Abstract
Cardiac excitation-contraction (EC) coupling, which links plasma membrane depolarization to activation of cardiomyocyte contraction, occurs at dyads, the nanoscopic microdomains formed by apposition of transverse (T)-tubules and junctional sarcoplasmic reticulum (jSR). In a dyadic junction, EC coupling occurs through Ca2+-induced Ca2+ release. Membrane depolarization opens voltage-gated L-type Ca2+ channels (LTCCs) in the T-tubule. The resulting influx of extracellular Ca2+ into the dyadic cleft opens Ca2+ release channels known as ryanodine receptors (RYRs) in the jSR, leading to the rapid increase in cytosolic Ca2+ that triggers sarcomere contraction. The efficacy of LTCC-RYR communication greatly affects a myriad of downstream intracellular signaling events, and it is controlled by many factors, including T-tubule and jSR structure, spatial distribution of ion channels, and regulatory proteins that closely regulate the activities of channels within dyads. Alterations in dyad architecture and/or channel activity are seen in many types of heart disease. This review will focus on the current knowledge regarding cardiac dyad structure and function, their alterations in heart failure, and new approaches to study the composition and function of dyads.
Keywords: Cardiac dyads, EC coupling, T-tubule, jSR
Introduction
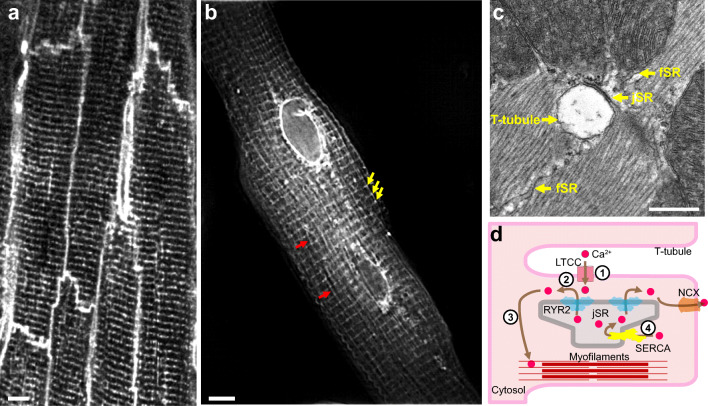
In cardiomyocytes, excitation-contraction (EC) coupling transduces electrical signals from the sarcolemma into intracellular Ca2+ transients that govern heart muscle contraction. Electrical signals in the form of the cardiomyocyte action potential are transmitted along the sarcolemma. In mammalian ventricular cardiomyocytes, the sarcolemma forms dense tubular invaginations, transverse (T)-tubules, that penetrate deep into the cell (Fig. 1A). Ca2+ is primarily released from the sarcoplasmic reticulum (SR) at specialized domains, the junctional SR (jSR; Fig. 1B and C). These two membrane systems interface at dyads, the fundamental structural unit that mediates normal EC coupling. In dyads, T-tubules form tight physical couplings with the terminal cisternae of jSR, with the membranes separated by a 12 ~ 18-nm-wide cytoplasmic microdomain, the dyadic cleft (Franzini-Armstrong et al. 1999) (Fig. 1C and D). This structural organization promotes EC coupling by facilitating rapid communication between voltage-gated L-type Ca2+ channels (LTCCs), concentrated on the T-tubule membrane (Brandt 1985), and Ca2+ release channels/ryanodine receptors (RYRs), clustered on the junctional sarcoplasmic reticulum (jSR) (Franzini-Armstrong et al. 1999), via Ca2+-induced Ca2+ release (CICR) (Fabiato and Fabiato 1975) (Fig. 1). Sarcolemmal depolarization activates LTCCs, which deliver a train of high local Ca2+ pulses (“Ca2+ sparklets”) (Wang et al. 2001) to the RYRs in the jSR. LTCC Ca2+ sparklets stochastically activate RYRs causing them to discharge “Ca2+ sparks” (Cheng et al. 1993) from different dyads, which ultimately summate into global Ca2+ transients that trigger coordinated sarcomere contraction across the cell (Bers 2002).
Fig. 1.
Anatomy of cardiac dyads. (A) T-tubule network of mature adult cardiomyocytes. The mouse heart was perfused with MM-4-64, which stains the sarcolemma, and optically sectioned and imaged using a confocal microscope. Bar = 5 μm. (B) Total sarcoplasmic reticulum (SR) structure of an adult cardiomyocyte. The dissociated adult mouse cardiomyocyte was stained using Fluo5N and visualized by structured illumination microscopy. Note the discrete junctional SR (jSR; yellow arrows) and the diffusible free SR (fSR; red arrows) and the much brighter perinuclear envelopes. Bar = 5 μm. (C) Longitudinal section of mouse left ventricular myocyte in a thin section transmission electron micrograph. A T-tubule, shown in cross section, is separated from pancake-shaped jSR cisternae by a narrow (~12 nm) junctional gap. Electron dense “foot” structures corresponding to the cytoplasmic domain of RYR2 protrude into the gap. fSR is contiguous with jSR. Bar = 0.5 μm. (D) Cartoon showing the process of EC coupling. At dyadic junctions, L-type Ca2+ channels (LTCCs) in T-tubules are coupled with RYR2 Ca2+ release channels in jSR. (1) The action potential depolarizes T-tubule membranes, opening LTCCs. (2) Extracellular Ca2+ enters through LTCCs into dyadic clefts, resulting in Ca2+-induced Ca2+ release through RYR2. (3) The resulting increase in [Ca2+]i causes sarcomere contraction. (4) [Ca2+]i is returned to basal levels by SERCA2a, which actively pumps Ca2+ back into SR, and by extrusion through the Na+/Ca2+ exchanger, NCX
EC coupling and CICR are fundamental to normal cardiac rhythm and contraction, and disorders of these processes contribute to both arrhythmia and heart failure (Connell et al. 2020). Understanding the molecular regulation of EC coupling and CICR is therefore of critical importance to understanding cardiac muscle function in both health and disease. In this review, we will discuss the architecture and function of cardiac dyads, its alterations in heart diseases, and new approaches to study the composition and function of dyads.
Dyad architecture
T-tubule structure
T-tubules were identified by electron microscopy (EM) as tubular invaginations of the sarcolemmal membrane (Franzini-Armstrong and Porter 1964). T-tubules communicate with the extracellular space and are composed of transversely oriented tubules (i.e., perpendicular to the long axis of cardiomyocytes; Fig. 1A), with interconnecting, longitudinally oriented tubules (Forssmann and Girardier 1970; Sperelakis and Rubio 1971). The transverse T-tubules are aligned with the sarcomere Z-lines and have a corresponding spacing of ~1.8 μm, whereas the longitudinal T-tubules have a spacing of ~0.5 μm (McNutt 1975; Wagner et al. 2012). In ventricular myocytes, the average diameter of T-tubules, measured by optical imaging, is 200–400 nm, depending on the species: mouse, ~200 nm (Wagner et al. 2012); rat, ~250 nm (Soeller and Cannell 1999); rabbit, ~400 nm (Savio-Galimberti et al. 2008); and human, ~400 nm (Cannell et al. 2006). Collectively, T-tubules occupy 1–3% of the volume of ventricular cardiomyocytes. Depending on the species, T-tubules are variably present in atrial cardiomyocytes (Shiels and Galli 2014; Caldwell et al. 2014).
The physiological function of T-tubules depends on a unique set of ion channels, scaffolding proteins, and cytoskeletal structural proteins localized at or neighboring the T-tubules. A key protein of the T-tubules is the LTCC. Eighty percent of sarcolemma LTCCs are localized at T-tubules (Pásek et al. 2008a). This localization puts LTCCs in the vicinity of RYR2 Ca2+ release channels in the SR membrane (see “Sarcoplasmic reticulum”). BIN1, a membrane scaffolding protein that localizes to T-tubules, is required for forward microtubule-dependent trafficking of LTCCs to T-tubules (Hong et al. 2010; Fu and Hong 2016). A cardiac-specific isoform of BIN1 is also required for sculpting of microfolds in T-tubules, creating microdomains enriched in LTCCs that attract RYR2 in the adjacent jSR, suggesting that BIN1 contributes to dyad organization (Hong et al. 2014; Caldwell et al. 2014; Hong and Shaw 2017). These BIN1-dependent microfolds create “slow diffusion zones” or “fuzzy space” within the T-tubule lumens for Ca2+, K+, and likely other ions, with important effects on membrane electrophysiology and cellular Ca2+ handling (Pásek et al. 2008b; Hong et al. 2014). Caveolin-3, which binds sarcolemmal membrane microdomains known as caveolae and contributes to their spheroid morphology, is enriched in T-tubules. Specific receptors and ion channels, including the β-adrenergic receptor, a subset of LTCCs, and K+ channels, localize to caveolae and contribute to their regulation (Wright et al. 2014). Recently, caveolae were noted to also be present within the T-tubular membranes, albeit at lower density than on the cell surface (Burton et al. 2017). However, the specific function of caveolae within the T-tubules remains to be determined.
In summary, the T-tubule network enables electrical excitation to rapidly propagate into the cell interior, where it synchronizes Ca2+ release from the Ca2+ store, the SR. Microdomains within the T-tubules compartmentalize ion handling proteins, most notably LTCCs, and signaling molecules to precisely tune EC coupling.
Sarcoplasmic reticulum
The SR is a continuous membrane-bounded organelle that occupies ~1–3.5% of the volume of cardiomyocytes (Bers 2001; Shiels and Galli 2014) (Fig. 1B). The SR comprises at least two functional elements: free or network SR and jSR. Free SR makes up most of the SR membrane and takes the form of interconnected, primarily longitudinal nanotubules distributed over most of the cell. Near sarcomere Z-lines, the SR becomes specialized, pancake-like cisterns, the jSR, with an average length of ~400 nm and a luminal width of ~30 nm (Brochet et al. 2005). At intracellular dyads, the jSR is separated by a narrow (~12 nm) junctional gap from the T-tubules (Fig. 1C and D) or, at “peripheral couplings,” by a ~30-nm cleft from the surface membrane (Takeshima 2002). In cardiomyocytes that lack T-tubules (e.g., atrial cardiomyocytes in some species), the corbular SR (non-junctional SR) containing RYR2 is distributed near sarcomere Z-lines and participates in CICR by amplifying Ca2+ that enters the cells through LTCC in the surface sarcolemma (Shiels and Galli 2014; Caldwell et al. 2014).
Transport channels and Ca2+ binding proteins exhibit fine anatomical segregation in the SR membrane and within the SR lumen that endows the SR with the power of orchestrating Ca2+ cycling. The jSR contains RYR2 intracellular Ca2+ release channels, which are separated from LTCCs on the T-tubules by the narrow dyadic gap. Individual RYR2 channels are homotetramers (MW 2.2 MDa) that occupy an area of about 30 × 30 nm2 (Baddeley et al. 2009). The cardiac SR Ca2+ is buffered by calsequestrin 2 (CASQ2), a low-affinity, high-capacity Ca2+ buffering protein that is localized nearly exclusively within the jSR lumen. In the presence of Ca2+, CASQ2 forms a linear polymer that binds 20–40 Ca2+ ions, and this Ca2+-dependent CASQ2 polymerization is essential for its localization within the jSR (Slupsky et al. 1987; Mitchell et al. 1988). CASQ2 binding to triadin and junctin, transmembrane proteins embedded in the jSR membrane that interact with RYR2, also likely contributes to CASQ2 retention in jSR (Zhang et al. 1997). This quaternary complex between RYR2, triadin, junctin, and CASQ2 mediates inhibitory interactions between CASQ2 and RYR2 that reduce spontaneous RYR2 opening (Knollmann 2010).
Whereas the macromolecular complex of RYR2 and its interacting proteins is localized to jSR, the SR Ca2+-ATPase, SERCA2a, is mainly localized on the longitudinal SR, although it is also present at lower levels in the jSR (Jorgensen et al. 1982; Vangheluwe et al. 2003). Thus, Ca2+ is released from the jSR, stimulates contraction of adjacent sarcomeres, and is returned to the SR along the longitudinal tubules, as well as the jSR. SERCA2a is regulated by phospholamban (PLB), a cardiac-specific endogenous SERCA2a inhibitor that binds to and colocalizes with SERCA2a in the longitudinal SR and jSR (He et al. 2020).
These fine, structurally specialized SR membrane complexes determine the spatiotemporal intermolecular Ca2+ signaling in the nanoscopic dyadic region.
Ca2+ signaling in dyads
In a single dyad, nanoscopic Ca2+ signaling is an elaborate local control process between LTCCs on the T-tubules and RYR2 on the jSR (Fig. 1D). During EC coupling, the opening of an individual Ca2+-permeant LTCC in response to membrane depolarization creates a Ca2+ microdomain within the dyadic cleft that activates RYR2 almost instantly (Ton ≤ 1 ms) (Gyorke and Fill 1993) at high-affinity cytosolic Ca2+ activation sites. The activated RYR2 cluster releases a much larger amount of Ca2+ into the dyadic cleft, in the form of a Ca2+ spark (Cheng et al. 1993), accompanied by local depletion of Ca2+ at the cluster’s jSR luminal side, a Ca2+ blink (Brochet et al. 2005). As a result, the merging of Ca2+ influx through LTCC and SR Ca2+ release through RYR2 builds up local Ca2+ gradients that arise abruptly in ~5 ms, attain peaks of ~100 μmol/L, and persist for ~15 ms (Acsai et al. 2011; Cannell et al. 2013). During full-fledged cardiac EC coupling, ~104 sparks are activated within a few tens of milliseconds in a single cardiomyocyte (Cannell et al. 1994), summating into a global Ca2+ transient of ~1 μmol/L and leading to activation of downstream contractile proteins.
RYR2 also has a stochastic probability of spontaneously opening to release Ca2+ in quiescent unstimulated myocytes at diastolic [Ca2+]i ~100 nM without EC coupling. These spontaneous sparks appear to be identical to the ones evoked by LTCC activation during EC coupling in terms of amplitude, kinetics, and spatial properties (Cheng and Lederer 2008).
RYR2 homotetramers are organized into clusters as two-dimensional, elongated paracrystalline arrays. Due to Ca2+-dependent interactions between adjacent RYR2 homotetramers, the dyad’s Ca2+ release properties depend on the geometry, density, and spacing of these clusters. Depending on species and measurement technique, the average array in ventricular cardiomyocytes contains 14–100 homotetramers (Baddeley et al. 2009; Shiels and Galli 2014). RYR2 channels within a cluster have been hypothesized to act synergistically as single Ca2+ release units, in an all-or-none mode (Stern 1992). However, this coupled gating (Marx et al. 2001) cannot explain the prompt termination of Ca2+ sparks and the fidelity and stability of intracellular signaling (Stern et al. 1999; Wang et al. 2004) and also sharply contrasts with the relatively constant and brief duration of Ca2+ sparks (Wang et al. 2002). Since RYR2 arrays located in intracellular jSR membranes are inaccessible to direct electrophysiological investigation, their activity has been measured using a nanosensor targeted to RYR2. This nanosensor, consisting of the Ca2+-sensitive genetically encoded sensor GCaMP6f fused to junctin or triadin, visualized wide variability of Ca2+ nanospark amplitude at a single dyad and between dyads, indicative of stochastic recruitment of different numbers or subclusters of RYR2 to yield Ca2+ sparks (Shang et al. 2014).
The development of dyads
Efficient EC coupling in mature mammalian ventricular cardiomyocytes requires dyads. However, dyads are not present in fetal cardiomyocytes and form postnatally. In rats and mice, T-tubules are absent at birth. At postnatal day 10 (P10), nascent T-tubules are visible as invaginations of the surface sarcolemma. The nascent tubular network expands from the periphery inward, with most tubules maintaining continuity with the cell surface (Maio et al. 2007). By P20, cardiomyocytes contain a fully mature T-tubular network (Ziman et al. 2010; Chen et al. 2013).
The SR component of dyads develops with a different time course. In fetal cardiomyocytes, the jSR docks to the plasma membrane and forms peripheral couplings. Initially, the junctions contain very few Ca2+-handling proteins such as RYR2, LTCC, and CASQ2 (Franzini-Armstrong et al. 2005). With further development, both the density of peripheral couplings and their content of Ca2+-handling proteins increase. Internal jSR/RYR2 Ca2+ release units develop after birth and form an extended network aligned with sarcomere Z-lines and are fully capable of Ca2+ release by P10, when T-tubules are just starting to form (Ziman et al. 2010). As the T-tubular network develops and matures over the next 10 days, dyadic coupling forms between LTCC on T-the tubules and RYR2 on the jSR. This formation of dyadic junctions is marked by the redistribution of junctophilin 2 (JPH2): at P10, JPH2 primarily localized to surface the sarcolemma and did not colocalize with RYR2 at the jSR, whereas by P20, JPH2 was primarily localized with RYR2 at the jSR (Ziman et al. 2010).
The factors that drive and regulate the remarkable process of dyad formation, involving the development of the jSR network, the T-tubule network, and the close physical coupling between the two, are poorly understood. The factors responsible for the formation of jSR and its localization within the cell at Z-discs early in postnatal life have not been reported, whereas several candidate T-tubule regulators have been reported. BIN1 promotes membrane curvature and is localized to the T-tubules (Lee et al. 2002). In Drosophila, ablation of the ortholog of BIN1 caused loss of flight and disrupted flight muscle T-tubules (Razzaq et al. 2001). However in mice, cardiac specific ablation of BIN1 did not prevent T-tubule formation and was compatible with normal heart function until 3 months, with slow progressive deterioration over the subsequent year (Hong et al. 2014; Laury-Kleintop et al. 2015). While not required for T-tubule formation, BIN1 is responsible for T-tubule folding, establishment of microdomains within T-the tubules, and LTCC trafficking to the T-tubules (Hong et al. 2010, 2014; Fu and Hong 2016). BIN1 was also sufficient to increase T-tubules and LTCC clustering near RYR2 within human embryonic stem cell–derived cardiomyocytes (Mata et al. 2019). JPH2 has attracted considerable interest in the regulation of T-tubule biogenesis and the coordination of EC coupling. A C-terminal transmembrane domain anchors JPH2 to the jSR, while the N-terminal membrane binding domains bind the sarcolemma. This structure enables JPH2 to bridge between the jSR and T-tubule membranes and stabilize dyads (Takeshima et al. 2000). JPH2 colocalizes with RYR2 at the jSR, and cardiac JPH2 knockdown using an shRNA-expressing transgene disrupted T-tubule formation in developing cardiomyocytes impaired intracellular Ca2+ handling and precipitated severe heart failure (Chen et al. 2013; Reynolds et al. 2013). However, a Cas9-based mosaic gene knockout strategy revealed that JPH2 is not essential for T-tubule formation in the normal heart, although it plays an important role in stabilizing T-tubules in the context of cardiac dysfunction (Guo et al. 2017). This study also identified RYR2 as being the cell autonomously required for maintenance of T-tubules. Recent work showed that nexilin (NEXN), a widely expressed F-actin binding protein (Ohtsuka et al. 1998), is specifically localized in cardiomyocytes to the jSR and is required for normal Ca2+ handling (Liu et al. 2019). Human NEXN mutations cause dilated cardiomyopathy (Mazzarotto et al. 2020), and in mice NEXN knockout caused rapidly lethal dilated cardiomyopathy (Liu et al. 2019). Within this context of significant heart failure, NEXN knockout abolished formation of nascent T-tubules in the neonatal heart (Liu et al. 2019).
In summary, dyads develop in the first weeks of postnatal life through extensive and coordinated reorganization of both the sarcolemma and the SR membranes and their associated proteins. The factors responsible for this intricate process remain largely unknown.
Dyad alterations in heart disease
Dyad alterations in heart failure
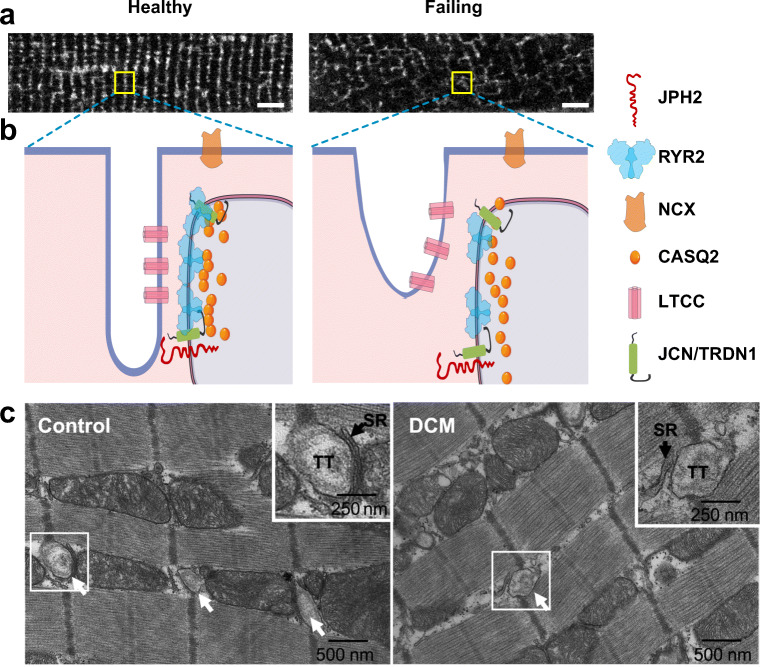
The dyad’s highly organized structure is essential for EC coupling and normal cardiac function. In dilated and ischemic cardiomyopathies, both the volume densities and the surface areas of T-tubules and jSR were largely decreased, leading to dramatically reduced dyads (by up to 60%) and coupling area (~17%) that impair the efficiency of EC coupling and heart function (Zhang et al. 2013). In failing hearts, T-tubules become disorganized and less tightly coupled to SR at dyads. Among the changes to T-tubules seen in failing hearts are (Fig. 2): decreased transverse and total T-tubules (He 2001; Wei et al. 2010a), increased fraction of longitudinal tubules (Kaprielian et al. 2000; Song et al. 2006), T-tubule displacement from Z-lines (Cannell et al. 2006), and the appearance of highly dilated (Crossman et al. 2017) or sheet-like (Seidel et al. 2017) T-tubules. This spatial dispersion, fracture, and loss of T-tubules alters the spatial distribution of LTCCs, causes their dislocation and separation from jSR (Hong et al. 2010), and creates orphaned RYR2 channels (Song et al. 2006). Heart failure also perturbs T-tubule microdomains and disorders the normal “fuzzy space” within the T-tubule lumens, which alters ion channel activity and electrophysiological properties and contributes to arrhythmias (Hong et al. 2014). Heart failure also impacts the nanoscale organization of RYR2 into clusters. In failing cardiomyocytes, RYR2 clusters have a more sparse, dispersed configuration: the number of clusters increases due to fragmentation of clusters into smaller adjacent subclusters and the number of RYR2 channels per dyad and per cluster decreases by 2/3 and 1/2, respectively (Kolstad et al. 2018).
Fig. 2.
Altered dyad architecture in heart failure. (A) Confocal micrographs of the T-tubule system revealed by MM4-64 membrane dye in healthy and diseased murine cardiomyocytes. Note the T-tubular disorganization in the diseased cardiomyocyte. (B) Schematic illustration of normal (left) and diseased (right) dyadic junctions. The diseased dyad architecture features T-tubule loss and remodeling, ion channel dislocation, junctional contact area shrinkage, LTCC-RYR2 uncoupling, RYR2 cluster redistribution, and disordered fuzzy space (see text). Bar = 5 μm. JCN, junctin; TRDN1, triadin 1. (C) Transmission electron micrographs of human LV myocardium from healthy control (left) or patient with dilated cardiomyopathy (DCM, right).Arrows point to T-tubules (TT). Boxed areas are enlarged in upper right corner. In control, jSR is separated from T-tubule by a narrow junctional gap. In DCM, the junctional gap is wider, and the contact area is reduced. Panel C was reproduced from Zhang et al. (2013) with permission from the publisher
Associated with these structural alterations, as well as changes in ion channel expression and activity, failing cardiomyocytes have impaired Ca2+ handling characterized by reduced Ca2+ transient amplitude, slower rate of decay of the Ca2+ transient, and decreased SR Ca2+ content (Piacentino et al. 2003). The reduction of the Ca2+ transient amplitude, which is directly linked to reduced cardiac contractility, is largely due to the decreased EC coupling resulting from the disrupted dyad architecture (Gomez 1997). The dispersed nanoscale organization of RYR2 also contributes to the slower Ca2+ transient upstroke and lower peak [Ca2+]i by slowing Ca2+ sparks and de-synchronizing global Ca2+ transients in heart failure (Kolstad et al. 2018). The disruption of T-tubules and uncoupling of LTCC from RYR2 also cause dyssynchronous Ca2+ release among dyads, Ca2+ instability (Song et al. 2006; Heinzel et al. 2008; Lyon et al. 2009), arrhythmogenic Ca2+ waves (Cheng et al. 1996), and delayed afterdepolarizations (Shiferaw et al. 2012; Fowler et al. 2020). Therefore, T-tubule pathology is a major factor that contributes to contractile dysfunction and propensity to arrhythmia in human heart failure (Crossman et al. 2015).
Although the mechanistic details of dyad remodeling in heart failure are still being elucidated, existing studies link this process to altered expression of junctional membrane complex proteins that construct dyads. In failing hearts from rodent models (Minamisawa et al. 2004; Wei et al. 2010b) and patients (Landstrom et al. 2011) (Frisk et al. 2016), JPH2 is profoundly downregulated. This progressive decrease in JPH2 protein level correlates with the loss and disruption of T-tubule and in turn is likely due to a combination of upregulation of microRNA-24, the immediate upstream suppressor (Xu et al. 2012), mislocation (Zhang et al. 2014), and calpain-mediated degradation (Guo et al. 2015). Interestingly, an N-terminal JPH2 fragment generated by calpain protease cleavage in heart failure translocates to the nucleus and regulates gene transcription to counteract deleterious changes that occur in heart failure (Guo et al. 2018a). JPH2 activity is also regulated by phosphorylation by SPEG (striated muscle preferentially expressed protein kinase), a recently described junctional kinase essential for junctional membrane complex integrity (Quick et al. 2017) (see “Proteomic mapping of the cardiac dyad”). SPEG is transcriptionally decreased by about 80% in patients with non-genetic forms of cardiomyopathy and heart failure (Quick et al. 2017).
However, disorganization of T-tubules in some experimental heart failure models is not associated with altered JPH2 level (Caldwell et al. 2014). Decreased BIN1 level is also found in heart failure (Hong et al. 2012; Caldwell et al. 2014), which aggravates overall T-tubule loss (Caldwell et al. 2014) and disrupts T-tubule morphology and microdomains (Hong et al. 2014) and BIN1-dependent LTCC trafficking to T-tubules (Hong et al. 2010). Triadin and junctin, components of the RYR2 macromolecular complex in jSR, are also markedly downregulated in failing human hearts (Gergs et al. 2007). Junctin knockout mice had structurally and functionally normal hearts but developed lethal ventricular arrhythmias associated with increased SR Ca2+ load and increased delayed afterdepolarizations (Yuan et al. 2007). Similarly, triadin knockout caused cardiac arrhythmias with preserved ventricular function. The jSR showed extensive structural remodeling and reduced T-tubule contacts, associated with reduced EC coupling; decreased expression of jSR proteins such as RYR2, junctin, and CASQ2; elevated SR Ca2+; and increased rate of spontaneous SR Ca2+ release (Chopra et al. 2009).
Dyad alterations caused by mutations in Ca2+ release unit genes
Mutations in genes that encode components of Ca2+ release units, such as RYR2, junctin, triadin, and CASQ2, cause an inherited arrhythmia known as catecholaminergic polymorphic ventricular tachycardia (Sumitomo 2016). These mutations increase diastolic SR Ca2+ release through RYR2 by directly or indirectly altering the Ca2+ release properties of the RYR2 macromolecular complex. Interestingly, these mutations also alter the ultrastructure of Ca2+ release units. In mouse models, CPVT-causing Ryr2 or Casq2 mutation (Rizzi et al. 2008; Denegri et al. 2012; Bongianino et al. 2017) decreased the number of dyads and the length of individual dyads while widening the jSR lumen and increasing the variability in jSR shape. The molecular link between CPVT-causing mutations and the ultrastructural abnormalities is not clear, but the similar effect of the Ryr2 and Casq2 mutations on jSR suggests a shared pathophysiological mechanism, such as abnormal intraluminal Ca2+ regulation.
New approaches to studying the dyad
The recently developed technologies promise to accelerate our understanding of the structure and function of the dyad in health in disease. Here we highlight two recently developed approaches.
Proteomic mapping of the cardiac dyad
The leading role of dyads in controlling EC coupling highlights a need for a more comprehensive dissection of the highly packed dyadic protein complexes. Application of the emerging proteomic approaches to dyads is providing new insights into the regulation of EC coupling and CICR in dyads. For instance, through a classic affinity purification/mass spectrometry proteomic analysis of immunoprecipitated RYR2 and JPH2 interactomes, SPEG was identified as a novel binding partner for both proteins in junctions, and its deficiency caused T-tubule loss, Ca2+ mishandling, and heart failure (Quick et al. 2017). T-tubule abnormalities temporally preceded cardiac dysfunction, suggesting that it was the cause rather than the result of heart failure. Mechanistically, SPEG bound and promoted phosphorylation of JPH2, and JPH2 phosphorylation, was reduced in SPEG ablated cardiomyocytes, suggesting that SPEG phosphorylation of JPH2 is required to maintain T-tubules and cardiac homeostasis.
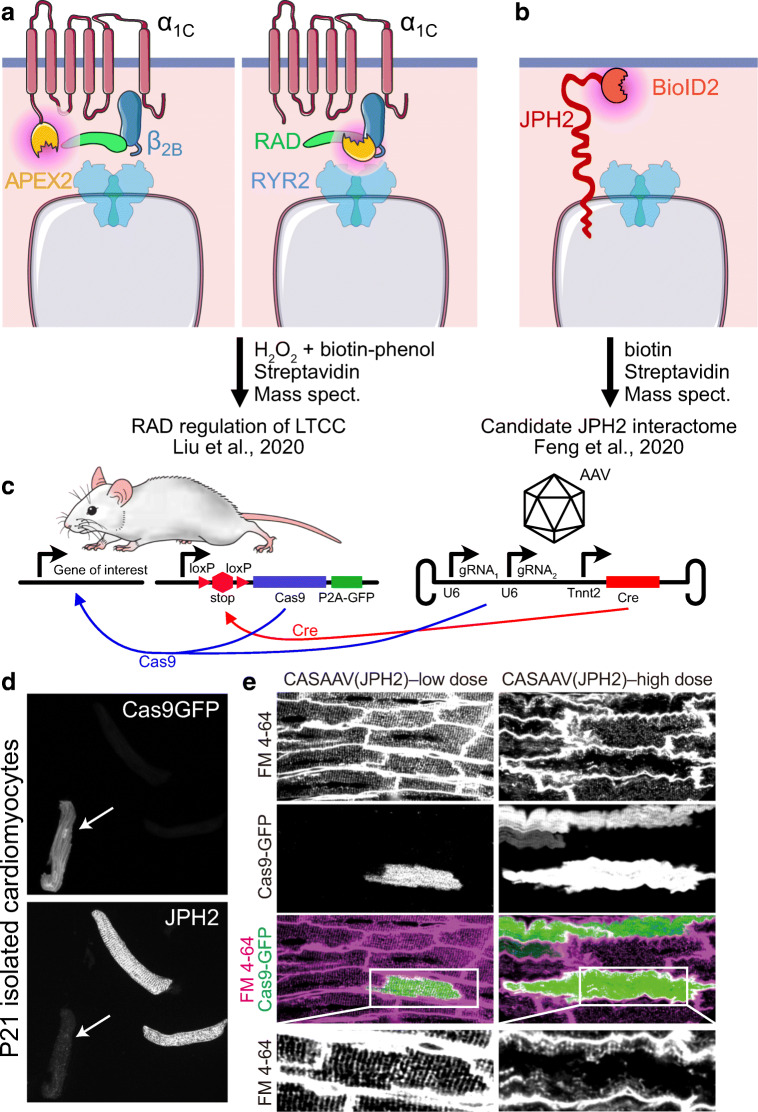
Recent advances in proximity labeling-based interactome mapping have been applied to describing the proteome of the dyad both in vitro and in vivo. Biotin-based proximity labeling techniques (Trinkle-Mulcahy 2019) such as APEX, based on an engineered ascorbate peroxidase, and BioID, based on an engineered, promiscuous biotin ligase, BirA*, have been applied to identify the cardiac dyad proteome. To address the long undefined molecular mechanism by which adrenergic stimulation increases Ca2+ entry through the LTCC, Marx and colleagues expressed APEX2 fused to the pore-forming transmembrane α1C or intracellular β2B subunits of LTCC and performed multiplexed quantitative proteomics to define proteins in the vicinity of LTCC with and without adrenergic stimulation (Liu et al. 2020) (Fig. 3A). These data showed that RAD, a monomeric G protein enriched in the LTCC microenvironment, acts as an inhibitor of LTCC under basal conditions. Stimulation of the β-adrenergic receptor activates PKA, which phosphorylates RAD and disengages it from LTCC.
Fig. 3.
New approaches to dissecting the structure and functional regulation of dyads. (A–B) Proximity proteomics applied to probe the dyadic proteome. Purple-shaded regions indicate labeling sphere determined by biotin free radical half-life. (A) APEX2-based proximity proteomics. Liu et al. (2020) fused APEX2 to components of the LTCC. Quantitative proteomics showed that β-adrenergic signaling regulated LTCC activity by controlling interaction of the LTCC β2B subunit with the novel dyad protein RAD. (B) Feng et al. (2020) fused endogenous JPH2 to BioID2 to detect nearby dyadic proteins. (C–E) Cas9 and AAV (CASAAV) strategy to generate somatic mutations in cardiomyocytes. AAV expressing gRNAs that target the gene of interest and cardiomyocyte-restricted Cre is administered to mice with genome-encoded, Cre-activated Cas9-P2A-GFP. Cre activates Cas9 and GFP, and Cas9 plus gRNAs inactivate the gene of interest. (D) Inactivation of JPH2 in Cas9-P2A-GFP expressing cardiomyocytes. Mice were treated with CASAAV vector targeting Jph2. At P21, dissociated cardiomyocytes were imaged to detect GFP and JPH2. Note the loss of JPH2 immunoreactivity in GFP+ cardiomyocyte (arrow). (E) Mosaic gene inactivation by CASAAV permits precise interpretation of results. CASAAV vector targeting JPH2 was given at lower or high doses. High dose, which caused heart failure, resulted in distorted T-tubule morphology in GFP+ and GFP− cells. Low dose, which did not cause heart failure, did not visibly disrupt T-tubules in GFP+ or GFP− cells. D–E were modified from Guo et al. (2017) with permission
Unlike APEX-based methods, BioID can be deployed in intact animals. To delineate the dyadic proteome proximal to JPH2, Chen et al. generated knock-in mice in which endogenous JPH2 is fused with BioID2 (Fig. 3B). In vivo BioID assay followed by mass spectrometry identified known proteins within the dyad (RYR2, LTCC α1C), along with some potential candidates that await experimental verification (Feng et al. 2020).
A more extensive application of novel proteomic approaches in normal and diseased states will expand our knowledge of the dyadic proteome and its changes in disease.
Mosaic, somatic gene inactivation to probe dyadic function
Studies of dyad development and function have been limited by the lack of in vitro model systems. Neonatal mammalian cardiomyocytes can be cultured for several days but lack dyads. Adult mammalian cardiomyocytes can be isolated and acutely studied, but rapidly dedifferentiate in culture, degrading dyad architecture and Ca2+ handling properties. Stem cell–derived cardiomyocytes for the most part lack T-tubules and mature junctions, although approaches to overcome these limitations are emerging (Parikh et al. 2017; Ronaldson-Bouchard et al. 2018; Guo and Pu 2020). As a result, the use of gain- and loss-of-function approaches to interrogate gene function in dyads has relied on genetically modified mice, which is both slow and resource intensive. Moreover, because standard genetic manipulation involves all cardiomyocytes, resolving direct from secondary effects of manipulations has often been difficult, confounding the interpretation of experiments (Guo and Pu 2018).
Somatic, cardiomyocyte-restricted mutagenesis induced by adeno-associated virus (AAV) in conjunction with CRISPR/Cas9 (CASAAV) is a powerful approach that overcomes these barriers (Guo et al. 2017, 2018b, 2019) (Fig. 3C). AAV9 efficiently transduces cardiomyocytes. AAV-mediated delivery of Cre, driven from a cardiomyocyte-specific promoter, and gene-specific guide RNA efficiently induce somatic loss-of-function mutations in targeted genes when combined with a Cre-activated Cas9 allele engineered into the mouse genome. Titration of AAV dose enables generation of genetic mosaics in which a low fraction (e.g., 10–20%) of cardiomyocytes are deficient in the targeted gene(s), permitting precise interpretation of the cell autonomous effect of gene inactivation within the context of normal cardiac function. In a proof-of-concept experiment, CASAAV was used to target Jph2, and over 70% of AAV-transduced cardiomyocytes lost JPH2 immunoreactivity (Fig. 3D). At higher AAV doses that transduced ~65% of cardiomyocytes, JPH2 depletion resulted in heart failure and T-tubule disorganization, both in AAV-transduced as well as non-AAV-transduced cardiomyocytes (Fig. 3E, high dose). At a lower AAV dose that transduced ~20% of cardiomyocytes, T-tubules were minimally affected in both transduced and non-transduced cardiomyocytes (Fig. 3E, low dose). Therefore, in a healthy myocardium, JPH2 is not cell autonomously required for T-tubule development, but it is required to stabilize T-tubules in the face of cardiac stress and dysfunction (Guo et al. 2017). When a floxed allele is available, titration of AAV-Cre can be used similarly to generate genetic mosaics to assess cell autonomous gene function (Prendiville et al. 2015; Zhang et al. 2017; Guo et al. 2018b, 2019). The AAV-based Cas9 and Cre/LoxP strategies complement each other: CASAAV can be used to rapidly screen the function of many different genes, followed by the detailed study of individual genes using AAV-Cre and a floxed allele.
Conclusions
There has been tremendous progress in understanding the physiological, architectural, and molecular basis of EC coupling and its perturbations in and contributions to heart disease. Despite these advances, many unanswered questions remain. What factors promote and coordinate postnatal formation of jSR at sarcomere Z-lines, formation of the T-tubular network, and intimate coupling of these systems at dyads? How can this process be stimulated in stem cell–derived cardiomyocytes so that they better recapitulate the physiology of native, mature cardiomyocytes? What are the molecular mechanisms that maintain homeostasis of this intricate architecture in the normal heart that lead to its disorganization and uncoupling in heart disease? What is the full complement of proteins in dyads and how do they impact dyad function? With the development of numerous powerful imaging techniques such as electron microscopy and super-resolution optical imaging, nanoscopic Ca2+ biosensors, a palette of proteomic technologies, and improved functional genomic strategies, we are well equipped to tackle these challenging questions that are fundamental to understanding cardiac physiology and disease.
Acknowledgments
The authors thank Kamillmaksymilian Prondzynsk for critical comments on the manuscript.
Funding information
FL was supported by a postdoctoral fellowship from the American Heart Association. WTP was supported by NIH grants R01 HL146634 and R21 HD094909.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acsai K, Antoons G, Livshitz L, et al. Microdomain [Ca2+] near ryanodine receptors as reported by L-type Ca2+ and Na+/Ca2+ exchange currents. J Physiol. 2011;589:2569–2583. doi: 10.1113/jphysiol.2010.202663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley D, Jayasinghe ID, Lam L, et al. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc Natl Acad Sci. 2009;106:22275–22280. doi: 10.1073/pnas.0908971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bers D (2001) Excitation-contraction coupling and cardiac contractile force. Springer Science & Business Media
- Bers DM. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Bongianino R, Denegri M, Mazzanti A, et al. Allele-specific silencing of mutant mRNA rescues ultrastructural and arrhythmic phenotype in mice carriers of the R4496C mutation in the ryanodine receptor gene (RYR2) Circ Res. 2017;121:525–536. doi: 10.1161/CIRCRESAHA.117.310882. [DOI] [PubMed] [Google Scholar]
- Brandt N. Identification of two populations of cardiac microsomes with nitrendipine receptors: correlation of the distribution of dihydropyridine receptors with organelle specific markers. Arch Biochem Biophys. 1985;242:306–319. doi: 10.1016/0003-9861(85)90506-5. [DOI] [PubMed] [Google Scholar]
- Brochet DXP, Yang D, Di Maio A, et al. Ca2+ blinks: rapid nanoscopic store calcium signaling. Proc Natl Acad Sci U S A. 2005;102:3099–3104. doi: 10.1073/pnas.0500059102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton RAB, Rog-Zielinska EA, Corbett AD, et al. Caveolae in rabbit ventricular myocytes: distribution and dynamic diminution after cell isolation. Biophys J. 2017;113:1047–1059. doi: 10.1016/j.bpj.2017.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell JL, Smith CER, Taylor RF, et al. Dependence of cardiac transverse tubules on the BAR domain protein amphiphysin II (BIN-1) Circ Res. 2014;115:986–996. doi: 10.1161/CIRCRESAHA.116.303448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannell MB, Cheng H, Lederer WJ (1994) Spatial non-uniformities in [Ca2+]i during excitation-contraction coupling in cardiac myocytes. Biophys J 67:1942–1956 [DOI] [PMC free article] [PubMed]
- Cannell MB, Crossman DJ, Soeller C. Effect of changes in action potential spike configuration, junctional sarcoplasmic reticulum micro-architecture and altered t-tubule structure in human heart failure. J Muscle Res Cell Motil. 2006;27:297–306. doi: 10.1007/s10974-006-9089-y. [DOI] [PubMed] [Google Scholar]
- Cannell MB, Kong CHT, Imtiaz MS, Laver DR. Control of sarcoplasmic reticulum Ca2+ release by stochastic RyR gating within a 3D model of the cardiac dyad and importance of induction decay for CICR termination. Biophys J. 2013;104:2149–2159. doi: 10.1016/j.bpj.2013.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Guo A, Zhang C, et al. Critical roles of junctophilin-2 in T-tubule and excitation–contraction coupling maturation during postnatal development. Cardiovasc Res. 2013;100:54–62. doi: 10.1093/cvr/cvt180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ. Calcium sparks. Physiol Rev. 2008;88:1491–1545. doi: 10.1152/physrev.00030.2007. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer MR, Lederer WJ, Cannell MB (1996) Calcium sparks and [Ca2+]i waves in cardiac myocytes. Am J Phys Cell Phys 270:C148–C159 [DOI] [PubMed]
- Chopra N, Yang T, Asghari P, et al. Ablation of triadin causes loss of cardiac Ca2+ release units, impaired excitation–contraction coupling, and cardiac arrhythmias. Proc Natl Acad Sci U S A. 2009;106:7636–7641. doi: 10.1073/pnas.0902919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connell P, Word TA, Wehrens XHT. Targeting pathological leak of ryanodine receptors: preclinical progress and the potential impact on treatments for cardiac arrhythmias and heart failure. Expert Opin Ther Targets. 2020;24:25–36. doi: 10.1080/14728222.2020.1708326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman DJ, Young AA, Ruygrok PN, et al. T-tubule disease: relationship between t-tubule organization and regional contractile performance in human dilated cardiomyopathy. J Mol Cell Cardiol. 2015;84:170–178. doi: 10.1016/j.yjmcc.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman DJ, Shen X, Jüllig M, et al. Increased collagen within the transverse tubules in human heart failure. Cardiovasc Res. 2017;113:879–891. doi: 10.1093/cvr/cvx055. [DOI] [PubMed] [Google Scholar]
- Denegri M, Avelino-Cruz JE, Boncompagni S, et al. Viral gene transfer rescues arrhythmogenic phenotype and ultrastructural abnormalities in adult calsequestrin-null mice with inherited arrhythmias. Circ Res. 2012;110:663–668. doi: 10.1161/CIRCRESAHA.111.263939. [DOI] [PubMed] [Google Scholar]
- Fabiato A, Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975;249:469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng W, Liu C, Spinozzi S, et al. Identifying the cardiac dyad proteome in vivo by a BioID2 knock-in strategy. Circulation. 2020;141:940–942. doi: 10.1161/CIRCULATIONAHA.119.043434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssmann WG, Girardier L. A study of the T system in rat heart. J Cell Biol. 1970;44:1–19. doi: 10.1083/jcb.44.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler ED, Wang N, Hezzell M, et al. Arrhythmogenic late Ca2 sparks in failing heart cells and their control by action potential configuration. Proc Natl Acad Sci. 2020;117:2687–2692. doi: 10.1073/pnas.1918649117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Porter KR. Sarcolemmal invaginations constituting the T system in fish muscle fibers. J Cell Biol. 1964;22:675–696. doi: 10.1083/jcb.22.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca2+ release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F, Tijskens P. The assembly of calcium release units in cardiac muscle. Ann N Y Acad Sci. 2005;1047:76–85. doi: 10.1196/annals.1341.007. [DOI] [PubMed] [Google Scholar]
- Frisk M, Ruud M, Espe EKS, et al. Elevated ventricular wall stress disrupts cardiomyocyte t-tubule structure and calcium homeostasis. Cardiovasc Res. 2016;112:443–451. doi: 10.1093/cvr/cvw111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Hong T. BIN1 regulates dynamic t-tubule membrane. Biochim Biophys Acta. 2016;1863:1839–1847. doi: 10.1016/j.bbamcr.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergs U, Berndt T, Buskase J, et al. On the role of junctin in cardiac Ca2+ handling, contractility, and heart failure. Am J Physiol Heart Circ Physiol. 2007;293:H728–H734. doi: 10.1152/ajpheart.01187.2006. [DOI] [PubMed] [Google Scholar]
- Gomez AM. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- Guo Y, Pu WT. Genetic mosaics for greater precision in cardiovascular research. Circ Res. 2018;123:27–29. doi: 10.1161/CIRCRESAHA.118.313386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Pu WT. Cardiomyocyte maturation: new phase in development. Circ Res. 2020;126:1086–1106. doi: 10.1161/CIRCRESAHA.119.315862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Hall D, Zhang C, et al. Molecular determinants of calpain-dependent cleavage of junctophilin-2 protein in cardiomyocytes. J Biol Chem. 2015;290:17946–17955. doi: 10.1074/jbc.M115.652396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, VanDusen NJ, Zhang L, et al. Analysis of cardiac myocyte maturation using CASAAV, a platform for rapid dissection of cardiac myocyte gene function in vivo. Circ Res. 2017;120:1874–1888. doi: 10.1161/CIRCRESAHA.116.310283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo A, Wang Y, Chen B et al (2018a) E-C coupling structural protein junctophilin-2 encodes a stress-adaptive transcription regulator. Science 362. 10.1126/science.aan3303 [DOI] [PMC free article] [PubMed]
- Guo Y, Jardin BD, Zhou P, et al. Hierarchical and stage-specific regulation of murine cardiomyocyte maturation by serum response factor. Nat Commun. 2018;9:3837. doi: 10.1038/s41467-018-06347-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Jardin BD, Sethi I, et al (2019) Sarcomeres regulate cardiomyocyte maturation through MRTF-SRF signaling. bioRxiv. 10.1101/824185 [DOI] [PMC free article] [PubMed]
- Gyorke S, Fill M (1993) Ryanodine receptor adaptation: control mechanism of Ca2+-induced Ca2+ release in heart. Science 260:807–809 [DOI] [PubMed]
- He J. Reduction in density of transverse tubules and L-type Ca2 channels in canine tachycardia-induced heart failure. Cardiovasc Res. 2001;49:298–307. doi: 10.1016/s0008-6363(00)00256-x. [DOI] [PubMed] [Google Scholar]
- He W, Huang D, Guo S, et al. Association with SERCA2a directs phospholamban trafficking to sarcoplasmic reticulum from a nuclear envelope pool. J Mol Cell Cardiol. 2020;143:107–119. doi: 10.1016/j.yjmcc.2020.04.025. [DOI] [PubMed] [Google Scholar]
- Heinzel FR, Bito V, Biesmans L, et al. Remodeling of T-tubules and reduced synchrony of Ca2 release in myocytes From chronically ischemic myocardium. Circ Res. 2008;102:338–346. doi: 10.1161/CIRCRESAHA.107.160085. [DOI] [PubMed] [Google Scholar]
- Hong T, Shaw RM. Cardiac T-tubule microanatomy and function. Physiol Rev. 2017;97:227–252. doi: 10.1152/physrev.00037.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T-T, Smyth JW, Gao D, et al. BIN1 Localizes the L-type calcium channel to cardiac T-tubules. PLoS Biol. 2010;8:e1000312. doi: 10.1371/journal.pbio.1000312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T-T, Smyth JW, Chu KY, et al. BIN1 is reduced and Cav1.2 trafficking is impaired in human failing cardiomyocytes. Heart Rhythm. 2012;9:812–820. doi: 10.1016/j.hrthm.2011.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong T, Yang H, Zhang S-S, et al. Cardiac BIN1 folds T-tubule membrane, controlling ion flux and limiting arrhythmia. Nat Med. 2014;20:624–632. doi: 10.1038/nm.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen AO, Shen AC, Daly P, MacLennan DH (1982) Localization of Ca2+ Mg2+ -ATPase of the sarcoplasmic reticulum in adult rat papillary muscle. J Cell Biol 93:883–892 [DOI] [PMC free article] [PubMed]
- Kaprielian RR, Stevenson S, Rothery SM, et al. Distinct patterns of dystrophin organization in myocyte sarcolemma and transverse tubules of normal and diseased human myocardium. Circulation. 2000;101:2586–2594. doi: 10.1161/01.cir.101.22.2586. [DOI] [PubMed] [Google Scholar]
- Knollmann BC. A “rough” journey to the sarcoplasmic reticulum--implications of altered calsequestrin trafficking for cardiac arrhythmia. J Mol Cell Cardiol. 2010;49:554–555. doi: 10.1016/j.yjmcc.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolstad TR, van den Brink J, MacQuaide N et al (2018) Ryanodine receptor dispersion disrupts Ca2 release in failing cardiac myocytes. eLife 7 [DOI] [PMC free article] [PubMed]
- Landstrom AP, Kellen CA, Dixit SS, et al. Junctophilin-2 expression silencing causes cardiocyte hypertrophy and abnormal intracellular calcium-handling. Circ Heart Fail. 2011;4:214–223. doi: 10.1161/CIRCHEARTFAILURE.110.958694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laury-Kleintop LD, Mulgrew JR, Heletz I, et al. Cardiac-specific disruption of Bin1 in mice enables a model of stress- and age-associated dilated cardiomyopathy. J Cell Biochem. 2015;116:2541–2551. doi: 10.1002/jcb.25198. [DOI] [PubMed] [Google Scholar]
- Lee E, Marcucci M, Daniell L, et al. Amphiphysin 2 (Bin1) and T-tubule biogenesis in muscle. Science. 2002;297:1193–1196. doi: 10.1126/science.1071362. [DOI] [PubMed] [Google Scholar]
- Liu C, Spinozzi S, Chen J-Y et al (2019) Nexilin is a new component of junctional membrane complexes required for cardiac T-tubule formation. Circulation. 10.1161/CIRCULATIONAHA.119.039751 [DOI] [PMC free article] [PubMed]
- Liu G, Papa A, Katchman AN, et al. Mechanism of adrenergic CaV1.2 stimulation revealed by proximity proteomics. Nature. 2020;577:695–700. doi: 10.1038/s41586-020-1947-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon AR, MacLeod KT, Zhang Y, et al. Loss of T-tubules and other changes to surface topography in ventricular myocytes from failing human and rat heart. Proc Natl Acad Sci. 2009;106:6854–6859. doi: 10.1073/pnas.0809777106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio AD, Di Maio A, Karko K, et al. T-tubule formation in cardiac myocytes: two possible mechanisms? J Muscle Res Cell Motil. 2007;28:231–241. doi: 10.1007/s10974-007-9121-x. [DOI] [PubMed] [Google Scholar]
- Marx SO, Gaburjakova J, Gaburjakova M, et al. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- Mata ADL, De La Mata A, Tajada S, et al. BIN1 induces the formation of T-tubules and adult-like Ca2 release units in developing cardiomyocytes. Stem Cells. 2019;37:54–64. doi: 10.1002/stem.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzarotto F, Tayal U, Buchan RJ, et al. Reevaluating the genetic contribution of monogenic dilated cardiomyopathy. Circulation. 2020;141:387–398. doi: 10.1161/CIRCULATIONAHA.119.037661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt NS. Ultrastructure of the myocardial sarcolemma. Circ Res. 1975;37:1–13. doi: 10.1161/01.res.37.1.1. [DOI] [PubMed] [Google Scholar]
- Minamisawa S, Oshikawa J, Takeshima H, et al. Junctophilin type 2 is associated with caveolin-3 and is down-regulated in the hypertrophic and dilated cardiomyopathies. Biochem Biophys Res Commun. 2004;325:852–856. doi: 10.1016/j.bbrc.2004.10.107. [DOI] [PubMed] [Google Scholar]
- Mitchell RD, Simmerman HK, Jones LR. Ca2+ binding effects on protein conformation and protein interactions of canine cardiac calsequestrin. J Biol Chem. 1988;263:1376–1381. [PubMed] [Google Scholar]
- Ohtsuka T, Nakanishi H, Ikeda W, et al. Nexilin: a novel actin filament-binding protein localized at cell-matrix adherens junction. J Cell Biol. 1998;143:1227–1238. doi: 10.1083/jcb.143.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh SS, Blackwell DJ, Gomez-Hurtado N, et al. Thyroid and glucocorticoid hormones promote functional T-tubule development in human-induced pluripotent stem cell-derived cardiomyocytes. Circ Res. 2017;121:1323–1330. doi: 10.1161/CIRCRESAHA.117.311920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pásek M, Brette F, Nelson A, et al. Quantification of t-tubule area and protein distribution in rat cardiac ventricular myocytes. Prog Biophys Mol Biol. 2008;96:244–257. doi: 10.1016/j.pbiomolbio.2007.07.016. [DOI] [PubMed] [Google Scholar]
- Pásek M, Šimurda J, Orchard CH, Christé G. A model of the guinea-pig ventricular cardiac myocyte incorporating a transverse–axial tubular system. Prog Biophys Mol Biol. 2008;96:258–280. doi: 10.1016/j.pbiomolbio.2007.07.022. [DOI] [PubMed] [Google Scholar]
- Piacentino V, Weber CR, Chen X, et al. Cellular basis of abnormal calcium transients of failing human ventricular myocytes. Circ Res. 2003;92:651–658. doi: 10.1161/01.RES.0000062469.83985.9B. [DOI] [PubMed] [Google Scholar]
- Prendiville TW, Guo H, Lin Z, et al. Novel roles of GATA4/6 in the postnatal heart identified through temporally controlled, cardiomyocyte-specific gene inactivation by adeno-associated virus delivery of Cre recombinase. PLoS One. 2015;10:e0128105. doi: 10.1371/journal.pone.0128105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quick AP, Wang Q, Philippen LE, et al. SPEG (striated muscle preferentially expressed protein kinase) is essential for cardiac function by regulating junctional membrane complex activity. Circ Res. 2017;120:110–119. doi: 10.1161/CIRCRESAHA.116.309977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaq A, Robinson IM, McMahon HT, et al. Amphiphysin is necessary for organization of the excitation-contraction coupling machinery of muscles, but not for synaptic vesicle endocytosis in Drosophila. Genes Dev. 2001;15:2967–2979. doi: 10.1101/gad.207801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JO, Chiang DY, Wang W, et al. Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc Res. 2013;100:44–53. doi: 10.1093/cvr/cvt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzi N, Liu N, Napolitano C, et al. Unexpected structural and functional consequences of the R33Q homozygous mutation in cardiac calsequestrin: a complex arrhythmogenic cascade in a knock in mouse model. Circ Res. 2008;103:298–306. doi: 10.1161/CIRCRESAHA.108.171660. [DOI] [PubMed] [Google Scholar]
- Ronaldson-Bouchard K, Ma SP, Yeager K et al (2018) Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 10.1038/s41586-018-0016-3 [DOI] [PMC free article] [PubMed]
- Savio-Galimberti E, Frank J, Inoue M, et al. Novel features of the rabbit transverse tubular system revealed by quantitative analysis of three-dimensional reconstructions from confocal images. Biophys J. 2008;95:2053–2062. doi: 10.1529/biophysj.108.130617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel T, Navankasattusas S, Ahmad A, et al. Sheet-like remodeling of the transverse tubular system in human heart failure impairs excitation-contraction coupling and functional recovery by mechanical unloading. Circulation. 2017;135:1632–1645. doi: 10.1161/CIRCULATIONAHA.116.024470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang W, Lu F, Sun T et al (2014) Imaging Ca2+ Nanosparks in heart with a new targeted biosensor. Circ Res 114:412–420 [DOI] [PubMed]
- Shiels HA, Galli GLJ. The sarcoplasmic reticulum and the evolution of the vertebrate heart. Physiology. 2014;29:456–469. doi: 10.1152/physiol.00015.2014. [DOI] [PubMed] [Google Scholar]
- Shiferaw Y, Aistrup GL, Andrew Wasserstrom J (2012) Intracellular Ca2+ waves, afterdepolarizations, and triggered arrhythmias. Cardiovasc Res 95:265–268 [DOI] [PMC free article] [PubMed]
- Slupsky JR, Ohnishi M, Carpenter MR, Reithmeier RA. Characterization of cardiac calsequestrin. Biochemistry. 1987;26:6539–6544. doi: 10.1021/bi00394a038. [DOI] [PubMed] [Google Scholar]
- Soeller C, Cannell MB. Examination of the transverse tubular system in living cardiac rat myocytes by 2-photon microscopy and digital image-processing techniques. Circ Res. 1999;84:266–275. doi: 10.1161/01.res.84.3.266. [DOI] [PubMed] [Google Scholar]
- Song L-S, Sobie EA, McCulle S, et al. Orphaned ryanodine receptors in the failing heart. Proc Natl Acad Sci. 2006;103:4305–4310. doi: 10.1073/pnas.0509324103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N, Rubio R. An orderly lattice of axial tubules which interconnect adjacent transverse tubules in guinea-pig ventricular myocardium. J Mol Cell Cardiol. 1971;2:211–220. doi: 10.1016/0022-2828(71)90054-x. [DOI] [PubMed] [Google Scholar]
- Stern MD. Theory of excitation-contraction coupling in cardiac muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern MD, Song LS, Cheng H, et al. Local control models of cardiac excitation-contraction coupling. A possible role for allosteric interactions between ryanodine receptors. J Gen Physiol. 1999;113:469–489. doi: 10.1085/jgp.113.3.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumitomo N. Current topics in catecholaminergic polymorphic ventricular tachycardia. J Arrhythm. 2016;32:344–351. doi: 10.1016/j.joa.2015.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima H. Intracellular Ca2+ store in embryonic cardiac myocytes. Front Biosci. 2002;7:d1642–d1652. doi: 10.2741/takeshim. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Komazaki S, Nishi M, et al. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Trinkle-Mulcahy L (2019) Recent advances in proximity-based labeling methods for interactome mapping. F1000Res 8. 10.12688/f1000research.16903.1 [DOI] [PMC free article] [PubMed]
- Vangheluwe P, Louch WE, Ver Heyen M, et al. Ca2+ transport ATPase isoforms SERCA2a and SERCA2b are targeted to the same sites in the murine heart. Cell Calcium. 2003;34:457–464. doi: 10.1016/s0143-4160(03)00126-x. [DOI] [PubMed] [Google Scholar]
- Wagner E, Lauterbach MA, Kohl T, et al. Stimulated emission depletion live-cell super-resolution imaging shows proliferative remodeling of T-tubule membrane structures after myocardial infarction. Circ Res. 2012;111:402–414. doi: 10.1161/CIRCRESAHA.112.274530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- Wang S-Q, Song L-S, Xu L, et al. Thermodynamically irreversible gating of ryanodine receptors in situ revealed by stereotyped duration of release in Ca2 sparks. Biophys J. 2002;83:242–251. doi: 10.1016/S0006-3495(02)75165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SQ, Stern MD, Rios E, Cheng H (2004) The quantal nature of Ca2+ sparks and in situ operation of the ryanodine receptor array in cardiac cells. Proc Natl Acad Sci 101:3979–3984 [DOI] [PMC free article] [PubMed]
- Wei S, Guo A, Chen B, et al. T-Tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S, Guo A, Chen B, et al. T-Tubule remodeling during transition from hypertrophy to heart failure. Circ Res. 2010;107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright PT, Nikolaev VO, O’Hara T, et al. Caveolin-3 regulates compartmentation of cardiomyocyte beta2-adrenergic receptor-mediated cAMP signaling. J Mol Cell Cardiol. 2014;67:38–48. doi: 10.1016/j.yjmcc.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Wu H-D, Li R-C, et al. Mir-24 regulates junctophilin-2 expression in cardiomyocytes. Circ Res. 2012;111:837–841. doi: 10.1161/CIRCRESAHA.112.277418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Q, Fan G-C, Dong M, et al. Sarcoplasmic reticulum calcium overloading in junctin deficiency enhances cardiac contractility but increases ventricular automaticity. Circulation. 2007;115:300–309. doi: 10.1161/CIRCULATIONAHA.106.654699. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kelley J, Schmeisser G, et al. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]
- Zhang H-B, Li R-C, Xu M, et al. Ultrastructural uncoupling between T-tubules and sarcoplasmic reticulum in human heart failure. Cardiovasc Res. 2013;98:269–276. doi: 10.1093/cvr/cvt030. [DOI] [PubMed] [Google Scholar]
- Zhang C, Chen B, Guo A et al (2014) Microtubule-mediated defects in junctophilin-2 trafficking contribute to myocyte transverse-tubule remodeling and Ca2+ handling dysfunction in heart failure. Circulation 129:1742–1750 [DOI] [PMC free article] [PubMed]
- Zhang D, Li Y, Heims-Waldron DA, et al. Mitochondrial cardiomyopathy caused by elevated reactive oxygen species and impaired cardiomyocyte proliferation. Circ Res. 2017;122:7. doi: 10.1161/CIRCRESAHA.117.311349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziman AP, Gómez-Viquez NL, Bloch RJ, Lederer WJ. Excitation-contraction coupling changes during postnatal cardiac development. J Mol Cell Cardiol. 2010;48:379–386. doi: 10.1016/j.yjmcc.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]