Background: Some patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have neurologic symptoms (1–3). Some observers propose that these symptoms are caused by viral infection of nerve cells (4), but the possibility exists that these symptoms might be produced by autoimmune mechanisms (1–4). Myasthenia gravis is an autoimmune disease in which antibodies bind to acetylcholine receptors (AChRs) or to functionally related molecules in the postsynaptic membrane at the neuromuscular junction (5).
Objective: To describe 3 patients without previous neurologic or autoimmune disorders who were diagnosed with myasthenia gravis after the onset of coronavirus disease 2019 (COVID-19).
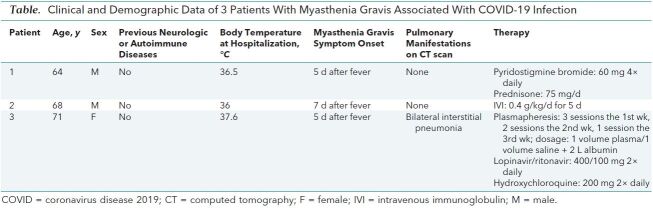
Case Report: Patient 1 was a 64-year-old man who had fever as high as 39 °C for 4 days. Five days after fever onset, he developed diplopia and muscular fatigability. Although his chest radiograph was normal, nasopharyngeal swab and real-time reverse transcriptase polymerase chain reaction (RT-PCR) testing for COVID-19 showed a positive result. We suspected myasthenia gravis because of his symptoms. His neurologic examination was unremarkable. Computed tomography (CT) of the thorax excluded thymoma. Repetitive stimulation of the facial nerve showed a 57% decrement, confirming involvement of the postsynaptic neuromuscular junction, and the concentration of AChR antibodies in his serum was elevated (22.8 pmol/L; normal value, <0.4 pmol/L). We administered pyridostigmine bromide and prednisone, and the patient had a response typical for someone with myasthenia gravis.
Patient 2 was a 68-year-old man who had fever as high as 38.8 °C for 7 days. On day 7, he developed general muscular fatigability, diplopia, and dysphagia. Although his chest CT scan was normal, nasopharyngeal swab and RT-PCR testing for COVID-19 yielded positive results. We suspected myasthenia gravis because of his symptoms. His neurologic examination was normal, and his chest CT scan excluded thymoma. Repetitive nerve stimulation showed a postsynaptic deficit of neuromuscular transmission of the facial (52%) and ulnar (21%) nerves. His serum AChR antibody level was elevated (27.6 pmol/L). He improved after 1 cycle of intravenous immunoglobulin treatment.
Patient 3 was a 71-year-old woman who had a cough and fever to 38.6 °C for 6 days. Nasopharyngeal swab and RT-PCR testing for COVID-19 showed a negative result. Five days after her symptoms began, she developed bilateral ocular ptosis, diplopia, and hypophonia. Thorax CT revealed bilateral interstitial pneumonia and excluded thymoma. One day later, she developed dysphagia and respiratory failure and was transferred to the intensive care unit, where she received mechanical ventilation through a tracheostomy. Repetitive nerve stimulation showed a postsynaptic deficit of neuromuscular transmission of the ulnar nerve (56%), and her serum AChR antibody level was elevated (35.6 pmol/L). Five days later, she had a second nasopharyngeal swab test for COVID-19, and the result was positive. Plasmapheresis was started; she improved and was extubated. This patient received hydroxychloroquine the day after the onset of her first neurologic symptoms (withdrawn a day later), so we do not believe that it caused her symptoms of myasthenia gravis.
Additional information about these patients is provided in the Table.
Table. Clinical and Demographic Data of 3 Patients With Myasthenia Gravis Associated With COVID-19 Infection.
Discussion: We describe what we believe are the first 3 reported cases of AChR antibody–positive myasthenia gravis after COVID-19. These observations are consistent with reports of other infections that induce autoimmune disorders, as well as with the growing evidence of other neurologic disorders with presumed autoimmune mechanisms after COVID-19 onset (1–3). We note that symptoms of myasthenia gravis appeared within 5 to 7 days after fever onset in all 3 patients, and the time from presumed infection with SARS-CoV-2 to the beginning of myasthenia gravis symptoms is consistent with the time from infection to symptoms in other neurologic disorders triggered by infections (2, 3). Several possible explanations exist. For example, antibodies that are directed against SARS-CoV-2 proteins may cross-react with AChR subunits, because the virus has epitopes that are similar to components of the neuromuscular junction; this is known to occur in other neurologic autoimmune disorders after infection. Alternatively, COVID-19 infection may break immunologic self-tolerance.
Footnotes
This article was published at Annals.org on 10 August 2020
References
- 1.Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS-CoV-2 infection [Letter]. N Engl J Med. 2020;382:2268-2270. [PMID: 32294339] doi:10.1056/NEJMc2008597 [DOI] [PMC free article] [PubMed]
- 2.Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? [Letter]. Lancet Neurol. 2020;19:383-384. [PMID: 32246917] doi:10.1016/S1474-4422(20)30109-5 [DOI] [PMC free article] [PubMed]
- 3.Toscano G, Palmerini F, Ravaglia S, et al. Guillain-barré syndrome associated with SARS-CoV-2 [Letter]. N Engl J Med. 2020;382:2574-2576. [PMID: 32302082] doi:10.1056/NEJMc2009191 [DOI] [PMC free article] [PubMed]
- 4.Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020;92:552-555. [PMID: 32104915] doi:10.1002/jmv.25728 [DOI] [PMC free article] [PubMed]
- 5.Gilhus NE. Myasthenia gravis. N Engl J Med. 2016;375:2570-2581. [PMID: 28029925] doi:10.1056/NEJMra1602678 [DOI] [PubMed]