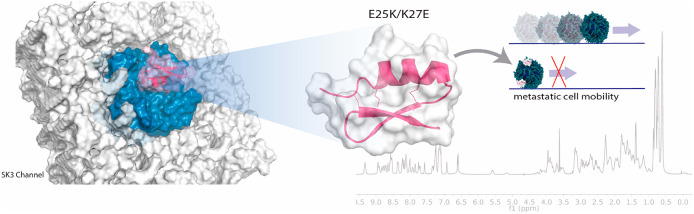
Abstract
Peptide-based therapy against cancer is a field of great interest for biomedical developments. Since it was shown that SK3 channels promote cancer cell migration and metastatic development, we started using these channels as targets for the development of antimetastatic drugs. Particularly, tamapin (a peptide found in the venom of the scorpion Mesobuthus tamulus) is the most specific toxin against the SK2 channel currently known. Considering this fact, we designed diverse tamapin mutants based on three different hypotheses to discover a new potent molecule to block SK3 channels. We performed in vitro studies to evaluate this new toxin derivative inhibitor of cancer cell migration. Our results can be used to generate a new tamapin-based therapy against cancer cells that express SK3 channels.
Keywords: SK3 channels, SK2 channels, scorpion toxins, tamapin, MDA-MB-435s, migration cells
A new research wave on toxin venoms from an array of creatures could seed future pharma development.1 Scorpion venoms consist of a large number of peptides that are found to act principally on the pore of ion channels, such as sodium, potassium, calcium, or chloride ion channels.2 Those active peptides are potent neurotoxins well suited for structure–activity studies.3 Ion channels constitute a varied class of membrane proteins with crucial roles in cellular physiology involved in neuronal signaling, hormone secretion, and muscle contractility and recently implicated in the development of cancers. Potassium channels are the most numerous and diverse ion channels in living organisms,4 and they are one important target for scorpion toxins. Among all families of K+ channels, there are the small conductance Ca2+-activated K+ channels which belong to the SKCa family (also named KCa2) that comprises three members: SK1 (KCa2.1), SK2 (KCa2.2), and SK3 (KCa2.3). In particular, it has been demonstrated that the SK3 channel promotes migration of various cancer cells including breast and melanoma.5−7 Functional SK3 and not SK2 channels are overexpressed on MDA-MB-435s, a highly metastatic cancer and invasive cancer cell line. The modulation of SKCa ion channel can be achieved mainly by three types of compounds including venom, small heterocyclic compounds,8 and, a discovery of the last ten years, amphiphilic compounds.9 Bee toxin apamin is a very specific ion channel blocker peptide for the SKCa family.10
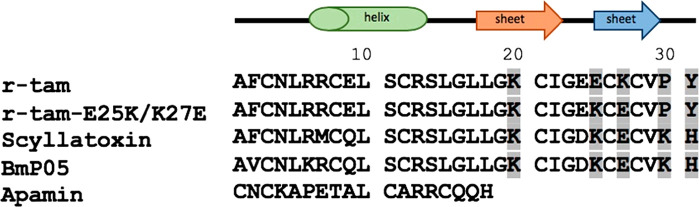
In 2002, Pedarzani demonstrated that tamapin, a toxin from the Indian red scorpion Mesobuthus tamulus venom, is one of the most specific scorpion toxins ever found against SK2 channels; the native tamapin (αKTx5.4) is a very selective blocker for the SK2 channels with an EC50 of 24 pM and 100 times less active for the SK3 channels with an EC50 of 1.7 nM;11 see Table 1. Native tamapin is amidated in the carboxyl-terminal. Recombinant tamapin, the not amidated r-tam, presents a typical cysteine stabilized α/β scaffold (CS-α/β) that comprises an α-helix and a double strand β-sheet connected by three disulfide bridges.12 The scyllatoxin, another αKTx5 toxin, is a slightly better blocker than tamapin for SK3, but not for SK2 channels; see Figure 1. In 2009,6 it was found that inhibition of SK3 channels decreases cell migration in the early stage of metastasis. Considering that tamapin is an excellent blocker of SK2 channels and its great similarity between SK2 and SK3 channels suggested us to employ the r-tam as the leading compound to create potent blocker for SK3 channels. Since the onco SK3 channels are composed of either heterotetrameric SK3/SK2 channels or homotetrameric SK3 channels, we propose to identify compounds acting on both SK3 and SK2 channels.
Table 1. Electrophysiological Activity of αKTx5 Toxin Family Reported against the SK2 and SK3 Channelsa.
| EC50 nM |
||||
|---|---|---|---|---|
| Toxin | SK2 | SK3 | % Identity | Reference |
| Tamapin | 0.024 | 1.7 | 100 | (11) |
| Scyllatoxin | 0.29 | 1.1 | 77 | (13) |
| BmP05 | 3.8 | 74 | (14) | |
| Apamin | 0.027 | 1.1 | NA | (15) |
Apamin is included as blocker reference of these channels. Identity column is with respect to r-tam.
Figure 1.
Alignment of αKTx5 scorpion toxin family and apamin. Sequence chain view of r-tam, r-tam-E25K/K27E, scyllatoxin, BmPO5, and apamin. The mutated amino acids are highlighted in gray.
To design this higher potency r-tam derivative blocker of onco SK3 channels, we designed several mutants with modifications on those positively charged and aromatic amino acids based on three different concepts. We report here the electrophysiological results of the best mutant with both SK3 and SK2 channels expressed in HEK cells, and we present the inhibition of migration produced by the most potent blocker r-tam mutant obtained. This new peptide could be used as a possible therapeutic compound to control specifically cancer cell migration.
Results and Discussion
The experimental procedure is available in the Supporting Information.
Design, Construction, and Expression of R-tam and Mutants
We adopted the following strategies to construct r-tam analogs. Five residues on a β-sheet were individually mutated to alanine and amino acids of opposite charge. Mutations are shown in Table 2. We considered three possible cases that could affect the current blockage: (1) C-terminal amidation of tamapin. (2) Comparison with high similar protein, scyllatoxin; see Figure 1. (3) Considering the functional dyad theory on scorpion’s toxins. We performed our study with the following mutations:
Table 2. Characterization by MALDI-TOF, PDB Codes, BMRD ID, and Electrophysiology Effect of Current Inhibition of R-tam and Mutantsa.
| r-tam mutant | Oxidized form, Theoretical Mass | Experimental Mass | PDB ID Code | BMRB ID | Case | Effect of SK2 current inhibition | Effect of SK3 current inhibition |
|---|---|---|---|---|---|---|---|
| r-tam | 3459.1 | 3459.0 | 2LU9 | 18513 | ⧫⧫⧫ | ⧫⧫ | |
| ΔP30 | 3362.0 | 3361.8 | 6D3T | 30454 | 1 | ⧫⧫ | ⧫ |
| ΔP30/In32N | 3476.1 | 3476.1 | 6D8Q | 30460 | 1 | ⧫⧫ | ⧫ |
| Y31H | 3433.1 | 3433.4 | 6D8Y | 30465 | 1 | ⧫⧫ | ⧫ |
| In32N | 3573.2 | 3573.1 | 6D8H | 30459 | 1 | ⧫⧫ | ⧫ |
| K20E | 3460.1 | 3460.2 | 6D8U | 30464 | 2 | ⧫⧫⧫ | ⧫ |
| E25K | 3458.2 | 3457.9 | 6D8R | 30461 | 2 | ⧫ | ⧫⧫ |
| K27E | 3460.1 | 3460.0 | 6D8S | 30462 | 2 | ⧫ | ⧫⧫ |
| E25K/K27E | 3459.1 | 3458.9 | 6D8T | 30463 | 2 | ⧫⧫ | ⧫⧫⧫ |
| K20A | 3402.0 | 3402.1 | 6VNZ | 30719 | 3 | ⧫⧫⧫ | – |
| E25A | 3401.1 | 3401.0 | 6D9O | 30467 | 3 | ⧫ | ⧫⧫ |
| K27A | 3402.0 | 3402.3 | 6D9P | 30468 | 3 | ⧫ | ⧫⧫ |
| Y31A | 3367.0 | 3367.0 | 6D93 | 30466 | 3 | ⧫⧫ | ⧫ |
We also include the case described to include the mutant in our study. We evaluated all mutants base on high (⧫⧫⧫, > 85%), medium (⧫⧫, 84–40), low (⧫, < 39%), or absence activity (−) under electrophysiology activity on SK2 and SK3 channels.
Case 1: We propose the insertion of an asparagine on the C-terminal; this modification could emulate the amide group and its interaction with the SK2 channel. The rationalization is if the increment of one amino acid could affect the activity and the Y31 could have more relevance than the P30, we decided to delete residue P30 and insert an asparagine, forming the mutant ΔP30InN32. In addition, we obtained the mutant InN32, ΔP30, and the Y31H to test if these amino acids could have some relevance.16
Case 2. Inside the family αKTx5, the scyllatoxin has slightly greater affinity for SK3 channels than tamapin. Mutations on tamapin in residues E25 and K27 could increase the blockage of channel SK3. Also, Andreotti stated that residue K20 could be important for the channel interaction.17 We obtained the mutants K20E, E25K, and K27E and the double mutant E25K/K27E.
Case 3. The functional dyad theory18 establishes that there should be a positively charged residue and an aromatic amino acid close in space at around 6.7 Å. We selected to mutate residues 20, 25, 27, and 31 (K20A, E25A, K27A, and Y31A) to test if one of these residues is relevant for the interaction.
According to the three cases considered above, we designed 12 r-tam mutants. The biosynthesizing procedure allows us to obtain recombinant peptides from E. coli with functional folding including the same cysteine connectivity for all the mutants. All mutants with their experimental and theoretical masses obtained by MALDI-TOF are shown in Table 2; also, PDB and BRMB ID’s and fast electrophysiology screening are listed.
NMR Mutant Assignments and Structure Calculation
We characterized and observed the impact of these mutations by NMR structure determination. There were slight differences in the NMR spectra as a result of changing amino acids. Despite all NMR difference data, most NOE signals were present in all peptides. The three-dimensional structures for all mutants were calculated in CYANA and refined using AMBER16.19 Examples of NMR experiments and Ramachandran statistics are summarized in the Supporting Information. These structures have low target functions value calculated with CYANA and obtained with no distance violations greater than 0.3 Å; those structures were refined by Molecular Dynamics using Amber. The structures of all mutants show a typical CS-α/β motif, stabilized by the same three disulfide bridges and with two flexible loops connecting the main secondary elements. The NMR structures of 12 r-tam mutants were deposited in both PDB and BRMD; see Table 2. This NMR evidence confirms that the tertiary structures for all mutants adopt the same overall structural topology. We show the 3D NMR structure superposition of r-tam and K27A mutant as an example that all mutants preserve the same structural motif; see Figure 2.
Figure 2.
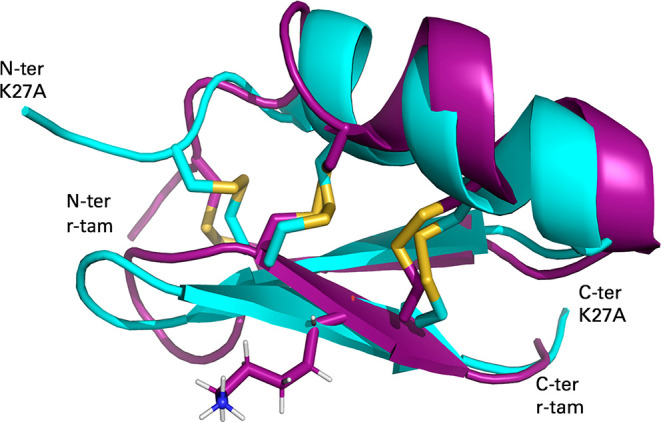
3D NMR structure of r-tam and K27A mutant. Alignment of mutant (cyan, PDB entry 6D9P) and r-tam (purple, PDB entry 2LU9) from amino acids C3-V29; the K27 amino acid is shown in sticks. K27A is the mutant with the greatest differences in HN protons compared to r-tam. Despite the difference in chemical shifts, both structures conserve the topology.
In Silico Interaction of SK2/SK3 Ion Channels with R-tam Mutants Using Molecular Dynamics Simulations
As a simple model, we performed molecular dynamics simulations to establish the interaction with a partial model of the channel (S5–S6 tetramer subunits) considering the KV1.1 as a homology model. We used molecular dynamics calculations as a better approach than docking calculations. For the r-tam and the analog peptides, no unfolding event was detected in any of the systems, even for values as high as ∼9 Å of structural deviations observed within the 1.5 μs of sampled time mainly in the terminal regions of the peptides. The PDB files obtained with r-tam and SK2 and SK3 channels can be found in the Supporting Information.
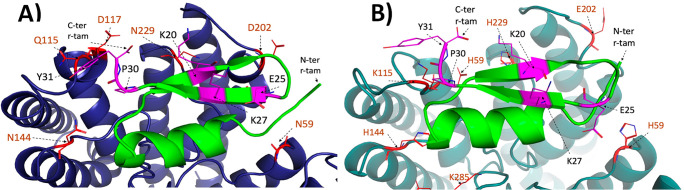
Molecular dynamic interactions of r-tam with SK2 and SK3 channels are shown in Figure 3. The computational results suggested that an interaction between the peptide mutants and SK2 and SK3 channels is possible. Also, the complex structures differ slightly from those reported by Andreotti.17
Figure 3.
Interactions of r-tam with (A) SK2 (dark blue) and (B) SK3 (cyan) channels explored by molecular dynamics. Toxin is colored in green. Residues which differ from channels SK2 to SK3 are colored in red. Residues mutated in this study are shown in pink. The PDB data to draw this figure is in the Supporting Information.
First Electrophysiology Screening
All mutants were screened to estimate the electrophysiological activity on homotetrameric SK2 and homotetrameric SK3 channels expressed in HEK cells. Since onco SK3 channels are composed of homotetrameric SK3 channels or heterotetrameric SK3/SK2 channels, we aim to develop blockers acting on both SK3 and SK2 channels. We sought two criteria to choose the best mutations: (1) Increase the activity of the compound on SK3 channels, and (2) decrease the activity of the compound on SK2 channels leading ideally to a SK2 and SK3 channel blocker with the same efficiency on both channels. With this in mind, the first electrophysiological tests were carried out at 10 nM of all the mutants on the SK2 and SK3 channels, taking r-tam at variable concentrations as a reference and apamin as a control blocker of SK2 and SK3 channels and 100 nM concentration that totally block SK2 and SK3 channels. The results are shown in Table 2. They indicate the effect found on both channels. Results are reported as high, medium, low, or no block. Modified mutants at the carboxyl terminus, Case 1, decrease their ability to block both channels, as does the Y31A mutant. Mutants generated from similarity to scyllatoxin, Case 2, show mixed results. The K20E and K20A mutant do not modify their blocking capacity on SK2 channels significantly, but the blocking on SK3 channels decreases greatly; this result is significant for later works which want to understand the blocking on the SK3 channel. The E25K, K27E, and E25K/K27E mutants decrease their activity on SK2 channels, but only the double mutant improves its activity on SK3 channels. For the last case studied, the E25A and K27A mutants decrease their activity on SK2 channels and maintain their activity on SK3 channels. The only mutant in this screening that complied with improving the blockade on the SK3 channels was the E25K/K27E; additionally, this mutant decreased its activity on SK2 channels. Given these results, we performed dose–response experiments of E25K/K27E mutant on both SK2 and SK3 channels in order to determine its blocking efficiency.
Efficiency of the E25K/K27E Mutant on SK2 and SK3 Channels by Patch-Clamp Experiments
We know the possible inhibitory capacity of the E25K/K27E mutant blocking SK2 and SK3 channels separately, which are stably expressed in HEK293 and HEK293T cells. Based on this, we suggest that E25K/K27E peptide would block homotetramer as well as heterotetramer SK2/SK3 channels which are reported in nonengineered and cancer cells. Engineered cells only produce homotetramer channels. Besides, the heterotetramer channel conformations are possible, as described in cardiac atrial myocytes.20 Nevertheless, the molecular composition of SK3 channels (homotetramer SK3 channel and/or heterotetramer SK2/SK3 channel) in cancer cells is still unknown.
Figure 4A and C shows typical whole cell currents recorded at membrane potentials varying from −100 to +100 mV for 500 ms before and after r-tam or E25K/K27E mutant applications. Apamin at 100 nM was applied as the toxin reference at the end of each experiment to fully block the residual SKCa currents. Consistent with the selectivity of r-tam toward SK2 and SK3 channels, the amplitude of SK2 currents was significantly more reduced than SK3 currents after an application of 1 nM r-tam. Figure 4B shows that r-tam reduced 86% of SK2 currents and only 25% for SK3 current. Interestingly, E25K/K27E mutant presents the same inhibition efficacy toward SK2 (80%) as for SK3 (73%) currents at the same concentration, suggesting that the mutations introduced into r-tam derivative enhance its binding affinity for SK3 channels; see Figure 4D. Next, we performed a concentration–response curve to compare the half-maximal inhibitory concentrations (EC50) of r-tam and E25K/K27E mutant for SK2 and SK3 currents. As shown in Figure 4E, the EC50 of r-tam for SK2 and SK3 were estimated at 16 pM and 2.6 nM, respectively, values closed to native tamapin.11 The concentration–response curves of SK2 and SK3 for E25K/K27E mutant are overlapped with an EC50 estimated at 0.28 nM and 0.38 nM, respectively. To conclude E25K and K27E mutations of r-tam have led to decrease the blocking efficiency toward SK2 channel (∼ −17-fold) and an increment of its blocking efficiency toward SK3 channels (∼7-fold). Thus, the simplified molecular dynamics model used gave a good electrophysiological data explanation with both channel interactions and the double mutant E25K/K27E.
Figure 4.
Effects of r-tam E25K/K27E mutant on SK2 and SK3 currents. (A, C) SK2 and SK3 current recordings in control condition (black trace), after application of 1 nM r-tam (green trace) or 1 nM E25K/K27E (blue trace) and 100 nM apamin (red trace) to inhibit residual SKCa currents. Whole-cell SK2 currents in HEK293 cells expressing recombinant rat SK2 and whole-cell SK3 currents in HEK293T cells expressing recombinant human SK3 were generated by ramp protocol from −100 to 100 mV for 500 ms from a constant holding potential of 0 mV at pCa 6. (B, D). Currents were analyzed at 0 mV, a membrane potential where the current is only carried by SKCa channels. Lines indicate the median; each point represents the percentage of SKCa currents sensitive to peptides (**p < 0.01, Mann–Whitney test). (E) Concentration–response curves of SK2/SK3 currents to r-tam and E25K/K27E mutant (means ± SEM, n = 3–8, GraphPad Prism). Currents were analyzed at 0 mV.
Antimigration Effect on Cancer Cells That Express Onco SK3 Channels
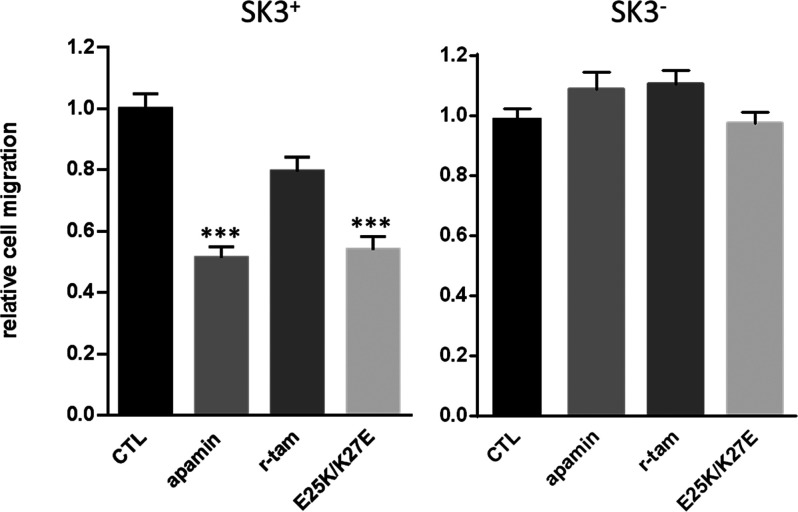
Onco SK3 channels (composed of homotetrameric SK3 channels or heterotetrameric SK2/SK3 channels) were found in cancer cells to promote cell migration and the development of metastases. In previous work, it has been shown that silencing SK3 protein in MDA-MB-435s cells totally abolishes SK3 channels-dependent cancer cell migration and reduces to 60% their global migration capacity leading to a large decrease of their ability to form bone metastases in vivo.21 We compared the potency of each peptide, at 100 nM, to reduce the migration of MDA-MB-435s that expressed (SK3+) or not SK3 channel (SK3–). Figure 5 shows that while r-tam has no significant effect on SK3+ cell migration, E25K/K27E mutant and apamin reduced to 45.9% and 48.6%, respectively, the capacity of SK3+ to migrate. R-tam still has no significant effect on SK3– cell migration; in these cells that did not express the SK3 channel, the effect of apamin and E25K/K27E was suppressed. Thus, the E25K/K27E mutant as apamin almost totally abolished the SK3-dependent cell migration (Table 3). Considering the high chemical stability of tamapin mutations, the E25K/K27E mutant could be a potential drug to decrease the metastasis process.
Figure 5.
Effect of E25K/K27E mutant, r-tam, and apamin on SK3-dependent cell migration. Histograms showing the effect of 100 nM E25K/K27E mutant, r-tam, and apamin on MDA-MB-435s migration expressing (SK3 +) or not SK3 channel (SK3 –). The normalized cell number corresponds to the ratio of the number of migrating cells in the presence of peptides over the number of migrating cells in control conditions (means ± SEM, N = 4, n = 12, *** p < 0.01, Kruskal–Wallis and post hoc tests).
Table 3. Efficiency of R-tam and E25K/K27E Compared to Apamin on SK3 Current and SK3-Dependent Cancer Cell Migrationa.
| Peptide | % inhibition of SK3 current | EC50 pM | total% inhibition of cell migration decreased on MDA-MB-435s | % inhibition of SK3 dependent cell migration |
|---|---|---|---|---|
| r-tam | 25 | 2600 | No effect | No effect |
| E25K/K27E | 73 | 380 | 45.9 | 76.5 |
| Apamin | Control* | 360 | 48.6 | 81 |
R-tam and E25K/K27E were used at 1 nM concentration, while apamin was used at 100 nM as a control*. The % of SK3 inhibition corresponds to the ratio of the amplitude of outward currents blocked by 1 nM compounds to the amplitude of the current in the absence of compounds. The % of SK3-dependent cell migration was determined on MDA-MB435s cells and corresponds to the ratio of the total number of migrating cells in the presence of compounds to the total number of migrating cells in control experiments of SK3+ cells and normalized to the full SK3-dependent capacity of cells to migrate (60%).
Our study shows that the relationship between SKCa channels and r-tam is unique for each isoform of SKCa channels. Establishing the residues of greater relevance for the interaction between SKCa potassium channels and their r-tam inhibitor mutant will help to lay the molecular bases that will be very useful to continue developing new derivatives aimed to specifically block SK3 and SK2 channels and thus of onco SK3 channels. For instance, mutant K20A deserves another deeper study to demonstrate its probable specific behavior. These results contribute to continuing designing high value metastasis inhibitors for the generation of highly selective compounds, as Lei-Dab7,22 and therapies.
Conclusions
Tamapin toxin is a highly specific peptide for SK2 channels. Mutations performed over r-tam preserve the CSα/β motif. Specific punctual mutations on r-tam toxin in residues E25 and K27, based on the activity of scyllatoxin increases by a factor of 7 the activity on the SK3 channels. At the same time, the E25K/K27E mutant activity over the SK2 channels was decreased by 17 times. This increased blockage of the SK3 channels and the block decrease on the SK2 channels, in comparison with the r-tam, allow us to modulate SK3 channels in an efficient manner by E25K/K27E. As we demonstrate that E25K/K27E mutant almost totally blocks SK3-dependent cancer cells, it is possible to assume that the E25K/K27E mutant of r-tam could be used as an inhibitor of the onset of metastasis and it is a starting point to study its pharmacology properties to be considered as a potential drug.
Acknowledgments
We thank E. García-Ramos, L. C. Márquez-Alonso, and L. M. Ríos-Ruiz for technical support. We also thank D. del Rio-Pulido for proofreading the article. M.M.F., C.M.M.M., and G.T.D. acknowledge the national council of science and technology (CONACyT) for scholarship numbers 289035, 596629, and 273490 respectively. We also thank Catherine Leroy for secretarial support. This work was partially supported by Dirección General de Asuntos del Personal Académico with grants DGAPA-PAPIIT number 210319 and Dirección General de Cómputo y de Tecnologías de la Información y Comunicación, Supercomputing Project LANCAD-UNAM-DGTIC-145 (F.R.P.). This study was made possible in part thanks for the computational time provided by the Center of High-Performance Computing at the University of Utah (R.G.M.). Part of this work was funded by University of Tours, the “Région Centre-Val de Loire” “INSERM”, Canceropôle Grand Ouest, the “Ligue Nationale Contre le Cancer”, the Association “CANCEN”, and Tours “Hospital oncology association ACORT”.
Glossary
Abbreviations
- BMRB
The Biological Magnetic Resonance Data Bank
- CPK
space-filling model
- CTL
control experiment
- CYANA
combined assignment and dynamics algorithm for NMR applications
- Kv 1.1
potassium voltage-gated channel subfamily A member 1
- r-tam
recombinant tamapin not amidated
- std. dev.
standard deviation
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsmedchemlett.0c00300.
Experimental section, NMR structure statistics, 1H NMR spectra of Y31A and K20A r-tam mutants, NOESY spectra of K25E r-tam mutant, cystein disulfide bond connectivities of r-tamapin and E25K/K27E mutant (PDF)
PDB data file of r-tam with SK2 channel (PDB)
PDB data file of r-tam with SK3 channel (PDB)
The authors declare no competing financial interest.
Supplementary Material
References
- McDermott A. News Feature: Venom Back in Vogue as a Wellspring for Drug Candidates. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (19), 10100. 10.1073/pnas.2004486117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luz Morales-Lazaro S.; Hernandez-Garcia E.; Serrano-Flores B.; Rosenbaum T. Organic Toxins as Tools to Understand Ion Channel Mechanisms and Structure. Curr. Top. Med. Chem. 2015, 15 (7), 581–603. 10.2174/1568026615666150217110710. [DOI] [PubMed] [Google Scholar]
- Hübner C. a; Jentsch T. J. Ion Channel Diseases. Hum. Mol. Genet. 2002, 11 (20), 2435–2445. 10.1093/hmg/11.20.2435. [DOI] [PubMed] [Google Scholar]
- Leanza L.; Doyle A.; Venturini E.; Zoratti M.; Szegezdi E.; Szabo I. Correlation between Potassium Channel Expression and Sensitivity to Drug-Induced Cell Death in Tumor Cell Lines. Curr. Pharm. Des. 2014, 20 (2), 189–200. 10.2174/13816128113199990032. [DOI] [PubMed] [Google Scholar]
- Girault A.; Haelters J.-P.; Potier-Cartereau M.; Chantome A.; Jaffres P.-A.; Bougnoux P.; Joulin V.; Vandier C. Targeting SKCa Channels in Cancer: Potential New Therapeutic Approaches. Curr. Med. Chem. 2012, 19 (5), 697–713. 10.2174/092986712798992039. [DOI] [PubMed] [Google Scholar]
- Chantome A.; Girault A.; Potier M.; Collin C.; Vaudin P.; Pagès J. C.; Vandier C.; Joulin V. KCa2.3 Channel-Dependent Hyperpolarization Increases Melanoma Cell Motility. Exp. Cell Res. 2009, 315 (20), 3620–3630. 10.1016/j.yexcr.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Potier M.; Joulin V.; Roger S.; Besson P.; Jourdan M. L.; LeGuennec J. Y.; Bougnoux P.; Vandier C. Identification of SK3 Channel as a New Mediator of Breast Cancer Cell Migration. Mol. Cancer Ther. 2006, 5 (11), 2946–2953. 10.1158/1535-7163.MCT-06-0194. [DOI] [PubMed] [Google Scholar]
- Brown B. M.; Shim H.; Christophersen P.; Wulff H. Pharmacology of Small- and Intermediate-Conductance Calcium-Activated Potassium Channels. Annu. Rev. Pharmacol. Toxicol. 2020, 60 (1), 219–240. 10.1146/annurev-pharmtox-010919-023420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffrès P. A.; Gajate C.; Bouchet A. M.; Couthon-Gourvès H.; Chantôme A.; Potier-Cartereau M.; Besson P.; Bougnoux P.; Mollinedo F.; Vandier C.. Alkyl Ether Lipids, Ion Channels and Lipid Raft Reorganization in Cancer Therapy. Pharmacology and Therapeutics; Elsevier Inc, September 1, 2016; pp 114–131. 10.1016/j.pharmthera.2016.06.003. [DOI] [PubMed] [Google Scholar]
- Lamy C.; Goodchild S. J.; Weatherall K. L.; Jane D. E.; Liégeois J. F.; Seutin V.; Marrion N. V. Allosteric Block of KCa2 Channels by Apamin. J. Biol. Chem. 2010, 285 (35), 27067–27077. 10.1074/jbc.M110.110072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedarzani P.; D’Hoedt D.; Doorty K. B.; Wadsworth J. D. F.; Joseph J. S.; Jeyaseelan K.; Kini R. M.; Gadre S. V.; Sapatnekar S. M.; Stocker M.; et al. Tamapin, a Venom Peptide from the Indian Red Scorpion (Mesobuthus Tamulus) That Targets Small Conductance Ca2+-Activated K+ Channels and Afterhyperpolarization Currents in Central Neurons. J. Biol. Chem. 2002, 277 (48), 46101–46109. 10.1074/jbc.M206465200. [DOI] [PubMed] [Google Scholar]
- Ramírez-Cordero B.; Toledano Y.; Cano-Sánchez P.; Hernández-López R.; Flores-Solis D.; Saucedo-Yáñez A. L.; Chávez-Uribe I.; Brieba L. G.; Del Río-Portilla F. Cytotoxicity of Recombinant Tamapin and Related Toxin-like Peptides on Model Cell Lines. Chem. Res. Toxicol. 2014, 27 (6), 960–967. 10.1021/tx4004193. [DOI] [PubMed] [Google Scholar]
- Auguste P.; Hugues M.; Mourre C.; Moinier D.; Lazdunski M.; Tartar A. Scyllatoxin, a Blocker of Ca2+-Activated K+ Channels: Structure-Function Relationships and Brain Localization of the Binding Sites. Biochemistry 1992, 31 (3), 648–654. 10.1021/bi00118a003. [DOI] [PubMed] [Google Scholar]
- Feng J.; Hu Y.; Yi H.; Yin S.; Han S.; Hu J.; Chen Z.; Yang W.; Cao Z.; De Waard M.; et al. Two Conserved Arginine Residues from the SK3 Potassium Channel Outer Vestibule Control Selectivity of Recognition by Scorpion Toxins. J. Biol. Chem. 2013, 288 (18), 12544–12553. 10.1074/jbc.M112.433888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolting A.; Ferraro T.; D’Hoedt D.; Stocker M. An Amino Acid Outside the Pore Region Influences Apamin Sensitivity in Small Conductance Ca2+-Activated K+ Channels. J. Biol. Chem. 2006, 282 (6), 3478–3486. 10.1074/jbc.M607213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauplais M.; Lecoq A.; Song J.; Cotton J.; Jamin N.; Gilquin B.; Roumestand C.; Vita C.; De Medeiros C. L. C.; Rowan E. G.; et al. On the Convergent Evolution of Animal Toxins. Conservation of a Diad of Functional Residues in Potassium Channel-Blocking Toxins with Unrelated Structures. J. Biol. Chem. 1997, 272 (7), 4302–4309. 10.1074/jbc.272.7.4302. [DOI] [PubMed] [Google Scholar]
- Andreotti N.; Di Luccio E.; Sampieri F.; De Waard M.; Sabatier J. M. Molecular Modeling and Docking Simulations of Scorpion Toxins and Related Analogs on Human SKCa2 and SKCa3 Channels. Peptides 2005, 26 (7), 1095–1108. 10.1016/j.peptides.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Zhu L.; Gao B.; Luo L.; Zhu S. Two Dyad-Free Shaker-Type K + Channel Blockers from Scorpion Venom. Toxicon 2012, 59 (3), 402–407. 10.1016/j.toxicon.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Salomon-Ferrer R.; Case D. A.; Walker R. C. An Overview of the Amber Biomolecular Simulation Package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013, 3 (2), 198–210. 10.1002/wcms.1121. [DOI] [Google Scholar]
- Tuteja D.; Rafizadeh S.; Timofeyev V.; Wang S.; Zhang Z.; Li N.; Mateo R. K.; Singapuri A.; Young J. N.; Knowlton A. A.; et al. Cardiac Small Conductance Ca2+-Activated K+ Channel Subunits Form Heteromultimers via the Coiled-Coil Domains in the C Termini of the Channels. Circ. Res. 2010, 107 (7), 851–859. 10.1161/CIRCRESAHA.109.215269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantôme A.; Potier-Cartereau M.; Clarysse L.; Fromont G.; Marionneau-Lambot S.; Guéguinou M.; Pagés J. C.; Collin C.; Oullier T.; Girault A.; et al. Pivotal Role of the Lipid Raft SK3-Orai1 Complex in Human Cancer Cell Migration and Bone Metastases. Cancer Res. 2013, 73 (15), 4852–4861. 10.1158/0008-5472.CAN-12-4572. [DOI] [PubMed] [Google Scholar]
- Shakkottai V. G.; Regaya I.; Wulff H.; Fajloun Z.; Tomita H.; Fathallah M.; Cahalan M. D.; Gargus J. J.; Sabatier J. M.; Chandy K. G. Design and Characterization of a Highly Selective Peptide Inhibitor of the Small Conductance Calcium-Activated K+ Channel, SkCa2. J. Biol. Chem. 2001, 276 (46), 43145–43151. 10.1074/jbc.M106981200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.