Abstract
Background
Recovery of motor dysfunction is important for patients with incomplete cervical spinal cord injury (SCI). To enhance the recovery of muscle strength, both research and treatments mainly focus on injury of upper motor neurons at the direct injury site. However, accumulating evidences have suggested that SCI has a downstream effect on the peripheral nervous system, which may contribute to the poor improvement of the muscle strength after operation. The aim of this study is to investigate the impact of early vs. delayed surgical intervention on the lower motor neurons (LMNs) distal to the injury site in patients with incomplete cervical SCI.
Methods
Motor unit number index (MUNIX) was performed on the tibialis anterior (TA), extensor digitorum brevis (EDB) and abductor hallucis (AH) in 47 patients with incomplete cervical SCI (early vs. delayed surgical-treatment: 17 vs. 30) and 34 healthy subjects approximately 12 months after operation. All patients were further assessed by American spinal injury association (ASIA) motor scales and Medical Research Council (MRC) scales.
Results
There are no difference of both ASIA motor scores and MRC scales between the patients who accepted early and delayed surgical treatment (P > 0.05). In contrast, the patients undergoing early surgical treatment showed lower MUSIX values in both bilateral EDB and bilateral TA, along with greater MUNIX values in both right-side EDB and right-side TA, compared to the patients who accepted delayed surgical treatment (P < 0.05).
Conclusions
Cervical SCI has a negative effect on the LMNs distal to the injury site. Early surgical intervention in Cervical SCI patients may improve the dysfunction of LMNs distal to the injury site, reducing secondary motor neuron loss, and eventually improving clinical prognosis.
Keywords: Spinal cord injury, Motor unit number index, Motor unit loss, Optimal timing for surgery, Trans-synaptic degeneration
Background
Recovery of motor dysfunction is important for patients with incomplete cervical spinal cord injury (SCI) since it is essential for improving health-related quality of life [1]. To enhance the recovery of muscle strength, both research and treatments mainly focus on both primary and secondary injury of upper motor neurons (UMN) at the direct injury site [2, 3]. In many different neuroprotective treatments, surgical decompression is demonstrated as one of the most important methods, and accumulating evidence has suggested that early operation can relieve both mechanical compression and microcirculation disturbance of cervical cord, therefore improving the clinical prognosis [3, 4].
The pathophysiology of cervical SCI is complex, and recently published studies revealed obvious electrophysiological abnormalities in distal paralyzed muscles in patients with SCI [1, 5–7], suggesting the degeneration of both spinal motoneurons and peripheral motor axons in regions caudal to the level of direct injury, which may contribute to the poor improvement of the muscle strength. Unfortunately, few studies have involved the effects of early surgical treatment on the loss/dysfunction of distal motor neurons, although this issue is important for clinician to establish suitable treatment of cervical SCI.
Motor unit number index (MUNIX) is a recently developed quantitative method that provides an estimated index of the number and size of the functional motor units in the tested muscle [8]. According to the previous studies, MUNIX was demonstrated to be very sensitive in detecting motor unit loss in many different neuromuscular diseases [8–11], and both Li et al. and Marciniak et al. demonstrated MUNIX detection can be used to assess the integrity of lower motor neuron in patients with SCI [12, 13].
The aim of the present study is to quantify the functional motor units in the lower limb muscles in patients with incomplete cervical SCI who accepted early (≤ 72 h) versus delayed (> 72 h) surgical treatment.
Methods
Subjects
A total of 47 patients with incomplete cervical SCI and 34 healthy subjects were analyzed retrospectively in this study. In the present study, seventeen patients with cervical SCI underwent early surgical treatment (≤ 72 h), and the other 30 patients underwent delayed surgical treatment (> 72 h) (Table 1). All subjects in cervical SCI patient group were recruited in SongJiang Hospital from December 2015 to June 2017. The study protocol was approved by Human Ethics Committees (Shanghai Songjiang District Central Hospital, KYLL2015–263). All subjects gave informed consent.
Table 1.
Characteristics of patients with cervical SCI in both surgical treatment groups
| Early surgical treatment group | Delayed surgical treatment group | |
|---|---|---|
| Number of subjects | 17 | 30 |
| Age range (years) | 45.0 ± 12.2 | 47.4 ± 13.1 |
| Height range (cm) | 164.7 ± 8.9 | 165.9 ± 9.2 |
| Gender (Male vs. Female) | 12 vs. 5 | 24 vs. 6 |
| Time from injury to surgery (days) | 1.9 ± 0.7 | 17.2 ± 7.9 |
| Severity of SCI | ||
| ASIA B | 2/17 (11.8%) | 2/30 (6.7%) |
| ASIA C | 10/17 (58.8%) | 13/30 (43.3%) |
| ASIA D | 5/17 (29.4%) | 15/30 (50%) |
| Imaging abnormalities (n/total patient (%)) | ||
| Cervical fracture | 5/17 (29.4%) | 8/30 (26.7%) |
| Intramedullary high-signal lesion | 7/17 (41.2%) | 18/30 (60.0%) |
| Mechanism of injury (n/total patient (%)) | ||
| Falls | 6/17 (35.3%) | 9/30 (30.0%) |
| Vehicle accidents | 11/17 (64.7%) | 21/30 (70.0%) |
| Surgical approach (n/total patient (%)) | ||
| Anterior | 5/17 (29.4%) | 7/30 (23.3%) |
| Posterior | 13/17 (76.5%) | 20/30 (66.7%) |
| Combined | 1/17 (5.9%) | 3/30 (10.0%) |
SCI Spinal cord injury, ASIA ASIA (American Spinal Injury Association) impairment scale
The subjects in normal control group were chosen based on the inclusion and exclusion criteria published previously [5]. The inclusion criteria for cervical SCI patients includes (1) a clear history of trauma; (2) different degrees of sensory and motor impairments in both upper limbs and/or the lower limbs with a variable effect on bladder function; (3) magnetic resonance imaging (MRI) and/or Computed tomography (CT) demonstrating SCI at the cervical segment without the injuries in the level of thoracic and lumbar spine injuries. The exclusion criteria for cervical SCI patients includes previous spinal surgery, polyneuropathies, radiculopathies, plexopathies, focal neuropathies, muscle disorders, diabetes, diseases of the central nervous system, syringomyelia, spinal cord tumour/inflammation/infection, spinal deformities and severe degenerative diseases of thoracic and lumbar segments.
Testing methods
Motor unit number index
The MUNIX detection was applied in both 47 patients with incomplete cervical SCI approximately 1 year after operation and 34 healthy subjects. The MUNIX method described by Nandedkar et al. was used in this study [8]. The maximal compound muscle action potential (CMAP) was recorded bilaterally from the tibialis anterior (TA), extensor digitorum brevis (EDB) and abductor hallucis (AH) in a belly-tendon montage (filters: 3 Hz-10 kHz) to supramaximal stimulation. Subsequently, surface interference pattern (SIP) for ten different force levels of isometric contraction was recorded in a 300-ms window (filters: 10 Hz–1000 Hz). According to these measurements, both MUNIX values and motor unit size index (MUSIX) values for these three muscles were measured.
For the evaluation of the reproducibility, left-side MUNIX measurements of 15 healthy subjects and 21 patients with cervical SCI were tested twice by the same examiner. The intervals between these 2 tests were longer than 60 min, and the electrodes were completely removed after the initial test.
All electrophysiological examinations were carried out by Keypoint EMG machine (Medtronic Dantec, Skovlunde, Denmark) with a skin temperature > 32 °C. MUNIX values cannot be measured when the following conditions occur: SIP area < 20 mV.ms, ideal case motor unit count (ICMUC) > 100, SIP area/CMAP area < 1, or CMAP amplitude < 0.5 mV.
Clinical function examination
All 47 patients with cervical SCI accepted American spinal injury association (ASIA) classification to identify the severity of SCI at the time of admission. All of these patients further underwent muscle strength examination in all tested muscles graded by the Medical Research Council (MRC) scales and American spinal injury association (ASIA) motor scores approximately 1 year after operation.
Statistical methods
The measurements were analyzed by SPSS 18.0 (IBM, Armonk, NY), and Medsci simple size tools (Shanghai, China) were used to calculate sample size. The measurements among healthy subjects and different cervical SCI patient groups were tested using one-way ANOVA (least significant difference correction). The preoperative and postoperative ASIA motor scores were compared by the paired t-test.
Pearson or Spearman correlation coefficient analysis (CCA) was used to evaluate the relationship between MUNIX values and MRC scales in each patient group. The test-retest reproducibility of MUNIX in healthy subjects and patients with cervical SCI was analyzed using interclass correlation coefficient (ICC) methods.
A P-value less than 0.05 was considered significant.
Results
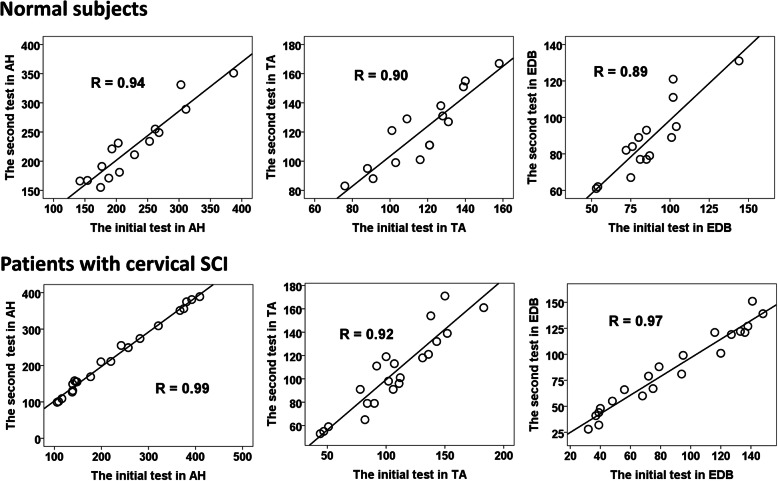
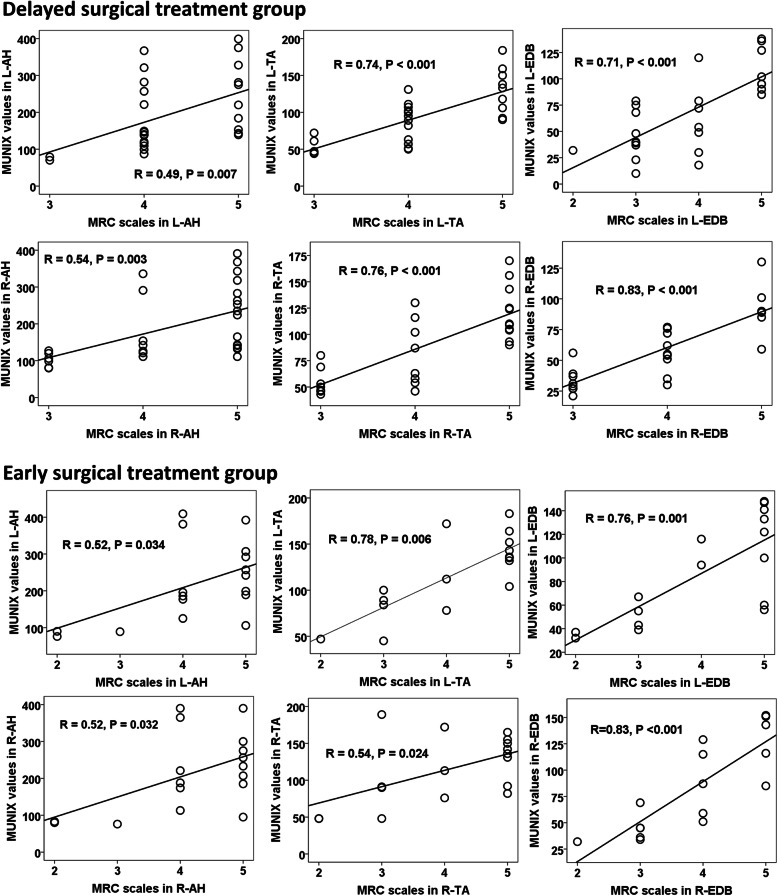
A significantly positive correlation of all MUNIX values between the initial and second tests was demonstrated in all tested muscles in both normal control and cervical SCI patient groups (P < 0.05, Fig. 1), and good reproducibility of all MUNIX measurements was further confirmed by ICC in both subject groups (Table 2). Furthermore, there is an obvious positive correlation between the MUNIX values and MRC scales in all tested muscles in both patient groups (P < 0.05, Fig. 2).
Fig. 1.
Correlations between MUNIX values of the initial and the second tests in both patients with cervical SCI (left side) and healthy subjects (left side). The graphs show that there is a strong positive correlation between the values of first and the second tests in all tested muscles in both cervical SCI patient and control groups. SCI: Spinal cord injury; MUNIX: motor unit number index; AH: abductor hallucis; EDB: extensor digitorum brevis; TA: tibialis anterior
Table 2.
Test-retest reproducibility of MUNIX in patients with SCI and healthy subjects
| Patients with cervical SCI | Healthy subjects | |||
|---|---|---|---|---|
| Number of cases | 21 | 15 | ||
| Age range (years) | 49.2 ± 11.8 | 45.0 ± 11.5 | ||
| Height range (cm) | 163.6 ± 9.2 | 166.2 ± 6.3 | ||
| ICC | CCA | ICC | CCA | |
| Abductor hallucis | ||||
| CMAP | 0.99 | 0.99 | 0.99 | 0.99 |
| MUNIX | 0.99 | 0.99 | 0.97 | 0.94 |
| MUSIX | 0.96 | 0.93 | 0.96 | 0.95 |
| Extensor digitorum brevis | ||||
| CMAP | 0.99 | 0.97 | 0.98 | 0.97 |
| MUNIX | 0.99 | 0.97 | 0.94 | 0.89 |
| MUSIX | 0.95 | 0.90 | 0.91 | 0.84 |
| Tibialis anterior | ||||
| CMAP | 0.99 | 0.98 | 0.97 | 0.94 |
| MUNIX | 0.96 | 0.92 | 0.94 | 0.90 |
| MUSIX | 0.90 | 0.84 | 0.87 | 0.78 |
MUNIX Motor unit number index, SCI Spinal cord injury, CMAP Compound muscle action potential, MUSIX Motor unit size index, ICC Intraclass correlation coefficient, CCA Correlation coefficient analysis
Fig. 2.
Correlations between MRC scales and MUNIX values in both cervical SCI patient groups. There was a significant relationship between the MRC scales and MUNIX values in all tested muscles in both cervical SCI patient groups. SCI: Spinal cord injury; MRC: medical research council; MUNIX: motor unit number index; AH: abductor hallucis; EDB: extensor digitorum brevis; TA: tibialis anterior; L: left side; R: right side
In the delayed surgical treatment group, the MUNIX values were not recorded from all tested muscles in two patients. Furthermore, absent MUNIX values were also observed in bilateral TA in one patient and in unilateral EDB in two patients. In addition, the other two patients in this group presented with absent MUNIX values in bilateral EDB, along with absent MUNIX values in left-side TA in one of these 2 patients. Therefore, seven (7/30, 23.3%) patients who accepted delayed surgical treatment presented with absent postoperative MUNIX values in at least one of the tested muscles in this study. In contrast, only one (1/17, 5.9%) patient in the early surgical treatment group presented with absent MUNIX values in bilateral EDB.
Compared with the normal controls, the patients who accepted early surgical treatment presented with increased MUSIX values in both AH and EDB on right side (P < 0.05, Table 3). Furthermore, the patients undergoing delayed surgical treatment showed increased MUSIX values in bilateral AH, as well as both reduced MUNIX values and increased MUSIX values in both TA and EDB on bilateral side (P < 0.05, Table 3). In addition, delayed surgical treatment group patients also presented with reduced CMAP amplitudes in left-side EDB (P < 0.05, Table 3).
Table 3.
Measurements of MUNIX detection and clinical function measures in both SCI patient and normal control groups
| Early surgical treatment group | Delayed surgical treatment group | Healthy subjects | ||||
|---|---|---|---|---|---|---|
| Number of subjects | 17 | 30 | 34 | |||
| Age range (years) | 45.0 ± 12.2 | 47.4 ± 13.1 | 44.0 ± 11.1 | |||
| Height range (cm) | 164.7 ± 8.9 | 165.9 ± 9.2 | 166.9 ± 5.7 | |||
| Left side | Right side | Left side | Right side | Left side | Right side | |
| MUNIX detection | ||||||
| CMAP-AH | 14.0 ± 4.7 | 14.1 ± 4.8 | 13.4 ± 4.7 | 13.3 ± 4.5 | 14.6 ± 4.9 | 14.2 ± 5.0 |
| MUNIX-AH | 218.4 ± 108.3 | 213.6 ± 106.0 | 195.9 ± 97.5 | 192.8 ± 96.0 | 240.8 ± 96.8 | 240.4 ± 103.5 |
| MUSIX-AH | 71.5 ± 20.3 | 74.8 ± 24.2* | 76.8 ± 14.1* | 77.1 ± 14.9* | 63.0 ± 12.5 | 62.7 ± 13.7 |
| CMAP-TA | 6.1 ± 1.3 | 6.1 ± 1.1 | 5.9 ± 1.8 | 5.9 ± 1.5 | 6.5 ± 1.0 | 6.5 ± 1.0 |
| MUNIX-TA | 117.3 ± 41.9 | 113.4 ± 45.6# | 95.3 ± 38.2* | 89.6 ± 37.2*# | 122.9 ± 31.7 | 119.6 ± 30.8 |
| MUSIX-TA | 58.1 ± 20.3# | 61.1 ± 22.2# | 70.6 ± 15.6*# | 75.7 ± 20.5*# | 55.2 ± 10.4 | 56.4 ± 11.3 |
| CMAP-EDB | 5.6 ± 2.2 | 5.6 ± 2.3 | 5.0 ± 2.0* | 5.0 ± 2.0 | 6.0 ± 1.5 | 5.9 ± 1.9 |
| MUNIX-EDB | 86.9 ± 42.8 | 84.3 ± 44.0# | 69.6 ± 37.4* | 60.4 ± 28.5*# | 94.8 ± 30.1 | 94.7 ± 34.4 |
| MUSIX-EDB | 73.8 ± 19.2# | 76.7 ± 18.3*# | 87.7 ± 30.2*# | 92.9 ± 20.0*# | 65.2 ± 13.6 | 63.7 ± 12.2 |
| Clinical function measures | ||||||
| MRC-AH | 4.2 ± 1.0 | 4.2 ± 1.0 | 4.1 ± 0.8 | 4.2 ± 1.0 | / | / |
| MRC-TA | 3.9 ± 1.2 | 4.0 ± 1.1 | 3.9 ± 1.0 | 3.9 ± 1.0 | ||
| MRC-EDB | 3.8 ± 1.3 | 3.7 ± 1.2 | 3.7 ± 1.0 | 3.7 ± 1.0 | / | / |
| ASIA motor score | 81.4 ± 15.2 | 78.6 ± 18.3 | / | |||
SCI Spinal cord injury, MUNIX Motor unit number index, CMAP Compound muscle action potential, MUSIX Motor unit size index, TA tibialis anterior, AH abductor hallucis, EDB Extensor digitorum brevis, MRC Medical research council score, ASIA American spinal injury association
* Statistical difference between the patient and control groups, P < 0.05
# Statistical difference between the early and delayed surgical treatment groups, P < 0.05
There is no difference of ASIA motor scores between the patients who accepted early and delayed surgical treatment, and MRC scales in all tested muscles were similar between the early and delayed surgical treatment groups (P > 0.05, Table 3). In contrast, the patients undergoing early surgical treatment showed lower MUSIX values in both bilateral EDB and bilateral TA, along with greater MUNIX values in both right-side EDB and right-side TA, compared to the patients who accepted delayed surgical treatment (P < 0.05, Table 3).
Discussion
The MUNIX method used in this study only requires a few numbers of electrical stimulation, and it usually takes approximately 30 min to measure six muscles in one subject, thus facilitating patient compliance. More importantly, according to the results of both ICC and CCA, we identified the excellent levels of reproducibility of MUNIX measurements in both the control and SCI patient groups. Therefore, the findings of this study are technically sound and reflect reliably functional motor units of the tested muscles.
In the present study, significant loss of motor units in the lumbosacral-innervated muscles in patients with cervical SCI, suggesting that peripheral nerve function is compromised following upper motor neuron lesion. Central disconnection of the second-order motor neurons is considered to be the likely reason for this condition, and many previous studies have provided evidence with regard to this disconnection may cause loss of lower motor neurons (LMNs) distal to the initial injury site, which may cause the motor axonal degeneration and an eventual reduction in motor units [1, 5, 12–14].
Although the measurements of MUNIX detection were obvious difference between the patients who accepted early and delayed surgical intervention approximately 1 year after operation, both CMAP amplitudes in both AH and TA and MRC scores in all tested muscles were similar between these two patient groups. This condition may be ascribed to these evaluation methods are relatively suboptimal to detect subtle difference between different treatments. Similar results were also reported in many previous studies [8, 11, 15–17], and collateral sprouting induced by longstanding neurodegeneration is likely the main reason. The remarkable increase in MUSIX values in the tested muscles in this study further identified the existence of the reinnervation from the surviving motor axons. According to previous studies, the reinnervation process can provide a functional compensatory mechanism to preserve muscle strength, and recently published study further indicated muscle strength usually can be preserved by reinnervation until 50% or more of motor units are lost [1, 18, 19]. Therefore, compared to current clinical biomarkers (e.g., CMAP amplitudes and muscle strength), MUNIX may be a more objective, sensitive and reliable method for monitoring the treatment outcomes of SCI. Although clinical function improvement is very important to the patients with SCI, the difference of MUNIX measurements between two different treatment group may provide additional unique information for guiding the doctors to select treatment modalities.
When reviewing these findings, one of the limitations is that MUNIX detection is easily influenced by both the examiner and the protocol (e.g., recording of maximum CMAP amplitude). Thus, a standard protocol and a fixed examiner were used in the current study [8]. Furthermore, another limitation is that the time cutoffs by which to identify early vs. delayed surgical treatment were different in various studies involving SCI (e.g., 12 h, 24 h, 4 days and 2 weeks) [20, 21]. Although the definition of early surgery as within the first 2 weeks is not the most common time cutoff, the recent systematic review demonstrated it is preferable to operate within 2 weeks compared to operation within 24 h [22]. Furthermore, few patients can accept surgical treatment within 24 h, due to both transportation issues and referral delays. The other clinical limitation of this study is low sample size, which may be ascribed to the fewer patients with incomplete cervical SCI have complete medical records and more patients lost to follow-up. More significant results might be achieved in future studies with an increased number of both cases and sub-groups.
Conclusion
Cervical SCI has a potential negative effect on the motor neurons distal to the level of injury. Equally important, different follow-up results of the MUNIX detection between the early vs. delayed surgical treatment group supported that early surgical intervention in some patients with SCI may relatively improve the dysfunction of LMNs and reduce the loss of motor neurons, eventually improving clinical prognosis.
Acknowledgements
Not applicable.
Abbreviations
- SCI
Spinal cord injury
- UMN
Upper motor neuron
- LMN
Lower motor neuron
- MUNIX
Motor unit number index
- TA
Tibialis anterior
- EDB
Extensor digitorum brevis
- AH
Abductor hallucis
- CMAP
Compound muscle action potential
- SIP
Surface interference pattern
- MUSIX
Motor unit size index
- MRC
Medical Research Council
- ASIA
American spinal injury association
- CCA
Correlation coefficient analysis
- ICC
Interclass correlation coefficient
Authors’ contributions
HE S and WANG D have made substantial contributions to conception and design; LI J and ZHU Y have made substantial contributions to acquisition of data, or analysis and interpretation of data; LI Y have been involved in drafting the manuscript or revising it critically for important intellectual content; all authors have given final approval of the version to be published.
Funding
All authors (HE S, WANG D, LI J, ZHU Y and LI Y) declare that Financial support from the Foundation of science and Technology Commission of Songjiang District, Shanghai (18sjgg9931).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
All authors (HE S, WANG D, LI J, ZHU Y and LI Y) declare that the study protocol was approved by Human Ethics Committees (Shanghai Songjiang District Central Hospital). All subjects gave informed consent through written.
Consent for publication
Not applicable.
Competing interests
All authors (HE S, WANG D, LI J, ZHU Y and LI Y) declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jun Li and Yancheng Zhu contributed equally to this work.
Contributor Information
Shisheng He, Email: heshisheng0321@126.com.
Deguo Wang, Email: wangdeguo0321@126.com.
References
- 1.Van De Meent H, Hosman AJ, Hendriks J, et al. Severe degeneration of peripheral motor axons after spinal cord injury: a European multicenter study in 345 patients. Neurorehabil Neural Repair. 2010;24:657–665. doi: 10.1177/1545968310368534. [DOI] [PubMed] [Google Scholar]
- 2.Yelamarthy PKK, Chhabra HS, Vaccaro A, et al. Management and prognosis of acute traumatic cervical central cord syndrome: systematic review and spinal cord society-spine trauma study group position statement. Eur Spine J. 2019;28:2390–2407. doi: 10.1007/s00586-019-06085-z. [DOI] [PubMed] [Google Scholar]
- 3.Divi SN, Schroeder GD, Mangan JJ, et al. Management of Acute Traumatic Central Cord Syndrome: a narrative review. Global Spine J. 2019;9:89S–97S. doi: 10.1177/2192568219830943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lenehan B, Fisher CG, Vaccaro A, et al. The urgency of surgical decompression in acute central cord injuries with spondylosis and without instability. Spine (Phila Pa 1976) 2010;35:S180–S186. doi: 10.1097/BRS.0b013e3181f32a44. [DOI] [PubMed] [Google Scholar]
- 5.Zheng C, Zhu Y, Lu F, et al. Trans-synaptic degeneration of motoneurons distal to chronic cervical spinal cord compression in cervical spondylotic myelopathy. Int J Neurosci. 2017;127:988–995. doi: 10.1080/00207454.2017.1287701. [DOI] [PubMed] [Google Scholar]
- 6.Zheng C, Nie C, Zhu Y, et al. Changes in central motor conduction time and its implication on dysfunction of distal upper limb in distal-type cervical Spondylotic Amyotrophy. J Clin Neurophysiol. 2019;36:52–59. doi: 10.1097/WNP.0000000000000532. [DOI] [PubMed] [Google Scholar]
- 7.Xiong GX, Zhang JW, Hong Y, et al. Motor unit number estimation of the tibialis anterior muscle in spinal cord injury. Spinal Cord. 2008;46:696–702. doi: 10.1038/sc.2008.7. [DOI] [PubMed] [Google Scholar]
- 8.Nandedkar SD, Barkhaus PE, Stålberg EV. Motor unit number index (MUNIX): principle, method, and findings in healthy subjects and in patients with motor neuron disease. Muscle Nerve. 2010;42:798–807. doi: 10.1002/mus.21824. [DOI] [PubMed] [Google Scholar]
- 9.Philibert M, Grapperon A, Delmont E, et al. Monitoring the short-term effect of intravenous immunoglobulins in multifocal motor neuropathy using motor unit number index. Clin Neurophysiol. 2017;128:235–240. doi: 10.1016/j.clinph.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Zheng C, Chen Z, Zhu Y, et al. Motor unit number index in quantitatively assessing motor root lesions and monitoring treatment outcomes in patients with lumbosacral radiculopathy. Muscle Nerve. 2020;61:759–766. doi: 10.1002/mus.26854. [DOI] [PubMed] [Google Scholar]
- 11.Zheng C, Song J, Zhu Y, et al. Motor unit number index (MUNIX) in the quantitative assessment of severity and surgical outcome in cervical spondylotic amyotrophy. Clin Neurophysiol. 2019;130:1465–1473. doi: 10.1016/j.clinph.2019.05.023. [DOI] [PubMed] [Google Scholar]
- 12.Mandeville RM, Brown JM, Sheean GL. A neurophysiological approach to nerve transfer to restore upper limb function in cervical spinal cord injury. Neurosurg Focus. 2017;43:E6. doi: 10.3171/2017.5.FOCUS17245. [DOI] [PubMed] [Google Scholar]
- 13.Li X, Jahanmiri-Nezhad F, Rymer WZ, et al. An examination of the motor unit number index (MUNIX) in muscles paralyzed by spinal cord injury. IEEE Trans Inf Technol Biomed. 2012;16:1143–1149. doi: 10.1109/TITB.2012.2193410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng C, Yu Q, Shan X, et al. Early surgical decompression ameliorates dysfunction of spinal motor neuron in patients with acute traumatic central cord syndrome: an Ambispective cohort analysis. Spine. 2020. 10.1016/j.wneu.2020.01.171. [DOI] [PubMed]
- 15.Escorcio-Bezerra ML, Abrahao A, de Castro I, et al. MUNIX: reproducibility and clinical correlations in amyotrophic lateral sclerosis. Clin Neurophysiol. 2016;127:2979–2984. doi: 10.1016/j.clinph.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Querin G, Lenglet T, Debs R, et al. The motor unit number index (MUNIX) profile of patients with adult spinal muscular atrophy. Clin Neurophysiol. 2018;129:2333–2340. doi: 10.1016/j.clinph.2018.08.025. [DOI] [PubMed] [Google Scholar]
- 17.Bas J, Delmont E, Fatehi F, et al. Motor unit number index correlates with disability in Charcot-Marie-tooth disease. Clin Neurophysiol. 2018;129:1390–1396. doi: 10.1016/j.clinph.2018.04.359. [DOI] [PubMed] [Google Scholar]
- 18.Fukada K, Matsui T, Furuta M, et al. The motor unit number index of subclinical abnormality in amyotrophic lateral sclerosis. J Clin Neurophysiol. 2016;33:564–568. doi: 10.1097/WNP.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 19.Zheng C, Zhu Y, Zhu D, et al. Motor unit number estimation in the quantitative assessment of severity and progression of motor unit loss in Hirayama disease. Clin Neurophysiol. 2017;128:1008–1014. doi: 10.1016/j.clinph.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Aarabi B, Akhtar-Danesh N, Chryssikos T, et al. Efficacy of ultra-early (< 12 h), early (12-24 h), and late (>24-138.5 h) surgery with magnetic resonance imaging-confirmed decompression in American spinal injury association impairment scale grades a, B, and C cervical spinal cord injury. J Neurotrauma. 2020;37:448–457. doi: 10.1089/neu.2019.6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson JR, Witiw CD, Badhiwala J, et al. Early surgery for traumatic spinal cord injury: where are we now? Global Spine J. 2020;10:84S–91S. doi: 10.1177/2192568219877860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anderson KK, Tetreault L, Shamji MF, et al. Optimal timing of surgical decompression for acute traumatic central cord syndrome: a systematic review of the literature. Neurosurgery. 2015;77:S15–S32. doi: 10.1227/NEU.0000000000000946. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.