Abstract
Heart disease represents a significant public health burden and is associated with considerable morbidity and mortality at the level of the individual. Current therapies for pathologies such as myocardial infarction, cardiomyopathy and heart failure are unable to repair damaged tissue to an extent that provides restoration of function approaching that of the pre-diseased state. Novel approaches to repair and regenerate the injured heart include cell therapy and the use of exogenous factors. Improved understanding of the role of growth factors in endogenous cardiac repair processes has motivated the investigation of their potential as therapeutic agents for cardiac pathology. Despite the disappointing performance of other growth factors in historical clinical trials, insulin-like growth factor 1 (IGF-1), neuregulin and platelet-derived growth factor (PDGF) have recently emerged as new candidate therapies. These growth factors elicit tissue repair through anti-apoptotic, pro-angiogenic and fibrosis-modulating mechanisms and have produced clinically significant functional improvement in preclinical studies. Early human trials suggest that IGF-1 and neuregulin are well tolerated and yield dose-dependent benefit, warranting progression to later phase studies. However, outstanding challenges such as short growth factor serum half-life and insufficient target-organ specificity currently necessitate the development of novel delivery strategies.
Keywords: Growth factor, Cardiac repair, Insulin-like growth factor 1, Neuregulin, Platelet-derived growth factor
Introduction
The mammalian heart possesses a very poor regenerative capacity following injury. This results in pathological structural changes such as cardiomyocyte loss and myocardial fibrosis that impair function (Frangogiannis 2012). Despite the significant societal burden associated with cardiac pathology (Mathers and Loncar 2006), we currently lack interventions capable of repairing or regenerating the injured heart to a degree that would significantly restore function to that of the pre-injury state. Regenerative cardiovascular medicine is an emerging field featuring both cell therapy and exogenous factor-based approaches. Growth factors are a subset of protein factors that offer therapeutic promise given their fundamental role as regulators of cellular functions such as proliferation, migration and adhesion in cardiac repair. In addition, the regenerative capacity of growth factors has been highlighted by cell therapy studies that demonstrate much of the benefit associated with stem cell delivery to injured myocardium can be attributed to the paracrine actions of stem cell-secreted growth factors (Tachibana et al. 2017; Gnecchi et al. 2008; Mirotsou et al. 2011).
During the late twentieth century, growth factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF) and human growth hormone (hGH) yielded a series of positive results in preclinical models of myocardial ischemia and infarction, prompting progression to clinical trials. Unfortunately, these growth factors performed disappointingly in human studies (Simons et al. 2002; Henry et al. 2003; Osterziel et al. 1998; Isgaard et al. 1998), which subsequently blunted interest in the field. However, improved understanding of the role of growth factors in cardioprotective and reparative processes has recently yielded several new candidate growth factors such as insulin-like growth factor-1 (IGF-1), epidermal growth factor family member neuregulin and platelet-derived growth factor (PDGF).
This review aims to assess the viability of growth factor therapy for cardiac pathology by identifying novel growth factor therapies and summarizing the current state of knowledge regarding their safety and efficacy in preclinical and early human studies.
Methods
OVID versions of EMBASE (1947 through to 2020 week 1), MEDLINE (1946 through to January 2020, week 1), and the Cochrane Central Register of Controlled Trials (Issue 1, January 2020) were systematically searched for relevant randomized controlled studies. Full-text manuscripts and abstracts were accepted and language restrictions were not imposed. The search terms used included keywords ‘growth factor’, ‘_gf’, ‘heart failure’, ‘dilated cardiomyopathy’, ‘ischaemic cardiomyopathy’, ‘ischemic cardiomyopathy’, ‘hypertrophic cardiomyopathy’, ‘myocardial infarction’, ‘myocardial infarct’, ‘heart attack*’ and MeSH terms ‘Intercellular Signaling Peptides and Proteins’, ‘Heart Failure’, ‘Cardiomyopathy, Dilated’, ‘Cardiomyopathy, Hypertrophic’, ‘Myocardial Infarction,’ which were combined using Boolean operators “AND” and “OR”. Reference lists of included articles and personal files were also scanned for relevant studies. Inclusion criteria were the therapeutic use of one or more growth factors to address a myocardial pathology and the measurement of at least one structural or functional cardiac outcome. Studies combining therapeutic growth factors with other forms of reparative treatment such as cell therapy were excluded.
The role of growth factors in mammalian endogenous cardiac repair—a rationale for growth factor therapy
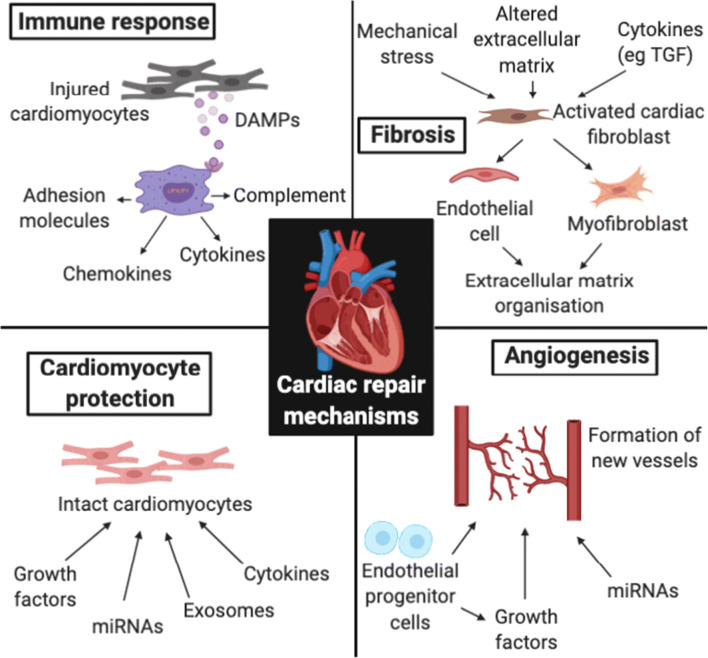
Prolonged myocardial ischemia induces cardiomyocyte and parenchymal cell death primarily not only through coagulative necrosis but also via autophagic and apoptotic pathways. Restoration of myocardial perfusion may exacerbate tissue injury through the production of reactive oxygen species (ROS) (Zhu et al. 2007) and complement pathway activation (Rossen et al. 1985; Vakeva et al. 1994). Damaged cells and extracellular matrix secrete danger-associated molecular patterns (DAMPs) that activate cognate pattern recognition receptors (PRRs) expressed by infiltrating immune cells and intact resident parenchymal cells (Fig. 1). Signal transduction pathways downstream of PRRs converge at the stimulation of transcription factors such as NF-kB, which upregulates a pro-inflammatory gene profile including cytokines, chemokines, adhesion molecules and components of complement pathways.
Fig. 1.
Acute immune response, fibrosis, angiogenesis and cardiomyocyte protection represent potential reparative mechanisms operating via distinct molecular pathways. These processes interact with one another, mediating the protection, modulation and regeneration of functional tissue. DAMPs, danger-associated molecular patterns; TGF, transforming growth factor. Notes: 1. Figure made using BioRender program with a paid subscription
Replacement fibrosis involves the deposition of fibrous scar tissue at the site of myocardial infarction (MI), which is critical for the stabilization of ventricular wall integrity during the proliferative phase of acute healing. In the first week following MI, resident cardiac fibroblasts undergo expansion in the infarct and border areas that peaks 2–4 days after injury (Fu et al. 2018) before assuming an activated phenotype. Activated cardiac fibroblasts exhibit multipotent competency and transdifferentiate into endothelial cells (Ubil et al. 2014) and myofibroblasts (Ubil et al. 2014) (Fig. 1). The conversion of cardiac fibroblasts to myofibroblasts is driven by an array of stimuli including mechanical stress (Fu et al. 2018), altered extracellular matrix (ECM) composition (Serini et al. 1998) and immune cell-derived cytokines such as transforming growth factor-β (TGF-β) (Serini et al. 1998). Recent lineage-tracing studies suggest that myofibroblasts constitute a transient network of support cells most active 3–10 days after injury (Ubil et al. 2014). Myofibroblasts serve to rapidly stabilize the ventricular wall through their ability to contract via α-smooth muscle actin as well as secrete ECM proteins and other non-structural matricellular proteins such as thrombospondins, tenascins and connective tissue growth factor (CTGF) that act contextually by regulating cellular responses to cytokines and growth factors (Frangogiannis 2012). Whilst the persistence of cardiac fibroblasts at the site of injury for many weeks after infarction is known to confer long-term scar stability (Fu et al. 2018), further work is required to characterize additional stages of fibroblast differentiation and the specific processes mediated by functional subsets of fibroblasts.
Following myocardial injury, ventricular wall stress as well as paracrine and hormonal factors mediate reactive fibrosis whereby fibrotic tissue deposition occurs in remote healthy myocardium. Whilst fibroblasts and myofibroblasts have been identified as key mediators of reactive fibrosis, the exact mechanisms involved remain largely unresolved. Mechanical stress in uninjured myocardium promotes the activation of fibrotic mediator TGF-β (Fu et al. 2018). In addition, pro-fibrotic factors secreted by myofibroblasts at the infarct border may diffuse into adjacent uninjured myocardium and drive the activation and proliferation of collagen-secreting fibroblasts (Fu et al. 2018). Reactive fibrosis stiffens the ventricular wall, impairing compliance and therefore cardiac output, which increases the risk of subsequent heart failure.
Angiogenesis involves the outgrowth of microvasculature from pre-existing vessels, which assists in the delivery of oxygen and nutrients and removal of waste products from tissue. This process is not only critical for the repair of myocardial injury, but also in the preservation of adjacent intact myocardium that can become threatened due to microvascular dysfunction (Yajima et al. 2019) or elevated metabolic demand secondary to mechanical stress (Vikhert and Cherpachenko 1974). Hypoxia promotes the upregulation of pro-angiogenic growth factors such as vascular endothelial growth factor (VEGF) (Shweiki et al. 1992) and platelet-derived growth factor (PDGF) (Kourembanas et al. 1990) driving the activation of previously quiescent endothelial cells. Plasma protein extravasation subsequent to enhanced microvascular permeability gives rise to a provisional extravascular matrix (Dobaczewski et al. 2006) onto which endothelium may migrate to form neovessels (Fig. 1) (Clark 1988). Endothelial progenitor cells (EPCs) are also recruited to sites of myocardial vascular injury (Fadini et al. 2009). EPCs mediate restitution of local microvasculature through both direct incorporation into new vessels (Ziebart et al. 2008) and the secretion of paracrine factors important for tprotection of mature endothelial cells against oxidative injury (Yang et al. 2010).
The native regenerative potential of adult mammalian myocardium is poor (Bergmann et al. 2015). Physiologically relevant cardiomyocyte proliferation occurs in the developing embryonic, but not adult, heart (Ieda et al. 2009; Porrello et al. 2011). Historically, regeneration of adult cardiomyocytes was hypothesised to occur through the recruitment and subsequent proliferation and differentiation of stem or progenitor cells. However, initial intense preclinical research into harnessing the regenerative potential of endogenous stem cells or delivering exogenous adult stem cells for cardiomyogenesis failed to deliver consistent results in clinical trials (Menasché et al. 2008; Duckers et al. 2011; Heeschen et al. 2004; Chen et al. 2004; Wang et al. 2006). These findings, along with recent stem cell lineage tracing studies demonstrating that any endogenous cardiac progenitor cell has a very limited cardiomyogenic capacity (van Berlo et al. 2014; Sultana et al. 2015; Liu et al. 2016; Li et al. 2018; Vagnozzi et al. 2018), have made the potential role for adult stem cells in cardiomyocyte regeneration highly contentious. Rather, benefits associated with adult stem cell therapy may instead be attributed to other mechanisms such as protection of intact cardiomyocytes and potentiation of the acute immune response (Vagnozzi et al. 2019) (Fig. 1) as opposed to the formation of new cardiomyocytes. This has implications for the proposed mechanisms underpinning cardiac growth factor therapy and will be discussed further below.
Growth factor therapy—a viable treatment for myocardial pathology?
Learning from the past
The poor historical performance of growth factors such as VEGF, bFGF and hGH in clinical trials (Simons et al. 2002; Henry et al. 2003; Osterziel et al. 1998; Isgaard et al. 1998) is likely related to a number of factors.
Delivery
Strategies for delivery of growth factors to the heart can be broadly categorized as either protein or gene therapy. Protein therapy generally involves the administration of a functional growth factor protein to injured myocardium via the circulation and is therefore conceptually tractable. In contrast, gene therapy (reviewed in Ishikawa et al. 2018; Kieserman et al. 2019) is less clear as it not only requires delivery of genetic material to target cells but also hosts cell machinery to translate the genetic material into a functional protein that can be secreted into the local microenvironment at therapeutic levels.
During the 1990s and early 2000s, a number of trials were performed whereby naked DNA plasmids coding for pro-angiogenic factors such as VEGF were directly injected into the myocardium of patients with chronic myocardial ischemia (Losordo et al. 1998; Symes et al. 1999) under the assumption that the plasmids would be spontaneously internalized by cardiomyocytes. Similar to any other cell membrane, the cardiomyocyte sarcolemma repels negatively charged DNA, rendering this approach largely ineffectual. Improvement in vector technology has given rise to cardiotropic adeno-associated virus (AAV) serotypes, which are now considered the gold standard for cardiomyocyte gene therapy due to their indefinite transgene expression (Penaud-Budloo et al. 2008). Refinement of AAV-mediated VEGF delivery is currently underway (Shi et al. 2020) and may offer cardiac VEGF gene therapy a renaissance if successful. Remaining challenges in AAV development include reducing immunogenicity and enhancing cardiac tropism to allow for intravenous as opposed to intracoronary delivery.
Major barriers to growth factor protein therapy include low protein stability, deactivation by enzymes, short serum half-life, poor target organ specificity and potential toxicity at high blood concentrations, which suggests growth factors may be poorly suited to the conventional delivery strategies used in past clinical trials. To address this, attention has turned toward bioengineering more sophisticated delivery strategies (reviewed in Ferrini et al. 2019) to enhance the therapeutic efficacy of growth factors that previously failed in human studies as well as factors such as neuregulin-1 (Cohen et al. 2014; Garbayo et al. 2016), which have only recently been used in clinical trials.
Trial design
Many of the early VEGF clinical trials for chronic myocardial ischemia that demonstrated treatment benefit had highly flawed uncontrolled open-label designs. The use of more rigorous study designs in subsequent randomized, double-blinded, placebo-controlled studies such as the EUROINJECT-ONE (Kastrup et al. 2005) and NORTHERN (Stewart et al. 2009) trials failed to replicate improvement in key outcomes such as perfusion, which significantly blunted enthusiasm in the field. It is therefore essential to ensure appropriate study design in future preclinical and clinical studies involving growth factor therapy.
Combination therapy
Historically, the biotechnology sector has prioritized developing novel cardiac monotherapies because combination therapies are associated with greater mechanistic complexity, increased regulatory demand and therefore delays in bringing products to market. However, considering the multifactorial nature of the cardiac repair, combining different growth factors or even growth factors with other therapeutic modalities may enhance the potential for improved outcomes (and therefore approval for clinical use).
Insulin-like growth factor 1
IGF-1 is a member of the IGF (somatomedin) family that comprises 70 amino acid residues with three cross-linking disulphide bridges (Rinderknecht and Humbel 1978). It is produced by the liver in response to growth hormone and is an important mediator of childhood growth and anabolism in adults.
The rationale for use in cardiac repair
A potential cardioprotective role for IGF-1 was first demonstrated by Buerke et al. who used a murine myocardial ischemia-reperfusion model to show IGF-1 pre-treatment decreases myocardial cell death and neutrophil accumulation (Buerke et al. 1995). Expression of the IGF-1 receptor was subsequently observed in human endothelial (Chisalita and Arnqvist 2004) and vascular smooth muscle cells (Chisalita et al. 2009). Given its role in promoting cell cycle progression in a number of cell types (Radcliff et al. 2005; Mairet-Coello et al. 2009), attention soon turned toward whether IGF-1 was a potential mediator of cardiomyocyte proliferation in the injured mammalian heart. This motivated studies showed that IGF-1 signalling increased proliferation of human embryonic stem cell-derived cardiomyocytes in vitro (McDevitt et al. 2005) and drove cardiomyocyte proliferation in the regenerating injured zebrafish heart (Huang et al. 2013).
Mechanism of action
IGF-1 is an important regulator of cardiomyocyte homeostasis following injury through both pro-survival and anti-apoptotic mechanisms (Table 1). Low-dose intracoronary IGF-1 administered 2 h following reperfusion in a porcine MI model was shown to induce phosphorylation of the IGF-1 receptor and downstream mediators of pro-survival signalling pathways such as protein kinase B (Akt) and extracellular signal-related kinase (ERK) in the ischemic border zone (O'Sullivan et al. 2011). Consistent with enhanced Akt activity, this was associated with enhanced phosphorylation (inactivation) of GSK-3β (O'Sullivan et al. 2011), which has been implicated in the pro-necrotic mitochondrial permeability transition pore (mPTP)–associated pathway (Gomez et al. 2008). This correlated to an increased ischemic zone cardiomyocyte count that was maintained at 2 months post-injury compared with saline-treated controls (O'Sullivan et al. 2011). Intracoronary IGF-1 mRNA has also been shown to amplify Akt and ERK phosphorylation as well as reduce the activity of pro-apoptotic enzyme caspase-9 in the ischemic border zone 24 h after MI in a murine model (Huang et al. 2015). Endothelial progenitor cells have emerged as an important source of IGF-1 with anti-IGF-1 neutralizing antibodies abrogating infarct border zone angiogenesis and improvement in left ventricular contractility 8 weeks after MI in pigs treated with endothelial progenitor cell-derived conditioned media (Hynes et al. 2013).
Table 1.
Mechanisms of cardiac repair induced by growth factors IGF-1, neuregulin and PDGF
| Growth factor | Mechanisms | References |
|---|---|---|
| IGF-1 | Anti-apoptosis | 30, 32, 36 |
| Angiogenesis | 33 | |
| Expansion of resident cardiac progenitor cells | 35 | |
| Anti-fibrosis | 36 | |
| Neuregulin | Cardiomyocyte proliferation | 46, 53 |
| Restoration of cardiomyocyte sarcomere alignment | 44, 45 | |
| Maintenance of cardiomyocyte intracellular calcium homeostasis | 47 | |
| PDGF | Anti-apoptosis | 60, 61 |
| Angiogenesis | 56, 57, 68 | |
| Pro-fibrosis | 62, 65–67 | |
| Fibrosis modulation | 68 |
IGF-1, insulin-like growth factor-1; PDGF, platelet-derived growth factor
In addition to regulating myocardial repair immediately following ischemic insult, IGF-1 may also confer benefit in established disease through anti-apoptotic and anti-fibrotic mechanisms. Intramuscular injection of adeno-associated virus 5 encoding IGF-1 in a murine heart failure model significantly reduced cardiomyocyte apoptosis and left ventricular myocardial fibrosis (measured using collagen fractional area) after 5 weeks (Lai et al. 2012). These histological changes correlated to functional benefit with IGF-1-treated animals exhibiting increased left ventricular ejection fraction (LVEF) and decreased left ventricular end-systolic volume (LVESV) (Lai et al. 2012).
Clinical testing
The RESUS-AMI pilot trial evaluated the safety and efficacy of low-dose (1.5 ng or 15 ng) intracoronary IGF-1 following percutaneous coronary intervention in patients with ST-elevation MI and LVEF < 40% (Table 2). There was no difference in the primary safety endpoint (freedom from hypoglycaemia) or secondary safety endpoints (freedom from significant hypotension or arrhythmia) 1 h post-PCI (Caplice et al. 2018). Furthermore, despite all groups achieving increased LVEF after 8 weeks, there was no significant difference in LVEF improvement between the treatment and control groups (Caplice et al. 2018). The doses of IGF-1 used in the RESUS-AMI trial were 100,000 times lower than the amount already proven to be safe in humans, which may explain the lack of efficacy observed. Whilst such low doses yielded promising results in an earlier porcine study (O'Sullivan et al. 2011), greater human disease heterogeneity and extent of inter-subject variability should necessitate higher dosing in future human studies. Another limitation of RESUS-AMI was that its relatively small sample size and high variability in baseline characteristics such as LVEF impaired the statistical power to measure differences in efficacy. Future studies should aim to achieve more homogenous baseline characteristics amongst study groups. Dosing and delivery modality also deserve further consideration. Given IGF-1 has a half-life of 14 min and cardiomyocyte death occurs over 24–72 h following ischemic insult, the use of sequential dosing or controlled release IGF-1 may confer longer-lasting cytoprotective benefit compared with single bolus regimens.
Table 2.
Randomized controlled trials evaluating IGF-1 and neuregulin for cardiac repair in humans
| Growth factor | Pathology | Intervention | Findings | References |
|---|---|---|---|---|
| IGF-1 | Acute ST-elevation myocardial infarction with LVEF ≤ 40% | Single intracoronary infusion of 1.5 ng or 15 ng rhIGF-1 during PCI |
• Well-tolerated • Dose-dependent modulation of post-MI myocardial remodelling after 8 weeks • Increase in LVEF lacked statistical significance compared to placebo |
37 |
| Neuregulin | Heart failure with reduced ejection fraction (LVEF ≤ 40%; New York Heart Association functional class II or III) | Continuous 10-h intravenous infusion of 0.3, 0.6 or 1.2 μg/kg/day rhNRG-1 for 10 consecutive days |
• Well-tolerated • Significantly increased LVEF after 30 days in 0.6 μg/kg/day group • Beneficial myocardial remodelling maintained after 90 days |
47 |
| Heart failure with reduced ejection fraction (LVEF ≤ 40%; New York Heart Association functional class II or III) | Single 30-min intravenous infusion of 0.007, 0.021, 0.063, 0.19, 0.38, 0.76 or 1.5 mg/kg NRG-1β3 |
• Well-tolerated except for one case of transient hyperbilirubinemia and elevated liver transaminases • Dose-dependent improvement in LVEF after 90 days |
49 |
IGF-1, insulin-like growth factor-1; rhIGF-1, recombinant human insulin-like growth factor-1; rhNRG-1, recombinant human neuregulin-1; NRG-1β3, neuregulin-1β3; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention
Neuregulin
The neuregulins comprise four structurally similar proteins (neuregulin-1, -2, -3, and -4) that are members of the epidermal growth factor family. Whilst neuregulin-1 is known to play an important role in the development of the nervous system, mammary tissue and heart (Britsch 2007), the roles of the other neuregulins remain relatively uncertain.
The rationale for use in cardiac repair
The neuregulin-1-erbB2/erbB4 signalling pathway is crucial in the myocardial trabeculation and endocardial cushion development stages of cardiac development (Gassmann et al. 1995). This piqued interest in neuregulin-1 as a potential mediator of cardiac repair. Upregulation of neuregulin was subsequently observed in acute myocardial stressors such as ischemia-reperfusion injury (Fang et al. 2010; Kuramochi et al. 2004) and ventricular pressure overload (Rohrbach et al. 1999) as well as in patients with advanced heart failure (Rohrbach et al. 2005). A seminal study performed by Bersell et al. demonstrated that activation of the neuregulin-1-erbB4 axis produces functionally significant regeneration of injured postnatal murine myocardium by increasing mononucleated cardiomyocyte cell cycle activity (Bersell et al. 2009). This has prompted the use of neuregulin-1 as an effector of cardiac repair in recent clinical trials.
Mechanism of action
Neuregulin is an important mediator of myocardial repair through numerous mechanisms (Table 1). In both embryonic and neonatal (Baliga et al. 1999) murine cardiomyocytes, neuregulin has been shown to regulate sarcomere alignment associated with dose-dependent amplification of MAP kinase, a downstream mediator of erbB. Neuregulin also induces cardiomyocyte proliferation through post-infarct differentiated adult mouse cardiomyocyte cell cycle re-entry in vivo (Bersell et al. 2009). This was associated with neuregulin-treated animals exhibiting sustained attenuation of pathological remodelling phenomena such as left ventricular dilatation and interventricular septum hypertrophy at 15 weeks after infarction as well as significantly increased LVEF compared with controls (Bersell et al. 2009). In addition, improvement in intracellular calcium homeostasis and cardiomyocyte contractility through downregulation of protein phosphatase in neuregulin-treated models of heart failure has also been observed (Gao et al. 2010).
Given the downregulation of erbB in established disease states such as heart failure, developing an improved understanding of the mechanisms underpinning its expression is important. Enhancing erbB expression in heart failure through the use of small molecules or gene therapy may enhance response to neuregulin. In fact, constitutive expression of erbB2 using a carcinogenic mutation has been demonstrated to extend the postnatal proliferative and regenerative potential of murine cardiomyocytes into adulthood (D'Uva et al. 2015). This produced 5-fold greater cardiomyocyte proliferation in post-infarct mice treated with neuregulin compared with those without erbB2 constitutive expression (D'Uva et al. 2015).
Clinical testing
Initial preclinical work has shown that 5-to-7-day treatment with intravenous recombinant human neuregulin-1 (rhNRG-1) produces early and sustained divergence in survival curves in murine models of ischemic, doxorubicin-induced and Coxsackie virus B3-related cardiomyopathies (Liu et al. 2006). Numerous studies have been subsequently performed evaluating rhNRG-1 in chronic heart failure. A single 30-min infusion of cimaglermin alfa, an isotype of recombinant neuregulin (NRG-1β3), was generally well-tolerated amongst patients with systolic heart failure except for transient headache and nausea and one case of transient hyperbilirubinemia and elevated liver transaminases at the highest dose (1.5 mg/kg) in a recent phase I study (Lenihan et al. 2016) (Table 2). Notably, dose-dependent improvement in LVEF was observed after 90 days (Lenihan et al. 2016). An earlier phase I trial also demonstrated that rhNRG-1 was well-tolerated (Jabbour et al. 2011). A phase II trial found that addition of rhNRG-1 (0.6 μg/kg/day) to standard heart failure therapy for 10 days produced significantly increased LVEF after 30 days, which was maintained after 90 days despite not being significantly different from placebo (Gao et al. 2010) (Table 2). This was accompanied by a significant reduction in LVESV at days 30 and 90 (Gao et al. 2010) suggestive of beneficial modulation of chronic myocardial remodelling. The authors hypothesised the synergistic benefit conferred by combining neuregulin with standard therapy may be due to standard therapies reducing cardiomyocyte stress and thereby maximizing their response to neuregulin (Gao et al. 2010). However, the 0.3 and 1.2 μg/kg/day treatment groups did not achieve significantly increased LVEF at 30 days, with only the 0.3 μg/kg/day group achieving significantly increased LVEF after 90 days (Gao et al. 2010). A large phase III study evaluating the effect of intravenous recombinant neuregulin-1β on mortality in patients with NYHA class II–III heart failure is currently underway.
Platelet-derived growth factor
The four PDGF isotypes PDGF-A, PDGF-B, PDGF-C and PDGF-D all exist as functional homodimers, except for PDGF-A and PDGF-B, which may exist as either homodimers or heterodimers (Chen et al. 2013). PDGF exhibits mitogenic action on mesenchymal cells and is currently used in clinical practice to promote the healing of chronic ulcers.
The rationale for use in cardiac repair
The mitogenic properties of PDGF both in non-cardiac tissue and in the developing heart (Kang et al. 2008; Smith et al. 2011) generated interest in its potential utility as an effector of cardiac regeneration in the adult heart. Normal murine myocardium expresses all four isotypes of PDGF, with PDGF-A and PDGF-D and PDGF receptors-α and β being upregulated after MI (Zhao et al. 2011). In the diseased adult human heart, Chong et al. identified a population of PDGF receptor-α-expressing progenitors that predominantly differentiated into endothelial and vascular smooth muscle cells with minimal contribution to the cardiomyocyte pool (Chong et al. 2013). This finding, along with the expression of PDGF following heart transplant (Sack et al. 2004; Koch et al. 2006), supports a potential angiogenic role for PDGF in cardiac repair, which has directed subsequent research.
Mechanism of action
PDGF appears to be an important regulator of angiogenesis (Table 1). Pre-treatment of a murine MI model with intracoronary PDGF-AB promotes angiogenesis and reduces infarct size (Edelberg et al. 2002). Whilst the mechanisms underpinning PDGF-related angiogenesis remain largely unresolved, in vitro overexpression of PDGF receptor-β in endothelial progenitor cells enhances their PDGF-BB-induced proliferation and migration via the PI3K/Akt signalling pathway (Wang et al. 2012). Furthermore, the discovery of high-affinity PDGF-VEGF receptor interactions (Mamer et al. 2017), along with findings that PDGF receptor antagonism does not attenuate the induction of angiogenesis 1 week post-MI (Liu et al. 2014), indicates that some of the PDGF pro-angiogenic effect may be mediated through activation of non-PDGF receptors. Protection of cardiomyocytes from apoptosis is another proposed cardioprotective mechanism conferred by PDGF (Table 1). Transient PDGF-BB delivery to cardiomyocyte monolayer and engineered cardiac tissue enhances contractility through increased cardiomyocyte count in the absence of hypertrophy or hyperplasia, which is suggestive of an anti-apoptotic effect (Vantler et al. 2010). In addition, the protection of cardiomyocytes from apoptosis in co-culture with endothelial cells is attenuated by anti-PDGF-BB or anti-PDGF receptor-β neutralizing antibodies but not anti-PDGF-A or anti-PDGF receptor-α (Hsieh et al. 2006), suggesting the PDGF-BB/PDGF receptor-β signalling pathway is an endothelium-derived cardiomyocyte anti-apoptotic pathway. A randomized murine study showed that the delivery of intramyocardial PDGF-BB using peptide nanofibres but not PDGF-BB alone reduced cardiomyocyte apoptosis and preserved systolic function following MI (Hsieh et al. 2006), reflecting the importance of negotiating the short PDGF serum half-life through sustained-release preparations. PDGF has also been implicated as a key mediator of fibrosis. PDGF is a regulator of epicardium-derived cell differentiation into mature fibroblasts during myocardial development (Smith et al. 2011) and the PDGF receptor is expressed by myofibroblasts and fibroblasts (Moore-Morris et al. 2014). In mice exposed to infarction-reperfusion injury, PDGF receptor blockade decreases infarct collagen content (Zymek et al. 2006), which may reduce star stability and thereby potentiate enhanced ventricular remodelling. However, PDGF-mediated fibrosis can also contribute to pathologies such as atrial fibrillation in pressure-loaded hearts (Liao et al. 2010) and dilated cardiomyopathy in transgenic rodents overexpressing PDGF-D (Ponten et al. 2005).
Preclinical testing
Recently, our research group has reported the effects of a 7-day recombinant human PDGF-AB (rhPDGF-AB) infusion in a clinically relevant porcine myocardial ischemia-reperfusion model. Compared with controls, rhPDGF-AB-treated animals exhibited increased infarct collagen anisotropy, maturation of collagen and increased vascular density (Thavapalachandran et al. 2020). Functionally, this was associated with a 12% improvement in LVEF in the rhPDGF-AB group 28 days post-injury, decreased inducible ventricular tachycardia and a 40% reduction in arrhythmic mortality compared to the control group, which was mostly attributed to reduced infarct heterogeneity (Thavapalachandran et al. 2020). Interestingly, these effects occurred despite there being no significant difference in infarct size between the study groups (Thavapalachandran et al. 2020). We found no evidence of off-target fibrotic or neoplastic phenomena (Thavapalachandran et al. 2020) suggesting the safety of rh-PDGF-AB. Moving forward, studies extending beyond the 28-day endpoint used in earlier murine and porcine studies (Thavapalachandran et al. 2020; Asli et al. 2019) are required to make observations on longer term safety and efficacy before rhPDGF-AB can be escalated to human trials. In addition, performing electrophysiological mapping studies to compare the nature of electrical conduction in control and rhPDGF-AB-treated infarcts should assist in elucidating how rhPDGF-AB treatment reduces the incidence of ventricular arrhythmia.
Growth factor therapy in the current landscape of novel cardiac therapies
The development of novel therapies from conception at the bench to ultimate clinical translation is enormously expensive and requires skillsets beyond the scope of traditional academic remits. This is particularly true for the “valley of death” stage where therapy has shown early preclinical promise but not yet shown consistent results in clinical trials. In this context, it is important to note that there has been major growth in the biotechnology sector developing novel therapies for myocardial ischemia and heart failure. As discussed above, growth factor therapies were able to recruit capital and industry support several decades ago (Osterziel et al. 1998; Isgaard et al. 1998; Laham et al. 2000; Henry et al. 2003). However, there is now increased competition from several other therapeutic modalities that were not conceived at that time. These include the use of non-coding RNAs (HAYA), stem cell therapies (Sana, Blue Rock) and cardiac cellular reprogramming (Tenaya). The emerging growth factor therapies described above may be “handicapped” by perceptions that recombinant technology is “old news”. Whether these translational efforts will be able to attract the investor interest and capital necessary for successful clinical translation remains to be seen.
Conclusion
Growth factor therapy for cardiac repair is currently at a crossroads. IGF-1, neuregulin and PDGF have emerged as novel candidates with a number of preclinical and early clinical trials yielding promising data regarding safety profile and therapeutic efficacy, warranting progression to later stage studies. Furthermore, the development of more sophisticated delivery techniques may potentially breathe new life into factors such as VEGF and bFGF that previously failed in clinical trials. However, the landscape of novel cardiac therapeutics has become flooded with alternative modalities such as non-coding RNAs, stem cell therapy and cardiac cellular reprogramming, which may ‘crowd out’ growth factor therapy. In addition, IGF-1, neuregulin and PDGF remain susceptible to barriers to efficacy such as short serum half-life and poor target-organ specificity that plagued earlier growth factor therapies. Therefore, it will remain imperative to continue to bioengineer novel delivery strategies to optimize the spatio-temporal delivery of these growth factors to target tissues. Given the historical failure of the previous wave of growth factor therapies in clinical trials, it would appear advisable to proceed with caution.
Authors’ contributions
James Chong conceived the research topic. Samuel White and James Chong designed the search strategy. Samuel White applied the search strategy to obtain relevant studies. Data analysis was performed by Samuel White and James Chong. Samuel White prepared an original draft, which was critically revised by James Chong.
Funding information
This work was supported by an NSW Health Cardiovascular Disease Clinician Scientist Grant and a National Foundation for Medical Research and Innovation Project Grant. JJHC was supported by a Future Leader Fellowship (ID 100463) from the National Heart Foundation of Australia and a Sydney Medical School Foundation Fellowship.
Compliance with ethical standards
Conflict of interest
James Chong is an inventor on PCT 2019/050617 filed by the University of Sydney that covers ‘cardiac treatment.’
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Asli NS, Xaymardan M, Patrick R, Farbehi N, Cornwell J, Forte E et al (2019) PDGFRα signaling in cardiac fibroblasts modulates quiescence, metabolism and self-renewal, and promotes anatomical and functional repair. bioRxiv
- Baliga RR, Pimental DR, Zhao YY, Simmons WW, Marchionni MA, Sawyer DB, et al. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Phys. 1999;277:H2026–H2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161(7):1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Bersell K, Arab S, Haring B, Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- Britsch S. The neuregulin-I/ErbB signaling system in development and disease. Adv Anat Embryol Cell Biol. 2007;190:1–65. [PubMed] [Google Scholar]
- Buerke M, Murohara T, Skurk C, Nuss C, Tomaselli K, Lefer AM. Cardioprotective effect of insulin-ike growth factor in myocardial ischemia followed by reperfusion. Proc Natl Acad Sci U S A. 1995;92:8031–8035. doi: 10.1073/pnas.92.17.8031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplice NM, DeVoe MC, Choi J, Dahly D, Murphy T, Spitzer E, et al. Randomized placebo controlled trial evaluating the safety and efficacy of single low-dose intracoronary insulin-like growth factor following percutaneous coronary intervention in acute myocardial infarction (RESUS-AMI) Am Heart J. 2018;200:110–117. doi: 10.1016/j.ahj.2018.03.018. [DOI] [PubMed] [Google Scholar]
- Chen S-L, Fang W-W, Ye F, Liu Y-H, Qian J, Shan S-J, et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am J Cardiol. 2004;94:92–95. doi: 10.1016/j.amjcard.2004.03.034. [DOI] [PubMed] [Google Scholar]
- Chen PH, Chen XY, He XL. Platelet-derived growth factors and their receptors: structural and functional perspectives. Biochimica Et Biophysica Acta-Proteins and Proteomics. 2013;1834:2176–2186. doi: 10.1016/j.bbapap.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisalita SI, Arnqvist HJ. Insulin-like growth factor I receptors are more abundant than insulin receptors in human micro- and macrovascular endothelial cells. Am J Physiol Endocrinol Metab. 2004;286:E896–E901. doi: 10.1152/ajpendo.00327.2003. [DOI] [PubMed] [Google Scholar]
- Chisalita SI, Johansson GS, Liefvendahl E, Back K, Arnqvist HJ. Human aortic smooth muscle cells are insulin resistant at the receptor level but sensitive to IGF1 and IGF2. J Mol Endocrinol. 2009;43:231–239. doi: 10.1677/JME-09-0021. [DOI] [PubMed] [Google Scholar]
- Chong JJH, Reinecke H, Iwata M, Torok-Storb B, Stempien-Otero A, Murry CE. Progenitor cells identified by PDGFR-alpha expression in the developing and disease human heart. Stem Cells Dev. 2013;22(13):1932–1943. doi: 10.1089/scd.2012.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RA. Overview and general considerations of wound repair. In: Clark RA, Henson PM, editors. The molecular and cellular biology of wound repair. New York: Plenum Press; 1988. pp. 3–33. [Google Scholar]
- Cohen JE, Purcell BP, MacArthur JW, Mu A, Shudo Y, Patel JB, et al. A bioengineered hydrogel system enables targeted and sustained intramyocardial delivery of neuregulin, activating the cardiomyocyte cell cycle and enhancing ventricular function in a murine model of ischemic cardiomyopathy. Circ Heart Fail. 2014;7:619–626. doi: 10.1161/CIRCHEARTFAILURE.113.001273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobaczewski M, Bujak M, Zymek P, Ren G, Entman ML, Frangogiannis NG. Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 2006;324(3):475–488. doi: 10.1007/s00441-005-0144-6. [DOI] [PubMed] [Google Scholar]
- Duckers HJ, Houtgraaf J, Hehrlein C, Schofer J, Waltenberger J, Gershlick A, et al. Final results of a phase IIa, randomised, open-label trial to evaluate the percutaneous intramyocardial transplantation of autologous skeletal myoblasts in congestive heart failure patients: the SEISMIC trial. EuroIntervention. 2011;6:805–812. doi: 10.4244/EIJV6I7A139. [DOI] [PubMed] [Google Scholar]
- D'Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, et al. ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation. Nat Cell Biol. 2015;17:627–638. doi: 10.1038/ncb3149. [DOI] [PubMed] [Google Scholar]
- Edelberg JM, Lee SH, Kaur M, Tang L, Feirt NM, McCabe S, et al. Platelet-derived growth factor-AB limits the extent of myocardial infarction in a rat model: feasibility of restoring impaired angiogenic capacity in the aging heart. Circulation. 2002;105:608–613. doi: 10.1161/hc0502.103672. [DOI] [PubMed] [Google Scholar]
- Fadini GP, Albiero M, Boscaro E, Agostini C, Avogaro A. Endothelial progenitor cells as resident accessory cells for post-ischemic angiogenesis. Atherosclerosis. 2009;204:20–22. doi: 10.1016/j.atherosclerosis.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Fang SJ, Wu XS, Han ZH, Zhang XX, Wang CM, Li XY, et al. Neuregulin-1 preconditioning protects the heart against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Chin Med J. 2010;123:3597–3604. [PubMed] [Google Scholar]
- Ferrini A, Stevens MM, Sattler S, Rosenthal N. Toward regeneration of the heart: bioengineering strategies for immunomodulation. Front Cardiovasc Med. 2019;6:26. doi: 10.3389/fcvm.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Khalil H, Kanisicak O, Boyer JG, Vagnozzi RJ, Maliken BD, et al. Specialized fibroblast differentiated states underlie scar formation in the infarcted mouse heart. J Clin Invest. 2018;128:2127–2143. doi: 10.1172/JCI98215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao R, Zhang J, Cheng L, Wu X, Dong W, Yang X, et al. A phase II, randomized, double-blind, multicenter, based on standard therapy, placebo-controlled study of the efficacy and safety of recombinant human neuregulin-1 in patients with chronic heart failure. J Am Coll Cardiol. 2010;55:1907–1914. doi: 10.1016/j.jacc.2009.12.044. [DOI] [PubMed] [Google Scholar]
- Garbayo E, Gavira JJ, de Yebenes MG, Pelacho B, Abizanda G, Lana H, et al. Catheter-based Intramyocardial injection of FGF1 or NRG1-loaded MPs improves cardiac function in a preclinical model of ischemia-reperfusion. Sci Rep. 2016;6:25932. doi: 10.1038/srep25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassmann M, Casagranda F, Orioli D, Simon H, Lai C, Klein R, et al. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature. 1995;378(6555):390–394. doi: 10.1038/378390a0. [DOI] [PubMed] [Google Scholar]
- Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L, Paillard M, Thibault H, Derumeaux G, Ovize M. Inhibition of GSK3beta by postconditioning is required to prevent opening of the mitochondrial permeability transition pore during reperfusion. Circulation. 2008;117:2761–2768. doi: 10.1161/CIRCULATIONAHA.107.755066. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, et al. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- Henry TD, Annex BH, McKendall GR, Azrin MA, Lopez JJ, Giordano FJ, et al. The VIVA trial: vascular endothelial growth factor in ischemia for vascular angiogenesis. Circulation. 2003;107:1359–1365. doi: 10.1161/01.cir.0000061911.47710.8a. [DOI] [PubMed] [Google Scholar]
- Hsieh PC, Davis ME, Gannon J, MacGillivray C, Lee RT. Controlled delivery of PDGF-BB for myocardial protection using injectable self-assembling peptide nanofibers. J Clin Invest. 2006;116:237–248. doi: 10.1172/JCI25878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Harrison MR, Osorio A, Kim J, Baugh A, Duan C. Igf signaling is required for cardiomyocyte proliferation during zebrafish heart development and regeneration. PLoS One. 2013;8(6):e67266. doi: 10.1371/journal.pone.0067266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CL, Leblond AL, Turner EC, Kumar AH, Martin K, Whelan D, et al. Synthetic chemically modified mrna-based delivery of cytoprotective factor promotes early cardiomyocyte survival post-acute myocardial infarction. Mol Pharm. 2015;12:991–996. doi: 10.1021/mp5006239. [DOI] [PubMed] [Google Scholar]
- Hynes B, Kumar AH, O'Sullivan J, Klein Buneker C, Leblond AL, Weiss S, et al. Potent endothelial progenitor cell-conditioned media-related anti-apoptotic, cardiotrophic, and pro-angiogenic effects post-myocardial infarction are mediated by insulin-like growth factor-1. Eur Heart J. 2013;34:782–789. doi: 10.1093/eurheartj/ehr435. [DOI] [PubMed] [Google Scholar]
- Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong T-T, Shaw RM, et al. Cardiac fibroblasts regulate myocardial proliferation through β1 integrin signaling. Dev Cell. 2009;16(2):233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isgaard J, Bergh CH, Caidahl K, Lomsky M, Hjalmarson A, Bengtsson BÅ. A placebo-controlled study of growth hormone in patients with congestive heart failure. Eur Heart J. 1998;19:1704–1711. doi: 10.1053/euhj.1998.1123. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Weber T, Hajjar RJ. Human cardiac gene therapy. Circ Res. 2018;123(5):601–613. doi: 10.1161/CIRCRESAHA.118.311587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabbour A, Hayward CS, Keogh AM, Kotlyar E, McCrohon JA, England JF, et al. Parenteral administration of recombinant human neuregulin-1 to patients with stable chronic heart failure produces favourable acute and chronic haemodynamic responses. Eur J Heart Fail. 2011;13:83–92. doi: 10.1093/eurjhf/hfq152. [DOI] [PubMed] [Google Scholar]
- Kang J, Gu Y, Li P, Johnson BL, Sucov HM, Thomas PS. PDGF-A as an epicardial mitogen during heart development. Dev Dyn. 2008;237(3):692–701. doi: 10.1002/dvdy.21469. [DOI] [PubMed] [Google Scholar]
- Kastrup J, Jorgensen E, Ruck A, Tagil K, Glogar D, Ruzyllo W, et al. Direct intramyocardial plasmic vascular endothelial growth factor-A165 gene therapy in patients with stable severe angina pectoris. A randomized double-blind placebo-controlled study: the Euroinject One Trial. J Am Coll Cardiol. 2005;45:982–988. doi: 10.1016/j.jacc.2004.12.068. [DOI] [PubMed] [Google Scholar]
- Kieserman JM, Myers VD, Dubey P, Cheung JY, Feldman AM. Current landscape of heart failure gene therapy. J Am Heart Assoc. 2019;8:e012239. doi: 10.1161/JAHA.119.012239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Palchyk E, Gassler N, Dengler TJ, Remppis A, Pritsch M, et al. Expression of platelet-derived growth factor and fibroblast growth factor in cryopreserved endomyocardial biopsies early and late after heart transplant. Ann Thorac Surg. 2006;81:1372–1378. doi: 10.1016/j.athoracsur.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Kourembanas S, Hannan RL, Faller DV. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990;86(2):670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramochi Y, Cote GM, Guo X, Lebrasseur NK, Cui L, Liao R, et al. Cardiac endothelial cells regulate reactive oxygen species-induced cardiomyocyte apoptosis through neuregulin-1beta/erbB4 signaling. J Biol Chem. 2004;279:51141–51147. doi: 10.1074/jbc.M408662200. [DOI] [PubMed] [Google Scholar]
- Laham RJ, Chronos NA, Pike M, Leimbach ME, Udelson JE, Pearlman JD. Intracoronary basic fibroblast growth factor (FGF-2) in patients with severe ischemic heart disease: results of a phase I open-label dose escalation study. J Am Coll Cardiol. 2000;36(7):2132–2139. doi: 10.1016/s0735-1097(00)00988-8. [DOI] [PubMed] [Google Scholar]
- Lai NC, Tang T, Gao MH, Saito M, Miyanohara A, Hammond HK. Improved function of the failing rat heart by regulated expression of insulin-like growth factor I via intramuscular gene transfer. Hum Gene Ther. 2012;23:255–261. doi: 10.1089/hum.2011.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenihan DJ, Anderson SA, Lenneman CG, Brittain E, Muldowney JAS, 3rd, Mendes L, et al. A phase I, single ascending dose study of cimaglermin alfa (Neuregulin 1beta3) in patients with systolic dysfunction and heart failure. JACC Basic Transl Sci. 2016;1:576–586. doi: 10.1016/j.jacbts.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, He L, Huang X, Bhaloo SI, Zhao H, Zhang S, et al. Genetic lineage tracing of nonmyocyte population by dual recombinases. Circulation. 2018;138(8):793–805. doi: 10.1161/CIRCULATIONAHA.118.034250. [DOI] [PubMed] [Google Scholar]
- Liao CH, Akazawa H, Tamagawa M, Ito K, Yasuda N, Kudo Y, et al. Cardiac mast cells cause atrial fibrillation through PDGF-A-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120:242–253. doi: 10.1172/JCI39942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Gu X, Li Z, Li X, Li H, Chang J, et al. Neuregulin-1/erbB-activation improves cardiac function and survival in models of ischemic, dilated, and viral cardiomyopathy. J Am Coll Cardiol. 2006;48:1438–1447. doi: 10.1016/j.jacc.2006.05.057. [DOI] [PubMed] [Google Scholar]
- Liu C, Zhao W, Meng W, Zhao T, Chen Y, Ahokas RA, et al. Platelet-derived growth factor blockade on cardiac remodeling following infarction. Mol Cell Biochem. 2014;397:295–304. doi: 10.1007/s11010-014-2197-x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Yang R, Huang X, Zhang H, He L, Zhang L, et al. Genetic lineage tracing identifies in situ kit-expressing cardiomyocytes. Cell Res. 2016;26(1):119–130. doi: 10.1038/cr.2015.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losordo DW, Vale PR, Symes JF, Dunnington CH, Esakof DD, Maysky M, et al. Gene therapy for myocardial angiogenesis: initial clinical results with direct myocardial injection of phVEGF165 as sole therapy for myocardial ischemia. Circulation. 1998;98:2800–2804. doi: 10.1161/01.cir.98.25.2800. [DOI] [PubMed] [Google Scholar]
- Mairet-Coello G, Tury A, DiCicco-Bloom E. Insulin-like growth factor-1 promotes G1/S cell cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J Neurosci. 2009;29(3):775–788. doi: 10.1523/JNEUROSCI.1700-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamer SB, Chen S, Weddell JC, Palasz A, Wittenkeller A, Kumar M, et al. Discovery of high-affinity PDGF-VEGFR interactions: redefining RTK dynamics. Sci Rep. 2017;7:16439. doi: 10.1038/s41598-017-16610-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDevitt TC, Laflamme MA, Murry CE. Proliferation of cardiomyocytes derived from human embryonic stem cells is mediated via the IGF/PI3-kinase/Akt signaling pathway. J Mol Cell Cardiol. 2005;39(6):865–873. doi: 10.1016/j.yjmcc.2005.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menasché P, Alfieri O, Janssens S, McKenna W, Reichenspurner H, Trinquart L, et al. The myoblast autologous grafting in ischemic cardiomyopathy (MAGIC) trial: first randomized placebo-controlled study of myoblast transplantation. Circulation. 2008;117:1189–1200. doi: 10.1161/CIRCULATIONAHA.107.734103. [DOI] [PubMed] [Google Scholar]
- Mirotsou M, Jayawardena TM, Schmeckpeper J, Gnecchi M, Dzau VJ. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol. 2011;50:280–289. doi: 10.1016/j.yjmcc.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, et al. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124(7):2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterziel KJ, Strohm O, Schuler J, Friedrich M, Hanlein D, Willenbrock R. Randomised, double-blind, placebo-controlled trial of human recombinant growth hormone in patients with chronic heart failure due to dilated cardiomyopathy. Lancet. 1998;351:1233–1237. doi: 10.1016/S0140-6736(97)11329-0. [DOI] [PubMed] [Google Scholar]
- O'Sullivan JF, Leblond AL, Kelly G, Kumar AH, Metharom P, Buneker CK, et al. Potent long-term cardioprotective effects of single low-dose insulin-like growth factor-1 treatment postmyocardial infarction. Circ Cardiovasc Interv. 2011;4:327–335. doi: 10.1161/CIRCINTERVENTIONS.110.960765. [DOI] [PubMed] [Google Scholar]
- Penaud-Budloo M, Le Guiner C, Nowrouzi A, Toromanoff A, Chérel Y, Chenuaud P, et al. Adeno-associated virus vector genomes persist as episomal chromatin in primate muscle. J Virol. 2008;82:7875–7885. doi: 10.1128/JVI.00649-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponten A, Folestad EB, Pietras K, Eriksson U. Platelet-derived growth factor D induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res. 2005;97:1036–1045. doi: 10.1161/01.RES.0000190590.31545.d4. [DOI] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, et al. Transient regenerative potential of the neonatal mouse heart. Science. 2011;331(6020):1078–1080. doi: 10.1126/science.1200708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radcliff K, Tang T-B, Lim J, Zhang Z, Abedin M, Demer LL. Insulin-like growth factor-I regulates proliferation and osteoblastic differentiation of calcifying vascular cells via extracellular signal-regulated protein kinase and phosphatidylinositol 3-kinase pathways. Circ Res. 2005;96(4):398–400. doi: 10.1161/01.RES.0000157671.47477.71. [DOI] [PubMed] [Google Scholar]
- Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253(8):2769–2776. [PubMed] [Google Scholar]
- Rohrbach S, Yan X, Weinberg EO, Hasan F, Bartunek J, Marchionni MA, et al. Neuregulin in cardiac hypertrophy in rats with aortic stenosis. Differential expression of erbB2 and erbB4 receptors. Circulation. 1999;100:407–412. doi: 10.1161/01.cir.100.4.407. [DOI] [PubMed] [Google Scholar]
- Rohrbach S, Niemann B, Silber RE, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium -- depressed expression and attenuated activation. Basic Res Cardiol. 2005;100:240–249. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]
- Rossen RD, Swain JL, Michael LH, Weakley S, Giannini E, Entman ML. Selective accumulation of the first component of complement and leukocytes in ischemic canine heart muscle: a possible initiator of an extra myocardial mechanism of ischemic injury. Circ Res. 1985;57:119–130. doi: 10.1161/01.res.57.1.119. [DOI] [PubMed] [Google Scholar]
- Sack FU, Vielfort TJ, Koch A, Haass M, Taylor S, Otto HF, et al. The role of platelet derived growth factor in endomyocardial biopsies shortly after heart transplantation in relation to postoperative course. Eur J Cardiothorac Surg. 2004;25:91–97. doi: 10.1016/j.ejcts.2003.09.025. [DOI] [PubMed] [Google Scholar]
- Serini G, Bochaton-Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L, et al. The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor-beta1. J Cell Biol. 1998;142(3):873–881. doi: 10.1083/jcb.142.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H, Xue T, Yang Y, Jiang C, Huang S, Yang Q, et al. Microneedle-mediated gene delivery for the treatment of ischemic myocardial disease. Sci Adv. 2020;6(25):eaaz3621. doi: 10.1126/sciadv.aaz3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shweiki D, Itin A, Soffer D, Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992;359:843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Simons M, Annex BH, Laham RJ, Kleiman N, Henry T, Dauerman H, et al. Pharmacological treatment of coronary artery disease with reocmbinant fibroblast growth factor-2: double-blind, randomized, controlled clinical trial. Circulation. 2002;105:788–793. doi: 10.1161/hc0802.104407. [DOI] [PubMed] [Google Scholar]
- Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ Res. 2011;108:e15–e26. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DJ, Kutryk MJ, Fitchett D, Freeman M, Camack N, Su Y, et al. VEGF gene therapy fails to improve perfusion of ischemic myocardium in patients with advanced coronary disease: results of the NORTHERN trial. Mol Ther. 2009;17:1109–1115. doi: 10.1038/mt.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana N, Zhang L, Yan J, Chen J, Cai W, Razzaque S, et al. Resident c-kit(+) cells in the heart are not cardiac stem cells. Nat Commun. 2015;6:8701. doi: 10.1038/ncomms9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symes JF, Losordo DW, Vale PR, Lathi KG, Esakof DD, Mayskiy M, et al. Gene therapy with vascular endothelial growth factor for inoperable coronary artery disease. Ann Thorac Surg. 1999;68:830–836. doi: 10.1016/s0003-4975(99)00807-3. [DOI] [PubMed] [Google Scholar]
- Tachibana A, Santoso MR, Mahmoudi M, Shukla P, Wang L, Bennett M, et al. Paracrine effects of the pluripotent stem cell-derived cardiac myocytes salvage the injured myocardium. Circ Res. 2017;121:e22–e36 ince. doi: 10.1161/CIRCRESAHA.117.310803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thavapalachandran S, Grieve SM, Hume RD, Le TYL, Raguram K, Hudson JE et al (2020) Platelet-derived growth factor-AB improves scar mechanics and vascularity after myocardial infarction. Sci Transl Med. 10.1126/scitranslmed.aay2140 [DOI] [PubMed]
- Ubil E, Duan J, Pillai IC, Rosa-Garrido M, Wu Y, Bargiacchi F, et al. Mesenchymal-endothelial transition contributes to cardiac neovascularization. Nature. 2014;514:585–590. doi: 10.1038/nature13839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi RJ, Sargent MA, Lin S-CJ, Palpant NJ, Murry CE, Molkentin JD. Genetic lineage tracing of Sca-1+ cells reveals endothelial but not myogenic contribution to the murine heart. Circulation. 2018;138:2931–2939. doi: 10.1161/CIRCULATIONAHA.118.035210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, et al. An acute immune response underlies the benefit of cardiac stem cell therapy. Nature. 2019;577:405–409. doi: 10.1038/s41586-019-1802-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakeva A, Morgan BP, Tikkanen I, Helin K, Laurila P, Meri S. Time course of complement activation and inhibitor expression after ischemic injury of rat myocardium. Am J Pathol. 1994;144:1357–1368. [PMC free article] [PubMed] [Google Scholar]
- van Berlo JH, Kanisicak O, Maillet M, Vagnozzi RJ, Karch J, Lin SC, et al. c-kit+ cells minimally contribute cardiomyocytes to the heart. Nature. 2014;509(7500):337–341. doi: 10.1038/nature13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantler M, Karikkineth BC, Naito H, Tiburcy M, Didie M, Nose M, et al. PDGF-BB protects cardiomyocytes from apoptosis and improves contractile function of engineered heart tissue. J Mol Cell Cardiol. 2010;48:1316–1323. doi: 10.1016/j.yjmcc.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Vikhert AM, Cherpachenko NM. Changes in metabolism of undamaged sections of myocardium following infarction. Circ Res. 1974;35(Suppl 3):182–191. [PubMed] [Google Scholar]
- Wang JA, Xie XJ, He H, Sun Y, Jiang J, Luo R-H, et al. A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:107–110. [PubMed] [Google Scholar]
- Wang H, Yin Y, Li W, Zhao X, Yu Y, Zhu J, et al. Over-expression of PDGFR-beta promotes PDGF-induced proliferation, migration, and angiogenesis of EPCs through PI3K/Akt signaling pathway. PLoS One. 2012;7:e30503. doi: 10.1371/journal.pone.0030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajima S, Miyagawa S, Fukushima S, Isohashi K, Watabe T, Ikeda H, et al. Microvascular dysfunction related to progressive left ventricular remodeling due to chronic occlusion of the left anterior descending artery in an adult porcine heart. Int Heart J. 2019;60(3):715–727. doi: 10.1536/ihj.18-346. [DOI] [PubMed] [Google Scholar]
- Yang Z, von Ballmoos MW, Faessler D, Voelzmann J, Ortmann J, Diehm N, et al. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211(1):103–109. doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
- Zhao W, Zhao T, Huang V, Chen Y, Ahokas RA, Sun Y. Platelet-derived growth factor involvement in myocardial remodeling following infarction. J Mol Cell Cardiol. 2011;51:830–838. doi: 10.1016/j.yjmcc.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Zuo L, Cardounel AJ, Zweier JL, He G. Characterization of in vivo tissue redox status, oxygenation, and formation of reactive oxygen species in postischemic myocardium. Antioxid Redox Signal. 2007;9(4):447–455. doi: 10.1089/ars.2006.1389. [DOI] [PubMed] [Google Scholar]
- Ziebart T, Yoon C-H, Trepels T, Wietelmann A, Braun T, Kiessling F. Sustained persistence of transplanted proangiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res. 2008;103(11):1327–1334. doi: 10.1161/CIRCRESAHA.108.180463. [DOI] [PubMed] [Google Scholar]
- Zymek P, Bujak M, Chatila K, Cieslak A, Thakker G, Entman ML, et al. The role of platelet-derived growth factor signaling in healing myocardial infarcts. J Am Coll Cardiol. 2006;48:2315–2323. doi: 10.1016/j.jacc.2006.07.060. [DOI] [PubMed] [Google Scholar]