SUMMARY
SETTING:
In Côte d’Ivoire, more than 2000 human immunodeficiency virus (HIV) infected children aged <15 years were started on antiretroviral therapy (ART) during 2004–2008.
OBJECTIVES:
To estimate tuberculosis (TB) incidence and determinants among ART enrollees.
DESIGN:
A nationally representative retrospective cohort study among 2110 children starting ART during 2004–2008 at 29 facilities.
RESULTS:
At ART initiation, the median age was 5.1 years; 82% had World Health Organization Stage III/IV, median CD4% was 11%, 42% were severely undernourished (weight-for-age Z-score [WAZ] <−3), and 150 (7%) were taking anti-tuberculosis treatment. Documentation of TB screening before ART declined from 63% to 46% during 2004–2008. Children taking anti-tuberculosis treatment at ART enrollment had a lower median CD4% (9.0% vs. 11.0%, P = 0.037) and a higher prevalence of WAZ <−3 (59% vs. 40%, P < 0.001). Among children considered TB-free at ART enrollment, TB incidence was 6.28/100 child-years during days 0–90 of ART, declining to 0.56/100 child-years after 180 days. Children with one unit higher WAZ at ART enrollment had 13% lower TB incidence (adjusted HR 0.87, 95%CI 0.77–1.00, P = 0.047).
CONCLUSIONS:
Ensuring clinician compliance with TB screening before ART and ensuring earlier ART initiation before children suffer from advanced HIV disease and nutritional compromise might reduce TB morbidity during ART.
Keywords: pediatric, incident tuberculosis, tuberculosis screening, Ivory Coast
RESUME
CONTEXTE :
En Côte d’Ivoire, plus de 2000 enfantsâgés de <15 ans positifs pour le virus de l’immuno-déficience humaine (VIH) ont débuté un traitement antirétroviral (ART) entre 2004 et 2008.
OBJECTIF :
Estimer l’incidence de la tuberculose (TB) et ses déterminants chez ces enfants sous ART.
SCHÉMA :
Une étude rétrospective de cohorte, représentative au niveau national, sur 2110 enfants ayant débuté leur ART entre 2004 et 2008 dans 29 établissements.
RÉSULTATS :
Lors de la mise en route du traitement, l’âge médian était de 5,1 ans, 82% étaient au Stade III-IV selon la classification de l’Organisation Mondiale de la Santé, le taux médian de CD4 était de 11%, 42% souffraient de malnutrition grave (score Z du rapport poids/âge [WAZ] <−3) et 7% (n = 150) recevaient un traitement antituberculeux. Le dépistage de la TB avant la mise en route de l’ART a décline de 63% à 46% de 2004 à 2008. Les enfants sous traitement anti-tuberculeux lors du démarrage de l’ART avaient un taux médian de CD4 plus bas (9% contre 11%; P = 0,037) et une prévalence plus élevée de malnutrition grave (<−3) (59% contre 40%; P < 0,001). Parmi les enfants considérés comme exempts de TB lors de l’inclusion, l’incidence de la TB a été de 6,28/100 enfants-années pendant les 90 premiers jours du ART, tombant à 0,56/100 enfants-années au-delà de 180 jours. Les enfants dont le WAZ était plus élevé d’un degré ont eu une incidence de TB inférieure de 13% (aHR 0,87 ; IC95% 0,77–1.00 ; P = 0,047).
CONCLUSION :
La morbidité de la TB pendant un ART pourrait être réduite à condition que le personnel de santé dépiste systématiquement la TB avant la mise en route du traitement et instaure le ART précocement avant que les enfants ne souffrent d’une infection à VIH avancée et que leur état nutritionnel ne soit compromis.
RESUMEN
MARCO DE REFERENCIA:
En Côte d’Ivoire, más de 2000 niños de <15 años de edad infectados por el virus de la inmunodeficiencia humana (VIH) iniciaron el tratamiento antirretrovírico (ART) entre el 2004 y el 2008.
OBJETIVO:
Calcular la incidencia de tuberculosis (TB) y los factores determinantes de la aparición de TB en los pacientes inscritos en el programa de ART.
MÉTODO:
Se llevó a cabo un estudio retrospectivo de cohortes representativo a escala nacional en 2110 niños que comenzaron el ART entre el 2004 y el 2008 en 29 centros.
RESULTADOS:
Al comienzo del ART la mediana de la edad fue 5,1 años; el 82% de los casos se encontraban en la fase III o IV según la clasificación de la Organización Mundial de la Salud; la mediana del recuento de células CD4 fue 11%; el 42% presentaba una desnutrición grave (puntuación Z del peso según la edad [WAZ] <−3); y 150 niños recibían tratamiento antituberculoso (7%). La documentación de una detección sistemática de la TB antes de iniciar el ART disminuyó de 63% a 46% durante el período del estudio. Los niños que recibían tratamiento antituberculoso en el momento de iniciar el ART presentaban una mediana más baja del recuento de células CD4 (9,0% contra 11,0%; P = 0,037) y una mayor prevalencia de WAZ <−3 (59% contra 40%; P < 0,001). En los niños que se consideraron sin TB en el momento de iniciar el ART, la incidencia de esta enfermedad fue 6,28 por 100 años-niño en los días 0 a 90 del tratamiento y disminuyó a 0,56 por 100 años-niño después de 180 días. La incidencia de TB en los niños con una unidad más alta en la WAZ al inicio del ART fue un 13% inferior (HR ajustado 0,87; IC95% 0,77 – 1,00; P = 0,047).
CONCLUSIÓN:
Es posible disminuir la morbilidad por TB durante la administración del ART cuando se logra el cumplimiento de la detección sistemática de la TB antes de iniciar el ART y se inician los medicamentos contra el virus de manera más temprana, antes de que los niños sufran una infeccion avanzada por el VIH y deterioro nutricional.
THE HUMAN IMMUNODEFICIENCY VIRUS (HIV) epidemic in sub-Saharan Africa has fuelled the pre-existing tuberculosis (TB) epidemic.1 The increased burden of TB among HIV-infected adults has been associated with increased transmission of TB to household members, including children.2,3 Post mortem studies suggest that TB is a leading cause of mortality in HIV-infected children, accounting for 12–18% of deaths.4,5 Even if TB is diagnosed and treated, incident TB in children can lead to chronic lung disease, including bronchiectasis,6 causing lifelong morbidity.
Evaluating the burden and predictors of TB among HIV-infected children starting ART, and clinician compliance with TB screening before ART, can help TB-HIV program managers identify program improvement opportunities.7 Previous studies in Côte d’Ivoire have focused on small cohorts of children in the capital city, Abidjan,8,9 limiting the usefulness of findings for national TB-HIV program managers.
We conducted a large, nationally representative retrospective cohort study among 2110 children starting ART in Côte d’Ivoire during 2004–2008 at 29 facilities to assess clinician compliance with TB screening guidelines, the prevalence and determinants of active TB among new pediatric ART enrollees, and incidence and predictors of TB during ART.
METHODS
ART eligibility
During 2004–2008, children aged 0–14 years diagnosed with World Health Organization (WHO) Stage IV disease, WHO Stage III disease if <12 months old, or with certain clinical conditions including TB, were eligible for ART regardless of CD4+ T-cell (CD4) % or count. Children not yet eligible by clinical criteria could be eligible for ART based on age-dependent CD4% or count criteria, as described in the 2006 WHO treatment guidelines.10 Recommended ART regimens for children with HIV-1 infection include two nucleoside reverse transcriptase inhibitors (NRTI) and either nevirapine (NVP) or efavirenz (EFV). For children with TB co-infection, NVP was replaced with either EFV or a third NRTI, depending on the age of the child. For first-line ART for HIV-2-infected children, two NRTIs and a ritonavir-boosted protease inhibitor were recommended.
ART monitoring
Côte d’Ivoire guidelines recommend that pediatric ART patients be seen frequently initially (at 2 weeks, monthly for 3 months and quarterly thereafter) until stable, and then at least every 6 months,10 to evaluate disease progression or improvement. At each visit, standard Ministry of Health (MOH) recommended medical records are completed. Documentation of whether TB screening was performed and results of any TB diagnostic tests is recommended at all clinic visits. Recommended TB screening practices for children included assessment for 1) poor weight gain, 2) current fever, 3) chronic cough, 4) TB contact history, and 5) night sweats. Recommended TB tests for all child TB suspects included 1) Mantoux skin test, 2) two sputum samples (or gastric aspirates) sent for smear microscopy, 3) one sputum (or gastric aspirate) sent for TB culture, and 4) chest X-ray. If extra-pulmonary TB was suspected, lymph node aspiration, lumbar puncture and abdominal ultra-sound were additional diagnostic tests available at some facilities. In reality, only two laboratories provided TB culture; very few TB suspects would therefore have had sputum samples or gastric aspirates sent for TB culture.
Study design and population
This was a nationally representative retrospective cohort study. By 1 January 2008, about 3000 children had initiated ARTat 64 health facilities.11 To improve study feasibility, only facilities with >10 pediatric ART enrollees by 1 January 2008 were considered eligible. Of 30 eligible facilities, 29 agreed to participate. According to MOH records, these 29 facilities had enrolled 2820 (94%) of all 3000 children enrolled nationally during 1998–2008. Because the medical records of ART enrollees before 2004 had considerable missing data, the protocol excluded 427/2820 enrollees (15%) who started treatment before 2004. A further 195 (7%) records could not be found, and 88 (3%) had been transferred with the child to another facility. All remaining 2110 records were included in the study. Data were collected from the MOH-recommended ART medical records by trained data abstractors from November 2009 to March 2010.
Treatment outcomes
Children taking anti-tuberculosis treatment at ART initiation were considered prevalent TB cases. Children starting anti-tuberculosis treatment (for pulmonary or extra-pulmonary TB) during ART were considered incident TB cases.
Exposure variables
All variables on MOH-recommended ART records were assessed as possible risk factors for TB (Table 1). Weight was recoded as weight-for-age Z-score (WAZ score), using Centers for Disease Control and Prevention (CDC) growth curves for children aged 5–14 years, and WHO curves for children aged 0–<5 years.
Table 1.
Patient- and site-level characteristics at ART initiation by tuberculosis treatment status, Côte d’Ivoire, 2004–2008
| All patients at enrollment (n = 2110) |
Receiving anti-tuberculosis treatment* (n = 150) | Not receiving anti-tuberculosis treatment* (n = 1960) | ||||
|---|---|---|---|---|---|---|
| Original‡ |
Imputed* | |||||
| n | % | % | % | % | P value† | |
| Age at enrollment, years, median [IQR] | 2110 | 5.1 [2.2–8.8] | 5.1 [2.2–8.8] | 6.7 [3.2–10.7] | 5.0 [2.1–3.2] | <0.001 |
| Sex | ||||||
| Male | 1146 | 54 | 54 | 54 | 54 | 0.952 |
| Females | 964 | 46 | 46 | 46 | 46 | |
| Maternal vital status | ||||||
| Mother alive | 1128 | 67 | 67 | 64 | 68 | 0.510 |
| Mother dead | 549 | 33 | 33 | 36 | 32 | |
| Missing | 433 | 21 | ||||
| Paternal vital status | ||||||
| Father alive | 1155 | 77 | 77 | 77 | 77 | 0.964 |
| Father dead | 348 | 23 | 23 | 23 | 23 | |
| Missing | 607 | 29 | ||||
| Type of HIV | ||||||
| HIV-1 | 2044 | 99 | 99 | 99 | 99 | 0.553 |
| HIV-2 or dual HIV-1 & 2 | 20 | 1 | 1 | 1 | 1 | |
| Missing | 46 | 2 | ||||
| WHO Stage | ||||||
| I/II | 329 | 19 | 19 | 4 | 20 | <0.001 |
| III | 939 | 53 | 53 | 24 | 55 | |
| IV | 510 | 29 | 29 | 72 | 25 | |
| Missing | 332 | 16 | ||||
| Weight-for-age Z-score | ||||||
| ≥ –1 (normal) | 372 | 19 | 20 | 13 | 21 | <0.001 |
| ≥ −2 — < −1 (mild) | 357 | 19 | 18 | 15 | 19 | |
| ≥ −3 — < −2 (moderate) | 383 | 20 | 20 | 14 | 20 | |
| < −3 (severe) | 798 | 42 | 42 | 59 | 40 | |
| Missing | 200 | 9 | ||||
| CD4 cell count %, median [IQR] | 1835 | 10.8 [5.4–15.0] | 11.0 [6.0–15.0] | 9.0 [4.0–13.0] | 11.0 [6.0–15.0] | 0.037 |
| Missing | 275 | 13 | ||||
| CD4 cell count, cells/μl, median [IQR] | 1894 | 333 [116–618] | 337 [122–621] | 261 [78–547] | 342 [126–624] | 0.089 |
| Missing | 216 | 10 | ||||
| Hemoglobin, g/dl, median [IQR] | 1759 | 9.3 [8.3–10.3] | 9.3 [8.3–10.4] | 9.5 [8.5–10.6] | 9.3 [8.3–10.3] | 0.246 |
| Missing | 351 | 17 | ||||
| ART site size | ||||||
| ≥ 100 ART children ever | 1210 | 57 | 57 | 69 | 56 | 0.072 |
| < 100 ART children ever | 900 | 43 | 43 | 31 | 44 | |
| ART site type | ||||||
| Primary | 1181 | 56 | 56 | 43 | 57 | 0.011 |
| Secondary | 231 | 11 | 11 | 6 | 11 | |
| Tertiary | 698 | 33 | 33 | 51 | 32 | |
| Any stock-out of ARVs (first- or second-line) in last year at ART site | ||||||
| Yes | 955 | 45 | 45 | 45 | 45 | 0.804 |
| No | 1155 | 55 | 55 | 55 | 55 | |
| Nutritional support at ART site? | ||||||
| Yes | 713 | 34 | 34 | 17 | 35 | 0.007 |
| No | 1397 | 66 | 66 | 83 | 65 | |
Analyses utilized imputed data sets.
Random effects bivariate logistic regression.
Original data before imputation.
ART = antiretroviral therapy; IQR = interquartile range; HIV = human immunodeficiency virus; WHO = World Health Organization; ARV = antiretroviral.
Analysis
Data were analyzed using Stata 11 (StataCorp, 2009, Stata Statistical Software, Release 11, College Station, TX, USA). Missing data are reported for each covariate of interest and were assumed to be missing at random (MAR). If <30% of observations were missing data for a baseline covariate of interest, multiple imputation with chained equations was used to impute the missing data.12 The ICE (imputation by chained equations)13 procedure in Stata was used to create 20 imputed data sets for the key outcome of interest: TB incidence. The imputation model included the event indicator, all study variables and the Nelson-Aalen estimate of cumulative hazard.14 In all analyses involving imputed data, estimates were combined across data sets according to Rubin’s rules.12 Using imputed data, associations between baseline covariates and prevalent TB were assessed using bivariate logistic regression, with random effects specified for each facility.
As ART medical records did not routinely document the date of anti-tuberculosis treatment cessation, time at risk for incident TB among prevalent TB cases could not be estimated. As in other studies, in time-to-event analysis, patients with prevalent TB were excluded from the cohort at risk.15 Patients who were included in the cohort at risk, but were not diagnosed as having incident TB during follow-up, were censored at the most recent visit, date of death if death occurred, or date of transfer if transferred.
Cox proportional hazards regression models were used to estimate non-adjusted and adjusted hazard ratios (HR and aHR), with random effects specified for ART facility. The proportional hazards assumption was assessed using visual methods and the Grambsch and Therneu test.16 Kaplan-Meier curves were used to examine the cumulative probability of remaining undiagnosed with TB over time stratified by baseline variables.
Ethics
This study was approved by the Ivorian Ethics Review Committee and the Institutional Review Boards of the United States CDC and the Harvard School of Public Health.
RESULTS
Patient characteristics at ART initiation
Among 2110 children starting ART during 2004–2008, the median age was 5.1 years, and 150 (7%) were receiving anti-tuberculosis treatment (prevalent TB cases). The median age of the prevalent TB cases was older than that of children considered TB-free (6.7 vs. 5.0 years, P < 0.001) (Table 1). The majority of the children (99%) were HIV-1-infected; only 15 (0.7%) were HIV-2-infected, and five (0.2%) were dually HIV-1 and −2 seroreactive. HIV type was not associated with prevalent TB (P = 0.553).
Markers of advanced HIV disease were common among ART enrollees; 29% had WHO Stage IV, 42% had severe under-nutrition (WAZ <−3), and median CD4% was 11%. Compared with children considered TB-free at ART initiation, children with prevalent TB were more likely to have WHO Stage IV disease (72% vs. 25%, P < 0.001), to have severe under-nutrition (59% vs. 40%, P < 0.001), and to have a lower median CD4% (9.0% vs. 11.0%, P = 0.037).
Compared with children assessed as TB-free, prevalent TB cases were more likely to start ART at tertiary care facilities (51% vs. 32%, P = 0.011) and at facilities lacking nutritional support programs (83% vs. 65%, P = 0.007).
TB screening
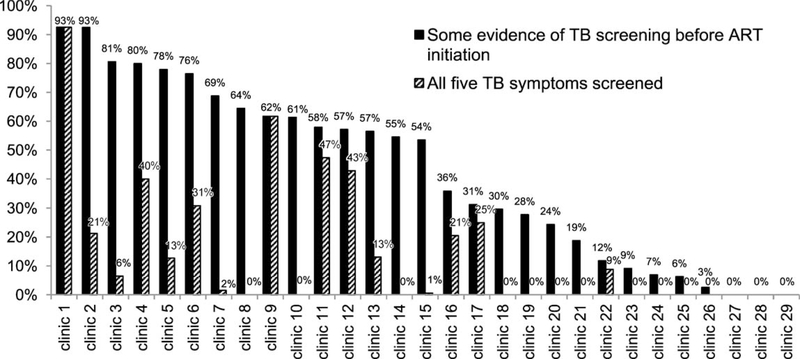
Overall, 1012 (48%) children had documentation of TB screening before ART initiation; 723 (34%) were screened for chronic cough, 565 (27%) for fever, 391 (19%) for TB contact, 366 (17%) for weight loss, and 228 (11%) for night sweats. Only 10% of children had documentation of screening for all five symptoms. The proportion of medical records with documentation of any TB screening before the start of ART declined from 63% to 46% during 2004–2008. The proportions of patients screened for at least one TB symptom before ART varied widely by clinic, from 0% to 93%. At 25/29 clinics, <80% of ART enrollees had some documentation of screening for TB (Figure 1).
Figure 1.
Clinic-level differences in the proportion of pediatric ART medical records with documentation of TB screening before ART initiation. *Some evidence of TB screening before ART initiation was defined as documentation of screening for at least one of the following: 1) poor weight gain, 2) current cough, 3) chronic fever, 4) TB contact, and 5) night sweats. Patients taking treatment at ART initiation were considered to have been screened for TB. TB = tuberculosis; ART = antiretroviral therapy.
TB incidence
Among the 1960 children assessed as TB-free at the start of ART, 56 (3%) were documented to start TB treatment during 4190 child-years (cy) of follow-up, at a rate of 1.34 cases/100 cy (95% confidence interval [CI] 1.03–1.74). Of 56 TB cases, 8 were extra-pulmonary TB (0.19 cases/100 cy, 95%CI 0.09–0.37) and 48 were pulmonary TB (1.14 cases/100 cy, 95%CI 0.86–1.52). The overall TB incidence rate was highest in days 0–90 of ART, at 6.28/100 cy (95%CI 4.31–9.16). This rate declined to 2.52/100 cy (95%CI 1.36–4.69) during days 91–180 of ART, and 0.56/100 cy (95%CI 0.36–0.89) thereafter.
Incident TB risk factors
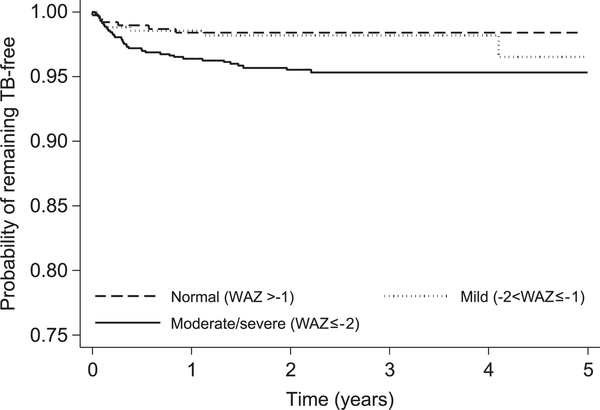
A 1-year increase in age was borderline associated with increased TB incidence (aHR 1.08, 95%CI 0.99–1.15, P = 0.067) (Table 2). Each one-unit increase in WAZ was associated with a 13% reduction in TB incidence (aHR 0.87, 95%CI 0.77–1.00, P = 0.047) (Figure 2). Maternal and paternal orphan status and severe anemia were not associated with TB incidence risk.
Table 2.
Patient- and site-level characteristics associated with TB incidence during ART among children starting ART, Côte d’Ivoire, 2004–2008
| Original n | Crude |
Adjusted |
||||||
|---|---|---|---|---|---|---|---|---|
| Rate (per 100 cy) | HR | (95%CI) | P value | aHR | (95%CI) | P value | ||
| Age at enrollment, per year increase | 1960 | — | 1.10 | (1.03–1.18) | 0.003 | 1.08 | (0.99–1.15) | 0.067 |
| Sex | ||||||||
| Male | 1065 | 1.03 | 1.00 | — | — | 1.00 | — | — |
| Females | 895 | 1.72 | 1.64 | (0.96–2.78) | 0.068 | 1.61 | (0.94–2.76) | 0.081 |
| Maternal vital status | ||||||||
| Mother alive | 1048 | 1.06 | 1.00 | — | — | 1.00 | — | — |
| Mother dead | 502 | 1.87 | 1.87 | (1.05–3.34) | 0.033 | 1.45 | (0.77–2.72) | 0.250 |
| Paternal vital status | ||||||||
| Father alive | 1067 | 1.31 | 1.00 | — | — | 1.00 | — | — |
| Father dead | 324 | 1.40 | 1.13 | (0.58–2.23) | 0.714 | 0.73 | (0.35–1.52) | 0.584 |
| Year of ART start, per increase in calendar year | 1960 | 0.98 | (0.79–1.21) | 0.841 | 0.88 | (0.69–1.13) | 0.313 | |
| WHO Stage | ||||||||
| I/II | 324 | 0.70 | 1.00 | — | — | 1.00 | — | — |
| III | 904 | 1.28 | 1.95 | (0.65–5.83) | 0.232 | 1.82 | (0.58–5.66) | 0.299 |
| IV | 410 | 2.09 | 3.04 | (0.98–9.41) | 0.054 | 2.00 | (0.58–6.93) | 0.271 |
| Undernutrition | ||||||||
| Per WAZ unit increase in severity | 1911 | — | 0.81 | (0.73–0.91) | ,0.001 | 0.87 | (0.77–1.00) | 0.047 |
| CD4 cell count, % | ||||||||
| >20 | 149 | 0.71 | 1.00 | — | — | 1.00 | — | — |
| 10–20 | 825 | 1.15 | 1.78 | (0.42–7.49) | 0.434 | 1.75 | (0.41–7.40) | 0.446 |
| <10 | 722 | 1.71 | 2.60 | (0.61–11.0) | 0.194 | 1.86 | (0.42–8.23) | 0.413 |
| Hemoglobin, g/dl | ||||||||
| ≥8 | 1327 | 1.21 | 1.00 | — | — | 1.00 | — | — |
| <8 | 300 | 2.01 | 1.56 | (0.78–3.13) | 0.210 | 1.24 | (0.58–2.66) | 0.572 |
| Site size | ||||||||
| >100 ART patients aged <15 years ever | 1106 | 1.16 | 1.00 | — | — | 1.00 | — | — |
| ≤100 ART patients aged <15 years ever | 854 | 1.75 | 1.22 | (0.71–2.067) | 0.471 | 1.26 | (0.49–3.24) | 0.996 |
| Site type | ||||||||
| Primary | 1117 | 1.51 | 1.00 | — | — | 1.00 | — | — |
| Secondary | 222 | 0.66 | 0.43 | (0.13–1.40) | 0.161 | 0.32 | (0.08–1.29) | 0.109 |
| Tertiary | 621 | 1.34 | 0.84 | (0.47–1.50) | 0.551 | 0.82 | (0.26–2.62) | 0.742 |
| Any stock-out of ARVs (first- or second-line) in last year | ||||||||
| No | 1073 | 0.91 | 1.00 | — | — | 1.00 | — | — |
| Yes | 887 | 2.18 | 2.00 | (1.17–3.43) | 0.012 | 1.47 | (0.60–3.59) | 0.398 |
| Nutritional support at site? | ||||||||
| Yes | 687 | 0.86 | 1.00 | — | — | 1.00 | — | — |
| No | 1273 | 1.66 | 1.73 | (0.95–3.18) | 0.075 | 1.42 | (0.55–3.68) | 0.467 |
| TB care provided onsite? | ||||||||
| Yes | 625 | 1.63 | 1.00 | — | — | 1.00 | — | — |
| No | 1335 | 1.27 | 0.89 | (0.51–1.57) | 0.700 | 1.09 | (0.45–2.67) | 0.844 |
TB = tuberculosis; ART = antiretroviral therapy; cy = child-years; HR = hazard ratio; CI = confidence interval; aHR = adjusted hazard ratio; WHO = World Health Organization; WAZ = weight-for-age Z-score; ARV = antiretroviral.
Figure 2.
Cumulative probability of remaining tuberculosis-free by WAZ. TB = tuberculosis; WAZ = weight-for-age Z-score.
DISCUSSION
This is the largest and first nationally representative evaluation of TB incidence among children starting ART in Côte d’Ivoire,8,9 and has several important findings.
TB burden
TB prevalence at enrollment on ART (7%) is higher than that reported from previous studies in Western Kenya (3.6%),17 Côte d’Ivoire (2–4%),8,9 and Zambia (5.7%),18 but is lower than that reported from Johannesburg, South Africa (29%).19 There could be many reasons for the variation in TB prevalence at ART initiation, including variations in TB screening and diagnostic algorithms,20 variations in the degree of immune suppression,7 and variations in background TB incidence in the general population.19 Earlier ART enrollment for HIV-infected children could reduce the burden of TB among ART enrollees.7,17 To facilitate earlier ART enrollment, expansion of early infant diagnostic and referral services would be needed.11
Our reported TB incidence (1.34/100 cy), equivalent to about 1340 per 100 000 population, is considerably higher than the national average of 399/100000.21 Our TB incidence rate is similar to previous Ivorian reports (1.3–2.1/100 cy),8,9 but lower than reported South African incidence rates among children starting ART (6.4/100 cy).22 Higher background TB incidence in South Africa (971 vs. 399/100 000),21 or better TB screening and diagnostic capability in South Africa, might explain the different rates.7
As in other studies,17,23,24 TB incidence in our cohort was highest in days 0–90 of ART, and declined thereafter. Several mechanisms might explain the high early TB incidence rates.24 Some early incident TB may be new active TB disease.24 However, the majority of TB cases detected and treated in early ART are probably due to 1) ‘unmasking’ of subclinical disease present at ART initiation, or 2) worsening of symptomatic disease that went unscreened or undiagnosed before the start of ART.24 Active disease may be subclinical at ART initiation because TB symptoms are dependent on both bacillary burden and immune response. The immune reconstitution associated with early ART can unmask new TB signs and symptoms, which in turn will initiate TB diagnostic or trial treatment protocols.24 Among symptomatic children who went undiagnosed through failure to screen or diagnose pediatric TB,7 ART could worsen TB symptoms, prompting more intensive TB diagnostics or a clinical trial of anti-tuberculosis treatment.24 As in other retrospective cohort studies,22 our reported TB prevalence and incidence rates may be underestimates due to failure to screen for and/or diagnose TB disease, or overestimates due to incorrect trials of anti-tuberculosis treatment in children with clinical TB diagnoses.
TB screening
TB screening practices were suboptimal, appeared to worsen over time, and varied widely by clinic. Operational research assessing TB screening practices among pediatric ART enrollees is limited; one Thai study reported documentation of TB screening before pediatric ART for 60–90% of patients.25 Poor documentation of, and declining clinician compliance with, TB screening are worrying, because undiagnosed TB at the start of ART is an important cause of morbidity and mortality.26 Targeting the 25 clinics where <80% of pediatric ART enrollees were screened for at least one TB symptom with intensified training and supervision could reduce TB-associated morbidity and mortality among children starting ART.20
TB risk factors
Markers of advanced HIV disease (advanced WHO stage, under-nutrition and low CD4%) were predictive of prevalent TB,8,17 and higher WAZ at enrollment was protective against incident TB. Earlier initiation of ART, before advanced HIV disease, could reduce the TB burden among HIV-infected children.17
The association between lower WAZ and incident TB has been reported previously.17,23 Possible explanations include the following: 1) active TB at ART enrollment, a possible cause of low WAZ, went undiagnosed due to insensitive diagnostic tests or failure to screen for TB;27 2) clinicians made presumptive diagnoses of TB after ART initiation because WAZ gain was suboptimal;17 or 3) under-weight TB-free children at ART initiation are more at risk of developing active TB during follow-up due to malnutrition-induced impairment of cell-mediated immunity.28
To address failure to diagnose active TB at start of ART, it is essential to monitor and ensure TB screening before ART initiation. In addition, the rollout of the GeneXpert® MTB/RIF assay (Xpert, Cepheid, Sunnyvale, CA, USA), whose sensitivity in diagnosing culture-positive TB in HIV-infected children is twice that of smear microscopy (76% vs. 38%), should be accelerated.27 To address the possibility that malnutrition is a root cause of the development of active TB during ART, there may be a role for expanding existing nutrition programs.28 In our study, lack of a nutrition program at the clinic was associated with prevalent TB at ART initiation (P = 0.007), and was marginally associated with TB incidence in crude (P = 0.075), but not adjusted (P = 0.323), analysis. Further evaluation of pediatric HIV food supplementation programs are needed to assess their benefit and cost-effectiveness.28
As in other studies,17 increased age was associated with prevalent TB and incident TB during follow-up. This may be due to more advanced immune suppression in older children at ART enrollment, or difficulties in diagnosing TB in younger children.17
Limitations
This report has several limitations. First, due to the lack of data on the timing of anti-tuberculosis treatment cessation among prevalent TB cases, prevalent TB cases were excluded from the cohort at risk to estimate TB incidence. However, in a sensitivity analysis (data not shown), estimates of TB incidence with all patients included in the cohort at risk (n= 2110) did not differ from our reported TB incidence rate (n= 1960). Second, as described earlier, reported TB prevalence and incidence rates may be underestimates or overestimates of true TB burden due to difficulties in diagnosing pediatric TB. Third, missing data on covariates probably introduced non- differential measurement error. Fourth, some incident TB may have been prevalent cases, for whom the results of diagnostic tests only returned after ART initiation. Finally, lack of information on which children screened positive for TB, and, among TB suspects, which children were tested for and diagnosed with TB, limits our ability to assess clinician compliance with TB diagnosis guidelines.
CONCLUSIONS
Among our cohort of pediatric ART enrollees, advanced HIV disease was common and was associated with both prevalent TB and incident TB. Earlier ART initiation could reduce the TB burden. In particular, initiation of ART at higher WAZ could reduce TB burden during ART. In addition, improved pre-ART clinician compliance with TB screening, and improved TB diagnostics (i.e., Xpert), could reduce TB incidence during ART.
Acknowledgements
This research has been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the US Centers for Disease Control and Prevention (CDC).
Footnotes
Disclaimer: Use of trade names is for identification only and does not imply endorsement by the United States CDC or the US Department of Health and Human Services. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the US CDC.
Conflict of interest: none declared.
References
- 1.Harries AD, Zachariah R, Corbett EL, et al. The HIV-associated tuberculosis epidemic—when will we act? Lancet 2010; 375: 1906–1919. [DOI] [PubMed] [Google Scholar]
- 2.Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: the need for age-specific interventions. Clin Infect Dis 2006; 42: 1040–1047. [DOI] [PubMed] [Google Scholar]
- 3.Middelkoop K, Bekker LG, Myer L, Dawson R, Wood R. Rates of tuberculosis transmission to children and adolescents in a community with a high prevalence of HIV infection among adults. Clin Infect Dis 2008; 47: 349–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chintu C, Mudenda V, Lucas S, et al. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 2002; 360: 985–990. [DOI] [PubMed] [Google Scholar]
- 5.Ansari NA, Kombe AH, Kenyon TA, et al. Pathology and causes of death in a series of human immunodeficiency virus-positive and -negative pediatric referral hospital admissions in Botswana. Pediatr Infect Dis J 2003; 22: 43–47. [DOI] [PubMed] [Google Scholar]
- 6.Jeena PM, Coovadia HM, Thula SA, et al. Persistent and chronic lung disease in HIV-1 infected and uninfected African children. AIDS 1998; 12: 1185–1193. [DOI] [PubMed] [Google Scholar]
- 7.Marais BJ, Graham SM, Cotton MF, Beyers N. Diagnostic and management challenges for childhood tuberculosis in the era of HIV. J Infect Dis 2007; 196 (Suppl 1): S76–S85. [DOI] [PubMed] [Google Scholar]
- 8.Elenga N, Kouakoussui KA, Bonard D, et al. Diagnosed tuberculosis during the follow-up of a cohort of human immunodeficiency virus-infected children in Abidjan, Cote d’Ivoire: ANRS 1278 study. Pediatr Infect Dis J 2005; 24: 1077–1082. [DOI] [PubMed] [Google Scholar]
- 9.Kouakoussui A, Fassinou P, Anaky MF, et al. Respiratory manifestations in HIV-infected children pre- and post-HAART in Abidjan, the Ivory Coast. Paediatr Respir Rev 2004; 5: 311–315. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Antiretroviral therapy of HIV infection in infants and children: towards universal access: recommendations for a public health approach. Geneva, Switzerland: WHO, 2006. http://www.who.int/hiv/pub/guidelines/paediatric020907.pdf. Accessed January 2014. [PubMed] [Google Scholar]
- 11.World Health Organization. Towards universal access: scaling up priority HIV/AIDS interventions in the health sector: progress report 2010. Geneva, Switzerland: WHO, 2010. http://wwwwhoint/hiv/pub/2010progressreport/full_report_en.pdf. Accessed January 2014. [Google Scholar]
- 12.Rubin DB Multiple imputation for nonresponse in surveys. New York, NY, USA: J Wiley & Sons, 1987: pp 291. [Google Scholar]
- 13.Royston P Multiple imputation of missing values: update of ice. Stata J 2005; 5: 527–536. [Google Scholar]
- 14.White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009; 28: 1982–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Auld AF, Mbofana F, Shiraishi RW, et al. Incidence and determinants of tuberculosis among adults initiating antiretroviral therapy—Mozambique, 2004–2008. PLOS ONE 2013; 8: e54665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika 1994; 81: 515–526. [Google Scholar]
- 17.Braitstein P, Nyandiko W, Vreeman R, et al. The clinical burden of tuberculosis among human immunodeficiency virus-infected children in Western Kenya and the impact of combination antiretroviral treatment. Pediatr Infect Dis J 2009; 28: 626–632. [DOI] [PubMed] [Google Scholar]
- 18.Bolton-Moore C, Mubiana-Mbewe M, Cantrell RA, et al. Clinical outcomes and CD4 cell response in children receiving antiretroviral therapy at primary health care facilities in Zambia. JAMA 2007; 298: 1888–1899. [DOI] [PubMed] [Google Scholar]
- 19.Meyers TM, Yotebieng M, Kuhn L, Moultrie H. Antiretroviral therapy responses among children attending a large public clinic in Soweto, South Africa. Pediatr Infect Dis J 2011; 30: 974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bakeera-Kitaka S, Conesa-Botella A, Dhabangi A, et al. Tuberculosis in human immunodeficiency virus infected Ugandan children starting on antiretroviral therapy. Int J Tuberc Lung Dis 2011; 15: 1082–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Estimates of tuberculosis incidence by country, 2009. Geneva, Switzerland: WHO, 2009. http://www.hpa.org.uk/web/HPAweb&HPAwebStandard/HPAweb_C/1195733837507. Accessed January 2014. [Google Scholar]
- 22.Martinson NA, Moultrie H, van Niekerk R, et al. HAART and risk of tuberculosis in HIV-infected South African children: a multi-site retrospective cohort. Int J Tuberc Lung Dis 2009; 13: 862–867. [PMC free article] [PubMed] [Google Scholar]
- 23.Walters E, Cotton MF, Rabie H, et al. Clinical presentation and outcome of tuberculosis in human immunodeficiency virus infected children on anti-retroviral therapy. BMC Pediatr 2008; 8: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawn SD, Wilkinson RJ, Lipman MC, Wood R. Immune reconstitution and ‘unmasking’ of tuberculosis during antiret- roviral therapy. Am J Respir Crit Care Med 2008; 177: 680–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lolekha R, Chunwimaleung S, Hansudewechakul R, et al. Pediatric HIVQUAL-T: measuring and improving the quality of pediatric HIV care in Thailand, 2005–2007. Jt Comm J Qual Patient Saf 2010; 36: 541–551. [DOI] [PubMed] [Google Scholar]
- 26.Lawn SD, Harries AD. Reducing tuberculosis-associated early mortality in antiretroviral treatment programmes in sub-Saharan Africa. AIDS 2011; 25: 1554–1555. [DOI] [PubMed] [Google Scholar]
- 27.Nicol MP, Workman L, Isaacs W, et al. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: a descriptive study. Lancet Infect Dis 2011; 11: 819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidence from studies in humans and experimental animals. Int J Tuberc Lung Dis 2004; 8: 286–298. [PubMed] [Google Scholar]