbic cortex; RMTg, rostromedial tegmentum; SNc, substantia nigra pars compacta; SNr, substantia nigra pars reticulata; STN, subthalamic nucleus; VP, ventral pallidum; VPdl, dorsolateral ventral pallidum; VPvm, ventromedial ventral pallidum; VPvl, ventrolateral ventral pallidum; VTA, ventral tegmental area
In recent years, we have learned much about the functional organization of these pathways. The role for VP in relapse depends on synaptic input from the nucleus accumbens core (AcbC) because selective inhibition of dorsomedial prefrontal cortex (dmPFC), AcbC, VP, or prelimbic cortex (PL) ➔ AcbC or AcbC ➔ VP pathways can reduce relapse to cocaine or alcohol seeking (Khoo, Gibson, Prasad, & McNally, 2015; Mahler et al., 2014; Stefanik et al., 2013; Stefanik, Kupchik, Brown, & Kalivas, 2013). Projections from the AcbC to VP emerge from both D1 (Drd1) and D2 (Drd2) receptor expressing neurons and these two populations of neurons innervate overlapping populations of the VP neurons (Heinsbroek et al., 2017; Kupchik et al., 2015; Lobo et al., 2010). There are distinct roles for these two inputs to the VP in relapse. Drd1 projections to the VP are essential for cue‐induced reinstatement of cocaine seeking (Pardo‐Garcia et al., 2019) as well as sensitization of locomotor responses (Creed, Ntamati, Chandra, Lobo, & Luscher, 2016), whereas Drd2 projections to the VP may contribute to cocaine‐induced negative affect (Creed et al., 2016) and suppress relapse (Heinsbroek et al., 2017). Moreover, these two Acb inputs to the VP undergo plasticity that contributes to their roles in promoting or preventing relapse (Creed et al., 2016; Heinsbroek et al., 2017).
These findings have direct relevance for understanding treatment mechanisms of alcohol‐use disorders. The opioid receptor antagonist naltrexone remains a key, first line pharmacotherapy for alcohol‐use disorders. The VP is one of the brain sites for naltrexone's therapeutic effectiveness. μ opioid receptors in the VP determine both affective/hedonic (i.e. liking) and motivational (i.e. wanting) impacts of rewards and tastes (Smith, Tindell, Aldridge, & Berridge, 2009; Tindell, Smith, Pecina, Berridge, & Aldridge, 2006). Microinjections of μ opioid receptor antagonists into the VP prevent multiple forms of relapse (context‐induced, primed reinstatement) to alcohol (Perry & McNally, 2013) or cocaine (primed reinstatement) seeking (Tang et al., 2005). Moreover, persistent activation of μ‐opioid receptors on Acb terminals in the VP contributes to changes in plasticity in this projection contributing to relapse (Heinsbroek et al., 2017; Kupchik et al., 2014). In humans, the VP is part of the limbic pallidum, which also includes the globus pallidus. Reduced globus pallidus volumes have been detected in heroin‐dependent individuals (Muller et al., 2019) and in binge drinkers (Maksimovskiy, Fortier, Milberg, & McGlinchey, 2019). Moreover, in one case study, infarcts of the globus pallidus after methadone overdose led to abstinence from opiates and alcohol use for 10 years (Moussawi, Kalivas, & Lee, 2016).
3. COMPLEXITIES PROMOTING HETEROGENEITY
Nonetheless, the role of the VP in relapse and aspects of drug addiction is more complex and interesting still. The VP is heterogeneous in terms of its anatomical organization, afferent and efferent connectivity and constituent cell types. As we will show in later sections, understanding this heterogeneity is essential to understanding both the mechanisms for and treatments of relapse. It also has important implications for understanding mental health problems which are frequently co‐morbid with alcohol‐use disorders.
Expression of substance P delineates the VP from adjacent regions including the bed nucleus of the stria terminalis and the interstitial nucleus of the posterior limb of the anterior commissure (Heimer, Zahm, Churchill, & Kalivas, 1991; Root, Melendez, Zaborszky, & Napier, 2015; Zahm & Heimer, 1990). Anatomically, the VP comprises dorsolateral (dl), ventromedial (vm), and ventrolateral (vl) subdivisions (Groenewegen, Berendse, & Haber, 1993; Heimer et al., 1991; Root et al., 2015; Zahm & Heimer, 1990). These anatomical subdivisions are distinguishable by their neurochemical signatures. The VPdl can be delineated by dense calbindin‐d28k and low neurotensin expression, the VPvm has dense neurotensin and little calbindin‐d28k expression, whereas the VPvl expresses neither calbindin‐d28k nor neurotensin (Root et al., 2015; Tripathi, Prensa, Cebrian, & Mengual, 2010; Tripathi, Prensa, & Mengual, 2013; Zahm, 1989; Zahm & Heimer, 1990). These subdivisions serve different roles in different forms of relapse and motivation. For example, Mahler et al. (2014) have shown that the VPvm is recruited during cue‐induced reinstatement of cocaine seeking, whereas the VPdl is recruited during cocaine‐primed reinstatement of cocaine seeking. Similarly, Berridge and colleagues have shown important differences in the role of opioid actions in these regions (hedonic “hot” and “cold” spots) in determining the hedonic impact of tastes (Smith et al., 2009; Smith & Berridge, 2005).
This compartmentalization of the VP is also reflected in the organization of its major afferents and efferents (Figure 1b). Most importantly for present purposes, the calbindin‐d28k rich VPdl receives extensive projections from the AcbC, whereas the neurotensin rich VPvm receives extensive projections from the nucleus accumbens shell (AcbSh) (Heimer et al., 1991; Zahm & Brog, 1992; Zahm & Heimer, 1990). Other inputs, including from the basolateral amygdala (BLA), ventral tegmental area (VTA) and the dorsal raphé, reach both regions (see Root et al., 2015, for review). Likewise, whereas the VPdl projects extensively to subthalamic nucleus (STN) and substantia nigra pars reticulata (SNr), the VPvm projects extensively to medial dorsal thalamus, lateral hypothalamus (LH) and VTA (Groenewegen et al., 1993; Tripathi et al., 2013; Zahm, 1989; Zahm, Williams, & Wohltmann, 1996). So there are distinct, segregated channels of information flow through the VP, including AcbSh ➔ VPvm ➔ LH/VTA and AcbC ➔ VPdl ➔ STN/substantia nigra channels (Groenewegen et al., 1993; Zahm & Heimer, 1990).
There are at least four major cell types in the VP:‐ GABAergic (GABA), glutamatergic (vGlut2), and cholinergic (ChAT), with at least one other class characterized by expression of parvalbumin (PV) and releasing either or both GABA and glutamate (Faget et al., 2018; Knowland et al., 2017; Root et al., 2015) (Figure 1c). These VP neurons express various peptides including calretinin, dynorphin, calbindin‐d28k, neuropeptide Y and somatostatin (Root et al., 2015). GABAergic neurons are especially abundant, comprising more than 70% of the VP neurons with lower but similar numbers (10%–15%) of glutamatergic and cholinergic neurons (Faget et al., 2018). The relative distribution of these neurons varies across the VP. GABAergic neurons are more abundant in the medial portion of the VP, glutamatergic neurons in the medial VP, whereas the VP cholinergic neurons tend to be more sparsely and heterogeneously dispersed (Faget et al., 2018; Knowland et al., 2017; Tooley et al., 2018). The VP cell types also differ in their long‐range connectivity. For example, the VP GABA neurons project to the LH and VTA, lateral habenula (LHb), VTA, substantia nigra pars compacta (SNc), VP parvalbumin neurons send dense and separate projections to the LHb and VTA, and can be both excitatory and inhibitory on their postsynaptic targets via release of both glutamate and GABA (Knowland et al., 2017), VP glutamatergic neurons project to the LHb, VTA and rostromedial tegmental nucleus (RMTg), while VP cholinergic neurons project to PFC and BLA (Faget et al., 2018; Knowland et al., 2017; Tooley et al., 2018).
4. PARSING THE CELL‐TYPE AND CIRCUIT‐SPECIFIC VP MECHANISMS FOR DIFFERENT FORMS OF RELAPSE
We have shown how different forms of relapse are assembled from these distinct cell types and their major projection targets using an animal model of distinct aspects of relapse to alcohol seeking (Figure 2a). In this model, rats are trained in a distinctive context to make a response (nosepoke) to receive a small amount (0.6 ml) of alcoholic beer for each response. The animals readily learn to self‐administer alcohol under these conditions, reach high levels of intake (25–30 ml·h−1) and show behavioural indices of intoxication (reduced locomotor activity, impaired righting reflex and somnolence). Then we extinguish this seeking behaviour in a second, distinctive context, and rats readily learn to reduce alcohol seeking. When rats are tested in the extinction context, they refrain from alcohol seeking. When rats are tested in the training context, they relapse to alcohol seeking even though no alcohol is available on test. This form of relapse is renewal or context‐induced reinstatement (Crombag, Bossert, Koya, & Shaham, 2008; Crombag, Grimm, & Shaham, 2002; Crombag & Shaham, 2002; Todd, 2013; Todd, Vurbic, & Bouton, 2014). It is a robust and reliable way of modelling and understanding how contexts and places associated with alcohol‐use provoke relapse (Gibson et al., 2018; Hamlin, Clemens, Choi, & McNally, 2009; Hamlin, Newby, & McNally, 2007; Marchant, Hamlin, & McNally, 2009). Importantly, because animals refrain from alcohol seeking when tested in the extinction context, this is also a robust and reliable way of modelling and understanding the voluntary refraining from alcohol seeking (Gibson et al., 2018; Millan, Furlong, & McNally, 2010; Millan, Marchant, & McNally, 2011; Millan & McNally, 2011). Finally, animals receive a single session of retraining of alcohol self‐administration. This contingent re‐exposure to alcohol causes a rapid return to pre‐extinction levels of responding and alcohol intake (Willcocks & McNally, 2011). This rapid reacquisition models the rapid transition from lapse (a single drinking episode) to relapse (a return to pre‐abstinence levels of drinking), characteristic of relapse in human drinkers and which is difficult to prevent (Marlatt, Baer, Donovan, & Kivlahan, 1988; Marlatt & Donovan, 2005).
FIGURE 2.
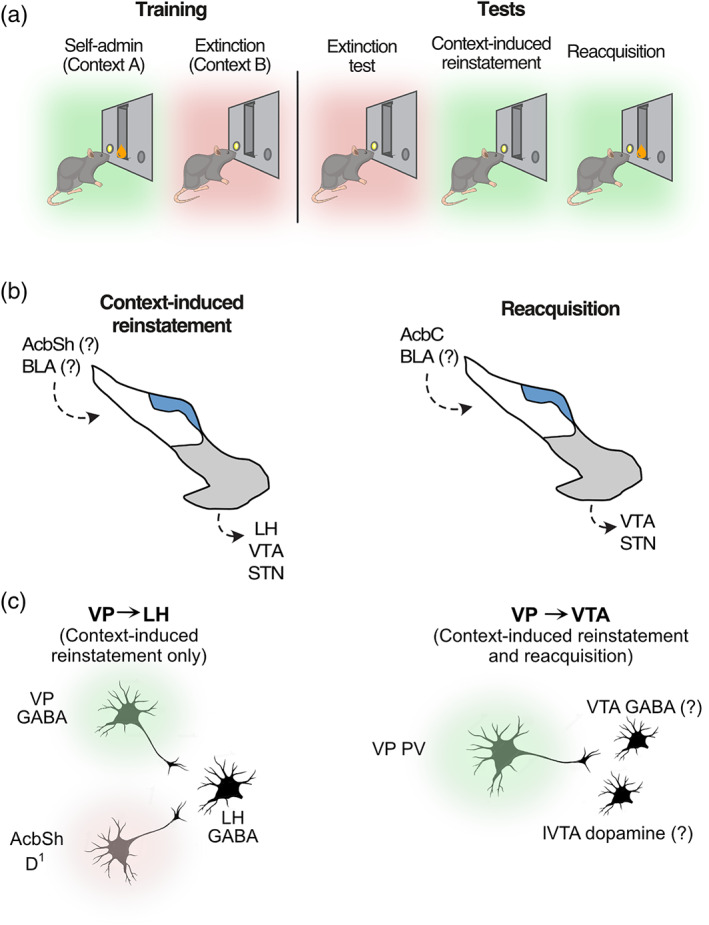
(a) Animal model of context‐induced reinstatement and reacquisition. (b) Transpallidal pathways mediating context‐induced reinstatement and reacquisition. (c) Cell‐type and circuit‐specific mechanisms for relapse. Context‐induced reinstatement is mediated by a VP GABA ➔ LH monosynaptic projection that converges with monosynaptic inputs from AcbSh Drd1 neurons onto the same LH GABA neurons, determining propensity to relapse (VP GABA ➔ LH) or refrain from relapse (AcbSh Drd1 ➔ LH). Context‐induced reinstatement and reacquisition are mediated by a VP PV ➔ VTA projection
Context‐induced reinstatement of alcohol seeking is associated with recruitment of the VP, as shown by expression of the Fos protein (Perry & McNally, 2013). This recruitment of the VP is causal to context‐induced reinstatement of alcohol seeking because relapse can be prevented by microinjections of μ receptor antagonists to the VP (Perry & McNally, 2013) as well as by chemogenetic inhibition of the VP neurons (Prasad et al., 2020). Importantly, the VP also contributes to other forms of reinstatement of alcohol seeking, including alcohol‐priming reinstatement (Perry & McNally, 2013) and rapid reacquisition of alcohol seeking and drinking (Khoo et al., 2015; Prasad et al., 2020; Prasad & McNally, 2016). This critical role for the VP in context‐induced and other forms of reinstatement of alcohol seeking is consistent with other findings, reviewed above, identifying the VP as obligatory for relapse.
We have mapped the neurocircuitry of these VP contributions to relapse (Figure 2b). Context‐induced reinstatement is associated with the selective recruitment of AcbC inputs to the VP, consistent with the location of the VP in a final common relapse pathway (Perry & McNally, 2013). However, other major afferents to the VP, including from caudal parts of the basolateral amygdala (BLA; Kelley, Domesick, & Nauta, 1982; Zaborszky, Leranth, & Heimer, 1984) are also recruited during this reinstatement (Perry & McNally, 2013), supporting other evidence that the VP serves an integrative, not just relay, role in motivated behaviour (Ottenheimer, Richard, & Janak, 2018; Richard, Ambroggi, Janak, & Fields, 2016; Richard, Castro, DiFeliceantonio, Robinson, & Berridge, 2013). Little is known about the causal roles of these VP inputs in context‐induced reinstatement. Despite being strongly recruited, the AcbC ➔ VP input is not necessary for context‐induced reinstatement because optogenetic inhibition of this input does not prevent reinstatement (Khoo et al., 2015). On the other hand, the AcbSh ➔ VP input is likely to be important because the AcbSh Drd1 neurons that project to the VP also collateralize to the VTA and these AcbSh Drd1 neurons mediate context‐induced reinstatement (Gibson et al., 2018).
The AcbC ➔ VP input is important for relapse during reacquisition of alcohol seeking (Khoo et al., 2015). Recall that the AcbSh targets VPvm, whereas AcbC targets VPdl, so this dissociation in the roles of AcbC ➔ VP pathway in context‐induced reinstatement versus reacquisition might reflect different roles of the VP channels in different forms of relapse, but other evidence reviewed below suggests that this is an oversimplification. Regardless, these structural differences in the VP inputs controlling two different forms of relapse in the same animal provide one mechanism for why medications may prove more (or less) effective against different forms of relapse.
Context‐induced reinstatement of alcohol seeking is associated with the selective recruitment of key the VP outputs. Studies combining retrograde tracing with expression of the c‐Fos protein show that the VP projections to the LH, VTA and STN are each recruited during context‐induced reinstatement (Prasad et al., 2020; Prasad & McNally, 2016). Each of these specific VP output pathways causally mediates context‐induced reinstatement because chemogenetic disconnection of VP ➔ STN, VP ➔ VTA, and VP ➔ LH each prevents reinstatement (Prasad et al., 2020; Prasad & McNally, 2016). There are again nuanced but important differences in how the VP outputs contribute to different forms of relapse. The VP ➔ VTA and VP ➔ STN pathways mediate context‐induced reinstatement and reacquisition as well as motivation to respond for and consume alcohol more generally (Prasad et al., 2020; Prasad & McNally, 2016), whereas the VP ➔ LH pathway mediates context‐induced reinstatement but not reacquisition (Prasad et al., 2020). A role for the VP ➔ VTA projections in relapse to alcohol seeking supports work by Mahler et al. (2014) using relapse to cocaine seeking. A role for the VP ➔ STN pathway in relapse is novel but well supported by other findings implicating STN in motivation to respond for drugs of abuse (Baracz, Everett, & Cornish, 2015; Baunez, Dias, Cador, & Amalric, 2005; Baunez, Yelnik, & Mallet, 2011; Lardeux & Baunez, 2008; Rouaud et al., 2010). Moreover, this identification of a key role for the VP ➔ STN pathway in different forms of relapse to alcohol seeking provides a mechanistic circuit basis for understanding the therapeutic effects of STN deep brain stimulation against relapse (Baunez et al., 2011; Hachem‐Delaunay et al., 2015; Pelloux & Baunez, 2013; Rouaud et al., 2010).
These findings suggest that the different roles for the VP in relapse are more complex than different forms of relapse being associated with different VP channels of information flow (i.e. AcbSh ➔ VPvm ➔ LH/VTA vs. AcbC ➔ VPdl ➔ STN/SN). The same channels or parts of channels are recruited across different forms of relapse. To further understand this, we have mapped the cellular architecture of the VP contributions to relapse (Figure 2c). This mapping has answered key questions about how the VP assembles relapse. Single molecule fluorescent in situ hybridization showed that context‐induced reinstatement is associated with the selective activation of the VP GABA and PV neurons (Prasad et al., 2020). Cell‐type and pathway‐specific chemogenetic disconnections showed that the VP PV neurons control both context‐induced reinstatement and reacquisition via their projections to VTA (Prasad et al., 2020). The VP PV neurons target lateral VTA where they synapse onto both GABA and dopamine neurons (Knowland et al., 2017). Both lateral VTA dopamine (Liu et al., 2020) and GABA (Gibson et al., 2018) neurons mediate context‐induced reinstatement and reacquisition, but whether this is due to the VP PV input to these cells is yet to be determined. In contrast, the VP GABA neurons mediate context‐induced reinstatement but not reacquisition. The VP GABA neurons mediate context‐induced reinstatement via their projections to the LH. So different forms of relapse depend on both distinct VP cell types and distinct VP output pathways. This underscores the important point that different medications may be more (or less) effective against different forms of relapse due to these important but subtle differences in how VP assembles different forms of relapse.
The VP GABAergic projection to the LH is of special interest. We and others have shown that VP GABAergic neurons provide monosynaptic input to LH GABA neurons (Jennings, Rizzi, Stam'takis, Ung, & Stuber, 2013; Prasad et al., 2020). This is important because AcbSh Drd1 neurons also provide monosynaptic inhibitory input to LH GABA neurons (Gibson et al., 2018; O'Connor et al., 2015). Whereas the VP GABA inputs to LH GABA neurons cause context‐induced reinstatement (Prasad et al., 2020), AcbSh Drd1 GABA inputs to LH GABA neurons prevent this reinstatement (Gibson et al., 2018). Moreover, using optogenetic‐assisted circuit mapping, we showed that a subpopulation of these LH GABA neurons receive converging monosynaptic inputs from both the VP GABA and AcbSh Drd1 neurons (Prasad et al., 2020). This provides the first known location of cellular convergence between the striatopallidal circuits controlling relapse and striato‐hypothalamic circuits controlling extinction.
This convergence of striatopallidal and striato‐hypothalamic pathways with opposing functions in promoting versus preventing relapse identifies LH GABA neurons as critical to controlling propensity to relapse. Single‐cell transcriptomic analyses have shown that LH GABA neurons are diverse and comprise at least 15 major clusters (Mickelsen et al., 2019). The critical cluster of LH GABA neurons that receive converging striatopallidal and striato‐hypothalamic inputs to bidirectionally control relapse are currently unknown. One attractive possibility is a cluster of LH GABA neurons that expresses Gal (encoding galanin) along with Nts (encoding neurotensin), Cartpt (encoding cocaine‐ and amphetamine‐regulated transcript) and Tac1 (encoding preprotachykinin‐1). The neuropeptide products of each of these genes, including galanin (Genders et al., 2019; Genders, Scheller, & Djouma, 2020; Wilson et al., 2018), neurotensin (Pandey, Badve, Curtis, Leibowitz, & Barson, 2019; Vadnie et al., 2014), CART (King, Furlong, & McNally, 2010; Moffett et al., 2006; Philpot & Smith, 2006; Rogge, Jones, Hubert, Lin, & Kuhar, 2008) and substance P/neurokinin A (Schank, 2020; Schank & Heilig, 2017; Thorsell, Schank, Singley, Hunt, & Heilig, 2010) have been identified by preclinical studies as important in reducing alcohol consumption and alcohol seeking. Moreover, neurokinin receptor antagonists have therapeutic potential, blunting spontaneous and challenge elicited alcohol cravings in detoxified human drinkers (George et al., 2008). However, the effects of individually manipulating these neuropeptide systems are often small. Further identification and functional characterization of the critical population of LH GABA neurons controlling propensity to relapse may allow for interventions with larger effects against craving and relapse.
5. VP AND AVERSIVE MOTIVATION
The VP has other functions important to understanding alcohol‐use disorders as well as some of the co‐morbidities that accompany these disorders. Major depressive disorder, anxiety disorders and stress‐related disorders are highly co‐morbid with alcohol‐use disorders (Castillo‐Carniglia, Keyes, Hasin, & Cerdá, 2019). The causes of these co‐morbidities are poorly understood but likely to be complex and heterogenous. These extensive co‐morbidities not only increase the personal and broader impacts of alcohol‐use disorders, but they also pose significant challenges for treatment.
Recent findings have strongly implicated the VP in key behavioural responses to stressors and aversive motivation, especially via projections to the VTA and LHb. For example, the VP PV neurons play an important role in responses to chronic social defeat stress (Knowland et al., 2017). Mice subjected to chronic social defeat show elevated activity of the VP PV neurons and this causes adverse behavioural and motivational effects. The VP PV ➔ VTA pathway mediates social withdrawal, whereas the VP PV ➔ LHb pathway mediates behavioural despair (struggling in a tail suspension test) in response to chronic social stress (Knowland et al., 2017). The VP glutamatergic neurons, on the other hand, have been strongly implicated in aversion, avoidance and anxiety. Stimulation of the VP vGlut2 neurons is aversive, supporting avoidance behaviours (Faget et al., 2018; Stephenson‐Jones et al., 2020) and this depends on the VP vGlut2 ➔ LHb pathway (Faget et al., 2018). This linking of specific VP cell types and their projections to distinct aspects of stress, anxiety, and aversive motivation is well supported by findings from non‐human primates with pharmacological manipulations (Saga et al., 2016; Saga, Ruff, & Tremblay, 2019) and single unit recordings (Kaplan, Mizrahi‐Kliger, Israel, Adler, & Bergman, 2020).
This role for the VP in aversion processing has important implications for understanding alcohol‐use disorders. First, this role provides a potential cellular basis for understanding changes in social behaviour, stress‐reactivity and mood associated with alcohol use. Social behaviours have a key role in reducing drug craving, protecting against relapse and maintaining abstinence (Heilig, Epstein, Nader, & Shaham, 2016; Venniro et al., 2018; Venniro, Caprioli, & Shaham, 2018). We now know that the same population of VP neurons (PV neurons) and projections (to VTA) that orchestrate relapse to alcohol seeking also orchestrate social withdrawal in response to stressors. The targeting of this specific circuit may have benefit. Second, the role for the VP in aversion processing also may help explain changes in aversive and risky decision making. Persistent alcohol use, despite the adverse consequences of this use, is a diagnostic criterion of substance‐use disorder and addictive disorders and the VP is essential for controlling drug seeking under adverse consequences (Farrell et al., 2019). Finally, although these roles for the VP in stress and aversion have been identified in alcohol and other drug free animals, there is emerging evidence that drug withdrawal profoundly alters neurotransmission in the VP cell types (e.g. vGlut2) essential for aversion processing (Heinsbroek et al., 2020; Inbar et al., 2020).
6. CONCLUSIONS
The ventral pallidum (VP) is a key node in the neural circuits controlling relapse to alcohol seeking. It is also a key target of pharmacotherapies for relapse prevention. The work reviewed here shows how distinct forms of relapse to alcohol seeking can be linked to the molecular, anatomical, pharmacological, and functional properties of the VP. Although the VP is obligatory for all forms of relapse that have been studied, different forms of relapse are assembled from different VP cellular and circuit architectures. It follows that the efficacy of relapse prevention medications will be determined, at least in part, by which component of these different architectures is targeted. These findings provide a mechanistic bridge to understanding heterogeneity in treatment responsiveness among individuals with alcohol‐use disorders because they begin to identify why and when relapse medications may be effective and ineffective. Moreover, they raise the important possibility of targeting different architectures to provide tailored interventions for different forms of relapse. Of course, how such selective targeting may be achieved remains to be determined. Finally, the recent insights into the cellular and circuit mechanisms for VP to aversion processing have important implications for understanding and remediating social, affective, and decision‐making processes in alcohol‐use disorders.
6.1. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, et al., 2019, Alexander, Keely, et al., 2019).
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGEMENTS
Preparation of this manuscript was supported by grants from the National Health and Medical Research Council of Australia to G.P.M. (GNT1164514 and GNT1138062) and the Australian Research Council (DE170100509) to A.A.P.
Prasad AA, McNally GP. The ventral pallidum and relapse in alcohol seeking. Br J Pharmacol. 2020;177:3855–3864. 10.1111/bph.15160
REFERENCES
- Agabio, R. , Sinclair, J. M. A. , Addolorato, G. , Aubin, H.‐J. , Beraha, E. M. , Caputo, F. , … Leggio, L. (2018). Baclofen for the treatment of alcohol use disorder: The Cagliari Statement. The Lancet Psychiatry, 5, 957–960. 10.1016/S2215-0366(18)30303-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton, R. F. , O'Malley, S. S. , Ciraulo, D. A. , Cisler, R. A. , Couper, D. , Donovan, D. M. , et al. (2006). Combined Pharmacotherapies and Behavioral Interventions for Alcohol‐Dependence. JAMA, 17, 2003–2017. [DOI] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Keely, E. , Mathie, A. , Peter, J. A. , Veale, E. L. , Armstrong, J. H. , … GTP collaborators . (2019). The concise guide to pharmacology 2019/2020: Introduction and other protein target. British Journal of Pharmacology, 176, S1–S20. 10.1111/bph.14747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peter, J. A. , … CGTP Collaborators . (2019). The concise guide to pharmacology 2019/2020: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baracz, S. J. , Everett, N. A. , & Cornish, J. L. (2015). The involvement of oxytocin in the subthalamic nucleus on relapse to methamphetamine‐seeking behaviour. PLoS ONE, 10, e0136132–e0136117. 10.1371/journal.pone.0136132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunez, C. , Dias, C. , Cador, M. , & Amalric, M. (2005). The subthalamic nucleus exerts opposite control on cocaine and 'natural' rewards. Nature Neuroscience, 8, 484–489. 10.1038/nn1429 [DOI] [PubMed] [Google Scholar]
- Baunez, C. , Yelnik, J. , & Mallet, L. (2011). Six questions on the subthalamic nucleus: Lessons from animal models and from stimulated patients. Neuroscience, 198, 193–204. 10.1016/j.neuroscience.2011.09.059 [DOI] [PubMed] [Google Scholar]
- Castillo‐Carniglia, A. , Keyes, K. M. , Hasin, D. S. , & Cerdá, M. (2019). Psychiatric comorbidities in alcohol use disorder. The Lancet Psychiatry, 6, 1068–1080. 10.1016/S2215-0366(19)30222-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed, M. , Ntamati, N. R. , Chandra, R. , Lobo, M. K. , & Luscher, C. (2016). Convergence of reinforcing and anhedonic cocaine effects in the ventral pallidum. Neuron, 92, 214–226. 10.1016/j.neuron.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag, H. S. , Bossert, J. M. , Koya, E. , & Shaham, Y. (2008). Context‐induced relapse to drug seeking: A review. Philosophical Transactions of the Royal Society of London: Series B, Biological Sciences, 363, 3233–3243. 10.1098/rstb.2008.0090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crombag, H. S. , Grimm, J. W. , & Shaham, Y. (2002). Effect of dopamine receptor antagonists on renewal of cocaine seeking by reexposure to drug‐associated contextual cues. Neuropsychopharmacology, 27, 1006–1015. 10.1016/S0893-133X(02)00356-1 [DOI] [PubMed] [Google Scholar]
- Crombag, H. S. , & Shaham, Y. (2002). Renewal of drug seeking by contextual cues after prolonged extinction in rats. Behavioral Neuroscience, 116, 169–173. 10.1037/0735-7044.116.1.169 [DOI] [PubMed] [Google Scholar]
- Faget, L. , Zell, V. , Souter, E. , McPherson, A. , Ressler, R. , Gutierrez‐Reed, N. , … Hnasko, T. S. (2018). Opponent control of behavioral reinforcement by inhibitory and excitatory projections from the ventral pallidum. Nature Communications, 9, 849 10.1038/s41467-018-03125-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell, M. R. , Ruiz, C. M. , Castillo, E. , Faget, L. , Khanbijian, C. , Liu, S. , … Mahler, S. V. (2019). Ventral pallidum is essential for cocaine relapse after voluntary abstinence in rats. Neuropsychopharmacology, 44, 2174–2184. 10.1038/s41386-019-0507-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genders, S. G. , Scheller, K. J. , & Djouma, E. (2020). Neuropeptide modulation of addiction: Focus on galanin. Neuroscience and Biobehavioral Reviews, 110, 133–149. 10.1016/j.neubiorev.2018.06.021 [DOI] [PubMed] [Google Scholar]
- Genders, S. G. , Scheller, K. J. , Jaehne, E. J. , Turner, B. J. , Lawrence, A. J. , Brunner, S. M. , … Djouma, E. (2019). GAL3 receptor knockout mice exhibit an alcohol‐preferring phenotype. Addiction Biology, 24, 886–897. 10.1111/adb.12641 [DOI] [PubMed] [Google Scholar]
- George, D. T. , Gilman, J. , Hersh, J. , Thorsell, A. , Herion, D. , Geyer, C. , … Heilig, M. (2008). Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science, 319, 1536–1539. 10.1126/science.1153813 [DOI] [PubMed] [Google Scholar]
- Gibson, G. D. , Prasad, A. A. , Jean‐Richard Dit Bressel, P. , Yau, J. O. Y. , Millan, E. Z. , Liu, Y. , et al. (2018). Distinct accumbens shell output pathways promote versus prevent relapse to alcohol seeking. Neuron, 98, 512, e516–520. [DOI] [PubMed] [Google Scholar]
- Groenewegen, H. J. , Berendse, H. W. , & Haber, S. N. (1993). Organisation of the output of the ventral striatopallidal system in the rat: Ventral pallidal efferents. Neuroscience, 57, 113–142. 10.1016/0306-4522(93)90115-V [DOI] [PubMed] [Google Scholar]
- Hachem‐Delaunay, S. , Fournier, M.‐L. , Cohen, C. , Bonneau, N. , Cador, M. , Baunez, C. , & le Moine, C. (2015). Subthalamic nucleus high‐frequency stimulation modulates neuronal reactivity to cocaine within the reward circuit. Neurobiology of Disease, 80, 54–62. 10.1016/j.nbd.2015.05.007 [DOI] [PubMed] [Google Scholar]
- Hamlin, A. S. , Clemens, K. J. , Choi, E. A. , & McNally, G. P. (2009). Paraventricular thalamus mediates context‐induced reinstatement (renewal) of extinguished reward seeking. European Journal of Neuroscience, 29, 802–812. 10.1111/j.1460-9568.2009.06623.x [DOI] [PubMed] [Google Scholar]
- Hamlin, A. S. , Newby, J. , & McNally, G. P. (2007). The neural correlates and role of D1 dopamine receptors in renewal of extinguished alcohol‐seeking. Neuroscience, 146, 525–536. 10.1016/j.neuroscience.2007.01.063 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR . (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46, D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilig, M. , Epstein, D. H. , Nader, M. A. , & Shaham, Y. (2016). Time to connect: Bringing social context into addiction neuroscience. Nature Reviews Neuroscience, 17, 592–599. 10.1038/nrn.2016.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer, L. , Zahm, D. S. , Churchill, L. , & Kalivas, P. W. (1991). Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience, 41, 89–125. 10.1016/0306-4522(91)90202-Y [DOI] [PubMed] [Google Scholar]
- Heinsbroek, J. A. , Bobadilla, A. C. , Dereschewitz, E. , Assali, A. , Chalhoub, R. M. , Cowan, C. W. , & Kalivas, P. W. (2020). Opposing regulation of cocaine seeking by glutamate and GABA neurons in the ventral pallidum. Cell Repoirts, 30, 2018–2027. 10.1016/j.celrep.2020.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsbroek, J. A. , Neuhofer, D. N. , Griffin, W. C. , Siegel, G. S. , Bobadilla, A.‐C. , Kupchik, Y. M. , et al. (2017). Loss of plasticity in the D2‐accumbens pallidal pathway promotes cocaine seeking. The Journal of Neuroscience, 37, 757–767. 10.1523/JNEUROSCI.2659-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inbar, K. , Levi, L. A. , Bernat, N. , Odesser, T. , Inbar, D. , & Kupchik, Y. M. (2020). Cocaine dysregulates dynorphin modulation of inhibitory neurotransmission in the ventral pallidum in a cell‐type‐specific manner. The Journal of Neuroscience, 40, 1321–1331. 10.1523/JNEUROSCI.1262-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings, J. H. , Rizzi, G. , Stam'takis, A. M. , Ung, R. L. , & Stuber, G. D. (2013). The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science, 341, 1517–1521. 10.1126/science.1241812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas, D. E. , Amick, H. R. , Feltner, C. , Bobashev, G. , Thomas, K. , Wines, R. , … Garbutt, J. C. (2014). Pharmacotherapy for Adults With Alcohol Use Disorders in Outpatient Settings: A Systematic Review and Meta‐analysis. JAMA, 311(18), 1889–1900. 10.1001/jama.2014.3628 [DOI] [PubMed] [Google Scholar]
- Kalivas, P. W. , & Volkow, N. D. (2005). The neural basis of addiction: A pathology of motivation and choice. American Journal of Psychiatry, 162, 1403–1413. 10.1176/appi.ajp.162.8.1403 [DOI] [PubMed] [Google Scholar]
- Kaplan, A. , Mizrahi‐Kliger, A. D. , Israel, Z. , Adler, A. , & Bergman, H. (2020). Dissociable roles of ventral pallidum neurons in the basal ganglia reinforcement learning network. Nature Neurosciencein press, 23, 556–564. 10.1038/s41593-020-0605-y [DOI] [PubMed] [Google Scholar]
- Kelley, A. E. , Domesick, V. B. , & Nauta, W. J. H. (1982). The rat amygdalostriatal projection in the rat—An anatomical study by anterograde and retrograde tracing methods. Neuroscience, 7, 615–630. 10.1016/0306-4522(82)90067-7 [DOI] [PubMed] [Google Scholar]
- Khoo, A. T. , Gibson, G. D. , Prasad, A. A. , & McNally, G. P. (2015). Role of the striatopallidal pathway in renewal and reacquisition of alcohol seeking. Behavioral Neuroscience, 129, 2–7. 10.1037/bne0000036 [DOI] [PubMed] [Google Scholar]
- King, B. J. , Furlong, T. M. , & McNally, G. P. (2010). Cocaine and amphetamine related transcript (CART) inhibits context induced reinstatement of reward seeking. Behavioral Neuroscience, 124, 423–427. 10.1037/a0019540 [DOI] [PubMed] [Google Scholar]
- Knowland, D. , Lilascharoen, V. , Pacia, C. P. , Shin, S. , Wang, E. H.‐J. , & Lim, B. K. (2017). Distinct ventral pallidal neural populations mediate separate symptoms of depression. Cell, 170, 284, e218–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik, Y. M. , Brown, R. M. , Heinsbroek, J. A. , Lobo, M. K. , Schwatrz, D. J. , & Kalivas, P. W. (2015). Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nature Neuroscience, 18, 1230–1232. 10.1038/nn.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupchik, Y. M. , Scofield, M. D. , Rice, K. C. , Cheng, K. , Roques, B. P. , & Kalivas, P. W. (2014). Cocaine dysregulates opioid gating of GABA neurotransmission in the ventral pallidum. The Journal of Neuroscience, 34, 1057–1066. 10.1523/JNEUROSCI.4336-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lardeux, S. , & Baunez, C. (2008). Alcohol preference influences the subthalamic nucleus control on motivation for alcohol in rats. Neuropsychopharmacology, 33, 634–642. 10.1038/sj.npp.1301432 [DOI] [PubMed] [Google Scholar]
- Litten, R. Z. , Ryan, M. L. , Falk, D. E. , Reilly, M. , Fertig, J. B. , & Koob, G. F. (2015). Heterogeneity of alcohol use disorder: understanding mechanisms to advance personalized treatment. Alcohol Clinical and Experimental Research, 39, 579–584. 10.1111/acer.12669 [DOI] [PubMed] [Google Scholar]
- Liu, S. , von Bressel, P. J. R. , Joy, Y. , Willing, A. , Prasad, A. A. , Power, J. M. , et al. (2020). The mesolimbic dopamine activity signatures of relapse to alcohol seeking. BioRxiv. 10.1101/2020.03.06.981605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo, M. K. , Covington, H. E. , Chaudhury, D. , Friedman, A. K. , Sun, H. , Damez‐Werno, D. , … Nestler, E. J. (2010). Cell type‐specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science, 330, 385–390. 10.1126/science.1188472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler, S. V. , Vazey, E. M. , Beckley, J. T. , Keistler, C. R. , McGlinchey, E. M. , Kaufling, J. , et al. (2014). Designer receptors show role for ventral pallidum input to ventral tegmental area in cocaine seeking. Nature Neuroscience, 17, 577–585. 10.1038/nn.3664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maksimovskiy, A. L. , Fortier, C. B. , Milberg, W. P. , & McGlinchey, R. E. (2019). A structural MRI study of differential neuromorphometric characteristics of binge and heavy drinking. Addictive Behavior Reports, 9, 100168 10.1016/j.abrep.2019.100168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant, N. J. , Hamlin, A. S. , & McNally, G. P. (2009). Lateral hypothalamus is required for context‐induced reinstatement of extinguished reward seeking. The Journal of Neuroscience, 1331–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt, G. A. , Baer, J. S. , Donovan, D. M. , & Kivlahan, D. R. (1988). Addictive behaviors: Etiology and treatment. Annual Review of Psychology, 39, 223–252. 10.1146/annurev.ps.39.020188.001255 [DOI] [PubMed] [Google Scholar]
- Marlatt, G. A. , & Donovan, D. M. (2005). Relapse prevention. New York: Guildford Press. [Google Scholar]
- Mickelsen, L. E. , Bolisetty, M. , Chimileski, B. R. , Fujita, A. , Beltrami, E. J. , Costanzo, J. T. , … Jackson, A. C. (2019). Single‐cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nature Neuroscience, 22, 642–656. 10.1038/s41593-019-0349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan, E. Z. , Furlong, T. M. , & McNally, G. P. (2010). Accumbens shell‐hypothalamus interactions mediate extinction of alcohol seeking. The Journal of Neuroscience, 30, 4626–4635. 10.1523/JNEUROSCI.4933-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan, E. Z. , Marchant, N. J. , & McNally, G. P. (2011). Extinction of drug seeking. Behavioural Brain Research, 217, 454–462. 10.1016/j.bbr.2010.10.037 [DOI] [PubMed] [Google Scholar]
- Millan, E. Z. , & McNally, G. P. (2011). Accumbens shell AMPA receptors mediate expression of extinguished reward seeking through interactions with basolateral amygdala. Learning & Memory, 18, 414–421. 10.1101/lm.2144411 [DOI] [PubMed] [Google Scholar]
- Moffett, M. , Stanek, L. , Harley, J. , Rogge, G. , Asnicar, M. , Hsiung, H. , & Kuhar, M. (2006). Studies of cocaine‐ and amphetamine‐regulated transcript (CART) knockout mice. Peptides, 27, 2037–2045. 10.1016/j.peptides.2006.03.035 [DOI] [PubMed] [Google Scholar]
- Mohammadkhani, A. , James, M. H. , Pantazis, C. B. , & Aston‐Jones, G. (2020). Persistent effects of the orexin‐1 receptor antagonist SB‐334867 on motivation for the fast acting opioid remifentanil. Brain Research, 1731, 146461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moussawi, K. , Kalivas, P. W. , & Lee, J. W. (2016). Abstinence from drug dependence after bilateral Globus Pallidus hypoxic ischemic injury. Biological Psychiatry, 80, 79–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, U. J. , Mawrin, C. , Frodl, T. , Dobrowolny, H. , Busse, S. , Bernstein, H. G. , et al. (2019). Reduced volumes of the external and internal globus pallidus in male heroin addicts: A postmortem study. European Archives of Psychiatry and Clinical Neuroscience, 269, 317–324. 10.1007/s00406-018-0939-6 [DOI] [PubMed] [Google Scholar]
- O'Connor, E. C. , Kremer, Y. , Lefort, S. , Harada, M. , Pascoli, V. , Rohner, C. , et al. (2015). Accumbal D1R neurons projecting to lateral hypothalamus authorize feeding. Neuron, 88, 553–564. 10.1016/j.neuron.2015.09.038 [DOI] [PubMed] [Google Scholar]
- Ottenheimer, D. , Richard, J. M. , & Janak, P. H. (2018). Ventral pallidum encodes relative reward value earlier and more robustly than nucleus accumbens. Nature Communications, 9, 4350 10.1038/s41467-018-06849-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey, S. , Badve, P. S. , Curtis, G. R. , Leibowitz, S. F. , & Barson, J. R. (2019). Neurotensin in the posterior thalamic paraventricular nucleus: Inhibitor of pharmacologically relevant ethanol drinking. Addiction Biology, 24, 3–16. 10.1111/adb.12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo‐Garcia, T. R. , Garcia‐Keller, C. , Penaloza, T. , Richie, C. T. , Pickel, J. , Hope, B. T. , … Heinsbroek, J. A. (2019). Ventral pallidum is the primary target for accumbens D1 projections driving cocaine seeking. The Journal of Neuroscience, 39, 2041–2051. 10.1523/JNEUROSCI.2822-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelloux, Y. , & Baunez, C. (2013). Deep brain stimulation for addiction: Why the subthalamic nucleus should be favored. Current Opinion in Neurobiology, 23, 713–720. 10.1016/j.conb.2013.02.016 [DOI] [PubMed] [Google Scholar]
- Perry, C. J. , & McNally, G. P. (2013). A role for the ventral pallidum in context‐induced and primed reinstatement of alcohol seeking. The European Journal of Neuroscience, 38, 2762–2773. 10.1111/ejn.12283 [DOI] [PubMed] [Google Scholar]
- Philpot, K. , & Smith, Y. (2006). CART peptide and the mesolimbic dopamine system. Peptides, 27, 1987–1992. 10.1016/j.peptides.2005.11.028 [DOI] [PubMed] [Google Scholar]
- Prasad, A. A. , & McNally, G. P. (2016). Ventral pallidum output pathways in context‐induced reinstatement of alcohol seeking. The Journal of Neuroscience, 36, 11716–11726. 10.1523/JNEUROSCI.2580-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad, A. A. , Xie, C. , Chaichim, C. , Nguyen, J. H. , McClusky, H. E. , Killcross, S. , et al. (2020). Complementary roles for ventral pallidum cell types and their projections in relapse. The Journal of Neuroscience, 40, 880–893. 10.1523/JNEUROSCI.0262-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, J. M. , Ambroggi, F. , Janak, P. H. , & Fields, H. L. (2016). Ventral pallidum neurons encode incentive value and promote cue‐elicited instrumental actions. Neuron, 90, 1165–1173. 10.1016/j.neuron.2016.04.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard, J. M. , Castro, D. C. , DiFeliceantonio, A. G. , Robinson, M. J. F. , & Berridge, K. C. (2013). Mapping brain circuits of reward and motivation: In the footsteps of Ann Kelley. Neuroscience and Biobehavioral Reviews, 37, 1919–1931. 10.1016/j.neubiorev.2012.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers, J. L. , Ghee, S. , & See, R. E. (2008). The neural circuitry underlying reinstatement of heroin‐seeking behavior in an animal model of relapse. Neuroscience, 151, 579–588. 10.1016/j.neuroscience.2007.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogge, G. , Jones, D. , Hubert, G. W. , Lin, Y. , & Kuhar, M. J. (2008). CART peptides: Regulators of body weight, reward and other functions. Nature Reviews Neuroscience, 9, 747–758. 10.1038/nrn2493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root, D. H. , Melendez, R. I. , Zaborszky, L. , & Napier, T. C. (2015). The ventral pallidum: Subregion‐specific functional anatomy and roles in motivated behaviors. Progress in Neurobiology, 130, 29–70. 10.1016/j.pneurobio.2015.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaud, T. , Lardeux, S. , Panayotis, N. , Paleressompoulle, D. , Cador, M. , & Baunez, C. (2010). Reducing the desire for cocaine with subthalamic nucleus deep brain stimulation. Proceedings of the National Academy of Sciences of the United States of America, 107, 1196–1200. 10.1073/pnas.0908189107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga, Y. , Richard, A. , Sgambato‐Faure, V. , Hoshi, E. , Tobler, P. N. , & Tremblay, L. (2016). Ventral pallidum encodes contextual information and controls aversive behaviors. Cerebral Cortex, 27, 2538–2543. [DOI] [PubMed] [Google Scholar]
- Saga, Y. , Ruff, C. C. , & Tremblay, L. (2019). Disturbance of approach‐avoidance behaviors in non‐human primates by stimulation of the limbic territories of basal ganglia and anterior insula. European Journal of Neuroscience, 49, 687–700. [DOI] [PubMed] [Google Scholar]
- Schank, J. R. (2020). Neurokinin receptors in drug and alcohol addiction. Brain Research, 1734, 146729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank, J. R. , & Heilig, M. (2017). Substance P and the Neurokinin‐1 Receptor: The new CRF. International Review of Neurobiology, 136, 151–175. 10.1016/bs.irn.2017.06.008 [DOI] [PubMed] [Google Scholar]
- Smith, K. S. , & Berridge, K. C. (2005). The ventral pallidum and hedonic reward: Neurochemical maps of sucrose “Liking” and food intake. The Journal of Neuroscience, 25, 8637–8649. 10.1523/JNEUROSCI.1902-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, K. S. , Tindell, A. J. , Aldridge, J. W. , & Berridge, K. C. (2009). Ventral pallidum roles in reward and motivation. Behavioural Brain Research, 196, 155–167. 10.1016/j.bbr.2008.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik, M. T. , Kupchik, Y. M. , Brown, R. M. , & Kalivas, P. W. (2013). Optogenetic evidence that pallidal projections, not nigral projections, from the nucleus accumbens core are necessary for reinstating cocaine seeking. The Journal of Neuroscience, 33, 13654–13662. 10.1523/JNEUROSCI.1570-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanik, M. T. , Moussawi, K. , Kupchik, Y. M. , Smith, K. C. , Miller, R. L. , Huff, M. L. , … LaLumiere, R. T. (2013). Optogenetic inhibition of cocaine seeking in rats. Addiction Biology, 18, 50–53. 10.1111/j.1369-1600.2012.00479.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson‐Jones, M. , Bravo‐Rivera, C. , Ahrens, S. , Furlan, A. , Xiao, X. , Fernandes‐Henriques, C. , & Li, B. (2020). Opposing contributions of GABAergic and glutamatergic ventral pallidal neurons to motivational behaviors. Neuron, 105, 921–933. 10.1016/j.neuron.2019.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, X. C. , McFarland, K. , Cagle, S. , & Kalivas, P. W. (2005). Cocaine‐induced reinstatement requires endogenous stimulation of μ‐opioid receptors in the ventral pallidum. The Journal of Neuroscience, 25, 4512–4520. 10.1523/JNEUROSCI.0685-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsell, A. , Schank, J. R. , Singley, E. , Hunt, S. P. , & Heilig, M. (2010). Neurokinin‐1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology, 209, 103–111. 10.1007/s00213-010-1775-1 [DOI] [PubMed] [Google Scholar]
- Tindell, A. J. , Smith, K. S. , Pecina, S. , Berridge, K. C. , & Aldridge, J. W. (2006). Ventral pallidum firing codes hedonic reward: When a bad taste turns good. Journal of Neurophysiology, 96, 2399–2409. 10.1152/jn.00576.2006 [DOI] [PubMed] [Google Scholar]
- Todd, T. P. (2013). Mechanisms of renewal after the extinction of instrumental behavior. Journal of Experimental Psychology: Animal Behavior Processes, 39, 193–207. 10.1037/a0032236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd, T. P. , Vurbic, D. , & Bouton, M. E. (2014). Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning. Neurobiology of Learning and Memory, 108, 52–64. 10.1016/j.nlm.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooley, J. , Marconi, L. , Alipio, J. B. , Matikainen‐Ankney, B. , Georgiou, P. , Kravitz, A. V. , & Creed, M. C. (2018). Glutamatergic ventral pallidal neurons modulate activity of the habenula‐tegmental circuitry and constrain reward seeking. Biological Psychiatry, 83, 1012–1023. 10.1016/j.biopsych.2018.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi, A. , Prensa, L. , Cebrian, C. , & Mengual, E. (2010). Axonal branching patterns of nucleus accumbens neurons in the rat. Journal of Comparative Neurology, 518, 4649–4673. 10.1002/cne.22484 [DOI] [PubMed] [Google Scholar]
- Tripathi, A. , Prensa, L. , & Mengual, E. (2013). Axonal branching patterns of ventral pallidal neurons in the rat. Brain Structure and Function, 218, 1133–1157. 10.1007/s00429-012-0451-0 [DOI] [PubMed] [Google Scholar]
- Vadnie, C. A. , Park, J. H. , Abdel Gawad, N. , Ho, A. M. , Hinton, D. J. , & Choi, D. S. (2014). Gut‐brain peptides in corticostriatal‐limbic circuitry and alcohol use disorders. Frontiers in Neuroscience, 8, 288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro, M. , Caprioli, D. , & Shaham, Y. (2018). Novel models of drug relapse and craving after voluntary abstinence. Neuropsychopharmacology, 44, 234–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venniro, M. , Zhang, M. , Caprioli, D. , Hoots, J. K. , Golden, S. A. , Heins, C. , … Shaham, Y. (2018). Volitional social interaction prevents drug addiction in rat models. Nature Neuroscience, 21, 1520–1529. 10.1038/s41593-018-0246-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford, H. A. , Degenhardt, L. , Rehm, J. , Baxter, A. J , Ferrari, A. J. , Erskine, H. E. , … Vos, T. (2013). Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. The Lancet, 382, (9904), 1575–1586. [DOI] [PubMed] [Google Scholar]
- Willcocks, A. L. , & McNally, G. P. (2011). The role of context in re‐acquisition of extinguished alcoholic beer‐seeking. Behavioral nNuroscience, 125, 541–550. 10.1037/a0024100 [DOI] [PubMed] [Google Scholar]
- Wilson, K.‐E. , Limburg, S. , Duggan, M. K. , Lawther, A. J. , Williams, S. J. , Lawrence, A. J. , … Djouma, E. (2018). The galanin receptor‐3 antagonist, SNAP 37889, inhibits cue‐induced reinstatement of alcohol‐seeking and increases c‐Fos expression in the nucleus accumbens shell of alcohol‐preferring rats. Journal of Psychopharmacology, 32, 911–921. 10.1177/0269881118780015 [DOI] [PubMed] [Google Scholar]
- Zaborszky, L. , Leranth, C. S. , & Heimer, L. (1984). Ultrastructural evidence of amygdalofugal axons terminating on cholinergic cells of the rostral forebrain. Neuroscience Letters, 52, 219–225. 10.1016/0304-3940(84)90165-4 [DOI] [PubMed] [Google Scholar]
- Zahm, D. S. (1989). The ventral striatopallidal parts of the basal ganglia in the rat ‐ II. Compartmentalisation of Ventral Pallidal Efferents. Neuroscience, 30, 33–50. [DOI] [PubMed] [Google Scholar]
- Zahm, D. S. , & Brog, J. S. (1992). On the significance of subterritories in the “accumbens” part of the rat ventral striatum. Neuroscience, 4, 751–767. [DOI] [PubMed] [Google Scholar]
- Zahm, D. S. , & Heimer, L. (1990). Two transpallidal pathways originating in the rat nucleus accumbens. The Journal of Comparative Neurology, 302, 437–446. 10.1002/cne.903020302 [DOI] [PubMed] [Google Scholar]
- Zahm, D. S. , Williams, E. , & Wohltmann, C. (1996). Ventral striatopallidothalamic projection: IV. Relative involvements of neurochemically distinct subterritories in the ventral pallidum and adjacent parts of the rostroventral forebrain. The Journal of Comparative Neurology, 364, 340–362. 10.1002/(SICI)1096-9861(19960108)364:240::AID-CNE11%3.0.CO;2-T [DOI] [PubMed] [Google Scholar]


