Abstract
Purpose
EGFR-mutation (EGFR-mt) is a major oncogenic driver mutation in lung adenocarcinoma (ADC) and is more often observed in Asian population. In lung ADC, some radiomics parameters of FDG PET have been reported to be associated with EGFR-mt. Here, the associations between EGFR-mt and PET parameters, particularly asphericity (ASP), were evaluated in Asian population.
Methods
Lung ADC patients who underwent curative surgical resection as the first treatment were retrospectively enrolled. EGFR mutation was defined as exon 19 deletion and exon 21 point mutation and was evaluated using surgical specimens. On FDG PET, image parameters of maximal standardized uptake value (SUVmax), metabolic tumor volume (MTV), total lesion glycolysis (TLG), and ASP were obtained. The parameters were compared between EGFR-mt and wild type (EGFR-wt) groups, and the relationships between these PET parameters and EGFR-mt were evaluated.
Results
A total of 64 patients (median age 66 years, M:F = 34:30) were included in the analysis, and 29 (45%) patients showed EGFR-mt. In EGFR-mt group, all the image parameters of SUVmax, MTV, TLG, and ASP were significantly lower than in EGFR-wt group (all adjusted P < 0.050). In univariable logistic regression, SUVmax (P = 0.003) and ASP (P = 0.010) were significant determinants for EGFR-mt, whereas MTV was not (P = 0.690). Multivariate analysis revealed that SUVmax and ASP are independent determinants for EGFR-mt, regardless of inclusion of MTV in the analysis (P < 0.05).
Conclusion
In Asian NSCLC/ADC patients, SUVmax, MTV, and ASP on FDG PET are significantly related to EGFR mutation status. Particularly, low SUVmax and ASP are independent determinants for EGFR-mt.
Electronic supplementary material
The online version of this article (10.1007/s13139-020-00646-7) contains supplementary material, which is available to authorized users.
Keywords: FDG PET/CT, Lung cancer, EGFR mutation, Radiogenomics, Asphericity
Introduction
Mutation of epidermal growth factor receptor (EGFR) is one of the major oncogenic driver mutations in non-small cell lung cancer (NSCLC), specifically adenocarcinoma (ADC). EGFR-mutation (EGFR-mt) is particularly important in Asians, because the prevalence of EGFR-mt in Asian-pacific patients with NSCLC/ADC is up to 47%, which is much higher than those of other ethnicities [1]. Systemic treatment with EGFR-targeting tyrosine kinase inhibitors (TKI) such as gefitinib and erlotinib has demonstrated effective survival gain, and currently, it is the first-line chemotherapy agent in advanced NSCLC/ADC with EGFR-mt. Additionally, EGFR-mt is an effective prognostic marker regardless of EGFR-TKI use [2].
Radiogenomics is a study for relationships between genetic status of a tumor and radiomics parameters. With radiogenomics knowledge, genetic status may be expected from image findings, and role of a gene can be comprehensively understood based on radiological phenotypic expression. On 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT), standardized uptake value (SUV) and metabolic tumor volume (MTV) are well-known image parameters, which reflect glucose metabolism and metabolic tumor burden. Recently, asphericity (ASP) that describes the shape of a tumor has been suggested as an effective radiomics parameter. ASP quantifies deviation of a tumor volume from the spherical shape [3] and has been shown to be effective in prediction of survival in NSCLC [4]. Furthermore, ASP on FDG PET is a radiomics parameter relatively robust to motion artifact [5].
In recent studies, ASP as well as maximum SUV (SUVmax) and MTV showed positive correlations with EGFR status [3, 6]. However, there are conflicting results on the relationship between SUVmax and EGFR status [7–9]. Thus, further consolidative studies are required on the relationships between EGFR status and radiomics parameters of FDG PET, particularly in Asian-pacific population.
The aim of this study is to investigate the relationship between EGFR mutation and radiomics parameters of FDG PET including ASP and SUVmax in Korean patients with NSCLC/ADC.
Materials and Methods
Patients
Among patients who received surgical resection of lung cancer between July 2013 and February 2015, those who met the following inclusion criteria were retrospectively enrolled: pathologically confirmed ADC, initial curative surgical resection, preoperative FDG PET/CT for staging work-up, and available data on EGFR status from surgical specimens. Patients with pathologic T1 stage were excluded because metabolic characteristics measured on FDG PET/CT can be affected by partial volume effect. Tumors with SUVmax < 2.5 were also excluded, due to difficulty in delineating MTV. EGFR mutation in exons 18, 19, 20, or 21 was evaluated by either peptide nucleic acid clamping or direct DNA sequencing. Patients with exon 19 deletion or exon 21 point mutation were regarded as EGFR-mt, and otherwise, EGFR-wild type (EGFR-wt). The present study was approved by the Institutional Review Board of Seoul National University Hospital (No. 2001-146-1097) and informed consent was waived.
FDG PET/CT Image Acquisition and Analysis
After fasting more than 4 h, patients were intravenously injected with 5.18 MBq/kg of FDG. After 1 h, PET image was acquired from the skull base to the proximal thigh using dedicated PET/CT scanners (Biograph mCT 40 or mCT 64, Siemens, Erlangen, Germany) for 1 min per bed. A Gaussian filter (FWHM 5 mm) was applied to reduce noise, and images were reconstructed using an ordered-subset expectation maximization algorithm (2 iterations and 21 subsets). Fusion PET and CT images were visually reviewed by two experienced nuclear medicine physicians for overall image quality and disease status.
For tumor delineation and PET parameter calculation, a freeware image analysis tool was used (CGITA v.1.4, Chang-Gung Memorial Hospital, Taiwan). Tumor volumes were delineated using absolute threshold method, with a cutoff value of SUV 2.5, based on previous reports [10–14]. From the delineated tumor volume, SUVmax and MTV were measured, and total lesion glycolysis (TLG) was calculated by multiplying MTV and average SUV. ASP was defined as follows [4, 15, 16]:
where S and V are surface and volume of MTV, respectively.
Statistics
Values are expressed in mean ± standard deviation. A commercial software package was used for statistical analysis (MedCalc v.19.1, MedCalc Software bv, Ostend, Belgium). Mann-Whitney U test was used to compare PET parameters between groups, and Bonferroni correction was used to adjust false discovery rate (FDR) on multiple comparison. Correlations between ASP and other parameters were analyzed by Pearson correlation coefficient. To find significant determinants for EGFR mutation, univariable and multivariable logistic regression tests were used, after patients were categorized by the optimal cutoff values obtained from receiver-operating characteristic (ROC) curve analyses. Two-tailed P value of 0.05 was regarded as statistically significant. Multiple comparison correction was performed with controlling false discovery rate smaller than 0.05.
Results
Patients
Initially, 99 patients who met the inclusion criteria were retrieved from our patient database. Among them, 64 patients (M:F = 34:30, median age 66 years, range 34–79 years) were finally included in the analysis by excluding 35 patients who had T1 tumors. Patient demographic data are summarized in Table 1. Twenty-five (39%) patients had smoking history, and the other 39 (61%) patients were never-smokers. EGFR-mt were observed in 29 (45%) patients, of which 10 (34%) were exon 19 deletion and 19 (66%) were exon 21 point mutation. There were no significant differences of smoking history and T-staging between EGFR-wt and EGFR-mt groups (Table 1). The tumor diameters of EGFR-mt and EGFR-wt groups were 3.2 ± 0.7 cm and 4.0 ± 1.8 cm, respectively (P = 0.025, Table 2).
Table 1.
Patient characteristics
| Characteristics | Overall | EGFR-mt | EGFR-wt |
|---|---|---|---|
| N | 64 | 29 | 35 |
| Sex | |||
| Male | 34 (53%) | 16 (55%) | 18 (51%) |
| Female | 30 (47%) | 13 (45%) | 17 (49%) |
| Smoking | |||
| Current smoker | 7 (11%) | 2 (7%) | 5 (14%) |
| Ex-smoker | 18 (28%) | 7 (24%) | 11 (31%) |
| Never-smoker | 39 (61%) | 20 (69%) | 19 (54%) |
| T-stage | |||
| T2a | 40 (63%) | 22 (76%) | 18 (51%) |
| T2b | 8 (13%) | 1 (3%) | 7 (20%) |
| T3 | 13 (20%) | 5 (17%) | 8 (23%) |
| T4 | 3 (5%) | 1 (3%) | 2 (6%) |
*Statistically significant (P < 0.05)
Table 2.
PET parameters according to EGFR mutation
| Parameters | EGFR-mt | EGFR-wt | P |
|---|---|---|---|
| Tumor size | 3.2 ± 0.7 | 4.0 ± 1.8 | 0.025 |
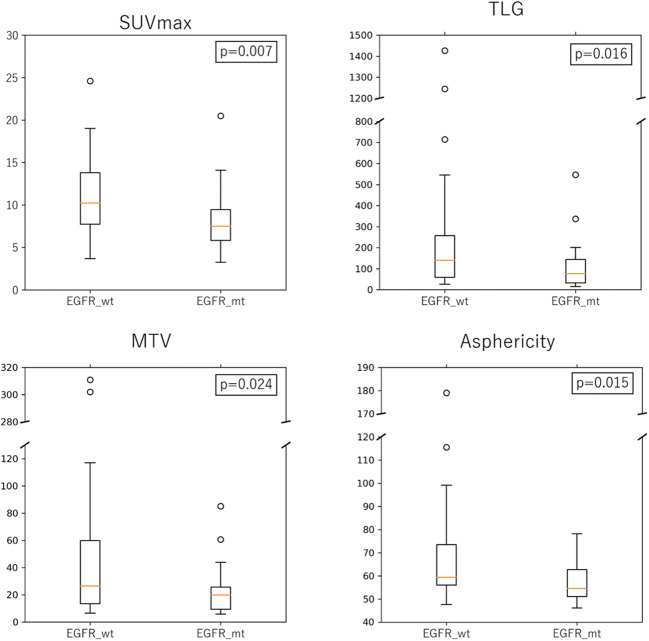
| SUVmax | 8.1 ± 3.7 | 11.0 ± 4.7 | 0.007 |
| MTV | 22.7 ± 17.8 | 50.5 ± 69.6 | 0.024 |
| TLG | 106.0 ± 111.1 | 269.1 ± 348.5 | 0.016 |
| ASP | 57.0 ± 8.4 | 68.6 ± 25.0 | 0.015 |
Relationship between PET Parameters and EGFR Mutation
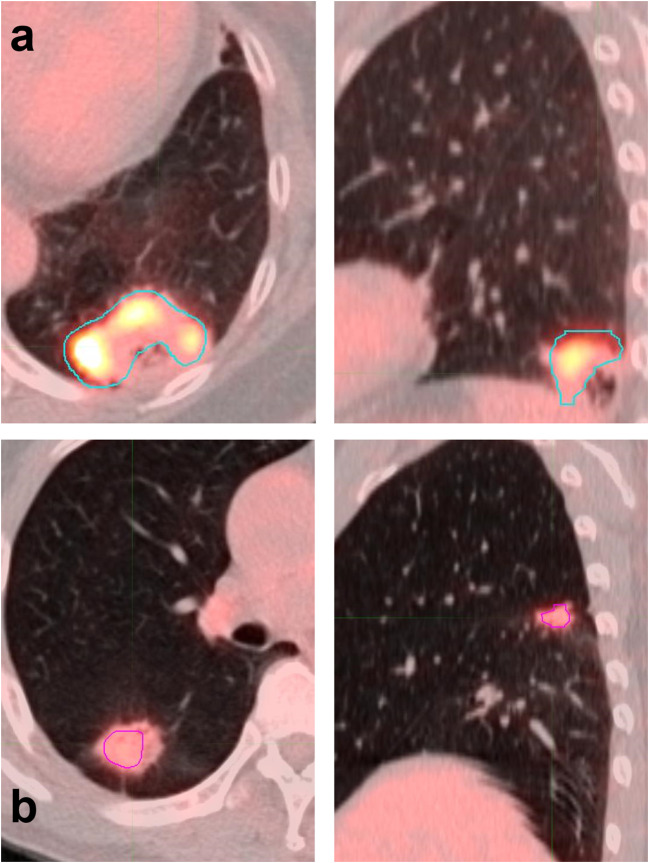
In overall patients, SUVmax, MTV, TLG, and ASP were 9.6 ± 4.5, 37.9 ± 54.3 mL, 195.2 ± 27.8 and 63.3 ± 20.1, respectively. There was no significant difference in any of the tested PET parameters between EGFR mutations of exon 19 deletion and exon 21 mutation (Supplemental Fig. 1). All the tested four parameters were lower in EGFR-mt group than in EGFR-wt group (Fig. 1) and the differences were statistically significant (P = 0.007, 0.024, 0.016, and 0.015, respectively; Table 2). In Bonferroni correction, all the parameters remained significant (all FDR < 0.05). With the optimal cutoff values determined on ROC curve analysis, sensitivities and specificities for predicting EGFR-mt were 79.3% and 57.1% for SUVmax, 75.9% and 51.4% for MTV, and 69.0% and 52.9% for ASP, respectively. Representative PET/CT images of each group are shown in Fig. 2.
Fig. 1.
The levels of PET parameters of (SUVmax, MTV, TLG, and ASP) of EGFR-wt and EGFR-mt groups, respectively
Fig. 2.
Two representative PET/CT scans of EGFR-wt and EGFR-mt patients. (a) EGFR-wt patient with SUVmax of 7.9 and ASP of 92.9. (b) EGFR-mt patient with SUVmax of 9.2 and ASP of 58.2
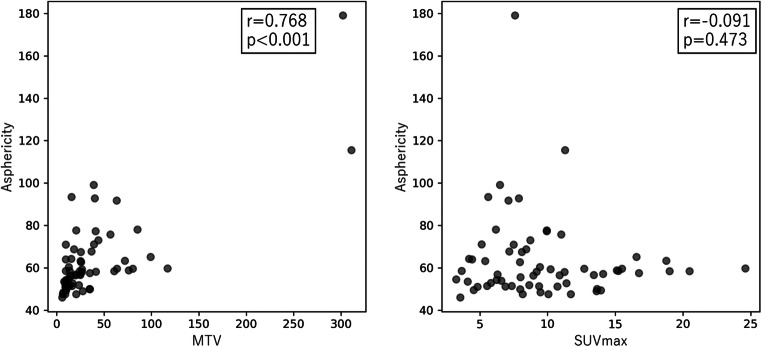
ASP exhibited a significant correlation with MTV (r = 0.768, P < 0.001), but not with SUVmax (r = − 0.091, P = 0.473) (Fig. 3).
Fig. 3.
Correlation of asphericity with other two PET parameters (SUVmax, MTV)
Uni- and Multivariable Analysis for Determinants of EGFR Mutation
Logistic regression analysis for determinants of EGFR mutation was performed, including PET parameters of SUVmax, MTV, and ASP, as well as clinical factors of sex and smoking history. TLG was not included because it is determined by SUV and MTV, and thus, it may have significant interactions with those two factors. Each lung cancer was classified as high or low SUVmax, MTV, and ASP group by the optimal cutoff values from ROC curve analysis (9.5, 24.7, and 58.5, respectively). In univariable analysis, sex and smoking were not significant for determining EGFR mutation (P = 0.765 and 0.229, respectively), whereas PET parameters of SUVmax and ASP were significant (P = 0.003 and 0.010, respectively, Table 3). MTV was not significant for determining EGFR mutation (P = 0.690). From the results of univariate analysis, multivariable analysis was conducted using two significant parameters, SUVmax and ASP (Model 1). Additional multivariable analysis was conducted with inclusion of MTV, to test possible interfering effect of MTV on the relationship between EGFR status and ASP (Model 2). Both of SUVmax and ASP were significant factors for determining EGFR mutation regardless of tested models (P = 0.004 and 0.011 for Model 1, P = 0.008 and 0.026 for Model 2, respectively; Table 3), while MTV was not significant factor in Model 2 (P = 0.617).
Table 3.
Logistic regression analysis for determinants of EGFR mutation
| Parameters | Univariable | Multivariable (Model 1) | Multivariable (Model 2) | |||
|---|---|---|---|---|---|---|
| OR (95% CI) |
P | OR (95% CI) |
P | OR (95% CI) |
P | |
| Sex |
0.86 (0.32–2.31) |
0.765 | – | – | – | – |
| Smoking |
0.53 (0.19–1.50) |
0.229 | – | – | – | – |
| SUVmax |
5.11 (1.67–15.67) |
0.003 |
6.09 (1.80–20.61) |
0.004 |
5.56 (1.57–19.72) |
0.008 |
| MTV |
3.33 (1.13–9.78) |
0.072 | – | – |
1.38 (0.39–4.95) |
0.617 |
| ASP |
3.76 (1.32–10.67) |
0.010 |
4.57 (1.43–14.62) |
0.011 | 4.08(1.18–14.10) | 0.026 |
Discussion
Radiogenomics is aimed at understanding the relationship between image findings and genetic status. Thus, role of a certain genetic property of a tumor can be understood based on image findings. In this study, it was demonstrated that ASP as well as other conventional PET parameters of SUVmax and MTV is significantly different according to EGFR status. The relationships of SUVmax and ASP to EGFR-mt were independent of other clinicopathologic factors. However, ASP was negatively correlated with EGFR-mt in this study, which is discordant with a previous report that there is a positive correlation between EGFR expression and ASP [3]. Multivariable logistic regression analyses using PET parameters showed significance of SUVmax and ASP as determinants for EGFR-mt.
The relationship between glucose metabolism and EGFR-mt in lung cancer has been widely investigated, although the results are somewhat controversial. Whereas most studies reported that EGFR-mt is related to low glucose metabolism [7, 8, 17–20], some other studies reported that EGFR-mt is related to high glucose metabolism [3, 6]. Some others reported no statistical difference between EGFR-mt and EGFR-wt groups [9, 21] (summarized in Supplemental Table 1). In these two studies, relatively larger proportion of EGFR-mt than that of usual population [22], difference in ethnicity, and uncontrolled other genetic mutation in EGFR-wt group may have caused bias in the results. In 3 studies that enrolled Korean patients, they all reported lower SUVmax in EGFR-mt than in EGFR-wt, which is in accordance with the results of the present study.
Because there are many factors that can affect glucose metabolism of a tumor, multifactorial analysis is necessary to appropriately evaluate the relationship between EGFR-mt and glucose metabolism. EGFR stimulation potently upregulates glycolysis by inducing translocation of glucose transporter (GLUT) [23]. Inhibition of EGFR induces dramatic decrease in cancer glucose metabolism in vitro and in vivo [23, 24]. However, glucose metabolism is also upregulated in lung cancer with other driver mutations. KRAS is another major driver mutation found in 25% of lung ADC, which is mutually exclusive with EGFR-mt [25]. KRAS mutation in lung cancer is well-known to upregulate GLUT [26]. A recent study reported that NSCLC cell lines with EGFR-mt show downregulation of the NOX4/ROS/GLUT1 axis compared with a KRAS-mt/EGFR-wt cell line, which may be a reason for relatively lower glucose metabolism in NSCLC with EGFR-mt [27]. NSCLC may be combined with various driver mutations that can affect glucose metabolism [28], which can be a cause to controversial results for glucose metabolism in EGFR-mt patients.
Radiomics has recently been studied in many clinical researches in terms of diagnosis, response evaluation, and prognosis prediction. Intratumoral heterogeneity assessed by radiomics was a significant prognostic factor for EGFR inhibitor therapy in NSCLC [20]. However, there is a limitation of radiomics study using FDG PET in lung cancer, that respiratory motion can affect PET radiomics parameters [29, 30]. ASP or sphericity is a radiomics parameter that has the least variability on conventional 3-dimensional PET as well as respiration-gated PET [5]. Because respiration gating is not widely performed in current clinical PET/CT studies, ASP may be an effective parameter for radiomics research in lung cancer. The present study showed a significant correlation between EGFR and ASP, which is discordant with a previous report [3]. The discrepancy may have been caused by a small proportion of EGFR-mt, because the proportion of EGFR-mt is as low as 17% in Caucasian patients [22]. In contrast, EGFR-mt is more frequent in Asian NSCLC patients, and the proportion has been reported to be up to 39%. In the present study, the proportion of EGFR-mt was 45%.
ASP is a parameter representing the “sphere-shapedness” of a tumor and has been investigated in several tumors [3, 15, 16, 31]. The results showed that high ASP, or high deviation from the sphere shape, is associated with advanced tumor stage and worse prognosis [15, 16]. ASP is also correlated with Ki-67 index, which is a marker for high proliferation rate and poor prognosis. It is speculated that tumors with higher growth rate show a more aberrant and irregular growth pattern, and consequently, higher ASP. High SUVmax of a tumor is also a well-known marker for high aggressiveness and poor prognosis [32, 33]. Thus, it is expected that EGFR-mt is related to low ASP and SUVmax, considering good prognosis of EGFR-mt. Although good prognosis of EGFR-mt NSCLC largely benefits from EGFR-TKI use [34], the radiogenomic results suggest that EGFR-mt itself is related to less aggressive nature.
In radiomics analysis of FDG PET, interference between ASP and MTV needs to be considered, because ASP and MTV are positively correlated with each other, especially in small tumors [15]. The present study also showed a significant positive correlation between ASP and MTV. In this study, MTV was significantly lower in EGFR-mt, and a similar tendency was observed in a previous study [3]. In another previous study on Asian patients, MTV was also significantly low in EGFR-mt [35]. Thus, MTV may be a confounding factor for analyzing correlation of EGFR status and ASP. In multivariate analysis of the present study, it was shown that ASP is a significant determinant for EGFR-mt regardless of MTV.
There are a few limitations in the present study. First, it is a retrospective study, and the sample size was relatively small. It can be a cause for sampling bias and low statistical power. Second, respiration gating and motion correction were not applied to PET/CT imaging, although ASP is relatively robust to motion artifact. Third, a fixed threshold method with cutoff SUV 2.5 was used for tumor delineation. Although this is frequently adopted in radiomics study, it would have not been optimal, particularly in low FDG-avid or small tumors.
Conclusion
In Asian NSCLC/ADC patients, SUVmax, MTV, and ASP on FDG PET are significantly related to EGFR mutation status. Particularly, low SUVmax and ASP are independent determinants for EGFR-mt. Based on the results, SUVmax and ASP may be considered as significant factors when EGFR-mt status is predicted by using PET parameters, and EGFR status may be considered as a significant interfering factor when ASP and SUVmax are analyzed in radiomics study of FDG PET in NSCLC/ADC.
Electronic supplementary material
(DOCX 245 kb)
Compliance with Ethical Standards
Conflict of Interest
Wonseok Whi, Seunggyun Ha, Sungwoo Bae, Hongyoon Choi, Jin Chul Paeng, Gi Jeong Cheon, Keon Wook Kang, and Dong Soo Lee declare that there is no conflict of interest. There is no source of funding.
Ethical Statement
All procedures followed were performed in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2013. The study design of the retrospective analysis and exemption of informed consent were approved by the Institutional Review Board of the Seoul National University Hospital (2001–146-1097). This manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Seunggyun Ha, Email: seunggyun.ha@gmail.com.
Jin Chul Paeng, Email: paengjc@snu.ac.kr.
References
- 1.Midha A, Dearden S, McCormack R. EGFR mutation incidence in non-small-cell lung cancer of adenocarcinoma histology: a systematic review and global map by ethnicity (mutMapII) Am J Cancer Res. 2015;5:2892. [PMC free article] [PubMed] [Google Scholar]
- 2.Takamochi K, Oh S, Matsunaga T, Suzuki K. Prognostic impacts of EGFR mutation status and subtype in patients with surgically resected lung adenocarcinoma. J Thorac Cardiovasc Surg. 2017;154:1768–1774. doi: 10.1016/j.jtcvs.2017.06.062. [DOI] [PubMed] [Google Scholar]
- 3.Apostolova I, Ego K, Steffen IG, Buchert R, Wertzel H, Achenbach HJ, et al. The asphericity of the metabolic tumour volume in NSCLC: correlation with histopathology and molecular markers. Eur J Nucl Med Mol Imaging. 2016;43:2360–2373. doi: 10.1007/s00259-016-3452-z. [DOI] [PubMed] [Google Scholar]
- 4.Apostolova I, Rogasch J, Buchert R, Wertzel H, Achenbach HJ, Schreiber J, et al. Quantitative assessment of the asphericity of pretherapeutic FDG uptake as an independent predictor of outcome in NSCLC. BMC Cancer. 2014;14:896. doi: 10.1186/1471-2407-14-896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oliver JA, Budzevich M, Zhang GG, Dilling TJ, Latifi K, Moros EGJTo Variability of image features computed from conventional and respiratory-gated PET/CT images of lung cancer. Transl Oncol. 2015;8:524–534. doi: 10.1016/j.tranon.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C-T, Yen R-F, Cheng M-F, Hsu Y-C, Wei P-F, Tsai Y-J, et al. Correlation of F-18 fluorodeoxyglucose-positron emission tomography maximal standardized uptake value and EGFR mutations in advanced lung adenocarcinoma. Med Oncol. 2010;27:9–15. doi: 10.1007/s12032-008-9160-1. [DOI] [PubMed] [Google Scholar]
- 7.Cho A, Hur J, Moon YW, Hong SR, Suh YJ, Kim YJ, et al. Correlation between EGFR gene mutation, cytologic tumor markers, 18F-FDG uptake in non-small cell lung cancer. BMC Cancer. 2016;16:224. doi: 10.1186/s12885-016-2251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Usuda K, Sagawa M, Motono N, Ueno M, Tanaka M, Machida Y, et al. Relationships between EGFR mutation status of lung cancer and preoperative factors-are they predictive. Asian Pac J Cancer Prev. 2014;15:657–662. doi: 10.7314/APJCP.2014.15.2.657. [DOI] [PubMed] [Google Scholar]
- 9.Putora PM, Früh M, Müller J. FDG-PET SUV-max values do not correlate with epidermal growth factor receptor mutation status in lung adenocarcinoma. Respirology. 2013;18:734–735. doi: 10.1111/resp.12083. [DOI] [PubMed] [Google Scholar]
- 10.Pan L, Cheng J, Zhou M, Yao Z, Zhang Y. The SUVmax (maximum standardized uptake value for F-18 fluorodeoxyglucose) and serum squamous cell carcinoma antigen (SCC-ag) function as prognostic biomarkers in patients with primary cervical cancer. J Cancer Res Clin Oncol. 2012;138:239–246. doi: 10.1007/s00432-011-1092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang Y, Li X, Wan H, Fang Y, Zheng R, Zhang W, et al. Prognostic value of volume-based metabolic parameters obtained by 18F-FDG–PET/CT in patients with locally advanced squamous cell cervical carcinoma. J Comput Assist Tomogr. 2018;42:429–434. doi: 10.1097/RCT.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 12.Hong JH, Min KJ, Lee JK, So KA, Jung US, Kim S, et al. Prognostic value of the sum of metabolic tumor volume of primary tumor and lymph nodes using 18F-FDG PET/CT in patients with cervical cancer. Medicine. 2016;95(9):e2992. [DOI] [PMC free article] [PubMed]
- 13.Du S, Sun H, Gao S, Xin J, Lu Z. Metabolic parameters with different thresholds for evaluating tumor recurrence and their correlations with hematological parameters in locally advanced squamous cell cervical carcinoma: an observational 18F-FDG PET/CT study. Quant Imaging Med Surg. 2019;9:440. doi: 10.21037/qims.2019.02.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung HH, Kim JW, Han KH, Eo JS, Kang KW, Park N-H, et al. Prognostic value of metabolic tumor volume measured by FDG-PET/CT in patients with cervical cancer. Gynecol Oncol. 2011;120:270–274. doi: 10.1016/j.ygyno.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Apostolova I, Steffen IG, Wedel F, Lougovski A, Marnitz S, Derlin T, et al. Asphericity of pretherapeutic tumour FDG uptake provides independent prognostic value in head-and-neck cancer. Eur Radiol. 2014;24:2077–2087. doi: 10.1007/s00330-014-3269-8. [DOI] [PubMed] [Google Scholar]
- 16.Hofheinz F, Lougovski A, Zöphel K, Hentschel M, Steffen IG, Apostolova I, et al. Increased evidence for the prognostic value of primary tumor asphericity in pretherapeutic FDG PET for risk stratification in patients with head and neck cancer. Eur J Nucl Med Mol Imaging. 2015;42:429–437. doi: 10.1007/s00259-014-2953-x. [DOI] [PubMed] [Google Scholar]
- 17.Mak RH, Digumarthy SR, Muzikansky A, Engelman JA, Shepard J-AO, Choi NC, et al. Role of 18F-fluorodeoxyglucose positron emission tomography in predicting epidermal growth factor receptor mutations in non-small cell lung cancer. Oncologist. 2011;16:319–326. doi: 10.1634/theoncologist.2010-0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byun BH, Kim KM, Cheon GJ, Choe DH, Koh JS, Lee DY, et al. 18F-FDG uptake and EGFR mutations in patients with non-small cell lung cancer: a single-institution retrospective analysis. Lung Cancer. 2010;67:76–80. doi: 10.1016/j.lungcan.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 19.Minamimoto R, Jamali M, Gevaert O, Echegaray S, Khuong A, Hoang CD, et al. Prediction of EGFR and KRAS mutation in non-small cell lung cancer using quantitative 18F FDG-PET/CT metrics. Oncotarget. 2017;8:52792. doi: 10.18632/oncotarget.17782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park S, Ha S, Lee S-H, Paeng JC, Keam B, Kim TM, et al. Intratumoral heterogeneity characterized by pretreatment PET in non-small cell lung cancer patients predicts progression-free survival on EGFR tyrosine kinase inhibitor. PLoS One. 2018;13:e0189766. doi: 10.1371/journal.pone.0189766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caicedo C, Garcia-Velloso MJ, Lozano MD, Labiano T, Diaz CV, Lopez-Picazo JM, et al. Role of [18 F] FDG PET in prediction of KRAS and EGFR mutation status in patients with advanced non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2014;41:2058–2065. doi: 10.1007/s00259-014-2833-4. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y-L, Yuan J-Q, Wang K-F, Fu X-H, Han X-R, Threapleton D, et al. The prevalence of EGFR mutation in patients with non-small cell lung cancer: a systematic review and meta-analysis. Oncotarget. 2016;7:78985. doi: 10.18632/oncotarget.12587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makinoshima H, Takita M, Matsumoto S, Yagishita A, Owada S, Esumi H, et al. Epidermal growth factor receptor (EGFR) signaling regulates global metabolic pathways in EGFR-mutated lung adenocarcinoma. J Biol Chem. 2014;289:20813–20823. doi: 10.1074/jbc.M114.575464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qiu W, Schönleben F, Li X, Ho DJ, Close LG, Manolidis S, et al. PIK3CA mutations in head and neck squamous cell carcinoma. Clin Cancer Res. 2006;12:1441–1446. doi: 10.1158/1078-0432.CCR-05-2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Momcilovic M, Bailey ST, Lee JT, Fishbein MC, Magyar C, Braas D, et al. Targeted inhibition of EGFR and glutaminase induces metabolic crisis in EGFR mutant lung cancer. Cell Rep. 2017;18:601–610. doi: 10.1016/j.celrep.2016.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerr EM, Martins CP. Metabolic rewiring in mutant Kras lung cancer. FEBS J. 2018;285:28–41. doi: 10.1111/febs.14125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Zhou Y, Tang X, Yang C, Tian Y, Xie R, et al. EGFR mutation decreases FDG uptake in non-small cell lung cancer via the NOX4/ROS/GLUT1 axis. Int J Oncol. 2019;54:370–380. doi: 10.3892/ijo.2018.4626. [DOI] [PubMed] [Google Scholar]
- 28.Steuer CE, Behera M, Berry L, Kim S, Rossi M, Sica G, et al. Role of race in oncogenic driver prevalence and outcomes in lung adenocarcinoma: results from the lung Cancer mutation consortium. Cancer. 2016;122:766–772. doi: 10.1002/cncr.29812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yip S, McCall K, Aristophanous M, Chen AB, Aerts HJ, Berbeco R. Comparison of texture features derived from static and respiratory-gated PET images in non-small cell lung cancer. PLoS One. 2014;9:e115510. doi: 10.1371/journal.pone.0115510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grootjans W, Tixier F, van der Vos CS, Vriens D, Le Rest CC, Bussink J, et al. The impact of optimal respiratory gating and image noise on evaluation of intratumor heterogeneity on 18F-FDG PET imaging of lung cancer. J Nucl Med. 2016;57:1692–1698. doi: 10.2967/jnumed.116.173112. [DOI] [PubMed] [Google Scholar]
- 31.Meißner S, Janssen J-C, Prasad V, Brenner W, Diederichs G, Hamm B, et al. Potential of asphericity as a novel diagnostic parameter in the evaluation of patients with 68 Ga-PSMA-HBED-CC PET-positive prostate cancer lesions. EJNMMI Res. 2017;7:85. doi: 10.1186/s13550-017-0333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European lung Cancer working party for the IASLC lung Cancer staging project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]
- 33.Higashi K, Ueda Y, Arisaka Y, Sakuma T, Nambu Y, Oguchi M, et al. 18F-FDG uptake as a biologic prognostic factor for recurrence in patients with surgically resected non–small cell lung cancer. J Nucl Med. 2002;43:39–45. [PubMed] [Google Scholar]
- 34.Kuan F-C, Kuo L-T, Chen M-C, Yang C-T, Shi C-S, Teng D, et al. Overall survival benefits of first-line EGFR tyrosine kinase inhibitors in EGFR-mutated non-small-cell lung cancers: a systematic review and meta-analysis. Br J Cancer. 2015;113:1519–1528. doi: 10.1038/bjc.2015.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang B, Wang QG, Lu M, Ge Y, Zheng YJ, Zhu H, et al. Correlations study between 18F-FDG PET/CT metabolic parameters predicting epidermal growth factor receptor mutation status and prognosis in lung adenocarcinoma. Front Oncol. 2019;9. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 245 kb)