Abstract
Studies of neonatal health risks of unconventional natural gas development (UNGD) have not included comprehensive assessments of environmental chemical exposures. We investigated a clustering of dysphagic cases in neonatal foals born between 2014–2016 in an area of active UNGD in Pennsylvania (PA),USA. We evaluated equine biological data and environmental exposures on the affected PA farm and an unaffected New York (NY) farm owned by the same proprietor. Dams either spent their entire gestation on one farm or moved to the other farm in late gestation. Over the 21-month study period, physical examinations and blood/tissue samples were obtained from mares and foals on each farm. Grab samples of water, pasture soil and feed were collected; continuous passive sampling of air and water for polycyclic aromatic hydrocarbons was performed. Dysphagia was evaluated as a binary variable; logistic regression was used to identify risk factors. Sixty-five foals were born,17 (all from PA farm) were dysphagic. Odds of dysphagia increased with the dam residing on the PA farm for each additional month of gestation (OR = 1.4, 95% CI 1.2, 1.7, p = 6.0E-04). Males were more likely to be born dysphagic (OR = 5.5, 95% CI 1.2, 24.5, p = 0.03) than females. Prior to installation of a water filtration/treatment system, PA water concentrations of 3,6-dimethylphenanthrene (p = 6.0E-03), fluoranthene (p = 0.03), pyrene (p = 0.02) and triphenylene (p = 0.01) exceeded those in NY water. Compared to NY farm water, no concentrations of PAHs were higher in PA following installation of the water filtration/treatment system. We provide evidence of an uncommon adverse health outcome (dysphagia) in foals born near UNGD that was eliminated in subsequent years (2017–2019) following environmental management changes. Notably, this study demonstrates that domestic large animals such as horses can serve as important sentinels for human health risks associated with UNGD activities.
Keywords: foals, dysphagia, fracking, PAHs, water, UNGD
1. Introduction
Between 2000 and 2013, the US EPA (2016) estimated that approximately 3,900 public water systems, serving 8.6 million people, had at least one hydraulically fractured (unconventional) well within 1 mile of their water source. An additional 3.6 million people obtained drinking water from non-public sources in counties that have at least one unconventional well (U.S. EPA 2016). A concern is that the rapid rate of UNGD is outpacing the scientific understanding of its health and environmental impacts (Steinzor et al. 2013). Detection of contaminated water sources may be difficult in the absence of routine screening or its linkage to a health disorder.
Of the 632 chemicals reported to be used in UNGD operations (2005–2009), 353 cause adverse health effects (Colborn et al. 2011). Human, animal and environmental exposures to these chemicals arise from contamination of soil and water resources (Adgate et al. 2014; Bamberger and Oswald 2012; Shonkoff et al. 2014; U.S. EPA 2016) and from emissions of volatile and semi-volatile organic compounds including polycyclic aromatic hydrocarbons (PAHs) (Colborn et al. 2014; Sommariva et al. 2014; Paulik et al. 2016, Paulik et al. 2018). Chemicals and chemical mixtures found in air, freshwater and wastewater near UNGD have been evaluated using in vitro and in vivo studies in animal models at environmentally relevant doses. These studies have demonstrated endocrine disrupting, developmental, behavioral and immunological effects (Cozzarelli et al. 2017; Kassotis et al. 2014, Kassotis et al. 2015, Kassotis et al. 2016a, Kassotis et al. 2016b; Mesquita et al. 2014; Nagel et al. 2020; Sapouckey et al. 2018). A review of epidemiological studies evaluating the human health effects of UNGD identified 25 studies that reported statistically significant associations between exposure and health outcomes including increased adverse pregnancy outcomes, hospitalizations, asthma exacerbations, cardiovascular disease indicators, rates of sexually transmitted diseases, and traffic accidents (Deziel et al. 2020).
The effects of UNGD on fetal and childhood development are worrisome. Several studies report an association between maternal residential proximity to UNGD operations and the presence of congenital defects (McKenzie et al. 2014), severity of preterm birth (Whitworth et al. 2017, Whitworth et al. 2018), reduced birth weights and APGAR scores (Currie et al. 2017; Hill 2018) in newborn infants. Prenatal PAH exposure is associated with reduced fetal growth, cognitive developmental delay, reduced IQ and behavioral disorders (Perera et al. 2009, Perera and Herbstman 2011).
PAHs have been identified in both the water and air near unconventional wells (Colborn et al. 2014; Paulik et al. 2018; Werner et al. 2015). Alkylated derivatives predominately make up the composition of PAHs associated with petrogenic sources, such as natural gas (Stogiannidis and Laane 2015; Zakaria et al. 2002). Analytical techniques for evaluating the presence of PAHs in the environment is a well-established methodology, however, traditional methods have primarily focused on EPA’s 16 priority parent PAHs (Martinez et al. 2004; Wise et al. 2015). In order to establish sourcing for a mixture of PAHs both parent and alkylated derivatives must be quantified (Stogiannidis and Laane 2015; Tobiszewski and Namieśnik 2012; Yunker et al. 2002). Additionally, alkylated and parent PAHs beyond EPA’s priority list have been associated with increased hazard (Andersson and Achten 2015; Scott et al. 2011; Shankar et al. 2019). To accurately identify source and adverse health effects associated with an environmental exposure to PAHs, a comprehensive analysis beyond EPA’s priority list must be conducted. Furthermore, concurrent qualitative and quantitative assessment of environmental chemical exposures and health outcomes is lacking making it difficult to link adverse health effects with UNGD operations.
In March of 2014, we investigated a cluster of neurological cases involving unrelated, full-term neonatal foals born at a horse breeding facility in northeastern PA, USA (PA farm). Five of the 10 foals born on site exhibited the uncommon condition of dysphagia (tracheal milk aspiration); they exhibited a strong suckle reflex and an altered, subdued mentation. (Prevalence of dysphagia, reported by farm managers and veterinarians, is typically <1 % of foal births). Extensive medical evaluations of the affected foals eliminated known causes of dysphagia and altered mentation (Burrows and Borchard 1982; Holcombe et al. 2012; Pearson et al. 2005). Further queries of the farm owner and farm managers by two of the study authors (K.R.M, D.M.A.) revealed that in 2012 and 2013, 2 of 8 and 2 of 6 neonatal foals born on site respectively, had been dysphagic. At another horse breeding facility owned by the same proprietor and located 420 kilometers east in New York, USA (NY farm), similar animal health problems (subdued, dysphagic foals) had never occurred over a 10-year period. As movement of pregnant mares (5–7 months gestation) between the two farms occurred, it is noteworthy that the dams of the dysphagic PA foals, when they had resided at the NY facility, never had an abnormal foal. Importantly, animals at both facilities were fed the same source hay and grain, but water and pasture sources differed. The PA farm is in an area of active UNGD; the NY farm is not.
We hypothesized that environmental chemical exposure was associated with the clinical syndrome observed in the foals on the PA farm and commenced a comprehensive animal health and environmental assessment of both farms utilizing multiple methodologies including passive sampling of air and water sources continuously over 21 months. Although several studies have documented the deleterious effects of air-borne PAH exposure during pregnancy on subsequent fetal or child development (Choi et al. 2012, Padula et al. 2014, Jedrychowski et al. 2015), this is the first study measuring water- and air-borne PAH exposures through the entire gestation period of an animal model. Utilizing a natural prospective study design with a crossover of participants between environments with and without UNGD exposure, we sought to (1) characterize the demographic, physical examination and laboratory findings associated with dysphagia, and (2) determine environmental exposures in this unique disease cluster.
2. Methods
2.1. Study design and approval
This prospective study (December 2014-August 2016) involved two Standardbred broodmare farms and entailed equine tissue collection and environmental sampling at approximately six-week intervals. The “main” data set also included information on 3 foals born in March 2014 (NY farm). Historical information on mare environmental exposures, foal gender and dysphagia were obtained from detailed farm and veterinary medical records for the entire 2014 foaling season (January 2014-June 2014). These data, combined with the same variables in the main data set, comprised a larger data set encompassing a longer period of time (“extended” data set) and were used to test the robustness of the statistical model for those same variables.
The investigation protocols were approved by the Cornell University Institutional Animal Care and Use Committees (2014–0030) and by the Oregon State University Institutional Animal Care and Use Committees (2015–0013). Written consent to investigate the farm environments and animal health parameters was obtained from the owner of the NY and PA farms prior to initiation of the study.
2.2. Environmental sampling
Environmental PAHs can exist as particulate-bound entities or in a vapor or dissolved phase (unbound fraction). The latter are able to diffuse across biological membranes and are suggested to be more biologically active (Allan et al. 2012; Paulik et al. 2018). Passive sampling of air and water for organic pollutants is a well-established methodology (Anderson et al. 2008; Huckins et al. 2006; Lohmann et al. 2012). Passive samplers sequester the unbound fraction of lipophilic compounds through diffusion in a time-integrated manner.
One stationary air and one water passive sampler were deployed at each farm location for continuous air and water monitoring during a 21-month sampling period starting December 2014 (air) and January 2015 (water). Each passive sampler consisted of five low-density polyethylene strips in metal cages located in a pasture stand (air) and in the post-water filtration system (Figures A.1, A.2). The air samplers were secured to broodmare pasture fencing at a height of 1.2 m. The PA air sampler was located on the corner of the pasture closest to the gas well pad (approximately 340 m away). Passive samplers were re-deployed at 6-week intervals by one of the study authors (K.R.M.) who replaced and packaged existing samplers in airtight polytetrafluoroethylene bags that were mailed to the Food Safety and Environmental Stewardship Lab, OSU in Corvallis, OR.
At those same 6-week intervals, samples of pasture soil (400 g) and feed (200 g hay, 400 g commercial grain) were collected in plastic freezer bags; samples of barn tap water (470 mL) were collected in plastic bottles. Samples were transported back to the lab and stored at −80°C until batch analyzed.
2.3. Experimental animals and farm descriptions
Information on the mare’s breeding date, breeding stallion, farm location of mare at the time of breeding and foaling, movement dates between farms, health problems and treatments of the mare or its foal were recorded. All mares were bred by artificial insemination using fresh cooled commercial semen (stallions off-site). Mares spent their gestation period either entirely on the PA farm or NY farm or moved between the PA and NY farms in mid-late gestation according to farm management decisions.
All foals born on the 2 farms during the study period were eligible for enrollment in the study. Dysphagia was defined as tracheal milk aspiration, suspected by abnormal gurgling noises made during nursing and confirmed via video tracheoscopy post-nursing by two of the study authors (D.M.A., K.R.M.) who are large animal internists. Foals diagnosed with neonatal encephalopathy, sepsis and prematurity (and their dams) were excluded from the study as these syndromes were distinct from that affecting our study population (Lyle-Dugas et al. 2017).
The PA (affected) farm is in a northeast region of the Marcellus shale formation (41.9678° N, 76.3922° W). Adjacent to the facility is one well pad containing a single gas well with a SPUD date of 3/23/2011; natural gas production commenced during the second half of 2012 (PA DEP Oil and Gas Reporting Website, 2018). Within 10 kilometers of the farm are 28 gas wells with earlier SPUD dates. The 85.5-acre farm supports 10–12 mares, their foals, 10–12 yearlings and contains residences for the 2 families working on the farm. Late pregnant mares (>300 days gestation) and mares with young foals are housed in individual stalls in a single barn. Each stall is bedded with wood shavings and straw and contains an automatic waterer. Water is sourced from 2 wells from which the output is combined. Well 1 is 38.4 meters below grade, open hole with 6.4 m of casing. Well 2 is 61 meters below grade, has 6.2 m of a 0.15 m diameter grouted steel casing and a pump set at 58 meters below grade. The main water line passes through a sediment filter and water softener and provides water to the families and horses. Between 8/11/2015–8/13/2015, a water filtration/treatment system containing carbon filters, UV light and chlorine injection was installed. (NB: When the water filtration/treatment system was installed in August 2015, the PA water sampler that was deployed on 8/7/2015 was removed on 8/11/2015 but maintained in its water-filled canister. It was re-deployed on 8/13/2015.) The nearest gas well pad is located approximately 522 m from Well 1 and 433 m from Well 2.
The NY (unaffected) farm is located 420 kilometers east in a region that has never been exposed to natural resource mining activities (41.8633° N, 73.6851° W). This 150-acre facility contains 15–20 mares, their foals, 15–20 yearlings and 3 residences for the 4 individuals employed on the farm. Drinking water for the residences and animals is provided by two separate wells. The broodmare barn and most of the paddocks/pastures are supplied by one well which was analyzed in the study. The water line passes through a sediment filter and water softener. Stalls, pastures and paddocks have automatic waterers. Late pregnant mares and mares with young foals are housed in individual stalls in a single barn. Stalls are bedded with wood shavings and straw (same supplier as utilized by PA farm). Horses at both farms are fed alfalfa hay (single supplier) and the same brand of commercial concentrate (Poulin Grain, Newport, VT).
2.4. Experimental procedures
2.4.1. Animal data collection and sample testing
All foalings were attended by trained farm managers who recorded the foal’s time and date of birth, a modified Apgar Score (Smith, 2015), time-to-nurse, birth weight, wither height, rectal temperature, pulse and respiratory rates, the presence of dysphagia and placental weight. Physical examinations of the 12- to 24-hour-old foals and dams were performed by the farm veterinarian; placental samples were placed in formalin for histopathological evaluation; jugular venous blood samples from each mare and foal were collected for laboratory analysis. Samples were kept cool and shipped overnight on cool packs (NY farm) or hand-delivered (PA farm) to a study author (D.M.A.).
Complete blood counts (CBC), serum amyloid A (SAA), biochemistry panels, IgG, selenium (Se), and vitamin E concentrations were determined; in the second year of the study, blood lead concentrations were also measured. Serum samples were stored at −80°C for batched analysis of serum elements and thyroid hormones. Washed red blood cells were stored at −80°C for batched measurement of glutathione peroxidase activity (GPX). The CBCs, biochemistry panels and concentrations of SAA, IgG, Vitamin E, Se, thyroid hormones, blood lead and glutathione peroxidase activity were measured at the Animal Health Diagnostic Center, Cornell University (AHDC CU), Ithaca, NY. Placental histopathological examination was performed by one pathologist (J.N.S.N.T.) at the AHDC CU, Ithaca, NY. Foal serum elements—aluminum, arsenic, barium, boron, calcium, chromium, cobalt, copper, iron, lead, lithium, magnesium, molybdenum, selenium, silicon, strontium and zinc—were measured at the Utah Veterinary Diagnostic Laboratory, Utah State University, Logan, UT.
Foals born on the PA farm requiring medical attention were examined at the Cornell University Hospital for Animals, Ithaca, NY (65 kilometers away); those from the NY farm were examined at the Rhinebeck Equine Hospital, Rhinebeck, NY (32 kilometers away). Dysphagic foals underwent detailed physical examinations, endoscopic evaluations of the upper and lower respiratory tract and ultrasonographic evaluations of the thoracic and abdominal cavities. Blood cultures were obtained at the discretion of the attending veterinarian. Foals received intensive care while hospitalized. The duration of dysphagia was determined by daily endoscopic evaluation of the foal’s trachea performed pre- and post-nursing.
2.4.2. Environmental testing
2.4.2.1. Soil and water elements, feed composition
Aluminum, arsenic, barium, boron, calcium, cadmium, chromium, cobalt, copper, iron, potassium, lead, lithium, magnesium, manganese, nickel, phosphorous, potassium, selenium, silicon (water only) sodium, sulfur, strontium, titanium, vanadium and zinc were measured at the Cornell Nutrient Analysis Laboratory (CNAL), CU, Ithaca, NY. Nutrient (hay and grain) and grain mycotoxin (aflatoxin B1, B2, G1, G2, vomitoxin, 3-acetyldeoxynivalenol, 15-acetyldeoxynivalenol, trichothecene and zearalenone) analyses were performed at the Dairy One Forage Testing Laboratory, Ithaca NY.
2.4.2.2. Passive sampler procedures and chemical analysis
Procedures used for conditioning, cleaning, and extraction of low-density polyethylene strips can be found elsewhere (Anderson et al. 2008; Tidwell et al. 2016). Prior to deployment, the low-density polyethylene strips were infused with performance reference compounds (PRCs, fluorene-d10, pyrene-d10, benzo[b]fluoranthene-d12) to determine in situ sampling rates and time integrated air and water concentrations (Huckins et al. 2006). Extracts from the low-density polyethylene strips were quantitatively analyzed for 62 PAHs (Table A.1) using an Agilent 7890A gas chromatograph interfaced with an Agilent 700 GC/MS-MS (Anderson et al. 2015).
Air (ng/m3) and water (ng/L) concentrations were calculated from instrument concentrations (pg/μL). Sampling rates were determined with an empirical uptake model using PRCs (Huckins et al. 2006) and, for each PRC, was estimated based on duration of deployment, initial amount of PRC, and temperature corrected sampler-air/water partition coefficient (Donald and Anderson 2017; Tidwell et al. 2017).
Quality control samples consisting of construction, field, trip, post-deployment cleaning and instrument blanks, made up approximately 40% of all samples analyzed. Pyrene-d10 was used as the performance reference compound for these samples, % fraction retained was used to determine air and water concentrations. Average % fraction retained for both water and air low density polyethylene strips was 56%. The analytical method used to quantify PAH concentrations was validated prior to use based on detection limits for calibration, precision, and accuracy (Anderson et al. 2015).
2.4.2.3. Forensic chemical analysis
In order to determine if a PAH mixture was predominantly petrogenic (petroleum-derived) or pyrogenic (derived from fossil fuel combustion), PAH isomer ratios (Table A.2) were calculated for air and water samples from each farm (Stogiannidis and Laane 2015; Tobiszewski and Namieśnik 2012; Yunker et al. 2002). To assess associations between natural gas production and PAH exposure, production records were obtained (PA DEP Oil and Gas Reporting Website, 2018). Mean gas production was calculated for each deployment period, by averaging production amount over months of deployment.
2.4.3. Statistical methods
All statistical analyses were conducted using R software (R Project for Statistical Computing [http://www.r-project.org/]). Dysphagia, the outcome of interest, was evaluated as a binary variable. 106 explanatory variables (main data set) were examined for possible associations with dysphagia in foals. Descriptive statistics were calculated for the outcome and explanatory variables. A few outliers in the toxicology variables were identified and upon discussion with the lab toxicologist, were considered as measurement errors (values reported were not compatible with life) and excluded from subsequent analyses.
Comparison of box-plots for dysphagic and unaffected foals was used for initial screening of continuous explanatory variables; cross-tabulation by the outcome of interest was applied for initial examination of categorical variables. For initial evaluation of possible interactions, boxplots for continuous variables and dysphagia by an additional categorical explanatory variable of interest (Foaling site, Gender and Foaling year) were examined. Associations at univariable level between dysphagia and continuous variables were evaluated by logistic regression and the Mann-Whitney U Test (Wilcoxon test); variables significantly associated with the outcome in the Mann-Whitney U Test but not in the logistic regression were median-dichotomized and considered further in the analysis as binary variables. These were subjected to the Chi-square/Fisher’s exact test to evaluate associations between dysphagia and categorical explanatory variables. Spearman correlation was used to quantify correlation among continuous explanatory variables; variables with correlation coefficient larger than abs(0.75) were considered in multivariable modeling one at a time starting with the variable with the lowest p-value. The correction for multiple comparisons in the univariate analyses was not conducted due to the exploratory nature of the investigation.
Variables statistically significantly associated with the outcome at the univariable level were considered in the multivariable logistic regression using forward stepwise selection procedure in increasing order of univariable p-values (among those with a p-value of <0.05). Likelihood ratio test (LRT) was conducted to assess the improvement in the model fit due to addition of a variable. Adjusted Odds Ratios (ORs), 95% confidence intervals and Wald-test p-values were presented based on the final model for each explanatory variable. The variance inflation factor (VIF) was used in the final model to diagnose collinearity. Diagnostic plots were used to assess the suitability of models that assume a linear relationship between the explanatory variable and the log odds of dysphagia. All analyses were first conducted on foals from both the NY and PA farm. As there were no dysphagic foals born on the NY farm during the study period, all unaffected foals (from PA and NY) were compared to the PA dysphagic foals. Given the small sample size, dysphagic and unaffected foals on the PA farm only were not compared. The unaffected foals of the NY and PA farm were compared to determine if there were systematic differences between the two farms.
Soil, water, grain and hay samples collected on the two farms were analyzed using Wilcoxon rank sum tests to determine if they differed between the two farms. P values <0.05 were considered statistically significant.
Analysis of PAHs in air and water identified above detection limits involved (1) examination of scatterplots and boxplots and (2) statistical testing using a Kruskal-Wallis test. For the air data, PAH compounds and forensic ratios (Table A.2) were compared between the two farms. For the water data, the interest was in the interaction between the sampling period (pre- and post-) water filtration/treatment system and farm (NY, PA) resulting in a 4-way comparison of groups: A significant Kruskal-Wallis test was followed with the Dunn’s test to control the false discovery rate using the Benjamini-Hochberg procedure. Spearman rho correlation was used to identify correlations between gas production and the summed total of the polycyclic aromatic hydrocarbons (ΣPAH) for a specific period.
3. Results
3.1.1. Overall prevalence of dysphagia and its duration
Of the 69 foals born during 2014–2016, 17 were dysphagic (all born in PA) and 48 were normal (11 born in PA, 37 born in NY; Table 1). Four foals (2 born in NY, 2 born in PA) diagnosed with neonatal encephalopathy (Lyle-Dugas et al. 2017) were excluded from analysis. Foal gender and dysphagia were strongly associated: 13/17 (76%) of dysphagic foals were colts; 19/48 (40%) of unaffected foals were colts (Chi-square Test, p = 0.009). In 2014, 26% of all foals (NY and PA) born were dysphagic compared to 41% and 13% in 2015 and 2016, respectively. Two management changes occurred during the study period that may have decreased the incidence of dysphagia. In August 2015 (after the foaling season), a water filtration/treatment system was installed on the PA farm; starting in January 2016, NY-bred mares that moved to the PA farm for foaling spent only their last 4–6 weeks pre-partum in PA, compared to previous years in which the time spent at the PA farm was typically 4–6 months.
Table 1.
Number of Dysphagic and Non-dysphagic Foals Born on the PA and NY Farms
| PA Farm | NY Farm | |||||||
|---|---|---|---|---|---|---|---|---|
| Normal | Dysphagic | Normal | Dysphagic | |||||
| Main | Extended | Main | Extended | Main | Extended | Total | Extended | |
| Female | 6 | 9 | 3 | 4 | 15 | 20 | 0 | 0 |
| Male | 0 | 2 | 9 | 13 | 6 | 17 | 0 | 0 |
| Total | 6 | 11 | 12 | 17 | 21 | 37 | 0 | 0 |
| 2014 | 0 | 5 | 0 | 5 | 3 | 9 | 0 | 0 |
| 2015 | 1 | 1 | 9 | 9 | 10 | 12 | 0 | 0 |
| 2016 | 5 | 5 | 3 | 3 | 8 | 16 | 0 | 0 |
Column “Main” refers to foal data in the main (prospective) data set; column “Extended” includes foals from the main data set and additional historic data on foals born in 2014 for which selected variables (dysphagia, gender) were available. For additional explanation, refer to methods.
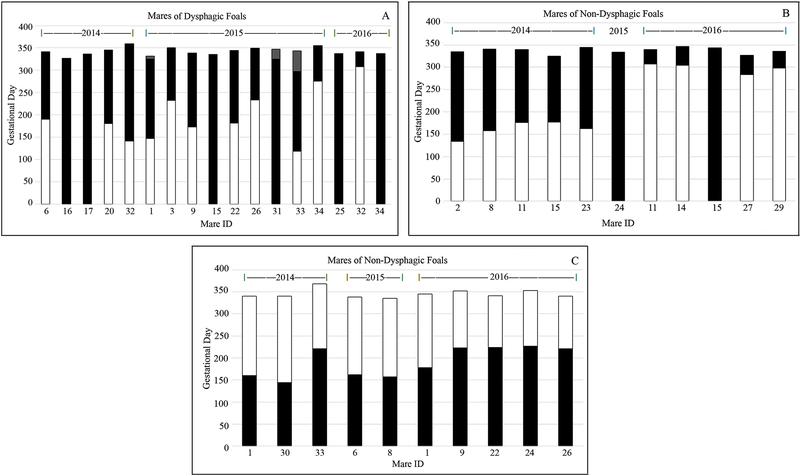
Of the 17 dysphagic foals, 6 were from mares that spent their entire gestation at the PA farm; 11 were born of mares that had moved from the NY to PA farm in mid-late gestation (Figure 1A). In 2015, 3 late-pregnant PA mares (1-, 3- and 6-weeks pre-partum) were relocated to another farm not near UNGD and still delivered dysphagic foals. Thirteen mares each had 1 dysphagic foal; 2 mares (M32, M34) each had 2 dysphagic foals. Of the 15 mares that had dysphagic foals, 6 delivered ≥ 1 normal foal in NY during the study period. One PA mare (M15) had a dysphagic foal in 2015, but normal foals in 2014 and 2016 (Figure 1A, 1B). Eleven non-dysphagic foals were born on the PA farm, 2 from mares that had spent their entire gestation in PA and 9 that had moved from the NY to PA farm during gestation (Figure 1B).
Figure 1.
Location of individual study mares during their gestational period (2014–2016). For each mare, the gestational days spent on the PA farm is indicated in black, those spent on the NY farm are in white. Gray (Panel A) indicates the gestational days spent on a different PA farm not near UNGD prior to parturition (3 mares). Mares that produced dysphagic foals are represented in panel A; those that produced non-dysphagic foals are shown panel B (having spent the latter part of gestation at the PA farm) or panel C (having spent the latter part of gestation at the NY farm). Not shown are mares that spent their entire gestation in NY where no dysphagic foals were born.
Of the 37 foals born on the NY farm, none were dysphagic: 27 of the mares had spent their entire gestation in NY and 10 had been bred in PA and moved to NY during late gestation (Figure 1C).
Analysis of breeding records (sire-dam matings) from both farms failed to demonstrate a genetic link between a given stallion and a dysphagic foal.
Following medical treatment, 14/17 dysphagic foals gained their ability to nurse effectively. Their median duration of dysphagia was 11 days. Three foals never gained their nursing ability after 16, 28 and 46 days of intensive care. They were weaned and fed milk from a pan and solid feed.
3.1.2. Characteristics of foals
Clinical and laboratory data were obtained for 12 dysphagic (PA farm) and 27 unaffected (6 from PA farm, 21 from NY farm) foals and their dams (n=39 mares). Univariate analyses of continuous variables comparing dysphagic and unaffected foals are shown in Tables 2, A.3 and A.4; in Table 3 are the categorical data. No differences were found in physical parameters (gestational age, birth weight, height, rectal temperature, pulse rate), time-to-nurse, serum immunoglobulin G (reflecting colostral transfer of maternal antibody), markers of inflammation (SAA, fibrinogen, CBC count) or oxidative stress (vitamin E, selenium, glutathione peroxidase activity), and thyroid hormone levels, with several minor exceptions (Tables 2 and A.3). The modified APGAR score was lower in dysphagic foals compared to unaffected but within the normal range. Dysphagic foals had a lower respiratory rate (24 bpm) at birth compared to unaffected foals (48 bpm) (p = 2.0E-03). Its cause, undetermined in the absence of arterial blood gas analyses, might have reflected neuromuscular weakness or impaired respiratory drive (Koterba et al. 1990). Farm and hospital veterinarians attending to dysphagic foals noted them to be subdued, consistent with central neurological dysfunction.
Table 2.
Demographic and Clinical Parameters of Newborn Foals and Mares
| Non-dysphagic (NY & PA Farm) | Dysphagic (PA Farm) | ||||||
|---|---|---|---|---|---|---|---|
| Median (IQR) | n | Median (IQR) | n | p-value | |||
| Foals: | |||||||
| Gestational age (days) | 341 | (338–347) | 27 | 343 | (339–347) | 12 | 0.93 |
| Foal Apgar Score* | 10 | (10–10) | 24 | 10 | (9–10) | 12 | 0.02 |
| Time to nurse (minutes) | 136 | (110–170) | 24 | 168 | (134–201) | 12 | 0.16 |
| Rectal temperature (°C) | 38.2 | (37.9–38.4) | 24 | 38.1 | (37.8–38.2) | 11 | 0.32 |
| Pulse rate (bpm) | 107 | (100–120) | 24 | 108 | (98–116) | 11 | 0.38 |
| Respiratory rate (bpm)* | 48 | (40–60) | 24 | 24 | (20–35)† | 11 | 2.0E-03 |
| Weight (kg) | 51 | (42–55) | 24 | 55 | (52–55) | 12 | 0.09 |
| Wither height (cm) | 103.5 | (100.2–107) | 22 | 105 | (101–105) | 9 | 0.97 |
| Mares: | |||||||
| PA Farm Duration of Expose (days)* | 34 | (0–201) | 27 | 177 | (151, 328) | 12 | 0.01 |
| PA Farm Exposure Total (%)* | 10 | (0–56) | 27 | 52 | (44, 95) | 12 | 0.01 |
| PA Farm Exposure 1st trimester (%) | 0 | (0–100) | 27 | 0 | (0, 100) | 12 | 0.80 |
| PA Farm Exposure 2nd trimester (%)* | 0 | (0–74) | 27 | 68 | (31, 100) | 12 | 0.02 |
| PA Farm Exposure 3rd trimester (%) * | 0 | (0–0) | 27 | 96 | (61, 100) | 12 | 2.4E-05 |
| Placental weight (kg) | 5.9 | (5.0–6.6) | 15 | 5.3 | (5.0, 5.7) | 9 | 0.40 |
IQR=interquartile range
Values were considered in the multivariate model. P-values based on Wilcoxon test.
Outside of the normal reference range for neonatal foals.
PA Farm Duration of Expose (days) = number of days of the mare’s pregnancy spent on the PA farm
PA Farm Exposure Total (%) = percentage of mare’s total pregnancy spent on the PA Farm
PA Farm Exposure 1st trimester (%) (or 2nd, 3rd) = percentage of that trimester spent on the PA Farm (1st trimester = days 0–115; 2nd trimester = days 116–231; 3rd trimester = days 232-foaling).
Table 3.
Categorical Explanatory Variables in Newborn Foals
| Categorical Explanatory Variable | Variable Level | Non-dysphagic |
Dysphagic |
OR | Lower | Upper | p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||||
| Foaling site* | NY | 21 | 78 | 0 | 0 | 1.00 | NA | NA | NA |
| PA | 6 | 22 | 12 | 100 | Inf | 6.65 | Inf | 4.8E-06 | |
| Gender* | Filly | 21 | 78 | 3 | 25 | 1.00 | NA | NA | NA |
| Colt | 6 | 22 | 9 | 75 | 9.73 | 1.75 | 74.58 | 3.5E-03 | |
| Bands (cells × 103/ μL)* | 0 | 27 | 100 | 7 | 58 | 1.00 | NA | NA | NA |
| >0 | 0 | 0 | 5 | 42 | Inf | 2.66 | Inf | 1.0E-03 | |
| Strontium (ppm)* | ≤0.0305 | 15 | 68 | 2 | 17 | 1.00 | NA | NA | NA |
| >0.0305 | 7 | 32 | 10 | 83 | 10.71 | 1.84 | 62.49 | 4.0E-03 | |
| Boron (ppm)* | ≤0.1265 | 8 | 36 | 9 | 75 | 1.00 | NA | NA | NA |
| >0.1265 | 14 | 64 | 3 | 25 | 0.19 | 0.04 | 0.91 | 0.03 | |
Analysis based upon main data set
Variables were considered in the multivariate model.
Bands = band neutrophils, NA = not applicable, Inf = infinite; p-values based on Fisher’s Exact test except for strontium and boron which were based on a Chi-squared tests. Bands, strontium and boron were median dichotomized before analysis.
Compared to unaffected, median band neutrophil counts were slightly higher (p = 4.5E-04); and median lymphocyte (p = 2.5E-04) and monocyte (p=0.02) counts were lower in dysphagic foals. These differences were attributed to inflammation associated with aspiration (band neutrophils) and stress (lymphocytes). Serum iron and iron saturation values were lower in dysphagic foals (p = 0.01, p = 0.01, respectively), but within reference ranges (Harvey et al. 1984). These changes likely reflected inflammation due to aspiration (Corradini et al. 2014) and were excluded from further analysis. Small differences between the two groups in serum sodium, potassium, anion gap, bicarbonate and SUN (Table A.3), were attributed to sample aging associated with shipping. These variables along with lymphocyte numbers were excluded from further analysis (Dupuy et al. 2018, Rendle et al. 2010).
Median serum concentrations of 5 elements (Table A.4) differed between the two groups of foals: Boron (p = 0.01), chromium (p = 4.0E-03) and silicon (p = 0.02) were lower; barium (p = 0.01) and strontium (p = 0.01) were higher in dysphagic foals compared to unaffected. Differences were small and all values were within the published reference ranges for horses (Puls, 1994).
Foal categorical data considered in the multivariable analysis are shown in Table 3. Band neutrophils, serum strontium and boron were median dichotomized for inclusion in the multivariable model.
3.1.3. Characteristics of mares
The demographic (Table 2) and clinical pathological data of the dams of the foals born in 2014–2016 (Table A.5) were examined. Median duration of exposure to the PA farm during pregnancy was greater for dams of dysphagic foals (177 days) compared to dams of unaffected foals (34 days, p = 0.01). Median percentage of mare’s total pregnancy spent on the PA farm was greater for dams of dysphagic (52%) compared to mares of unaffected foals (10%, p = 0.01). Exposure during the second (68% vs 0%, p = 0.02) and third (96% vs 0%, p = 2.0E-05) trimesters was significantly greater for dams of dysphagic vs. unaffected foals. Significant differences in serum markers of inflammation (SAA, fibrinogen, WBC count), oxidative stress (vitamin E, selenium) or renal and hepatic dysfunction were not evident between the two groups of mares. Differences in serum potassium between the two groups (p = 1.6E-4) were attributed to sample aging due to shipping of NY sample and it was excluded from further analysis (Dupuy et al.2018; Rendle et al. 2010).
Placental weights did not differ between dysphagic and unaffected foals (Table 2). Histopathological examinations were performed on 12 placentas: 3 were from healthy NY foals born in 2016; 9 were from foals born in PA (1 healthy, 6 dysphagic foals born in 2015, 2 healthy foals born in 2016). All examinations were normal.
3.1.4. Final multivariable model for dysphagic and non-dysphagic foals
Significant continuous and categorical variables from the univariable analysis were used to a construct a multivariable logistic regression model. Variables retained in the final model included PA Farm Exposure 3rd trimester and Foal Gender (Table 4). The robustness of the model was tested utilizing the extended data set and the best fit model included PA Farm Duration of Exposure and Foal Gender. This highlighted the findings from the prospective study period that odds of dysphagia were associated with mares residing on the PA farm for a longer percentage of gestation. The syndrome also appeared to be gender-associated with colts having an increased risk of dysphagia compared to fillies.
Table 4.
Final Multivariable Logistic Regression Model
| Estimate | Std. Error | z value | Pr(>|z|) | OR | OR-lower CI | OR-upper CI | |
|---|---|---|---|---|---|---|---|
| Main data set (n=39) | |||||||
| (Intercept) | −3.7839 | 1.1268 | −3.3580 | 0.0008 | 0.02 | 0.00 | 0.21 |
| PAExpos.p3rd | 0.0456 | 0.0149 | 3.0682 | 0.0022 | 1.05 | 1.02 | 1.08 |
| FoalGenderColt | 2.4728 | 1.2069 | 2.0488 | 0.0405 | 11.86 | 1.11 | 126.27 |
| Extended data set (n=65) | |||||||
| (Intercept) | −3.7128 | 0.9178 | −4.0452 | 0.0001 | 0.02 | 0.00 | 0.15 |
| PADurExpose.(mon) | 0.3412 | 0.0998 | 3.4206 | 0.0006 | 1.41 | 1.16 | 1.71 |
| FoalGenderColt | 1.6991 | 0.7649 | 2.2213 | 0.0263 | 5.47 | 1.22 | 24.49 |
OR = Odds Ratio; CI= Confidence Interval
PAExpos.p3rd = percentage of 3rd trimester (days 232-foaling) mare spent on the PA farm. FoalGenderColt = gender with filly as the baseline.
3.1.5. Characteristics of non-dysphagic foals
To test for a systematic farm level difference, non-dysphagic foals born in PA (n = 6) were compared to non-dysphagic foals born in NY (n = 21). The only variable significant at the univariable level in the logistic regression was serum gamma glutamyl transferase (p = 0.01). Additional continuous variables identified in the Wilcoxon test (serum aspartate aminotransferase, creatine kinase, aluminum, molybdenum, and strontium) were median dichotomized for testing with Fisher’s exact test. None were significant. Therefore, the only variable that differed between non-dysphagic foals born in NY and PA was serum gamma glutamyl transferase (OR for PA vs NY foals = 0.84, 95% CI: 0.71–0.98, p-value = 0.03). A systematic difference between the two farms was not identified.
3.2. Environmental samples
3.2.1. Feed analyses
Samples of alfalfa hay collected from the NY (n = 6) and PA (n = 7) farms did not differ in nutrient components (Table A.6). Concentrate samples from the NY (n = 6) and PA (n = 7) farms did not differ in the presence of nutrient composition with the exception of small differences in % phosphorous (p = 0.03) and zinc ppm (p = 0.03) (Table A.7). These small differences were not considered clinically significant (NRC, 2007).
3.2.2. Pasture soil and water element concentrations
Analysis of soil grab samples collected from the NY (n = 7) and PA (n = 6) farms (Table A.8) demonstrated higher concentrations of arsenic (p = 5.0E-03), barium (p = 2.0E-03), cadmium (p = 0.03), cobalt (p = 0.02), sodium (p = 4.0E-03), sulfur (p = 8.0E-03), vanadium (p = 0.01) and zinc (p = 0.01) in the PA soil. Molybdenum (p = 0.04) and titanium (p = 3.0E-03) concentrations were lower in the PA farm soil. All element concentrations were below recommended maximum soil trace element concentrations for garden soils in the Northeast US (Cornell Waste Management Institute 2015, Gough et al. 1979).
Analysis of water grab samples collected from NY (n = 12) and PA (n = 12) farms (Table A.9) demonstrated higher concentrations of barium (p = 1.6E-4), boron (p = 2.7E-5), calcium (p = 1.2E-4), iron (p = 0.02), lithium (p = 9.8E-3), magnesium (p = 1.2E-4), potassium (p = 9.7E-5), sulfur (p = 4.8E-4), and strontium (p = 0.04) in the PA water. Molybdenum (p = 1.9E-6) and sodium concentrations (p = 5.9E-4) were lower on the PA farm. All water mineral levels were within primary and secondary drinking water standards (US EPA, 2019). That serum barium and strontium were significantly higher in dysphagic foals possibly reflected the mare’s consumption of water containing higher concentrations of these elements. Sources of barium contamination in drinking water include discharge of drilling waste, discharge from metal refineries, and erosion of natural deposits (US EPA 2016). At high doses (>20 ppm) of the diet, barium is associated with skeletal and cardiac muscle paralysis, salivation and diarrhea. Naturally occurring strontium is found in groundwater and bedrock aquifers. Provided the diet is adequate in calcium, animals can tolerate up to 2,000 ppm dietary strontium. Toxicity is associated with reduced growth rates and rickets (Puls, 1994) which were not evident in the foals.
3.2.3. Well water PAHs
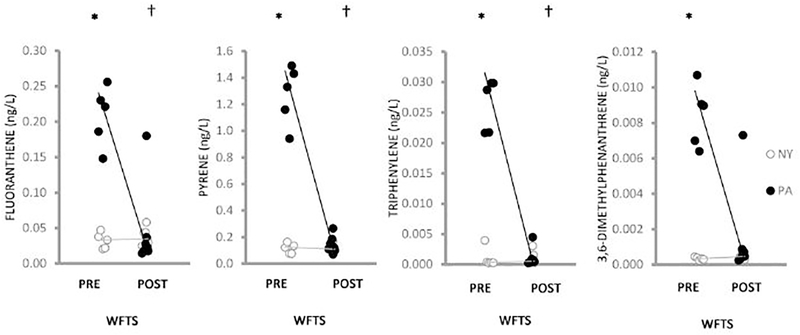
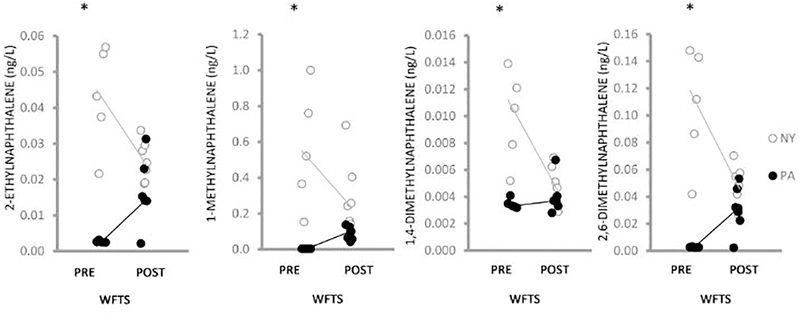
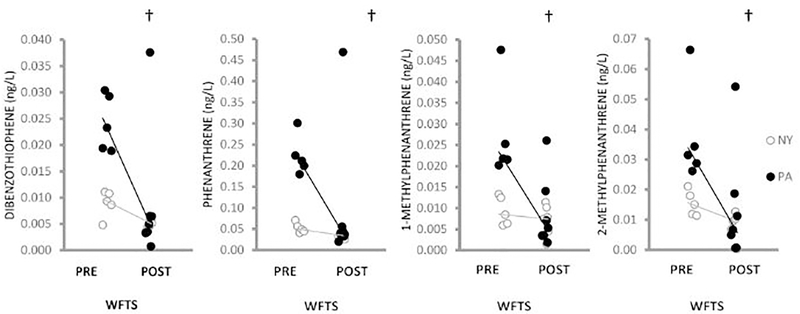
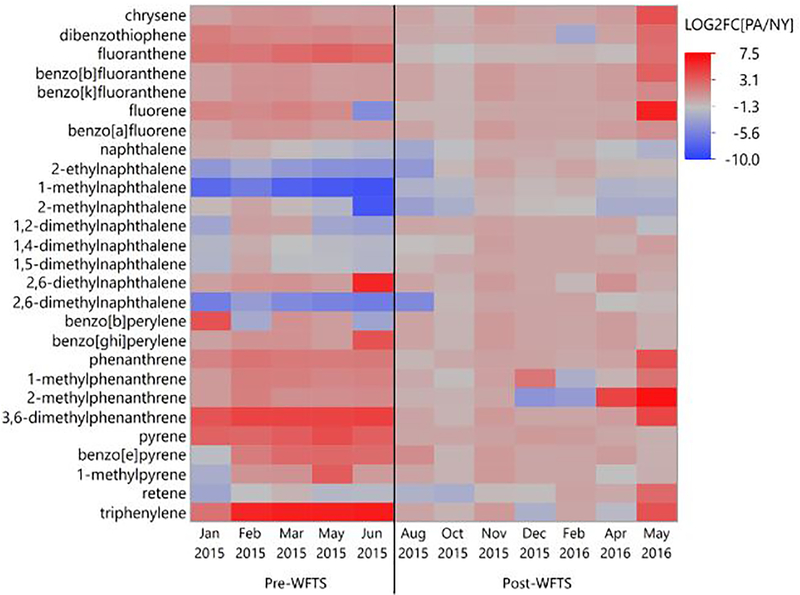
Continuous passive sampling of well water in NY (n = 12) and PA (n = 12) identified 27 PAHs above detection limits (Tables A.10, A.11). Prior to the installation of the water filtration/treatment system, PA water concentrations of fluoranthene (p = 0.03), pyrene (p = 0.02), triphenylene (p = 0.01), and 3,6-dimethylphenanthrene (p = 5.6E-03) exceeded those in NY water (Figure 2, Table A.11). NY water concentrations of 2-ethylnaphthalene (p = 1.2E-03), 1-methylnaphthalene (p = 6.0E-04), 1,4-dimethylnaphthalene (p = 6.1E-03) and 2,6-dimethylnaphthalene (p = 1.3E-03) exceeded those in PA water (Figure 3). Following installation of the water filtration/treatment system, PA water concentrations of fluoranthene (p = 3.5E-3), pyrene (p = 0.02) and triphenylene (p = 0.02) (Figure 2) in addition to dibenzothiophene (p = 0.01), phenanthrene (p = 0.03), 1-methylphenanthrene (p = 0.01), 2-methylphenanthrene (p = 0.02) (Figure 4) and naphthalene (p = 0.049) (Figure A.3), were reduced compared to pre-water filtration/treatment system PA concentrations. The effect of the installation of the water filtration/treatment system is further demonstrated by comparing Log2 fold changes in PAH concentrations in PA vs. NY by sample period (Figure 5). In the post-water filtration/treatment system sampling interval, no PAH concentration in PA water was significantly higher than that in the NY water (Table A.11), although in May 2016, there was an increase in the concentration of several PAHs in PA compared to NY (Figure 5). During this time, gas production from the adjacent well had stopped leading us to hypothesize that there was an association between production ceasing and the spike in PAH concentrations.
Figure 2.
Scatterplot of 4 PAHs (ng/L) detected in higher concentrations in PA (black circles) compared to NY (open circles) well water prior to (pre) or following (post) installation of the WFTS over the 21-month sampling. Individual data points were jittered to enhance visualization. Lines connect medians. *indicates a significant difference in the pre-WFTS samples between the PA and NY farms. Fluoranthene (p = 0.03), pyrene (p = 0.02), triphenylene (p = 0.01) and 3,6-dimethylphenanthrene, (p = 5.6E-03) concentrations were greater in PA well water. † indicates a significant difference between the pre and post WFTS samples on the PA farm: Fluoranthene (p = 3.5E-03); pyrene (p = 0.02) and triphenylene (p = 0.02) concentrations were significantly lower compared to pre-WFTS PA concentrations.
Figure 3.
Scatterplot of 4 PAHs (ng/L) detected in higher concentrations in NY (open circles) compared to PA (black circles) well water pre and post-installation of the WFTS over the 21-month sampling. Individual data points were jittered to enhance visualization. Lines connect medians. *indicates pre-WFTS NY concentrations of 2-ethylnaphthalene (p = 1.2E-03), 1-methylnaphthalene (p = 0.6.0E-04), 1,4-dimethylnaphthalene (p = 6.1E-03) and 2,6-dimethylnaphthalene (p = 1.3E-03) were significantly greater than PA concentrations. Post-WFTS, no differences were found between the 2 farms in these PAHs.
Figure 4:
Scatterplot of 4 PAHs (ng/L) detected in higher concentrations in PA (black circles) well water pre-WFTS compared to PA post-WFTS over the 21-month sampling period. NY (open circles) concentrations are also shown. Jittering was used to visualize individual data points more clearly. Lines connect medians. With installation of the WFTS, PA water concentrations of dibenzothiophene (p = 0.01), phenanthrene (p = 0.03), 1-methylphenanthrene (p = 0.01) and 2-methylphenanthrene (p = 0.02) were significantly reduced compared to the PA pre-WFTS concentrations and did not differ from NY water concentrations post-WFTS.
Figure 5:
Heatmap of Log2 fold changes of concentrations of PAHs in well water in PA compared to NY (LOG2FC[PA/NY]) by sampling period (Jan 2015-May 2016). Values >0 (<0) are shown in red (blue) and indicate the concentration of the PAH was higher (lower) in PA compared to NY for the same sampling period. The black vertical line indicates when the WFTS was installed in PA.
The ΣPAH in the PA water post-filtration/treatment system (15.97 ng/L) was less (p = 0.03) than the ΣPAH of NY water (40.0 ng/L) for the same sampling period; it was also less than the PA ΣPAH pre-water filtration/treatment system (26.59 ng/L, p = 0.02). Gas production by sampling period was neither correlated with PA ΣPAH nor to specific PAHs by sampling period.
Two forensic sourcing ratios (Fluoranthene/Pyrene, Fluroanthene/(Fluoranthene + Pyrene)) were available for both farms and suggested a petrogenic source for well water PAHs (Table A.12). Prior to installation of the water filtration/treatment system, the two PA farm ratios were less than NY farm ratios (p = 0.08, p = 0.08, respectively); post-installation of the water filtration/treatment system, this difference became significant (p = 0.02, p = 0.02, respectively).
3.2.4. Air Polycyclic Aromatic Hydrocarbons
Stationary air monitoring at the PA (n = 13) and NY (n = 13) farms identified 40 PAHs above detection limits (Tables A.13 and A.14). Initial analysis identified a significant difference between farms for 2-methylanthracene (p = 0.03) however, after removing an outlier, the difference was no longer significant suggesting absence of a true difference. Reported ΣPAH for PA (789 ng/m3) and NY (631ng/m3) did not differ between locations (p = 0.66).
Ten individual ratios were used for forensic sourcing (Table A.15) with both locations indicating a predominant pyrogenic signature. Only two ratios, fluoranthene/pyrene (p = 0.01) and fluoranthene/(fluoranthene + pyrene) (p = 0.02) were significantly different between farms. There was no significant correlation between gas production by sampling period, PA ΣPAH and PA forensic ratios (p > 0.05).
4. Discussion
4.1. Association between environmental PAH exposure and neonatal dysphagia
We report a syndrome of dysphagia in neonatal foals born on a farm in PA that was associated with increased length of environmental exposure for late-pregnant mares. Colts were also more likely than fillies to be affected. The unique study design allowed us to document that dams of the dysphagic PA foals, when they foaled at the NY farm, never had an abnormal foal, even if they had resided in PA for the first half of gestation. Comprehensive analysis of the well water, air, soil and feedstuffs revealed significantly higher levels of several PAHs in the PA farm water. When a water treatment/filtration system was installed and mares experienced reduced environmental exposure to PAHs, neonatal dysphagia on the PA farm was eliminated: None of the 29 foals born on the PA farm premises from 2017–2019 were dysphagic (personal communication, PA Farm manager).
Equine neonatal dysphagia is an uncommon disorder. In a study of 1,065 neonatal foals admitted to an equine ICU, 5% had a primary diagnosis of dysphagia (Giguere et al. 2017). Our detailed physical examinations and laboratory investigation allowed us to eliminate known causes of foal dysphagia including oropharyngeal and esophageal malformations, prematurity, septicemia, selenium deficiency, lead toxicity and neonatal encephalopathy (Burrows and Borchard 1982; Holcombe et al. 2012; Pearson et al. 2005). Primary dysphagia may result from oropharyngeal or esophageal muscular dysfunction; central or peripheral, motor or sensory neurological dysfunction; or some combination thereof (Holcombe et al. 2012; Jadcherla 2016; Matsuda et al. 2010).
We identified higher concentrations of 3,6-dimethylphenanthrene, fluoranthene, pyrene and triphenylene in the PA water compared to the NY farm. With installation of the water filtration/treatment system—which has been shown to degrade or remove PAHs from water—the PAH concentrations were reduced to levels detected in NY well water (Beltran et al. 1995; Bertilsson and Widenfalk 2002; Kalmykova et al. 2014). Previous studies have identified phenanthrenes and pyrene in flowback waters or in ambient air near unconventional wells (Orem et al. 2014; Paulik et al. 2016). However these PAHs are also abundant environmental pollutants found in indoor air (Messier et al. 2019), coal tar, wood smoke, or diesel exhaust (Cereceda-Balic et al. 2012; Correa and Arbilla 2006; Taylor and Jones 2001; Williams et al. 1986). Nevertheless, with the exception of the May 2016 sample, the majority of the forensic ratios for the PA well water samples reflected a petrogenic signature supporting UNGD activities as a possible source.
The highly lipophilic PAHs can cross the placental barrier; they also can be isolated from human breast milk (Drwal et al. 2019; Pulkrabova et al. 2016). Epidemiological studies demonstrate in utero exposure to PAHs affects neurodevelopment (Perera et al. 2006, Perera et al. 2009, Perera et al. 2012; Tang et al. 2008, Tang et al. 2014). Proposed mechanisms for toxicity are numerous and include endocrine disruption: decreased oxygen and nutrient exchange (PAH binding to placental growth factors) and induction of cytochrome P450 enzymes via Ah receptor binding. Other potential mechanisms are DNA damage from activation of apoptotic pathways, epigenetic effects, or oxidative stress due to inhibition of brain antioxidant scavenging systems; and N-methyl-D-aspartate glutamate receptor-mediated loss of neuronal activity (Chepelev 2015; Perera et al. 2009; Saunders et al. 2006). In an in vitro model, some alkylated PAHs as well as dibenzothiophene, induced neuronal cell damage via oxidative stress at higher levels than parent compounds, suggesting that toxicity of less well characterized alkylated PAHs should be considered in environmental risk assessments (Sarma et al. 2017).
In experimental studies, rats receiving oral doses of fluoranthene had significant decreases in motor activity and in responses to sensorimotor stimuli. They exhibited weakness, loss of coordination and had altered autonomic functions (increased urination and defecation). As males were more severely affected than females, Saunders et al. (2003) proposed that the differential responses may reflect females having higher levels of phase I and phase II detoxification enzymes and/or dissimilarities in the regulation of antioxidant systems or metabolic enzymes (the latter by hormones such as estrogen). Additional experimental studies demonstrate embryonic, perinatal and adult exposures to pyrene, phenanthrene, and mixtures of PAHs adversely affected motor and behavioral functions including disruption of cranial innervation patterns, decreased muscle coordination, learning and responses to startle stimuli, and swimming velocities (Gauthier et al. 2016; Geier et al. 2018; He et al. 2012; Luis and Guilhermino 2012). In our study, we found that males were significantly more likely to be dysphagic than females. The reason for this difference is unknown but is consistent with the observations in the rodent studies of Saunders et al. (2003).
The subdued mentation present in the dysphagic foals in our study further suggests a neurological basis for this syndrome. Although no differences in plasma glutathione peroxidase activity were found, we cannot dismiss the possibility of altered brain antioxidant scavenging systems. While it is unknown if PAH exposure was responsible for the observed clinical syndrome in the foals, a possible source of PAHs was contamination of the well water on the PA farm. UNGD is a known source of well water contamination (DiGiulio and Jackson 2016; U.S. EPA 2016). Interestingly, dysphagia in this study was associated with duration of exposure to the PA farm and exposure during mid to late gestation, but not during the first trimester. This contrasts with other studies in which early prenatal exposures to environmental toxins are most critical (Drwal et al. 2019; Whitworth et al. 2018). However, an in vitro model of neurotoxicity of a PAH mixture suggests that the window of vulnerability likely extends from fetal brain development through early childhood (Slotkin et al. 2017).
PAHs are rapidly metabolized, and in experimental studies, neurobehavioral effects are short-lived with normal behavior returning 48–72 hours after a single oral exposure (Saunders et al. 2002, Saunders et al. 2003). Fourteen of the 17 dysphagic foals in this study recovered when moved off-site to a university veterinary ICU. However, the long recovery time (up to 37 days with one foal remaining unable to suckle effectively after 46 days) does call into question whether foals continued to be exposed via ingestion of the dam’s milk which was fed via pan or a nasogastric tube. Additionally, prolonged fetal exposure may require a more prolonged recovery period.
4.2. Study limitations
These include the small sample size and potential selection bias related to enrollment of only two farms. However, common ownership and the participant crossover on the two farms provided a unique strength in this study because comparable populations, evidenced by comparison of healthy foals born on the two farms, could be assessed. As there were no other commercial livestock farms in the immediate vicinity of the PA farm, we could not determine the extent of neonatal dysphagia in the area. After installation of the water filtration/treatment system, PAHs in PA water declined and became similar to levels found in NY farm water. This may have played a role in the reduced prevalence of dysphagia in foals born in the spring of 2016 and its elimination in subsequent years (2017–2019). Similarly, since 2016, mares that had been bred on the NY farm and moved to the PA farm for foaling have spent only their last 4–6 weeks pre-partum in PA, compared to previous years in which the time spent was 4–6 months. This reduced environmental exposure may also have played a role in the reduced prevalence of dysphagia observed in 2016. Natural gas production was also variable during the study period and could not be controlled. It is unknown if and how this affected the results. Information bias, if any, would have been non-systematic resulting in a bias towards the null, meaning that the identified risk factors would have had even stronger true associations with dysphagia (Dohoo et al., 2010). The possibility of a confounding bias was controlled through measurement and analysis of a large number of explanatory variables; however, a possibility of a confounding bias related to any unmeasured variable could not be eliminated.
5. Conclusions
This study is the first investigation of a multi-year outbreak of equine neonatal dysphagia significantly associated with duration of pregnancy spent on a particular farm; males were more affected than females. We systematically eliminated known causes of dysphagia and performed a comprehensive toxicological investigation of biological and environmental samples from both farms. A similar study of these environmental variables would be nearly impossible to undertake in humans. Extensive environmental analysis determined that well water contained elevated levels of several PAHs that were diminished with installation of a WFTS. Future studies are needed to quantify PAHs in biologic samples in animals that live in areas with active UNGD, to evaluate the toxicity of the mixture of PAHs at the concentrations identified here in relevant models, and to establish if there are long-term health effects (other than dysphagia) of fetal exposures for both humans and animals.
Supplementary Material
Highlights.
A high prevalence of dysphagia (milk aspiration) in neonatal foals born near UGND occurred.
Continuous passive sampling of well water demonstrated high concentrations of PAHs.
Installation of a water filtration/treatment system eliminated the neurodevelopmental defect.
Horses are sentinels of health risks in areas of natural resource mining.
Acknowledgements
The authors are grateful to the following individuals for their contributions to the study including Jeff Hall DVM PhD Dipl ABVT Toxicology Section, Utah State Veterinary Diagnostic Lab; Jimmy NSN Tran BVSc Dipl ACVP Anatomic Pathology Section, Cornell AHDC; Erica Behling-Kelly DVM PhD Dipl ACVP Clinical Pathology Section, Cornell AHDC; Ned Place PhD, MD Diagnostic Endocrinology Lab, Cornell ADHC; Barbara Schanbacher DVM Diagnostic Endocrinology Lab, Cornell AHDC; James Mort DVM and Megan Cox DVM (referring veterinarians for NY and PA farms, respectively); Christina Moore DVM; M.J. Sun DVM; Robert Oswald PhD (Dept Molecular Medicine, Cornell CVM); Steve Eicker DVM MSc Valley Ag Software; the farm managers and the owner of the participating farms.
Funding: This work was supported by the National Institute of Environmental Health Sciences/National Institutes of Health [grant numbers R21ES026398, P42 ES012016465, P30 ES0000210 and P30 ES006096]. B.N.R. was supported in part by a NIEHS Fellowship [T32 ES007060].
Footnotes
Declaration of competing interests
The following authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary material: Appendix A
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adgate JL, Goldstein BD, McKenzie LM. 2014. Potential public health hazards, exposures and health effects from unconventional natural gas development. Environ Sci Technol 48(15):8307–8320, 10.1021/es404621. [DOI] [PubMed] [Google Scholar]
- Anderson KA, Sethajintanin D, Sower G, Quarles L. 2008. Field trial and modeling of uptake rates of in situ lipid-free polyethylene membrane passive sampler. Environ Sci Technol 42(12):4486–4493, 10.1021/es702657n. [DOI] [PubMed] [Google Scholar]
- Andersson JT, Achten C. 2015. Time to say goodbye to the 16 EPA PAHs? Toward an up-to-date use of PACs for environmental purposes. Polycycl Aromat Compd 35(2–4):330–354. 10.1080/10406638.2014.991042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan SE, Smith BW, Anderson KA. 2012. Impact of the deepwater horizon oil spill on bioavailable polycyclic aromatic hydrocarbons in gulf of mexico coastal waters. Environ Sci Technol 46(4):2033–2039, 10.1021/es202942q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KA, Szelewski MJ, Wilson G, Quimby BD, Hoffman PD. 2015. Modified ion source triple quadrupole mass spectrometer gas chromatograph for polycyclic aromatic hydrocarbon analyses. J Chromatogr A 1419:89–98, 10.1016/j.chroma.2015.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger M, Oswald RE. 2012. Impacts of gas drilling on human and animal health. New Solutions 22(1):51–77, 10.2190/NS.22.1.e. [DOI] [PubMed] [Google Scholar]
- Beltran FJ, Ovejero G, Garcia-Araya JF, Rivas J. 1995. Oxidation of polynuclear aromatic hydrocarbons in water. 2. Uv radiation and ozonation in the presence of uv radiation. Ind Eng Chem Res 34:1607–1615. [Google Scholar]
- Bertilsson S, Widenfalk A. 2002. Photochemical degradation of PAHs in freshwaters and their impact on bacterial growth–influence of water chemistry. Hydrobiologia 469 (1–3):23–32, 10.1023/A:1015579628189. [DOI] [Google Scholar]
- Burrows GE, Borchard RE. 1982. Experimental lead toxicosis in ponies: comparison of the effects of smelter effluent-contaminated hay and lead acetate. Am J Vet Res 43(12):2129–33. [PubMed] [Google Scholar]
- Cereceda-Balic F, Fadic X, Llanos AL, Domínguez AM, Guevara JL, Vidal V, et al. 2012. Obtaining polycyclic aromatic hydrocarbon concentration ratios and molecular markers for residential wood combustion: Temuco, a case study. J Air Waste Manag Assoc 62(1):44–51. [DOI] [PubMed] [Google Scholar]
- Chepelev NL, Moffat ID, Bowers WJ, Yauk CL. 2015. Neurotoxicity may be an overlooked consequence of benzo[a]pyrene exposure that is relevant to human health risk assessment. Mutat Res Rev Mutat Res 764(Apr-Jun):64–89, 10.1016/j.mrrev.2015.03.001. [DOI] [PubMed] [Google Scholar]
- Choi H, Wang L, Lin X, Spengler JD, Perera FP. 2012. Fetal window of vulnerability to airborne polycyclic aromatic hydrocarbons on proportional intrauterine growth restriction. PLoS ONE 7(4): e35464, doi: 10.1371/journal.pone.035464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T, Kwiatkowski C, Schultz K, Bachran M. 2011. Natural gas operations from a public health perspective. Human Ecol Risk Assess 17(5):1039–1056, 10.1080/10807039.2011.605662. [DOI] [Google Scholar]
- Colborn T, Schultz K, Herrick L, Kwiatkowski C. 2014. An exploratory study of air quality near natural gas operations. Human Ecol Risk Assess 20(1):86–105, 10.1080/10807039.2012.749447. [DOI] [Google Scholar]
- Cornell Waste Management Institute 2015. Healthy Soils, Healthy Communities. Metals in urban garden soils. http://ecommons.cornell.edu [Accessed April 2019]. [Google Scholar]
- Corradini I, Armengou L, Viu J, Rodríguez-Pozo ML, Cesarini C, Jose-Cunilleras E. 2014. Parallel testing of plasma iron and fibrinogen concentrations to detect systemic inflammation in hospitalized horses. J Vet Emerg Crit Care (San Antonio) 24(4):414–20, 10.1111/vec.12189. [DOI] [PubMed] [Google Scholar]
- Correa SM, Arbilla G. 2006. Aromatic hydrocarbons emissions in diesel and biodiesel exhaust. Atmospheric Environ 40(35):6821–6826. 10.1016/j.atmosenv.2006.05.068. [DOI] [Google Scholar]
- Cozzarelli IM, Skalak KJ, Kent DB, Engle MA, Benthem A, Mumford AC, et al. 2017. Environmental signatures and effects of an oil and gas wastewater spill in the Williston Basin, North Dakota. Sci Total Environ 579:1781–1793, 10.1016/j.scitotenv.2016.11.157. [DOI] [PubMed] [Google Scholar]
- Currie J, Greenstone M, Meckel K. 2017. Hydraulic fracturing and infant health: New evidence from Pennsylvania. Sci Adv 3(12):e1603021, 10.1126/sciadv.1603021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deziel NC, Brokovich E, Grotto I, Clark CJ, Barnett-Itzhaki Z, Broday D, et al. 2020. Unconventional oil and gas development and health outcomes: A scoping review of the epidemiological research. Environ Res Mar;182:109124, 10.1016/j.envres.2020.109124. [DOI] [PubMed] [Google Scholar]
- DiGiulio DC, Jackson RB. 2016. Impact to underground sources of drinking water and domestic wells from production well stimulation and completion practices in the Pavillion, Wyoming, Field. Environ Sci Technol 50(8):4524–36, 10.1021/acs.est.5b04970. [DOI] [PubMed] [Google Scholar]
- Dohoo IR, Martin W, Stryhn H. 2010. Veterinary epidemiologic research. 2nd edition. Atlantic Veterinary College Inc., University of Prince Edward Island, Prince Edward Island, Canada, p. 257. [Google Scholar]
- Donald CE, Anderson KA. 2017. Assessing soil-air partitioning of PAHs and PCBs with a new fugacity passive sampler. Sci Total Environ 596–597:293–302, 10.1016/j.scitotenv.2017.03.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drwal E, Rak A, Gregoraszczuk EL. 2019. Review: Polycyclic aromatic hydrocarbons (PAHs)-Action on placental function and health risks in future life of newborns. Toxicology 411:133–142, 10.1016/j.tox.2018.10.003. [DOI] [PubMed] [Google Scholar]
- Dupuy AM, Cristol JP, Vincent B, Barbnoux AS, Mednes M, Philbert P, et al. 2018. Stability of routine biochemical analytes in whole blood and plasma/serum: Focus on potassium stability from lithium heparin. Clin Chem Lab Med 56(3): 413–421. 10.1515/cclm-2017-0292. [DOI] [PubMed] [Google Scholar]
- Gauthier PT, Norwood WP, Prepas EE, Pyle GG. 2016. Behavioural alterations from exposure to Cu, phenanthrene, and Cu-phenanthrene mixtures: linking behaviour to acute toxic mechanisms in the aquatic amphipod, Hyalella azteca. Aquat Toxicol 170:377–383, 10.1016/j.aquatox.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Geier MC, James Minick D, Truong L, Tilton S, Pande P, Anderson KA, et al. 2018. Systematic developmental neurotoxicity assessment of a representative PAH Superfund mixture using zebrafish. Toxicol Appl Pharmacol 354:115–125, 10.1016/j.taap.2018.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère S, Weber EJ, Sanchez LC. 2017. Factors associated with outcome and gradual improvement in survival over time in 1065 equine neonates admitted to an intensive care unit. Equine Vet J 49(1):45–50, 10.1111/evj.12536. [DOI] [PubMed] [Google Scholar]
- Gough LP, Shacklette HT, Case AA. 1979. Element concentrations toxic to plants, animals, and man. Geological Survey Bulletin 1466, USDA, US Government Printing Office, Washington DC. [Google Scholar]
- Harvey JW, Asquith RL, McNulty PK, Kivipelto J, Bauer JE. 1984. Haematology of foals up to one year old. Equine Vet J 16(4):347–53. [DOI] [PubMed] [Google Scholar]
- He C, Wang C, Li B, Wu M, Geng H, Chen Y, et al. 2012. Exposure of Sebastiscus marmoratus embryos to pyrene results in neurodevelopmental defects and disturbs related mechanisms. Aquat Toxicol 116–117:109–15, 10.1016/j.aquatox.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Hill EL. 2018. Shale gas development and infant health: Evidence from Pennsylvania. J Health Econ 61:134–150, 10.1016/j.jhealeco.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holcombe SJ, Hurcombe SD, Barr BS, Schott HC 2nd. 2012. Dysphagia associated with presumed pharyngeal dysfunction in 16 neonatal foals. Eq Vet J Suppl 41:105–8, 10.1111/j.2042-3306.2011.00451.x. [DOI] [PubMed] [Google Scholar]
- Huckins JN, Petty JD, Booij K. 2006. Monitors of organic chemicals in the environment: Semipermeable membrane devices:Springer Science & Business Media.
- Jadcherla S 2016. Dysphagia in the high-risk infant: Potential factors and mechanisms. Am J Clin Nutr 103(2):622S–8S, 10.3945/ajcn.115.110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski WA, Perera FP, Camann D, Spengler J, Butscher M et al. 2015. Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res 22: 3631–3639, https://doi10.1007/s11356-014-3627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmykova Y, Moona N, Strömvall A-M, Björklund K. 2014. Sorption and degradation of petroleum hydrocarbons, polycyclic aromatic hydrocarbons, alkylphenols, bisphenol A and phthalates in landfill leachate using sand, activated carbon and peat filters. Water Res 56:246–257, 10.106/j.watres.2012.11.054. [DOI] [PubMed] [Google Scholar]
- Kassotis CD, Bromfield JJ, Klemp KC, Meng CX, Wolfe A, Zoeller RT, et al. 2016a. Adverse reproductive and developmental health outcomes following prenatal exposure to a hydraulic fracturing chemical mixture in female C57Bl/6 mice. Endocrinology 157(9):3469–3481, 10.1210/en.2016-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassotis CD, Iwanowicz LR, Akob DM, Cozzarelli IM, Mumford AC, Orem WH, et al. 2016b. Endocrine disrupting activities of surface water associated with a West Virginia oil and gas industry wastewater disposal site. Sci Total Environ 557–558:901–910, 10.1016/j.scitotenv.2016.03.113. [DOI] [PubMed] [Google Scholar]
- Kassotis CD, Klemp KC, Vu DC, Lin CH, Meng CX, Besch-Williford CL, et al. 2015. Endocrine-disrupting activity of hydraulic fracturing chemicals and adverse health outcomes after prenatal exposure in male mice. Endocrinology 156(12): 4458–4473, 10.1210/en.2015-1375. [DOI] [PubMed] [Google Scholar]
- Kassotis CD, Tillitt DE, Davis JW, Hormann AM, Nagel SC. 2014. Estrogen and androgen receptor activities of hydraulic fracturing chemicals and surface and ground water in a drilling-dense region. Endocrinology 155(3):897–907, 10.1210/en.2013-1697. [DOI] [PubMed] [Google Scholar]
- Koterba AM, Drummond WH, Kosch PC, eds. 1990. Equine Clinical Neonatology. Philadelphia: Lea and Febiger. [Google Scholar]
- Lohmann R, Booij K, Smedes F, Vrana B. 2012. Use of passive sampling devices for monitoring and compliance checking of pop concentrations in water. Environ Sci Poll Res Int. 19:1885–1895. [DOI] [PubMed] [Google Scholar]
- Luís LG, Guilhermino L. 2012. Short-term toxic effects of naphthalene and pyrene on the common prawn (Palaemon serratus) assessed by a multi-parameter laboratorial approach: mechanisms of toxicity and impairment of individual fitness. Biomarkers 17(3):275–85, 10.3109/1354750X.2012.666765. [DOI] [PubMed] [Google Scholar]
- Lyle-Dugas J, Giguère S, Mallicote MF, Mackay RJ, Sanchez LC. 2017. Factors associated with outcome in 94 hospitalised foals diagnosed with neonatal encephalopathy. Equine Vet J 49(2):207–210, 10.1111/evj.12553. [DOI] [PubMed] [Google Scholar]
- Martinez E, Gros M, Lacorte S, Barceló D. 2004. Simplified procedures for the analysis of polycyclic aromatic hydrocarbons in water, sediments and mussels. J Chromatogr A 1047:181–188. 10.1016/j.chroma.2004.07.003 [DOI] [PubMed] [Google Scholar]
- Matsuda K, Qiu Y, Furuse T, Kawamura Y, Yokoyama D, Kato A, Taniyama H. 2010. Bronchogenic and esophageal cyst with laryngeal malformations in a Thoroughbred foal. Vet Pathol 47(2):351–3, 10.1177/0300985809359319. [DOI] [PubMed] [Google Scholar]
- McKenzie LM, Guo R, Witter RZ, Savitz DA, Newman LS, Adgate JL. 2014. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environ Health Perspect 122:412–417, 10.1289/ehp.1306722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita SR, van Drooge BL, Reche C, Guimarães L, Grimalt JO, Barata C, et al. 2014. Toxic assessment of urban atmospheric particle-bound PAHs: Relevance of composition and particle size in Barcelona (Spain). Environ Pollut 184:555–562, 10.1016/j.envpol.2013.09.034. [DOI] [PubMed] [Google Scholar]
- Messier KP, Tidwell LG, Ghetu CG, Rohlman D, Scott RP, Bramer LM et al. 2019. Indoor versus outdoor air quality during wildfires. Environ Sci Tech Letters 6:696–701. 10.1021/acs.estlett.9b00599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel SC, Kassotis CD, Vandenberg LN, Lawrence BP, Robert J, Balise VD. 2020. Developmental exposure to a mixture of unconventional oil and gas chemicals: A review of effects on adult health, behavior, and disease. Mol Cell Endocrinol 2:110722, 10.1016/j.mce.2020.110722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NRC (National Research Council) Nutrient Requirement of Horses Sixth Revised Edition 2007. https://nrc88.nas.edu/nrh/ [Accessed 11 August 2019].
- Orem W, Tatu C, Varonka M, Lerch H, Bates A, Engle M, et al. 2014. Organic substances in produced and formation water from unconventional natural gas extraction in coal and shale. Int J Coal Geology 126:20–31. 10.1016/j.coal.2014.01.003. [DOI] [Google Scholar]
- Padula AM, Noth EM, Hammond SK, Lurmann FW, Yang W, Tager IB et al. 2014. Exposure to airborne polycyclic aromatic hydrocarbons during pregnancy and risk of preterm birth. Environ Res 135: 221–226. doi: 10.1016/j.envres.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulik LB, Donald CE, Smith BW, Tidwell LG, Hobbie KA, Kincl L, et al. 2016. Emissions of polycyclic aromatic hydrocarbons from natural gas extraction into air. Environ Sci Technol 50(14):7921–9, 10.1021/acs.est.6b02762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulik LB, Hobbie KA, Rohlman D, Smith BW, Scott RP, Kincl L, et al. 2018. Environmental and individual PAH exposures near rural natural gas extraction. Environ Pollut 241:397–405, 10.1016/j.envpol.2018.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PA DEP (Pennsylvania Department of Environmental Production) Oil and Gas Reporting Website 2018. https://www.paoilandgasreporting.state.pa.us/publicreports/Modules/Welcome/Welcome.aspx [Accessed 5 November 2018].
- Pearson EG, Snyder SP, Saulez MN. 2005. Masseter myodegeneration as a cause of trismus or dysphagia in adult horses. Vet Rec 156(20):642–646. [DOI] [PubMed] [Google Scholar]
- Perera F, Herbstman J. 2011. Prenatal environmental exposures, epigenetics, and disease. Reprod Toxicol 31(3):363–73, 10.1016/j.reprotox.2010.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Li Z, Whyatt R, Hoepner L, Wang S, Camann D, et al. 2009. Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics 124(2):e195–202, 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang D, Diaz D, et al. 2006. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect 114(8):1287–92, 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, et al. 2012. Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect 120(6):921–6, 10.1289/ehp.1104315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puls R 1994. Mineral Levels in Animal Health: Diagnostic Data. 2nd ed. Aldergrove, BC, Canada: Sherpa International. [Google Scholar]
- Pulkrabova J, Stupak M, Svarcova A, Rossner P, Rossnerova A, Ambroz A, et al. 2016. Relationship between atmospheric pollution in the residential area and concentrations of polycyclic aromatic hydrocarbons (PAHs) in human breast milk. Sci Total Environ 562: 640–647, doi: 10.1016/j.scitotenv.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Rendle DI, Heller J, Hughes KJ, Innocent GT, Durham AE. 2010. Stability of common biochemistry analytes in equine blood stored at room temperature. Eq Vet J 41: 428–432, 10.2746/042516409X370928. [DOI] [PubMed] [Google Scholar]
- Sapouckey SA, Kassotis CD, Nagel SC, Vandenberg LN. 2018. Prenatal exposure to unconventional oil and gas operation chemical mixtures altered mammary gland development in adult female mice. Endocrinology 1;159(3):1277–1289, 10.1210/en.2017-00866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma SN, Blais JM, Chan HM. 2017. Neurotoxicity of alkylated polycyclic aromatic compounds in human neuroblastoma cells. J Toxicol Environ Health A 80(5):285–300, 10.1080/15287394.2017.1314840. [DOI] [PubMed] [Google Scholar]
- Saunders CR, Das SK, Ramesh A, Shockley DC, Mukherjee S. 2006. Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol 26(5):427–38, 10.1002/jat.1157. [DOI] [PubMed] [Google Scholar]
- Saunders CR, Ramesh A, Shockley DC. 2002. Modulation of neurotoxic behavior in F-344 rats by temporal disposition of benzo(a)pyrene. Toxicol Lett 129(1–2):33–45, 10.1016/S0378-4274(01)00467-2. [DOI] [PubMed] [Google Scholar]
- Saunders CR, Shockley DC, Knuckles ME. 2003. Fluoranthene-induced neurobehavioral toxicity in F-344 rats. Int J Toxicol 22(4):263–76, 10.1080/10915810305114. [DOI] [PubMed] [Google Scholar]
- Scott JA, Incardona JP, Pelkki K, Shepardson S, Hodson PV. 2011. Ahr2-mediated, CYP1A-independent cardiovascular toxicity in zebrafish (Danio rerio) embryos exposed to retene. Aquat Toxicol 101(1):165–174. 10.1016/j.aquatox.2010.09.016 [DOI] [PubMed] [Google Scholar]
- Shankar P, Geier MC, Truong L, McClure RS, Pande P, Waters KM, et al. 2019. Coupling genome-wide transcriptomics and developmental toxicity profiles in zebrafish to characterize polycyclic aromatic hydrocarbon (PAH) hazard. Int J Mol Sci 20(10):2570 10.3390/ijms20102570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonkoff SB, Hays J, Finkel ML. 2014. Environmental public health dimensions of shale and tight gas development. Environ Health Perspect 122:787–795, 10.1289/ehp.1307866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Skavicus S, Card J, Giulio RT, Seidler FJ. 2017. In vitro models reveal differences in the developmental neurotoxicity of an environmental polycyclic aromatic hydrocarbon mixture compared to benzo[a]pyrene: Neuronotypic PC12 Cells and embryonic neural stem cells. Toxicology 377:49–56, 10.1016/j.tox.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BP, ed. 2015. Large Animal Internal Medicine, 5th ed. St. Louis, MO: Elsevier. [Google Scholar]
- Sommariva R, Blake RS, Cuss RJ, Cordell RL, Harrington JF, White IR, et al. 2014. Observations of the release of non-methane hydrocarbons from fractured shale. Environ Sci Technol 48(15):8891–8896, 10.1021/es502508w. [DOI] [PubMed] [Google Scholar]
- Steinzor N, Subra W, Sumi L. 2013. Investigating links between shale gas development and health impacts through a community survey project in Pennsylvania. New Solut 23(1):55–83, 10.2190/NS.23.1.e. [DOI] [PubMed] [Google Scholar]
- Stogiannidis E, Laane R. 2015. Source characterization of polycyclic aromatic hydrocarbons by using their molecular indices: An overview of possibilities. In: Reviews of environmental contamination and toxicology:Springer, 49–133. [DOI] [PubMed] [Google Scholar]
- Tang D, Lee J, Muirhead L, Li TY, Qu L, Yu J, 1 et al. 2014. Molecular and neurodevelopmental benefits to children of closure of a coal burning power plant in China. PLoS One 9(3):e91966, 10.1371/journal.pone.0091966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Li TY, Liu JJ, Zhou ZJ, Yuan T, Chen YH, et al. 2008. Effects of prenatal exposure to coal burning pollutants on children’s development in China. Environ Health Perspect 116(5):674–679, 10.1289/ehp.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor L, Jones D. 2001. Bioremediation of coal tar PAH in soils using biodiesel. Chemosphere 44:1131–1136, 10.1016/S0045-6535(00)00344-1. [DOI] [PubMed] [Google Scholar]
- Tidwell LG, Allan SE, O’Connell SG, Hobbie KA, Smith BW, Anderson KA. 2016. PAH and OPAH flux during the deepwater horizon incident. Environ Sci Technol 50:7489–7497, 10.1021/acs.est.6b02784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidwell LG, Paulik LB, Anderson KA. 2017. Air-water exchange of PAHs and OPAHs at a superfund mega-site. Sci Total Environ 603:676–686. 10.1016/j.scitotenv.2017.01.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiszewski M, Namieśnik J. 2012. PAH diagnostic ratios for the identification of pollution emission sources. Environ Pollut 162:110–119, 10.1016/j.envpol.2011.10.025. [DOI] [PubMed] [Google Scholar]
- U.S. EPA (U.S. Environmental Protection Agency). 2016. Assessment of potential impacts of hydraulic fracturing for oil and gas on drinking water resources in the United States: Final report. EPA/600/R-16/236F. Washington, DC: U.S. EPA; https://cfpub.epa.gov/ncea/hfstudy/recordisplay.cfm?deid=332990. [accessed 5 January 2019]. [Google Scholar]
- U.S. EPA. National Primary Drinking Water Regulations, https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations#Inorganic [accessed 5 January 2019].
- Werner AK, Vink S, Watt K, Jagals P. 2015. Environmental health impacts of unconventional natural gas development: A review of the current strength of evidence, doi: 10.1016/j.scitotenv.2014.10.084. [DOI] [PubMed]
- Whitworth KW, Marshall AK, Symanski E. 2017. Maternal residential proximity to unconventional gas development and perinatal outcomes among a diverse urban population in Texas. PLoS ONE 12(7): e0180966, 10.1371/journal.pone.0180966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KW, Marshall AK, Symanski E. 2018. Drilling and production activity related to unconventional gas development and severity of preterm birth. Environ Health Perspect 126(3):037006, 10.1289/EHP2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PT, Bartle KD, Andrews GE. 1986. The relation between polycyclic aromatic compounds in diesel fuels and exhaust particulates. Fuel 65:1150–1158. 10.1016/0016-2361(86)90184-5. [DOI] [Google Scholar]
- Wise SA, Sander LC, Schantz MM. 2015. Analytical methods for determination of polycyclic aromatic hydrocarbons (PAHs) — a historical perspective on the 16 U.S. EPA priority pollutant PAHs. Polycycl Aromat Compds 35:187–247. 10.1080/10406638.2014.970291. [DOI] [Google Scholar]
- Yunker MB, Macdonald RW, Vingarzan R, Mitchell RH, Goyette D, Sylvestre S. 2002. PAHs in the Fraser River basin: A critical appraisal of PAH ratios as indicators of PAH source and composition. Org Geochem 33:489–515. 10.1016/S0146-6380(02)00002-5. [DOI] [Google Scholar]
- Zakaria MP, Takada H, Tsutsumi S, Ohno K, Yamada J, Kouno E, et al. 2002. Distribution of polycyclic aromatic hydrocarbons (PAHs) in rivers and estuaries in Malaysia: A widespread input of petrogenic PAHs. Environ Sci & Technol 36:1907–1918. 10.1021/es011278. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.