Abstract
Myocardial fibrosis is recognized as a key pathological process in the development of cardiac disease and a target for future therapeutics. Despite this recognition, the assessment of fibrosis is not a part of routine clinical practice. This is primarily due to the difficulties in obtaining an accurate assessment of fibrosis non-invasively. Moreover, there is a clear discrepancy between the understandings of myocardial fibrosis clinically where fibrosis is predominately studied with comparatively low-resolution medical imaging technologies like MRI compared with the basic science laboratories where fibrosis can be visualized invasively with high resolution using molecularly specific fluorescence microscopes at the microscopic and nanoscopic scales. In this article, we will first review current medical imaging technologies for assessing fibrosis including echo and MRI. We will then highlight the need for greater microscopic and nanoscopic analysis of human tissue and how this can be addressed through greater utilization of human tissue available through endomyocardial biopsies and cardiac surgeries. We will then describe the relatively new field of molecular imaging that promises to translate research findings to the clinical practice by non-invasively monitoring the molecular signature of fibrosis in patients.
Keywords: Myocardial fibrosis, Echocardiography, Magnetic resonance, Endomyocardial biopsy, Histology, Confocal, Super resolution, Molecular imaging
Introduction
Myocardial fibrosis (MF) is an established pathological process in the development of cardiovascular diseases with proven prognostic value in progression to the development of heart failure. These cardiovascular diseases include conditions associated with pressure overload such as, hypertension and aortic stenosis (AS), volume overload as in aortic regurgitation (AR), myocardial ischemia, and genetic disorders associated with cardiomyopathies. Various myocardial insults result in structural changes in the myocardial fabric (Stephane Heymans 2015). Higher degrees of fibrotic accumulation, lesion size, and transmurality are implicated in worse long-term survival (Everaars 2020; Gulati et al. 2018; Ørn et al. 2007). Ultimately, this causes contractile dysfunction and arrhythmogenicity. For example, as post myocardial infarction (MI) mortality rates fall, we face the challenge of new-onset heart failure (HF) developing in the surviving population. A study conducted on 7733 patients hospitalized for MI found that 75% of this population developed HF in the 5 years post MI (Ezekowitz et al. 2009).
The estimated prevalence of HF is 38 million worldwide; it is one of the leading causes of morbidity and mortality globally and the most rapidly growing cardiovascular disease with rising burden on healthcare systems. In the USA, healthcare costs associated with cardiovascular disease were estimated to be $300 billion annually (Voicu 2019; Benjamin 2018). It is therefore, important to distinguish how myocardial fibrosis is addressed. Clinically, MF is often assessed indirectly by measuring the myocardial function or by holistic visualization of the macroscopic changes in the myocardium by echocardiology (echo), magnetic resonance imaging (MRI), and computer tomography (CT), and rarely endomyocardial biopsies are indicated for identification of the underlying disease process when a cause for function deterioration is difficult to identify. In contrast, laboratory assessments of the fibrotic process tend to be more direct, looking at the myocardial mesh changes under microscopes through immunologic labeling which enables isolation of the different contributors to the fibrotic changes and monitoring the evolution of the fibrotic tissue over time.
Despite substantial literature on specific pathophysiologic mechanisms highlighting potential new treatment targets, clinically treatment protocols tend to focus on the resulting deterioration in myocardial function, rather than the underlying fibrotic process, hence the uniform management post MI with angiotensin-converting enzyme inhibitors or mineralocorticoid receptor antagonists (Bahit et al. 2018; Pfeffer et al. 2003; Møller et al. 2004). It is not surprising, therefore, that outcomes vary significantly from one class of patients to the next. The paucity of patient-customized treatment regimens can be attributed primarily to the deficiency of safe, specific, and accessible diagnostic tools to investigate the pathological process of MF in humans and the shortage of validated animal model studies.
Attempts to address the fibrotic changes more directly are underway; pirfenidone as an example is an antifibrotic agent approved as a treatment option for idiopathic pulmonary fibrosis (IPF) (Nathan et al. 2017) and shows promise in treating other fibrotic conditions such as focal segmental glomerulosclerosis (Cho et al. 2007). It is being investigated as a treatment option for myocardial fibrosis given the overlap in the pathophysiology underlying the fibrotic process in these conditions (Graziani et al. 2018). Pirfenidone was able to attenuate the fibrotic process in the canine atria (Lee et al. 2006) and was also able to reduce the ventricular scar size post MI in rat studies (Nguyen et al. 2010; Li et al. 2017). Current clinical trials involving pirfenidone are mostly investigating its role in ameliorating lung fibrosis, with some studies investigating its role in hepatic fibrosis, renal fibrosis and only one cardiac trial; these have been recently reviewed by Aimo et al. (2020). Similarly, other antifibrotic agents targeting renin-angiotensin-aldosterone system, inflammatory modulators, TGF-β inhibitors, and endothelin inhibitors have demonstrated promising effects in reducing the fibrotic process in animals (Fang et al. 2017). However, clinical trials investigating the same agents have failed to validate their use in human, and these clinical trials have been reviewed by Fang et al. (2017).
The exciting increase in potential therapeutic agents in the preclinical models is facing the challenge of validation in human studies. The discrepancy between animal and human studies maybe related to interspecies physiological and genetic differences, and potentially additional pathways that influence these disappointing results. Moreover, clinical trials may be improved with clearer identification of suitable patients, with appropriate treatment endpoints and dosing regimens (Fang et al. 2017). We argue that the process of fibrosis in humans is poorly understood in comparison with what is known from animal models and that this knowledge gap is holding up the development of antifibrotic treatments. For example, high-resolution molecular specific fluorescence microscopy (e.g., confocal) is common place in the research of fibrosis in animal models but is rarely used on human tissue, where histological stains (e.g., Masson’s trichrome) predominate (Crossman et al. 2015). This level of knowledge at population scale is required to develop a robust picture of the fibrotic process in the different forms of human HF, which can then be used to identify and develop suitable animal models of HF. This knowledge deficit is partly due to the difficulty in obtaining human tissue for research particularly in the early stages of disease process where treatments would likely have the most benefit. However, this objective is not impossible particularly with the ability to obtain tissue safely from endomyocardial biopsy (EMB). For routine clinical application, there is a heightened need for safe, reliable diagnostic tools for appropriate patient selection for wide-scale clinical trials and to enable monitoring of the disease progression under the effect of these antifibrotic agents. Ideally, any diagnostic modality would be expected to accurately identify, quantify, characterize, and monitor MF of any etiology, while minimizing complications and invasiveness.
This literature review will provide an evaluation of the currently available and developing diagnostic, prognostic, and research imaging modalities for myocardial fibrosis as a key first step in developing patient-tailored treatments.
Definition of myocardial fibrosis
MF has been loosely defined as the process occurring in pathological remodeling of the myocardium due to excessive production and deposition of extracellular matrix (ECM) proteins, including collagen. This reduces tissue compliance and accelerates progression to heart failure as well as affecting the electrical properties of the myocytes resulting in arrhythmias (Schisler 2019). Tissue fibrosis is the final outcome of the healing process after injury in many tissues. This process starts initially with inflammation, followed by new tissue formation which starts 2–10 days following injury and finally by tissue remodeling which begins 2–3 weeks after injury; in the remodeling phase, the ECM consists mostly of collagen and becomes devoid of cellular components as they undergo apoptosis (Gurtner 2008).
Although overall, 28 types of collagen are discovered so far (Fischer 2012). The most frequently studied types are collagen I and collagen III. Collagen III forms 11% of the total collagen in the heart, deposits to form the backbone of the new extracellular matrix, and is gradually replaced by collagen I which forms 85% of the total collagen in the heart. The processes of collagen formation deposition, maturation, and crosslinking have been reviewed by Bodh (2003). The quantity and the proportions of these collagen depositions vary in different cardiac tissue injury, for example collagen I exhibits more prolonged upregulation than collagen III in post MI and in hypertensive animal models (Mukherjee 1993; Whittaker 1989). Furthermore, collagen VI has been implicated in the remodeling process occurring in the t-tubules and was found to profoundly stimulate myofibroblast transdifferentiation in human studies (Crossman et al. 2017; Naugle et al. 2006). It is therefore conceivable that other types of collagen and proteins may also contribute to the alterations in the myocardial mesh following myocardial injury; this is an area worthy of further investigation.
Substantial advancements in understanding the pathological processes influencing myocardial remodeling have been made from animal models (Frangogiannis 2019) however, attempts to utilize this knowledge in the clinical setting are hindered, primarily because MF is addressed as a generic term in the clinical setting. It is diagnosed and monitored macroscopically in most cases, and when measured microscopically, this is usually done by a one-off tissue sample which is histologically stained to assess collagen prevalence, dismissing other molecular details such as the types of collagen, the level of maturation, and the cross linkage of collagen, and disregarding the non-collagen contributors to cardiac remodeling. The lack of serial sampling also restricts the ability to monitor the disease progression. These shortcomings are likely due to the lack of clinical tools to monitor these features in humans.
Classification of MF
The current understanding of MF classification is mostly obtained from murine, canine, and swine model studies.
Reparative (replacement) fibrosis is defined as the development of an organized scar after MI. It occurs immediately following myocyte death by apoptosis, necrosis, and autophagy as a result of insufficient tissue perfusion. This triggers an immense inflammatory cascade involving numerous cellular and molecular agents causing excessive ECM expansion (Baines 2011; Kong 2014). This response is required acutely to mechanically stabilize the evolving tissue defect, thereby preventing ventricular dilatation and rupture, but can result in adverse sequelae in the long term as the contractile function is lost and replaced by collagen which has the tensile strength of steel (Gandhi et al. 2011). Reactive fibrosis on the other hand appears to develop from different pathological processes. It results in unique structural and metabolic changes, with a qualitatively different distribution of collagen as compared with reparative fibrosis (De Boer et al. 2019). ECM and collagen deposition in reactive fibrosis is mostly observed in perivascular and interstitial regions of the myocardium. Reactive fibrosis has been studied mainly in animal models, and is attributed to prolonged myocardial stress in the form of pressure overload, such as in aortic stenosis and prolonged hypertension (Hill 2008; Bacmeister et al. 2019), volume overload, as in aortic regurgitation (Borer et al. 2002), or in cardiomyopathies, including hypertrophic cardiomyopathy (Prinz et al. 2011; Raman 2018), dilated cardiomyopathy (Vergaro et al. 2015), and diabetic cardiomyopathy (Boudina 2007) reactive fibrosis is also seen post MI, in the non-infarct regions (Borne 2008) as well as in heart failure with preserved ejection fraction (HFpEF) (Mohammed 2012; Erberto 2016) and has been reported in aging hearts without HF (Annoni et al. 1998; Capasso 1990; Dhalla 2012). Infiltrative interstitial fibrosis is another type of myocardial fibrosis which results from progressive deposition of non-degradable material and has been reported in amyloidosis and Fabry disease (Shah 2006; Zarate 2008).
Notably, regardless of the etiology, the injured myocardium often harbors all types of fibrosis at different stages of the disease. Mature scar tissue from reparative fibrosis is devoid of inflammatory cells and is a non-reversible process; however, reactive and interstitial myocardial fibrosis may be reversible and accordingly is the treatment target of post myocardial injury (Weber 2005; Zannad et al. 2000).
Current and future clinical imaging of fibrosis
Current and potential future clinical imaging modalities for measuring myocardial fibrosis include echo, late gadolinium enhancement cardiac MRI, T1 mapping, EMB, and molecular cardiac imaging. Although plain X-ray and cardiac CT scans are used in the clinical setting, their value in portraying myocardial fibrosis is limited and they will not be discussed. Serum biomarkers, albeit usefulness in early identification of cardiovascular disease, remain an indirect tool in assessing the myocardial fabric. Some of these markers have been reviewed by Omland (2017). For the purposes of this review, they will not be discussed.
Echocardiography
Echo is an ultrasonography of the heart. It is ubiquitous, and is typically utilized as a first step for assessment of myocardial function and structure, albeit these assessments being an indirect way for obtaining information on the myocardial scar. Echo has a favorable safety profile. It is minimally invasive, acceptable to most patients, and portable. It allows for expeditious and detailed information at the bedside, at comparatively low cost (Sadeghpour 2018a).
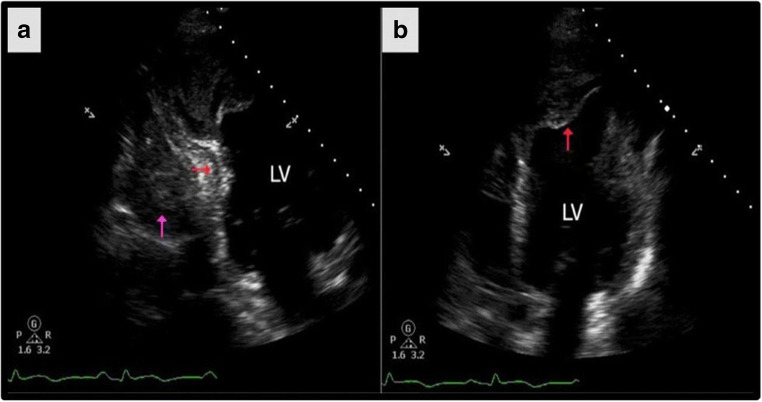
In MI, echo is often utilized in the acute setting for rapid detection of the macroscopic mechanical complications following myocardial ischemia including free wall rupture, papillary muscle rupture, and the resulting mitral regurgitation (MR) as well as ventricular septal defects (VSD) (Sadeghpour 2018b) (Fig. 1). Subsequently, as myocardial remodeling occurs, echo is used to track the progressive ventricular dilation. Echo studies can also detect mitral regurgitation following spherical remodeling and papillary muscle displacement as well as monitoring the reduction in function (LVEF reduction) and the resulting exacerbation of heart failure (Hung et al. 2010; Esmaeilzadeh 2013).
Fig. 1.
Echocardiogram in the acute setting is capable of demonstrating post MI heamopericardium (purple arrow) and inferior wall intramyocardial dissection (red arrow). However, there is poor characterization of the injured myocardium. Adapted from Garg, Abdel-Rahman, Greenwood, and Plein (2015)
In a study conducted on 626 patients with their first acute MI, evaluations of LVEF were employed as a monitoring tool for LV remodeling. Counter-intuitively, a higher initial LVEF at time of MI was found to correspond with adverse LV remodeling. The study also describes a 3-fold increased risk of all-cause mortality associated with the absence of LVEF recovery post MI (Ndrepepa et al. 2007).
Attempts to increase the utility of echo to reliably detect the degree of myocardial deformation in other conditions have been investigated. Speckle tracking echo for example is a newly emerging echo technique which analyzes the motion of speckles in the 2D ultrasonic image, allowing a non-Doppler, angle-independent, objective analysis of myocardial deformation, with the possibility to quantify thickening, shortening, and rotation dynamics of cardiac function (Matteo Cameli 2017). In hypertrophic cardiomyopathy (HCM), a disease known for its associated regional myocardial fibrosis and increased LV wall thickness (Chang 2012), employing speckle tracking echo techniques, demonstrated correlation between the regional impairment of myocardial function as assessed by echo and the degree of myocardial fibrosis as shown by cardiac magnetic resonance (CMR) imaging or EMB in several studies (Chang 2012; Popović et al. 2008; Reant et al. 2016). Similarly, in the setting of HFpEF, the global longitudinal strain as assessed by speckle tracking echo was found to be a reliable tool in assessing the functional status of the myocardium which is affected by the underlying fibrotic remodeling and increased ventricular stiffness as assessed by CMR and T1 maps (Erberto 2016; Mordi 2018).
In light of these findings, echo proves to be a useful monitoring tool for myocardial function. It can indirectly track the remodeling process post myocardial injury through assessment of the ventricular size, shape, and volume. Its strength lies in its ability to prognosticate cardiac morbidity and mortality, rather than in diagnosis of MF itself.
Limitation
Despite echo’s benefit as a qualitative diagnostic and prognostic tool, it is inadequate for direct identification and quantification of the type and extent of MF. As a tool for functional assessment, it falls short in its ability to identify the underlying fibrotic patterns resulting in this functional derangement and therefore cannot be utilized independently to measure and monitor the degree and progression of myocardial fibrosis. It is dependent on acoustic windows (the location from which an ultrasound probe makes its scan) is variable and affected by the operator’s skill making it prone to errors leading to potentially inaccurate results and poor reproducibility.
Late gadolinium enhancement cardiac magnetic resonance
Cardiac MRI is a robust tool capable of providing macroscopic representation of the changes in the injured myocardium. The use of MRI scans to identify and quantify MF has witnessed significant developments in the past 20 years. The underlying principle is that all myocardial fibrosis, irrespective of the underlying cause, will ultimately result in expansion of the extracellular matrix. Gadolinium is an extracellular agent accumulating in areas of interstitial expansion due to myocardial fibrosis, edema, or infiltration. Three phases can be assessed after gadolinium injection: the first pass (immediate imaging at rest or during stress), early enhancement (first 5 min), and late enhancement (5–20 min after injection; Parsai et al. 2012).
Late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) is a widely available tool. It enables non-invasive quantification of myocardial mass, volume, and function to identify macroscopic myocardial fibrosis. It is cost-effective and is acceptable to patients (Sandstede et al. 2000). LGE-CMR is therefore, considered the gold standard for characterizing chronic MIs. Measurements of the infarct size and transmurality were found to correspond closely with histological findings in patients with an old MI (Yan 2015; Kali et al. 2015a, b; Yang 2018) (Fig. 2).
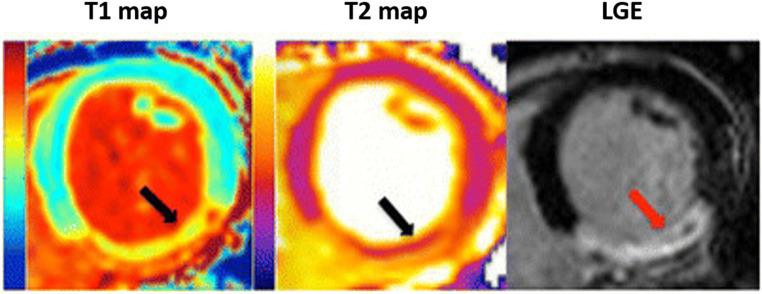
Fig. 2.
A comparison between T1, T2 mapping, and LGE images demonstrating a transmular MI in the right coronary artery territory. T1 and T2 maps delineating an area of myocardial edema (black arrows). T2 and LGE images showing some microvascular obstruction (black and red arrows respectively). Adapted from Bulluck et al. (2015)
LGE-CMR’s ability to characterize and track the myocardial scar is of substantial prognostic value. It was found to be superior to echo in predicting adverse cardiac outcomes such as arrhythmias (Guo D 2019).
In acute MI, LGE-CMR is a robust tool, able to identify and quantify myocardial tissue damage with a high degree of accuracy and reproducibility (Everaars 2020). Its utility extends to recognizing microinfarctions occurring during interventional procedures (Ricciardi et al. 2001). It is capable of distinguishing the reversibility of the ischemic injury to the myocardium regardless of the infarct age in canine models (Kim et al. 1999; Fieno et al. 2000). Furthermore, LGE-CMR can identify the transmural extent of the infarction and the remaining viable myocardium in human studies (Flacke et al. 2001). This is critical to prognosis following revascularisation as higher levels of transmural involvement and the infarction size negatively correlate with the myocardial contractile function and the overall major adverse cardiac events (MACE) (Everaars 2020; Barclay et al. 2007; Choi et al. 2001; Kim et al. 2000; Ingkanisorn 2004). Last, it is a useful tool for risk stratification which is necessary for optimizing treatments and allocating healthcare resources (Wong et al. 2013).
LGE-CMR is able to identify the different patterns of myocardial fibrosis in a range of cardiac conditions beyond ischemic cardiomyopathies including hypertrophic cardiomyopathy, non-ischemic dilated cardiomyopathy, cardiomyopathy-associated pressure overload as in aortic stenosis and hypertension, as well as diabetic cardiomyopathy and amyloidosis (Mewton 2011).
Myocardial fibrosis as evaluated by LGE-CMR was found to be of substantial prognostic value in patients with clinically suspected myocarditis, predicting all-cause death, cardiovascular death, resuscitated cardiac arrest, transplantation, appropriate implantable cardioverter-defibrillator (ICD) shock, rehospitalization following a cardiac event, recurrent acute myocarditis, and requirement for extracorporeal membrane oxygenation (ECMO) or ventricular assist device (VAD) regardless of the LVEF (Yang et al. 2020).
Limitation
The use of gadolinium-based contrast agents (GBCA) has been a concern for many clinicians due to the suspected risk of developing nephrogenic systemic fibrosis (NFS), a condition inciting fibrocytic stimulation and resulting in systemic fibrosis which may be fatal (Thomsen et al. 2013). However, more recent studies suggest that this concern is overestimated. These studies propose decreasing the dose of gadobutrol (Monti et al. 2020) or using macrocyclic GBCA e.g., gadoteric acid which appears to be safe even in patients receiving hemodialysis, with no significant reduction in the image quality (Alfano et al. 2020; Woolen et al. 2020; Costelloe 2020). Further safety concerns are related to the identification of gadolinium deposit in the brain of patients receiving multiple doses of GBCA. The data available on the clinical implications of these deposits are scarce; it is indeed an area requiring further prospective clinical research (Stanescu et al. 2020; Kanda et al. 2015; Rossi Espagnet 2017; Layne 2020).
An additional constraint in utilizing LGE-CMR has been cardiac implantable electronic devices (CIEDs). Until recently, these were considered an absolute contraindication to MRI, owing to the risk of changing the device parameters in the MRI environment. Modern development of MR-conditional CIEDs provides a partial solution to the safety concerns (Nazarian et al. 2017). However, the quality of the images obtained is compromised by the artifact created by these devices; ongoing attempts to optimize this are underway (Rajiah 2020).
Undoubtedly, LGE-CMR has proven to be a sensitive and specific qualitative diagnostic and prognostic tool for replacement fibrosis commonly seen in ischemic cardiomyopathy. However, in this condition, LGE-CMR may overestimate the necrotic myocardium because of the increased extracellular space in the peri-infarct area acutely. This can result in erroneous stratification of low-risk patients into a high-risk group (Dall’Armellina K. T. 2010; Dallʼarmellina et al. 2011). LGE-CMR falls short in identifying reactive or infiltrative fibrosis with high specificity. This is owing to the fact that it relies on a region of normal myocardium for reference and in these types of fibrosis, the reference regions of normal myocardium can be unrecognizable (Aherne 2019; Salerno 2013; Rutherford E 2016; Xu et al. 2020). Validation against endomyocardial biopsies (EMB) has indicated that significant myocardial fibrosis is often missed by LGE-CMR in non-ischemic cardiomyopathies (Vöhringer 2007; Sibley 2012). It is suggested that T1 mapping and EMB remain superior to LGE-CMR in distinguishing all types of MF and pinpointing the different etiologies of cardiomyopathy in these conditions (Arora et al. 2012).
Myocardial mapping by MRI
T1 and T2-weighted sequences are commonly used MRI protocols capable of highlighting different tissue components. MRI images can be obtained by measuring T1 relaxation times (T1 spin-lattice relaxation time) of soft tissue with no contrast (native T1) or following gadolinium administration. Alternatively, images are obtained by measuring T2 relaxation times (T2 spin-spin relaxation time). The relaxation time is dependent on the tissue type, the water content, and the pathological status of the tissue (e.g., edema, inflammation, ischemia, and fibrosis). Multiple techniques have been developed to enhance the interrogation of myocardial tissue by mapping the relaxation times in milliseconds and quantifying the signal of each voxel on a standardized scale (Granitz 2019). These techniques are well covered by Aherne (2019).
T1 maps
A T1 map is a quantity pixel-by-pixel depiction of T1 relaxation times which are specific to different tissue compositions. Normal myocardium has a predictable T1 relaxation time that deviates from the normal range when the tissue is edematous or fibrotic (Pfeifer 1984; Messroghli 2003; Bull W. P. 2013; Fernandes 2015; Liu et al. 2016).
T1 mapping can clearly differentiate acute from chronic MI owing to its sensitivity to water content in the tissue i.e., the edema resulting from acute injury. It is capable of assessing the severity of myocardial ischemia and in delineating the area at risk of irreversible damage after reperfusion interventions. All of these are central to appropriate risk stratification and prognostication of patients at a very early stage of the disease (Aherne 2012; Vanessa et al. 2012; Liu et al. 2017; Alkhalil et al. 2020). The ability to stratify patients early in the disease course allows appropriate patient recruitment for clinical trials to precisely assess the effectiveness of new interventions.
T1 versus T2 in acute MI
Early diagnosis of acute MI and distinguishing new versus old MI are unquestionably vital to timely intervention (Antman et al. 2002; Abdel-Aty et al. 2004). Ischemic myocardial injury results in myocytic edema which precedes the necrotic process (Abdel-Aty H. C. 2009) and has been found even to precede cardiac biomarker elevation in many cases (Kwong et al. 2003; Cury et al. 2008). In view of this, edema-sensitive T2 imaging has been proposed as an alternative technique for early accurate identification of the immediate post ischemic tissue edema and the regional distribution of the area at risk (Cury et al. 2008; Plein et al. 2004; Kwong et al. 2003; Verheugt 2006; Garg 2018) (Fig. 2). Employing cardiac MRI with the addition of T2 protocols has proven to be 85% sensitive and 96% specific in identifying signs of acute injury as shown by a study conducted on 87 patients presenting to the ED with chest pain. The same study highlights an improvement in the accuracy of identifying signs of acute myocardial damage to 93% when compared with previously employed CMR protocols devoid of T2 imaging (accuracy of 84%) (Cury et al. 2008). Evidently, bothT1 and T2 protocols are of comparable sensitivity in detecting myocardial edema. However, there seems to be less variability in the results obtained when employing T1 protocols (Aherne 2012).
T1 maps in subacute to chronic MI
Post gadolinium T1 maps permit measurement of the infarction size and transmurality (Liu et al. 2017; Perez-Terol et al. 2019; Alkhalil et al. 2020). Furthermore, extracellular volume (ECV) fraction maps can be extrapolated from these, which reflect the increased extracellular edema and fibrosis correlating histologically with increased collagen deposition (Flett 2010) taking into account that the calculation of ECV relies on the difference between precontrast and postcontrast T1 relaxation times and on hematocrit (Lurz et al. 2016; Radunski et al. 2017). As the infarction advances to the chronic stages, T1 maps become commensurately more efficient in denoting the myocardial tissue changes. This is likely due to the subsiding myocardial water content in chronic MI (Dastidar et al. 2019). Even when used without gadolinium contrast (native T1), it can reliably differentiate between normal, infarcted, ischemic, and remote myocardium at rest and during adenosine/dobutamine stress (Kali et al. 2014; Liu et al. 2016; Dastidar et al. 2017).
In contrast to LGE-CMR, T1 mapping can also identify the diffuse type of MF as validated by histologic biopsies as in patients with deteriorating cardiac function (Iles et al. 2008; Sibley 2012; Iles et al. 2015; Siepen et al. 2015; Haaf et al. 2016). T1 mapping promises early detection and quantification of the disease severity and to provide prognostic insights into certain conditions. It can monitor the healing process and therapy response without the need for contrast agents. This aids risk stratification in different stages of cardiomyopathy. If used routinely, it could reduce the number of EMBs required for surveillance following cardiac transplant and other conditions (Imran et al. 2019; Siepen et al. 2015; Bulluck 2015; Bohnen et al. 2017; Xu et al. 2020).
In conclusion, T1 mapping is independent, non-invasive, and accessible. It is able to identify and quantify the extent of myocardial damage on a segmental level and monitor the progression of MF at a reasonable cost benefit value. In post MI, T1 mapping seems to recognize changes to the myocardium before they become visible by LGE-CMR. As noted earlier, T1 mapping may be used for quantifying the degree of ECM expansion in cases difficult to distinguish by LGE-CMR (Höke et al. 2017). Treatment options for MI hinge on quantifying viable myocardium and this is a point of strength of T1 mapping (Kim and Manning 2004).
Limitations
There are several limitations to T1 mapping; first, CMR is not suitable for all patients; metallic implants and intracardiac devices are still considered an absolute contraindication to this modality when MRI-conditioned devices are not utilized. In addition, comprehensive cardiac MRI in acute infarct patients can be challenging because of the multiple breath-holds required, developing dyspnea in supine position, and relatively long acquisition times. However, free breathing cardiac T1 mapping sequences, which may improve clinical feasibility, are being developed (Garg P. S. 2018; Chow K 2016; de Jong et al. 2011). Even outside of the acute setting, a patient’s ability to breath-hold and symptoms of claustrophobia may make this technique unsuitable for some.
Image quality may be degraded by signal drop-out, bright signals from slow blood flow, or in tachyarrhythmias (Friedrich et al. 2009; Kellman 2007; de Jong et al. 2011). Moreover, timing of the MRI exam after gadolinium administration is critical. A too short delay between gadolinium administration and scanning may result in insufficient clearance of the contrast agents, whereas a too long delay will result in poor signal-to-noise and ultimately in an underestimation of the scarred tissue (de Jong et al. 2011). Although attempts to standardize T1 mapping were made (Moon et al. 2013), these have not been universally adopted and therefore, there is considerable inter-center and inter-scanner variability in the results (Bauner et al. 2012). Last, data validating the myocardial image as obtained from T1 maps against the microscopic picture as obtained from tissue biopsies are mostly from animal models and further research in human cohorts is required (Bull et al. 2013; Andreas Kammerlander 2015; Spath et al. 2018; Flett et al. 2010).
The rising need for a closer look at myocardial fibrosis
Microscopic analysis of MF
There is more to myocardial fibrosis than meets the eye; the macroscopic images obtained by the previously discussed modalities are hindered by the lack of resolution to view cellular and subcellular features and the specificity to characterize the molecular players that influence the journey post myocardial injury. The resolution achieved by macroscopic and microscopic imaging modalities is summarized in Table 1.
Table 1.
The resolutions achieved by different imaging modalities of the myocardium (Eugene Lin 2009)
| Imaging modality | Spatial resolution full width at half maximum (FWHM) |
|---|---|
| Echocardiography | ~ 0.5–2 mm |
| CT | 0.5–0.625 mm |
| MRI | 1–2 mm |
| PET | 4–10 mm |
| SPECT | 4–15 mm |
| Electron microscope | 1–10 nm |
| Confocal microscope | 5–50 nm |
When analyzing fibrotic tissue at a molecular level, collagen deposits are implicated. In healthy myocardium, collagen is distributed homogenously which is necessary for maintaining the integrity of the myocardium. However, as a result of any myocardial insult, the deposition of collagen increases leading to myocardial stiffness. This in turn impedes the physiologic contractile function and electrical properties of the myocardium and may result in heart failure and arrhythmias (Pellman 2016). Direct identification, quantification, and monitoring of collagen in the clinical setting will influence the treatment guidelines and further our understanding of the pathological process in human for better prognostic outcomes (de Jong 2011). However, to get this information, at least initially, it will require the use of invasive techniques to directly sample tissue from patients for in vitro imaging.
There is currently a paucity of data on the microscopic analysis of the fibrotic process in the human heart in comparison with the body of detailed knowledge that has been gained from research on animal models of heart failure. This is likely a result of the difficulties in obtaining and preserving human tissue in a timely manner that preserves microstructure and antigenicity. As already highlighted, the use of basic science imaging techniques, including confocal and super resolution, could help translate knowledge from animal models to humans. Although these tools have potential for diagnostics (Crossman et al. 2015), their greatest benefit is likely in pathological research. The objective of more microscopy-based analysis of human tissue could be met with greater utilization of tissue that can become available from EMB and cardiac surgery. In the next two sections, we will focus on the potential of imaging this tissue with fluorescence methods using focused examples from the literature. We will not cover electron microscopy (EM) that provides the highest resolution (~ 1 nm) as it has been used clinically and for pathological research for decades (Schaper 1983; Mudhar 2001; Kanzaki 2010; Saito 2015). Moreover, the down side of EM is that is that it has low contrast and is difficult to label with molecularly specific probes and is usually restricted to small regions and thin volumes in comparison with fluorescence methods that provide good contextual overview (Pullman 2019).
Endomyocardial biopsy
EMB is an invasive procedure that involves introducing a catheter through the femoral or jugular veins and advancing the catheter through the tricuspid valve into the right ventricle to obtain samples from the right interventricular septum. Samples are then visualized under electron microscopy, or labeled with special stains such as Congo red stain to identify amyloidosis, elastic trichrome stain to detect collagen, and elastic tissue and Perls’ stain to assess iron (Fig. 3; Khan et al. 2017). Techniques common in basic science such as immunohistochemistry labeling and confocal (Fig. 4) and super-resolution microscopy (Fig. 5) enable direct visualization of the unique cellular and molecular patterns contributing to the fibrosis seen in different myocardial diseases (Crossman et al. 2015). Although employing these tools for studying human tissue over time would be of substantial value in ascertaining and transferring the knowledge obtained from the animal models, it remains an underutilized tool due to the paucity of human tissue samples which is caused by the perceived risk associated with EMB.
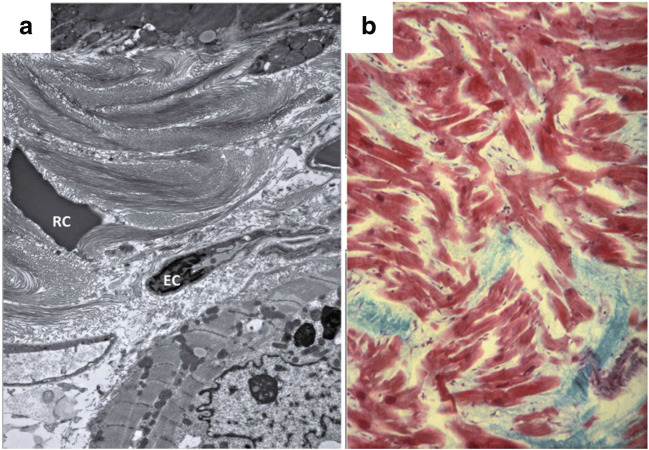
Fig. 3.
Electron and light microscopy of myocardial fibrosis. a EM characterization of the extracellular matrix post MI in rat heart showing dense collagen deposition, RC: extravasated red blood cell and EC: endothelial cell. Adapted from De Haas et al. (2014). b Histological section from the ventricle of human heart with hypertrophic cardiomyopathy showing myocyte disarray (red) and fibrosis (blue) stained by Masson’s trichrome stain. Adapted from Ho (2009)
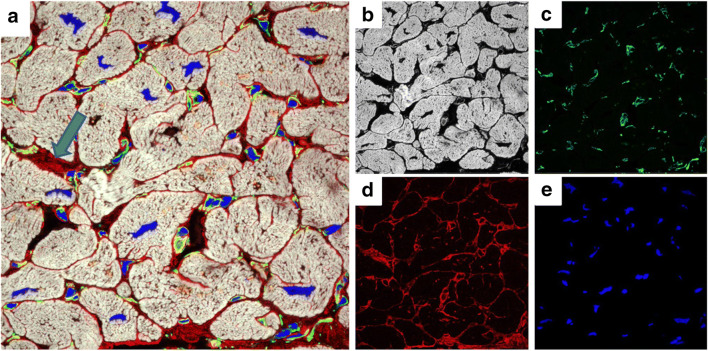
Fig. 4.
Immunohistochemistry labeling and confocal microscopy images of the myocardial structure post MI in human biopsy sample. a Four channel image showing labeling for f-actin, vementin, collagen VI, and nuclei. b Transversely orientated myocytes labeled with f-actin. c Fibroblasts labeled for vementin. d Collagen VI labeling. e Nuclei. Blue arrow indicates area of interstitial fibrosis. Summer Hassan unpublished data
Fig. 5.
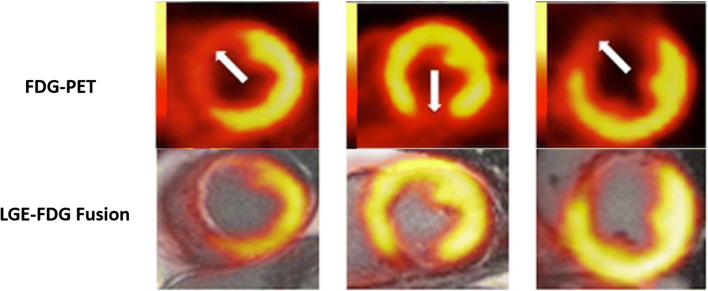
Hybrid positron emission tomography–magnetic resonance (PET-MR) showing area of subendocardial infarction (white arrows). 18F-fluorodeoxyglucose (FDG) uptake is decreased demonstrating abnormal glucose metabolism in the infarct region. The decrease in FDG uptake extends both transmurally and radially beyond the areas of late gadolinium enhancement (LGE). Adapted from Bulluck et al. (2016)
Current guidelines state that EMB is the “gold standard” diagnostic tool for diagnosing myocardial fibrosis in general and for quantification of diffuse MF specifically (Zheng et al. 2017; Mewton 2011; Moon James 2013). Specific guidance from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology advises that EMB is only indicated for monitoring cardiac allograft rejection or identifying a cause in a patient with unexplained new-onset heart failure of less than 2 weeks duration, or in patients with unexplained new-onset HF of 2 weeks–3 months duration associated with a dilated left ventricle and new ventricular arrhythmias, or finally when the patient fails to respond to usual care within 1–2 weeks (Cooper et al. 2007). Furthermore, EMB is a useful tool in certain clinical settings when other evaluations are not conclusive. This may occur in myocarditis, amyloidosis, and sarcoidosis (Mewton 2011).
Overall, EMB is a diagnostic and prognostic tool which allows direct microscopic assessment of the myocardial components and fibrotic changes, particularly when a definitive diagnosis is needed to guide treatment. Having access to human tissue biopsies provides a unique opportunity for research as it enables direct understanding of the pathophysiologic mechanisms of MF in humans, thereby validating the knowledge obtained from animal models.
Limitations
It is understandable that clinicians veer away from obtaining cardiac tissue samples to affirm diagnoses, due to the potential-associated risk e.g., tricuspid valve injury, transient complete heart block, right bundle branch block, and pericardial effusion (Daly 2012). More drastic adverse events such as myocardial perforations or deaths are significantly low but remain a concern hindering the decision to obtain endomyocardial biopsies. However, the complications associated with EMB may be overestimated. When assessed in a study conducted in Japan on 9508 patients, this study concluded that serious cardiac complications of EMB were rare, and that higher hospital volume was associated with lower complication rate (Isogai 2015). In another study investigating the risk associated with diagnostic cardiac catheterization conducted on 53,964 patients, this confirmed that the risk of MI and/or stroke associated with this procedure is 0.06% (Al-Hijji 2018). Furthermore, when investigating the development of clinically significant pericardial effusion, heamopericardium, or tricuspid valve injury post EMB, it was determined that the risk of such complications was so low that routine post EMB echo is no longer indicated in some centers (McElhinney 2018).
An additional limitation to obtaining tissue samples is sampling error, restricting the diagnostic accuracy in cases of localized fibrosis; the biopsies obtained are generally too small for reliable representation of collagen volume (Mewton 2011). Lastly, EMB is a non-reliable tool in detecting replacement type fibrosis mostly due to the epicardial location of scar which cannot be detected by biopsies taken from the endomyocardium; CMR is therefore a superior diagnostic tool in conditions associated with replacement fibrosis (Marra et al. 2013).
Surgical cardiac tissue
Human cardiac tissue for research can be obtained from coronary artery bypass surgery (Krul et al. 2015), left ventricle assist device implantation (LVAD) (Farris 2017), and cardiac transplant operations (Crossman et al. 2015).
The importance of microscopic fibrosis data has been highlighted in a study of left atrial appendages (LAA) obtained from patients undergoing procedures for atrial fibrillation (Krul et al. 2015). The authors used optical mapping to record electric activation of the tissue followed by microscopic analysis of fibrosis using picrosirius red labeling. Notably, electrical characteristics were uncorrelated with total amount of fibrosis per se but were correlated with the presence of thick interstitial collagen strands. LAA containing thick interstitial collagen fibers displayed higher longitudinal conduction velocity but slowed overall activation time that was associated with lines of conduction block. Moreover, the thick collagen stands were associated with increased fibroblasts identifying these cells as potential therapeutic target (Krul et al. 2015). Another interesting ex vivo preparation with potential for fibrotic research is the living myocardial slice preparations originally developed from animal hearts that have emerged as a viable method to record functional data (electrical activation, Ca2+, and force) of human ventricular tissue obtained from transplant operations (Pitoulis 2019; Kang 2016). For example, conduction remodeling in the failing human heart was linked to increased fibrosis measured by Masson’s trichrome and downregulation of connexion 43 expression and phosphorylation measured by confocal and westerns (Glukhov 2012).
The benefit of tissue obtained from LVAD surgeries is subsequently the same hearts that can often be resampled when the patient receives cardiac transplant allowing the effect of mechanical unloading to be examined. For example, microscopic assessment of collagen by picrosirius red labeling in the myocardium demonstrated no change in labeling due to mechanical unloading indicating that the fibrotic process remains persistent despite the known benefits of LVADs (Farris 2017). In another study of LVADs, the relationship between coronary artery fibrosis and hemodynamics was examined by obtaining tissue from cardiac transplant patients with and without previous implantation of continuous flow LVAD. As well as using standard histological assessments, the authors employed 2-photon microscopy to measure the intrinsic second-harmonic generation signal of native collagen and were able to identify a substantial increase in adventitial coronary fibrosis in the patients with LVADs identifying a potential worrying complication for the new continuous flow devices despite their overall better outcomes (Ambardekar et al. 2018).
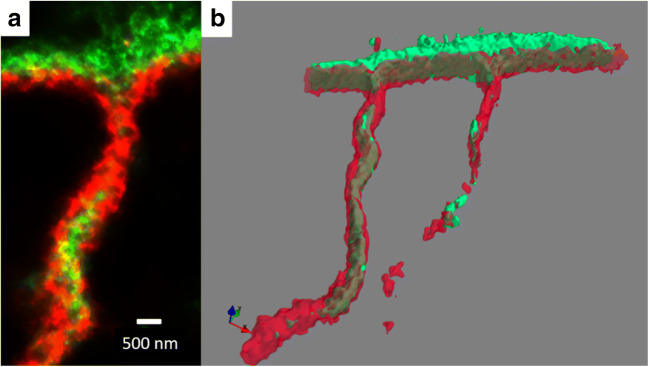
In our own work, we have used both confocal microscopy with ~ 250 nm resolution and super-resolution microscopy with ~ 30 nm resolution to identify both microscopic and nanoscopic changes in fibrosis that likely impair contraction in the failing human heart. We are particularly interested in collagen VI, a glycosylated protein we identified by proteomics and western blotting, as being substantially elevated in heart failure (Crossman et al. 2017). Confocal microscopy was able to demonstrate that the usual basement membrane location of collagen VI in the normal myocardium shifted to an interstitial location in the failing heart, indicating aberrant expression (Crossman et al. 2017) (Fig. 4). Collagen VI was also present at the transverse (t)-tubules but confocal did not have the required resolution to adequately resolve this labeling. T-tubules are nanoscale invaginations of the plasma membrane, on average around 350 nm in diameter in human ventricular myocytes, that allow conduction of the action potential deep within the myocyte that facilitate a synchronous Ca2+ release and contraction. In earlier work, we identified that loss of the transverse elements of the t-tubules was strongly correlated to reduced contractility within the failing human heart as determined by tagged MRI (Crossman et al. 2015). By using super-resolution microscopy, we were able to demonstrate that dilated t-tubules in the failing heart had increased amounts of collagen VI leading us to hypothesize that fibrosis is responsible for driving t-tubule remodeling in heart failure (Crossman et al. 2017) (Fig. 5). This data indicates that changes in the extracellular matrix can have a direct impact on cellular remodeling as reviewed by Crossman et al. (2017). One of the advantages of super-resolution imaging is the ability to provide nanoscale distribution of relevant protein targets mapped to cellular structure. This is demonstrated in Fig. 5 where 3D super-resolution imaging reveals that the distribution of collagen VI is interstitial with respect to the basement membrane.
An example of an innovative basic biomedical imaging technique that could be applied to clinical samples is illustrated in a study carried out by Smaill and colleagues where they explored the role of infarct site border zone structure in generating arrhythmia (Rutherford 2012). In this work, confocal imaging of a large volume of rat myocardium containing an infarct was collected (Fig. 6). From this image, areas of viable myocytes were identified allowing measurement of fiber orientation and network connectivity around the border zone. This high-resolution data was then used to model the relationship between border zone structure and the generation of re-entrant arrhythmias. It can be envisaged that similar approach could be applied to human tissue obtained from transplants. It would be a challenge to upscale this from rodent heart with a ventricle thickness ~ 2 mm to a human heart with a ventricle thickness of ~ 10 mm. However, the advent of light sheet imaging and optical clearing methods (Ding et al. 2018) makes this an achievable goal.
Fig. 6.
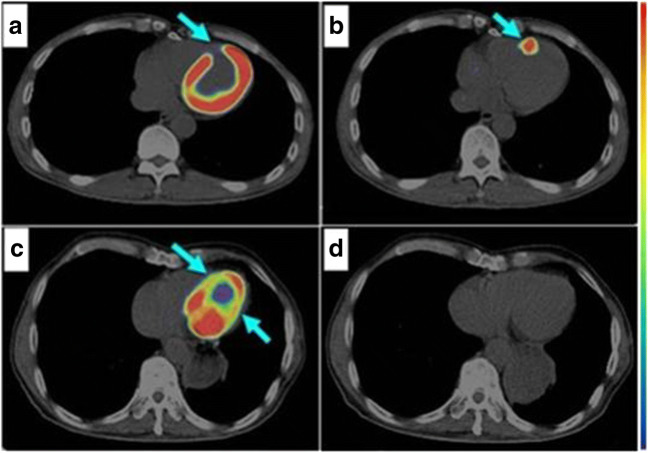
PET/CT scan of human heart 6 days post MI demonstrating area of infarction as indicated by the lack of 18F-FDG uptake in the infarct region (a, arrow) and the presence of active angiogenesis as indicated by the uptake of 68Ga-PRGD2 (b, arrow). Two years post MI 18F-FDG uptake is decreased (c, arrow) and no angiogenesis as demonstrated by 68Ga-PRGD2 accumulation (d). Adapted from Sun et al. (2014)
Limitations
The greatest limitation of this type of imaging is the difficulties of obtaining and preserving human tissue in a condition required for molecular analysis. The tissue obtained is incidental to cardiac surgery and this can make it logistically difficult to organize. Although autopsy samples can be used in research, the delay in obtaining tissue will cause sample degradation that will preclude many of the high-resolution imaging techniques. Moreover, large amounts of surgical tissue are generally only available from transplant operations from patients with end-stage heart failure. Note that tissue from early stages can be obtained from EMB which is the topic of the previous section. However, despite these limitations, this is an incredibly valuable resource for research. Unfortunately, this tissue is not always collected due to limited resources. Notably tissue often becomes available after hours that can be a logistical challenge. Moreover, obtaining non-failing or normal human tissue for comparison is relatively rare although infrequently tissue becomes available from healthy donor hearts that cannot be utilized for transplantation. This is where tissue banks as exemplified by the Sydney Heart Bank play an important role (Remedios et al. 2017). Tissue banks allow the collection and cryogenic storage of research tissue over the long term allowing a large collection of samples to be obtained facilitating studies on a clinical scale where hundreds of samples can be analyzed improving the ability to draw statistically robust conclusions.
Molecular imaging: potential to address the limitations
In the above section, we detailed how fluorescence methods can provide a high-resolution molecularly specific imaging of the fibrotic process in human tissue. This paradigm has great potential to yield insight into disease mechanisms and identify therapeutic targets. However, to be of benefit in the clinic, these features need to be monitored routinely in patients. Although EMB is used for diagnosis in reality, its wide-spread use is unlikely to occur due to its invasive nature. In this section, we will discuss the developing field of molecular imaging that has the potential to identify and monitor fibrosis in vivo and leverage molecularly specific details identified from imaging of excised tissue.
SPECT and PET
Single-photon emission computed tomography (SPECT) and positron emission tomography (PET) work by identifying injectable labels which are consumed by the myocardium. SPECT is able to construct 3D images and is frequently put in use to investigate the myocardial perfusion at rest and under stress (rest/stress test), this is achieved by injecting radioactive tracers such as thallium-201 (201Tl) and technetium-99m (99mTc) sestamibi or (99mTc) tetrofosmin and imaging their uptake by cardiomyocytes while the patient is at rest or excising/dobutamine injection (Underwood et al. 2004). PET on the other hand utilizes biologically active tracers including 13N-ammonia, 15O-water, and 82rubidium for myocardial perfusion and 18F-fluorodeoxyglucose (FDG) allowing assessment of cardiomyocyte metabolism and viability (Fig. 7; Ylä-Herttuala 2019; Ghosh 2010). PET has a higher resolution compared with SPECT and was found to be of superior sensitivity of 84% and specificity of 92% relative to SPECT with sensitivity of 81% and specificity of 85% in detecting myocardial fibrosis (De Haas 2014; Jaarsma et al. 2012).
Fig. 7.
T-tubule from the left ventricle of idiopathic dilated failing human heart imaged by super-resolution microscopy. a 2D imaging showing labeling for collagen VI (green) and collagen IV red. b 3D isosurface projection of the same t-tubule. David Crossman, unpublished data
Molecular imaging using SPECT, PET, or MRI
The principle behind molecular imaging is to expand the use of SPECT and PET to trace key biological molecules of interest by combining them with a detectable label such as a radiotracer. Similarly, tracers that are detectable by MRI e.g., gadolinium chelates have been developed. Hybrid systems PET/CT, SPECT/CT, and most recently PET/MRI are becoming more available enabling acquisition of images that represent different cellular and molecular elements of myocardial remodeling in vivo.
The pathogenesis of MF is highly intricate involving numerous cellular and molecular components that culminates in collagen deposition. Under healthy conditions, collagen forms 5% of the dry weight of the human myocardium (Chello 1996; Weber et al. 1988). During the healing process, the concentration of particularly type 1 is upregulated up to 10-fold (Jugdutt 1996). The produced collagen then resides in the injured tissue for a prolonged duration due to its long half-life (Jugdutt 1996; Whittaker 1989; Mukherjee 1993). This makes collagen an excellent target for radiotracers; indeed, its excessive accumulation in the extracellular matrix of injured tissue increases its uptake of the radiotracers and obviates the need for labeling agents to penetrate the cells (Helm et al. 2008).
Radiolabeled probes targeting collagen correlated with obtained biopsies have been shown to yield good insight to the degree of fibrosis in the injured tissue in multiple murine studies (Caravan et al. 2007; Helm et al. 2008; Zheng et al. 2017). Moreover, several probes designed for molecular imaging have been utilized in animal models to target some of the implicated suspects in excessive collagen deposition and have been reviewed by De Haas et al. (2014) and by Ylä-Herttuala (2019).
Multiple human studies were able to corroborate the adequacy of SPECT in the risk stratification of patients with acute MI, and predicting their propensity for MACE (Cabrera-Rodríguez et al. 2013; Sharir et al. 1999; Messerli 2006).
Angiogenesis imaging, particularly imaging of αvβ3 integrin which is preferentially expressed by the newly formed endothelial cells and not the stable endothelium of mature vasculature, allows monitoring of the healing process following myocardial injury. In a study investigating the utility of αvβ3 integrin imaging using 68Ga-PRGD2 PET/CT in a patient population with confirmed MI following percutaneous coronary intervention, valuable information was obtained regarding the timeline trends of the angiogenesis process at the infarct region (Sun et al. 2014) (Fig. 8). Targeting αvβ3 integrin by PET scan is an example of how employing this versatile diagnostic modality can aid in affirming the obtained knowledge from animal research with special value in monitoring the progress of the healing myocardium under the influence of different therapeutics to stimulate angiogenesis within the infarct zone.
Fig. 8.
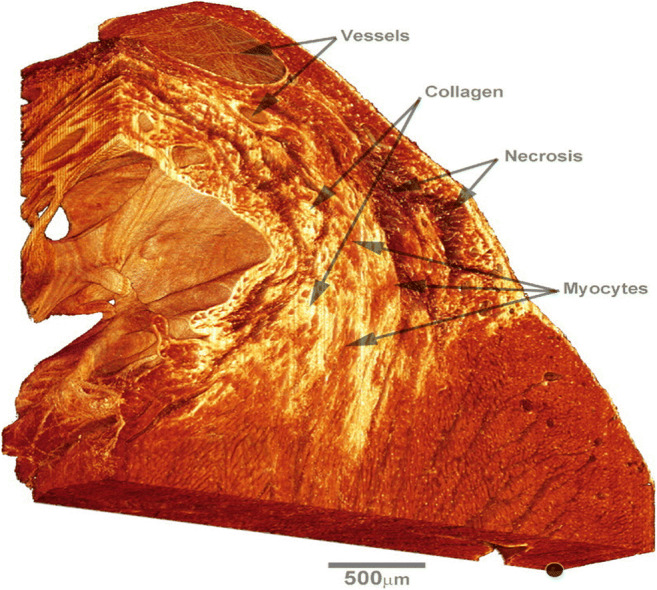
3D reconstruction of confocal microscopy volume of picrosirius red labeling of the infarction border zone in a rat model showing detailed characterization of the myocardial injury including the location and extent of necrotic tissue and the degree of collagen deposition as well as the blood vessels and myocyte distribution. The marker, bottom right, indicates the basal subepicardium and is used as a fiducial reference. Adapted from Rutherford et al. (2012)
Judicious use of molecular imaging may be the gateway to further understanding of the disease-specific cellular functions and molecular pathways that underlie the development of myocardial fibrosis in vivo in the clinic. This tool holds great potential in efficiently identifying and quantifying collagen as a hallmark of fibrosis, alongside a host of other markers of the disease. It clearly delineates the progression of events in a variety of myocardial insults. Employing this modality in the preclinical settings can guide future management decisions, and its capacity to non-invasively track the changes in the myocardium will allow direct therapeutic monitoring. Ultimately, this brings us a step closer towards personalizing treatments to enhance patients’ outcomes.
Limitations
The obvious limitation of molecular imaging methods is the low resolution relative to the cellular and subcellular detail provided by fluorescence methods. However, this is offset with their ability for in vivo imaging of molecules in patients providing the ability to diagnose and then monitor response to therapeutic interventions. A more pressing limitation is that molecular imaging is still in its infancy. There is a clear deficiency in large human studies addressing fibrosis in the myocardium specifically. Supplemental information on diagnostic and prognostic capacity of molecular imaging in non-ischemic cardiomyopathy is an area awaiting further work. Using SPECT to monitor the progression of fibrosis is constrained by the risk associated with recurrent exposure to ionizing radiation. This is being ameliorated by the rapid advancement in developing MRI tracers as well as introducing the PET/MRI hybrid systems. Additionally, these systems also have the advantage of compensating for cardiac and respiratory motion artifact (Ylä-Herttuala 2019). In a human and canine study comparing the diagnostic capacity of LGE-CMR and SPECT in detecting small subendocardial infarcts (partial wall thickness infarctions), it was found that SPECT is of significantly inferior sensitivity (sensitivity of 92% for LGE-CMR versus 28% for SPECT) (Wagner et al. 2003).
Modeling new and alternative radiotracers to target the multitude of cellular and molecular elements involved in the cardiac remodeling is an ever evolving area, highlighting the need for further studies evaluating the available tracers against each other for standardization of practice. Molecular imaging is expensive and yet to become a readily available modality in clinical practice, particularly in the emergency setting. In acute MI, utilizing SPECT or PET requires administration of the appropriate radiotracer during the ischemic episode in ED to allow for high-quality imaging after the patient is stabilized (Aletras et al. 2006). However, protocols pertaining to appropriate stocking and administration of the radiotracers in acute settings are lacking.
Summary
Knowledge is deficient in humans
The vast knowledge achieved in understanding the cellular and molecular interactions influencing the development of myocardial fibrosis is obtained—for the most part—from animal studies. Understandably, there is a deficiency in human tissue biopsies of the heart available for research and when obtained, these samples are often at the end stage of the disease prior to cardiac transplants, limiting the ability to track the progression of events leading to the end stage of the disease.
Additional human studies would allow us to affirm the data obtained from the animal models. It would also offer a direct pathway for investigating potential new treatments by tracking these cellular and molecular contributors. It is notable that in human studies, a large proportion of the data available is obtained from retrospective studies and the available prospective studies have a low number of subjects. There is a need for more prospective studies on large portion of the population at risk for further affirmation of the knowledge obtained thus far and to guide the development of diagnostic protocols in different cardiac diseases (Gannon 2019).
A comparison of the diagnostic modalities
The review sheds light on the extensive efforts made in diagnosing one of the most morbid and prevalent cardiovascular conditions in the world. In this review, multiple diagnostic modalities for MF have been evaluated. In summary, echo is a safe and accessible tool which will undoubtedly continue to be used for functional assessment and monitoring following myocardial injury. However, it has limited utility in quantifying and differentiating etiologies of MF.
LGE-CMR is recognized as a one of the best tools in identifying acute replacement fibrosis, although it overestimates the lesional size acutely. In addition, it is ineffective in differentiating various types of diffuse MF and therefore less useful in identifying non-ischemic causes of MF. T1 mapping on the other hand is a reliable modality when investigating diffuse MF. Evidence suggests that it is likely the best tool for monitoring the progression of MF. However, it is underutilized in the clinical practice (Patriki et al. 2018; Gannon 2019). As highlighted previously, there is room for improvement on standardization of T1 normal reference ranges, particularly when this modality is utilized as a monitoring measure.
EMBs are the current gold standard for quantifying collagen deposition and diagnosing diffuse myocardial fibrosis. They have specific utility in establishing the etiology of heart failure when other means are not successful. The clear limitation is the risk imposed by this procedure as well as the propensity for sampling errors. Using confocal microscopy in the lab setting provides detailed characterization of the myocardial fabric and allows for direct comparison between healthy myocardium and injured myocardial e.g., following MI (Crossman et al. 2015; White 2015).
Molecular imaging is a modality that holds great potential in diagnosing all types of MF, as well as tracking changes in the myocardial fabric over time. Its distinguishing feature is its ability to isolate and follow specific cellular and molecular agents in vivo, non-invasively, adding valuable insight to the pathophysiologic mechanisms and potential treatment targets for MF. Recent developments in diagnostic modalities for MF hold potential for tailored treatments targeting patients’ specific underlying pathology.
Conclusion
Developing effective treatment plans, tailored to patients’ pathological process, is the solution to the high toll of cardiovascular disease. As a first measure, myocardial fibrosis needs to be addressed as a less generic term, factoring to the underlying pathological mechanism. This requires validation of the mechanisms identified from animal models. More human studies are needed; this will require more studies on human tissue obtained from EMB and cardiac surgeries. Once these mechanisms are validated in human, the emerging molecular imaging modalities provide the promise to translate this knowledge to the clinic by enabling minimally invasive in vivo tracking.
There is an unmet need for clinical trials investigating alternative treatment options for MF; including antifibrotic agents and investigating current management protocols by tracking the changes in the myocardial fabric prospectively at a molecular level, current diagnostic tools rely on identification of the macroscopic changes of the myocardium. These changes may be preventable by directing our research efforts to the molecular pathways and interfering with the cascades that result in irreversible MF. In addition, there is room for improvement on standardization of LGE-CMR and MRI protocols to aid accurate reproducible detection and quantification of MF and to allow appropriate risk stratification for patients and implementing the most beneficial treatment options.
Acknowledgments
We wish to thank Auckland City Hospital and St Vincent’s Hospital staff for the assistance in obtaining tissue and the transplant recipients and donor families for donating tissue.
Funding information
Research funding was provided by the Auckland Medical Research Foundation and Health Research Council of New Zealand.
Footnotes
The original online version of this article was revised: Table 1 of the original article was published with an error in the “Confocal microscope”. The correct data should be "250 nm" instead of "5–50 nm".
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
11/19/2021
A Correction to this paper has been published: 10.1007/s12551-021-00911-9
References
- Abdel-Aty HC. Edema as a very early marker for acute myocardial ischemia. J Am Coll Cardiol. 2009;53(14):1194–1201. doi: 10.1016/j.jacc.2008.10.065. [DOI] [PubMed] [Google Scholar]
- Abdel-Aty JH, Zagrosek GA, Schulz-Menger GJ, Taylor GA, Messroghli GD, Kumar GA, et al. Delayed enhancement and T2-weighted cardiovascular magnetic resonance imaging differentiate acute from chronic myocardial infarction. Circulation. 2004;109(20):2411–2416. doi: 10.1161/01.CIR.0000127428.10985.C6. [DOI] [PubMed] [Google Scholar]
- Aherne CC. Cardiac T mapping: techniques and applications. J Magn Reson Imaging. 2019;51(5):1336–1356. doi: 10.1002/jmri.26866. [DOI] [PubMed] [Google Scholar]
- Aherne CK. Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction. J Cardiovasc Magn Reson. 2012;14(1):15. doi: 10.1186/1532-429X-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimo A, Cerbai E, Bartolucci G, Adamo L, Barison A, Lo Surdo G, et al. Pirfenidone is a cardioprotective drug: mechanisms of action and preclinical evidence. Pharmacol Res. 2020;155(5):104694. doi: 10.1016/j.phrs.2020.104694. [DOI] [PubMed] [Google Scholar]
- Aletras HA, Tilak SG, Natanzon MA, Hsu FL-Y, Gonzalez EF, Hoyt ER, Arai EA. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: Histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113(15):1865–1870. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- Alfano G, Fontana F, Ferrari A, Solazzo A, Perrone R, Giaroni F, et al. Incidence of nephrogenic systemic fibrosis after administration of gadoteric acid in patients on renal replacement treatment. Magn Reson Imaging. 2020;70(1):1–4. doi: 10.1016/j.mri.2020.02.012. [DOI] [PubMed] [Google Scholar]
- Al-Hijji RL. Abstract 14914: safety and risk of diagnostic cardiac catheterization. Circulation. 2018;12(7):e007791. doi: 10.1161/CIRCINTERVENTIONS.119.007791. [DOI] [PubMed] [Google Scholar]
- Alkhalil M, Borlotti A, De Maria G, Wolfrum M, Dawkins S, Fahrni G, et al. Hyper-acute cardiovascular magnetic resonance T1 mapping predicts infarct characteristics in patients with ST elevation myocardial infarction. J Cardiovasc Magn Reson. 2020;22(1):3. doi: 10.1186/s12968-019-0593-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambardekar VA, Weiser-Evans CM, Li NM, Purohit BS, Aftab SM, Reece ST, Moulton SK. Coronary artery remodeling and fibrosis with continuous-flow left ventricular assist device support. Circ Heart Fail. 2018;11(5):e004491. doi: 10.1161/CIRCHEARTFAILURE.117.004491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreas Kammerlander CT. Extracellular matrix expansion by cardiac magnetic resonance T1 mapping- validation with myocardial biopsy. J Cardiovasc Magn Reson. 2015;9(1):14–23. [Google Scholar]
- Annoni G, Luvarà G, Arosio B, Gagliano N, Fiordaliso F, Santambrogio D, et al. Age-dependent expression of fibrosis-related genes and collagen deposition in the rat myocardium. Mech Ageing Dev. 1998;101(1–2):57–72. doi: 10.1016/s0047-6374(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Antman EM, Cooper HA, Gibson CM, de Lemos JA, Mccabe CH, Giugliano RP, et al. Determinants of improvement in epicardial flow and myocardial perfusion for ST elevation myocardial infarction; insights from TIMI 14 and InTIME-II. Eur Heart J. 2002;23(12):928–933. doi: 10.1053/euhj.2001.2964. [DOI] [PubMed] [Google Scholar]
- Arora G, Ohaji C, Lavalley M, Zhao Y, Berk J, Sanchorawala V, et al. Global T1 mapping and late gadolinium enhancement cardiac MR in myocardial fibrosis compared with amyloid cardiomyopathy. Circulation. 2012;126(21):Suppl S. [Google Scholar]
- Bacmeister L, Schwarzl M, Warnke S, Stoffers B, Blankenberg S, Westermann D, Lindner D. Inflammation and fibrosis in murine models of heart failure. Basic Res Cardiol. 2019;114(3):19. doi: 10.1007/s00395-019-0722-5. [DOI] [PubMed] [Google Scholar]
- Bahit MC, Kochar A, Granger CB. Post-myocardial infarction heart failure. JACC: Heart Failure. 2018;6(3):271–279. doi: 10.1016/j.jchf.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Baines CP. How and when do myocytes die during ischemia and reperfusion: the late phase. J Cardiovasc Pharmacol Ther. 2011;16(3-4):239–243. doi: 10.1177/1074248411407769. [DOI] [PubMed] [Google Scholar]
- Barclay JL, Egred M, Kruszewski K, Nandakumar R, Norton MY, Stirrat C, et al. The relationship between transmural extent of infarction on contrast enhanced magnetic resonance imaging and recovery of contractile function in patients with first myocardial infarction treated with thrombolysis. Cardiology. 2007;108(4):217–222. doi: 10.1159/000096781. [DOI] [PubMed] [Google Scholar]
- Bauner KU, Biffar AM, Theisen DM, Greiser AP, Zech CJ, Nguyen ET, et al. Extracellular volume fractions in chronic myocardial infarction. Investig Radiol. 2012;47(9):538–545. doi: 10.1097/RLI.0b013e3182631c37. [DOI] [PubMed] [Google Scholar]
- Betty Raman RA. Progression of myocardial fibrosis in hypertrophic cardiomyopathy: mechanisms and clinical implications. Eur Heart J Cardiovasc Imaging. 2018;20(2):157–167. doi: 10.1093/ehjci/jey135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodh Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation: Journal of the American Heart Association. 2003;108(11):1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- Bohnen S, Radunski U, Lund G, Ojeda F, Looft Y, Senel M, et al. Healing of myocarditis can be monitored by native myocardial T1 and T2 measurements without the need for contrast media. Both native myocardial T1 and T2 provide an excellent performance for assessing the stage of myocarditis by CMR. Eur Heart J Cardiovasc Imaging. 2017;8(7):744–751. [Google Scholar]
- Borer SJ, Truter MS, Herrold JE, Falcone ND, Pena FM, Carter AJ, et al. Myocardial fibrosis in chronic aortic regurgitation: molecular and cellular responses to volume overload. Circulation: Journal of the American Heart Association. 2002;105(15):1837–1842. doi: 10.1161/01.cir.0000014419.71706.85. [DOI] [PubMed] [Google Scholar]
- Borne SW. Molecular imaging of interstitial alterations in remodeling myocardium after myocardial infarction. J Am Coll Cardiol. 2008;52(24):2017–2028. doi: 10.1016/j.jacc.2008.07.067. [DOI] [PubMed] [Google Scholar]
- Boudina S, EA Diabetic cardiomyopathy revisited. Circulation. 2007;115(25):3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- Bull S, White SK, Piechnik SK, Flett AS, Ferreira VM, Loudon M, et al. Human non-contrast T1 values and correlation with histology in diffuse fibrosis. Heart. 2013;99(13):932–937. doi: 10.1136/heartjnl-2012-303052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulluck H, White SK, Rosmini S, Bhuva A, Treibel TA, Fontana M, .Hausenloy D (2015) T1 mapping and T2 mapping at 3T for quantifying the area-at-risk in reperfused STEMI patients. 17(1):73 [DOI] [PMC free article] [PubMed]
- Bulluck KH, White MS, Fröhlich GG, Casson MS, O’meara CC, Newton MA, Grov (2016) Quantifying the aSrea at risk in reperfused ST-segment–elevation myocardial infarction patients using hybrid cardiac positron emission tomography–magnetic resonance imaging. 9(3):e003900 [DOI] [PMC free article] [PubMed]
- Bulluck MR-G-D. Myocardial T1 mapping-hope or hype. Circulation. 2015;79(3):487–494. doi: 10.1253/circj.CJ-15-0054. [DOI] [PubMed] [Google Scholar]
- Cabrera-Rodríguez LO, Peix AT, Padrón KM, Chacón D, Carrillo R, Fernández Y, Mena E. Prognostic value of gated SPECT after reperfusion for acute myocardial infarction. MEDICC Review. 2013;15(2):20–25. doi: 10.37757/MR2013V15.N2.5. [DOI] [PubMed] [Google Scholar]
- Capasso PO. Severe myocardial dysfunction induced by ventricular remodeling in aging rat hearts. Valhalla: Department of Pathology, New York Medical College; 1990. pp. 1086–1096. [DOI] [PubMed] [Google Scholar]
- Caravan P, Das B, Dumas S, Epstein F, Helm P, Jacques V, et al. Collagen-targeted MRI contrast agent for molecular imaging of fibrosis. Angew Chem. 2007;46(43):8171–8173. doi: 10.1002/anie.200700700. [DOI] [PubMed] [Google Scholar]
- Chang S-A, Lee S-C, Choe Y, Hahn H-J, Jang S, Park S-J et al (2012) Effects of hypertrophy and fibrosis on regional and global functional heterogeneity in hypertrophic cardiomyopathy. The International Journal of Cardiovascular Imaging [DOI] [PubMed]
- Chello MM. Collagen network remodelling and left ventricular function in constrictive pericarditis. Heart. 1996;75(2):184–189. doi: 10.1136/hrt.75.2.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho ME, Smith DC, Branton MH, Penzak SR, Kopp JB. Pirfenidone slows renal function decline in patients with focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2007;2(5):906–913. doi: 10.2215/CJN.01050207. [DOI] [PubMed] [Google Scholar]
- Choi MK, Kim JR, Gubernikoff DG, Vargas MJ, Parker MM, Judd MR. Transmural extent of acute myocardial infarction predicts long-term improvement in contractile function. Circulation: Journal of the American Heart Association. 2001;104(10):1101–1107. doi: 10.1161/hc3501.096798. [DOI] [PubMed] [Google Scholar]
- Chow K YY. Robust free-breathing SASHA T1 mapping with high-contrast image registration. J Cardiovasc Magn Reson Journal of Cardiovascular MRI. 2016;18(1):47. doi: 10.1186/s12968-016-0267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease. J Am Coll Cardiol. 2007;116(19):2216–2233. doi: 10.1161/CIRCULATIONAHA.107.186093. [DOI] [PubMed] [Google Scholar]
- Costelloe CM. Risks and benefits of gadolinium-based contrast enhanced MRI. Semin Ultrasound CT MR. 2020;41(2):170–182. doi: 10.1053/j.sult.2019.12.005. [DOI] [PubMed] [Google Scholar]
- Crossman DJ, Young AA, Ruygrok PN, Nason GP, Baddelely D, Soeller S, Cannell MB. T-tubule disease: relationship between t-tubule organization and regional contractile performance in human dilated cardiomyopathy. J Mol Cell Cardiol. 2015;84:170–178. doi: 10.1016/j.yjmcc.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossman DJ, Shen X, Jüllig M, Munro M, Hou Y, Middleditch M, et al. Increased collagen within the transverse tubules in human heart failure. Cardiovasc Res. 2017;113(8):879–891. doi: 10.1093/cvr/cvx055. [DOI] [PubMed] [Google Scholar]
- Cury CR, Shash TK, Nagurney DJ, Rosito HG, Shapiro JM, Nomura FC, et al. Cardiac magnetic resonance with T2-weighted imaging improves detection of patients with acute coronary syndrome in the emergency department. Circulation. 2008;118(8):837–844. doi: 10.1161/CIRCULATIONAHA.107.740597. [DOI] [PubMed] [Google Scholar]
- Dallʼarmellina CE, Karia DN, Lindsay DA, Karamitsos MT, Ferreira DV, Robson PM, et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circulation. 2011;4(3):228–236. doi: 10.1161/CIRCIMAGING.111.963421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Armellina KT. CMR for characterization of the myocardium in acute coronary syndromes. Nat Rev Cardiol. 2010;7(11):624–636. doi: 10.1038/nrcardio.2010.140. [DOI] [PubMed] [Google Scholar]
- Daly MV. Endomyocardial biopsy and selective coronary angiography are low-risk procedures in pediatric heart transplant recipients: results of a multicenter experience. Clinical Key. 2012;31(4):398–409. doi: 10.1016/j.healun.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastidar AG, Harries I, Pontecorboli G, Bruno VD, De Garate E, Moret C, et al. Native T1 mapping to detect extent of acute and chronic myocardial infarction: comparison with late gadolinium enhancement technique. The international journal of cardiovascular imaging. 2019;35(3):517–527. doi: 10.1007/s10554-018-1467-1. [DOI] [PubMed] [Google Scholar]
- Dastidar AG, Pontecorboli G, Harries I, Moret C, Morgan G, Garate ED, et al. Non-contrast assessment of myocardial viability in chronic myocardial infarction by native T1 and T2 mapping at 1.5T CMR: comparison with late gadolinium enhancement technique. Heart. 2017;103(10):1. [Google Scholar]
- De Boer RA, De Keulenaer G, Bauersachs J, Brutsaert D, Cleland JG, Diez J, et al. Towards better definition, quantification and treatment of fibrosis in heart failure. A scientific roadmap by the Committee of Translational Research of the Heart Failure Association (HFA) of the European Society of Cardiology. Eur J Heart Fail. 2019;21(3):272–285. doi: 10.1002/ejhf.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haas JH. Molecular imaging of the cardiac extracellular matrix. Circulation. 2014;114(5):903–915. doi: 10.1161/CIRCRESAHA.113.302680. [DOI] [PubMed] [Google Scholar]
- de Jong SZ. Direct detection of myocardial fibrosis by. MRI. 2011;51(6):974–979. doi: 10.1016/j.yjmcc.2011.08.024. [DOI] [PubMed] [Google Scholar]
- de Jong S, Zwanenburg JJ, Visser F, Der Nagel Rv, van Rijen HV, Vos MA, Luijten PR (2011) Direct detection of myocardial fibrosis by MRI. J Mol Cell Cardiol 51(6):974–979 [DOI] [PubMed]
- Dhalla NR. Cardiac remodeling and subcellular defects in heart failure due to myocardial infarction and aging. Heart Fail Rev. 2012;17(4-5):671–681. doi: 10.1007/s10741-011-9278-7. [DOI] [PubMed] [Google Scholar]
- Ding Y, Ma J, Langenbacher A, Baek K, Lee J, Chang C, Hsiai T (2018) Multiscale light-sheet for rapid imaging of cardiopulmonary system. JCI Insight 3(16):e121396 [DOI] [PMC free article] [PubMed]
- Benjamin EJ, SS Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67–e492. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- Erberto BP. Fibrosis assessment by integrated backscatter and its relationship with longitudinal deformation and diastolic function in heart failure with preserved ejection fraction. The International Journal of Cardiovascular Imaging. 2016;32(7):1071–1080. doi: 10.1007/s10554-016-0881-5. [DOI] [PubMed] [Google Scholar]
- Esmaeilzadeh MP. The role of echocardiography in coronary artery disease and acute myocardial infarction. The journal of Tehran Heart Center. 2013;8(1):1–13. [PMC free article] [PubMed] [Google Scholar]
- Eugene Lin AA. What are the basic concepts of temporal, contrast, and spatial resolution in cardiac CT? Journal of Cardiovascular Computed Tomography. 2009;3(6):403–408. doi: 10.1016/j.jcct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaars H v. Cardiac magnetic resonance for evaluating nonculprit lesions after myocardial infarction: comparison with fractional flow reserve. JACC Cardiovasc Imaging. 2020;13(3):715–728. doi: 10.1016/j.jcmg.2019.07.019. [DOI] [PubMed] [Google Scholar]
- Ezekowitz J, Kaul P, Bakal J, Armstrong P, Welsh R, Mcalister F. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J Am Coll Cardiol. 2009;53(1):13–20. doi: 10.1016/j.jacc.2008.08.067. [DOI] [PubMed] [Google Scholar]
- Fang L, Murphy A, Dart A. A clinical perspective of anti-fibrotic therapies for cardiovascular disease. Front Pharmacol. 2017;8:186. doi: 10.3389/fphar.2017.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris FM-H-O. Heart failure with preserved ejection fraction and skeletal muscle physiology. Heart Fail Rev. 2017;134(1):73–90. doi: 10.1007/s10741-017-9603-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes R. T1 Mapping. Clinical Key. 2015;23(1):25–34. doi: 10.1016/j.mric.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Fieno DS, Kim RJ, Chen EL, Lomasney JW, Klocke FJ, Judd RM. Contrast-enhanced magnetic resonance imaging of myocardium at risk: distinction between reversible and irreversible injury throughout infarct healing. J Am Coll Cardiol. 2000;36(6):1985–1991. doi: 10.1016/s0735-1097(00)00958-x. [DOI] [PubMed] [Google Scholar]
- Fischer VL. Characterization of fibrillar collagen types using multi-dimensional multiphoton laser scanning microscopy. Int J Cosmet Sci. 2012;34(2):209. doi: 10.1111/j.1468-2494.2012.00705.x. [DOI] [PubMed] [Google Scholar]
- Flacke SJ, Fischer SE, Lorenz CH. Measurement of the gadopentetate dimeglumine partition coefficient in human myocardium in vivo: normal distribution and elevation in acute and chronic infarction. Radiology. 2001;218(3):703–710. doi: 10.1148/radiology.218.3.r01fe18703. [DOI] [PubMed] [Google Scholar]
- Flett Andrew S, HM Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122(2):138–144. doi: 10.1161/CIRCULATIONAHA.109.930636. [DOI] [PubMed] [Google Scholar]
- Frangogiannis NG. Cardiac fibrosis: cell biological mechanisms, molecular pathways and therapeutic opportunities. Mol Asp Med. 2019;65(1):70–99. doi: 10.1016/j.mam.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, et al. Cardiovascular magnetic resonance in myocarditis. J Am Coll Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi M, Kamalov G, Shahbaz A, Bhattacharya S, Ahokas R, Sun Y, et al. Cellular and molecular pathways to myocardial necrosis and replacement fibrosis. Heart Fail Rev. 2011;16(1):23–34. doi: 10.1007/s10741-010-9169-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gannon MP. State of the art: evaluation and prognostication of myocarditis using cardiac MRI. J Magn Reson Imaging. 2019;49(7):e122–e131. doi: 10.1002/jmri.26611. [DOI] [PubMed] [Google Scholar]
- Garg PP, Abdel-Rahman PS-E-D, Greenwood PJ, Plein PS. Free-wall rupture post–reperfused acute myocardial infarction: insights from multimodality cardiovascular imaging. Circulation. 2015;132(21):e245–e247. doi: 10.1161/CIRCULATIONAHA.115.018932. [DOI] [PubMed] [Google Scholar]
- Garg PS. Role of cardiac T1 mapping and extracellular volume in the assessment of myocardial infarction. Anatolian Journal Of Cardiology. 2018;19(6):404–411. doi: 10.14744/AnatolJCardiol.2018.39586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh NR. Assessment of myocardial ischaemia and viability: role of positron emission tomography. Eur Heart J. 2010;31(24):2984–2995. doi: 10.1093/eurheartj/ehq361. [DOI] [PubMed] [Google Scholar]
- Glukhov FR. Conduction remodeling in human end-stage nonischemic left ventricular cardiomyopathy. Circulation. 2012;125(15):1835–1847. doi: 10.1161/CIRCULATIONAHA.111.047274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granitz LJ. Comparison of native myocardial T1 and T2 mapping at 1.5T and 3T in healthy volunteers. Wien Klin Wochenschr. 2019;131(7):143–155. doi: 10.1007/s00508-018-1411-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani F, Varone F, Crea F, Richeldi L. Treating heart failure with preserved ejection fraction: learning from pulmonary fibrosis. Eur J Heart Fail. 2018;20(10):1385–1391. doi: 10.1002/ejhf.1286. [DOI] [PubMed] [Google Scholar]
- Gulati GA, Japp PA, Raza AS, Halliday AB, Jones RD, Newsome GS, et al. Absence of myocardial fibrosis predicts favorable long-term survival in new-onset heart failure: a cardiovascular magnetic resonance study. Circulation: Cardiovascular Imaging. 2018;11(9):1. doi: 10.1161/CIRCIMAGING.118.007722. [DOI] [PubMed] [Google Scholar]
- Guo D, HH Value of myocardial scar in predicting malignant ventricular arrhythmia in patients with chronic myocardial infarction. J ZheJiang Univ (Medical Science) 2019;48(5):511–516. doi: 10.3785/j.issn.1008-9292.2019.10.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner Wound repair and regeneration. Nature. 2008;453(7193):314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Haaf P, Garg P, Messroghli D, Broadbent D, Greenwood J, Plein S. Cardiac T1 mapping and extracellular volume (ECV) in clinical practice: a comprehensive review. J Cardiovasc Magn Reson. 2016;18(1):89. doi: 10.1186/s12968-016-0308-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helm P, Caravan P, French B, Jacques V, Shen L, Xu Y, et al. Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology. 2008;247(3):788–796. doi: 10.1148/radiol.2473070975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill a E. Cardiac plasticity. N Engl J Med. 2008;358(13):1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- Ho SY (2009) Anatomy and myoarchitecture of the left ventricular wall in normal and in disease. 10(8):iii3–7 [DOI] [PubMed]
- Höke U, Khidir MJ, van Der Geest RJ, Schalij MJ, Bax JJ, Delgado V, Ajmone Marsan N. Relation of myocardial contrast-enhanced T1 mapping by cardiac magnetic resonance to left ventricular reverse remodeling after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. Am J Cardiol. 2017;199(9):1456–1462. doi: 10.1016/j.amjcard.2017.01.023. [DOI] [PubMed] [Google Scholar]
- Hung C, Verma A, Uno H, Shin S, Bourgoun M, Hassanein A, et al. Longitudinal and circumferential strain rate, left ventricular remodeling, and prognosis after myocardial infarction. J Am Coll Cardiol. 2010;118(18):S684–S684. doi: 10.1016/j.jacc.2010.06.044. [DOI] [PubMed] [Google Scholar]
- Iles L, Ellims A, Llewellyn H, Hare J, Kaye D, Mclean C, Taylor A. Histological validation of cardiacmagnetic resonance analysis of regional and diffuse interstitial myocardial fibrosis. European Heart Journal-Cardiovascular Imaging. 2015;265(3):724–732. doi: 10.1093/ehjci/jeu182. [DOI] [PubMed] [Google Scholar]
- Iles L, Pfluger H, Phrommintikul A, Cherayath J, Aksit P, Gupta SN, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52(19):1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- Imran M, Wang L, McCrohon J, Yu C, Holloway C, Otton J, et al. Native T1 mapping in the diagnosis of cardiac allograft rejection. JACC. 2019;12(8):1618–1628. doi: 10.1016/j.jcmg.2018.10.027. [DOI] [PubMed] [Google Scholar]
- Ingkanisorn WR. Gadolinium delayed enhancement cardiovascular magnetic resonance correlates with clinical measures of myocardial infarction. J Am Coll Cardiol. 2004;43(12):2253–2259. doi: 10.1016/j.jacc.2004.02.046. [DOI] [PubMed] [Google Scholar]
- Isogai YH. Hospital volume and cardiac complications of endomyocardial biopsy: a retrospective cohort study of 9508 adult patients using a nationwide inpatient database in Japan. Clin Cardiol. 2015;38(3):164–170. doi: 10.1002/clc.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaarsma C, Leiner T, Bekkers SC, Crijns HJ, Wildberger JE, Nagel E, et al. Diagnostic performance of noninvasive myocardial perfusion imaging using single-photon emission computed tomography, cardiac magnetic resonance, and positron emission tomography imaging for the detection of obstructive coronary artery disease. J Am Coll Cardiol. 2012;59(19):1719–1728. doi: 10.1016/j.jacc.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Jugdutt BI. Rate of collagen deposition during healing and ventricular remodeling after myocardial infarction in rat and dog models. Circulation. 1996;94(1):94–101. doi: 10.1161/01.cir.94.1.94. [DOI] [PubMed] [Google Scholar]
- Kali AC-Y, Cokic I, Yang H-J, Tighiouart M, Conte AH, Li D, et al. Native T1 mapping by 3-T CMR imaging for characterization of chronic myocardial infarctions. JACC Cardiovasc Imaging. 2015;8(9):1019–1030. doi: 10.1016/j.jcmg.2015.04.018. [DOI] [PubMed] [Google Scholar]
- Kali A, Choi E-Y, Sharif B, Kim YJ, Bi X, Spottiswoode B, et al. Native T1 mapping by 3-T CMR imaging for characterization of chronic myocardial infarctions. JACC: Cardiovascular Imaging; 2015. [DOI] [PubMed] [Google Scholar]
- Kali YA, Choi JE, Sharif JB, Kim SY, Bi WX, Spottiswoode JB, et al. Abstract 13610: native T1-based cardiovascular magnetic resonance imaging for characterizing chronic myocardial infarctions in patients. Circulation. 2014;130(Suppl):2. [Google Scholar]
- Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015;276(1):228–232. doi: 10.1148/radiol.2015142690. [DOI] [PubMed] [Google Scholar]
- Kang . Science Letter. 2016. Human organotypic cultured cardiac slices: new platform for high throughput preclinical human trials; p. 646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanzaki TO. Three-dimensional architecture of cardiomyocytes and connective tissue in human heart revealed by scanning electron microscopy. Circulation. 2010;122(19):1973–1974. doi: 10.1161/CIRCULATIONAHA.110.979815. [DOI] [PubMed] [Google Scholar]
- Kellman PA. T 2 -prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57(5):891–897. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan T, Selvakumar D, Trivedi S, Rao K, Harapoz M, Thiagalingam A, et al. The value of endomyocardial biopsy in diagnosis and guiding therapy. Pathology. 2017;49(7):750–756. doi: 10.1016/j.pathol.2017.08.004. [DOI] [PubMed] [Google Scholar]
- Kim JR, Manning JW. Viability assessment by delayed enhancement cardiovascular magnetic resonance. Circulation. 2004;109(21):2476–2479. doi: 10.1161/01.CIR.0000130730.63776.69. [DOI] [PubMed] [Google Scholar]
- Kim JR, Fieno SD, Parrish BT, Harris PK, Chen JE-L, Simonetti MO, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100(19):1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- Kim RJ, Wu E, Rafael A, Chen E-L, Parker MA, Simonetti O, et al. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N Engl J Med. 2000;343(20):1445–1453. doi: 10.1056/NEJM200011163432003. [DOI] [PubMed] [Google Scholar]
- Kong PC. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71(4):549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krul PS, Berger RW, Smit WN, Van Amersfoorth CS, Driessen HA, Van Boven JW, et al. Atrial fibrosis and conduction slowing in the left atrial appendage of patients undergoing thoracoscopic surgical pulmonary vein isolation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2015;8(2):288–295. doi: 10.1161/CIRCEP.114.001752. [DOI] [PubMed] [Google Scholar]
- Kwong YR, Schussheim EA, Rekhraj HS, Aletras FA, Geller SN, Davis EJ, et al. Detecting acute coronary syndrome in the emergency department with cardiac magnetic resonance imaging. Circulation. 2003;107(4):531–537. doi: 10.1161/01.cir.0000047527.11221.29. [DOI] [PubMed] [Google Scholar]
- Layne KA. Gadolinium-based contrast agents-what is the evidence for ‘gadolinium deposition disease’ and the use of chelation therapy? Clinical toxicology (Philadelphia, Pa) 2020;58(3):151–160. doi: 10.1080/15563650.2019.1681442. [DOI] [PubMed] [Google Scholar]
- Lee WK, Everett HT, Rahmutula MD, Guerra EJ, Wilson EE, Ding EC, Olgin EJ. Pirfenidone prevents the development of a vulnerable substrate for atrial fibrillation in a canine model of heart failure. Circulation. 2006;114(16):1703–1712. doi: 10.1161/CIRCULATIONAHA.106.624320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Han R, Kang L, Wang J, Gao Y, Li Y, Tian J (2017) Pirfenidone controls the feedback loop of the AT1R/p38 MAPK/renin-angiotensin system axis by regulating liver X receptor-alpha in myocardial infarction-induced cardiac fibrosis. Sci Rep 7(0):40523 [DOI] [PMC free article] [PubMed]
- Liu A, Wijesurendra RS, Francis JM, Robson MD, Neubauer S, Piechnik SK, Ferreira VM. Adenosine stress and rest T1 mapping can differentiate between ischemic, infarcted, remote, and normal myocardium without the need for gadolinium contrast agents. JACC Cardiovasc Imaging. 2016;9(1):27–36. doi: 10.1016/j.jcmg.2015.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LD, Borlotti DA, Viliani DD, Jerosch-Herold DM, Alkhalil DM, De Maria DG, et al. CMR native T1 mapping allows differentiation of reversible versus irreversible myocardial damage in ST-segment–elevation myocardial infarction: an OxAMI study (Oxford Acute Myocardial Infarction) Circulation: Cardiovascular Imaging. 2017;10(8):e005986. doi: 10.1161/CIRCIMAGING.116.005986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurz P, Luecke C, Eitel I, Föhrenbach F, Frank C, Grothoff M, et al. Comprehensive cardiac magnetic resonance imaging in patients with suspected myocarditis. J Am Coll Cardiol. 2016;67(15):1800–1811. doi: 10.1016/j.jacc.2016.02.013. [DOI] [PubMed] [Google Scholar]
- Marra M, De Lazzari M, Rizzo S, Zilio F, Vettor G, Calore C, et al. The diagnostic and prognostic value of myocardial fibrosis in nonischemic dilated cardiomyopathy: a study by endomyocardial biopsy and cardiac magnetic resonance. Circulation. 2013;17(1):34. [Google Scholar]
- Matteo Cameli SM (2017) Speckle tracking echocardiography: a practical guide. G Ital Cardiol (Rome) 18(4):253–269 [DOI] [PubMed]
- McElhinney DM. Utility of the routine screening echocardiogram after endomyocardial biopsy for identification of hemopericardium or tricuspid valve injury. Pediatr Transplant. 2018;69(21):2579–2589. doi: 10.1111/petr.13275. [DOI] [PubMed] [Google Scholar]
- Messerli FH. Guidelines on the management of stable angina pectoris: executive summary: the Task Force on the Management of Stable Angina Pectoris of the European Society of Cardiology. Eur Heart J. 2006;27(23):2902–2903. doi: 10.1093/eurheartj/ehl308. [DOI] [PubMed] [Google Scholar]
- Messroghli DN-M. T 1 mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson. 2003;5(2):353–359. doi: 10.1081/jcmr-120019418. [DOI] [PubMed] [Google Scholar]
- Mewton LC. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011;57(8):891–903. doi: 10.1016/j.jacc.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed MS. Age independent myocardial fibrosis in heart failure with preserved ejection fraction. Circulation. 2012;12(11):2291–2301. [Google Scholar]
- Møller JE, Dahlström U, Gøtzsche O, Lahiri A, Skagen K, Andersen GS, Egstrup K (2004) Effects of losartan and captopril on left ventricular systolic and diastolic function after acute myocardial infarction: results of the Optimal Trial in Myocardial Infarction with Angiotensin II antagonist losartan (optimaal) echocardiographic substudy. Am Heart J 147(3):494–501 [DOI] [PubMed]
- Monti CB, Codari M, Cozzi A, Alì M, Saggiante L, Sardanelli F, Secchi F. Image quality of late gadolinium enhancement in cardiac magnetic resonance with different doses of contrast material in patients with chronic myocardial infarction. European radiology experimental. 2020;4(1):21. doi: 10.1186/s41747-020-00149-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon James C, T. T. T1 mapping for diffuse myocardial fibrosis: a key biomarker in cardiac disease? J Am Coll Cardiol. 2013;62(14):1288–1289. doi: 10.1016/j.jacc.2013.05.077. [DOI] [PubMed] [Google Scholar]
- Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, et al. Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2013;15(1):92. doi: 10.1186/1532-429X-15-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mordi AS. Comprehensive echocardiographic and cardiac magnetic resonance evaluation differentiates among heart failure with preserved ejection fraction patients, hypertensive patients, and healthy control subjects. JACC Cardiovasc Imaging. 2018;11(4):577–585. doi: 10.1016/j.jcmg.2017.05.022. [DOI] [PubMed] [Google Scholar]
- Mudhar WS. Electron microscopy of myocardial tissue. A nine year review. J Clin Pathol. 2001;54(4):321. doi: 10.1136/jcp.54.4.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee SS. Alteration of cardiac collagen phenotypes in hypertensive hypertrophy: role of blood pressure. J Mol Cell Cardiol. 1993;25(2):185. doi: 10.1006/jmcc.1993.1021. [DOI] [PubMed] [Google Scholar]
- Nathan SD, Albera C, Bradford WZ, Costabel U, Glaspole I, et al (2017) Effect of pirfenidone on mortality: pooled analyses and meta-analyses of clinical trials in idiopathic pulmonary fibrosis, Lancet Respiratory medicine, 5(1):33–41 [DOI] [PubMed]
- Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, et al. Type VI collagen induces cardiac myofibroblast differentiation: implications for postinfarction remodeling. Am J Phys. 2006;290(1):323–330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- Nazarian S, Hansford R, Rahsepar AA, Weltin V, Mcveigh D, Gucuk Ipek E, et al. Safety of magnetic resonance imaging in patients with cardiac devices. N Engl J Med. 2017;377(26):2555–2564. doi: 10.1056/NEJMoa1604267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndrepepa G, Mehilli J, Martinoff S, Schwaiger M, Schömig A, Kastrati A (2007) Evolution of left ventricular ejection fraction and its relationship to infarct size after acute myocardial infarction. J Am Coll Cardiol [DOI] [PubMed]
- Nguyen DT, Ding C, Wilson E, Marcus GM, Olgin JE. Pirfenidone mitigates left ventricular fibrosis and dysfunction after myocardial infarction and reduces arrhythmias. Heart Rhythm. 2010;7(10):1438–1445. doi: 10.1016/j.hrthm.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Omland TW. State of the art: blood biomarkers for risk stratification in patients with stable ischemic heart disease. Clin Chem. 2017;63(1):165–176. doi: 10.1373/clinchem.2016.255190. [DOI] [PubMed] [Google Scholar]
- Ørn S, Manhenke C, Anand IS, Squire I, Nagel E, Edvardsen T, Dickstein K. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am J Cardiol. 2007;99(8):1109–1114. doi: 10.1016/j.amjcard.2006.11.059. [DOI] [PubMed] [Google Scholar]
- Parsai C, O’Hanlon R, Prasad S, Mohiaddin R. Diagnostic and prognostic value of cardiovascular magnetic resonance in non-ischaemic cardiomyopathies. J Cardiovasc Magn Reson. 2012;14(1):54. doi: 10.1186/1532-429X-14-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriki D, Gresser E, Manka R, Emmert MY, Lüscher TF, Heidecker B. Approximation of the incidence of myocarditis by systematic screening with cardiac magnetic resonance imaging. JACC: Heart Failure. 2018;6(7):573–579. doi: 10.1016/j.jchf.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Pellman ZS. 3Article Myocyte-fibroblast communication in cardiac fibrosis and arrhythmias: mechanisms and model systems. JMCC. 2016;94(1):22–31. doi: 10.1016/j.yjmcc.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Terol I, Rios-Navarro C, de Dios E, Morales J, Gavara J, Perez-Sole N, et al. Magnetic resonance microscopy and correlative histopathology of the infarcted heart. Sci Rep. 2019;9(1):20017. doi: 10.1038/s41598-019-56436-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer M, Mcmurray J, Velazquez E, Rouleau J, Kober L, Maggioni A, Califf R (2003) Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med 349(20):1893-906 [DOI] [PubMed]
- Pfeifer PA (1984) A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1–100 MHz: dependence on tissue type, NMR frequency, temperature, species, excision, and age. Medical Physics Medical physics 11(4):425–448 [DOI] [PubMed]
- Pitoulis W (2019) Myocardial slices come to age: an intermediate complexity in vitro cardiac model for translational research. Cardiovasc Res 1(1) [DOI] [PMC free article] [PubMed]
- Plein S, Greenwood JP, Ridgway JP, Cranny G, Ball SG, Sivananthan MU. Assessment of non–ST-segment elevation acute coronary syndromes with cardiac magnetic resonance imaging. J Am Coll Cardiol. 2004;44(11):2173–2181. doi: 10.1016/j.jacc.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Popović ZB, Kwon DH, Mishra M, Buakhamsri A, Greenberg NL, Thamilarasan M et al (2008) Association between regional ventricular function and myocardial fibrosis in hypertrophic cardiomyopathy assessed by speckle tracking echocardiography and delayed hyperenhancement magnetic resonance imaging. J Am Soc Echocardiogr [DOI] [PubMed]
- Prinz C, van Buuren F, Faber L, Bitter T, Bogunovic N, Burchert W, Horstkotte D. In hypertrophic cardiomyopathy myocardial fibrosis is associated with biventricular dysfunction. Circulation. 2011;29(4):438–444. doi: 10.1111/j.1540-8175.2011.01588.x. [DOI] [PubMed] [Google Scholar]
- Pullman (2019) New views of the glomerulus: advanced microscopy for advanced diagnosis. Frontiers In Medicine 6 [DOI] [PMC free article] [PubMed]
- Radunski U, Lund G, Säring D, Bohnen S, Stehning C, Schnackenburg B, et al. T1 and T2 mapping cardiovascular magnetic resonance imaging techniques reveal unapparent myocardial injury in patients with myocarditis. Clin Res Cardiol. 2017;106(1):10–17. doi: 10.1007/s00392-016-1018-5. [DOI] [PubMed] [Google Scholar]
- Rajiah KF. Cardiac magnetic resonance in patients with cardiac implantable electronic devices. J Thorac Imaging. 2020;34(12):1682–1686. doi: 10.1097/RTI.0000000000000462. [DOI] [PubMed] [Google Scholar]
- Reant P, Mirabel M, Lloyd G, Peyrou J, Lopez Ayala J-M, Dickie S et al (2016) Global longitudinal strain is associated with heart failure outcomes in hypertrophic cardiomyopathy. Heart [DOI] [PubMed]
- Remedios C, Lal S, Li A, McNamara J, Keogh A, Macdonald P, Velden J (2017) The Sydney Heart Bank: improving translational research while eliminating or reducing the use of animal models of human heart disease 9(4), 431–441 [DOI] [PMC free article] [PubMed]
- Ricciardi JM, Wu JE, Davidson MC, Choi JK, Klocke OF, Bonow MR, et al. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation. Circulation. 2001;103(23):2780–2783. doi: 10.1161/hc2301.092121. [DOI] [PubMed] [Google Scholar]
- Rossi Espagnet MB-T. Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol. 2017;47(10):1345–1352. doi: 10.1007/s00247-017-3874-1. [DOI] [PubMed] [Google Scholar]
- Rutherford E TM (2016) Defining myocardial tissue abnormalities in end-stage renal failure with cardiac magnetic resonance imaging using native T1 mapping. Kidney Int Kidney Int 90(4):845–852 [DOI] [PMC free article] [PubMed]
- Rutherford TS (2012) High-resolution 3-dimensional reconstruction of the infarct border zone: impact of structural remodeling on electrical activation. 113(3), 301–311 [DOI] [PubMed]
- Sadeghpour AA (2018a) Case-based textbook of echocardiography, Cham, Switzerland
- Sadeghpour AA. Echocardiography in coronary artery disease and acute myocardial infarction. Cham: Springer; 2018. [Google Scholar]
- Saito AS (2015) Ultrastructural features of cardiomyocytes in dilated cardiomyopathy with initially decompensated heart failure as a predictor of prognosis. aito, Tsunenori; Asai, Kuniya; Sato, Shigeru; Takano, Hitoshi; Mizuno, Kyoichi; Shimizu, Wataru 36(724–732):724–732 [DOI] [PubMed]
- Salerno K. Advances in parametric mapping with CMR imaging. Jacc-Cardiovascular Imaging. 2013;6(7):806–822. doi: 10.1016/j.jcmg.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstede J, Lipke C, Beer M, Hofmann S, Pabst T, Kenn W, et al. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. Eur Radiol. 2000;1(1):7–21. doi: 10.1007/s003300050072. [DOI] [PubMed] [Google Scholar]
- Schaper S. Ultrastructural correlates of reduced cardiac function in human heart disease. Eur Heart J. 1983;4:35–42. doi: 10.1093/eurheartj/4.suppl_a.35. [DOI] [PubMed] [Google Scholar]
- Schisler MS (2019) Fibrosis in disease: an organ-based guide to disease pathophysiology and therapeutic considerations. Humana Press, Cham, Switzerland
- Shah YI. Amyloidosis and the heart: a comprehensive review. Arch Intern Med. 2006;166(17):1805–1813. doi: 10.1001/archinte.166.17.1805. [DOI] [PubMed] [Google Scholar]
- Sharir T, Germano G, Kavanagh PB, Lai S, Cohen I, Lewin HC, et al. Incremental prognostic value of post-stress left ventricular ejection fraction and volume by gated myocardial perfusion single photon emission computed tomography. Circulation. 1999;100(10):1035–1042. doi: 10.1161/01.cir.100.10.1035. [DOI] [PubMed] [Google Scholar]
- Sibley RA. T1 mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Cardiac Imaging. 2012;265(3):724–732. doi: 10.1148/radiol.12112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siepen F, Buss SJ, Messroghli D, Andre F, Lossnitzer D, Seitz S, et al. T1 mapping in dilated cardiomyopathy with cardiac magnetic resonance: quantification of diffuse myocardial fibrosis and comparison with endomyocardial biopsy. Eur Heart J Cardiovasc Imaging. 2015;16(2):210–216. doi: 10.1093/ehjci/jeu183. [DOI] [PubMed] [Google Scholar]
- Spath NB, Tavares A, Gray GA, Dweck MR, Newby DE, Yang PC, et al. 10 manganese-enhanced T1 mapping in myocardial infarction: validation with 18 F-FDG PET/MR. Heart. 2018;104(Suppl 5):A9. [Google Scholar]
- Stanescu AL, Shaw DW, Murata N, Murata K, Rutledge JC, Maloney E, Maravilla KR. Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation. Pediatr Radiol. 2020;50(3):388–396. doi: 10.1007/s00247-019-04535-w. [DOI] [PubMed] [Google Scholar]
- Stephane Heymans AG-A. Searching for new mechanisms of myocardial fibrosis with diagnostic and/or therapeutic potential. Eur J Heart Fail. 2015;17(9):764–771. doi: 10.1002/ejhf.312. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zeng Y, Zhu Y, Feng F, Xu W, Wu C, Zhu Z (2014) Application of (68)Ga-PRGD2 PET/CT for αvβ3-integrin imaging of myocardial infarction and stroke. Theranostics 4(8):778–786 [DOI] [PMC free article] [PubMed]
- Thomsen H, Morcos S, Almén T, Bellin M-F, Bertolotto M, Bongartz G, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23(2):307–318. doi: 10.1007/s00330-012-2597-9. [DOI] [PubMed] [Google Scholar]
- Underwood S, Anagnostopoulos C, Cerqueira M, Ell P, Flint E, Harbinson M, et al. Myocardial perfusion scintigraphy: the evidence. Eur J Nucl Med Mol Imaging. 2004;31(2):261–291. doi: 10.1007/s00259-003-1344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanessa F, K PS, Erica D, D KT, M FJ, P CR et al (2012) Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson [DOI] [PMC free article] [PubMed]
- Vergaro G, Del Franco A, Giannoni A, Prontera C, Ripoli A, Barison A, et al. Galectin-3 and myocardial fibrosis in nonischemic dilated cardiomyopathy. Int J Cardiol. 2015;10(3):184–196. doi: 10.1016/j.ijcard.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Verheugt FG. Aborted myocardial infarction: a new target for reperfusion therapy. Eur Heart J. 2006;27(8):901–904. doi: 10.1093/eurheartj/ehi829. [DOI] [PubMed] [Google Scholar]
- Vöhringer MY. Significance of late gadolinium enhancement in cardiovascular magnetic resonance imaging (CMR) Herz Kardiovaskuläre Erkrankungen. 2007;32(2):129–137. doi: 10.1007/s00059-007-2972-5. [DOI] [PubMed] [Google Scholar]
- Voicu, MD (2019) Hypertension and heart failure, epidemiology, mechanisms and treatment. Cham, Switzerland: Springer. 2019
- Wagner A, Mahrholdt H, Holly TA, Elliott MD, Regenfus M, Parker M, et al. MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. Lancet. 2003;1;361(9355):374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- Weber KT. Are myocardial fibrosis and diastolic dysfunction reversible in hypertensive heart disease? Congestive Heart Failure. 2005;11(6):322–324. doi: 10.1111/j.1527-5299.2005.04479.x. [DOI] [PubMed] [Google Scholar]
- Weber TK, Janicki SJ, Shroff GS, Pick MR, Chen IR, Bashey IR. Collagen remodeling of the pressure overloaded, hypertrophied nonhuman primate myocardium. Circulation. 1988;62(4):757–765. doi: 10.1161/01.res.62.4.757. [DOI] [PubMed] [Google Scholar]
- White RCD (2015) Confocal scanning microscopy in assessment of cardiac allograft rejection-a pilot study 47(8):2513–2516 [DOI] [PubMed]
- Whittaker DR. Analysis of healing after myocardial infarction using polarized light microscopy. Am J Pathol. 1989;134(4):879–893. [PMC free article] [PubMed] [Google Scholar]
- Wong TC, Piehler K, Puntil KS, Moguillansky D, Meier CG, Lacomis JM, et al. Effectiveness of late gadolinium enhancement to improve outcomes prediction in patients referred for cardiovascular magnetic resonance after echocardiography. Cardiovascular Magnetic Resonance. 2013;15(1):6. doi: 10.1186/1532-429X-15-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolen SA, Shankar PR, Gagnier JJ, MacEachern MP, Singer L, Davenport MS. Risk of nephrogenic systemic fibrosis in patients with stage 4 or 5 chronic kidney disease receiving a group II gadolinium-based contrast agent. JAMA Intern Med. 2020;180(2):223–230. doi: 10.1001/jamainternmed.2019.5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H-Y, Yang Z-G, Zhang Y, Peng W-L, Xia C-C, Li Z-L, et al. Prognostic value of heart failure in hemodialysis-dependent end-stage renal disease patients with myocardial fibrosis quantification by extracellular volume on cardiac magnetic resonance imaging. BMC Cardiovasc Disord. 2020;20(1):12. doi: 10.1186/s12872-019-01313-2. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Yan LF (2015) Identification of right ventricular myocardial infarction with late gadolinium enhancement in chronic ischemic heart disease: comparison with pathological findings. Circulation 132(supplement 3):A10958
- Yang F, Wang J, Li W, Xu Y, Wan K, Zeng R, Chen Y. The prognostic value of late gadolinium enhancement in myocarditis and clinically suspected myocarditis: systematic review and meta-analysis. Eur Radiol. 2020;30(5):2616–2626. doi: 10.1007/s00330-019-06643-5. [DOI] [PubMed] [Google Scholar]
- Yang PC. T1-mapping in myocardial disease: principles and applications. Cham, Switzerland: Springer; 2018. [Google Scholar]
- Ylä-Herttuala ES-H. Molecular imaging to monitor left ventricular remodeling in heart failure. Current cardiovascular imaging reports. 2019;24(2):574–590. [Google Scholar]
- Zannad F, Alla F, Dousset B, Perez A, Pitt B. Limitation of excessive extracellular matrix turnover may contribute to survival benefit of spironolactone therapy in patients with congestive heart failure: insights from the Randomized Aldactone Evaluation Study (RALES) Circulation. 2000;102(22):2700–2706. doi: 10.1161/01.cir.102.22.2700. [DOI] [PubMed] [Google Scholar]
- Zarate RH. Fabry’s disease. Lancet. 2008;372(9647):1427–1435. doi: 10.1016/S0140-6736(08)61589-5. [DOI] [PubMed] [Google Scholar]
- Zheng L, Ding X, Liu K, Feng S, Tang B, Li Q, et al. Molecular imaging of fibrosis using a novel collagen-binding peptide labelled with 99mTc on SPECT/CT. Amino Acids. 2017;49(1):89–101. doi: 10.1007/s00726-016-2328-7. [DOI] [PubMed] [Google Scholar]