Chronic kidney disease (CKD) affects almost 4 million subjects older than 64 in the US Medicare population and was responsible for 25% of all Medicare expenditures (~ $120 billion) in 2017.1 Its major etiologies are hypertension and diabetes mellitus. A major advance in its management took place in the early 1990s, after the description of a role for the systemic and renal renin-angiotensin systems (RAS) in progression of renal dysfunction in experimental animals. Clinical trials demonstrated slowing of such progression with inhibitors of the angiotensin converting enzyme (ACE) and blockers of the angiotensin (Ang) II AT1 receptor. Although they are now standard of therapy, they slow down but do not completely arrest progression of the disease. For example, in diabetic nephropathy, the decrease in the decline of glomerular filtration rate was 11%/year for captopril versus 17%/year with placebo,2 whereas that for losartan was 4.4 ml/min/year versus 5.2 ml/min/year with placebo,3 both treated rates remaining 4 to 10-fold higher than the 1%/year expected with aging alone. It has been extensively discussed whether this indicates participation of other mechanisms in the progression of CKD versus an inability of the currently available drugs to completely inhibit the RAS.
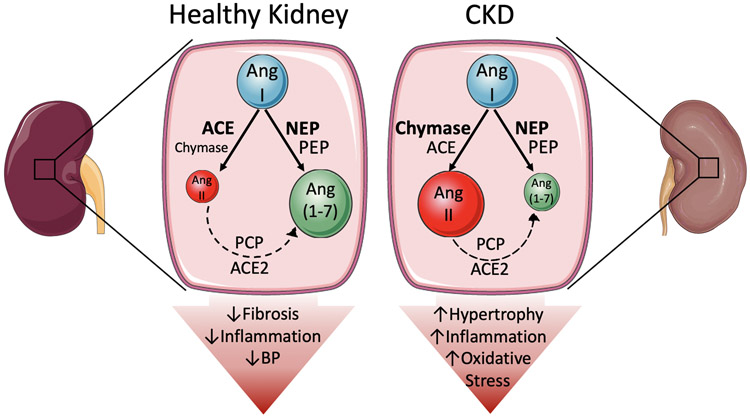
In the current issue of Circulation Research, Kaltenecker et al4 describe the enzymatic processing leading to generation of the vasoconstrictor Ang II and vasodilatory Ang 1-7 in kidney biopsies of normal subjects and patients with CKD (Figure 1). They used mass spectrometry to measure Ang products before and after specific enzyme blockers, immunohistochemistry to study localization of the expression of renal enzymes, and isolated murine perfused kidney to reproduce results. A major finding is that in normal kidneys, Ang I is predominantly converted to Ang 1-7 (70%) via neprilysin (NEP) with lower generation of Ang II (30%) via ACE, and that Ang II contributes little to total Ang 1-7 by further ACE2 processing. In contrast, in CKD kidneys, 1) generation of Ang 1-7 was diminished (53%) and that of Ang II increased (47%), tilting the overall system towards a pro-vasoconstrictor, pro-growth and pro-inflammatory balance, 2) Ang 1-7 generation was still mostly NEP-dependent; however, there might be some participation of prolyl-endopeptidase, 3) generation of Ang II was shifted from ACE to chymase, likely produced by infiltrating mast cells, and 4) generation of Ang 1-7 from Ang II was still minimal but may involve some participation of prolyl-carboxypeptidase supporting ACE2. A major strength of the study is the confirmation of enzyme effects in the murine isolated perfused kidney. These make it clear that results in human biopsies are not attributable to release of enzymes from the membrane or cell compartments in the homogenates, with consequent artificial exposure to the substrates. Weaknesses such as lack of study of other Ang peptides, lack of classification of diseases leading to CKD, and lack of study of the impact of gender on results are acknowledged by the authors and are inherent to studies that depend on invasive interventions, usually conducted in small numbers of patients.
Figure 1: Proposed Model of the differential metabolism of the renin-angiotensin system in normal and chronic kidney disease.
A. In a healthy kidney, most Ang I is converted to Ang-(1-7) by neprilysin (NEP), and to a lesser extent PEP, whereas it conversion to Ang II is quantitatively less important and preferentially by ACE. This results in a tilting of the renin-angiotensin system towards protective effects such as decreasing blood pressure, pro-inflammatory molecule production, and extracellular matrix expansion. B. In CKD patients, less Ang I is converted to Ang 1-7, whereas chymase takes over ACE for generation of excess Ang II. This reversal of the tilt of the RAS promotes oxidative stress, inflammation, and tissue hypertrophy.
Findings in CKD are novel and clinically relevant. The decrease in protective renal Ang 1-7 levels reflects NEP-rather than ACE2-deficiency, which may be a consequence of NEP inhibition by accumulation of natriuretic peptides. Low NEP activity becomes paradoxically predictive of cardiac events in CKD5 but is not actionable since there are no available agonists and their theoretical use might be counterproductive by reducing compensatory natriuretic peptides. In contrast, the shift of production of detrimental Ang II from ACE to chymase may have therapeutic implications. Chymase is rapidly inactivated in the circulation by proteases. Therefore, its major role as an Ang II-generating enzyme occurs in tissues, particularly in pathological conditions with macrophage infiltration.6 Major roles for this enzyme in ischemic heart disease, post-ischemic cardiomyopathy and aneurysm formation have been demonstrated by use of chymase inhibitors in experimental models.7 In the kidney, chymase inhibitors improve perfusion during salt-induced hypertension and in Goldbaltt clipped kidneys, despite no effect on blood pressure.8 These observations, together with those of Kaltenecker et al raise the possibility of targeting chymase for better slowing of renal dysfunction in CKD, a topic worth further investigation.
There is another, incidental but important and timely implication for the study of Kaltenecker et al. It has become apparent, during the current COVID-19 pandemic, that the kidney is a frequent target of the SARS-CoV-2 virus. Acute kidney injury (AKI) and proteinuria are common in subjects with severe disease and also in those with previous CKD. Two autopsy series involving 53 subjects9,10 showed that in addition to changes typical of AKI or those related to preceding renal illness, there were also distinct podocyte, tubular and endothelial lesions that are likely a consequence of the viral infection. Electron microscopic examination showed clusters of coronavirus-like particles with the typical spikes in the tubular epithelium and podocytes.
The initial concern that inhibitors of the RAS could enhance viral infection by inducing overexpression of its receptor (ACE2) seems now dispelled by observational studies in more than 40,000 subjects in whom these agents do not affect prognosis or actually improve it.11 Owing to the fact that the RAS balance is tilted towards the deleterious Ang II-AT1R pathway in COVID-19, an opposite therapeutic approach has been proposed, i.e., interventions that enhance the activity of the ACE2-Ang 1-7 pathway. This could theoretically be achieved by use of recombinant human ACE2,12 gene-delivery of ACE2, Ang 1-7 analogs, Mas receptor agonists,13 or AT1R ß-arrestin biased agonists.14 In view of the finding of Kaltenecker et al that the contribution of ACE2 to the generation of Ang 1-7 is minor, agents that directly enhance availability of Ang 1-7 or stimulate the Mas receptor may exert more renal benefit than those that enhance ACE2 activity.
Our understanding of the complexity of the RAS has been growing exponentially, with elucidation of the feedback loops mediated by the AT2 receptor, the Ang 1-7 / Mas receptor pathway, the action of shorter peptides such as Ang IV in the brain, and the generation of Ang II from non-angiotensinogen substrates (i.e., Ang 1-12). As the current publication by Kaltenecker et al demonstrates, continued study of the nuances of the system will improve and expand our ability to manipulate it for therapeutic purposes.
Acknowledgments
Supported by the National Institutes of Health grants K01HL130497 and T32HL144446.
Footnotes
Disclosures
The authors declare no conflict of interest to disclose.
References
- 1.Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2017 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2018;71(suppl 1):Svii,S1–S672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD for the Collaborative Study Group. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. N Engl J Med. 1993;329:1456–1465. [DOI] [PubMed] [Google Scholar]
- 3.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving HH, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, for the RENAAL study investigation. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–869. [DOI] [PubMed] [Google Scholar]
- 4.Kaltenecker CC, Domeni O, Kopecky C, et al. Critical role of neprilysin in kidney angiotensin metabolism. Circ Res. 2020;xx:xxxx-xxxx. (editorial office to complete) [DOI] [PubMed] [Google Scholar]
- 5.Emrich IE, Vodovar N, Feuer L, Untersteller K, Nougue H, Seiler-Mussler S, Fliser D, Launay JM, Heine GH. Do plasma neprilysin activity and plasma neprilysin concentration predict cardiac events in chronic kidney disease patients. Nephrol Dial Transplant. 2019;34:100–108. [DOI] [PubMed] [Google Scholar]
- 6.Urata H, Kinoshita A, Misono KS, Bumpus FM, Husain A. Identification of a highly specific chymase as the major angiotensin II-forming enzyme in the human heart. J Biol Chem. 1990;265:22348–22357. [PubMed] [Google Scholar]
- 7.Miyazaki M, Takai S, Jin D, Muramatsu M. Pathological roles of angiotensin II produced by mast cell chymase and the effects of chymase inhibition in animal models. Pharmacol Ther. 2006;112:668–676. [DOI] [PubMed] [Google Scholar]
- 8.Roszkowska-Chojecka MM, Walkowska A, Gawrys O, Baranowska I, Kalisz M, Litwiniuk A, Martynska L, Kompanowska-Jezierska E. Effects of chymostatin, a chymase inhibitor, on blood pressure, plasma and tissue angiotensin II, renal haemodynamics and renal excretion in two models of hypertension in the rat. Exp Physiol. 2015;100;1093–1105. [DOI] [PubMed] [Google Scholar]
- 9.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kid Int. 2020. April 9 epub ahead of print 10.1016/j.kint.2020.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puelles VG, Lutgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020. May 13 epub ahead of print DOI: 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elijovich F, Laffer CL. What kind of evidence is needed to dictate practice regarding inhibitors of the renin-angiotensin system in COVID-19. Hypertension. 2020. (in press) [DOI] [PubMed]
- 12.Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double-edged sword. Circulation. 2020. March 26 epub ahead of print DOI: 10.1161/CIRCULATIONAHA.120.047049 [DOI] [PubMed] [Google Scholar]
- 13.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, Raizada MK, Grant MB, Oudit GY. Angiotensin-coverting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ Res. 2020;126:1456–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manglik A, Wingler LM, Rockman HA, Lefkowitz RJ. β-arrestin-biased angiotensin II receptor agonists for COVID-19. Circulation. 2020. May 15 epub ahead of print DOI: 10.1161/CIRCULATIONAHA.120.048723 [DOI] [PMC free article] [PubMed] [Google Scholar]