Abstract
Introduction
The new initiative by the Department of Health and Human Services (DHHS) aims to decrease new HIV infections in the U.S. by 75% within 5 years and 90% within 10 years. Our objective was to evaluate whether the U.S. military provides a good example of the benefits of such policies.
Materials and methods
We conducted an analysis of a cohort of 1,405 active duty military personnel with HIV enrolled in the Natural History Study who were diagnosed between 2003 and 2015 at six U.S. military medical centers. The study was approved by institutional review boards at the Uniformed Services University of the Health Sciences and each of the sites. We evaluated the impact of Department of Defense (DoD) HIV care policies, including screening, linkage to care, treatment eligibility, and combined antiretroviral therapy (cART) initiation on achieving viral suppression (VS) within 3 years of diagnosis. As a secondary outcome, we evaluated the DoD’s achievement of UNAIDS 90-90-90 targets.
Results
Nearly all (99%) were linked to care within 60 days. Among patients diagnosed in 2003–2009, 77.5% (95% confidence intervals (CI) 73.9–80.6%) became eligible for cART within 3 years of diagnosis, 70.6% (95% CI 66.6–74.1%) overall initiated cART, and 64.2% (95% CI 60.1–68.0%) overall achieved VS. Among patients diagnosed in 2010–2015, 98.7% (95% CI 96.7–99.5%) became eligible for cART within 3 years of diagnosis, 98.5% (95% CI 96.4–99.4%) overall initiated cART, and 89.8% (95% CI 86.0–92.5%) overall achieved VS.
Conclusions
U.S. military HIV policies have been highly successful in achieving VS goals, exceeding the UNAIDS 90-90-90 targets. In spite of limitations, including generalizability, this example demonstrates the feasibility of the DHHS initiative to decrease new infections through testing, early treatment, and retention in care.
INTRODUCTION
With the introduction of combined antiretroviral therapy (cART), there have been marked reductions in both transmission of human immunodeficiency virus (HIV) and poor clinical outcomes among individuals with HIV.1,2 However, even within resource-rich countries, many individuals with HIV remain unaware of their status because of lack of universal screening. Since 1985, the U.S. military has been testing potential recruits for HIV and has implemented a force-wide active duty HIV screening program, with testing approximately every 2 years to assure delivery of appropriate medical care and protect the military’s unique walking blood bank.3,4 This policy has facilitated early diagnosis of HIV infection; specialist care linkage within 60 days for newly diagnosed personnel is directed by another policy.5–7 Together, these policies provide individual-level and population-based focus to maximize health and reduce transmission.
In the United States, HIV care in civilian populations can be complicated by differences in health status, healthcare access, insurance, and delayed presentation to care. Few U.S. populations offer the opportunity to study the effects of broad testing and a universal healthcare system, though recent research evaluating whether patients with HIV within the Kaiser Permanente system met outcome measures provides a detailed examination of a well-defined patient population.8 Healthcare access is universal for active duty military personnel, and the baseline health status of military personnel is generally without chronic medical conditions. Furthermore, because of biennial screening, the HIV status of all service members is largely known and new infections are identified relatively quickly.
Globally, the United Nations Joint Program on HIV/AIDS (UNAIDS) has promoted the 90-90-90 approach to end the AIDS epidemic, which aims to diagnose 90% of all infected individuals and notify them of their status, initiate cART for 90% of those with a known HIV diagnosis and achieve viral suppression (VS) in 90% of those initiating cART worldwide.9 Mathematical models suggest that if 73% (ie 90 × 90 × 90) of individuals with HIV worldwide have viral load of HIV RNA < 1,000 copies/mL, the basic reproductive number (R0) will fall below 1.0 and HIV transmission will gradually be extinguished.9 Achieving the UNAIDS target requires public health policymakers and clinicians to increase efforts of identifying, tracking, and retaining patients in care and demands patients adhere to treatment and remain engaged in care.9 Centers for Disease Control and Prevention recently estimated that about 80% of new HIV transmissions in the Unites States are from individuals who are either not linked to care or unaware of their status, while no transmissions were identified from virologically suppressed individuals.10 A continued effort to improving care linkages, treatment initiation, and achieving VS will prove vital to ending the epidemic.9
However, the analytical approach in evaluating the HIV cascade of care stages, including identification, treatment initiation, and VS, may have an impact on the findings. In fact, analyses of cohorts and their achievement in reaching the UNAIDS targets often do not account for mortality or attrition from the cascade of care. The UNAIDS 90-90-90 targets use a conditional cross-sectional analysis approach.11 While analyzing subsequent stages conditioned on achievement of previous stages follows the strategy employed by UNAIDS models,9 prior research has suggested that cross-sectional analyses of the cascade of care can lead to misleading inference.12
While studies on engagement in care have been conducted on U.S. veteran and military communities13,14 there have been no detailed studies aimed at quantifying the effectiveness of military HIV care at achieving the UNAIDS 90-90-90 targets. In this study, we quantified the percentage of participants with HIV who were linked to care, were eligible for and initiated cART, and achieved VS within 3 years from date of diagnosis. Furthermore, to address the inherent limitations of cross-sectional approaches in evaluating achievements in meeting UNAIDS 90-90-90 targets, we also implemented a longitudinal approach to the cascade based on the rate at which people transition through stages.
Methods
Study Population
The study population was all active duty military personnel with HIV in the U.S. Military HIV Natural History Study (NHS) diagnosed between 2003 and 2015. As previously described, NHS is an open-enrollment cohort with patient evaluation approximately every 6 months.15 All participants provide written informed consent. The protocol was approved by the institutional review board at the Uniformed Services University of the Health Sciences and the six participating sites (Madigan Army Medical Center, Naval Medical Center San Diego, Tripler Army Medical Center, San Antonio Military Medical Center, Naval Medical Center Portsmouth, and Walter Reed National Military Medical Center). We censored participants upon discharge from active duty or last study visit.
Setting
Department of Defense (DoD) policy mandates annual medical evaluation of active duty personnel with HIV,4,16 and each command is responsible to ensure its personnel are compliant with the policy.16 HIV care for personnel follows Department of Health and Human Services (DHHS) and other U.S. guidelines.17 Though there are small differences in service delivery models, the standards of care across all military branches are similar. Retention in military service is based on the service member’s health and ability to perform duties.
Data Collection
HIV-1 plasma viral load and CD4 counts are measured at study visits and captured from interim clinical encounters through extraction from the centralized Military Healthcare System Electronic Medical Record. Additionally, we collected data from the NHS database and Military Healthcare System records on demographics, cART history, and clinical outcomes.18
Statistical Analyses
We considered the following items as sequential stages: HIV infection, linkage to care, eligibility for cART based on DHHS guidelines, cART initiation, and VS (Table I). Each participant’s treatment eligibility was time varying from late 2009 to late 2015, based on a participant’s last-known CD4 or a history of an opportunistic infection, and determined by the guidelines at the time.
Table I.
Cascade of Care Stage Definitions
| Cascade stage | |
|---|---|
| HIV diagnosis | Due to universal, at least biennial HIV testing, the knowledge of HIV status is nearly 100%. |
| Linkage to care | Record of an infectious disease clinic visit within 60 days of the first positive HIV test. |
| cART eligibility (based on guidelines) | Before October 31, 2009: Any CD4 measure < 200 or a history of OI. |
| October 31, 2009–December 31, 2012: Any CD4 measure < 350 or a diagnosis of OI. (19, 20) | |
| January 1, 2013–August 31, 2015: Any CD4 measure < 500 or a diagnosis of OI. (21, 22) | |
| September 1, 2015 and later: All HIV+ considered cART eligible. (19, 23) | |
| cART initiation | Eligible for cART with a record of cART prescribed after eligibility. |
| Viral suppression | cART prescribed and one or more viral load measures < 200 copies/mL. |
cART: combined antiretroviral therapy; OI: opportunistic infections
We analyzed the cascade of care stages by using two approaches as recommended by the World Health Organization.11 First, we performed a cross-sectional cascade analysis in which all participants with HIV who were diagnosed within a given era were included in the denominator for each stage. An advantage of this analysis is high internal consistency.24 In this approach, we are using a denominator-numerator linkage and we assumed the quality of the linked data as high because they are derived from electronic medical databases for military personnel. Furthermore, no data were self-reported but rather obtained from NHS visits and medical records, thus limiting the biases from conditional linkages.24 Treatment guidelines changed frequently after 2009, affecting the proportion of participants eligible for treatment; therefore, we stratified the estimates into 2003–2009 and 2010–2015 eras. Specifically, we used the last-known cascade stage reached at the end of each era (ie 2003–2009 and 2010–2015) to calculate the proportion of personnel reaching that stage, ie we divided the total number of personnel with HIV who have reached each stage by the total number of active duty personnel with HIV who contributed follow-up to the era.
We also conducted a longitudinal cascade analysis using an approach previously described by Haber and colleagues12 in which we used Kaplan-Meier estimates of the proportion of personnel who within 3 years of diagnosis reached each stage (as recommended by the World Health Organization11, secondary analyses explored outcomes within 1 and 2 years), censoring due to time end of data collection and loss to follow-up because of discharge. Kaplan-Meier estimates were also used to help illustrate potentially misleading inference obtained from the cross-sectional cascade approach. To be considered eligible for a stage, we assumed sequential success. For example, to be included in the cART-initiation stage, the individual also needed to be eligible for cART, as per the treatment initiation guidelines at the time. Because participants often transitioned through multiple stages on the same day (eg linked to care, cART eligibility identified, and cART initiated), they were included in the denominator of each stage. This approach has been previously employed12 to maintain the conditional linkages between cascade stages.
We defined VS as < 200 copies/mL per DHHS standards,19 though this is more rigid than the World Health Organization definition of < 1,000 copies/mL.11 For the cross-sectional cascade analysis, the last viral load prior to the end of each era was used to determine VS. For the longitudinal cascade analysis, the lowest viral load observed during follow-up after cART initiation was used to determine VS.
In an attempt to determine whether the military met UNAIDS 90-90-90 targets, we used a conditional cross-sectional approach according to international guidelines.11 However, previous studies have demonstrated that cross-sectional analyses of the cascade of care can lead to misleading inference about the location and severity of issues across the cascade, related both to accumulation of events over time and to mortality-shrinking denominators in the cascade.12 To address this, we implemented a longitudinal approach to the cascade based on the rate at which people transition through stages. This approach utilizes Kaplan-Meier estimates of time to transitions between stages, censoring at the point of discharge and/or end of data collection.
We report Kaplan-Meier estimates with 95% CI. If we report medians, we also report the respective interquartile range (IQR). We performed all analyses in R.25 Longitudinal cascades were generated and estimated using the package longitudinalcascade.26
Results
From 2003 to 2015, 1,405 active duty personnel with HIV were newly diagnosed and enrolled in the NHS. Most participants were male (97.0%) and either African-American (42.8%) or Caucasian (36.4%) (Table II). Nearly 90% were enlisted (88.7%), and the median age at diagnosis was 27 years (IQR: 23–34). Of the participants who were diagnosed from 2003 to 2009 (n = 860), the median time from diagnosis to first HIV clinic visit was 21 days (IQR = 12–38), median time to cART initiation from eligibility determination was 3 days (IQR = 0–198), and the median time to VS was 81 days (IQR = 28–168) from treatment initiation. Among participants diagnosed between 2010 and 2015 (n = 545), the median time from diagnosis to first HIV clinic visit was 16 days (IQR = 9–28), median time to cART initiation from eligibility determination was 0 days (IQR = 0–30), and the median time to VS was 61 days (IQR = 6–145) from cART initiation.
Table II.
Demographics of U.S. Military HIV Natural History Study (n = 1,405)
| Characteristic | n (%) |
|---|---|
| Gender | |
| Male | 1,363 (97.0) |
| Female | 42 (3.0) |
| Race | |
| African American | 601 (42.8) |
| Caucasian | 512 (36.4) |
| Hispanic | 175 (12.5) |
| Other | 117 (8.3) |
| Rank | |
| Enlisted | 1,246 (88.7) |
| Officer/warrant officer | 151 (10.7) |
| Missing/unknown | 8 (<1.0) |
| Age (years) at diagnosis, median (IQR) | 27 (23–34) |
| Service | |
| Navy | 588 (41.9) |
| Air Force | 319 (22.7) |
| Army | 314 (22.3) |
| Marines | 127 (9.0) |
| Other | 56 (4.0) |
| 2003–2009 (n = 860) | |
| Median timea (IQR) to first ID visit, days | 21 (12–38) |
| Median timea (IQR) to cART initiation (eligibles), days | 3 (0–198) |
| Median timea (IQR) to VS (those initiating cART), days | 81 (28–168) |
| 2010–2015 (n = 545) | |
| Median timea (IQR) to first ID visit, days | 16 (9–28) |
| Median timea (IQR) to cART initiation (eligibles), days | 0 (0–30) |
| Median timea (IQR) to VS (those initiating cART), days | 61 (6–145) |
cART: combined antiretroviral therapy; IQR: interquartile range
aTime measured in days from previous stage.
Cascade of Care
Cross-Sectional Cascade Analysis
Among participants diagnosed from 2003 to 2009, 99% (850/860) were linked to care, 67% (575/860) met criteria for cART initiation by the end of 2009, 56% (481/860) initiated cART by the end of 2009, and 44% (381/860) achieved VS (Fig. 1, panel a). In contrast, among participants diagnosed in 2010–2015, 100% (545/545) were linked to care, 91% (495/545) met criteria for cART initiation by the end of 2015, 87% (476/545) initiated cART by the end of 2015, and 81% (440/545) achieved VS (Fig. 1, panel a).
Figure 1.
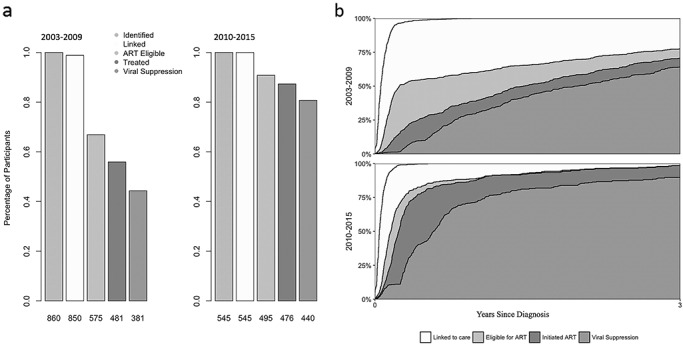
a. Cross-sectional cascade of HIV care trends over time by year of diagnosis contrasted with longitudinal Kaplan-Meier estimates, U.S. Military HIV Natural History Study Cohort, 2003–2015(n = 1405). Note: Kaplan-Meier estimates with 95% confidence intervals from the longitudinal cascade analysis displayed for reference in 2003–2009 and 2010–2015 time periods. b. Longitudinal cascade of HIV Care Trends Since Diagnosis Among U.S. Military HIV Natural History Study Cohort (n = 1405). Note: The curves represent Kaplan-Meier estimates of time to event from the start time of diagnosis, censoring at time of discharge and last date of data collection for the cohort. Each color represents the cumulative incidence of reaching a particular cascade stage following the identification.
Longitudinal Cascade Analysis
All study participants were linked to care within 3 years of diagnosis. We illustrate the speed at which VS was achieved from time of diagnosis in Figure 1, panel b and Table III. The participants diagnosed between 2010 and 2015 had better outcomes than the participants diagnosed between 2003 and 2009. We estimated 77.5% (95% CI: 73.9, 80.6%) of those diagnosed from 2003 to 2009 became eligible for cART within 3 years of diagnosis, while 98.7% (95% CI: 96.7, 99.5%) of those diagnosed from 2010 to 2015 became eligible for cART within 3 years of diagnosis. About, 70.6% (95% CI: 66.6, 74.1%) of those diagnosed from 2003 to 2009 initiated cART, and 98.5% (95% CI: 96.4, 99.4%) of those diagnosed from 2010 to 2015 initiated cART within 3 years. Lastly, 64.2% (95% CI: 60.1, 68.0%) of participants diagnosed from 2003 to 2009 achieved VS within 3 years, while 89.8% overall (95% CI: 86.0, 92.5%) of those diagnosed from 2010 to 2015 achieved VS within 3 years.
Table III.
Longitudinal Cascade of HIV Care Trends Over Time by Year of Diagnosis (2003–2015), U.S. Military HIV Natural History Study Cohort (n = 1,405)
| % Who reached stage within 3 years of diagnosis (95% CI) | ||
| 2003–2009 a | 2010–2015 a | |
| Linked to care | 100 (NA) | 100 (NA) |
| cART eligible | 77.5 (73.9–80.6) | 98.7 (96.7–99.5) |
| cART initiation | 70.6 (66.6–74.1) | 98.5 (96.4–99.4) |
| Viral suppression | 64.2 (60.1–68.0) | 89.8 (86.0–92.5) |
| % Who reached stage within 1 year of diagnosis (95% CI) | ||
| 2003–2009 a | 2010–2015 a | |
| Linked to care | 100 (99.0–100) | 99.8 (98.7–100) |
| cART eligible | 60.2 (56.4–63.7) | 89.2 (86.0–91.7) |
| cART initiation | 39.5 (35.7–43.2) | 88.0 (84.6–90.6) |
| Viral suppression | 30.5 (26.8–34.0) | 71.7 (67.3–75.6) |
| % Who reached stage within 2 years of diagnosis (95% CI) | ||
| 2003–2009 a | 2010–2015 a | |
| Linked to care | 100 (99.0–100) | 100 (98.7–100) |
| cART eligible | 69.7 (65.9–73.0) | 95.4 (92.9–97.1) |
| cART initiation | 58.5 (54.4–62.2) | 94.9 (92.2–96.6) |
| Viral suppression | 50.2 (46.0–54.0) | 84.6 (80.7–87.7) |
cART: combined antiretroviral therapy; CI: confidence interval
aKaplan-Meier estimates.
We also evaluated cascade achievement within 1 and 2 years of diagnosis using the Kaplan-Meier estimates (Table III). Nearly all subjects were linked to care within 1 year. Among participants diagnosed from 2003 to 2009, 60.2% (95% CI: 56.4, 63.7%) became cART eligible within 1 year of diagnosis. However, among participants diagnosed from 2010 to 2015, 89.2% (95% CI: 86.0, 91.7%) became cART eligible within 1 year of diagnosis. Among participants diagnosed from 2003 to 2009, 39.5% (95% CI: 35.7, 43.2%) initiated cART, while 88.0% (95% CI: 84.6, 90.6%) diagnosed from 2010 to 2015 initiated cART. Lastly, 30.5% (95% CI: 26.8, 34.0%) of participants diagnosed from 2003 to 2009 achieved VS within 1 year of HIV diagnosis, though 71.7% (95% CI: 67.3, 75.6%) of participants diagnosed from 2010 to 2015 achieved VS within 1 year of HIV diagnosis. Within 2 years of diagnosis, 69.7% (95% CI: 65.9, 73.0%) of participants diagnosed from 2003 to 2009 became eligible for cART; among participants diagnosed from 2010 to 2015, 95.4% (95% CI: 92.9, 97.1%) became eligible for cART within 2 years of diagnosis. Among those diagnosed from 2003 to 2009, 58.5% (95% CI: 54.4, 62.2%) initiated cART, though 94.9% (95% CI: 92.2, 96.6%) of those diagnosed from 2010 to2015 initiated cART. Lastly, 50.2% (95% CI: 46.0, 54.0) of participants diagnosed from 2003 to 2009 achieved VS within 2 years, while 84.6% (95% CI: 80.7, 87.7) of participants diagnosed from 2010 to 2015 achieved VS within 2 years.
To identify health system gaps over time, we illustrate the cumulative incidence of transitioning across and between stages in Supplementary Figure 1. The y-intercept represents the proportion of skipped and simultaneous transitions across multiple stages. The rates of transition were faster in the 2010–2015 era. Patients diagnosed in the 2003–2009 era were usually linked to care within a few weeks (median = 21 days; IQR: 12–38); patients diagnosed from 2010 to 2015 were typically linked to care within approximately 2 weeks (median = 16 days; IQR: 9–28). For patients diagnosed from 2003 to 2009, from linkage to eligibility for cART usually occurred within 1 month (median = 29 days; IQR: 14–386). Similarly, among those diagnosed in the 2010–2015 era, from linkage to care to eligibility for cART usually occurred within 1 month (median = 29 days; IQR: 14–65). Additionally, most patients who were eligible for cART in 2003–2009 usually initiated treatment within a few days of eligibility (median = 3 days; IQR: 0–198), and patients who were eligible for cART in the 2010–2015 era usually initiated cART immediately upon determination of eligibility (median = 0 days; IQR: 0–30). Lastly, patients who initiated cART in the 2003–2009 era usually achieved VS within 3 months (median = 81 days; IQR: 28–168), while patients who initiated cART between 2010 and 2015 typically achieved VS within about 2 months (median = 61 days; IQR: 6–145).
UNAIDS 90-90-90 Targets
To determine whether the military met UNAIDS 90-90-90 targets, we focused specifically on the identification, cART initiation, and VS stages among active duty personnel on the last day of each era. Using the cross-sectional data and conditionalities defined by UNAIDS,9 by the end of 2009 56% of those diagnosed initiated cART (481/860) and of those on cART by the end of 2009 79% achieved VS (381/481) (Fig. 1, panel a). In contrast, 87% of those diagnosed with HIV by the end of 2015 initiated cART (476/545), and a larger proportion of treated participants in the 2010–2015 era achieved VS by the end of 2015 (92%; 440/476). Using a different analytical approach, by including time in the analyses using Kaplan-Meier estimates, we demonstrate rapid transitions between stages. Specifically, 98.5% (95% CI: 96.4, 99.4%) of those diagnosed from 2010–2015 initiated cART and 90.3% (95% CI: 87.3, 92.5%) of those who initiated cART achieved VS within 3 years.
Discussion
In this cohort of newly diagnosed active duty military personnel with HIV, DoD HIV care policies have resulted in linkage to care within 60 days for nearly everyone. cART was initiated promptly among a large proportion of eligible participants, and military practices were updated to reflect guidelines for earlier cART initiation. Treatment has been highly successful; the achievement of VS in this cohort has continued to increase by multiple measures and, as of 2015, approached or exceeded the UNAIDS target of 90-90-90.
The strategies for prevention of HIV and treatment employed to optimize the cascade of care within the DoD may prove valuable in civilian populations. Some of these strategies in the DoD include pre-exposure prophylaxis services27 and policy-based linkage to universal, open access to care. U.S. communities have experienced decreased HIV incidence has decreased in U.S. communities after implementing treat-all policies,28,29 a treatment as prevention strategy with strong evidence established from HPTN 05230 and observational study data.2 Furthermore, the principle of undetectable (viral load) = untransmittable (U=U) was recently highlighted by Fauci and colleagues31 and is the roadmap for the new initiative by DHHS to decrease new infections by 75% within 5 years and 90% within 10 years.31 This ambitious strategy is the first of its kind in the Unites States, with coordination from multiple federal agencies focused on populations of interest at higher risk for HIV from which most new cases are diagnosed or on rural areas with higher than expected incidence. To achieve this objective, four pillars have been established: (1) diagnosing HIV early in all infected, (2) initiating treatment rapidly to achieve VS, (3) introducing or expanding pre-exposure prophylaxis particularly to those who are at high risk, and (4) identifying and responding to HIV clusters.31 U.S. military HIV care policies reflect these principles and may provide an archetype of what is possible to accomplish in achieving these pillars nationwide.
We found that the analytical approach substantially changed the interpretation and inference made from our results, highlighting some of the key limitations in the cross-sectional construction of the UNAIDS 90-90-90 goals. Using the cross-sectional recommendations for the UNAIDS 90-90-90 targets, we found that by the end of 2015, 87% of those diagnosed with HIV initiated cART, and of those on cART, 92% achieved VS. Using this analysis, we might conclude that the cohort did not meet those goals and that there are remaining gaps in healthcare coverage related to HIV/AIDS. However, that conclusion may largely be an artifact of analyses of outcomes that fail to consider time, as demonstrated by the rapid transitions observed in analyses, which include time. Kaplan-Meier estimates suggest that among participants diagnosed in 2010–2015, 98.5% initiated cART and 90.3% of those who initiated cART achieved VS within 3 years. The high rate of treatment initiation and early transition may be partly because of the military’s obligation to remain mission ready as well as the health-seeking behaviors of an active duty population.
Limitations
Our study has limitations. First, a participant could have achieved VS before this was ascertained in the cohort setting. Thus, all estimated survival functions for the time until VS gives a conservative upper bound. Though the NHS study schedule follows the standard military HIV care schedule, there may be differences in motivation with regard to health-seeking behaviors between those who choose to enroll in NHS and those who do not. NHS participants who leave the military prior to retirement (~3% per year) are not eligible for continued care or study visits with the DoD and may differ in health-related behaviors compared with those who remain.
Conclusion
In this cohort of active duty military personnel newly diagnosed with HIV, military care policies have resulted in linkage to care within 60 days for nearly everyone. cART was initiated promptly for more than 98% of personnel, and the rates of VS have continued to increase over time, exceeding 90% among those treated in 2010–2015. Regardless of metric, U.S. military care has approached or exceeded the UNAIDS targets. The benefits of focused testing and universal access to healthcare, as evidenced in the military’s integrated, open-access healthcare system, provide an example of what might be achieved at a national level with similar policies (see the Supplementary Panel for an overview of the study objectives, findings, and future considerations).
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank Xiuping Chu for her support during the data collection and analysis phases. We would also like to thank Camille Estupigan for her editorial assistance during preparation of this manuscript. This study was conducted by the Infectious Disease Clinical Research Program (IDCRP), a DoD program executed by the USUHS through a cooperative agreement with the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. (HJF).
We thank the following members of the Infectious Disease Clinical Research Program HIV Working Group for collecting and reviewing study data and ensuring effective protocol operations:
Brooke Army Medical Center, Fort Sam Houston, TX: S. De Leon; S. Merritt; T. Merritt; Lt Col J. Okulicz; T. Sjoberg.
Madigan Army Medical Center, Joint Base Lewis McChord, WA: C. Baker; S. Chambers; R. Colombo; COL T. Ferguson; LTC A. Kunz; C. Schofield; M. Stein.
National Institute of Allergy and Infectious Diseases, Bethesda, MD: J. Powers; COL (Ret.) E. Tramont.
Naval Medical Center Portsmouth, Portsmouth, VA: S. Banks; CAPT K. Kronmann; T. Lalani; R. Tant; T. Warkentien.
Naval Medical Center San Diego, San Diego, CA: S. Cammarata; N. Kirkland; CAPT R. Maves; CAPT (Ret.) G. Utz.
Tripler Army Medical Center, Honolulu, HI: COL M. Price.
Uniformed Services University of the Health Sciences, Bethesda, MD: B. Agan; X. Chu; W. Horton; H. Hsieh; A. Noiman; E. Parmelee; D. Tribble; X. Wang; S. Won.
Walter Reed Army Institute of Research, Silver Spring, MD: T. Crowell; S. Peel.
Walter Reed National Military Medical Center, Bethesda, MD: I. Barahona; LTC J. Blaylock; C. Decker; A. Ganesan; COL R. Ressner; D. Wallace.
Contact: Andrew Anglemyer, PhD, Department of Operations Research, Naval Postgraduate School, 1 University Circle, Monterey, CA 93943. Email: atanglem@nps.edu
The views expressed are those of the authors and do not reflect the official views of the Uniformed Services University of the Health Sciences, Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc., Brooke Army Medical Center, Walter Reed National Military Medical Center, Naval Medical Center San Diego, Madigan Army Medical Center, Naval Medical Center Portsmouth, Armed Forces Health Surveillance Branch, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of Defense, or the Departments of the Army, Navy or Air Force. Mention of trade names, commercial products, or organizations does not imply endorsement by the U.S. Government. The investigators have adhered to the policies for protection of human subjects as prescribed in 45CRF46.
Funding
This work was supported in whole, or in part, with federal funds from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH), under Inter-Agency Agreement Y1-AI-5072. The funding source was not involved in the design, collection, analysis, or interpretation of the data.
References
- 1. Cain, Cole S, Chmiel J et al. : Effect of highly active antiretroviral therapy on incident AIDS using calendar period as an instrumental variable. Am J Epidemiol. 2009; 163(4): 310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anglemyer A, Horvath T, Rutherford G: Antiretroviral therapy for prevention of HIV transmission in HIV-discordant couples. JAMA 2013; 310: 1619–20. [DOI] [PubMed] [Google Scholar]
- 3. Department of Defense Instruction on medical standards for appointment, enlistment, or induction into the military services. 2018. Available at https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/DODI_6130.03_JUL12.pdf; accessed March 4, 2020.
- 4. Department of Defense Department of defense instruction on HIV screening. 2013. Available at http://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/648501p.pdf. ; accessed March 4, 2020.
- 5. Department of the Army Identification, surveillance, and administration of personnel infected with human immunodeficiency virus. Army Regulation 600–110. Headquarters, Department of the Army, 2014. Available at https://www.army.mil/e2/downloads/rv7/r2/policydocs/r600_110.pdf; accessed March 4, 2020.
- 6. Department of the Navy Management of human immunodeficiency virus, hepatitis b virus, and hepatitis c virus infections in the navy and marine corps. Instruction 5300.30E. Department of the Navy, 2012. Available at http://www.public.navy.mil/surfor/IDCorpsmen_Docs/Hepatitis_B_C_HIV_INST_5300.30E.pdf; accessed March 4, 2020.
- 7. Secretary of the Air Force Human Immunodeficiency Virus Program. Instruction 44–178. Headquarters, US Air Force, 2014. Available at http://static.e-publishing.af.mil/production/1/af_sg/publication/afi44-178/afi44-178.pdf; accessed March 4, 2020.
- 8. Horberg M, Blank J, Rubenstein K et al. : Beyond viral suppression: broadening quality measures (QM) for Total HIV patient care. CROI 2018; Poster, 1127 Available at http://www.croiconference.org/sites/default/files/uploads/croi2018-abstract-ebook.pdf. Accessed March 4, 2020. [Google Scholar]
- 9. UNAIDS. 90-90-90 An ambitious treatment target to help end the AIDS epidemic. 2014. Available at http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf; accessed March 4, 2020.
- 10. Li Z, Purcell D, Sansom S et al. : Vital signs: HIV transmission along the continuum of care—United States, 2016. MMWR Morb Mortal Wkly Rep 2019; 68(11): 267–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization HIV strategic information for impact: cascade data use manual: to identify gaps in HIV and health services for programme improvement: user manual. 2018. Available at https://www.who.int/hiv/pub/toolkits/hiv-cascade-data-use-manual/en/; accessed March 4, 2020.
- 12. Haber N, Tanser F, Bor J et al. : From HIV infection to therapeutic response: a population-based longitudinal HIV cascade-of-care study in KwaZulu-Natal, South Africa. Lancet HIV 2017; 4: e223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Marconi VC, Grandits G, Weintrob A et al. : Research outcomes of highly active antiretroviral therapy in the context of universal access to healthcare: the US military HIV natural history study. AIDS Research and Therapy 2010; 7: 14–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mangal JP, Rimland D, Marconi VC: The continuum of HIV Care in a Veterans’ affairs clinic. AIDS Res Hum Retroviruses 2014; 30: 409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emuren L, Welles S, Evans A et al. : Health-related quality of life among military HIV patients on antiretroviral therapy. PLOS One 2017; 12: e0178953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Department of Defense Individual medical readiness (IMR). 2014. Available at: https://health.mil/Reference-Center/Glossary-Terms/2014/06/09/Individual-Medical-Readiness-6025-19; accessed March 4, 2020.
- 17. US Department of Health and Human Services Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2018. Available at https://aidsinfo.nih.gov/guidelines/html/1/adult-and-adolescent-arv/0; accessed March 4, 2020.
- 18. Agan BK, Ganesan A, Byrne M et al. The U.S. military HIV natural history study: informing military HIV care and policy for over 30 years. Mil Med. 2019; 184(Supplement_2): 6–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Department of Health and Human Services : Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the Use of Antiretroviral Agents in HIV-1-Infected Adults and Adolescents 2016; Available at https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003510.pdf; accessed March 4, 2020. [Google Scholar]
- 20. World Health Organization Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach. 2010. Available at http://www.ncbi.nlm.nih.gov/books/NBK138540/; accessed March 4, 2020. [PubMed]
- 21. Department of Health and Human Services Panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2013. Available at https://aidsinfo.nih.gov/contentfiles/AdultandAdolescentGL003371.pdf; accessed March 4, 2020.
- 22. World Health Organization Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach, 2013. Available at http://www.ncbi.nlm.nih.gov/books/NBK195400/; accessed March 4, 2020.. [PubMed]
- 23. World Health Organization Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015. Available at http://www.ncbi.nlm.nih.gov/books/NBK327115/; accessed March 4, 2020. [PubMed]
- 24. Haber N, Pillay D, Porter K et al. : Constructing the cascade of HIV care: methods for measurement. Curr Opin HIV AIDS 2016; 11: 102–8. [DOI] [PubMed] [Google Scholar]
- 25. R Core Team R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing; Available at https://www.R-Project.org/; 2020. [Google Scholar]
- 26. Haber N: Longitudinalcascade: longitudinal cascade generator. R Package Version 2019; 111. [Google Scholar]
- 27. Blaylock JM, Hakre S, Okulicz J et al. : HIV Preexposure prophylaxis in the US military services—2014–2016. MMWR Morb Mortal Wkly Rep 2018; 67: 569–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. San Francisco Department of Public Health HIV Epidemiology Annual Report 2017. San Francisco, Department of Public Health, Population Health Division, 2018Available at https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/AnnualReport2017-Green-20180904-Web.pdf; accessed March 4, 2020. [Google Scholar]
- 29. New York City Department of Health and Mental Hygiene HIV surveillance annual report. 2017. Available at https://www1.nyc.gov/assets/doh/downloads/pdf/dires/hiv-surveillance-annualreport-2017.pdf.
- 30. Cohen MS, Chen Y, McCauley M et al. : Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 2011; 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fauci AS, Redfield RR, Sigounas G et al. : Ending the HIV epidemic: a plan for the United States. JAMA 2019; 321(9): 844–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


