Doxorubicin-induced cardiomyopathy (DiCM) remains a significant cause of heart failure in cancer patients. For example, a recent clinical trial confirmed that 14.5% of doxorubicin-treated breast cancer patients experience a decrease in left ventricular ejection fraction of greater or equal to 10 percent1. DiCM can occur either as an early (during the first year after treatment) or late consequence, with the latter manifestation thought to be a long-term consequence of the acute doxorubicin insult2. It is known that DiCM is a result of cardiomyocyte cell death3, assessed by an increase in troponins in the peripheral blood. The mechanism for how doxorubicin causes cardiomyocyte cell death is thought to be a combination of DNA damage and the generation of reactive oxygen species (ROS) that triggers mitochondrial dysfunction. Despite the known link between ROS and DiCM, antioxidant therapies such as N-acetylcyteine have failed in patients4. Thus, uncovering mechanisms associated with DiCM is of high clinical significance.
Cardiomyopathy in general is increasingly associated with inflammation and evidence of activated immune cells within the myocardium, including innate macrophages. During ischemic cardiomyopathy, distinct populations of recruited versus resident cardiac macrophages contribute to myocardial inflammation and reparative processes that regulate ventricular remodeling. The origin of these immune subpopulations is key, as we now appreciate that macrophage ontogeny imprints macrophage function. For example and during ischemic insult, monocyte-derived macrophage subsets populate the heart from the circulation and are pro-inflammatory5. In contrast, resident cardiac macrophages originate from yolk sac-derived erythromyeloid progenitors, self-renew locally, and exhibit non-overlapping cardioprotective functions after cardiac ishemia6. During nonischemic cardiomyopathy, resident cardiac macrophages have been implicated in the heart’s adaptive response to pressure overload7. In an animal model of DiCM, a role for macrophages had been shown where NLRP3 deficiency reduced macrophage anti-inflammatory cytokine IL-10, in association with enhanced susceptibility to DiCM8. However beyond this, very little has been understood of the extent, contribution, and potential source of cardiac macrophage subsets during DiCM. Taken together, therefore it is possible that a patient’s predisposition to DiCM may be tied to how the heart handles the initial doxorubicin insult, combined with crosstalk with the innate immune response.
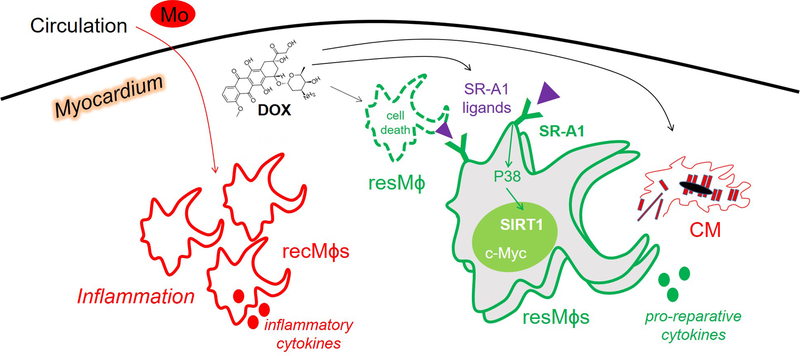
In this issue of Circulation Research, Zhang and colleagues newly explore the effects of doxorubicin on resident cardiac macrophage subpopulations 9. To do this, the research team utilized complementary cutting edge approaches, including macrophage lineage tracing and parabiosis in experimental mice in order to track and discover a protective role for cardiac resident macrophages after administration of doxorubicin. Although the findings suggest that peripheral pro-inflammatory monocyte-derived myocardial macrophages predominate during DiCM, resident cardiac macrophages ultimately mobilize and self-renew (Figure 1) in response to doxorubicin, and function to reduce adverse cardiac remodeling.
Figure 1. Working model.
Depicted is a schematic of recruited (“rec”) versus resident (“res”) macrophages (Mɸs) within the myocardium during doxorubicin (DOX)-induced cardiomyopathy (DiCM). Recruited Mɸs predominate in number and are derived from circulating monocytes (Mo), whereas resident Mɸs initially die, then proliferate and are cardioprotective, including though the potential clearance of dying cardiomyocytes (CMs). This proliferative response requires the Mɸ surface receptor SR-A1, which is activated by SR-A1 ligands, the latter of which may be triggered by DOX. SR-A1 initiates a P38, SIRT1, c-Myc signaling axis that promotes Mɸ proliferation.
The molecular mechanism by which cardiac resident macrophages resist cardiotoxicty by doxorubicin is through the phagocyte cell surface Scavenger Receptor A1, or SR-A1 (also known as MSR1 and CD204), which was both necessary and sufficient for cardioprotection. This is because SR-A1 was required to activate the transcription factor cellular-Myc (c-Myc) in order to promote resident cardiac macrophage proliferation. SR-A1 had been linked to macrophage proliferation in atherosclerosis10, however underlying intracellular molecular mechanisms were unclear. Furthermore, prior studies have shown that SR-A1 is critical for cardiac repair after myocardial infarction (MI) and that deficiency of SR-A1 predisposes experimental mice to cardiac rupture after MI11. In the case of permanent coronary occlusion MI, many resident cardiac macrophages die12 and therefore the rescue of surviving macrophages may also depend on SR-A1 in this scenario. As to what may be triggering SR-A1 signaling during DiCM, SR-A1 ligands can be generated by products of doxorubicin-induced lipid peroxidation in the heart. For example, lipid peroxidation adduct malondialdehyde is capable of modifying proteins for recognition by SR-A1 on phagocytic cells13. Furthermore, elevated cell death in the myocardium may stimulate phagocytic signaling by SR-A1.
Macrophage proliferation is known to be triggered by multiple cytokine receptor pairings that include macrophage colony-stimulating factor (M-CSF) signaling, interleukin 4 (IL-4) receptor activation, and granulocyte-macrophage colony-stimulating factor (GM-CSF) pathway activation 14. M-CSF-induced proliferation is linked to activation of c-Myc. In the case of Zhang et al., forced lentiviral expression of c-Myc was able to stimulate macrophage proliferation and improve cardiac function in doxorubicin-impaired experimental mice. The investigators propose that c-Myc works cooperatively with NAD-dependent deacetylase sirtuin-1 (SIRT1), and is activated by a TAK1-MKK4-P38 mitogen-activated protein kinase signaling cascade. Thus, newly elucidated is SR-A1 as a regulator of macrophage proliferation via activation of c-Myc intracellular signaling.
Despite our current appreciation of the importance of immune cells to cardiac disease, as well as the clinical importance of DiCM, our mechanistic understanding of how doxorubicin affects cardiac immunology remains vague. The studies of Zhang et al. are a good start, nevertheless a number of important and outstanding questions remain. This includes why select patients experience cardiotoxicity whilst others may tolerate higher chemotherapy doses. It is interesting to speculate that genetic predisposition or pre-existing metabolic state may influence the mobilization of cardiac immune cells to doxorubicin. Also, to what extent do innate immune cell subpopulations activate the adaptive arm of the immunity in order to calibrate cardiac inflammation during DiCM? Taken together, the findings of Zhang et al. point to a new cardioprotective role of cardiac resident macrophages during DiCM. Future endeavors may consider immunomodulatory strategies that promote cell survival of those resident cardiac macrophages, which initially succumb to cell death by doxorubicin or aging. In addition, higher resolution approaches such as single cell sequencing and in patients, may reveal yet additional cell subpopulations that uniquely tune the severity of DiCM.
Footnotes
Disclosures: None
Literature Cited
- 1.Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr., das Dores Cruz F, Goncalves Brandao SM, Rigaud VOC, Higuchi-Dos-Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, et al. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. J Am Coll Cardiol. 2018;71:2281–2290. [DOI] [PubMed] [Google Scholar]
- 2.Lipshultz SE, Lipsitz SR, Sallan SE, Dalton VM, Mone SM, Gelber RD and Colan SD. Chronic progressive cardiac dysfunction years after doxorubicin therapy for childhood acute lymphoblastic leukemia. J Clin Oncol. 2005;23:2629–36. [DOI] [PubMed] [Google Scholar]
- 3.Burridge PW, Diecke S, Matsa E, Sharma A, Wu H and Wu JC. Modeling Cardiovascular Diseases with Patient-Specific Human Pluripotent Stem Cell-Derived Cardiomyocytes. Methods Mol Biol. 2016;1353:119–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo SH, Kim LS, Kim SA, Kim HS, Han SJ, Park WJ and Choi YJ. Evaluation of Short-Term Use of N-Acetylcysteine as a Strategy for Prevention of Anthracycline-Induced Cardiomyopathy: EPOCH Trial - A Prospective Randomized Study. Korean Circ J. 2013;43:174–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, van der Laan AM, et al. Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circulation research. 2014;115:284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol. 2019;20:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao X, Shen Y, Zhang R, Sugi K, Vasudevan NT, Alaiti MA, Sweet DR, Zhou L, Qing Y, Gerson SL, et al. Distinct roles of resident and nonresident macrophages in nonischemic cardiomyopathy. Proc Natl Acad Sci U S A. 2018;115:E4661–e4669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi M, Usui F, Karasawa T, Kawashima A, Kimura H, Mizushina Y, Shirasuna K, Mizukami H, Kasahara T, Hasebe N, et al. NLRP3 Deficiency Reduces Macrophage Interleukin-10 Production and Enhances the Susceptibility to Doxorubicin-induced Cardiotoxicity. Scientific reports. 2016;6:26489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang H, Xu A, Sun X, Yang Y, Zhang L, Bai H, Ben J, Zhu X, Li X, Yang Q Self-Maintenance of Cardiac Resident Reparative Macrophages Attenuates Doxorubicin-Induced Cardiomyopathy Through the SR-A1-c-Myc Axis. Circ Res 2020xx–xxx. [DOI] [PubMed] [Google Scholar]
- 10.Robbins CS, Hilgendorf I, Weber GF, Theurl I, Iwamoto Y, Figueiredo JL, Gorbatov R, Sukhova GK, Gerhardt LM, Smyth D, et al. Local proliferation dominates lesional macrophage accumulation in atherosclerosis. Nature medicine. 2013;19:1166–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsujita K, Kaikita K, Hayasaki T, Honda T, Kobayashi H, Sakashita N, Suzuki H, Kodama T, Ogawa H, Takeya M. Targeted deletion of class A macrophage scavenger receptor increases the risk of cardiac rupture after experimental myocardial infarction. Circulation. 2007;115:1904–11. [DOI] [PubMed] [Google Scholar]
- 12.Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, et al. Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. The Journal of experimental medicine. 2012;209:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Canton J, Neculai D and Grinstein S. Scavenger receptors in homeostasis and immunity. Nature reviews Immunology. 2013;13:621–34. [DOI] [PubMed] [Google Scholar]
- 14.Sieweke MH and Allen JE. Beyond stem cells: self-renewal of differentiated macrophages. Science. 2013;342:1242974. [DOI] [PubMed] [Google Scholar]