Abstract
Programs including the ToxCast project have generated large amounts of in vitro high‒throughput screening (HTS) data, and best approaches for the interpretation and use of HTS data, including for chemical safety assessment, remain to be evaluated. To fill this gap, we conducted case studies of two indirect food additive chemicals where ToxCast data were compared with in vivo toxicity data using the RISK21 approach. Two food contact substances, sodium (2-pyridylthio)-N-oxide and dibutyltin dichloride, were selected, and available exposure data, toxicity data, and model predictions were compiled and assessed. Oral equivalent doses for the ToxCast bioactivity data were determined by in-vitro in-vivo extrapolation (IVIVE). For sodium (2-pyridylthio)-N-oxide, bioactive concentrations in ToxCast assays corresponded to low-and no-observed adverse effect levels in animal studies. For dibutyltin dichloride, the ToxCast bioactive concentrations were below the dose range that demonstrated toxicity in animals; however, this was confounded by the lack of toxicokinetic data, necessitating the use of conservative toxicokinetic parameter estimates for IVIVE calculations. This study highlights the potential utility of the RISK21 approach for interpretation of the ToxCast HTS data, as well as the challenges involved in integrating in vitro HTS data into safety assessments.
Keywords: RISK21, ToxCast, In-vitro in-vivo extrapolation, High-throughput screening, Food additive, Dibutyltin dichloride, Sodium (2-pyridylthio)-N-Oxide (pyrithione sodium)
1. Introduction
Current established methods of chemical safety testing are based on use of animal bioassays, and are time and resource intensive. Large numbers of chemicals are in use commercially in the United States and globally, and many have little to no toxicological data and await evaluation by these methods. Recently, a strategy was developed to shift toxicity testing towards a more mechanistic, high-throughput basis to enable testing of more chemicals more quickly and cost effectively (National Research Council, 2007). Part of this plan is the development of in vitro high-throughput screening (HTS) assays that will evaluate biological pathway perturbations to evaluate chemicals for toxicity. HTS assay development, chemical screening, and data generation have been significant, particularly by the Tox21 effort (Kavlock et al., 2012; Tice et al., 2013). Interpretation of HTS data and development of best practices for the use of such data in chemical safety assessment remains in progress (Groh and Muncke, 2017). HTS data are already being proposed as a means by which to prioritize chemicals for further testing (USEPA, 2014); however, comparison of HTS data with in vivo animal data for predictivity assessments of human toxicity are ongoing (Judson et al., 2010; Liu et al., 2017; Rotroff et al., 2013). Ultimately, the goal is to use in vitro data to predict in vivo human effects, reducing the reliance on animal testing (NCATS, 2016; NTP, 2016). The EPA’s ToxCast program, which is part of the larger Tox21 interagency collaboration, has provided a wealth of HTS data to the toxicology community, generating data on more than 3000 chemicals across 1000 assay endpoints, with the goal of generating screening data that could be used for prioritizing chemicals for further testing (Dix et al., 2007; Richard et al., 2016). So far, much of the work with the ToxCast data has focused on identifying endocrine disrupting chemicals, with some additional studies done to identify chemicals that potentially target specific pathways (D. Filer et al., 2014; Janesick et al., 2016; Pham et al., 2016; Reif et al., 2010). Little work has been done on a chemical-specific level, where the goal is based on identifying a mode of action or a point of departure for a specific chemical lacking hazard or safety data using this battery of assays (Shah et al., 2016).
Risk assessment is the process in which toxicity data, which are used to determine hazard, are combined with exposure data to determine the risk to a population for a particular chemical and use/exposure scenario. RISK21 is a flexible risk assessment framework that provides a transparent process to assess and visualize risk (Pastoor et al., 2014). This iterative process allows the user to evaluate risk (both hazard and exposure information) at each step of the process, refining information and estimates in a tiered manner, until there is enough precision to address the particular decision context (Embry et al., 2014). This can be visualized using the RISK21 webtool, providing a transparent way to present risk at each stage (HESI, 2017).
Chemicals having any use associated with food are of particular concern for chemical safety evaluation, as human exposure to chemicals in food is direct and frequent, and it is estimated that thousands of chemicals are added to foods in the US, either directly or indirectly (Neltner et al., 2011). The ToxCast screening library includes many chemicals having a use associated with food, and many of these chemicals show bioactivity in ToxCast assays (Karmaus et al., 2017, 2016). In the current study, we conduct case studies of two indirect food additives where we compare the animal and ToxCast data for both chemicals, using the RISK21 approach to provide an example and gain insight into a possible use of ToxCast HTS data. The purpose is to give context to HTS data, highlight a potential method for using HTS data in food safety assessment, and identify the challenges involved.
2. Materials and methods
2.1. Selection of chemicals for case studies
Two chemicals were selected for the case studies from the indirect food additives identified in ToxCast (Karmaus et al., 2017, 2016). Initially, the 20 indirect food additives with the highest number of active endpoints in the ToxCast data, after cytotoxicity filtering, were considered. Of these twenty, the two selected chemicals were ultimately picked based on the availability of animal toxicity data and initial exposure estimates (Karmaus et al., 2016). The two chemicals selected were sodium (2-pyridylthio)-N-oxide (also commonly referred to as pyrithione sodium or sodium omadine, abbreviated here as SPO, CASRN 3811-73-2; DTXSID3042390) and dibutyltin dichloride (abbreviated here as DBTC, CASRN 683-18-1; DTXSID8027292). SPO is an antimicrobial used on food contact surfaces and in adhesives used to make food packaging. DBTC is a catalyst and a heat stabilizer in polymers used in plastics (21 CFR§177.1680) (Forsyth, D S et al., 1993; Harper et al., 2005; Kannan et al., 1999; Quevauviller et al., 1991). DBTC is one of a group of organotin chemicals including dibutyltin diacetate, dibutyltin oxide, dibutyltin dilaurate, and the tributyltins and monobutyltins that are often considered together for toxicity (European Food Safety Authority Panel, 2004; Harper et al., 2005; WHO, 2006). These chemicals are amenable to grouping as the moieties attached to the dibutyltin (dichloride, diacetate, oxide, dilaurate) are relatively weakly bonded to the tin atom and easily interchanged, and thus the chemicals can interconvert (Fisch et al., 1999; Poller, 1978; WHO, 2006). For the purpose of integrating as much in vivo animal toxicity and exposure data as possible, we have chosen to include data across this group of organotin chemicals for this analysis.
2.2. Exposure
Registered uses of both chemicals were found in FDA and EPA databases by searching for CAS registry numbers and common names as listed in ChemBook (ChemicalBook, 2016). Databases and resources that were used and/or checked are listed in Supplemental Table 1. DBTC was listed in the Code of Federal Regulations (CFR, 21 CFR§177.1680), and SPO was listed in the CFR (21 CFR§175.105) and in the Inventory of Effective Food Contact Substance (FCS) Notifications database (FCN#1659, FCN #175) from the FDA. Other organotins, including dibutyltin dilaurate, dibutyltin dioxide, and dibutyltin diacetate, are also listed in the CFR as catalysts in various polymers (21 CFR§175.105, 21 CFR§175.300, 21 CFR177.1680, 21 CFR§177.2420). For estimates of exposure, reports from various regulatory agencies were reviewed, including the FDA’s CEDI database (U.S.FDA, 2012), the EPA’s rapid chemical exposure and dose research (ExpoCast) project (U.S.EPA, 2014), the MIGRESIVES project (European Commission, 2010), and the FACET project (EU, 2012). For SPO, exposure estimates from the CEDI database (U.S.FDA, 2012) and the ExpoCast project (Wambaugh et al., 2014) were used, which are publicly available at the EPA’s Computational Toxicology Chemistry Dashboard (U.S.EPA, 2016).
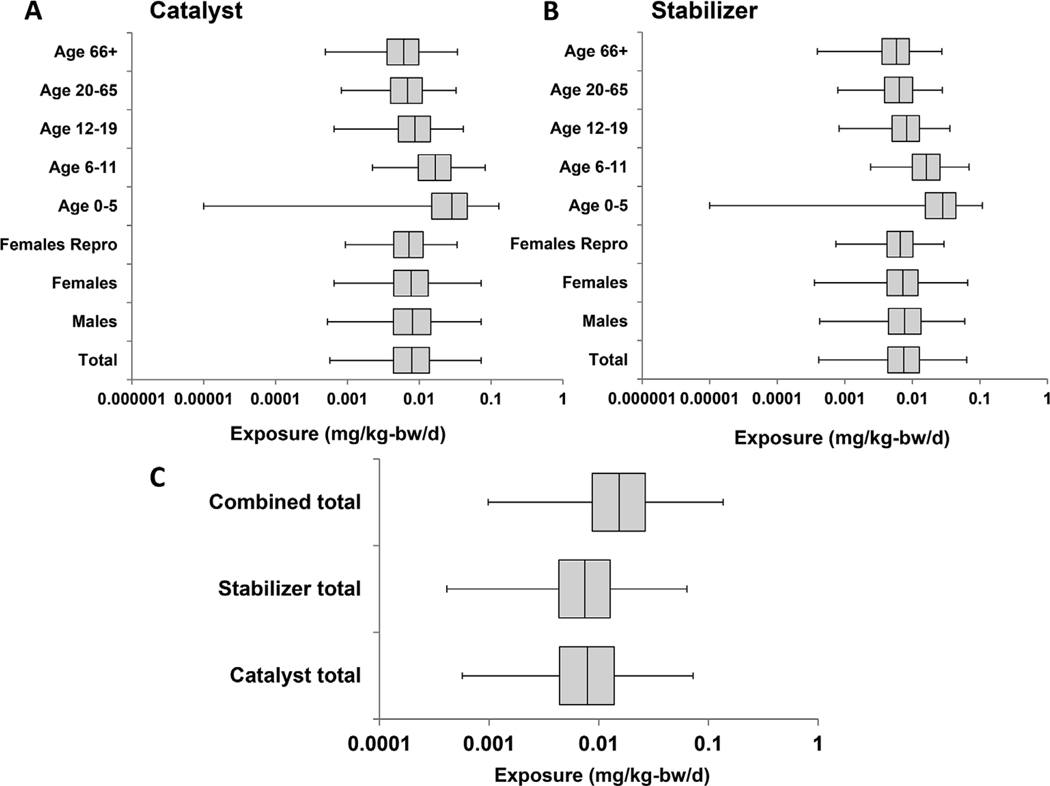
DBTC had an exposure estimate in ExpoCast, but was lacking a CEDI database entry or other exposure estimate. Accordingly, to increase confidence in the exposure estimates for DBTC, exposure modeling was conducted using the approach developed by Biryol et al. (2017) for chemicals in polymer food packaging (note this model was not applicable to SPO since it is not used as a polymer additive). This HTS-level modeling approach estimates exposure for chemicals used in food packaging. It combines physicochemical properties of chemicals (log of the octanol water partition coefficient, molecular weight, and solubility) with known uses and levels of chemicals in food packaging polymers to parameterize a model of migration from a food’s packaging material into various food types under different storage conditions (Biryol et al., 2017). DBTC physicochemical properties were obtained from 2006 WHO CICAD #73 and Chembook (ChemicalBook, 2016; WHO, 2006). These migration estimates are then combined with food consumption data from NHANES food diaries within EPA’s High]Throughput Stochastic Human Exposure and Dose Simulation (SHEDS-HT) (Isaacs et al., 2014) model to estimate exposure to foods containing the chemicals used in packaging (USDA, 2015). DBTC is listed for use as a catalyzer in polyurethanes (21 CFR§177.1680), but is also listed in the literature as a heat stabilizer in PVC and has been detected in plastics in addition to polyurethanes such as PVC (Forsyth, D S et al., 1993; Papaspyrou et al., 2007; Rosenberg, 2013). Accordingly, a SHEDS-HT exposure simulation was run with DBTC as a catalyst and another with DBTC as a heat stabilizer in PVC. Amounts of DBTC allowed for use in polymers are 0.5–1.5% as a heat stabilizer in PVC, and 0.001–0.5% as a catalyst in polymers, and these values were used to parameterize the exposure model simulations for the two uses (WHO, 2006). Because several dibutyltins are registered for use in different plastics, it was conservatively assumed that DBTC was used in all types of plastics as a catalyst in lieu of additional data. First, migration of DBTC from polymer packaging into food was estimated using the physicochemical properties of DBTC and the initial concentration in the polymer for various food categories, which were broken into aqueous, fatty, alcoholic, and acidic groups stored at various temperatures (U.S.FDA, 2007). This gave predictions of DBTC concentrations in foods. Secondly, exposure to food containing the migrated chemical was estimated using US food consumption data from NHANES food diaries. This results in a range of exposures to DBTC from the diet for a population of people. This is a conservative model, meant for chemical screening or prioritization, and makes several assumptions designed to favor over-estimation of exposure to the chemical, including the assumptions that dibutyltin is used in all of the polymers in which it allowed (full market penetration), the assumption that the polymer packing is a monolayer in direct contact with the food, and the assumption that the concentration of dibutyltin in foods is the same across the foods in a specific food category for the purposes of modeling individual consumption.
2.3. In vivo toxicity data
In vivo animal data for both chemicals were gathered from various sources. Searches were done for summary documents from regulatory agencies across the globe (Supplemental Table 1), and in databases such as ToxLine and PubMed. Data were collated and summarized. Reported NOEL, NOAEL and LOAEL values from animal bioassays were collected from reports from agencies and organizations involved in chemical safety, and used in this study as doses shown to cause activity in whole animal models (European Food Safety Authority Panel, 2004; Harper et al., 2005; OECD, 2008; U.S.EPA, 1996; WHO, 2006). Modes of action for both chemicals were identified if possible.
2.4. In vitro ToxCast data analysis
Assay-specific ToxCast data for both chemicals were downloaded from the ToxCast dashboard version 2 (U.S.EPA, 2015). Background and control assays, determined as assays that had “background measurement” or “null” as the intended target family, were removed. For SPO, 550 assays were obtained from the ToxCast Dashboard, and 447 remained for analysis after this removal. For DBTC, 325 assays were obtained from the ToxCast Dashboard and 228 remained for analysis after this removal. The remaining assays run were evaluated for activity using the hit calling criteria defined by the ToxCast Pipeline (tcpl) (D. L. Filer et al., 2014; Filer et al., 2017). If the assay was labeled a hit, it was labeled as an active assay for our purposes; all other tested assays were termed negative assays. A hit in ToxCast indicates that the response caused by the chemical in the assay exceeded the background noise of the assay and that a concentration-dependent trend (indicated by a best-fit model of Hill or gain-loss) could be fit to the data. For eachactive assay, a measure of potency in the form of an AC50 value (representing the concentration (in μM) at which half of the maximal activity/efficacy is achieved with the chemical in the assay) was reported as well as a value for efficacy represented as the maximal magnitude of response for the compound in the modeled concentration-response active assay, a measure of potency in the form of an AC50 value (representingthe concentration (in μM) at which half of the maximal activity/efficacy is achieved with the chemical in the assay) was reported as well as a value for efficacy represented as the maximal magnitude ofresponse for the compound in the modeled concentration-response curve. For each chemical, active assays were organized by AC50 value from low to high. Summary statistics were compiled for each chemical to find the mean and various percentiles across the AC50 values for assays in which the chemical was active. The cytotoxicity center was calculated as the median AC50 value among active cytotoxicity assays. The cytotoxicity limit (the concentration three global cytotoxicity median absolute deviations below the cytotoxicity center for a given compound) is reported on the ToxCast dashboard and was used to filter the results for potential confounding effects of cytotoxicity or cell stress (Judson et al., 2016). Active assays with an AC50 value less than the cytotoxicity limit were counted separately as active assays after cytotoxicity filtering; cytotoxicity assays below the cytotoxicity limit were excluded from this count.
2.5. Estimation of steady state concentrations (Css) and oral equivalent doses (OEDs)
First, steady state concentrations (Css) for the two chemicals were estimated in order to compare the ToxCast AC50 values with in vivo-derived NOAEL, NOEL, and LOAEL values. These Css values were used in conjunction with the ToxCast data, utilizing a process known as reverse toxicokinetics (rTK), to estimate oral equivalent doses (OEDs) (Wetmore et al., 2012). OEDs are defined as the external dose required to achieve plasma concentrations of the chemical equal to the in vitro-derived ToxCast AC50 values (in μM). For SPO, in vivo toxicokinetic (TK) data from the literature was used to calculate Css; and for DBTC, an in-vitro-in-vivo extrapolation (IVIVE) approach was used (Mitoma et al., 1983).
In the case of SPO, in vivo toxicokinetic parameters such as the elimination rate, half-life, and volume of distribution were available for three animal species (rat, rabbit, and rhesus monkey) and were used to estimate the clearance (where the clearance equals the volume of distribution multiplied by the elimination constant) and Css (where Css is the rate of administration divided by the clearance) assuming a 1 mg/ kg-bw/day chemical administration (Mitoma et al., 1983).
To calculate the OEDs, the following equation was employed.
This equation assumes a daily oral exposure of 1 mg/kg-bw/day. OEDs were calculated for each ToxCast assay endpoint, for each chemical respectively.
With the absence of reliable in vivo toxicokinetic data for DBTC, we modified an approach used by Wetmore et al. to estimate TK parameters using parameters measured in vitro, and came up with several scenarios for DBTC to estimate the Css (Wetmore et al., 2015). This model estimates Css by combining estimates of nonmetabolic renal clearance (estimated as a function of glomerular filtration of the unbound fraction of the chemical in the kidney) with estimates of hepatic clearance based on clearance of the chemical by hepatocytes in culture:
Where ko = chemical exposure rate, QH = mean hepatic blood flow approximated as 90 L/h (Davies and Morris, 1993), Fub = unbound fraction of parent chemical in the blood, ClintH = hepatic intrinsic metabolic clearance; and GFR = glomerular filtration rate. ClintH estimate is scaled to represent whole organ clearance (in liters/h) from a Cluin vitro measurement of chemical clearance by hepatocytes in culture in (ml/(min × million cells)) based on the following:
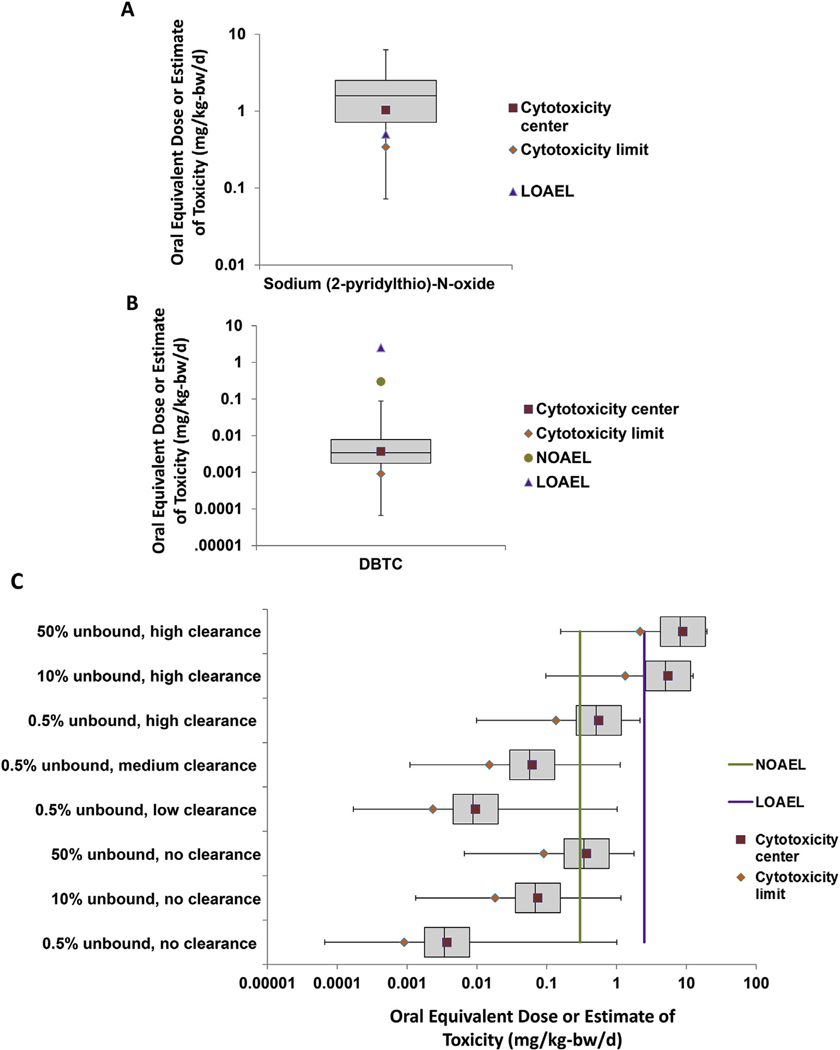
Where HPGL is the hepatocytes per gram liver (110 million cells/g liver (Barter et al., 2007);) and V1 is the liver volume (1596 g (Johnson et al., 2005);). As there were no experimental measurements of plasma protein binding or intrinsic hepatic clearance for DBTC (and metals are outside the domain of applicability for in silico prediction tools), DBTC Css was estimated using different binding and clearance scenarios as a surrogate. Plasma protein binding can theoretically range from 0% (none bound) to 100% (all of the chemical bound), and DBTC binding to plasma proteins was set to three different values (95.5% bound, 90% bound, 50% bound). Similarly, one of four possible liver clearance rates were also employed (e.g., no, low, medium or high clearance), which were chosen following review of the range of values reported for the over 400 chemicals that have been analyzed in in vitro hepatic clearance assays to date (Wetmore, 2015; Wetmore et al., 2012). These values were used to calculate Css as previously described (Wetmore et al., 2012). The OEDs were then calculated as described for SPO, but across a range of scenarios (Fig. 4C).
Fig. 4.
Comparison of ToxCast and Traditional Toxicology Data. ToxCast assay AC50 values were converted to oral equivalent doses (OED) and plotted as a distribution across the 5th, 25th, 50th, 75th, and 95th percentiles for all active assays for SPO and DBTC. The LOAEL/NOAEL values from the in vivo animal data, the cytotoxicity center, and cytotoxicity limit OEDs are plotted as point estimates. A) OEDs for SPO were determined using available toxicokinetic data. B) OEDs for DBTC were determined using the worst-case toxicokinetic parameter estimates. C) For DBTC, a range of toxicokinetic parameter estimates were used to determine OEDs from the AC50 values for the 5th, 25th, 50th, 75th, and 95th percentiles of all active assays, the cytotoxicity limit, and the cytotoxicity center. The in vivo NOAEL and LOAEL data are plotted as point estimates shown as green and purple lines respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.6. Generation of graphs and RISK21 plots
RISK21 plots were developed using the RISK21 webtool (HESI, 2017). Input data were generated as described in the section above. Other graphs were generated using SigmaPlot software (Systat, Chicago, IL).
3. Results
3.1. Estimates of exposure
A variety of sources and tools were mined for exposure estimates. Registered food uses of both chemicals were identified by CAS registry number and common names (ChemicalBook, 2016). SPO (CAS# 3811-73-2) is listed for use as an antimicrobial agent for use on food contact materials and as an antimicrobial in adhesives used to make packaging (21 CFR§175.105, FCN#1659, FCN #175) (U.S.EPA, 2011). DBTC(CAS# 683-18-1) is used as a catalyst in polymers used to make plastic packaging. DBTC is registered for use in polyurethanes (21 CFR§177.1680), but other dibutyltins which have the ability to interconvert (dibutyltin oxide, dibutyltin dilaurate, dibutyltin diacetate) are registered for use in other polymers (21 CFR§175.105, 21 CFR§175.300, 21 CFR177.1680, 21 CFR§177.2420). DBTC is also reported to be used as a heat stabilizer in polymers such as PVC, though this use was not found in the CFR (Forsyth, D S et al., 1993; Papaspyrou et al., 2007; Rosenberg, 2013).
SPO has an exposure estimate in the CEDI (Cumulative Estimated Daily Intake) database of 0.00016 mg/kg-bw/d (U.S.FDA, 2012). DBTC did not have an entry in the CEDI database, or in the other databases used in this study.
Both chemicals had a screening-level exposure estimate available from the EPA’s ExpoCast project. The ExpoCast approach computationally estimates exposure using a high-throughput heuristic model to get a broad estimate of exposure to use to prioritize chemicals for further study, particularly intended to be used to estimate exposure for chemicals when no other data are available (Wambaugh et al., 2014). It uses the production volume of a chemical and four simple use “heuristics” to estimate exposure via a regression against exposures inferred from biomonitoring data. In the case of SPO, the ExpoCast prediction corresponding to the upper 95% credible interval around the geometric mean, was below the CEDI value. Therefore, the median ExpoCast value for the whole population was taken as the low estimate of exposure for our analysis, and the CEDI value as the high estimate of exposure (Table 1).
Table 1.
Human exposure estimates.
| Compound | Exposure: Lower Bound (mg/kg-bw/d) | Exposure: Upper Bound (mg/kg-bw/d) |
|---|---|---|
|
| ||
| Sodium (2-pyridylthio)-N-oxide | 2.39E-08 (ExpoCast)a | 0.00016 (CEDI)b |
| Dibutyltin Dichloride | 1.07E-07 (ExpoCast)a | 0.015 (SHEDS-HT)c |
ExpoCast predictions are from a high-throughput model based on production volume and listed uses providing broad estimate of exposure (Wambaugh et al., 2014).
Cumulative Estimated Daily Intake (CEDI) is a database maintained by the US FDA on exposure to food-use chemicals.
SHEDS-HT estimates dietary exposure to chemicals in food contact materials by modeling migration of the chemical from the food contact substance polymer into different foods, and then modeling exposure to the food containing the chemical using NHANES food intakes, and is a very conservative model, meant for prioritization (Biryol et al., 2017).
Since only the ExpoCast exposure estimate was available for DBTC, an additional estimate of exposure to DBTC from migration from plastic packaging into foods was estimated as described in the Methods. Several dibutyltin chemicals, which have the ability to interconvert, are currently in use in plastic packaging with the relative usage amounts unknown (OECD, 2008). Therefore, we conservatively assumed dibutyltin use as both a heat stabilizer and a catalyst in a variety of polymers. This resulted in a range of exposures to dibutyltin in foods across the population, due to differences in food type consumption and body weights (Fig. 1). The estimates of exposure derived from this method were higher than the ExpoCast predictions, which is expected as the food contact exposure model makes a number of conservative assumptions including full market penetration of dibutyltin in the polymers for which it is permitted. For comparison with other data, the median values for the total population estimate for both the catalyst and stabilizer uses were added together and used as the high estimate of exposure (Table 1).
Fig. 1.
DBTC Estimate of Exposure. Estimates of exposure for DBTC were generated by modeling the migration of dibutyltin from plastic food packing into foods based on physicochemical parameters and initial amounts of the chemical in the packaging. Human exposure to foods containing dibutyltin was estimated using food diaries from NHANES. The 1st, 25th, 50th, 75th, and 99th percentiles of exposure were calculated for the total population and for various subgroups. Estimates were computed and plotted separately when considering different uses for DBTC as A) a catalyst or B) a heat stabilizer. C) Exposure values for the total population were plotted for use of DBTC as a catalyst, heat stabilizer, or combined use.
3.2. In vivo data (Traditional Toxicology Data)
Both chemicals were selected because of the availability of traditional in vivo toxicology data reviewed by various agencies and organizations involved with chemical safety (European Food Safety Authority Panel, 2004; Harper et al., 2005; OECD, 2008; U.S.EPA, 1996; WHO, 2006). The available data are summarized in Table 2.
Table 2.
Traditional toxicological data.
| Compound | NOAEL/LOAEL | Study type | Endpoint | Limit | Source |
|---|---|---|---|---|---|
|
| |||||
| Sodium (2-pyridylthio)-N-oxide | 0.5mg/kgbw/d (NOEL) | 2yr chronic rat (oral) | Neurotoxicity (hind limb paralysis) | 0.005mg/kg-bw/d | USEPA, 1996 |
| Dibutyltin Dichloride | 2mg/kgbw/d (NOAEL) | 90d rat (oral) | Reduced body weight and food intake, hematological effects | None determined | OECD SIDS, 2006 |
| Dibutyltin Dichloride | 5mg/kgbw/d (LOAEL) | 6wk rat (oral) | Immunotoxicity | 0.005mg/kg-bw/d | ATSDR, 2005 |
| Dibutyltin Dichloride | 2.5mg/kgbw/d (LOAEL) | 6wk rat (oral) | Immunotoxicity | 0.003mg/kg-bw/d | WHO, 2006 |
| Organotin Compounds | 0.025mg/kgbw/d (NOAEL)a | 2yr chronic rata | Immunotoxicity | 0.00025mg/kg-bw/db | EFSA, 2004 |
This study was conducted using tributyltin.
This limit is for a group of organotin compounds including dibutyltin dichloride. EFSA considered the effects dibutyltin, tributyltin, dioctyltin, and triphenyltin to be similar enough to warrent a group limit, and derived the limit from a 2 year chronic study with tributyltin and shorter studies with all four compounds individually.
SPO is registered with the EPA as a pesticide due to its antimicrobial properties and as such, the data on SPO comes through the EPA (U.S.EPA, 2011, 1996). The EPA established reference dose for SPO is 0.005 mg/kg-bw/d based on a NOEL of 0.5 mg/kg-bw/d (U.S.EPA, 1996). The most sensitive endpoint observed with SPO was neurotoxicity, comprising hind limb paralysis and skeletal muscle atrophy (U.S.EPA, 1996). SPO is an ionophore, but beyond this a mode of action has not been determined (Kim et al., 1999; Knox et al., 2004; Kondoh et al., 2002; Lind et al., 2009).
Several groups have reviewed DBTC, either alone or as a one of a number of organotin chemicals (European Food Safety Authority Panel, 2004; Harper et al., 2005; OECD, 2006; WHO, 2006). DBTC is an immunotoxicant, with changes in blood cell counts and hematocrit as the most often noted effects (OECD, 2006). Higher doses have been shown to cause reproductive toxicity as well as damage to the liver and pancreas. A definitive mode of action is unknown, but DBTC has been shown to induce apoptosis (Matsushita et al., 2012). DBTC also interacts with thiol-containing compounds, and may disrupt cell membranes and cytoskeletons (Ali et al., 1990).
DBTC and other dibutyltin chemicals are made and used on their own, but are also metabolites of tributyltin, and can break down into monobutyltin (Kimmel et al., 1977; Ueno et al., 1994). In addition, the various dibutyltin chemicals can interconvert under certain conditions, such as acidic conditions seen in the stomach (OECD, 2008). Therefore, some groups consider these chemicals together in a group (European Food Safety Authority Panel, 2004). Tributyltin is the most well studied of the group, and all three have been shown to act similarly in the body. Several groups have come up with tolerable daily allowances for DBTC and other organotin compounds (Table 2). The European Foods Safety Authority (EFSA) holds the lowest limit that is applied across the group of organotin chemicals after deeming them to possess a similar mode of action due to their similarity in structure and in effects observed in short-term assays (European Food Safety Authority Panel, 2004). EFSA set the limit based on the most sensitive endpoint effect level from a longer term tributyltin study (European Food Safety Authority Panel, 2004).
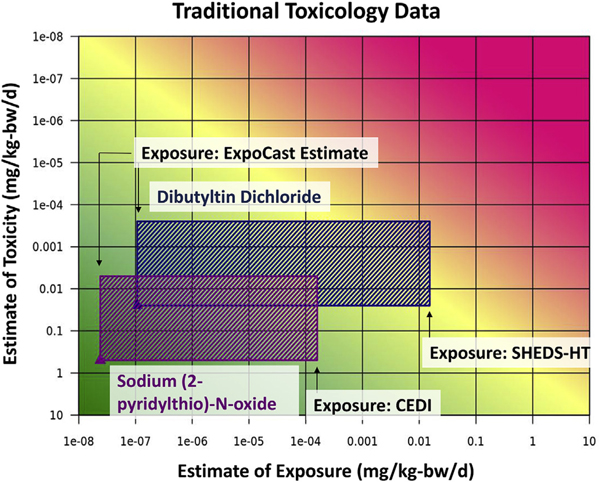
The exposure data were combined with the in vivo data for both chemicals to generate a plot using the RISK21 risk visualization matrix (Fig. 2). The matrix plot is an integration of traditional in vivo animal study toxicity data (Table 2) and exposure estimates (Table 1). For each chemical, the maximum and minimum exposure estimates were plotted on the X-axis, and the reference dose or tolerable daily intake was plotted on the Y-axis as a point estimate (using the NOEL for SPO and the NOAEL from the 2 year chronic rat study for DBTC in Table 2) plus the uncertainty factor of 100 to account for interspecies and intraspecies variation.
Fig. 2.
RISK21. Estimates of exposure from Table 1, and the most sensitive NOEL/NOAEL value from Table 2 were used to develop a RISK21 plot using the RISK21 webtool. The ExpoCast estimates of exposure are used as the lower estimate of exposure for both DBTC and SPO, and the upper estimates of exposure are the CEDI value for SPO and the SHEDS-HT value for DBTC. Estimates of toxicity (a NOEL of 0.5 mg/kgbw/d for SPO and a NOAEL of 0.025mg/kgbw/d for DBTC) were plotted as point estimates with an uncertainty factor of 100 to define the reference dose or tolerable daily intake established limit for each compound. The top of the colored boxes per chemical denote the reference dose or the tolerable daily intake, and the bottom of the box is the dose given to the animal used to determine that limit.
3.3. ToxCast data
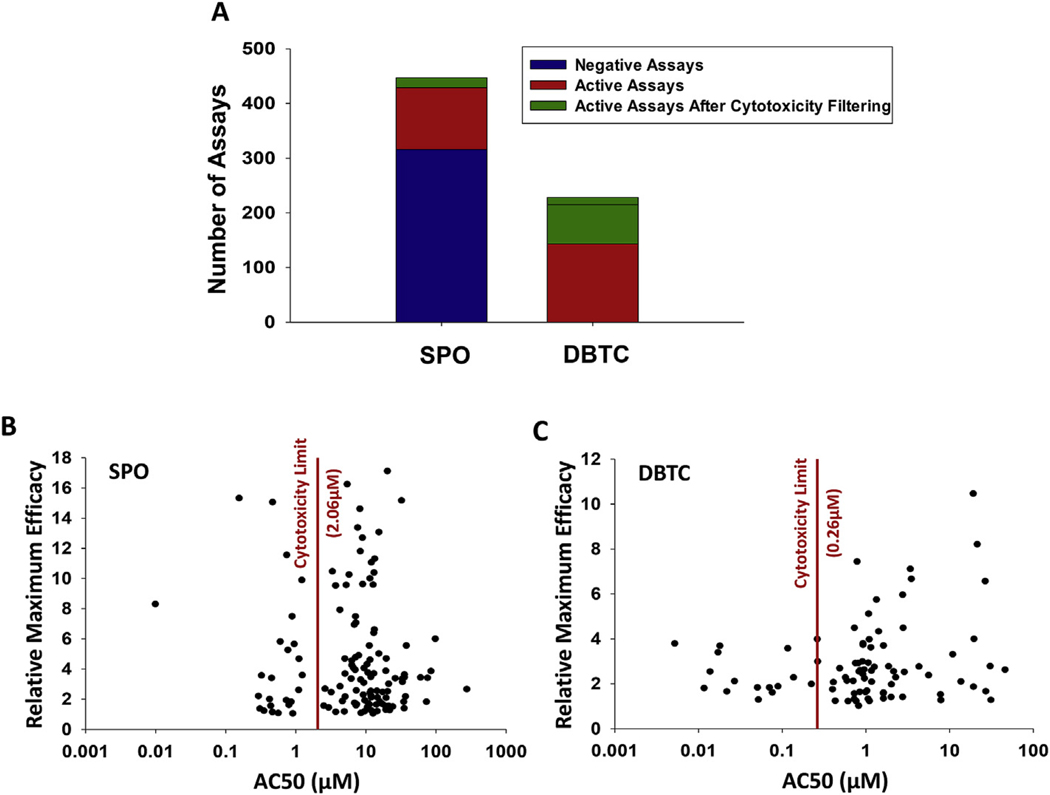
ToxCast data were processed to remove background and control assays, resulting in SPO having been evaluated across 447 assays, of which 131 were reported as active. The cytotoxicity center and cytotoxicity limit for filtering, determined as described in the methods, were6.23 μM and 2.06 μM respectively. After comparing the AC50 values from active assays to the cytotoxicity limit, there were 18 active assays that were not considered cytotoxicity-confounded based on our approach (Fig. 3A and B). These 18 assays are listed in Table 3 and reflect many target groups, with no specific biological targets based on intended target as listed in ToxCast appearing as distinctly sensitive to SPO.
Fig. 3.
Summary of the ToxCast data for DBTC and SPO. Assay-specific data for both chemicals were pulled from the ToxCast Dashboard. A) ToxCast hit calls were used to define negative and active assays. Cytotoxicity filtering was done by applying the cytotoxicity limit as a cutoff value wherein assays having an AC50 below the cytotoxicity limit remained active after filtering. B, C) All assays determined to be active for either compound were plotted as AC50 versus the modlTp (maximum efficacy of modeled concentration-response curve) parameter from the ToxCast data, for each assay, with the cytotoxicity limit plotted as a straight line.
Table 3.
Assay remaining after cytotoxicity filtering.
| Sodium (2-pyridylthio)-N-oxide | Dibutyltin Dichloride | ||
|---|---|---|---|
|
| |||
| Assay | AC50 (μM) | Assay | AC50 (μM) |
|
| |||
| ACEA_T47D_80hr_Positive | 0.4427 | ATG_PPRE_CIS_up | 0.1376 |
| APR_HepG2_OxidativeStress_24h_up | 0.8647 | TOX21_AR_BLA_Antagonist_ratio | 0.0272 |
| APR_HepG2_StressKinase_24h_up | 0.8041 | TOX21_AR_LUC_MDAKB2_Antagonist | 0.0896 |
| BSK_CASM3C_Thrombomodulin_up | 0.9144 | TOX21_ARE_BLA_agonist_ratio | 0.0052 |
| BSK_hDFCGF_CollagenIII_down | 1.2514 | TOX21_Aromatase_Inhibition | 0.1174 |
| BSK_hDFCGF_IL8_down | 0.4710 | TOX21_ERa_BLA_Antagonist_ratio | 0.0172 |
| BSK_hDFCGF_IP10_down | 0.7484 | TOX21_FXR_BLA_antagonist_ratio | 0.0181 |
| BSK_hDFCGF_MCSF_down | 0.6056 | TOX21_GR_BLA_Antagonist_ratio | 0.0220 |
| BSK_hDFCGF_MIG_down | 0.1566 | TOX21_PPARd_BLA_antagonist_ratio | 0.0118 |
| BSK_hDFCGF_MMP1_down | 0.8950 | TOX21_PPARg_BLA_antagonist_ratio | 0.0709 |
| BSK_hDFCGF_PAI1_down | 1.1242 | TOX21_TR_LUC_GH3_Antagonist | 0.0138 |
| BSK_hDFCGF_TIMP1_down | 0.9571 | TOX21_VDR_BLA_antagonist_ratio | 0.0767 |
| BSK_hDFCGF_VCAM1_down | 0.7786 | ||
| TOX21_AR_BLA_Antagonist_ratio | 0.2953 | ||
| TOX21_Aromatase_Inhibition | 0.3265 | ||
| TOX21_FXR_BLA_antagonist_ratio | 0.4553 | ||
| TOX21_PPARd_BLA_antagonist_ratio | 0.3095 | ||
| TOX21_TR_LUC_GH3_Antagonist | 1.1110 | ||
For DBTC, 228 assays were run after removal of control assays, and 85 of these were reported as active. Similar to SPO, the assays determined to be active span a range of target groups, and no one group emerges as a most sensitive target group to DBTC. The cytotoxicity center for DBTC was 1.07 μM, with a cytotoxicity limit of 0.26 μM, and 12 assays remain after cytotoxicity filtering (Fig. 3 A, C). The assays remaining after cytotoxicity filtering are listed in Table 3. For both chemicals, there is no group of most sensitive assays, and many of the assays after cytotoxicity filtering are loss-of-signal assays. For SPO, many of the active assays after cytotoxicity filtering are BioSeek assays investigating inflammatory and angiogenic endpoints or Tox21 nuclear receptor assays, and for DBTC many of the active assays after cytotoxicity filtering are Tox21 nuclear receptor antagonist assays. However, the BioSeek assays were not run for DBTC, and the difference in the types of assays hit may be influenced by the assays run for each chemical in ToxCast.
OEDs were calculated from the ToxCast AC50 values, and the calculated OEDs were plotted alongside the NOAEL and LOAEL levels from the animal data for both chemicals (Fig. 4). For both compounds, OEDs were calculated for several different AC50 values to get the range of possible OEDs, including the cytotoxicity center, the cytotoxicity limit, and the 5th, 25th, 50th, 75th, and 95th percentile AC50 values for all active assays. For SPO, the OEDs lined up well with the values from the ToxCast data. The LOAEL falls between the cytotoxicity limit and cytotoxicity center OED values, indicating that bioactivity in the ToxCast assays occurs at concentrations that align with doses seen to cause effects in animal bioassays (Fig. 4 A). For DBTC, the relationship was not as clear. In lieu of measured TK parameters, when the most conservative rTK estimates were used (no hepatic clearance and 95.5% of the chemical bound to plasma proteins), IVIVE-based calculations to estimate OEDs from bioactivity in ToxCast were well below doses needed to cause effects in the animal bioassays (Fig. 4 B). When the rTK estimates were varied to include different possible plasma protein binding levels and hepatic clearance levels that span the range of hepatic clearance values measured in vitro for over 400 chemicals for this method (Fig. 4 C), it was found that the calculated OEDs for any given ToxCast parameter shifted by three orders of magnitude. Thus, for DBTC, the lack of TK data limits the conclusions that can be drawn from the data, and future work to generate rTK parameters could significantly improve estimates.
4. Discussion
This study presents case studies of two indirect food additive chemicals for which in vitro HTS data from ToxCast were compared to in vivo toxicity data using the RISK21 risk assessment-based decision framework. The RISK21 framework is a problem-formulation based exposure driven risk assessment tool that visually represents risk by graphically plotting exposure and hazard data. One of the main challenges facing incorporation of HTS data in chemical risk evaluation is the ability to relate an in vitro bioactive concentration to an external dose. Our study directly addressed the challenge of converting concentrations from in vitro assays into comparable units of doses given in vivo. For one chemical used in this study, SPO, conversion of concentrations that elicit bioactivity in ToxCast to OEDs showed good correlation to in vivo animal-derived LOAELs. For the other chemical, DBTC, this was not the case. However, while SPO had available TK data from animal studies, DBTC did not have in vivo TK parameters. As an alternative, we utilized worst-case TK parameter estimates for IVIVE with DBTC, and found that this resulted in the concentrations that elicited bioactivity in ToxCast mapping to OEDs well below those shown to cause effects in animal studies. This is similar to what would be done on a first round risk assessment where, in the absence of data, worst case estimates would be used to see if an adequate decision could be made using those data. Comparing predictions from the worst-case scenario estimates against those derived using less conservative TK input parameters revealed a shift in OED potency of three orders of magnitude for DBTC. These OED values range from well below the dose shown to cause NOAEL/LOAEL effects in vivo to slightly above the LOAEL dose level, indicating that the more conservative estimates would result in lower levels relative to current data, and that refinement of the TK parameters has the potential to bring OEDs calculated from the ToxCast data into line with doses shown to cause effects in vivo. This finding underscores the importance in generating or refining uncertain TK information to ensure productive comparisons can be made between in vitro and in vivo datasets, as well as the potential utility of in vitro TK estimates for IVIVE.
As seen with DBTC, without adequate TK data IVIVE is challenging, but this difficulty can start to be overcome by employing worst-case estimates. Many chemicals besides DBTC are lacking toxicity data including TK data, and gaining these data would be of great significance for this type of work (Judson et al., 2009; Neltner et al., 2013). Work is ongoing in the estimation of TK parameters using in vitro assays as well as in the development of predictive models (Ingle et al., 2016; Wetmore, 2015; Wetmore et al., 2013). Expansion and refinement of these datasets and tools will be of great importance for studies such as this one. For our study, we wanted to show what would result from using rough estimates for IVIVE, as the most conservative values are often used in risk assessment when more specific data are not available(Embry et al., 2014; Pastoor et al., 2014). Our findings highlight that having even basic TK estimates such as in vitro measures of protein binding and liver clearance can greatly improve results and produce more accurate estimates.
For both chemicals, a mechanism of action was not readily evident based solely on the ToxCast data. While for some biological outcomes HTS assay coverage is robust, for others it is not, or data across the full assay battery may not be generated for all chemicals (Silva et al., 2015). If the chemical does not hit one of these well covered outcomes, it is difficult to determine the mechanism of action for the chemical, and to predict what toxicity would be seen in vivo. As demonstrated by other researchers, case studies done with chemicals with known mechanisms of action including endosulfan and methidathion, or ortho-phthalate chemicals, were able to match up some in vivo effects with ToxCast data, but were not able to match other in vivo endpoints, demonstrating that more needs to be done connecting in vitro bioactivity to in vivo outcomes (Pham et al., 2016; Silva et al., 2015). Both of the chemicals used in our study were rather potently cytotoxic in the ToxCast assays, and had relatively few hits below the cytotoxicity limit. Chemical concentrations that cause cytotoxicity in HTS assays show a burst activity in a number of assays, which is believed to be associated with cell stress and cytotoxicity responses as opposed to a single specific biomolecular interactions (Judson et al., 2016). It is possible that these chemicals do not have a specific cellular target and mechanism of action, but cause general cell stress and cytotoxicity, thus leading to toxicity. In this case, these results from the ToxCast assays are informative if the cell stress and cytotoxicity is the means by which these chemicals cause toxicity; however, without more assays and improved interpretation of existing assays, this is impossible to ascertain with certainty.
One exciting finding from this study is that for SPO, even though a mechanism of action could not be found using the ToxCast data, the values aligned well with the animal data, and would give a similar risk assessment outcome. Whether this is true for other chemicals is uncertain, and could be evaluated by doing this type of analysis on additional chemicals in ToxCast or other HTS data sets.
Another challenge is the lack of exposure data available for chemicals with any use associated with foods. From the RISK21 plot in Fig. 2, it is evident that the estimate of exposure needs refinement for both chemicals, especially DBTC, while the estimates of toxicity using traditional animal-based methods are adequate. For many chemicals, estimates of exposure are either non-existent, or are determined at a broad screening level (Wambaugh et al., 2014). This works well to prioritize chemicals for further study, but is not always adequate from a risk assessment perspective. The exposure modeling done with DBTC demonstrates one method of dealing with this data gap. However, even when such exposure model predictions are available, they may be too uncertain or overly conservative for use in final risk evaluations. Efforts to calibrate such conservative models with monitoring data or to refine information on the use of various chemicals in food applications (and corresponding migration and consumption information) can reduce this uncertainty. Data gaps in exposure and differences in exposure prediction approaches are highlighted in this paper by the large distance between the ExpoCast prediction and the CEDI or SHEDS-HT exposure estimates for both compounds. This discrepancy is due to differences in how these two exposure estimates are generated. To predict likely exposure, ExpoCast uses the amount of the chemical produced and 4 chemical use categories: pesticide active use, pesticide inert use, chemical/industrial process use with no consumer use, and consumer use and chemical/industrial process use. The ExpoCast model used here (which provides the current ExpoCast exposures in the CompTox Chemistry Dashboard) uses a regression model based on 5 heuristics as predictors of likely exposure. It takes into consideration the production volume of the chemical, and if the chemical has use as a pesticide inert ingredient, a pesticide active ingredient, consumer and industrial/ commercial use, and industrial/commercial but no consumer use (important note for this is that the consumer and industrial use and industrial but no consumer use categories do not include food uses). The model median and its uncertainty are estimated via a calibration again the NHANES population median and is thus reflective of the exposure to the chemical via all pathways for an average member of the population. It is not designed to be conservative per se, but rather the best model estimate for the true population median (with associated uncertainty). CEDI and SHEDS-HT, on the other hand, take a different approach to estimating exposure – both are predicting food-specific use by taking the known use of the chemical in food contact substances (FCS), the amounts allowed in the FCS, and then predicting migration from the FCS into the food and consumption of the food. These mechanistic pathway-specific models make numerous conservative assumptions (Section 2.2), and thus may reflect a worst-case estimate of the population median. The SHEDS model predictions are not (as presented here) calibrated against biomonitoring data (as are the ExpoCast values). A comparison of the SHEDS values with biomonitoring data in Biryol et al. (2017) demonstrated that the SHEDS-HT values were likely over-estimates by at least two orders of magnitude. Recent work has integrated multiple SHEDS models (as well as other mechanistic exposure models) with NHANES biomonitoring data in an evaluation framework called the Systematic Empirical Evaluation of Models (SEEM)(Ring et al., 2019). These efforts will allow for the integration of food contact pathways into next-generation ExpoCast exposure values while simultaneously correcting over-conservative models for individual pathways. Future work should also address the data gaps in the mechanistic food contact pathway exposure models that result in the use of overly-conservative assumptions. Such improvements will increase the predictive power of the food contact models within the SEEM framework, and hopefully reduce the resulting uncertainty in future ExpoCast predictions.
The case of DBTC highlights additional considerations needed for chemical groups since there is no standardized way of dealing with related chemicals that can interconvert and have similar effects (Groh and Muncke, 2017). Some agencies consider all members of such groups individually, whereas other agencies consider members of the group together. Clarity in the best practices for dealing with these types of chemicals are needed.
This study shows the potential for HTS data to be used in toxicity testing and food safety analysis, while also highlighting the need for more toxicokinetic data and increased breadth of HTS assay coverage. Though specific mechanisms of action were not identified from the ToxCast data for either compound, for SPO the OEDs calculated from the ToxCast data correlated with doses shown to cause effects in vivo and would lead to similar risk assessment conclusions. OEDs calculated for DBTC varied greatly depending on the TK parameter estimates of plasma protein binding and hepatic clearance used for IVIVE, but were generally lower than or included doses shown to cause effects in vivo. Great strides have been made in generating HTS data across commercial chemicals, and equally important will be availability of TK and exposure data to facilitate use of these data in chemical safety assessments.
Supplementary Material
Acknowledgements
The authors would like to thank the ILSI-North America Food and Chemical Safety Committee for their guidance and support. The authors would also like to thank Matt Martin formerly from the U.S. EPA’s NCCT, as well as U.S. FDA Center for Food Safety and Applied Nutrition (CFSAN) scientists, including Suzanne Fitzpatrick, Kirk Arvidson, Kristi Jacobs, Mike DiNovi, Mary Ditto, and Toni Mattia for their input.
Funding
AET was supported by the ILSI-North America Food and Chemical Safety Committee Summer 2016 Fellowship. ILSI North America is a public, nonprofit foundation that provides a forum to advance understanding of scientific issues related to the nutritional quality and safety of the food supply. ILSI North America receives support primarily from its industry membership.
Abbreviations
- AC50
Active concentration 50
- CFR
Code of Federal Regulations
- DBTC
Dibutyltin dichloride
- EPA
Environmental Protection Agency
- FDA
Food and Drug Administration
- HTS
High-throughput screening
- IVIVE
in-vitro in-vivo extrapolation
- LOAEL
Low observed adverse effect level
- NOAEL
No observed adverse effect level
- NOEL
No observed effect level
- NTP
National Toxicology Program
- OED
Oral equivalent dose
- PVC
Polyvinyl chloride
- rTK
Reverse toxicokinetics
- RISK21
Risk Assessment in the 21st Century
- SHEDS-HT
High-Throughput Stochastic Human Exposure and Dose Simulation
- SPO
sodium (2-pyridylthio)-N-oxide
- TK
Toxicokinetics
Footnotes
Publisher's Disclaimer: Disclaimer
The views expressed in this paper are those of the authors and do not necessarily reflect the views of their affiliations or policies of the US Environmental Protection Agency.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fct.2019.110819.
References
- Ali AA, Upreti RK, Kidwai AM, 1990. Assessment of di-and tri-butyltin interaction with skeletal muscle membranes agents. Bull. Environ. Contam. Toxicol 44, 29–38. [DOI] [PubMed] [Google Scholar]
- Barter Z, Bayliss M, Beaune P, Boobis A, Carlile D, Edwards R, Brian Houston J, Lake B, Lipscomb J, Pelkonen O, Tucke G, Rostami-Hodjegan A, 2007. Scaling factors for the extrapolation of in vivo metabolic Drug clearance from in vitro data: reaching a consensus on values of human micro-somal protein and hepatocellularity per gram of liver. Curr. Drug Metabol 8, 33–45. 10.2174/138920007779315053. [DOI] [PubMed] [Google Scholar]
- Biryol D, Nicolas CI, Wambaugh J, Phillips K, Isaacs K, 2017. High-throughput dietary exposure predictions for chemical migrants from food contact substances for use in chemical prioritization. Environ. Int 108, 185–194. 10.1016/j.envint.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ChemicalBook, 2016. Chembook. [WWW Document]. URL. https://www.chemicalbook.com/.
- Davies B, Morris T, 1993. Physiological parameters in laboratory animals and humans. harm. Res 10.1023/A:1018943613122. [DOI] [PubMed] [Google Scholar]
- Dix DJ, Houck KA, Martin MT, Richard AM, Setzer RW, Kavlock RJ, 2007. The toxcast program for prioritizing toxicity testing of environmental chemicals. Toxicol. Sci 95, 5–12. 10.1093/toxsci/kfl103. [DOI] [PubMed] [Google Scholar]
- Embry MR, Bachman AN, Bell DR, Boobis AR, Cohen SM, Dellarco M, Dewhurst IC, Doerrer NG, Hines RN, Moretto A, Pastoor TP, Phillips RD, Rowlands JC, Tanir JY, Wolf DC, Doe JE, 2014. Risk assessment in the 21st century: roadmap and matrix. Crit. Rev. Toxicol 44 (Suppl. 3), 6–16. 10.3109/10408444.2014.931924. [DOI] [PubMed] [Google Scholar]
- EU, 2012. Flavourings, additives and food contact materials exposure task (FACET). [WWW Document]. URL. http://www.ucd.ie/facet/. [Google Scholar]
- Commission European, 2010. MIGRESIVES: research programme on migration from adhesives in food packaging materials in support of European legislation and standardisation. [WWW Document]. URL. https://www.migresives.eu/index.php. [DOI] [PubMed]
- European Food Safety Authority Panel, E., 2004. Opinion of the Scientific Panel on Contaminants in the Food Chain on a request from the Commission to assess the health risks to consumers associated with exposure to organotins in foodstuffs. EFSA J. 102, 1–119. [Google Scholar]
- Filer D, Patisaul HB, Schug T, Reif D, Thayer K, 2014a. Test driving ToxCast: endocrine profiling for 1858 chemicals included in phase II. Curr. Opin. Pharmacol 19, 145–152. 10.1016/j.coph.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filer DL, Kothiya P, Setzer RW, Judson RS, Martin MT, 2017. Tcpl: the ToxCast pipeline for high-throughput screening data. Bioinformatics 33, 618–620. 10.1093/bioinformatics/btw680. [DOI] [PubMed] [Google Scholar]
- Filer DL, Kothiya P, Setzer WR, Judson RS, Martin MT, 2014b. The ToxCast Analysis Pipeline : an R Package for Processing and Modeling Chemical Screening Data. [Google Scholar]
- Fisch MH, Bacaloglu R, Biesiada K, Brecker LR, 1999. Mechanism of organotin stabilization of poly(vinyl chloride). 2: significance for PVC stabilization of structure and equilibria of alkyltin thioglycolates/chlorides. J. Vinyl Addit. Technol 5, 45–51. [Google Scholar]
- Forsyth DS, Dabeka R, Sun WF, Dalglish K, 1993. Speciation of organotins in poly (vinyl chloride) products. Food Addit. Contam 10, 531–540. [DOI] [PubMed] [Google Scholar]
- Groh KJ, Muncke J, 2017. In vitro toxicity testing of food contact materials: state-of-the-art and future challenges. Compr. Rev. Food Sci. Food Saf 10.1111/1541-4337.12280.00. [DOI] [PubMed] [Google Scholar]
- Harper C, Llados F, Diamond G, Chappell LL, 2005. Toxicological Profile for Tin and Tin Compounds. Agency for Toxic Substances and Disease Registry. [PubMed] [Google Scholar]
- HESI, 2017. The RISK21 webtool. [WWW Document]. URL. http://risk21.org/the-risk21-webtool-v-2-0/.
- Ingle BL, Veber BC, Nichols JW, Tornero-Velez R, 2016. Informing the human plasma protein binding of environmental chemicals by machine learning in the pharmaceutical space: applicability domain and limits of predictability. J. Chem. Inf. Model 56, 2243–2252. 10.1021/acs.jcim.6b00291. [DOI] [PubMed] [Google Scholar]
- Isaacs KK, Glen WG, Egeghy P, Goldsmith MR, Smith L, Vallero D, Brooks R, Grulke CM, Özkaynak H, 2014. SHEDS-HT: an integrated probabilistic exposure model for prioritizing exposures to chemicals with near-field and dietary sources. Environ. Sci. Technol 48, 12750–12759. 10.1021/es502513w. [DOI] [PubMed] [Google Scholar]
- Janesick AS, Dimastrogiovanni G, Vanek L, Boulos C, Chamorro-Garcia R, Tang W, Blumberg B, 2016. On the utility of ToxCast and ToxPi as methods for identifying new obesogens. Environ. Health Perspect 124, 1214–1226. 10.1289/ehp.1510352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TN, Tucker GT, Tanner MS, Rostami-Hodjegan A, 2005. Changes in liver volume from birth to adulthood: a meta-analysis. Liver Transplant. 11, 1481–1493. 10.1002/lt.20519. [DOI] [PubMed] [Google Scholar]
- Judson R, Houck K, Martin M, Richard AM, Knudsen TB, Shah I, Little S, Wambaugh J, Woodrow Setzer R, Kothya P, Phuong J, Filer D, Smith D, Reif D, Rotroff D, Kleinstreuer N, Sipes N, Xia M, Huang R, Crofton K, Thomas RS, 2016. Analysis of the effects of cell stress and cytotoxicity on in vitro assay activity across a diverse chemical and assay space. Toxicol. Sci 152, 323–339. 10.1093/toxsci/kfw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson R, Richard A, Dix DJ, Houck K, Martin M, Kavlock R, Dellarco V, Henry T, Holderman T, Sayre P, Tan S, Carpenter T, Smith E, 2009. The toxicity data landscape for environmental chemicals. Environ. Health Perspect 117, 685–695. 10.1289/ehp.0800168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judson RS, Houck KA, Kavlock RJ, Knudsen TB, Martin MT, Mortensen HM, Reif DM, Rotroff DM, Shah I, Richard AM, Dix DJ, 2010. In vitro screening of environmental chemicals for targeted testing prioritization: the toxcast project. Environ. Health Perspect 118, 485–492. 10.1289/ehp.0901392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan K, Senthilkumar K, Giesy JP, 1999. Occurrence of butyltin compounds in human blood. Environ. Sci. Technol 33, 1776–1779. 10.1021/es990011w. [DOI] [Google Scholar]
- Karmaus AL, Filer DL, Martin MT, Houck KA, 2016. Evaluation of food-relevant chemicals in the ToxCast high-throughput screening program. Food Chem. Toxicol 92, 188–196. 10.1016/j.fct.2016.04.012. [DOI] [PubMed] [Google Scholar]
- Karmaus AL, Trautman TD, Krishan M, Filer DL, Fix LA, 2017. Curation of food-relevant chemicals in ToxCast. Food Chem. Toxicol 103, 174–182. 10.1016/j.fct.2017.03.006. [DOI] [PubMed] [Google Scholar]
- Kavlock R, Chandler K, Houck K, Hunter S, Judson R, Kleinstreuer N, Knudsen T, Martin M, Padilla S, Reif D, Richard A, Rotro D, Sipes N, Dix D, 2012. Update on EPA’s ToxCast program: providing high throughput decision support tools for chemical risk management. Chem. Res. Toxicol 25, 1287–1302. [DOI] [PubMed] [Google Scholar]
- Kim CH, Kim JH, Moon SJ, Chung KC, Hsu CY, Seo JT, Ahn YS, 1999. Pyrithione, a zinc ionophore, Inhibits NF-kB Activation. Biochem. Biophys. Res. Commun 509, 505–509. [DOI] [PubMed] [Google Scholar]
- Kimmel EC, Fish RH, Casida JE, 1977. Bioorganotin Chemistry. Metabolism of organotin compounds in microsomal monooxygenase systems and in mammals. J. Agric. Food Chem 25, 1–9. [DOI] [PubMed] [Google Scholar]
- Knox RJ, Magoski NS, Wing D, Barbee SJ, Kaczmarek LK, 2004. Activation of a calcium entry pathway by sodium pyrithione in the bag cell neurons of Aplysia. J. Neurobiol 60, 411–423. 10.1002/neu.20029. [DOI] [PubMed] [Google Scholar]
- Kondoh M, Tasaki E, Araragi S, Takiguchi M, Higashimoto M, Watanabe Y, Sato M, 2002. Requirement of caspase and p38MAPK activation in zinc-induced apoptosis in human leukemia HL-60 cells. Eur. J. Biochem 269, 6204–6211. 10.1046/j.1432-1033.2002.03339.x. [DOI] [PubMed] [Google Scholar]
- Lind SE, Park JS, Drexler JW, 2009. Pyrithione and 8-hydroxyquinolines transport lead across erythrocyte membranes. Transl. Res 154, 153–159. 10.1016/j.trsl.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Liu J, Patlewicz G, Williams AJ, Thomas RS, Shah I, 2017. Predicting organ toxicity using in vitro bioactivity data and chemical structure. Chem. Res. Toxicol 30, 2046–2059. 10.1021/acs.chemrestox.7b00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Mizushima T, Shirahige A, Tanioka H, Sawa K, Ochi K, Tanimoto M, Koide N, 2012. Effect of taurine on acinar cell apoptosis and pancreatic fibrosis in dibutyltin dichloride-induced chronic pancreatitis. Acta Med. Okayama 66, 329–334. [DOI] [PubMed] [Google Scholar]
- Mitoma C, Steeger T, Rogers J, Thomas D, Wedic JH, 1983. Metabolic disposition of pyrithiones. Fundam. Appl. Toxicol 3, 256–263. 10.1093/toxsci/3.4.256. [DOI] [PubMed] [Google Scholar]
- National Research Council, 2007. Toxicity Testing in the 21st Century: A Vision and a Strategy. The National Academies Press, Washington, D.C. [Google Scholar]
- NCATS, 2016. Toxicology in the 21st century (Tox21). [WWW Document]. URL. https://ncats.nih.gov/tox21.
- Neltner TG, Alger HM, Leonard JE, Maffini MV, 2013. Data gaps in toxicity testing of chemicals allowed in food in the United States. Reprod. Toxicol 42, 85–94. 10.1016/j.reprotox.2013.07.023. [DOI] [PubMed] [Google Scholar]
- Neltner TG, Kulkarni NR, Alger HM, Maffini MV, Bongard ED, Fortin ND, Olson ED, 2011. Navigating the U.S. Food additive regulatory program. Compr. Rev. Food Sci. Food Saf 10, 342–368. 10.1111/j.1541-4337.2011.00166.x. [DOI] [Google Scholar]
- NTP, 2016. Toxicology in the 21st century (Tox21). [WWW Document]. URL. https://ntp.niehs.nih.gov/results/tox21/index.html.
- OECD, 2008. Dibutyltin Dicloride SIDS Dossier. [Google Scholar]
- OECD, 2006. SIDS Initial Assessment Summary: Dibutyltin Dichloride and Selected Thioglycolate Esters. 23. Siam, pp. 6. [Google Scholar]
- Papaspyrou SD, Thomaidis NS, Lampi EN, Lioupis A, 2007. Determination of migration of n-butyltins and n-octyltins to food simulants by gas chromatography-mass spectrometry. Appl. Organomet. Chem 21, 412–424. 10.1002/aoc. [DOI] [Google Scholar]
- Pastoor TP, Bachman AN, Bell DR, Cohen SM, Dellarco M, Dewhurst IC, Doe JE, Doerrer NG, Embry MR, Hines RN, Moretto A, Phillips RD, Rowlands JC, Tanir JY, Wolf DC, Boobis AR, 2014. A 21st century roadmap for human health risk assessment. Crit. Rev. Toxicol 44 (Suppl. 3), 1–5. 10.3109/10408444.2014.931923. [DOI] [PubMed] [Google Scholar]
- Pham N, Iyer S, Hackett E, Lock BH, Sandy M, Zeise L, Solomon G, Marty M, 2016. Using ToxCast to explore chemical activities and hazard traits: a case study with ortho-phthalates. Toxicol. Sci 151, 286–301. [DOI] [PubMed] [Google Scholar]
- Poller RC, 1978. Stabilization of PVC by organotin compounds. J. Macromol. Sci, Part A 12, 373–378. 10.1080/00222337808061386. [DOI] [Google Scholar]
- Quevauviller P, Bruchet A, Donard O, 1991. Leaching of organotin compounds from poly(vinyl chloride) (PVC) material. Appl. Organomet. Chem 5, 125–129. [Google Scholar]
- Reif DM, Martin MT, Tan SW, Houck KA, Judson RS, Richard AM, Knudsen TB, Dix DJ, Kavlock RJ, 2010. Endocrine profling and prioritization of environmental chemicals using toxcast data. Environ. Health Perspect 118, 1714–1720. 10.1289/ehp.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I, Yang C, Rathman J, Martin MT, Wambaugh JF, Knudsen TB, Kancherla J, Mansouri K, Patlewicz G, Williams AJ, Little SB, Crofton KM, Thomas RS, 2016. ToxCast chemical landscape: paving the road to 21st century toxicology. Chem. Res. Toxicol 29, 1225–1251. 10.1021/acs.chemrestox.6b00135. [DOI] [PubMed] [Google Scholar]
- Ring CL, Arnot JA, Bennett DH, Egeghy PP, Fantke P, Huang L, Isaacs KK, Jolliet O, Phillips KA, Price PS, Shin HM, Westgate JN, Setzer RW, Wambaugh JF, 2019. Consensus modeling of median chemical intake for the U.S. Population based on predictions of exposure pathways. Environ. Sci. Technol 53, 719–732. 10.1021/acs.est.8b04056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg E, 2013. Organotin Compounds in Foods, Persistent Organic Pollutants and Toxic Metals in Foods. Woodhead Publishing Limited; 10.1533/9780857098917.2.430. [DOI] [Google Scholar]
- Rotroff DM, Dix DJ, Houck KA, Knudsen TB, Martin MT, McLaurin KW, Reif DM, Crofton KM, Singh AV, Xia M, Huang R, Judson RS, 2013. Using in vitro high throughput screening assays to identify potential endocrine-disrupting chemicals. Environ. Health Perspect 121, 7–14. 10.1289/ehp.1003280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah I, Woodrow Setzer R, Jack J, Houck KA, Judson RS, Knudsen TB, Liu J, Martin MT, Reif DM, Richard AM, Thomas RS, Crofton KM, Dix DJ, Kavlock RJ, 2016. Using toxcast data to reconstruct dynamic cell state trajectories and estimate toxicological points of departure. Environ. Health Perspect 124, 910–919. 10.1289/ehp.1409029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M, Pham N, Lewis C, Iyer S, Kwok E, Solomon G, Zeise L, 2015. A comparison of ToxCast test results with in vivo and other in vitro endpoints for neuro, endocrine, and developmental toxicities: a case study using endosulfan and methidathion. Birth Defects Res. Part B Dev. Reproductive Toxicol 104, 71–89. 10.1002/bdrb.21140. [DOI] [PubMed] [Google Scholar]
- Tice RR, Austin CP, Kavlock RJ, Bucher JR, 2013. Improving the human hazard characterization of chemicals: a Tox21 update. Environ. Health Perspect 121, 756–765. 10.1289/ehp.1205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S.EPA, 2016. Chemistry dashboard [WWW Document]. URL. https://comptox.epa.gov/dashboard.
- U.S.EPA, 2015. ToxCast dashboard. [WWW Document]. URL. https://www.epa.gov/chemical-research/toxcast-dashboard.
- U.S.EPA, 2014. Rapid chemical exposure and dose research. [WWW Document]. URL. https://www.epa.gov/chemical-research/rapid-chemical-exposure-and-dose-research.
- U.S.EPA, 2011. Sodium Omadine Summary Document : Registration Review : Initial Docket December 2011. [Google Scholar]
- U.S.EPA, 1996. Sodium Omadine Reregistration, Reregistration Eligibility Decision. U.S.FDA, 2012. Cumulative estimated daily intake database. [WWW Document]. URL. https://www.fda.gov/Food/IngredientsPackagingLabeling/PackagingFCS/CEDI/default.htm. [Google Scholar]
- U.S.EDA, 2007. Guidance for industry: preparation of premarket submissions for food contact substances: Chemistry recommendations. [WWW Document]. URL. https://www.fda.gov/Food/GuidanceRegulation/ucm081818.htm. [Google Scholar]
- Ueno S, Susa N, Furukawa Y, Sugiyama M, 1994. Comparison of hepatotoxicity caused by mono-, di-and tributyltin compounds in mice. Arch. Toxicol. 69, 30–34. 10.1007/s002040050133. [DOI] [PubMed] [Google Scholar]
- USDA, 2015. What we eat in America. [WWW Document]. URL. https://data.nal.usda.gov/dataset/what-we-eat-america-wweia-database. [Google Scholar]
- USEPA, 2014. Integrated Bioactivity and Exposure Ranking : A Computational Approach for the Prioritization and Screening of Chemicals in the Endocrine Disruptor Screening Program. FIFRA SAP December 2–5, 2014. [Google Scholar]
- Wambaugh JF, Wang A, Dionisio KL, Frame A, Egeghy P, Judson R, Setzer RW, 2014. High throughput heuristics for prioritizing human exposure to environmental chemicals. Environ. Sci. Technol 48, 12760–12767. 10.1021/es503583j. [DOI] [PubMed] [Google Scholar]
- Wetmore BA, 2015. Quantitative in vitro-to-in vivo extrapolation in a high-throughput environment. Toxicology 332, 94–101. 10.1016/j.tox.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Allen B, Ferguson SS, Sochaski MA, Setzer RW, Houck KA, Strope CL, Cantwell K, Judson RS, LeCluyse E, Clewell HJ, Thomas RS, Andersen ME, 2015. Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol. Sci 148, 121–136. 10.1093/toxsci/kfv171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Ferguson SS, Li L, Clewell HJ, Judson RS, Freeman K, Bao W, Sochaski MA, Chu TM, Black MB, Healy E, Allen B, Andersen ME, Wolfinger RD, Thomas RS, 2013. Relative impact of incorporating pharmacokinetics on predicting in vivo hazard and mode of action from high-throughput in vitro toxicity assays. Toxicol. Sci 132, 327–346. 10.1093/toxsci/kft012. [DOI] [PubMed] [Google Scholar]
- Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS, 2012. Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol. Sci 125, 157–174. 10.1093/toxsci/kfr254. [DOI] [PubMed] [Google Scholar]
- WHO, W.H.O., 2006. Mono-and disubstituted methyltin, butyltin and octyltin compounds. In: Concise international chemical assessment document. 73. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.