Abstract
Medicinal plant production is most important than other agricultural plants due to their phytochemical compounds effects on human health. Paying attention to plant nutrition requirement is so important. In order to assess the effect of nitrate (NO3−) dosage supplies from two types of fertilizers on growth and phytochemical properties of Echinacea purpurea rhizomata cum radicibus, an experiment with completely simple design was carried out under greenhouse conditions. Two types of fertilizers (new invented nitrogen (N) slow release fertilizer and urea chemical fertilizer) at three dosages (50, 100, and 150 mM) were applied. Plant growth parameters and total phenolic (TPC), total flavonoids (TFC), polysaccarides content, essential oil content, caffeic acid derivatives, and anti-radical scavenging activities of E. purpurea were assessed. The results showed the significant (p ≤ 0.01) differences among treatments, both in growth and phytochemical properties. Using of N slow release, especially in 150 mM dosage, significantly increased all the plant growth and phytochemical properties. The dried E. purpurea rhizomata cum radicibus contained more caftaric acid (max 12.56 mg g−1 DW) and chicoric acid (max 7.56 mg g−1 DW) than other derivatives. Despite the impact of heavy metals on yield and growth of E. purpurea, the concentration of all heavy metals and micronutrients (boron (B), cadmium (Cd), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), lead (Pb), and zinc (Zn)) in studied soil and fertilizer samples was less than United States Environmental Protection Agency (USEPA) limits of contamination. Based on the results, using of N slow release fertilizers can improve phytochemical properties of the plant due to its polymeric structure and can be a suitable substitution of chemical fertilizers, especially in medicinal plants growth.
Subject terms: Plant sciences, Environmental sciences
Introduction
Many of researchers are pay attention to herbal and medicinal plants as natural remedies due to their bioactive compounds recently1,2. Echinacea purpurea (L.) Moench is one of the most important medical plants of Asteraceae family with various pharmacological effects3. The major three species of Echinacea are known as Echinacea angustifolia, Echinacea purpurea, and Echinacea pallida. E. purpurea is native to much of the United States and its morphological properties are also known as purple coneflower with orange and cone-shaped flowering head that change to purple, rose, or white petals during June to September4. It has a narrow leaves and three feet height stem with dark, thick and pungent rhizomata cum radicibus5. In the environment, E. purpurea grows randomically along water canals with low densities for affective harvest for commercial purposes6.
Comparing of other Echinacea species, E. purpurea is the most able species to adjust to new conditions. It is resistance to heat or cold weather conditions, easy to grow, and flaunt high efficiency7,8. Numerous scientific researches demonstrated the useful effect of Echinacea immunomodulatory functions and human health9,10. The doctors explored that the alcohol extract of Echinacea rhizomata cum radicibus immediately increased phagocytic activity and leads to high production of white blood cells, as well as, various in vitro researches has confirmed the phagocytosis, and antibody-dependent cellular cytotoxicity effects of E. purpurea rhizomata cum radicibus extracts4. Decreasing of severity and symptoms of the common cold by using E. purpurea extracts was reported in several clinical studies11. According to immunological studies, E. purpurea enhanced activation of the nonspecific cellular and increased humoral immunity by increasing the activation and production of monocytes, lymphocytes, leukocytes and cytokines12. Based on the studies, the immune‐stimulatory property is the most important property of the species that involves the immune system based on the dose level13. Although the rhizomata cum radicibus of E. purpurea is very beneficial, purple coneflower of the species was known to be useful for the improvement of the various illnesses, such as sore throats, coughs, and snake bite8. Meanwhile, E. purpurea has been used to treat viral, bacterial and fungal infections14 and the healing of the burns and wounds15.
Various chemical components of E. purpurea were associated with its biological activities and medicinal effects3,16. For instance, the main phenolic compounds of E. purpurea were found as caffeic acid esters and chicoric acid, as well as, polysaccarides was dominant in E. purpurea rhizomata cum radicibus extracts17. Recent studies demonstrated that the polysaccharide content leads to enhance the macrophage activity and the cytokine production18. Also, the antifungal and antiviral properties of E. purpurea were related to certain groups of the phenolic compounds and alkamides18–20. Scavenging of the free radicals and lipid peroxidation assay were related to the antioxidant properties of leaves and rhizomata cum radicibus extracts of E. purpurea21. Higher concentration of isobutyl amide was affects the Echinacea’s anti‐inflammatory property17.
Overall, good manufacturing of high quality medicinal plants is depend on precise aware of plant nutrient requirement. The study in Hungary demonstrated that application of 60–80 kg ha−1 N, 40–60 kg ha−1 phosphorus (P) and 80–100 kg ha−1 potassium (K+) lead to increase of biological yield and phenolic compounds of E. purpurea22. The optimum nutrients ratio for plant growth depends on species and environmental conditions23 and soil pollution with chemical pollutants such as various heavy metals24. Several studies demonstrated that soil heavy metals pollution caused to decrease of E. purpurea yield under greenhouse condition14. Among all nutrients, N, due to cooperation in phytochemical compound’s structures had very critical effect on Echinacea yield and medicinal properties13. So, attention to N supply has key role in chemical and medicinal composition of Echinacea. Previous researches illustrated that the various plant growth parameters of E. purpurea are generally improved with optimum mixture ratio of NO3− and ammonium (NH4+) in comparison with either N form alone16,17. The NO3− dosage can affect not only rhizomata cum radicibus morphological properties, but also the overall rhizomata cum radicibus biomass25. The results of Verma et al. (2019) showed that phenolic compounds (caftaric acid, cholorogenic acid, cynarin, echinacoside and chicoric acid) were significantly affected by NO3− dosage.
Urea is most popular conventional fertilizers in agriculture which is partially absorbed by plants. This results in reduce N usage efficiency for crops and environmental pollution26. In recent years, slow release fertilizer has been developed. Slow release fertilizer releases nutrients according to plants requirement for physiological functions during the long time, results in increase of fertilizer efficiency27.
Although there are many previous reports about growing medicinal plants in different culture media and NO3− dosages, there is no prior research on comparison of phytochemical properties of E. purpurea growing in the presence of novel invited N slow release fertilizer and common chemical fertilizer. So the goal of this research was to compare the growth properties and phytochemical compounds of E. purpurea growing with various amounts of NO3− dosage supplied from slow release and urea fertilizers.
Materials and methods
Experimental background
The experiment was carried out in a greenhouse complex at Urmia University, West of Azarbaijan Province, Iran. Seeds of E. purpurea obtained from Pakan Bazr Esfahan, an Iranian private joint stock company, on April. 15, 2019. The seeds were put into a mixture of perlite and peat moss substrates for initial growth. Irrigation operation was performed regularly as needed depending on greenhouse condition. Seedlings were harvested after one month when they were at 3–4 true leaves stage and transplanted in the experimental pots which had one plant grown in a pot (2.5 L) containing a mixture of soil (2-mm sieved) and fine sand for better aeration and leaching, under greenhouse conditions. The density of production system was chosen according to Waidyanatha et al. (2020). The set point for the greenhouse temperature and humidity were 19–21 °C (night–day) and 75% respectively. Fertigation was performed based on E. purpurea nutrition need, shown in Table 1.
Table 1.
Nutrition requirement of E. purpurea (Seif Sahandi et al.21).
| Nutrient | Attribute (mM) |
|---|---|
| Nitrogen (N) | 150.0 |
| Phosphorus (P) | 2.0 |
| Potassium (K) | 6.2 |
| Calcium (Ca) | 3.9 |
| Magnesium (Mg) | 2.0 |
| Sulfur (S) | 4.2 |
| Iron (Fe) | 50.0 |
| Manganese (Mn) | 9.0 |
| Zinc (Zn) | 0.8 |
| Copper (Cu) | 0.8 |
| Boron (B) | 18.0 |
| Molybdenum (Mo) | 0.5 |
| pH | 6.5–7.5 |
| EC (dS m−1) | < 2 |
All essential elements except N were supplied from the soil adequately, but two types of N fertilizers were used for supply of enough N nutrition need including slow release N fertilizer, and Urea. Chemical composition of used various fertilizers were analysed according to standard methods28,29. The amount of N application was determined based on the differences of plant N requirement and soil N, according to fertilizer type. In this study 50 (less than adequate), 100 (moderate), and 150 (adequate) mM N were provided from each fertilizer types. Slow release fertilizer used in this study, was invited based on a novel composition at science and technology center of Hamedan province, Iran and registered at Iranian Patent Office, Tehran on Jan. 30, 2017 (patent number 139550140003013815). Details of the novel N fertilizer and its Figure were shown in Table 2 and Fig. 1 respectively.
Table 2.
Main properties of invited slow release fertilizer.
| Properties | Details |
|---|---|
| Physical shape | Compact and tablet form |
| Weight (g) | 2.3 |
| Diameter (cm) | 1.5 |
| Height (cm) | 0.6 |
| Harness and solubility | 6.4/10 |
| Adhesion strength (%) | 100 |
| Surface area (mg g−1) | 200 |
| Colour | White |
| Main composition | Powdered cotton seed, Semi-solid sucrose, and water |
| Heavy metals | None |
Figure 1.
Nitrogen slow release fertilizer.
The fertilizer can control the N release during the plant grow period based on complex formation between the chelator agent and the element. The main difference that distinguish this novel fertilizer from usual and commercial slow release fertilizers is its chelator agent that is more efficient and environmental friendly. As well as, urea fertilizer was chosen as common N fertilizer among Iranian farmers. All experimental treatments were repeated as three replicates with less than 1% standard deviation. Plants were fertigated for 240 days, every 10 days, under control conditions in greenhouse. The rhizome with (rhizomata cum radicibus) of plants were harvested after growing period and dried at 23–25 °C (at room temperature), then phytochemical analyses were performed.
Soil analysis
Soil chemical and physical properties were measured based on standard methods. Soil pH and electrical conductivity (EC) were measured using 1:5 w/v soil to water ratio suspension11. Organic matter (OM) was determined after oxidation of soil organic fraction with potassium dichromate (K2Cr2O7) and sulfuric acid (H2SO4)30. Cation exchange capacity (CEC) was determined with 1 mol L−1 sodium acetate solution (pH 8.2) and 96% ethanol14. Calcium carbonate (CaCO3) was measured after boiling of 2.5 g soil with 25 mL of 0.5 N hydrochloric acid (HCl)12. Three fractions (sand, silt, and clay) of soil particles were determined following the pipette method15.
Soil calcium (Ca2+) and magnesium (Mg2+) were measured titrimetrically, K+ was determined by flame photometry, sulfate (SO42−) by spectrophotometric and turbidimetric methods, NO3− and phosphate (PO43−) by colorimetry with an ultraviolet–visible (UV–Vis) spectrophotometer11. Available micronutrients (Cu, Fe, Mn, Mo, and Zn) and heavy metals (Cd, Ni, Pb) were extracted with a solution containing 0.005 mol L−1 diethylene triamine penta acetic acid (DTPA), 0.01 mol L−1 calcium chloride, and 0.1 mol L−1 (pH 7.3) triethanolamine (TEA), as well as, boron (B) concentration was determined based on hot water method26,31. The concentration of micronutrients were measured by Atomic Absorption Spectrophotometer (AAS) (Model Varian Spectra-220).
Fertilizer analysis
The urea and sloe release fertilizer were passed through a 0.5-mm sieve after air-dried at 25 ± 1 °C. Total N, NH4+ and NO3− concentrations in samples were determined according to Kjeldahl method27. Heavy metals and trace elements (Cd, Cu, Fe, Mn, Ni, Pb and Zn) content in samples were determined after digestion with aqua regia based on standard method18,32.
Plant growth parameters
After harvesting the plants, different growth parameters such as plant height (cm) and total fresh leave weight (g plant−1), fresh rhizomata cum radicibus weight (g plant−1), chlorophylls a and b (mg g−1 FW), and leaf area (cm2) were measured. Weighted 0.5 g of dry sample was homogenized with 10 mL acetone23. Homogenized samples were centrifuged at 10,000 rpm for 15 min. The supernatant was separated and the absorbance spectra were measured at 400–700 nm. Maximum absorbance of chlorophylls a and b were measured in 662 nm and 645 nm, respectively29. So that6,10,
| 1 |
| 2 |
Essential oil content
Essential oils were extracted from 75 g dry weight of rhizomata cum radicibus of the plant17. The samples were oven-dried at 25 °C for 3–5 days. Rhizomata cum radicibus samples was mixed with 1,000 mL deionized water in a 2-L round-bottomed flask and the essential oil was extracted based on hydrodistillation method by clevenger trap17.
Polysaccharide
The standard method was followed for determine of rhizomata cum radicibus polysaccharides. The 0.6 mL of sugar solution was mixed with the 0.3 mL phenolic solution in screw cap, capped and vortex-stirred tubes. 1.5 mL of H2SO4 (1 M) was added slowly down the side of the tube. The tubes were incubated for 30 min at room temperature (20 °C)11,26. The absorbances was determined at 490 nm using distilled water as blank in a UV–Vis spectrophotometer26,33.
Total phenolic content
Dry and powder rhizomata cum radicibus was analysed for caftaric acid, cholorogenic acid, chicoric acid, cynarin and echinacoside based on standard method13,30. As, 40 mg of sample was transferred into a 15-mL centrifuge vial. The five bio active compounds were extracted with 4 mL of 70% methanol for 15 min using an ultrasonic sonicator13. The vials were centrifuged at 3,000 rpm for 10 min30. After filtration of supernatant by 0.2 μm nylon syringe filter, 300 μL of the filtered extract from each sample were transferred into a glass auto-sampler high performance liquid chromatography (HPLC) vial13,34. The HPLC system consisting of a controller, auto-injector, and a column oven. Samples were injected onto a Phenomenex Luna Qg column (5.0 μm; 4.6 × 150 mm) with a C18 guard column (4 × 3 mm)30. The mobile phase was 0.1% phosphoric acid (A) and acetonitrile (B) at a constant flow rate of 1.3 mL min−113. The standard concentrations of 5, 25, 50, 100, and 200 μg mL−1 was used for standard curves plotting as a function of peak area in HPLC chromatograms13. The quantifications of caftaric acid, cholorogenic acid, chicoric acid, cynarin, and echinacoside were performed on the basis of the peak area of UV absorption at 330 nm with comparison to the standard curves of the authentic sample13.
Total flavonoids content
The analysis of total flavonoid content in rhizomata cum radicibus extracts was performed by colorimetric method35. Briefly, 30 μL of the extract was added to 150 μL of sodium nitrate (5% W/V) followed by the addition of 3 mL of aluminum chloride hexahydrate (10% W/V) and incubated for 5 min35. The 1 mL of NaOH (1.0 M) was added to the mixture and diluted to the mark with distilled water. After incubation at room temperature in dark place for 30 min, the absorbance of the solution was measured at 415 nm by spectrophotometer. For the quantification of TFC, the quercetin (QE) was used as an external standard35.
Anti-radical scavenging activity
The anti-radical scavenging activity of samples was evaluated based on the colorimetric method12. Briefly, 15 μL of methanolic extract was added to 2.0 mL of the 2, 2-diphenyl-1-picrylhydrazyl (DPPH) solution and the mixture was incubated in dark place at 20 °C for 30 min12. Then the absorbance was measured at 517 nm. The following equation was used to calculation of DPPH inhibition12:
| 3 |
where Acontrol and Asample are the absorbance of the control and the sample respectively.
Super oxide anti-radical scavenging activity
Super oxide anti-radical scavenging activity of samples was determined according to standard method23,29. As, 9 mL of 5 mM HCl buffer (pH 8.2) was mixed with 1 mL of the extract. Then, 40 μL of 4.5 mM pyrogallol was added to the mixture. The mixture was shaken for 3 min and the absorbance of the solution was measured at 420 nm by spectrophotometer23. The percentage of scavenging effect was expressed as2:
| 4 |
where A0 is the HCl buffer absorbance and A1is the extract absorbance.
Nitric oxide anti-radical scavenging activity
Nitric oxide anti- radical inhibition can be estimated by using Griess Illosvoy reaction according to standard method19. Briefly, 3 mL of 10 mM sodium nitroprusside and 0.5 mL of phosphate buffer saline solution was incubated at 25 °C for 150 min19. Then, 0.5 mL of the solution mixed with 1 mL of sulfanilic acid reagent and left for 5 min for completing diazotization. A pink coloured chromophore is formed after addition of 1 mL of naphthyl ethylene diamine dihydrochloride in diffused light19. The absorbance of these solutions were measured at 540 nm against. The nitric oxide radical inhabitation was expressed as following equation14:
| 5 |
where Acontrol and Asample are the control and the extract absorbance respectively.
2,2′-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) test
ABTS test is also a spectrophotometric method which is carried out using an improved ABTS decolourisation assay18. It is applicable for both lipophilic and hydrophilic compounds. ABTS+ was generated by oxidation of ABTS with potassium persulfate18. Three milliliter of ABTS cation solution were added to 30 mL methanol extract solution in 1 cm path length disposable micro cuvette and the decrease of absorption was measured during 6 min18.
Statistical analysis
The statistical scheme was a completely simple design, including six treatments (50, 100, 150 mM slow release fertilizer and 50, 100, 150 mM urea) with three replications. Differences among means of treatments were analysed by Duncan’s multiple comparison at p ≤ 0.01. Statistical analysis was performed using statistical analysis software (SAS). All determinations were carried out in triplicate.
Results
Main physiochemical properties of soil sample was given in Table 3. According to results, the soil had neutral reaction without salinity effect (EC less than 2 dS m−1) which is suitable for wide range of nutrients availability20. As well as, it had calcareous nature (more than 5% CaCO3 at topsoil) with sandy clay loam texture. Soil organic matter was at moderate level (1–1%) in agricultural calcareous soils.
Table 3.
Physiochemical properties of studied soil.
| pH | EC | OM | CEC | CaCO3 | Sand | Silt | Clay | Soil texture | |
|---|---|---|---|---|---|---|---|---|---|
| (dS m−1) | (%) | (cmolc kg−1) | (%) | (%) | |||||
| Soil sample | 7.20 | 1.03 | 1.12 | 17.51 | 12.26 | 63 | 16 | 21 | Sandy clay loam |
Macro and micro nutrient concentrations in soil solution were shown in Tables 4 and 5.
Table 4.
Soluble anions and cations concentration in studied soil extract (1:20 soil to distilled water ratio).
| Ca2+ | Mg2+ | K+ | SO42− | PO43− | NH4+ | NO3− | |
|---|---|---|---|---|---|---|---|
| (meq L−1) | |||||||
| Soil sample | 5.50 | 3.32 | 2.51 | 3.78 | 4.93 | 0.73 | 2.45 |
Table 5.
Available concentration and total content of trace elements in studied soil sample.
| B | Cd | Cu | Fe | Mn | Mo | Ni | Pb | Zn | |
|---|---|---|---|---|---|---|---|---|---|
| (mg kg−1) | |||||||||
| Available | 0.65 | 0.10 | 5.04 | 102.50 | 81.57 | 0.15 | 4.36 | 1.09 | 10.72 |
| Total | 1.19 | 0.21 | 46.52 | 2,300.54 | 520.63 | 1.68 | 20.68 | 8.36 | 43.65 |
The results relevant that the different heavy metals content varied depending on the element and fertilizer type. Among heavy metals, Zn had the highest content (maximum 3.1 mg kg−1), whereas Cd showed the lowest (maximum 0.21 mg kg−1) in two types of N fertilizers. The results were in accordance with previous studies13,36. Iron generally does not cause toxicity to plants. However, a high concentration of Fe can decrease P plant availability due to the formation of iron-phosphate salts and it is therefore harmful to plants indirectly24. The content of various heavy metals in all N-fertilizer types were as fallowed: Fe > Mn > Zn > Ni > Cu > Pb > Cd. Among these heavy metals, Cu, Mn, Zn, and Fe are considered as nutritionally essential elements, Ni is classified as an element with possible beneficial health effects22, and Cd and Pb are regarded as elements with potentially toxic and environmentally hazardous effects19. Slow release fertilizer contains more essential element contents than urea (Table 6). The knowledge of trace element and heavy metals content in different chemical fertilizers is useful for estimation of the environmental potential risks resulting from high loading application of fertilizers8. In Table 7, the background mean content of trace elements and heavy metals in worldwide soils are presented21.
Table 6.
Content of trace elements in different types of fertilizers.
| Fertilizer | Cd | Cu | Ni | Pb | Zn | NO3− |
|---|---|---|---|---|---|---|
| (mg kg−1) | (%) | |||||
| Urea | 0.21 | 1.5 | 2.6 | 0.5 | 3.1 | 46 |
| Slow release | 0.01 | 2.1 | 3.8 | 0.06 | 5.4 | 32 |
Table 7.
Mean background contents of trace elements in surface soils (Khalaf et al.12).
| Heavy metals | Background content in soils |
|---|---|
| (mg kg−1) | |
| Cd | 0.41 |
| Cu | 38.90 |
| Fe | 45,000 |
| Mn | 488 |
| Ni | 29 |
| Pb | 27 |
| Zn | 70 |
Cumulative release of NO3− from slow release fertilizer at different concentrations during the time is shown in Fig. 2. Passing the time up to 200 h resulted in an increasing release of NO3− from slow release fertilizer. Increasing the NO3− concentration leads to increase of release rate over time. The highest (130 mM) amount of NO3− released was observed in 150 mM slow release fertilizer (Fig. 2). However, NO3− from the urea chemical fertilizer showed a maximum release (125 mM) within 1 to 2 h (data not shown).
Figure 2.
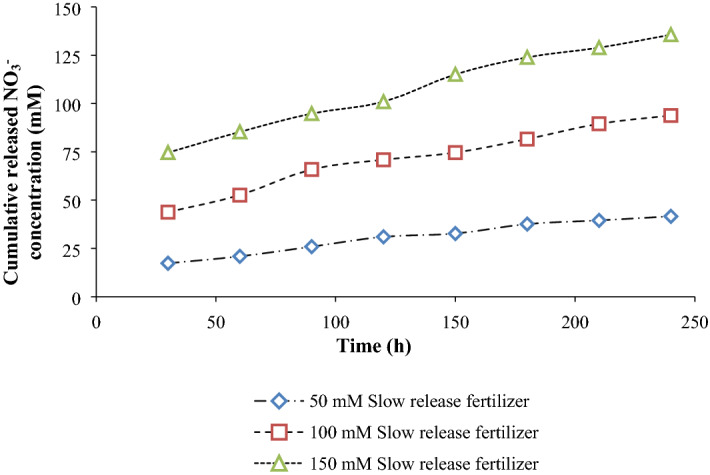
Kinetics of NO3− release from treated soil with slow release fertilizer.
Plant grow parameters of E. purpurea under different two types of fertilizers were shown in Table 8. Increasing of NO3− dosage caused to increase in plant height and rhizomata cum radicibus weight significantly (Table 8). All plant growth parameters were significantly more in slow release fertilizer treatments than urea.
Table 8.
Some morphological properties of E. purpurea growing under different fertilizer treatments.
| Property | Slow release fertilizer | Urea | ||||
|---|---|---|---|---|---|---|
| 50 mM | 100 mM | 150 mM | 50 mM | 100 mM | 150 mM | |
| Height (cm) | 74.60c ± 2.53 | 83.10b ± 2.82 | 105.20a ± 3.21 | 49.30e ± 2.10 | 66.80d ± 2.25 | 71.50c ± 2.60 |
| Total fresh leave weight (g plant−1) | 21.86bc ± 0.28 | 25.67b ± 0.56 | 33.19a ± 1.13 | 14.22d ± 0.11 | 19.23c ± 0.15 | 23.14b ± 0.49 |
| Fresh rhizomata cum radicibus weight (g plant−1) | 41.29c ± 1.15 | 53.20b ± 1.22 | 65.43a ± 1.40 | 16.32e ± 0.75 | 24.10d ± 0.90 | 29.36d ± 0.98 |
| Chlorophyll a (mg g−1 FW) | 12.36c ± 1.23 | 15.24b ± 1.32 | 20.10a ± 1.56 | 9.89e ± 0.62 | 12.10c ± 0.84 | 16.35d ± 0.55 |
| Chlorophyll b (mg g−1 FW) | 4.13bc ± 0.14 | 5.21b ± 0.16 | 7.54a ± 0.26 | 3.10d ± 0.11 | 4.56bc ± 0.14 | 5.19b ± 0.19 |
| Leaf area (cm2) | 38c ± 1.26 | 43b ± 2.26 | 55a ± 2.96 | 22e ± 0.59 | 30d ± 0.91 | 36c ± 1.52 |
Data were expressed as the mean of three replicates ± standard deviation (n = 3).
Values within a row followed by different letters are significantly different at the 0.01 probability level.
The effects of slow release and chemical fertilizers on total phenolic and flavonoid contents, polysaccarides and essential oil content of E. purpurea rhizomata cum radicibus are shown in Table 9. The significant differences were obtained among phytochemical properties in slow release and chemical fertilizers treatments. The highest total phenol was recorded in the 150 mM slow release fertilizer treatment. Increasing of NO3− concentration in slow release fertilizer significantly leads to increase of total phenolics, flavonoids, polysaccarides, and essential oil content.
Table 9.
Phytochemical analysis of E. purpurea rhizomata cum radicibus under various treatments.
| Slow release fertilizer | Urea | |||||
|---|---|---|---|---|---|---|
| 50 mM | 100 mM | 150 mM | 50 mM | 100 mM | 150 mM | |
| Total phenolic compound (GAE g−1 DW) | 47.3c ± 0.31 | 51.6b ± 0.30 | 60.2a ± 0.56 | 12.3f ± 0.52 | 30.6e ± 0.60 | 43.5d ± 0.15 |
| Total flavonoid content (QE g−1 DW) | 19.2cd ± 0.37 | 24.9b ± 0.48 | 32.8a ± 0.21 | 10.5e ± 0.17 | 14.6de ± 0.31 | 22.6bc ± 0.20 |
| Polysaccarides (mg g−1 DW) | 27.6c ± 0.42 | 38.4b ± 0.11 | 50.5a ± 0.75 | 13.9d ± 0.53 | 28.6c ± 0.18 | 36.9b ± 0.47 |
| Essential oil content (% w w−1) | 0.09de ± 0.001 | 0.18c ± 0.01 | 0.29a ± 0.01 | 0.06e ± 0.001 | 0.13d ± 0.01 | 0.24b ± 0.01 |
Data were expressed as the mean of three replicates ± standard deviation (n = 3).
Values within a row followed by different letters are significantly different at the 0.01 probability level.
The HPLC profile of a standard mixture of caffeic acid derivatives is shown in Fig. 3. Results of caffeic acid derivatives content as affected by slow release and chemical fertilizers in dry rhizomata cum radicibus of E. purpurea are shown in Table 10. Caffeic acid derivatives content was affected by fertilizer types and dosages. The dried E. purpurea rhizomata cum radicibus contained more cichoric acid and caftaric acid than other derivatives (Table 10). These results are in agreement with previous reports18,19.
Figure 3.
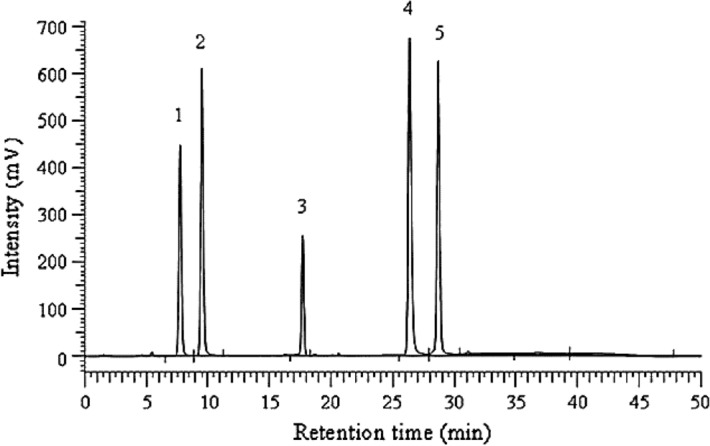
HPLC profile of a standard mixture of caffeic acid derivatives. Peak 1, caftaric acid; 2, chlorogenic acid; 3, echinacoside; 4, chicoric acid; and 5, cynarin.
Table 10.
Caffeic acid derivatives of E. purpurea rhizomata cum radicibus under various treatments.
| Caffeic acid derivatives | Slow release fertilizer | Urea | ||||
|---|---|---|---|---|---|---|
| 50 mM | 100 mM | 150 mM | 50 mM | 100 mM | 150 mM | |
| Caftaric acid (mg g−1 DW) | 2.31d ± 0.11 | 7.13b ± 0.15 | 12.26a ± 0.02 | 0.78e ± 0.02 | 1.06de ± 0.03 | 3.83c ± 0.06 |
| Chlorogenic acid (mg g−1 DW) | 0.23cd ± 0.07 | 0.95b ± 0.02 | 1.36a ± 0.02 | 0.16e ± 0.01 | 0.43d ± 0.01 | 0.73c ± 0.01 |
| Echinacoside acid (mg g−1 DW) | 0.89bc ± 0.01 | 1.32b ± 0.06 | 2.66a ± 0.01 | 0.29d ± 0.01 | 0.75cd ± 0.01 | 0.96bc ± 0.01 |
| Chicoric acid (mg g−1 DW) | 1.23e ± 0.06 | 4.56c ± 0.08 | 7.65a ± 0.65 | 0.74f ± 0.01 | 2.03d ± 0.04 | 5.44b ± 0.09 |
| Cynarin (mg g−1 DW) | 0.68c ± 0.01 | 2.88b ± 0.03 | 5.26a ± 0.04 | 0.43c ± 0.01 | 0.98c ± 0.02 | 3.28b ± 0.05 |
Data were expressed as the mean of three replicates ± standard deviation (n = 3).
Values within a row followed by different letters are significantly different at the 0.01 probability level.
The effects of various different fertilizers on anti-radical scavenging activity of E. purpurea rhizomata cum radicibus were shown in Table 11. The nitric oxide, DPPH, super oxide and ABTS anti-radicals were assesses in the present study.
Table 11.
Different anti-radical scavenging activity of E. purpurea rhizomata cum radicibus under various treatments.
| Anti-radical | Slow release fertilizer | Urea | ||||
|---|---|---|---|---|---|---|
| 50 mM | 100 mM | 150 mM | 50 mM | 100 mM | 150 mM | |
| DPPH (%) | 25.36b ± 0.95 | 28.95ab ± 0.36 | 31.22a ± 0.33 | 12.62d ± 0.36 | 20.31c ± 0.18 | 26.12b ± 0.26 |
| Super oxide (%) | 15.39cd ± 0.73 | 19.21bc ± 0.55 | 23.66b ± 0.65 | 12.36d ± 0.98 | 13.65d ± 0.42 | 28.76a ± 0.14 |
| Nitric oxide (%) | 28.24bc ± 0.68 | 32.19b ± 0.48 | 40.36a ± 0.15 | 19.21d ± 0.23 | 26.25c ± 0.36 | 31.42b ± 0.36 |
| ABTS (%) | 10.23d ± 0.59 | 15.23b ± 0.67 | 20.95a ± 0.29 | 8.26d ± 0.51 | 12.84c ± 0.11 | 16.63b ± 0.47 |
Data were expressed as the mean of three replicates ± standard deviation (n = 3).
Values within a row followed by different letters are significantly different at the 0.01 probability level.
Although there are characterized by excellent reproducibility, but all of them were different in their response to antioxidants under certain conditions. The highest nitric oxide (40.36%), DPPH (31.22%), super oxide (23.66%), and ABTS (20.95%) anti-radical scavenging activities were found in the 150 mM slow release fertilizer treatment, which could be due to higher phenolic content, whereas the lowest various anti-radical scavenging activities were obtained in the 50 mM urea treatment.
Discussion
Previous researches showed that soil CEC was varied as affected by organic matter content4. In fact, increasing of soil CEC results from the increasing of negative charge surface sites on soil colloids due to decomposition of organic matter in pH value above 3 (point of zero charge (PZC) of organic matter)30. Increasing of soil CEC leads to increase of nutrients retention at active soil exchangeable sites which plays critical role in nutrition release and their availability for plant15.
In comparison with E. purpurea nutrition requirement (Table 1), most of essential nutrients (except NO3−) in soil solution were in at adequate range which can supply plant essential macro and micro nutrition requirement without adding any fertilizer during the time. However, NO3− concentration in soil solution was noticeably (more than 3 times) less than E. purpurea requirement that indicates the need to NO3− fertilizers apply in order to increase NO3− level in the studied soil.
Nitrate leaching from agricultural soils is common due to anionic nature of NO3− ion and non-adsorption on negatively charged soil exchangeable sites, which leads to apply of different N fertilizers to soil7. In present study, the soil N deficiency was calculated with regard to E. purpurea N-requirement and provided the optimum level of NO3−concentration for the plant growth by using different N fertilizers containing various percentages of NO3−. Numerous research have reported the harmful influence of soil pollution and heavy metals concentration on E. purpurea growth and medicinal properties32. According to Table 5, the studied soil contains different heavy metals available concentration less than (more than 4 times) dangerous limit in agricultural soils30, which reported the 0.2, 13.0, 6.0, 16.0, and 22.0 mg kg−1 as acceptable limit for available Cd, Cu, Ni, Pb, and Zn in soil respectively.
The data show the lower total concentration of heavy metals in studied soil sample than USEPA limits, which reported the 1.5, 200, 50, 200, and 400 mg kg−1 for Cd, Cu, Ni, Pb, and Zn respectively. So, in the present study, it can be mention that there is no risk of heavy metal pollution for E. purpurea growth. The application of mineral fertilizers which have contaminants of trace elements may impose concern regarding the entry and toxic accumulation of these elements in agro ecosystems37. So, the chemical composition of 3 types of N fertilizers was analyzed for their content of NO3−, HN4+, and heavy metals (Table 6).
By comparing these values with the contents of trace elements in different N fertilizer types (Table 6), it is apparent that the content of all heavy metals in used N fertilizers in present study are lower than their natural background in soils. Therefore, in soils receiving these fertilizers, heavy metals cannot be enriched. So, in present study, the main source of these trace elements are the soil and only the soil can supply these elements for the plant growth mainly. Inspite of micro nutrients, N content of different studied fertilizers was shown in Table 6. Based on obtained results, urea (46% N) had higher NO3− content followed by slow release fertilizer (32%). Different NO3− fertilizers varied significantly in their N content that can affect the plant growth. However, NH4+ content in all N fertilizers changed in narrow range, so at the relatively constant NH4+ content in the soil, the soil NO3− content affects the plant phytochemical properties.
The presence of polymeric compounds used in the slow release fertilizer structure leads to the gradual maintenance and release of NO3− from the fertilizer and these compounds are not present in the urea fertilizer. Gradual release of NO3− from slow release fertilizers results in degradation of NO3− availability for the plant and prevention of leaching and loss of soil.
It is well known that the elasticity of the cell wall plays an important role in cell division6. The osmotic pressure in the cell increase by increasing of NO3− concentration, leading to sufficient swelling pressure for cell division that affect the morphological properties14. Meanwhile, the NO3− dosage can influence growing substrate pH, especially in nutrient recirculating systems18. It has been found that an increase of NO3− dosage caused a significant increase of medicinal and phytochemical compounds of E. purpurea due to improve of rhizomata cum radicibus growth31. Similar results were found by Sidhiq et al. (2020) in E. purpurea plants grown in floating raft system for 21 weeks. Nitrogen is a predominant element in chlorophyll structure. Increasing of NO3− dosage significantly affects the chlorophylls content21. Increasing of chlorophyll content caused to increase of leaf area due to increase of light adsorption36. The results were in consistent with Attarzadeh et al. (2019) who found that higher NO3− dosage resulted in higher leaf area, rhizomata cum radicibus and total biomass.
According to Saeed et al. (2018), accumulation of phenolic compounds in the plant rhizomata cum radicibus prevents NO3− toxicity in plants. There are some reports showing that stress conditions increase either phenylalanine ammonia lyase (PAL) synthesis or activity in plants6,11,12,14,32; other authors24 have found that some stress treatments delay the increase in wound-induced PAL activity. Phenylalanine Ammonia-Lyase activity was found to vary greatly with the stage of plant development18. The results were in accommodation with Zaushintsena et al. (2019). Variation between and among the herb species also exists due to geographical location, stage of development, time of harvest, and growth conditions1.
Caffeic acid derivatives are sensitive to NO3− dosage, and increasing NO3− concentration will result in a significant increase of caffeic acid derivatives in E. purpurea (Table 10). Results showed high caffeic acid derivatives content with slow release fertilizers than urea. It may be due to slow release of NO3− during the time and more availability for plants.
Increasing of NO3− dosage in slow release fertilizer leads to significant increase of all anti-radical scavenging activities. Rhizomata cum radicibus extract of E. purpurea can entrap hydroxyl radicals. This antioxidant mechanism is described as elimination of free radicals and chelating metal ions38. Effective compounds of E. purpurea are alkylamids, polysacharides, glicoproteins and chicoric acid. Chicoric acid is the main phenolic compound with antioxidant effect in E. purpurea. Phenolic compounds like caffeic acid and chlorogenic acid are the most efficient naturally occurring antioxidants. As the result of the presence of chicoric acid in leaf extraction of E. purpurea, this species has the most antioxidant effect among all other4. Beside chicoric acid, typical constituents of E. purpurea extracts are echinacoside, chlorogenic acid, cynarin and caftaric acid. All of them are able affect the free radical production and lipid peroxidation6. Recent studies also relevant that echinacoside and caffeic acid derivative have weak anti-bacterial and antiviral effects and are protectants against reactive oxygen species20,39. The antioxidant activity could be ascribed to the polyphenolic components13, such as flavonoids34, phenolic acids35 or phenolic diterpenes9.
It was found that the concentration of all heavy metals in soil samples and fertilizers studied was less than the USEPA limits of contamination. With this point of view, there were significant differences in morphological and phytochemical properties of E. purpurea with respect to slow release and chemical N fertilizers containing different dosages of NO3−. All morphological and phytochemical properties were higher in the presence of slow release fertilizer than urea chemical fertilizer. It may be due to the polymeric structure of slow release fertilizer that control the N release during the time. Increasing of morphological and phytochemical properties, caffeic acid derivations and anti-radical scavenging activities were observed by increasing of NO3− dosage. With regard to future growth conditions of the plant, it is important to make operational additional research projects for medicinal plants and flowers grown under greenhouse conditions, with the objective of building further confidence in the advantages of the plant cultivation and importance of NO3− concentration control in environmental systems.
Acknowledgements
The authors are thankful to the Office of Vice Chancellor for Research and Technology, Urmia University. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
F.A. conceived the idea and wrote the paper. A.S. and A.R. reviewed the collected data. F.A. was responsible for editing, original data and text preparation. All authors took responsibility for the integrity of the data that is present in this study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Fatemeh Ahmadi, Email: fa.ahmadi@urmia.ac.ir.
Amir Rahimi, Email: e.rahimi@urmia.ac.ir.
References
- 1.Attarzadeh M, Balouchi H, Rajaie M, Dehnavi MM, Salehi A. Growth and nutrient content of Echinacea purpurea as affected by the combination of phosphorus with arbuscular mycorrhizal fungus and Pseudomonas florescent bacterium under different irrigation regimes. J. Environ. Manage. 2019;231:182–188. doi: 10.1016/j.jenvman.2018.10.040. [DOI] [PubMed] [Google Scholar]
- 2.Balciunaite G, et al. Identification of Echinacea purpurea (L.) Moench root LysM lectin with nephrotoxic properties. Toxins. 2020;12:88–105. doi: 10.3390/toxins12020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pretorius TR, Charest C, Kimpe LE, Blais JM. The accumulation of metals, PAHs and alkyl PAHs in the roots of Echinacea purpurea. PLoS ONE. 2018;13:e0208325. doi: 10.1371/journal.pone.0208325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cadiz MP, Schara MR, Kemp BH, Gibbons Johnson RM. Echinacea purpurea root extract increases tumor necrosis factor production by concanavalin A-activated murine splenocytes. J. Med. Food. 2019;3:156–169. doi: 10.1089/jmf.2019.0065. [DOI] [PubMed] [Google Scholar]
- 5.Darvizheh H, Zahedi M, Abbaszadeh B, Razmjoo J. Changes in some antioxidant enzymes and physiological indices of purple coneflower (Echinacea purpurea L.) in response to water deficit and foliar application of salicylic acid and spermine under field condition. Sci. Horticult. 2019;247:390–399. doi: 10.1016/j.scienta.2018.12.037. [DOI] [Google Scholar]
- 6.Khorasaninejad S, Hemmati K. Effects of silicon on some phytochemical traits of purple coneflower (Echinacea purpurea L.) under salinity. Sci. Horticult. 2020;2:114–123. [Google Scholar]
- 7.De Rosa N, et al. Effect of immunomodulatory supplements based on Echinacea Angustifolia and Echinacea Purpurea on the posttreatment relapse incidence of genital condylomatosis: A prospective randomized study. Biomed. Res. Int. 2019;3:245–255. doi: 10.1155/2019/3548396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demirci T, Akçay UÇ, Baydar NG. Effects of 24-epibrassinolide and L-phenylalanine on growth and caffeic acid derivative production in hairy root culture of Echinacea purpurea L. Moench. Acta Physiol. Plant. 2020;42:66–102. doi: 10.1007/s11738-020-03055-7. [DOI] [Google Scholar]
- 9.Haron MH, et al. Plant microbiome-dependent immune enhancing action of Echinacea purpurea is enhanced by soil organic matter content. Sci. Rep. 2019;9:215–230. doi: 10.1038/s41598-018-36907-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoang CK, et al. Impact of nitrogen fertilizer on the mycorrhizal inoculating potential and fungal community structure in rhizosphere of medicinal plant Curcuma longa L. Geomicrobiol. J. 2019;36:385–395. doi: 10.1080/01490451.2018.1556751. [DOI] [Google Scholar]
- 11.Li Y, Kong D, Fu Y, Sussman MR, Wu H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020;148:80–90. doi: 10.1016/j.plaphy.2020.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Khalaf AA, et al. Protective effect of Echinacea purpurea (Immulant) against cisplatin-induced immunotoxicity in rats. DARU. 2019;9:189–199. doi: 10.1007/s40199-019-00265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahmood A, Kataoka R. Metabolite profiling reveals a complex response of plants to application of plant growth-promoting endophytic bacteria. Microbiol. Res. 2020;234:126–135. doi: 10.1016/j.micres.2020.126421. [DOI] [PubMed] [Google Scholar]
- 14.Maggini V, et al. Tissue specificity and differential effects on in vitro plant growth of single bacterial endophytes isolated from the roots, leaves and rhizospheric soil of Echinacea purpurea. BMC Plant Biol. 2019;19:284–296. doi: 10.1186/s12870-019-1890-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samuel DS, Priyadarshoni SP. Echinacea purpurea-A potent medicinal herb. Drug Invent. Today. 2019;11:143–158. [Google Scholar]
- 16.Sidhiq DF, Widiyastuti Y, Subositi D, Pujiasmanto B, Yunus A. Morphological diversity, total phenolic and flavonoid content of Echinacea purpurea cultivated in Karangpandan, Central Java, Indonesia. J. Biol. Divers. 2020;21:225–234. [Google Scholar]
- 17.Sun Y, et al. Negative effects of the simulated nitrogen deposition on plant phenolic metabolism: A meta-analysis. Sci. Total Environ. 2020;19:137–142. doi: 10.1016/j.scitotenv.2020.137442. [DOI] [PubMed] [Google Scholar]
- 18.Sheshbahreh MJ, Dehnavi MM, Salehi A, Bahreininejad B. Effect of irrigation regimes and nitrogen sources on biomass production, water and nitrogen use efficiency and nutrients uptake in coneflower (Echinacea purpurea L.) Agric. Water Manage. 2019;213:358–367. doi: 10.1016/j.agwat.2018.10.011. [DOI] [Google Scholar]
- 19.Soltanian B, Moghaddam PR, Asili J. Effects of water deficit stress and fertilizer sources on morphological characteristics and phenolic compounds in medicinal plant purple coneflower (Echinacea purpurea L.) Iran. J. Med. Aromat. Plants. 2020;36:105–120. [Google Scholar]
- 20.Senica M, Mlinsek G, Veberic R, Mikulic-Petkovsek M. Which plant part of purple coneflower (Echinacea purpurea (L.) Moench) should be used for tea and which for tincture? J. Med. Food. 2019;22:102–108. doi: 10.1089/jmf.2018.0026. [DOI] [PubMed] [Google Scholar]
- 21.Seif Sahandi M, Mehrafarin A, Khalighi-Sigaroodi F, Sharifi M. Changes in essential oil content and composition of peppermint (Mentha piperita L.) in responses to nitrogen application. J. Med. Plants. 2019;2:81–97. [Google Scholar]
- 22.Vidović S, Nastić N, Gavarić A, Cindrić M, Vladić J. Development of green extraction process to produce antioxidant-rich extracts from purple coneflower. Sep. Sci. Technol. 2019;54:1174–1181. doi: 10.1080/01496395.2018.1527854. [DOI] [Google Scholar]
- 23.Verma PPS, Padalia RC, Singh VR, Kumar A, Agri BK. Effect of nitrogen, phosphorus and potassium levels on growth and yield of turmeric (Curcuma longa L.) Under the Katyur valley of western Himalayan region of Uttarakhand. J. Med. Plants. 2019;7:117–122. [Google Scholar]
- 24.Sheshbahreh MJ, Dehnavi MM, Salehi A, Bahreininejad B. Physiological and yield responses of purple coneflower (Echinacea purpurea (L.) Moench) to nitrogen sources at different levels of irrigation. Physiol. Mol. Biol. Pla. 2019;25:177–187. doi: 10.1007/s12298-018-0611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bensalem N, et al. The interactive effect of nitrate/ammonium ratio and sodium chloride on Tunisian medicinal plant (Nigella sativa L) J. Plant Nutr. 2020;4:1–13. [Google Scholar]
- 26.Yetilmezsoy K, Kocak E, Akbin HM, Özçimen D. Utilization of struvite recovered from high-strength ammonium-containing simulated wastewater as slow-release fertilizer and fire-retardant barrier. Environ. Technol. 2020;41:153–170. doi: 10.1080/09593330.2018.1491642. [DOI] [PubMed] [Google Scholar]
- 27.Ye HM, et al. Degradable polyester/urea inclusion complex applied as a facile and environment-friendly strategy for slow-release fertilizer: Performance and mechanism. Chem. Eng. J. 2020;381:122–130. doi: 10.1016/j.cej.2019.122704. [DOI] [Google Scholar]
- 28.Rudmin M, et al. Mechanochemical preparation of slow release fertilizer based on glauconite-urea complexes. Minerals. 2019;9:507–516. doi: 10.3390/min9090507. [DOI] [Google Scholar]
- 29.Zhou Z, Pan S, Wu S. Modulation of the growth performance, body composition and nonspecific immunity of crucian carp Carassius auratus upon Enteromorpha prolifera polysaccharide. Int. J. Biol. Macromol. 2020;14:152–163. doi: 10.1016/j.ijbiomac.2020.01.065. [DOI] [PubMed] [Google Scholar]
- 30.Merajipoor M, Dehnavi MM, Salehi A, Yadavi A. Improving grain yield, water and nitrogen use efficiency of Nigella sativa with biological and chemical nitrogen under different irrigation regimes. Sci. Horticult. 2020;260:108–123. doi: 10.1016/j.scienta.2019.108869. [DOI] [Google Scholar]
- 31.Wang C, et al. Slow-release fertilizer improves the growth, quality, and nutrient utilization of wintering chinese chives (Allium tuberosum Rottler ex Spreng.) Agronomy. 2020;10:381–392. doi: 10.3390/agronomy10030381. [DOI] [Google Scholar]
- 32.Kilic S, Soylak M. Determination of trace element contaminants in herbal teas using ICP-MS by different sample preparation method. J. Food Sci. Technol. Mys. 2020;57:927–933. doi: 10.1007/s13197-019-04125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.El-Serafy RS, El-Sheshtawy AA. Effect of nitrogen fixing bacteria and moringa leaf extract on fruit yield, estragole content and total phenols of organic fennel. Sci. Horticult. 2020;265:105–123. doi: 10.1016/j.scienta.2020.109209. [DOI] [Google Scholar]
- 34.Mahmoed E, et al. Effect of Chlorella vulgaris enriched diet on growth performance, immune response, antioxidant and transcriptomics RNA sequences disorders caused by deltamethrin toxicity in Nile tilapia (Oreochromis niloticus) Fish Shellfish Immunol. 2020;12:56–68. doi: 10.1016/j.fsi.2020.04.061. [DOI] [PubMed] [Google Scholar]
- 35.Wold CW, Gerwick WH, Wangensteen H, Inngjerdingen KT. Bioactive triterpenoids and water-soluble melanin from Inonotus obliquus (Chaga) with immunomodulatory activity. J. Funct. Foods. 2020;71:104–115. doi: 10.1016/j.jff.2020.104025. [DOI] [Google Scholar]
- 36.Waidyanatha S, et al. A strategy for test article selection and phytochemical characterization of Echinacea purpurea extract for safety testing. Food Chem. Toxicol. 2020;11:525–602. doi: 10.1016/j.fct.2020.111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sikora J, et al. Assessment of the efficiency of nitrogen slow-release fertilizers in integrated production of carrot depending on fertilization strategy. Sustainability. 2020;12:198–206. doi: 10.3390/su12051982. [DOI] [Google Scholar]
- 38.Yao L, et al. Screening and evaluation of adventitious root lines of Panax notoginseng by morphology, gene expression, and metabolite profiles. Appl. Microbiol. 2019;103:4405–4415. doi: 10.1007/s00253-019-09778-z. [DOI] [PubMed] [Google Scholar]
- 39.Cui XH, et al. Effect of nutritional factors on the accretion of secondary metabolites in Malaysian ginseng adventitious root cultures. Plant Biotechnol. Rep. 2019;15:1–6. [Google Scholar]