 We investigated the potential of sulfonamides derived from carvacrol as candidates for treatment of Alzheimer's disease. The results are very promising for the molecular target investigated as well as for the phenotypic assays.
We investigated the potential of sulfonamides derived from carvacrol as candidates for treatment of Alzheimer's disease. The results are very promising for the molecular target investigated as well as for the phenotypic assays.
Abstract
Five synthetic sulfonamides derived from carvacrol, a natural product and a small molecule with druglike properties, were evaluated with respect to their effects on the cognitive deficits of animals with streptozotocin (STZ)-induced Alzheimer's disease (AD). Memory, ambulation, anxiety and oxidative stress were evaluated. In vitro assays were performed to assess the inhibition of acetylcholinesterase (AChE), and the data were combined with molecular docking for the establishment of structure–activity relationships. The memories of animals treated with the compounds derived from morpholine (1), hydrazine (3) and 2-phenol (5) were improved. Compound 3 was the most promising, yielding excellent results in the inhibitory avoidance test. Moreover, the compounds did not exhibit any deleterious effects on the animals' ambulation in the open field test. Molecular docking confirmed the results obtained in the AChE inhibition assay. In short, compounds 1, 3 and 5 can reduce STZ-induced deficits and show potential for the treatment of Alzheimer's. In addition, these agents produce significant anxiolytic and antioxidant effects.
Introduction
Alzheimer's disease (AD) is a neurodegenerative disorder, characterized by irreversible and progressive memory loss, which decreases daily task performance and reduces speech abilities and visual perception, ultimately culminating in total dementia.1,2 AD affects thousands of people worldwide regardless of ethnicity or socioeconomic conditions. Currently, 35.6 million people are affected by AD, and it is most common in the population above 60 years of age, making it more prevalent in Latin American countries (8.5%) and less prevalent in South Africa (2–4%).1,3,4
Commonly, the first cerebral structure to be affected by AD is the hippocampus. As the disease progresses, other cerebral structures are damaged, leading to symptoms such as impulsiveness, incoherent judgment, and difficulty in learning, talking and writing.5,6
Some of the medicines available for the treatment of AD are acetylcholinesterase (AChE) inhibitors, such as rivastigmine, donepezil and galantamine. AChE is an enzyme responsible for acetylcholine (ACh) hydrolysis. By inhibiting the enzyme, those drugs increase cerebral ACh levels.7,8 In some patients, the adverse and/or collateral effects are so intense that they lead to poor or no adherence at all to the treatment.9,10 Therefore, molecules with anti-Alzheimer's potential that act not only by attenuating the symptoms but also the characteristics of the disease, for example on its progression, are urgently needed. In this context, diverse chemotypes have been studied with regard to their therapeutic potential against AD.9
Natural products and their molecular frameworks have a long tradition as valuable starting points for medicinal chemistry and drug discovery.10,11 In this context, carvacrol (2-methyl-5-isopropyl-phenol), a small molecule with druglike properties and no stereogenic centers, is a monoterpene phenol found in the essential oils of the family Labiatae, including Origanum, Satureja, Thymbra, Thymus, and Corydothymus species, which can serve as an interesting basic scaffold onto which substituents can be introduced.12,13 Carvacrol is characterized by possessing several biological properties, among which are antioxidant14 and AChE inhibitory abilities.15–17
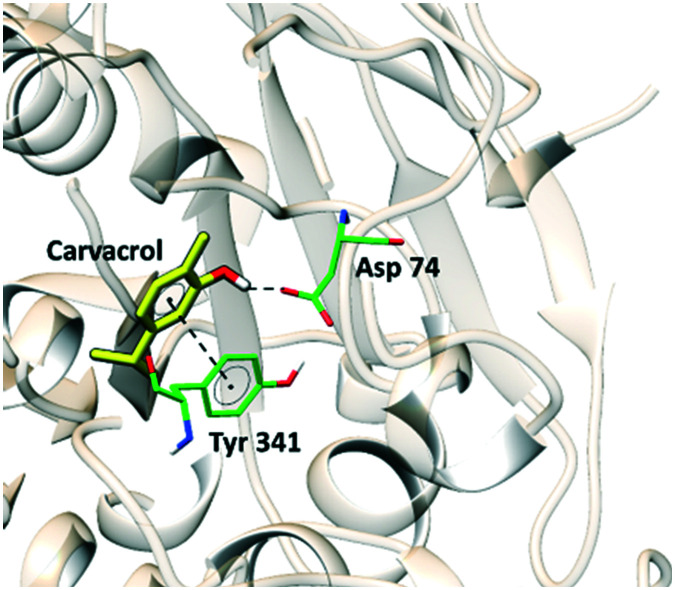
To carry out the study reported in this article, molecular docking studies were conducted to clarify the inhibition mode of the active compounds using carvacrol and the structure of human AChE (PDB ID 4M0E). Fig. 1 shows the binding conformation of carvacrol in the active site of AChE.
Fig. 1. Intermolecular interactions of carvacrol in the enzymatic cavity of the AChE enzyme.
Carvacrol forms a hydrogen bond between the hydroxyl group and the side chain of ASP74 and a π–π interaction with the side chain of TYR341. Although it is a low potency inhibitor with low affinity for AChE, carvacrol is a good compound for the study of structure–activity relationships, since it does not present any violation of Lipinski's rules (mlog P = 3.81; MW = 150.22; HBA = 1; HBD = 1). To find compounds potentially more active than carvacrol in enzymatic inhibition and based on the experience of the group in synthesis, the production of a series of sulfonamides was planned.
The choice of the synthesis of sulfonamides derived from this natural product is justified by the fact that new therapeutic activities have been discovered for sulfonamides, in addition to their use as probes with photoaffinity, prevention of beta secretase from acting, inhibition of the formation and aggregation of beta-amyloids, and free radical stabilization.18 In regard to AD, Brodney et al.19 demonstrated that heterocyclic sulfonamides could reduce β-secretase action, the enzyme responsible for incorrect amyloid precursor protein (APP) cleavage; this activity culminates in the production of β amyloid peptides, which are senile plaque constituents. Recently, a novel series of multifunctional anti-Alzheimer's agents based on N-substituted aryl sulfonamides were designed and synthesized.20 This series of compounds exhibited in vivo activity, which was correlated with the modulation of some selected biochemical markers of AD during behavioral assessment.20
Considering the AChE in vitro activity of the compounds synthesized herein, the use of similar agents in AD therapy, and the literature findings on the new pharmacologic properties of the sulfonamides, we have investigated compounds 1, 2, 3, 4 and 5 with respect to their protective effects against cognitive deficits on animal models of AD. In addition to the in vivo assays, we have correlated the enzymatic inhibitory activity with molecular docking data to rationalize the predominant structure–activity relationships.
Results and discussion
Chemistry
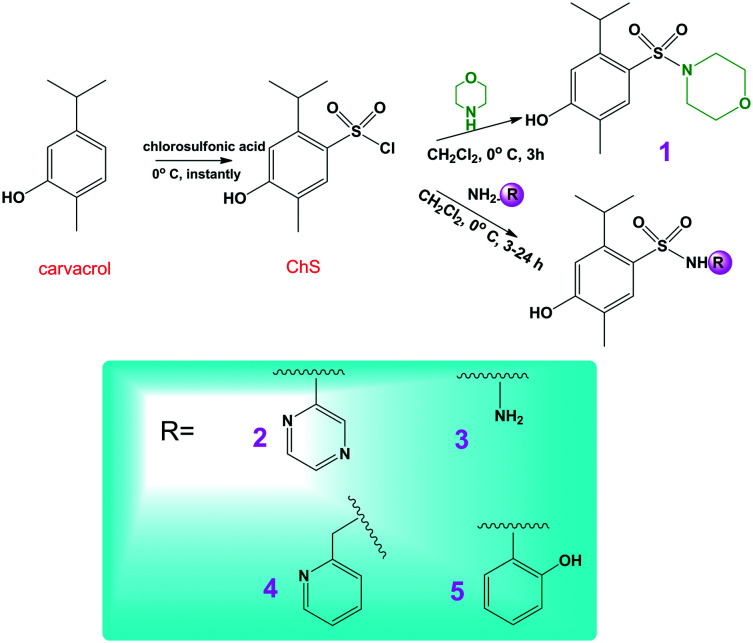
The purpose of this article is not to report the synthesis of sulfonamides derived from carvacrol, since this procedure has already been published recently by our research group.21 A series of 35 compounds were synthesized, of which 5 compounds were selected that exhibited greater activity in in vitro assays (antioxidant and acetylcholinesterase inhibition) especially as a measure to rationalize the number of animals, in line with international protocols. The chosen compounds were synthesized in good yields (83–93%) using the procedure illustrated in Scheme 1.
Scheme 1. Synthesis of sulfonamides derived from carvacrol.
The sulfonyl chloride of carvacrol (ChS) was obtained by reacting carvacrol with six equivalents of chlorosulfonic acid. The excess of acid in reactions of this type, chlorosulfonation of aromatic systems, has been studied previously due to the reversibility that exists in the mechanisms proposed for this reaction. It was also verified that the excess of six equivalents is the ideal quantity to obtain the best yields.21
AChE inhibitory activity
All studied compounds were evaluated as AChE inhibitors. The concentration of the tested compounds that inhibits substrate hydrolysis by 50% (IC50 – Table 1) was determined by plotting the inhibition percentage against the sample solution concentration. The sulfonamides showed high percentages of inhibition of AChE, with all of them being more active than galantamine (Reminyl®, a drug used for the treatment of AD), but all had lower inhibition compared to donepezil. These results demonstrate the potential of these compounds as novel AChE inhibitors.
Table 1. AChE inhibitory activity for sulfonamides.
| Comp. | IC50 a (μM) |
| 1 | 5.64 ± 0.33 |
| 2 | 7.34 ± 0.31 |
| 3 | 9.24 ± 0.12 |
| 4 | 6.99 ± 0.28 |
| 5 | 8.74 ± 0.02 |
| Carvacrol | 283.60 ± 0.12 |
| Galantamine | 17.10 ± 0.23 |
| Donepezil | 0.006 ± 0.001 |
aThe assays were performed in triplicate and the values were obtained from the means.
The most active compound in the series was compound 1, which is derived from morpholine and was 50 times more active than carvacrol; the least active was compound 3, which was derived from hydrazine and is still approximately 30 times more active than carvacrol. All the compounds were able to inhibit the catalytic activity of the enzyme. For a better understanding of the activity observed in the AChE assay, molecular modeling studies were performed, the results of which are shown below.
Molecular modelling
Structure–activity relationship (SAR) studies were carried out with compounds 1, 2, 3, 4 and 5. To that end, molecular docking runs were conducted using the structure of human AChE (PDB ID ; 4M0E).22 The inhibitors were docked into the same binding site occupied by the cocrystallized ligand dihydrotanshinone.
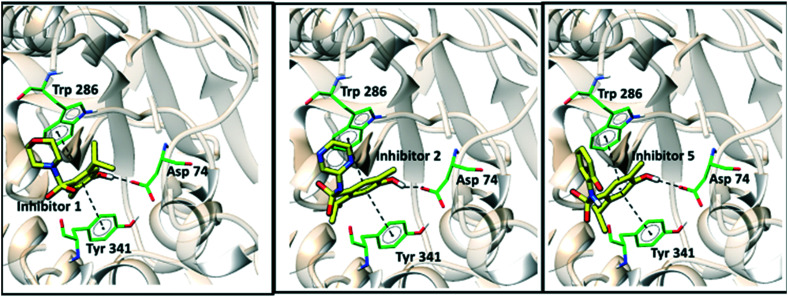
Fig. 2 shows the binding conformations of sulfonamides 1, 2, and 5 in the active site of AChE. These inhibitors were observed to maintain similar binding patterns, including a hydrogen bond between the ligands' hydroxyl groups and the side chain of Asp74 and a π–π interaction with the side chains of Tyr341 and Trp286. Fig. 3 illustrates the intermolecular interactions of compounds 3 and 4 in the active site of AChE.
Fig. 2. Intermolecular interactions of compounds 1, 2 and 5 in the enzymatic cavity of the AChE enzyme.
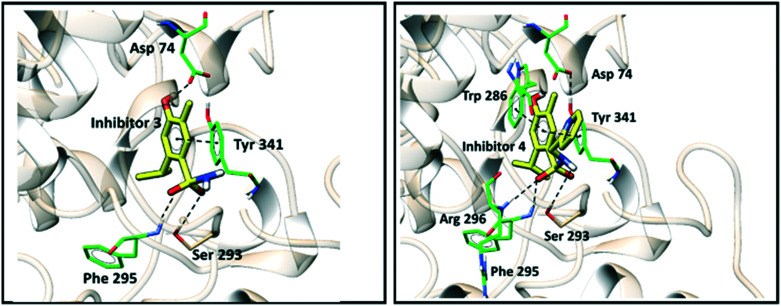
Fig. 3. Intermolecular interactions of compounds 3 and 4 within the AChE active site.
For compound 3, the same interactions described previously for the side chains of Tyr341 and Asp 74 are observed. Additional hydrogen bonds were observed for the side chains of Phe295 and Ser293. Analogously, for compound 4, all interactions predicted for compounds 1, 2, 3 and 5 were maintained, in addition to an additional interaction of hydrogen bonds with Arg296.
The differences in the IC50 values measured in the AChE assay were not substantial. Substitution of the cyclic substituents at the sulfonamide group in compounds 1, 2, 4 and 5 by the amine in compound 3 decreased the biological activity against the enzyme by approximately 2-fold. Overall, the molecular docking results point out that the binding of the inhibitors to the AChE active site is mainly driven by π–π contacts and hydrogen bonds.
In vivo assays
Open field test
Table 2 shows that none of these compounds or galantamine when administered to animals produced changes in behavioral parameters such as crossings and rearing when compared to the control group (p > 0.05). These results indicate that there were no sedative or stimulant effects in mice, as assessed by the open field apparatus (Table 2).
Table 2. Effect of the administration of compounds (30 mg kg–1, and galantamine at 0.5 mg kg–1, i.p.) on animal behaviour in the open field test.
| Compound | Crossing a | Rearing a |
| Vehicle | 114.00 ± 3.12 | 53.60 ± 1.62 |
| Sham | 112.20 ± 2.14 | 47.10 ± 2.20 |
| Naive | 123.56 ± 1.81 | 42.23 ± 3.18 |
| 1 | 116.30 ± 2.34 | 43.80 ± 1.31 |
| 2 | 108.34 ± 1.67 | 50.17 ± 2.22 |
| 3 | 98.45 ± 4.20 | 44.18 ± 2.21 |
| 4 | 102.45 ± 1.17 | 46.17 ± 3.21 |
| 5 | 107.00 ± 2.51 | 44.56 ± 4.31 |
| Galantamine | 122.50 ± 1.45 | 54.23 ± 2.31 |
aData are presented as the means ± S.E.M. N = 8–10 per group. The data for compounds do not differ from the vehicle.
These effects on locomotor activity were investigated to rule out the possibility that the effect of the compounds on the animals subjected to the inhibitory avoidance task could be a consequence of increased locomotor activity. However, it should be emphasized that drugs that induce hyperlocomotion may produce a false positive effect in this test, whereas drugs that decrease locomotion may produce a false negative result.23
Elevated plus maze test
The effects of the sulfonamides on the anxiety behavioral parameters of the animals subjected to the elevated plus maze test are presented in Table 3. Interestingly, treatment with the sulfonamides caused detectable emotional changes in this test, which is frequently used to detect and evaluate the anxiolytic/anxiogenic properties of drugs.24 These results are consistent with data reported in the literature on the anxiolytic potential of imidic compounds.25,26 These results are important for molecules with anti-Alzheimer's potential because patients with this pathology develop neuropsychiatric disorders such as anxiety and depression as the disease progresses.27–29 The thiocholinesterase agents used in the treatment have no effect on such disturbances. The results demonstrate that all the compounds produced anxiolytic-like effects by increasing the open arm permanency time when compared to the vehicle group (p < 0.01 and p < 0.001). Sham and naive did not have pharmacology profiles distinct from the vehicle group.
Table 3. Effects of treatment on animals subjected to the elevated plus maze test.
| Compound | Open arm frequency a (%) | Open arm time permanency a (%) |
| 1 | 25.11 | 14.30 |
| 2 | 47.70 | 19.30 |
| 3 | 43.31 | 17.10 |
| 4 | 69.44*** | 76.00** |
| 5 | 41.90** | 42.00** |
| Sham | 79.40** | 52.60*** |
| Naive | 67.34** | 38.7** |
| Vehicle | 51.17** | 37.6*** |
| Galantamine | 22.30 | 6.60 |
aEffects of compounds 1, 2, 3, 4 and 5 (30 mg kg–1 i.p.) and galantamine (0.5 mg kg–1 i.p.) after inducing AD with STZ on mice tested in the elevated plus maze test. Asterisks denote significant differences when compared to the vehicle group (**p < 0.01, ***p < 0.001).
Effects of the compounds on the behaviors of animals with streptozotocin-induced Alzheimer's disease
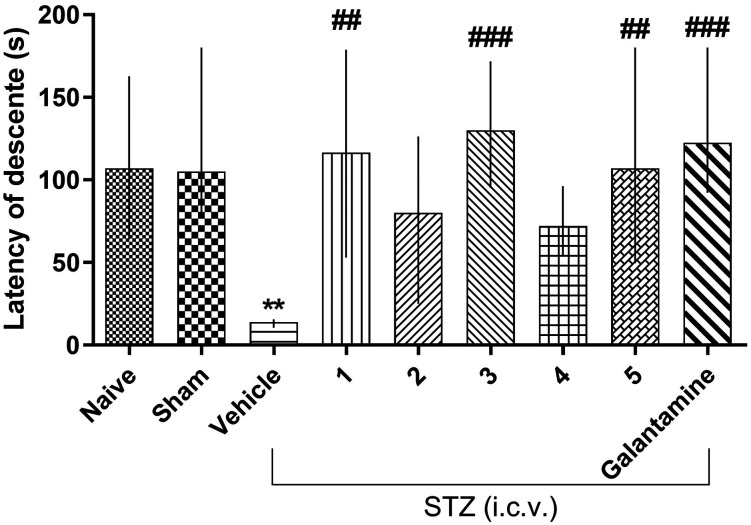
The effects of the compounds on the memory of the animals with Alzheimer's disease induced by streptozotocin are shown in Table 4 and Fig. 4. The results demonstrate that compounds 1, 3 and 5 (Fig. 4) promoted a statistically significant reversal of the cognitive deficit induced by STZ when compared to the vehicle group (p < 0.01 and p < 0.001) in the inhibitory avoidance test session. Furthermore, the results also show that the animals treated with the vehicle did not learn the inhibitory avoidance task, proving that STZ generated cognitive deficits in the mice. Additionally, galantamine used as a positive control was effective in reverting those deficits, as expected (p < 0.001). The results also point out that sham and naive animals exhibited the same behavioral profile in the inhibitory avoidance test; in other words, they learned the task because there were significant training and test session differences. Equally important, the chirurgical practice for STZ i.c.v. administration did not interfere in the animals' behavioral patterns, as demonstrated by the sham group. In addition, the result demonstrates that compound 3 has a nootropic activity similar to galantamine.
Table 4. Inhibitory avoidance test session descent latency of animals treated with the test compounds and/or vehicle submitted to a streptozotocin-induced Alzheimer's model.
| Compound | Median of avoidance latency descent a (s) |
| Vehicle | 14.00 (08–16) |
| 1 | 116.50 (53–178) |
| 2 | 80.00 (25–126.3) |
| 3 | 130.00 (87–180) |
| 4 | 72.00 (33–96.25) |
| 5 | 107.00 (49–180) |
| Galantamine | 122.50 (57–180) |
aMedian data following interquartile intervals (25% and 75%).
Fig. 4. Effects of compounds 1, 2, 3, 4 and 5 (30 mg kg–1 i.p.) and galantamine (0.5 mg kg–1 i.p.) after inducing AD with STZ on mice tested in the inhibitory avoidance test. Asterisks denote significant differences when compared to the same treatment training and test sessions. Hash symbols denote statistically significant differences when compared to the control (vehicle) in the test session. Each column represents the experiment median followed by the interquartile interval (25–27). The last chart represents the results referring only to the test session and comparing all treatments to the vehicle, and G is the utilized test model representation.
Effect on brain oxidative stress
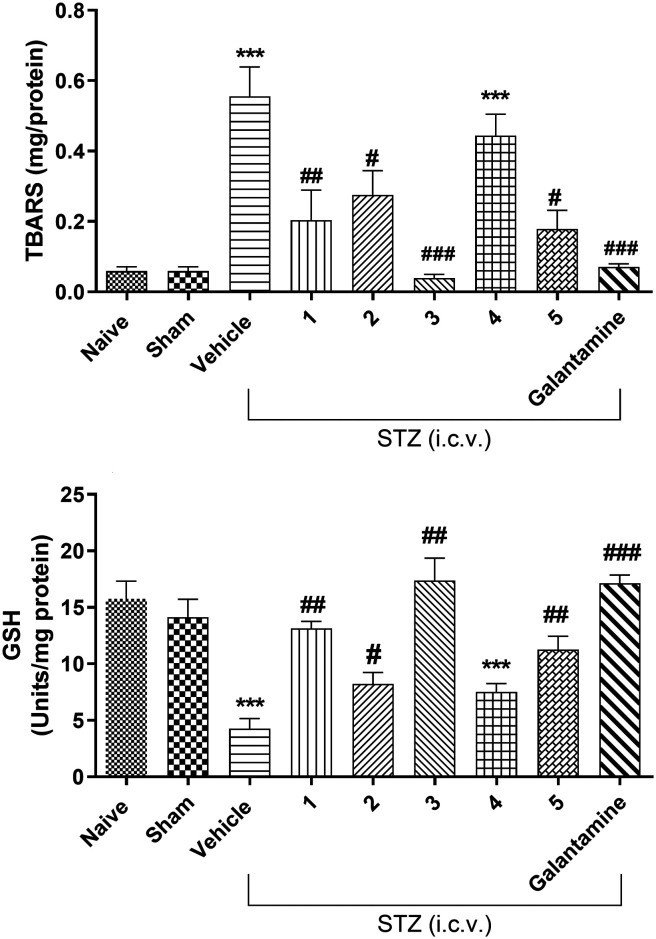
The results demonstrate that STZ i.c.v. markedly enhances the oxidative stress due to the increase of TBARS (thiobarbituric acid reactive substances) and reduction of the GSH levels in the brains of the animals (Fig. 5) when compared to the naïve group. As can be observed, galantamine and all investigated compounds (except for 4) significantly decrease TBAR levels and increase GSH levels when compared to the vehicle (Fig. 5).
Fig. 5. Effects of compounds 1, 2, 3, 4 and 5 (30 mg kg–1 i.p.) and galantamine (0.5 mg kg–1 i.p.) on brain TBARS levels and GSH activity. Bars represent means ± S.E.M. N = 8–10 per group; ***p < 0.001 compared with naive, and ##p < 0.01 or ##p < 0.001 compared with the vehicle. STZ infusion leads to significant alterations in the activities of the antioxidant enzyme (GSH) and TBARS in the hippocampus. Pre-treatment of compounds and galantamine significantly increased the activity of this enzyme and decreased lipid peroxidation. Sham = group of animals that received no treatment with STZ.
As previously reported, AD is a neurodegenerative disease characterized by progressive loss of memory, cognitive impairment, decline of language function, and several behavioral changes including paranoia, delusions, and impaired social functioning.1,2 Particularly, this pathology characteristically presents four alterations that have been studied to obtain a possible treatment: senile plaques originated from the deposition of abnormally produced beta-amyloid (Aβ) protein, neurofibrillary tangles30 created by the hyperphosphorylation of tau protein,31,32 drastic changes of cholinergic neurotransmission, neuroinflammation and oxidative stress.33
Genetic and environmental factors seem to contribute to the development of AD.34 Age, traumatic brain injury, depression, diabetes, exposure to toxic substances, and deficiency of neurotrophic factors may also be determinants for the development of this pathology.35
Most pharmacological therapies currently available are based on anticholinesterase drugs. These drugs only ease the symptoms and do not revert the pathologic condition. In addition, therapeutic approaches produce collateral effects caused by increased levels of ACh, not only in the peripheral and central areas but also in locations that have compatible cholinergic receptors. Occasionally, such adverse effects are so intense that they lead patients to abandon the treatment. In this context, the discovery of new anti-AD agents is urgently needed. Compounds with nootropic and antioxidant properties and that inhibit AChE-derived ACh degradation, as observed for the sulfonamides investigated herein, have the most suitable profile for further development.
Several pharmacological models have been used for the study of potential AD therapeutic agents. The AD model adopted in this study used streptozotocin. STZ is a nitrosourea-glucosamine compound originally identified as an antibiotic. Its administration into animals through routes such as i.c.v. causes them to develop insulin-resistant brains, which causes beta-amyloid aggregates, tau protein deposits, and cognition reduction, a pathologic condition similar to human AD.36 In addition to the symptoms and biochemical and histopathological changes common to AD, behavioral and emotional changes such as anxiety are observed in this animal model.37 In the present study, animals submitted to this AD model had significant cognitive deficits and anxiety. Parameters of oxidative stress were also observed, such as increased lipid peroxidation and decreased GSH activity.
AD diagnosis is mainly based on gradual memory loss. Initially, the patient has spatial memory loss, since the hippocampus is the first cerebral structure to be compromised. With the disease's progression, other memory types such as episodic declarative and semantic memories are affected.6 To evaluate the cognitive deficits in a preclinical study, some tests on animals are used.7
The memory model used in the present study is that of inhibitory avoidance. This test occurs over two days, training being the first one, when the animal is placed in a platform above bars and the latency of its complete descent is timed. This is only considered when the animal places its four paws in the bronze grid; when this happens, the mouse receives electric shocks (0.4 mA0) enduring for 2 seconds until it returns to the platform. On the next day, the test session is conducted while repeating the same procedure already described, except with the absence of shocks. The differences between both sessions are considered the memory index – the longer the animal takes to climb down from the platform on the second day, the better the effect of the tested compound on that animal's memory. The model is broadly used in memory preclinical tests because of its simple execution. In addition, it requires only one training session, which is advantageous compared to the Morris water maze model, for example.38
The obtained results in the inhibitory avoidance test demonstrated memory improvement of animals with STZ-induced Alzheimer's upon treatment with compounds 1, 3 and 5. These outcomes were expected, since these compounds exhibited in vitro anticholinesterase and oxidant effects.39 Compound 3 presented the best results.
During AD progression, several psychiatric symptoms can manifest, among which anxiety is the most common. Recent studies show that the higher the beta-amyloid accumulation, the more severe the anxiety degree will be.40 This factor can be used as an AD preclinical diagnosis; that is, early use, which would make the treatment significantly more effective, could slow and considerably decrease the disease's damage. In relation to the compounds' effects on anxiety, the plus maze test was applied. The model explores the conflict between the rodents' natural tendency to explore new environments and aversion based on natural fear that they show of open spaces and heights.22,23 The studied compounds had anxiolytic-like effects, 3 being the one that exhibited the best results regarding this aspect.
Cyclic imides have been studied in relation to this property and have produced promising results in anxiety treatment, which would be very advantageous not only to the patients with AD but also to those suffering from Parkinson's disease. Indeed, the anxiolytic effects of imidic compounds have already been reported in the literature; for example, Aazza and collaborators (2011)16 obtained positive results for this property and anticonvulsant characteristics in specific pharmacology experiments. Hassanzadeh and collaborators41 also detected these effects in a phthalimide series. Another consideration that can be related to the results for compounds 1, 2, 3 and 5 in the memory tests is their chemical structures. All the studied compounds that manifested change in streptozotocin-induced cognitive deficit and anxiolytic effects have electron donating groups, which can influence the anxiolytic activity and, consequently, the anti-Alzheimer's effect.
Calculation of molecular properties
To further evaluate the pharmacokinetic potential of compounds 1 through 5, their physicochemical and topological properties were calculated. Table 5 presents the octanol–water partition coefficients, mlog P, polar surface area (tPSA), number of atoms, molecular weight (MW), number of H-bond acceptors (HBA), number of H-bond donors (HBD), number of rotatable bonds (NRB), number of violations of Lipinski's rules and molecular volume. The descriptors obtained in silico were compared with the filters for the prediction of the solubility and permeability of drug candidates after oral administration as described by Lipinski,55 Oprea56 and Veber.57
Table 5. Molecular properties of compounds 1 through 5.
| Properties | 1 | 2 | 3 | 4 | 5 |
| mlog P | 2.43 | 2.45 | 1.72 | 3.14 | 3.77 |
| tPSA (Å2) | 66.84 | 92.18 | 92.42 | 79.29 | 86.62 |
| N atoms | 20 | 21 | 16 | 21 | 22 |
| MW | 299.39 | 307.38 | 244.32 | 306.39 | 321.40 |
| HBA | 5 | 6 | 5 | 5 | 5 |
| HBD | 1 | 2 | 4 | 2 | 3 |
| N violations | 0 | 0 | 0 | 0 | 0 |
| NRB | 3 | 4 | 3 | 4 | 4 |
| Molar volume (Å3) | 268.14 | 265.50 | 213.69 | 269.66 | 281.83 |
The results show that all of the sulfonamides evaluated are in accordance with Lipinski's rules: molecular weight (MW) ≤ 500, HBD ≤ 5, HBA ≤ 10 and log P ≤ 5.0 (Table 5). According to Lipinski, a compound whose data do not adhere to the rule will likely be poorly bioavailable because of poor absorption or permeation.55 The values for the compounds were MW 344.32–321.40 Da, HBD 1–4 and HBA 5–6.
The sulfonamides derived from carvacrol also satisfied Oprea's criteria, which additionally include: the number of rings ≤5 and mlog P in the range of –2.0 to 4.5. The compounds possessed between one and two rings, and their mlog P values were in the range of 1.72–3.77. To complement the previously mentioned results, Veber proposed a filter of two properties: the number of HBD and HBA ≤ 12 (tPSA ≤ 140 Å2), and the number of rotatable bonds (NRB) ≤ 10. All sulfonamides (1 to 5) meet the above criteria. It is postulated that limited molecular flexibility, expressed as the NRB, and low polar surface area (tPSA) are important predictors of oral bioavailability, independent of the molecular weight. The results reported herein show that the compounds analyzed would have favorable pharmacokinetics on application; that is, the compounds exhibit solubility and permeability after the oral administration of drug candidates.
Conclusions
As previously reported, AD is a neurodegenerative disease characterized by progressive loss of memory, cognitive impairment, decline of language function, and several behavioral changes including paranoia, delusions, and impaired social functioning.1,2 Particularly, this pathology characteristically presents four alterations that have been studied to obtain a possible treatment: senile plaques originated from the deposition of abnormally produced beta-amyloid (Aβ) protein, neurofibrillary tangles30 created by the hyperphosphorylation of tau protein,31,32 drastic changes of cholinergic neurotransmission, neuroinflammation and oxidative stress.33 Genetic and environmental factors seem to contribute to the development of AD.34 Age, traumatic brain injury, depression, diabetes, exposure to toxic substances, and deficiency of neurotrophic factors may also be determinants for the development of this pathology.35
Most pharmacological therapies currently available are based on anticholinesterase drugs. These drugs only ease the symptoms and do not revert the pathologic condition. In addition, therapeutic approaches produce collateral effects caused by increased levels of ACh, not only in the peripheral and central areas but also in locations that have compatible cholinergic receptors. Occasionally, such adverse effects are so intense that they lead patients to abandon the treatment. In this context, the discovery of new anti-AD agents is urgently needed. Compounds with nootropic and antioxidant properties and that inhibit AChE-derived ACh degradation, as observed for the sulfonamides investigated herein, have the most suitable profile for further development.
Considering the findings of this study, the evaluated sulfonamide compounds have promising effects that could be beneficial in the treatment of AD. All the sulfonamides synthesized were more active than galantamine and more active than the natural product carvacrol, which was used in synthesis. The results obtained in the in vivo experiments are consistent with previous in vitro investigations on the anticholinesterase and antioxidant characteristics that are essential to AD treatment. Moreover, some pharmacokinetic parameters were calculated, and all the investigated sulfonamides derived from carvacrol would have favorable pharmacokinetics on application and possess drug-like properties.
Experimental section
Chemistry
The preparation of sulfonamides derived from carvacrol, whose detailed description is not the objective of this article, was described recently by our research group,21 was performed in two steps, and all the solvents used were analytically pure.
AChE inhibitory activity
The enzymatic inhibition was measured using the method described by Oliveira and collaborators.39 Briefly, 90 μL of 50 mmol L–1 Tris-HCl buffer pH 8.0 and 30 μL of a buffer solution containing the compound (0.1 mg mL–1) dissolved in MeOH and 15 μL of an AChE solution containing 0.25 U mL–1 were dissolved in 50 mmol L–1 Tris-HCl pH 8 buffer containing 0.1% bovine serum albumin, and then the solution was incubated for 15 min. Then, 25 μL of an acetylthiocholine iodide solution (15 mmol in water) and 140 μL of Ellman's reagent (3 mmol L–1 in Tris-HCl pH 8.0 buffer containing 0.1 mol L–1 NaCl and 0.02 mol L–1 MgCl2) were added, and the final mixture was incubated for another 30 min at 28 °C. The absorbance of the mixture was measured at 405 nm. The same solvent in which the sample was dissolved, considered to have 100% AChE activity, was used as the negative control. The inhibition (%) was calculated as follows, in which Asample is the absorbance of the sample and Acontrol is the absorbance without the sample:I(%) = (100 – Asample/Acontrol) × 100
Animals and treatment
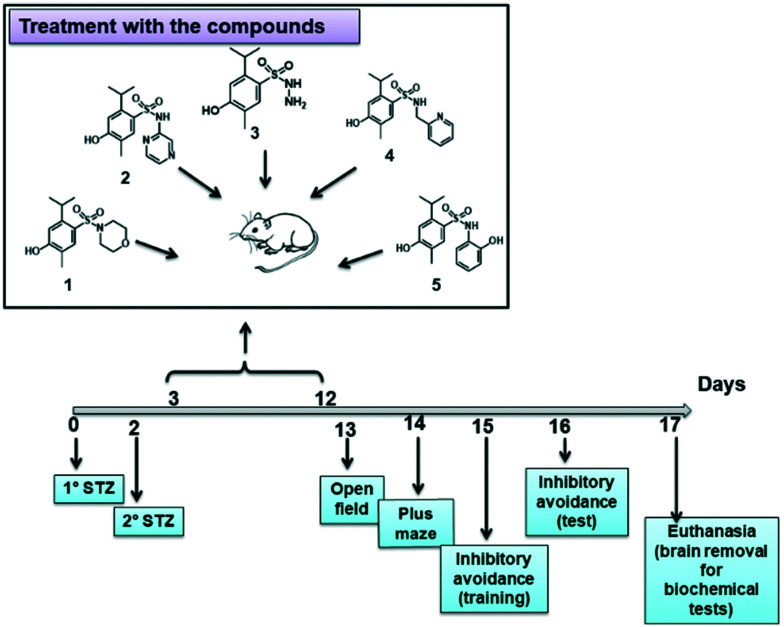
All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of “Laboratório de Farmacologia Experimental in vivo” from the “University of Vale do Itajaí” and approved by the Animal Ethics Committee under the number 045/14 CEUA/Univali. The testing was conducted according to Fig. 6. In the pharmacology experiments, 3 month old female mice (25.0–30.0 g) were used, and they were maintained at 22–27 °C with free water and food access with a light/dark cycle of 12 : 12 hours, except during the tests. The number (N) of animals for each experimental group was approximately 8 to 10.
Fig. 6. Diagram of the experiments performed.
Streptozotocin Alzheimer's induction model
The streptozotocin model was used to induce Alzheimer's disease. The procedures were realized as described by Pinton,24 with some modifications. The mice, previously anesthetized with xylazine/ketamine (1.0 mg mL–1 i.p.), were subjected to a small chirurgic procedure in order to remove the cutaneous tissue, exposing the cranium. For this purpose, before the procedure, a local anaesthetic agent with epinephrine vasoconstrictor (Xylestesin 2% s.c.) was administered. Posteriorly, the animals were subjected to the intracerebroventricular infusion of 3 μL of STZ (2.5 mg mL–1) solution. After 48 hours of the first STZ infusion, the method was repeated. The infusion location was based on the bregma fissure, wherein the injection site was 1 mm to the right or left from the cranial fissure central point, directly in the cerebral ventricle. The instrumentation utilized for the i.c.v. injection constituted one hypodermic needle coupled in a 5 μL Hamilton syringe. After the induction period, the animals were treated and evaluated in the memory, ambulation and anxiety test models.
Effects of compounds on behaviors of animals with streptozotocin induced Alzheimer's disease
Subsequent to the AD induction, the animals were separated into distinct groups and treated until the 12th day. The following were designated as experimental groups: naïve group, animals that received no STZ i.c.v. infusion; sham group, animals that receive no STZ i.c.v. infusion but suffered the surgical procedure; 5 groups that received STZ i.c.v. infusion and were treated with compounds 1, 2, 3, 4 and 5 (30 mg kg–1, i.p.); a positive control group that received STZ i.c.v. infusion and was treated with galantamine (0.5 mg kg–1, i.p.). After the last treatment day, the animals were subjected to the behavioural tests as shown in Fig. 6.
Open field test
To verify the effects of treatment with compounds upon locomotor activity, the animals were placed for 5 min in the open field arena. The apparatus, made of wood, had a black floor of 30 cm × 30 cm (divided by white lines into nine squares of 10 cm × 10 cm) and transparent walls, which were 15 cm high. The experiments were conducted in a sound-attenuated room under low-intensity light (12 lx). Each mouse was placed in the center of the open field, and the numbers of squares crossed (crossings) and exploratory behaviors (rearings) were registered.42
Elevated plus maze test
With the objective of verifying whether the animals' anxiety rates could influence the compound effects in the memory tests, the animals were also tested in the elevated plus maze (EPM).24 The animals were individually placed in the EPM center, pointed to one of the closed arms and observed for 5 min. During this time, the period that the animals spent exploring the open and closed arms, and their entry frequencies were registered. The entrances were considered as when a mouse was positioned with all four paws on the visited arm.43
Inhibitory avoidance test
The tool used for normal animal memory evaluation was a 50 cm length, 25 cm width and 25 cm height box, with a grid base of 1 mm diameter and space of 1 cm bronze bars. In the training session, the animal was placed on the platform and the latency that it took to climb down from it (with four paws on the bronze grid) was timed. When that occurred, the animal received an electric shock (0.4 mA°) for 2 seconds and was waited until it returned to the platform. On the next day, each trained animal was subjected to the same procedure already described, but omitting the electric shocks, and the time of latency to descent the platform was measured.23
Biochemical determinations
One day after the last behavioural analysis, the animals were killed by cervical dislocation; the brains were removed and homogenized in phosphate buffer (pH of 7.4). The homogenates were then centrifuged at 3000g for 15 min. The supernatants of the homogenates were used for biochemical estimations using the methods described below.
Estimation of the brain thiobarbituric acid reactive species (TBARS) level
Lipid peroxidation is measured by the production of malondialdehyde (MDA), achieved spectrophotometrically by the method of Ohkawa et al. (1979)44 with some modifications,45 using 1,1,3,3-tetraethoxypropane as the standard and expressed as nmol per mg protein. The reagents, 1.5 ml acetic acid (20%) at pH 3.5, 1.5 ml thiobarbituric acid (0.8%) and 0.2 ml sodium dodecylsulfate (8.1%), were added to 0.1 ml of the processed sample. This mixture was then heated at 100 °C for 60 min, then cooled under tap water, and 5 ml of n-butanol : pyridine (15 : 1% v/v) with 1 ml of distilled water was added. The mixture was shaken vigorously using a vortex. After centrifugation at 4000g for 10 min, the organic layer was withdrawn, and absorbance was measured at 532 nm.
Estimation of the brain reduced glutathione (GSH) level
The whole brain GSH level was measured by the method of Beutler et al. (1963),46 with slight modifications (Amoah et al., 2015).45 The absorbance was measured at 412 nm (DU640B spectrophotometer, Beckman Coulter Inc., CA, USA). Different concentrations of the GSH standard were processed similarly to prepare a standard curve (5–50 μg). Results were expressed as nmol of GSH/mg of protein.
Computational modeling
Ligand preparation
The 2D structural representations were constructed on Canvas.47 To simulate the molecular docking, it is relevant to prepare the ligands in their 3D-dimensional conformation. Thus, LigPrep48 was used to convert the 2D representations into 3D. First, the protonation states of the ligands at pH 8.0 were predicted by Epik.49 Next, the energy minimization process was performed by applying water as a solvent and the OPLS3 force field.50 The OPLS3 force field was chosen because it has a good charge model and good accuracy in the torsion parameters compared to the conventional force fields.50
Preparation of human AChE
In molecular docking studies, the structure of human acetylcholinesterase (PDB ID 4M0E,22 2 Å resolution) was selected. The selection of this structure was based on the presence of a cocrystallized compound at the active site and on the resolution of the structure. Molecular docking was used to generate the poses of the ligands in the active site and to extract the information relevant to the structure–activity relationship (SAR) studies. The protein was prepared using the Protein Preparation Wizard module.51 The high occupancies were selected. The preparation of the protonation states of the amino acid side chains was carried out by the Jensen method52 at pH 8.0. Finally, the macromolecule was minimized using the OPLS3 force field.50
Molecular docking
In the molecular docking runs, GOLD 5.2 (Cambridge Crystallographic Center, Cambridge) was used.53 The software uses a genetic algorithm to generate the binding conformations within the binding site.53 Based on the centroid of the cocrystallized compound, the active site was defined as a sphere of radius 4 Å. The previously prepared ligands were submitted to genetic algorithm parameters such as population size, selection pressure, operation number, island number, niche size, crossing frequencies and mutation, and migration frequency of 100, 1.1, 100 000, 5, 2, 95 and 10, respectively. The predicted docking modes were evaluated by the force-field-based fitness function GoldScore.54 The top conformations were considered by considering the best scoring values and were analyzed in the SAR studies.
Calculation of molecular properties
The ChemSketch tool was used to draw all the molecules. Multiple tools and online servers such as Molinspiration (http://www.molinspiration.com/) and Molsoft (; http://www.molsoft.com/mprop/) were employed to check the chemoinformatics properties of these compounds.
Statistical analysis
For the parametric tests (open field and EPM), the obtained data were presented as the means followed by the respective standard error of the mean, and the data were subjected to variation analysis (ANOVA); when necessary, Tukey's multiple comparison tests were utilized through the GraphPad INSTAT® software. For the non-parametric test (inhibitory avoidance), the Mann–Whitney post hoc test was employed. The results were presented as median values followed by the interquartile intervals. p values smaller than 0.05 (p < 0.05) were considered as statistically significant.45
Conflicts of interest
There are no conflicts to declare.
Acknowledgments
The authors wish to thank Central Analysis (Department of Chemistry-UFSC) for spectroscopic analysis. The authors acknowledge the National Council for Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher Education Personnel (CAPES), and the Sao Paulo Research Foundation (FAPESP, CIBFar grant 2013/07600-3), Brazil, for financial support.
References
- Price M., Ghercet M. and Prina M., The Epidemiology and Impact of Dementia: Current State and Future Trends. WHO Thematic Briefing, World Health Organization, 2015, pp. 1–4.
- Kim D. H., Yeo S. H., Park J. M., Choi J. Y., Lee T. H., Park S. Y., Ock M. S., Eo J., Kim H. S., Cha H. J. Gene. 2014;545:185–193. doi: 10.1016/j.gene.2014.05.031. [DOI] [PubMed] [Google Scholar]
- Teixeira J. B., Souza Junior P. R. B., Higa J., Theme Filha M. M. Cad. Saude Publica. 2015;31:850–860. doi: 10.1590/0102-311x00144713. [DOI] [PubMed] [Google Scholar]
- Sindi S., Calov E., Fokkens J., Ngandu T., Soininen H., Tuomilehto J., Kivipelto M. Alzheimers. Dement. 2015;1:328–333. doi: 10.1016/j.dadm.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner J., Rupsingh R., Smith M., Wells J. L., Borrie M. J., Bartha R. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:104–110. doi: 10.1016/j.pnpbp.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Hollands C., Bartolotti N., Lazarov O. Front. Neurosci. 2016;10:178–182. doi: 10.3389/fnins.2016.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epis R., Gardoni F., Marcello E., Genazzani A., Canonico P. L., Di Luca M. Eur. J. Pharmacol. 2010;626:57–63. doi: 10.1016/j.ejphar.2009.10.020. [DOI] [PubMed] [Google Scholar]
- Pereira M. E., Adams A. I. H., Silva N. S. Toxicol. Lett. 2004;146:269–274. doi: 10.1016/j.toxlet.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Kulshreshtha A., Piplani P. Neurol. Sci. 2016;37:1403–1435. doi: 10.1007/s10072-016-2625-7. [DOI] [PubMed] [Google Scholar]
- Gonçalves J. C. R., Oliveira F. S., Benedito R. B., De Sousa D. P., Almeida R. N., Araffljo D. A. M. Biol. Pharm. Bull. 2008;31:1017–1020. doi: 10.1248/bpb.31.1017. [DOI] [PubMed] [Google Scholar]
- Quintans Júnior L. J., Almeida J. R. G. S., Lima J. T., Nunes X. P., Siqueira J. S., de Oliveira L. E. G., Almeida R. N., de Athayde-Filho P. F., Barbosa-Filho J. M. Rev. Bras. Farmacogn. 2008;18:798–819. [Google Scholar]
- Turgut K., Tugrul Ay S. Afr. J. Tradit., Complementary Altern. Med. 2009;6:355. [Google Scholar]
- Cavalcante Melo F. H., Rios E. R., Rocha N. F., do Citó Mo C., Fernandes M. L., de Sousa D. P., de Vasconcelos S. M., de Sousa F. C. F. J. Pharm. Pharmacol. 2012;64(12):1722–1729. doi: 10.1111/j.2042-7158.2012.01552.x. [DOI] [PubMed] [Google Scholar]
- Guimarães A. G., Oliveira G. F., Melo M. S., Cavalcanti S. C., Antoniolli A. R., Bonjardim L. R., Silva F. A., Santos J. P., Rocha R. F., Moreira J. C., Araújo A. A., Gelain D. P., Quintans-Júnior L. J., Guimarães A. G. Basic Clin. Pharmacol. Toxicol. 2010;107(6):949–957. doi: 10.1111/j.1742-7843.2010.00609.x. [DOI] [PubMed] [Google Scholar]
- Maksimovic M., Milos M., Milos M. Phytother. Res. 2007;21(3):259–261. doi: 10.1002/ptr.2063. [DOI] [PubMed] [Google Scholar]
- Aazza S., Lyoussi B., Miguel M. G. Molecules. 2011;16:7672–7690. doi: 10.3390/molecules16097672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt B. Z., Gazioglu I., Dag A., Salmas R. E., Kayık G., Durdagi G. S., Sonmez F. Bioorg. Med. Chem. 2017;15(25):1352–1363. doi: 10.1016/j.bmc.2016.12.037. [DOI] [PubMed] [Google Scholar]
- Crump C. J., Murrey H. E., Ballard T. E., Am Ende C. H., Wu X., Gertsik N., Johnson D. S., Li Y. ACS Chem. Neurosci. 2016;7:1166–1173. doi: 10.1021/acschemneuro.6b00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodney M. A., Barreiro G., Ogilvie K., Hajos-Korcsok E., Murray J., Vajdos F., Ambroise C., Christoffersen C., Fisher K., Lanyon L., Liu J., Nolan C. E., Withka J. M., Borzilleri K. A., Efremov I., Oborski C. E., Varghese A., O'Neill B. T. J. Med. Chem. 2012;55:9224–9239. doi: 10.1021/jm3009426. [DOI] [PubMed] [Google Scholar]
- Masand N., Gupta S. P., Khosa R. L. Curr. Comput.-Aided Drug Des. 2018;14(4):338–348. doi: 10.2174/1573409914666180604115425. [DOI] [PubMed] [Google Scholar]
- de Oliveira A. S., Llanes L. C., Brighente I. M. C., Nunes R. J., Yunes R. A., Máximo Junior N. M., Baumgart A. M. K., Aust A. N., Cruz A. B. J. Biosci. Med. 2016;4:105–114. [Google Scholar]
- Cheung J., Gary E. N., Shiomi K., Rosenberry T. L. ACS Med. Chem. Lett. 2013;4(11):1091–1096. doi: 10.1021/ml400304w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mury F. B., da Silva W. C., Barbosa N. R., Mendes C. T., Bonini J. S., Sarkis J. E., Cammarota M., Izquierdo I., Gattaz W. F., Dias-Neto E. Eur. Arch. Psychiatry Clin. Neurosci. 2016;266(7):607–618. doi: 10.1007/s00406-015-0665-2. [DOI] [PubMed] [Google Scholar]
- Pinton S., da Rocha J. T., Zeni G., Nogueira C. W. Neurosci. Lett. 2010;472:56–60. doi: 10.1016/j.neulet.2010.01.057. [DOI] [PubMed] [Google Scholar]
- Hassanzadeh F., Rabbani M., Khodarahmi G. A., Moosavi M. Iran. J. Pharm. Res. 2012;11(1):109–115. [PMC free article] [PubMed] [Google Scholar]
- Kossakowski J., Jarocka-Wierzba M. Acta Pol. Pharm. 2003;60(5):367–374. [PubMed] [Google Scholar]
- Gulpers B. J. A., Oude Voshaar R. C., van Boxtel M. P. J., Verhey F. R. J., Köhler S. Am. J. Psychiatry. 2019;27(1):42–52. doi: 10.1016/j.jagp.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Forlenza O. V., Loureiro J. C., Pais M. V., Stella F. Curr. Opin. Psychol. 2017;30(2):151–158. doi: 10.1097/YCO.0000000000000309. [DOI] [PubMed] [Google Scholar]
- Moraros J., Nwankwo C., Patten S. B., Mousseau D. D. Depression Anxiety. 2017;34(3):217–226. doi: 10.1002/da.22584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozin S. A., Barykin E. P., Mitkevich V. A., Makarov A. A. Biochemistry. 2018;83(9):1057–1067. doi: 10.1134/S0006297918090079. [DOI] [PubMed] [Google Scholar]
- Congdon E. E., Sigurdsson E. M. Nat. Rev. Neurol. 2018;14(7):399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pchitskaya E. I., Zhemkov V. A., Bezprozvanny I. B. Biochemistry. 2018;83(9):1068–1074. doi: 10.1134/S0006297918090080. [DOI] [PubMed] [Google Scholar]
- Grossberg S. Front. Neural Circuits. 2017;11(82):1–25. doi: 10.3389/fncir.2017.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas G., Veltman J. A. Acta Neuropathol. 2019;137:183–207. doi: 10.1007/s00401-018-1939-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird T. D., GeneReviews®, 1998, pp. 1993–2019. [Google Scholar]
- Ravelli K. G., Rosário B. D., Camarini R., Hernandes M. S., Britto L. R. Neurotoxic. Res. 2017;31(3):327–333. doi: 10.1007/s12640-016-9684-7. [DOI] [PubMed] [Google Scholar]
- Pinton S., da Rocha J. T., Gai B. M., Nogueira C. W. Behav. Brain Res. 2011;30(223):1–6. doi: 10.1016/j.bbr.2011.04.014. [DOI] [PubMed] [Google Scholar]
- Crystal J. D. J. Exp. Anal. Behav. 2016;105(1):56–67. doi: 10.1002/jeab.173. [DOI] [PubMed] [Google Scholar]
- Oliveira A. S., Meier L., Zapp E., Brondani D., Brighente I. M. C., Sá M. M. J. Braz. Chem. Soc. 2019;30(5):1045–1054. [Google Scholar]
- España J., Giménez-Llort L., Valero J., Miñano A., Rábano A., Rodriguez-Alvarez J., LaFerla F. M., Saura C. A. Biol. Psychiatry. 2010;67(6):513–521. doi: 10.1016/j.biopsych.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Hassanzadeh F., Rabbani M., Khodarahmi G. A., Moosavi M. Iran. J. Pharm. Res. 2012;11(1):109–115. [PMC free article] [PubMed] [Google Scholar]
- Tolardo R., Zetterman L., Bitencourtt D. R., Mora T. C., de Oliveira F. L., Biavatti M. W., Amoah S. K., Bürger C., de Souza M. M. J. Ethnopharmacol. 2010;128:63–70. doi: 10.1016/j.jep.2009.12.026. [DOI] [PubMed] [Google Scholar]
- File S. E., Lippa A. S., Beer B., Lippa M. T. Curr. Protoc. Neurosci. 2004;26:8.3.1-8.3.22. doi: 10.1002/0471142301.ns0803s26. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Amoah S. K., Dalla Vecchia M. T., Pedrini B., Carnhelutti G. L., Gonçalve A. E., dos Santos D. A., Biavatti M. W., de Souza M. M. Eur. J. Pharmacol. 2015;15(769):195–202. doi: 10.1016/j.ejphar.2015.11.018. [DOI] [PubMed] [Google Scholar]
- Beutler E., Duron O., Kelly B. M. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- Canvas, Schrödinger Release 2016-3, 2016.
- LigPrep, Schrödinger Release 2016-3, 2016.
- Shelley J. C., Cholleti A., Frye L. L., Greenwood J. R., Timlin M. R., Uchimaya M. J. Comput.-Aided Mol. Des. 2007;21:681–691. doi: 10.1007/s10822-007-9133-z. [DOI] [PubMed] [Google Scholar]
- Harder E., Damm W., M J., Wu C., Reboul M., Xiang J. Y., Wang L., Lupyan D., Dahlgren M. K., Knight J. L., Kaus J. W., Cerutti D. S., Krilov G., Jorgensen W. L., Abel R., Friesner R. A. J. Chem. Theory Comput. 2016;12:281–296. doi: 10.1021/acs.jctc.5b00864. [DOI] [PubMed] [Google Scholar]
- Protein-Preparation-Wizard. Schrödinger Release 2016-3, 2016.
- Rostkowski M., Olsson M. H., Søndergaard C. R., Jensen J. H. BMC Struct. Biol. 2011;11:1–6. doi: 10.1186/1472-6807-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G., Willett P., Glen R. C., Leach A. R., Taylor R. J. Mol. Biol. 1997;267:727–748. doi: 10.1006/jmbi.1996.0897. [DOI] [PubMed] [Google Scholar]
- Verdonk M. L., Cole J. C., Hartshorn M. J., Murray C. W., Taylor R. D. Proteins: Struct., Funct., Genet. 2003;52:609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
- Lipinski C. A., Lombardo F., Dominy B. W., Feeney P. J. Adv. Drug Delivery Rev. 1997;23:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- Oprea T. I., Gottfries J., Sherbukhin V., Svensson V., Kuhler T. C. J. Mol. Graphics Modell. 2000;18:512. doi: 10.1016/s1093-3263(00)00066-8. [DOI] [PubMed] [Google Scholar]
- Veber D. F., Johnson S. R., Cheng H. Y., Smith B. R., Ward K. W., Kopple K. D. J. Med. Chem. 2002;45:2615. doi: 10.1021/jm020017n. [DOI] [PubMed] [Google Scholar]