Abstract
Mass spectrometry imaging (MSI) is routinely used to visualize the distributions of bio-molecules in tissue sections. In plants, MSI of metabolites is commonly observed, but the imaging of larger molecules is less frequently performed despite the importance of proteins and endogenous peptides to the plant. Here, we describe a matrix-assisted laser desorption/ionization MSI method for the imaging of peptides in Medicago truncatula root nodules. Sample preparation steps, including embedding in gelatin, sectioning, and matrix application are described. The method described was employed to determine the spatial distribution of hundreds of peptide peaks.
Keywords: MALDI, MSI, peptides, Medicago truncatula, imaging
1. Introduction
Matrix-assisted laser desorption/ionization mass spectrometry imaging (MALDI-MSI) is a powerful tool to visualize the distribution of molecules in a tissue [1]. In MALDI-MSI, a laser is fired at discrete positions, or pixels, across a matrix-covered tissue. At each pixel, a mass spectrum is collected. Once the instrument collects mass spectra at all of the pixels, software programs extract the ion intensity for a particular m/z across all pixels to create an image, or heatmap, for that m/z. In this way, hundreds of images can be generated from a single instrument run. To prepare a sample for analysis, the general sample preparation steps are flash freezing and embedding, sectioning, and applying a suitable matrix. Sample preparation is a critical step to preserve the sample and to achieve good signal of the chosen analytes [2,3]. For example, the matrix coating, which assists in ionizing analyte molecules in the tissue section, can influence the type of analytes in your sample that will ionize and the spatial resolution of the imaging experiment. MALDI-MSI has been applied to many different analyte types, including metabolites [4,5], neuropeptides [6], and proteins [7] in many different organisms. However, applications of the technique to plants have focused on small molecules [8], with only a few focusing on larger molecules [9-12].
Here, we provide a detailed protocol focusing on applying MALDI-MSI to investigate peptides present on the root nodules of Medicago truncatula (Medicago) [9]. Medicago forms specialized organs, called root nodules, on its roots as a result of a symbiotic relationship with rhizobia bacteria for biological nitrogen fixation. Plant peptides are involved in the formation of the nodule on the roots of the plant, as well as in plant growth and development in general [13,14]. For example, nodule-specific cysteine-rich peptides are involved in the differentiation of bacteria into bacteroids in the root nodules [15], and CLAVATA3/embryo-surrounding region (CLE) peptides are involved in autoregulation of nodulation [16,17]. Thus, the protocol here aims to give a method that can be used to determine the spatial distribution of plant peptides via MALDI-MSI to further our understanding about these important biomolecules.
2. Materials
2.1. Embedding Nodules
Plant material: Medicago truncatula plants inoculated with Sinorhizobium meliloti (Rm1021)
Embedding Media: 100 mg/mL gelatin
Plastic embedding containers suitable for storage in the −80°C
Dry ice
2.2. MALDI-MSI Sample Preparation
Optimal cutting temperature (OCT) compound
25 x 75 mm glass slides
50% Methanol: HPLC-grade methanol, MilliQ water (50:50 v:v)
50% Methanol 0.1% FA: HPLC-grade methanol, MilliQ water (50:50 v:v), 0.1% formic acid (FA)
DHB matrix solution: 40 mg/mL 2,5-dihydroxybenzoic acid (DHB) in 50% methanol 0.1% FA. Sonicate the matrix until completely dissolved.
50% Acetonitrile: HPLC-grade acetonitrile, MilliQ water (50:50 v:v)
50% Acetonitrile 0.1% FA: HPLC-grade acetonitrile, MilliQ water (50:50 v:v), 0.1% formic acid
CHCA matrix solution: 5 mg/mL α-cyano-4-hydroxycinnamic acid (CHCA) in 50% acetonitrile 0.1% FA. Sonicate the matrix until completely dissolved.
SA matrix solution: 5 mg/mL sinapic acid (SA) in 50% acetonitrile 0.1% FA. Sonicate the matrix to completely dissolve it.
3. Methods
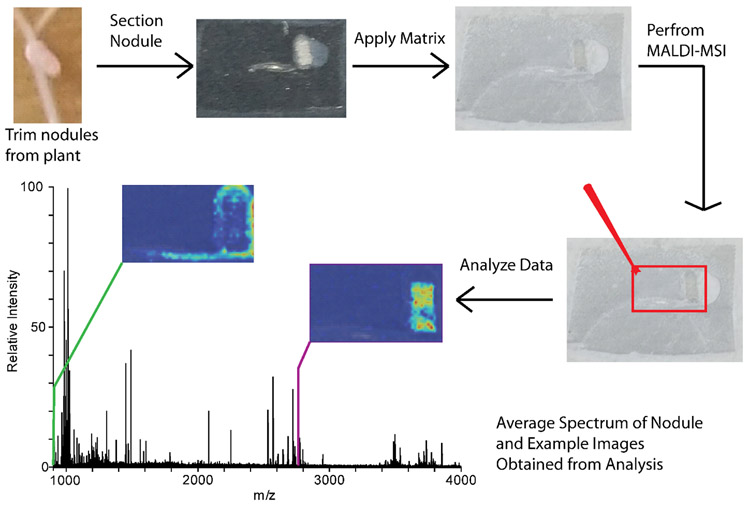
Sample preparation for MALDI-MSI is a critical step to obtain the best results during the MALDI-MSI analysis. Sample preparation steps, such as sample preservation, washing, matrix choice, and matrix application method will all influence the sample analysis. Here, we describe sample preparation steps for flash freezing the nodules and embedding in gelatin, followed by matrix application with a TM Sprayer automatic sprayer system (HTX Technologies). The sample is analyzed on the MALDI LTQ Orbitrap XL (Thermo Scientific) equipped with a nitrogen laser, and data analysis is performed in ImageQuest (Thermo Scientific) and MSiReader [18]. Figure 1 demonstrates the sample workflow for MALDI-MSI of Medicago root nodules.
Figure 1.
MALDI-MSI scheme showing the sample preparation, instrument analysis, and data analysis steps for a typical experiment.
3.1. Embedding Nodules
Trim nodules from the plant with about 2-4 mm of surrounding roots (see note 1).
Place nodule in a plastic cup or similar holding container of appropriate size for your sample (for example a 5 mm x 5 mm x 5 mm square plastic cup for very small samples) with a drop of 100 mg/mL gelatin (see note 2).
Place on dry ice and wait for nodule and gelatin to freeze. The gelatin will turn white when frozen.
Once the nodule is frozen, fill the embedding container with 100 mg/mL gelatin. Wait for the entire embedding container with gelatin to freeze. Once the gelatin is completely white, the nodule can be saved at −80°C (see note 3).
3.2. MALDI-MSI Sample Preparation
Take the embedded nodule and trim sample to rectangle with a couple mm of gelatin surrounding the sample on all sides. Do this quickly to minimize the time the sample is at room temperature.
Attach sample to a cryostat chuck with a drop of OCT compound (see note 4).
Allow sample on the chuck to equilibrate in the cryostat at −20°C for 15 minutes.
Align the sample so that the cryostat is cutting sections evenly across the root and root nodule. This can be done by taking about five sections and adjusting the chuck if part of the sample is being missed. For plant root nodules, our lab typically uses 16 μm, but other sections thickness between 8-20 μm, or approximately the thickness of a typical cell, can be used.
Once the center of the nodule (or other desired depth) is reached, thaw mount sections onto a glass slide by warming the back of the slide against your hand and then placing the front of the slide gently onto your tissue section.
Continue until desired number of sections across the z stack of the root nodule are obtained.
Keep the sections in a dry environment (i.e. dry box) while preparing the TM Sprayer for matrix application (see note 5).
Turn nitrogen gas on TM Sprayer to 10 psi, and the solvent pump to 0.25 mL/min. The solvent for the pump should be whatever your matrix is dissolved in (without the FA), so for DHB this would be 50% methanol and for CHCA this would be 50% acetonitrile. Turn on the TM Sprayer and laptop (see note 6).
Set the temperature on the software to the appropriate temperature for your solvent and TM Sprayer system (see note 7). For our lab, 80°C is the appropriate temperature for 50% methanol.
Load the dissolved matrix (i.e. DHB, CHCA, SA see note 8) into the sample loop with the knob in the load position.
Load the TM Sprayer method and manually change gas pressure and flow rate if method differs from the initial parameters of 10 psi and 0.25 mL/min. The TM Sprayer has recommended methods for specific matrices and analyte types, although method parameters may need to be optimized for a specific application. For DHB imaging of peptides, method parameters typically used in our lab are 1250 velocity, 0.1 mL/min, 12 passes, 30s dry time, rotate and offset (cc pattern), 10 psi, 80°C. For CHCA and SA imaging of peptides, the method parameters to start from are 1100 velocity, 0.2 mL/min, 8 passes, 30 s dry time, rotate and offset (cc pattern), 10 psi, 85°C (see note 9).
Once the TM Sprayer has reached the appropriate temperature, add slides containing sample to the sample holder. Secure slides in place as necessary to prevent movement during matrix application.
Switch the sample loop knob to the spray position. Once matrix is coming out of the nozzle, start the TM Sprayer program.
After the matrix application is finished, cool down the system while flushing with the solvent the matrix is dissolved in (for DHB, this would be 50% methanol) at 0.25 mL/min. Rinse the sample loop 3 times with solvent and toggle the knob. Once the system is below 50°C, the system can be turned off.
Store the sample in a dry box at −20°C if running on the instrument the following day.
3.3. Instrument analysis on the MALDI LTQ Orbitrap XL
Place glass slide(s) with sample into the slide adapter. If importing the image of the glass slide, scan the slide in the adapter with a scanner. Then add the backing plate and insert the plate into the instrument. Alternatively, the slide can be scanned after inserting the plate into the instrument with the camera in the instrument (see note 10).
Open the plate image in the MALDI source dialog box in the Tune software. Zoom in as necessary to see sample, depending on sample size. Draw boxes around the areas that you want to image (see note 11). Save this as a MALDI position file. For MS1 imaging, using a rectangle box and raster motion works best. Also set your desired spatial step size (75 μm is the smallest raster size without oversampling).
In Xcaliber, set up the sequence by adding the file name, path location, instrument method, and MALDI position file. The instrument method contains parameters controlling the mass resolution, mass range, and centroid/profile data. The instrument method also requires a tune file, which controls the laser energy and the microscans (microscans/step is controlled in the instrument file). The microscans and microscans/step should match to ensure that one pixel is one mass spectrum in the data file.
Check the laser energy by shooting the laser on a matrix only area that is not being imaged and checking the signal level. You can adjust the laser energy in your tune file as necessary to get the optimal signal.
Start the sequence.
3.4. Data Processing
Once the data is collected, the data can be viewed in ImageQuest, or exported to another software program. To visualize the data in ImageQuest, use the average spectra within a selected area tool to view an average spectrum of a certain area of your sample. In the bottom window of ImageQuest, there should be a spectrum from your sample. Figure 2 shows example spectra averaged over the nodules for peptide imaging results with DHB, CHCA, and SA matrices.
Look through the peaks in your spectrum, zooming in as appropriate, and when you want to visualize the distribution of a certain peak in your tissue, select add new data set. You will want a single dataset with plot type Mass Range/TIC. Use the m/z for the mass range and select the desired tolerance window (i.e. 5 ppm). Repeat as necessary to visualize the m/z in your sample. Under the 2D tab, there are other color bar options as well as smoothing options.
To view in MSiReader [18], export the data in ImageQuest into an imzML format, keeping the data in profile.
Load the imzML file into MSiReader and select the mass tolerance, image smoothing, and color bar. Insert a m/z that is localized across the sample to visualize the sample (you can find a good m/z for this in ImageQuest). Normalize to the total ion count (TIC). To pull out m/z unique to the sample, use the polygon tool to create interrogated and reference zones. Outline around the sample to create an interrogated zone, then create a matrix only region for the reference zone.
Use the extract peaks unique to the interrogated zone tool to create a list of m/z present in your image. You will need to set percentage numbers for the threshold a m/z needs to be above in the interrogated zone and the threshold a m/z needs to be below in the reference zone to be added to the list. Also set the algorithm for peak centroid calculation (typically parabolic centroid works well).
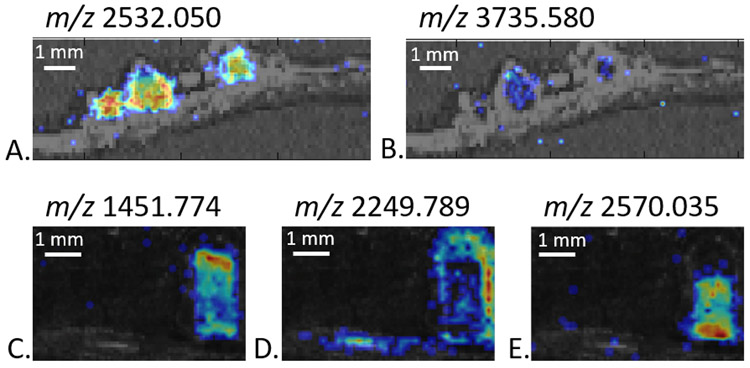
Once the list has been created, use the generate an image for each peak in a list tool to create images for all the m/z. Manually go through the images and remove any bad images (i.e. images that have signal in the matrix as well as the sample or do not appear to have any signal anywhere). Figure 3 shows example MALDI-MSI images generated from peptide imaging of root nodules with either DHB or CHCA as the matrix. Different distributions across the root and root nodules are observed.
Figure 2.
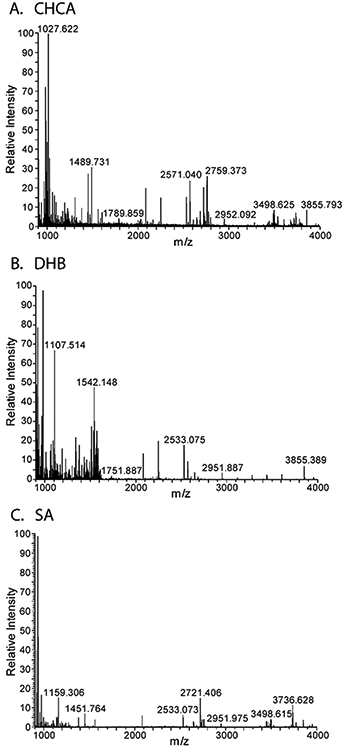
Example spectra average over the entire root nodules for MALDI-MSI on the root nodules with different matrices. The matrices are CHCA (A), DHB (B), SA (C).
Figure 3.
MALDI-MSI images of peptides with either DHB (A, B) or CHCA (C, D, E) as the matrix. The images are generated at +/− 5 ppm.
Footnotes
For best results, select nodules that are red in color and elongated rod in shape rather than round. These are the nodules that where the symbiosis is well developed.
To make the sectioning process easier, ensure that the nodule is as flat as possible with the root in line with the nodule. This will help to get both the root and the nodule in the same plane when sectioning.
If the nodule is not completely frozen when covered in gelatin, it will not stick to the bottom of the cup and instead will float up to the middle or top of the cup. This makes the nodule harder to find and may result in the positioning of the nodule being lost. After adding the gelatin, the cup should be kept level while waiting for the rest of the gelatin to freeze. If the gelatin freezes at an angle, it will be harder to level the nodule while sectioning to get both the root and root nodule in a single section. Avoid air bubbles close to the nodule when adding the gelatin, as this also will make the nodules harder to section.
OCT compound is a polymeric species and will suppress analyte signal if it comes into contact with the sample. Thus, care should be taken to ensure that the compound does not come into contact with the sample or with the blade or stage of the cryostat.
After sectioning and before matrix application, washing steps to remove high abundant lipid species can increase signal intensity and observed protein peaks [19]. For protein imaging, ethanol washes and potentially a Carnoy wash are typically used to remove the lipid species that can suppress protein signal. For endogenous peptide imaging, washes may (or may not) remove the target peptides, depending on the chemical properties of the peptides. Thus, care should be taken when using washing techniques with peptides to ensure that they are not being removed in the washing steps.
Here the TM Sprayer is used to apply the matrix to evenly across the sample. It is important that the matrix is applied in a homogenous manner at all points on the tissue so that matrix inhomogeneity does not skew the results. A matrix application method should be reproducible run-to-run to ensure that results remain consistent. Other pneumonic sprayers can be used (i.e. home-build or the Bruker ImagePrep). Other matrix application techniques include the airbrush and sublimation [20]. Airbrush application can be achieved easily with minimal expense, however, user-to-user variation can be high and reproducibility can be a challenge. Sublimation provides very small crystal size and good imaging results for metabolomics studies, but due to the dry application, the method requires further re-crystallization steps for analysis of larger molecules (i.e. peptides and proteins) [21].
The temperature of the TM Sprayer should be about 5°C below the temperature at which the “puffing” sound starts. This sound indicates that the matrix is not being sprayed in a consistent manner. If run at a temperature when the solvent is “puffing” the matrix will not cover the sample homogeneously, which will negatively affect results.
There are many different matrices to choose from. DHB and CHCA are both common matrices and can be used for a variety of analytes. Other matrices may be used primarily for larger peptides and proteins (i.e. SA) or primarily for negative mode (i.e. 9-aminoacrilamide). Matrices other than DHB and CHCA may work well depending on your desired analyte.
If this method is too wet, you can cut the flow rate in half and double the number of passes to achieve the same matrix density, but with a drier spray.
The preferred scanning method depends on the sample and time considerations. For the nodules, scanning in with the camera on the instrument provides good alignment and image quality, but this takes 25 minutes per slide. For larger tissues, the scanner separate from the instrument works well and saves time.
To check the alignment of the image to the slide in the instrument you can click a point on the image and check the cursor position on the camera box on the tune page to see where the actual position is. It can also be helpful to check the outside of the boxes to ensure the sample is not being cut-off.
5 References
- 1.Caprioli RM, Farmer TB, Gile J (1997) Molecular imaging of biological samples: localization of peptides and proteins using MALDI-TOF MS. Anal Chem 69 (23):4751–4760 [DOI] [PubMed] [Google Scholar]
- 2.Goodwin RJ, Pennington SR, Pitt AR (2008) Protein and peptides in pictures: imaging with MALDI mass spectrometry. Proteomics 8 (18):3785–3800. doi: 10.1002/pmic.200800320 [DOI] [PubMed] [Google Scholar]
- 3.Buchberger AR, DeLaney K, Johnson J, Li L (2018) Mass Spectrometry Imaging: A Review of Emerging Advancements and Future Insights. Anal Chem 90 (1):240–265. doi: 10.1021/acs.analchem.7b04733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ye H, Gemperline E, Venkateshwaran M, Chen R, Delaux PM, Howes-Podoll M, Ane JM, Li L (2013) MALDI mass spectrometry-assisted molecular imaging of metabolites during nitrogen fixation in the Medicago truncatula-Sinorhizobium meliloti symbiosis. Plant J 75 (1):130–145. doi: 10.1111/tpj.12191 [DOI] [PubMed] [Google Scholar]
- 5.Gemperline E, Jayaraman D, Maeda J, Ane JM, Li L (2015) Multifaceted investigation of metabolites during nitrogen fixation in Medicago via high resolution MALDI-MS imaging and ESI-MS. J Am Soc Mass Spectrom 26 (1):149–158. doi: 10.1007/s13361-014-1010-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen RB, Li LJ (2010) Mass spectral imaging and profiling of neuropeptides at the organ and cellular domains. Analytical and Bioanalytical Chemistry 397 (8):3185–3193. doi: 10.1007/s00216-010-3723-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaurand P, Norris JL, Cornett DS, Mobley JA, Caprioli RM (2006) New developments in profiling and imaging of proteins from tissue sections by MALDI mass spectrometry. Journal of Proteome Research 5 (11):2889–2900. doi: 10.1021/pr060346u [DOI] [PubMed] [Google Scholar]
- 8.Lee YJ, Perdian DC, Song Z, Yeung ES, Nikolau BJ (2012) Use of mass spectrometry for imaging metabolites in plants. Plant J 70 (1):81–95. doi: 10.1111/j.1365-313X.2012.04899.x [DOI] [PubMed] [Google Scholar]
- 9.Gemperline E, Keller C, Jayaraman D, Maeda J, Sussman MR, Ane JM, Li L (2016) Examination of Endogenous Peptides in Medicago truncatula Using Mass Spectrometry Imaging. J Proteome Res 15 (12):4403–4411. doi: 10.1021/acs.jproteome.6b00471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poth AG, Mylne JS, Grassl J, Lyons RE, Millar AH, Colgrave ML, Craik DJ (2012) Cyclotides associate with leaf vasculature and are the products of a novel precursor in petunia (Solanaceae). J Biol Chem 287 (32):27033–27046. doi: 10.1074/jbc.M112.370841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavatorta V, Sforza S, Mastrobuoni G, Pieraccini G, Francese S, Moneti G, Dossena A, Pastorello EA, Marchelli R (2009) Unambiguous characterization and tissue localization of Pru P 3 peach allergen by electrospray mass spectrometry and MALDI imaging. J Mass Spectrom 44 (6):891–897. doi: 10.1002/jms.1562 [DOI] [PubMed] [Google Scholar]
- 12.Grassl J, Taylor NL, Millar AH (2011) Matrix-assisted laser desorption/ionisation mass spectrometry imaging and its development for plant protein imaging. Plant Methods 7:11. doi: 10.1186/1746-4811-7-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tavormina P, De Coninck B, Nikonorova N, De Smet I, Cammue BP (2015) The Plant Peptidome: An Expanding Repertoire of Structural Features and Biological Functions. Plant Cell 27 (8):2095–2118. doi: 10.1105/tpc.15.00440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batut J, Mergaert P, Masson-Boivin C (2011) Peptide signalling in the rhizobium-legume symbiosis. Curr Opin Microbiol 14 (2):181–187. doi: 10.1016/j.mib.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 15.Van de Velde W, Zehirov G, Szatmari A, Debreczeny M, Ishihara H, Kevei Z, Farkas A, Mikulass K, Nagy A, Tiricz H, Satiat-Jeunemaitre B, Alunni B, Bourge M, Kucho K, Abe M, Kereszt A, Maroti G, Uchiumi T, Kondorosi E, Mergaert P (2010) Plant peptides govern terminal differentiation of bacteria in symbiosis. Science 327 (5969):1122–1126. doi: 10.1126/science.1184057 [DOI] [PubMed] [Google Scholar]
- 16.Mortier V, Den Herder G, Whitford R, Van de Velde W, Rombauts S, D'Haeseleer K, Holsters M, Goormachtig S (2010) CLE Peptides Control Medicago truncatula Nodulation Locally and Systemically. Plant Physiology 153 (1):222–237. doi: 10.1104/pp.110.153718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mortier V, De Wever E, Vuylsteke M, Holsters M, Goormachtig S (2012) Nodule numbers are governed by interaction between CLE peptides and cytokinin signaling. Plant Journal 70 (3):367–376. doi: 10.1111/j.1365-313X.2011.04881.x [DOI] [PubMed] [Google Scholar]
- 18.Robichaud G, Garrard KP, Barry JA, Muddiman DC (2013) MSiReader: An Open-Source Interface to View and Analyze High Resolving Power MS Imaging Files on Matlab Platform. Journal of the American Society for Mass Spectrometry 24 (5):718–721. doi: 10.1007/s13361-013-0607-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seeley EH, Oppenheimer SR, Mi D, Chaurand P, Caprioli RM (2008) Enhancement of protein sensitivity for MALDI imaging mass spectrometry after chemical treatment of tissue sections. Journal of the American Society for Mass Spectrometry 19 (8):1069–1077. doi: 10.1016/j.jasms.2008.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gemperline E, Rawson S, Li LJ (2014) Optimization and Comparison of Multiple MALDI Matrix Application Methods for Small Molecule Mass Spectrometric Imaging. Analytical Chemistry 86 (20):10030–10035. doi: 10.1021/ac5028534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Caprioli RM (2011) Matrix sublimation/recrystallization for imaging proteins by mass spectrometry at high spatial resolution. Anal Chem 83 (14):5728–5734. doi: 10.1021/ac200998a [DOI] [PMC free article] [PubMed] [Google Scholar]