ABSTRACT
Objective:
The aim of this study was to investigate the effect and risk management of early mobilization in the intensive care unit (ICU) with multidisciplinary collaboration and daily goal planning.
Methods:
Rehabilitation of ICU patients in our hospital between April 1, 2019, and September 30, 2019, was investigated retrospectively. The following factors were evaluated: age and sex of the subjects; diseases; the total number of early mobilization therapy sessions done at a lowered goal level; the clinical course of the step-down sessions; reasons for lowering goal levels that corresponded to the cancellation criteria from the officially issued guidelines of the Japanese Association of Rehabilitation Medicine, the expert consensus on ICU, or other reasons for step down; and the rate of planned goals that were achieved.
Results:
Of the 1908 overall rehabilitation sessions carried out during the period of investigation, 9.6% had the planned level lowered; changes in vital signs accounted for 54.6% of the reasons for lowering the level. Of the step-down sessions, 92.3% corresponded with the cancellation criteria of rehabilitation. Early mobilization in the ICU in accordance with daily goal planning via collaboration within the multidisciplinary team during rounds was accomplished in 90.4% of sessions. No serious mobilization-related adverse events were noted during the study period.
Conclusion:
Early mobilization should be performed with daily goal planning by a multidisciplinary team during rounds and should be governed by the cancellation criteria of rehabilitation.
Keywords: early mobilization, ICU, protocol, risk management
INTRODUCTION
An evidence-based, multicomponent, and interprofessional team management strategy has been developed for optimizing intensive care unit (ICU) recovery and outcomes.1,2) The strategy is called the ABCDEF bundle, which is derived from “Assess pain, Both SAT/SBT, Choice of drugs (analgesia and sedation), Delirium monitoring and management, Early mobility and exercise, and Family engagement and empowerment.” This protocol facilitates the adoption of the Pain, Agitation/Sedation, Delirium, Immobility and Disrupted Sleep Guidelines for comprehensive results in ICU management,3,4,5) recommended by the Society of Critical Care Medicine.
The activities of daily living (ADL) or quality of life of patients after discharge from the ICU remains low, although the survival rate of clinically severe patients in the ICU has increased. Muscle weakness and neuropathy have been recognized as ICU-acquired conditions and are believed to result from sustained immobilization, multiorgan dysfunction, and hyperglycemia, as well as the use of steroids and muscle relaxants.6,7) Needham et al. reported that, after patients have had 4 days of bed rest, it is common for critically severe ICU patients worldwide to be under the care of a multidisciplinary team focused on reducing heavy sedation. Moreover, increasing ICU staffing to include full-time physical and occupational therapists with new consultation guidelines is not uncommon. Using a quality improvement process, ICU delirium, physical rehabilitation, and functional mobility were markedly improved and were associated with a decreased length of stay.8) Schweickert et al. reported that, for patients who started early mobilization within 72 h of intubation in the ICU, the Barthel index score had significantly increased at the time of discharge; they concluded that hyperacute rehabilitation at the ICU was effective in improving ADL function.9)
To conduct early mobilization in the ICU, risk management during rehabilitation is considered particularly important. Complications in ICU patients are profoundly serious and potentially life threatening; these complications include acute respiratory distress, acute exacerbation of chronic respiratory distress, acute cardiac failure after major surgery, and post-cardiopulmonary arrest, among others. Consequently, because priority must be given to improving the function of all organs and controlling the general condition of the patient, early mobilization and active exercise during the early phase are sometimes contraindicated. However, excessive bed rest promotes the development of immobilization syndrome and increases the risk for various complications, and in such cases, early mobilization and active exercise during the early phase are advisable. In the ICU, the patient’s fundamental condition may change drastically, or the intensive care situation may change suddenly; as result, early mobilization and active exercise during the early phase needs to be carefully assessed.
Bailey et al. reported that less than 1% of patients who were admitted to the respiratory intensive care unit and required mechanical ventilation for >4 days suffered activity-related adverse events. Such events included falling to the knees without injury, feeding tube removal, high systolic blood pressure (>200 mm Hg), low systolic blood pressure (<90 mm Hg), and oxygen desaturation (<80%).10) Bourdin reported that an adverse event occurred in 3% of interventions in patients who had been in the ICU for more than 1 week, but none resulted in harmful consequences. These reports gave no clear criteria for starting early mobilization.11)
In 2014, Hodgson et al. developed the expert consensus safety standards on active mobilization, and, recently, several protocols and cancellation criteria have been proposed.12,13) In 2017 in Japan, development of the expert consensus on early mobilization and active exercise in the early phase was begun by the Japanese Society of Intensive Care Medicine.14)
During rehabilitation treatment, the adoption of cancellation criteria is necessary for risk management,15) and there are several cancellation criteria for rehabilitation.16,17) To effectively and safely execute early mobilization by applying the ABCDEF bundle, setting the protocol for cancellation criteria is fundamental. Multidisciplinary rounds were assumed to be necessary for the ABCDEF bundle and were therefore adopted in our hospital.
Recently, barriers to and strategies for early mobilization in the ICU and analysis of outcomes have been reported.18,19,20) However, to our knowledge, studies with protocol and cancellation criteria in Japan that particularly examined the safety and course of early mobilization and active exercise in the early phase in the ICU are limited.21) Consequently, we aimed to investigate the risk management and the specific method for early mobilization and active exercise for ICU patients using protocol and cancellation criteria within multidisciplinary rounds.
SUBJECTS AND METHODS
Hospital Setting
Our hospital is an 814-bed acute phase-specific hospital in Tokyo with a 12-bed semi-closed ICU. Admissions to the ICU are either from the emergency room or from hospital wards. All admissions to the ICU result either from unplanned emergency critical illnesses in outpatients or from planned postoperative or unplanned emergency conditions that develop in the wards.
Daily multidisciplinary rounds were performed every morning from Monday to Friday, and the team members consisted of physicians (chief, ICU, and rehabilitation physicians), physiotherapists, nurses, medical engineers, nutritionists, and pharmacists who collaborated to accomplish early mobilization.
During every morning round, the day’s specific rehabilitation goals were set using the protocol and with consideration of the patient’s condition on that day. Assessment of the patient’s condition included the pain level, respiratory or circulatory conditions, and the scaleRASS (delirium) or Glasgow scale score indicating consciousness and the level of sedation; these factors were used in association with the day’s planned treatments and the previous days’ accomplished mobilization goals.
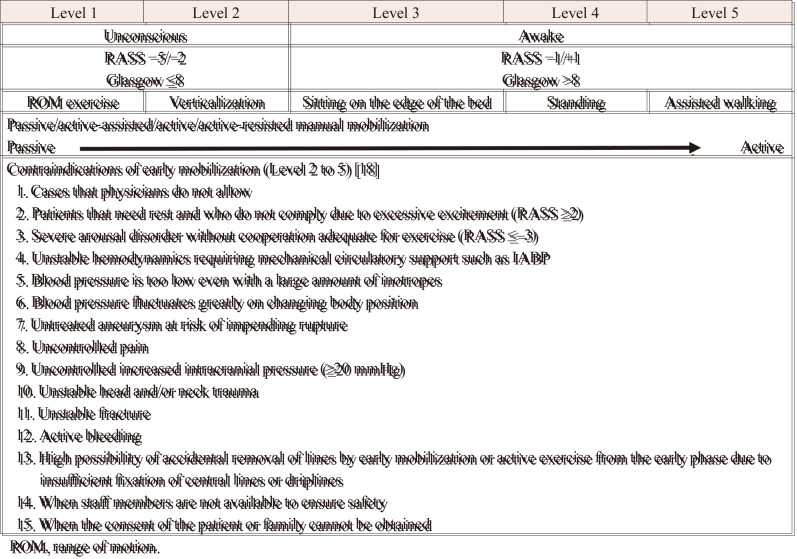
If a patient was unable to follow commands or actively participate in mobilization (RASS score range from −5 to −2 or the Glasgow scale ≤8), the level was set at 1 or 2, according to the patient’s general condition. If the patient was sentient and was able to follow commands (RASS score range from −1 to +1 or the Glasgow scale >8), active mobilization at a level of 3–5 (more than sitting on the edge of the bed) was set based on the patient’s general condition and on the goal accomplished on the previous day. After the goal of the day was set, the sedation level, respiratory mode, and the time of day for administering other treatments were adjusted as much as possible by members of the multidisciplinary team to facilitate accomplishment of the rehabilitation goal.
To execute early mobilization, the protocol is set in accordance with “the contraindication of early mobilization and active exercises from the early phase” established by the expert consensus of the Japanese Society of Intensive Care Medicine (Table 1).14) No physical therapists are assigned to the ICU, so they are brought from the rehabilitation center for each therapy session. The early mobilization and rehabilitation sessions are done with consideration of the cancellation criteria. The cancellation criteria were consistent with “the cancellation criteria in the early mobilization and active exercise in early phase in ICU” proposed in the expert consensus of the Japanese Society of Intensive Care Medicine14) and Guidelines for Safety Management and Promotion prepared by the Clinical Practice Guidelines Committee of the Japanese Association of Rehabilitation Medicine (Tables 2, 3).17)
Table 1. Early mobilization protocol at the Tokyo Medical and Dental University Hospital.
ROM, range of motion.
Table 2. Guidelines for Safety Management and Promotion of the Clinical Practice Guidelines Committee of the Japanese Association of Rehabilitation Medicine17).
|
Cases in which aggressive rehabilitation
should be avoided • Pulse rate at rest ≤40 beats/min or ≥120 beats/min • Systolic blood pressure at rest ≤70 mmHg or ≥200 mmHg • Diastolic blood pressure at rest ≥120 mmHg • Effort angina • Significant bradycardia or tachycardia in patients with atrial fibrillation • Poor cardiovascular hemodynamics in patients immediately after myocardial infarction • Significant arrhythmia • Chest pain at rest • Presence of palpitations, shortness of breath, or chest pain prior to rehabilitation • Dizziness, cold sweats, nausea, etc., in a sitting position • Body temperature at rest ≥38°C • Oxygen saturation at rest ≤90% |
Cases in which rehabilitation should be
canceled • Higher than moderate degree of shortness of breath, dizziness, nausea, angina pectoris, headache, a strong feeling of fatigue, etc.• Heart rate >140 beats/min • Systolic blood pressure on exercise rises by 40 mmHg or more or diastolic blood pressure rises by 20 mmHg or more • Tachypnea (≥30 cycles/min) or shortness of breath • Increased arrhythmia with exercise • Presence of bradycardia • Worsening level of consciousness |
|
Cases in which rehabilitation should be
stopped and may be restarted after recovery • Heart rate exceeds that before exercise by 30%: cancel or change to very light exercise if it does not return to less than 10% before exercise after 2 min of rest • Heart rate >120 beats/min • Ten or more premature ventricular contractions in 1 min • Presence of mild palpitation or shortness of breath |
Cases in which other precautions are
required • Presence of hematuria • Increase in amounts of sputum• Increase in body weight • Presence of fatigue • Loss of appetite or fasting• Worsening edema of the lower extremity |
Table 3. The Cancellation Criteria for Early Mobilization and Active Exercise in the Early Phase in the ICU proposed by the expert consensus of the Japanese Society of Intensive Care Medicine14).
| Category | Elements index | Value of decision criteria or condition | Remarks |
| General Nervous system |
Reaction Expression Consciousness Agitation Voluntary movement of extremities Posture adjustment |
Occurrence of obvious poor reactions Agony expression, the occurrence of pallor or cyanosis The occurrence of mild or more impaired consciousness The occurrence of dangerous behavior The occurrence of limb weakness Rapid increase in assistance Posture cannot be held, fall |
Drowsiness, stupor in response to call |
| Subjective symptoms | Dyspnea Fatigue |
Complaint of sudden dyspnea Appearance of forced breathing Unbearable fatigue Patient wants to stop |
Pneumothorax, PTE Modified Borg scale 5–8 |
| Respiratory system | Respiratory rate SpO2 Respiration pattern Ventilator |
<5 per min or >40 per min <88% Appearance of sudden expiratory or inspiratory effort breathing Desynchronization, bucking |
Except for transient symptoms assess with the findings of airway obstruction, for example by auscultation |
| Circulatory system | Heart rate Electrocardiograms Blood pressure |
Decreased heart rate or bradycardia after starting
exercise <40 bpm or >130 bpm New rhythm disorders Suspected myocardial ischemia Systolic blood pressure <65 mmHg or >180 mmHg 20% decrease of systolic or diastolic blood pressure Mean atrial pressure >110 mmHg |
Except for transient symptoms |
| Devices | Artificial airway Nasogastric tube, central venous catheter Chest drain, wound drain, bladder catheter |
Risks or occurrences of accidental removal | |
| Others | Patient refusal Action for suspension Suggestion of active bleeding Surgical wound condition |
Appearance of drain waste Risk of wound opening |
Patients
To evaluate the effectiveness of early mobilization with daily multidisciplinary rounds in promoting patient recovery after critical illness, all consecutive ICU patients aged at least 18 years who were treated between April 1, 2019, and September 31, 2019, were assessed. We extracted and analyzed the following patient data: age, sex, category of rehabilitation, type of surgery, intubation, assisted circulation [including percutaneous cardiopulmonary support (PCPS), extra-corporeal membrane oxygenation (ECMO), intra-aortic balloon pump (IABP), and ventricular assist device (VAD)], the planned goal of early mobilization established on the round, the current level of early mobilization, the intubation rates for each level, the number of sessions stepped down from the planned goal, the reason why the goal was not achieved, the number of sessions stepped down according to the cancellation criteria, the planned goal for the next day after the step down, the number of stepped-up sessions that needed to be lowered according to the cancellation criteria, the planned goal for the following day after the step down, and the rate of level up for the next day.
Data Analyses and Statistical Tests
The median age of the step-down patients in each category was assessed (Table 4). The Kruskal-Wallis test was used to compare the ages among the categories. The chi-squared test was used to compare the step-down rates among the categories. The chi-squared test was also used to compare the step-down rates among the planned levels (Table 5).
Table 4. Patient characteristics.
| Sessions (n) |
Patients (n) |
Sex | Age (years) |
ICU stay (days) |
Intubation (n, %) |
Assisted circulation (PCPS, VAD, ECMO, IABP) (n, %) |
Step-down sessions (n, %) |
||||||||||
| M/F | Median | Range | Pa | Median | Range | n | % | n | % | n | % | Pb | |||||
| Surgery | Cardiovascular | 691 | 107 | 70 | 37 | 70 | 21–89 | P<0.01* | 5 | 2–117 | 171 | 24.7 | 47 | 6.80 | 76 | 11a | P<0.01* |
| Brain | 382 | 150 | 80 | 70 | 63.5 | 20–81 | 2 | 2–23 | 133 | 34.8 | 0 | 0 | 36 | 9.4 | |||
| Liver/gastrointestinal | 227 | 60 | 49 | 11 | 74.5 | 42–89 | 3 | 2–19 | 95 | 41.9 | 0 | 0 | 20 | 8.8 | |||
| Respiratory | 64 | 22 | 18 | 4 | 68.5 | 46–81 | 2 | 2–38 | 37 | 27.8 | 0 | 0 | 4 | 6.2 | |||
| Others (orthopedic, urologic gynecologic, vascular) |
122 | 34 | 21 | 13 | 69 | 33–88 | 2 | 2–28 | 55 | 45.1 | 0 | 0 | 7 | 5.8 | |||
| Total | 1486 | 373 | 238 | 135 | 69 | 21–89 | 3 | 2–117 | 491 | 33.0 | 47 | 3.16 | 143 | 9.6 | |||
| Internal medicine | Circulation | 143 | 31 | 19 | 12 | 74 | 31–86 | 3 | 2–31 | 74 | 51.8 | 17 | 11.9 | 19 | 13.2 | ||
| Respiratory | 83 | 6 | 5 | 1 | 66.5 | 51–79 | 6 | 2–34 | 30 | 36.1 | 2 | 2.4 | 12 | 14.5 | |||
| Others (e.g., gastrointestinal, collagen, renal, neurological, blood) |
196 | 22 | 12 | 10 | 69 | 19–83 | 7 | 3–29 | 81 | 41.3 | 0 | 0 | 9 | 4.6 | |||
| Total | 422 | 59 | 36 | 23 | 70.5 | 19–86 | 4.5 | 2–34 | 185 | 43.8 | 19 | 4.50 | 40 | 9.5 | |||
| Total | 1908 | 432 | 274 | 158 | 70 | 19–89 | 3 | 2–117 | 686 | 40.0 | 66 | 3.46 | 183 | 9.6 | |||
aKruskal-Wallis test to compare the ages among disease categories
bChi-squared test to compare the step-down rates among disease categories
*Significantly different among disease categories
Table 5. The course of early mobilization.
| Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Total | P | ||
| ROM exercise | Verticalization | Sitting on the edge of the bed | Standing | Walking | ||||
| Planned sessions (n) | 848 | 379 | 197 | 222 | 262 | 1908 | P<0.01a | |
| Patients accomplished the planned level (n (%)) | 848 (100) | 334 (89.1) | 162 | 184 | 196 | 1725 | ||
| (82.2) | (82.9) | (74.8) | (90.4) | |||||
| Sessions actually doneb (n) | 901 | 388 | 178 | 246 | 196 | 1908 | ||
| Intubation (n) | 450 | 80 | 38 | 18 | 0 | 686 | ||
| Assisted circulation (PCPS, VAD, ECMO, IABP, etc.) (n) | 70 | 30 | 10 | 35 | 43 | 188 | ||
| Time of decision of step down | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Total | ||
| Sessions (n (%)) | 45 | 35 | 37 | 66 | 183 | |||
| At the start of rehabilitation (n (%)) | 45 (100) | 28 (80) | 15 (40.5) | 10 (15.2) | 98 (53.6) | |||
| During rehabilitation (n (%)) | 0 (0) | 7 (20) | 22 (59.5) | 56 (84.8) | 85 (46.4) | |||
| Reason for step down | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Total | ||
| under criteria | ||||||||
| Sessions (n (%)) | 42 (93.3) | 34 (97.1) | 33 (89.2) | 60 (90.1) | 169 (92.3) | |||
| Vital sign(+) | Blood pressure (n) | 13 | 7 | 14 | 22 | 56 | ||
| Tachycardia, bradycardia, arrhythmia (n) | 7 | 4 | 3 | 3 | 17 | |||
| Respiration (n) | 3 | 4 | 3 | 0 | 10 | |||
| O2 saturation (n) | 0 | 7 | 3 | 3 | 13 | |||
| Fever (n) | 1 | 0 | 0 | 3 | 4 | |||
| Total (n (%)) | 24 | 22 | 23 | 31 | 100 (54.6) | |||
| Vital sign(–) | Consciousness, delirium (n) | 4 | 3 | 3 | 4 | 14 | ||
| General fatigue (n) | 0 | 0 | 1 | 16 | 17 | |||
| Nausea, vomiting (n) | 0 | 0 | 4 | 7 | 11 | |||
| Convulsion (n) | 8 | 1 | 0 | 0 | 9 | |||
| Bleeding risk (n) | 3 | 1 | 0 | 0 | 4 | |||
| Chest pain or other pain (n) | 0 | 3 | 0 | 2 | 5 | |||
| Device (n) | 3 | 4 | 2 | 0 | 9 | |||
| Total (n (%)) | 18 | 12 | 10 | 29 | 69 (37.7) | |||
| Others | ||||||||
| Cerebrospinal fluid leak (n) | 2 | 0 | 0 | 0 | 2 | |||
| Graft ischemia (n) | 1 | 1 | 2 | 0 | 4 | |||
| Muscle weakness (n) | 0 | 0 | 2 | 6 | 8 | |||
| Total (n (%)) | 3 | 1 | 4 | 6 | 14 (7.7) | |||
| The level of the previous day | Level 1 | Level 2 | Level 3 | Level 4 | Level 5 | Total | ||
| The average of next day’s goal | Total (level) | 2.65 | 2.61 | 3.58 | 4.57 | 4.93 | 3.23 | |
| (1–5) | (1–5) | (1–5) | (1–5) | (1–5) | (1–5) | |||
| Step-down session (level) | 2.02 | 3.44 | 3.78 | 5 | 3.44 | |||
| (1–5) | (1–5) | (1–5) | (1–5) | (1–5) | ||||
| Next day: level up(+/–) | 50/16 | 7/38 | 4/14 | 54/0 | 156/27 | |||
| Total | 66 | 45 | 18 | 54 | 183 |
aChi-squared test to compare the step-down rate among the levels
bAt some mobilization levels, the number of sessions actually done was higher than the planned number because drop-down sessions were added.
All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of the R Commander designed to add statistical functions frequently used in biostatistics.22) The level of statistical significance was set at P <0.05.
This study was conducted with the approval of the Research Ethics Committee at the Tokyo Medical and Dental University (M2018-073) and in accordance with the World Medical Association Declaration of Helsinki. Written informed consent was obtained from all individual participants included in the study.
RESULTS
Within the study period, 1908 early mobilization therapy sessions involving 432 patients were investigated (Table 4).
The ages among the disease categories were significantly different (P <0.01). The patients in the cardiovascular surgery group, the liver and gastrointestinal surgery group, and the circulatory category were older than the patients in the brain surgery group. The ages of the stepped-down patients of each category were not significantly different. The step-down rate was significantly different among the disease categories (P <0.01). The step-down rates of the cardiovascular surgery and the circulatory and respiratory categories were high.
In 1725 sessions (90.4%) the planned goal was accomplished, whereas in 183 sessions (9.6%) the planned level was lowered (Table 5). Early mobilization, i.e., more than sitting up in bed, was accomplished in 630 sessions (33.0%). The step-down rates significantly increased at the higher levels.
The time of step down was decided either at the start of the rehabilitation session or during the session. Judgment of whether to step down was performed at the start of rehabilitation for the lower levels, whereas for the higher levels, judgment was done during the session.
Of the step-down sessions, 92.3% were attributed to events corresponding to the cancellation criteria of rehabilitation, and 54.6% of discontinuations were due to vital sign factors. Reasons other than the cancellation criteria accounted for 7.7% of the sessions where step down was deemed appropriate; these reasons included new conditions necessitating immobilization, such as cerebrospinal fluid leakage, graft ischemia, or muscle weakness.
The following day’s rehabilitation level was increased based on the current level, and it was increased at all levels, including for the step-down sessions. In this investigation period, no serious adverse events related to rehabilitation in the ICU were reported.
DISCUSSION
To reduce the incidence of post-ICU syndrome, early mobilization in the ICU is recommended in the ABCDEF bundle, and we attempted to conduct early mobilization in accordance with the cancellation criteria and with team collaboration during the multidisciplinary round.
By setting goals daily, during the 6-month study period, we estimated that most planned rehabilitation sessions (90.4%) in the ICU were accomplished to the level presumed to be possible during the round. Moreover, no serious adverse events occurred during performance of those therapies. However, the planned goal levels were lowered in 9.6% of sessions. Reasonable goals were set during the multidisciplinary round using the protocol according to the condition of the patient on the day, the day’s planned treatments, and the previous day’s accomplished goals.
Leveling down of the rehabilitation was decided either at the start or during rehabilitation sessions. In some cases, the general condition of the patient had changed between the multidisciplinary assessment in the morning and the time of initiation of rehabilitation. In other cases, the preparations for rehabilitation planned by the multidisciplinary members on the round, such as the reduction of sedation, pain management, or a change of respiratory mode, had to be delayed, and, therefore, the assessment to level down was made at the beginning of the therapy session. Additionally, lowering of the level was decided either according to the cancellation criteria or for other reasons. In six cases, new reasons for immobilization occurred: two cases of cerebrospinal fluid leakage and four cases of graft ischemia. In the other cases, during the rehabilitation therapies, the judgment to discontinue was done according to changes in vital signs or the occurrence of subjective symptoms such as general fatigue or nausea.
The rate of lowered sessions was significantly different depending on the general level. Overall, 92.3% of the reasons for the decision to lower the level of early mobilization were associated with conditions corresponding to the cancellation criteria of rehabilitation. The setting of cancellation criteria is said to be vital in rehabilitation therapy for acute stage patients.15) Such criteria are particularly important when determining whether to continue with therapy or whether a step up to the next level is safe and to address risk management against excessive exercise stress during rehabilitation in the ICU.
The level was lowered in nine sessions due to the cancellation criteria associated with newly added devices or delayed removal of devices after the morning round or because treatment did not permit patients to verticalize or to leave the bed because of IABP, ECMO, or continuous hemodialysis from an inguinal blood access. However, it should be noted that in the ABCDEF bundle in the ICU, the patient is not always prohibited to verticalize or to leave the bed, even when supported by PCPS, ECMO, IABP, or continuous hemodiafiltration or if a Swan-Ganz catheter is being used.23,24) In patients with implantable VADs, early mobilization is necessary to obtain higher levels of ADL, thereby facilitating the transition to waiting for a heart transplantation when the patient is at home.25,26) However, the conditions vary, and individual consideration is necessary for placing or stabilizing blood access to avoid kinking or disconnection. Furthermore, for patients with unstable circulation, conducting early mobilization has negative effects and poses new risks to the patient’s general condition and overall treatment.
Additionally, the rehabilitation level should be planned according to the characteristics and posttreatment of the disease. Overall, lowering of the planned level was done based on reasons other than the cancellation criteria in 7.7% of sessions. The levels of six sessions were lowered because bed rest was necessary due to changes in posttreatment for the original disease, such as cerebrospinal fluid leakage or graft ischemia. The posttreatment can also be changed during the course of the disease. Treatments were regularly altered depending on the patient’s condition, and judgment was consistently required that not only considered the patient’s general condition but also the specific disease symptoms and their corresponding treatments.
Muscle weakness was another reason for lowering the mobilization level. In nine patients at level 4 or 5, standing or walking was impossible because of muscle weakness, although their general condition was good. Six were post-operative cases (five intestine and one brain surgery), and three were internal medicine cases (one circulation and two others). The step down was appropriate to mitigate against falls or excessive exercise stress. The acute progression of muscle weakness is frequently seen in the ICU, and muscle strength should be considered when deciding whether to level up the day’s therapy. Moreover, the judgment for leveling down should be done considering fall risk management in patients whose muscle strength is inadequate for higher level mobilization even with assistance.
A total of 32.5% of early mobilization rehabilitation sessions involved more than sitting up in bed. In general, the rate of early mobilization or its course depends on the severity of the conditions of patients in the ICU and the particular hospital. The levels of the rehabilitation therapies on the following day were increased compared with the previous day even in the step-down sessions. Trials of early mobilization can be planned, performed, and evaluated according to the protocol. Although the progress of the course in early mobilization in the ICU depends on the severity of the patient’s condition, we could evaluate early mobilization individually and comprehensively.
This study has several limitations. First, we do not have comparative data for this study, although using the protocol made it easy to set definite, standardized, and appropriate goals via assessment of the patient’s level of consciousness or general condition before the rehabilitation therapy and to prepare patients effectively based on their condition before initiation of the actual rehabilitation therapy. These preparations included consideration of pain control, reduction of sedation, time adjustment of other treatments, or even the acquisition of compatible factors to evaluate the rehabilitation therapies themselves. Second, the investigation period was limited and covered 6 months only (from April to October) and did not include the winter or colder seasons. Finally, the diseases included varied widely: further investigation of specific diseases is necessary.
In conclusion, a relatively low number (9.6%) of the overall rehabilitation sessions in the ICU were stepped down during the investigation period, and 92.3% of the reasons for stepping down were associated with the cancellation criteria. Furthermore, all the reasons corresponded to cancellation of sessions during rehabilitation therapy. No serious adverse events were observed during the 6-month study period. Therefore, this study suggests that early mobilization in the ICU can be performed effectively and safely with consideration of the cancellation criteria and in accordance with specified daily goals as determined by collaboration among the multidisciplinary team members in accordance with appropriate protocols.
ACKNOWLEDGMENTS
We sincerely thank Dr. Hidenobu Shigemitsu, Professor of Intensive Care Medicine, Tokyo Medical and Dental University, who kindly checked the back translation of the cancellation criteria of rehabilitation in the Guidelines for Safety Management and Promotion of the Japanese Association of Rehabilitation Medicine. We also thank Ms. Masako Akiyama, URA, Research Administration Division, Tokyo Medical and Dental University, who provided a statistical review and comments on our data.
Footnotes
CONFLICTS OF INTEREST: The authors declare that there are no conflicts of interest regarding the publication of this article.
REFERENCES
- 1.Pandharipande P,Banerjee A,McGrane S,Ely EW: Liberation and animation for ventilated ICU patients: the ABCDE bundle for the back-end of critical care. Crit Care 2010;14:157. 10.1186/cc8999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balas MC,Vasilevskis EE,Olsen KM,Schmid KK,Shostrom V,Cohen MZ,Peitz G,Gannon DE,Sisson J,Sullivan J,Stothert JC,Lazure J,Nuss SL,Jawa RS,Freihaut F,Ely EW,Burke WJ: Effectiveness and safety of the awakening and breathing coordination, delirium monitoring/management, and early exercise/mobility bundle. Crit Care Med 2014;42:1024–1036. 10.1097/CCM.0000000000000129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jacobi J,Fraser GL,Coursin DB,Riker RR,Fontaine D,Wittbrodt ET,Chalfin DB,Masica MF,Bjerke HS,Coplin WM,Crippen DW,Fuchs BD,Kelleher RM,Marik PE,Nasraway SA Jr,Murray MJ,Peruzzi WT,Lumb PD, Task Force of the American College of Critical Care Medicine (ACCM) of the Society of Critical Care Medicine (SCCM), American Society of Health-System Pharmacists (ASHP), American College of Chest Physicians: Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002;30:119–141. 10.1097/00003246-200201000-00020 [DOI] [PubMed] [Google Scholar]
- 4.Barr J,Fraser GL,Puntillo K,Ely EW,Gélinas C,Dasta JF,Davidson JE,Devlin JW,Kress JP,Joffe AM,Coursin DB,Herr DL,Tung A,Robinson BR,Fontaine DK,Ramsay MA,Riker RR,Sessler CN,Pun B,Skrobik Y,Jaeschke R, American College of Critical Care Medicine: Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 2013;41:263–306. 10.1097/CCM.0b013e3182783b72 [DOI] [PubMed] [Google Scholar]
- 5.Devlin JW,Skrobik Y,Gélinas C,Needham DM,Slooter AJ,Pandharipande PP,Watson PL,Weinhouse GL,Nunnally ME,Rochwerg B,Balas MC,van den Boogaard M,Bosma KJ,Brummel NE,Chanques G,Denehy L,Drouot X,Fraser GL,Harris JE,Joffe AM,Kho ME,Kress JP,Lanphere JA,McKinley S,Neufeld KJ,Pisani MA,Payen JF,Pun BT,Puntillo KA,Riker RR,Robinson BR,Shehabi Y,Szumita PM,Winkelman C,Centofanti JE,Price C,Nikayin S,Misak CJ,Flood PD,Kiedrowski K,Alhazzani W: Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 2018;46:e825–e873. 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 6.Stanojcic M,Finnerty CC,Jeschke MG: Anabolic and anticatabolic agents in critical care. Curr Opin Crit Care 2016;22:325–331. 10.1097/MCC.0000000000000330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wischmeyer PE: Are we creating survivors…or victims in critical care? Delivering targeted nutrition to improve outcomes. Curr Opin Crit Care 2016;22:279–284. 10.1097/MCC.0000000000000332 [DOI] [PubMed] [Google Scholar]
- 8.Needham DM,Davidson J,Cohen H,Hopkins RO,Weinert C,Wunsch H,Zawistowski C,Bemis-Dougherty A,Berney SC,Bienvenu OJ,Brady SL,Brodsky MB,Denehy L,Elliott D,Flatley C,Harabin AL,Jones C,Louis D,Meltzer W,Muldoon SR,Palmer JB,Perme C,Robinson M,Schmidt DM,Scruth E,Spill GR,Storey CP,Render M,Votto J,Harvey MA: Improving long-term outcomes after discharge from intensive care unit. Crit Care Med 2012;40:502–509. 10.1097/CCM.0b013e318232da75 [DOI] [PubMed] [Google Scholar]
- 9.Schweickert WD,Pohlman MC,Pohlman AS,Nigos C,Pawlik AJ,Esbrook CL,Spears L,Miller M,Franczyk M,Deprizio D,Schmidt GA,Bowman A,Barr R,McCallister KE,Hall JB,Kress JP: Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet 2009;373:1874–1882. 10.1016/S0140-6736(09)60658-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bailey P,Thomsen GE,Spuhler VJ,Blair R,Jewkes J,Bezdjian L,Veale K,Rodriquez L,Hopkins RO: Early activity is feasible and safe in respiratory failure patients. Crit Care Med 2007;35:139–145. 10.1097/01.CCM.0000251130.69568.87 [DOI] [PubMed] [Google Scholar]
- 11.Bourdin G,Barbier J,Burle JF,Durante G,Passant S,Vincent B,Badet M,Bayle F,Richard JC,Guérin C: The feasibility of early physical activity in intensive care unit patients: a prospective observational one-center study. Respir Care 2010;55:400–407. [PubMed] [Google Scholar]
- 12.Hodgson CL,Stiller K,Needham DM,Tipping CJ,Harrold M,Baldwin CE,Bradley S,Berney S,Caruana LR,Elliott D,Green M,Haines K,Higgins AM,Kaukonen KM,Leditschke IA,Nickels MR,Paratz J,Patman S,Skinner EH,Young PJ,Zanni JM,Denehy L,Webb SA: Expert consensus and recommendations on safety criteria for active mobilization of mechanically ventilated critically ill adults. Crit Care 2014;18:658. 10.1186/s13054-014-0658-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sommers J,Engelbert RH,Dettling-Ihnenfeldt D,Gosselink R,Spronk PE,Nollet F,van der Schaaf M: Physiotherapy in the intensive care unit: an evidence-based, expert driven, practical statement and rehabilitation recommendations. Clin Rehabil 2015;29:1051–1063. 10.1177/0269215514567156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Evidence-based expert consensus for early rehabilitation in the intensive care unit. Nihon Shuchu Chiryo Igakukai Zasshi 2017;24:255–303. (in Japanese) 10.3918/jsicm.24_255 [DOI] [Google Scholar]
- 15.Sakai T,Jinno T,Hoshino C,Okawa A: Cancellation criteria of acute rehabilitation: rehabilitation risk management. Prog Rehabil Med 2019;4:n/a 10.2490/prm.20190013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doi Y: Risk and countermeasures. Medicina (B Aires) 1976;138:168. [Google Scholar]
- 17.Guidelines for Safety Management and Promotion in Rehabilitation Medicine. Japan Rehabilitation Medical Society Clinical Practice Guidelines Committee. Ishiyaku Syuppan, 2006.
- 18.Paton M,Lane R,Hodgson CL: Early mobilization in the intensive care unit to improve long-term recovery. Crit Care Clin 2018;34:557–571. 10.1016/j.ccc.2018.06.005 [DOI] [PubMed] [Google Scholar]
- 19.Bakhru RN,McWilliams DJ,Wiebe DJ,Spuhler VJ,Schweickert WD: Intensive care unit structure variation and implications for early mobilization practices. An international survey. Ann Am Thorac Soc 2016;13:1527–1537. 10.1513/AnnalsATS.201601-078OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dubb R,Nydahl P,Hermes C,Schwabbauer N,Toonstra A,Parker AM,Kaltwasser A,Needham DM: Barriers and strategies for early mobilization of patients in intensive care units. Ann Am Thorac Soc 2016;13:724–730. 10.1513/AnnalsATS.201509-586CME [DOI] [PubMed] [Google Scholar]
- 21.Liu K,Ogura T,Takahashi K,Nakamura M,Ohtake H,Fujiduka K, Abe E Oosaki H, Miyazaki D, Suzuki H, Nishikimi M, Lefor AK, Mato T. The safety of a novel early mobilization protocol conducted by ICU physicians: a prospective observational study. J Intensive Care 2018;6:10. doi: . eCollection 2018. 10.1186/s40560-018-0281-0 [DOI] [PMC free article] [PubMed]
- 22.Kanda Y: Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013;48:452–458. 10.1038/bmt.2012.244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiagarajan RR,Teele SA,Teele KP,Beke DM: Physical therapy and rehabilitation issues for patients supported with extracorporeal membrane oxygenation. J Pediatr Rehabil Med 2012;5:47–52. 10.3233/PRM-2012-0195 [DOI] [PubMed] [Google Scholar]
- 24.Damluji A,Zanni JM,Mantheiy E,Colantuoni E,Kho ME,Needham DM: Safety and feasibility of femoral catheters during physical rehabilitation in the intensive care unit. J Crit Care 2013;28:535.e9–535.e15. 10.1016/j.jcrc.2013.01.006 [DOI] [PubMed] [Google Scholar]
- 25.Morrone TM,Buck LA,Catanese KA,Goldsmith RL,Cahalin LP,Oz MC,Levin HR: Early progressive mobilization of patients with left ventricular assist devices is safe and optimizes recovery before heart transplantation. J Heart Lung Transplant 1996;15:423–429. [PubMed] [Google Scholar]
- 26.Guidelines for device therapy: implantable left ventricular assist device for patients with severe heart failure. (JCS/JSCV 2013) www.j-circ.or.jp/guideline/pdf/JCS2013_kyo_h.pdf