Abstract
The focus of this paper is to briefly discuss the major advances in scientific thinking regarding: a) processes governing the fate and transport of mercury in the environment; b) advances in measurement methods; and c) how these advances in knowledge fit in within the context of the Minamata Convention on Mercury. Details regarding the information summarized here can be found in the papers associated with this Virtual Special Issue of STOTEN.
Keywords: Minamata Convention, mercury isotopes, methylmercury, fluxes, archives, wildlife
1. Introduction
Mercury (Hg) is a unique element, being one of two that exist as a liquid at ambient conditions, the other being bromine. Because of the presence of Hg as a liquid, it is volatilized to the atmosphere from terrestrial and aquatic surfaces. Elemental Hg is generally considered to be somewhat inert and is globally transported via the atmosphere. However, elemental Hg can be deposited to the surfaces relatively quickly once emitted (Miller and Gustin, 2013; Gustin et al., 2013). Elemental Hg is oxidized by a variety of compounds and Hg (II) compounds have a higher deposition velocity than elemental Hg (Ariya et al., 2015). Mercury is ubiquitous in the atmosphere and atmospheric concentrations have increased substantially since the onset of civilization (Amos et al., 2013).
Given the volatility of elemental Hg and the lack of long-term sinks, once an atom of Hg is released to the atmosphere it can remain in circulation for > 1000 years (Figure 1). The only true sink is burial in ocean sediment and soil (Amos et al., 2013). Prior to development of human populations, major sources of Hg to the air included volcanic eruptions, emissions from rocks and soil enriched in Hg, and re-emission of that deposited to surfaces, coal burning events (Burger et al., 2019), and forest fires (Webster et al., 2016). Prior to humans, the major sink would have been uptake by plants. With the appearance of humans, Hg mining activities began, creating a novel and important source of Hg to the biogeochemical cycle.
Figure 1.
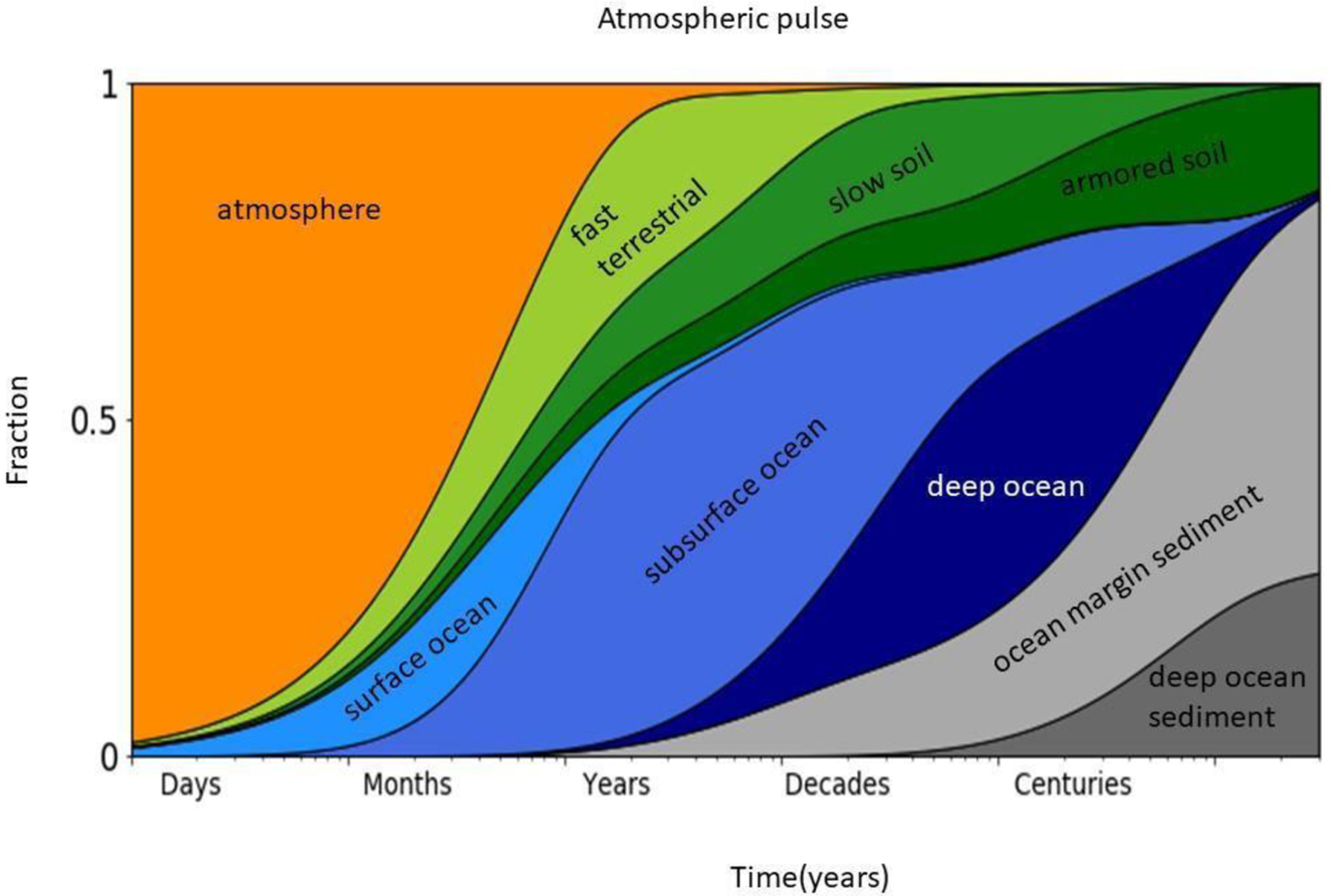
Temporal movement dynamics of Hg through different environmental reservoirs. The y-axis represents a unit pulse of Hg released to the atmosphere and the x-axis depicts how it partitions over time. This conceptual model represents a unit pulse of Hg as the perturbation, thus is independent of historical emission estimates and is meant to illustrate the relevant temporal scales involved in Hg movement through different environmental reservoirs. Re-drawn and modified from Amos et al. (2013).
Values in archives before 2000 BC, as proposed by Amos et al. (2013), are considered to reflect pre-anthropogenic emissions. After this time, civilizations utilizing Hg and biomass burning, potentially caused by humans, would have released Hg to the air. Hg has been used by societies for ≥ 2,500 years and is found in Egyptian tombs & amalgams dating back to ca. 500 BC. Greeks & Romans used it for medicine and cosmetics, and China’s first emperor (Qin Shi Huang 221–210 BCE) was buried within a moat of Hg. Prior to the Industrial Revolution major sources were silver, gold, and cinnabar mining (Outridge et al. 2018). Primary anthropogenic emissions of Hg to the atmosphere are currently considered to be artisanal gold mining>fossil fuel combustion> nonferrous metal and cement production (UNEP Global Mercury Assessment, 2013).
The biogeochemical cycle of an atom of Hg starts with the emission from natural and anthropogenic sources. Once emitted an atom enters the global atmospheric pool. This atom may be deposited to surfaces and then re-emitted. It also may be actively taken up by plants that sequester an estimated 1/3rd of the Hg emitted each year (Arnold et al., 2018). Hg also may be oxidized in the air promoting deposition. This Hg may be sequestered, converted to methyl- or di-methylmercury, or reduced and re-emitted back to the atmosphere.
Mercury pollution is an important environmental and public health issue. In August 2017, the United Nation’s Minamata Convention on Mercury entered into force. The Minamata Convention on Mercury is legally binding and is the main international policy instrument for managing anthropogenic Hg emissions (air) and releases (water) to the environment, and to also protect human health (Minamataconvention.org). The foundation for successful implementation of the Minamata Convention on Mercury is the use of recent policy relevant scientific advances that have furthered our understanding of Hg biogeochemical cycling and its impacts on humans and terrestrial and marine ecosystems (Bank, 2020). Here we synthesize the recent scientific advances (discussed in detail in this issue) in understanding Hg biogeochemical cycling and outline critical topics and research avenues to guide future investigations and efforts.
2.0. Results and Discussion
2.1. Utility of stable mercury isotopes for understanding sources and processes
Due to the pioneering work of Professor Joel Blum, and others in the early 2000s, Hg stable isotopes have become an important tool used to understand source apportionment dynamics of Hg contaminating any component of an ecosystem and processes affecting the fate and transport of Hg in the environment. Several review papers have recently been published on this topic (e.g., Bergquist and Blum, 2009; Yin et al., 2010; Blum et al., 2011; Kritee et al., 2013; Blum et al., 2014; Yin et al., 2014; Sun et al., 2016; Blum and Johnson, 2017; Buchachenko, 2018). See Tsui et al. (2020) for basic systematics for Hg isotopes and discussion of fractionation processes as well as advancements in measurements.
The utility of stable Hg isotopes is greater than many other common isotope tracers such as stable isotopes of carbon and nitrogen, primarily because mass-dependent fractionation (MDF) and mass independent fractionation (MIF) can change the natural abundance of isotope ratios that can be used to understand complex biogeochemical processes affecting Hg cycling. MDF occurs during many biogeochemical processes such as abiotic and biotic redox reactions, changes in Hg chemistry, and phase changes (e.g., volatilization). MIF mainly occurs during photochemical transformations and Hg(II) thiol complexation. Large-magnitude odd mass MIF (199Hg, 201Hg) occurs due to photochemical reactions (e.g., photoreduction and photodemethylation) mediated by the magnetic isotope effect while small-magnitude odd mass MIF may occur in the dark associated with the nuclear volume effect; even mass MIF (200Hg, 204Hg) originates from photochemical reactions potentially occurring in the upper atmosphere. The latter is not well understood; however, it is thought to be due to gas phase photochemical oxidation of elemental Hg (Berquist 2018). With an understanding of processes causing fractionation and the signature of specific sources, Hg isotope mixing models can be used to determine the contribution of Hg from different sources occurring in soils, sediments, and surface waters, etc. Since Hg isotopes often undergo both MDF and MIF in the environment, this provides a unique opportunity to use dual (i.e., MDF and odd-MIF) or even triple (i.e., MDF, odd-MIF, and even-MIF) isotope signatures that can be used as tracers of Hg in the environment. A current limitation is that the concentrations needed to do isotope analyses are in the 5 to 10 ng range. Lowering this concentration through further development of analytical methods should be a research emphasis.
More recently, an increasing number of studies have measured Hg isotope ratios in aquatic and terrestrial consumers in food webs and inferred the isotope ratios to represent those of MeHg. Besides answering questions for the sources and transformation of MeHg in the specific ecosystems, these studies showed that stable Hg isotopes can have the potential, yet largely unexplored, for addressing fundamental ecological questions such as how energy flows across ecosystem interfaces, and as a means of tracking animal migrations and movements (Tsui et al., 2020). Tsui et al. (2020) reviews the use of Hg isotopes in ecology and biogeochemistry. This paper provides important background information and provides suggestions for future work to use Hg isotopes to tackle research questions unrelated to Hg.
As described throughout this special issue, Hg isotopes are useful in understanding many biogeochemical processes and have significantly expanded our understanding of Hg cycling in the environment, which provides novel information to supplement the concentration and speciation analyses of Hg currently utilized in the majority of Hg research studies.
2.2. Advances in understanding mercury fluxes
Measurement of concentrations are useful; however, concentrations alone do not allow one to understand processes. For this flux needs to be measured. Flux is the rate of movement of a material to or from a surface or through a specific area. Measurements of fluxes from surfaces to the atmosphere and vice versa are done using flux chambers and micrometeorological methods. This has been done primarily for gaseous elemental Hg. For details see Sommar et al. (2020).
Novel dynamic flux chamber (DFCs) designs (Lin et al., 2012) producing a uniform surface friction velocity that enables rescaling of the measured gaseous elemental Hg flux taking the effect of atmospheric turbulence into account. Results from novel DFC measurement campaigns were comparable with independent non-intrusive micro-meteorological (MM) flux methods (Zhu et al., 2015; Osterwalder et al., 2018).
A single-detector relaxed eddy accumulation system for synchronous measurement of gaseous elemental flux at one height has been constructed to mitigate for potentially large uncertainties deriving from intermittent sampling or sequential concentration measurements at different heights (Osterwalder et al., 2017). Recent work using this method has yielded unexpected results, such as emission as being the current dominant flux from bogs (Osterwalder et al., 2017). Hg0 dry deposition is now recognized as the major component of the total Hg deposition in remote areas. Dry deposition of Hg0 via plant uptake displays profound dominance in terrestrial vegetated ecosystems with divalent Hg in gaseous or particulate phase making a minor contribution (Jiskra et al., 2018; Obrist et al., 2017; Wang et al., 2016).
There are developmental advances in implementing eddy covariance, the preferred and only direct MM method, to determine terrestrial Hg0 fluxes over background (Osterwalder et al., 2019) and contaminated sites (Pierce et al., 2015). Relatively low-cost Hg passive air samplers (MerPAS) have been deployed to capture vertical Hg0 concentration gradients in various landscapes to qualitatively address their role as Hg0 sources or sinks (Jeon and Cizdziel, 2019).
A new method has been developed for measuring gaseous oxidized Hg fluxes using field chambers (Miller et al., 2018). In addition, measurement of dry deposition of oxidized Hg can be done using passive samplers providing information on deposition velocities that provides a pathway for better understanding chemistry of GOM compounds (Huang et al., 2015b, 2017). Recent improvement in quantification and compound identification of reactive mercury (RM) in the atmosphere using the University of Nevada, Reno-Reactive Mercury Active System (UNR-RMAS 2.0) will help to better constrain RM deposition rates (Gustin et al., 2019; Luippold et al., 2020).
Improved process-based understanding of the gaseous elemental Hg flux has been achieved by using stable Hg isotope fingerprinting (Enrico et al., 2016; Jiskra et al., 2019b; Jiskra et al., 2015; Obrist et al., 2017; Yuan et al., 2019). Day-resolved sampling of gaseous elemental Hg isotopic ratio in ambient air is now feasible (Fu et al., 2015; Jiskra et al., 2019a). Hg stable isotope ratios of samples involved in atmosphere-terrestrial interaction provide, in combination with concentration and/or flux measurements provide novel constraints to quantitatively and qualitatively assess bi-directional gaseous elemental fluxes (Obrist et al., 2017; Yuan et al., 2019).
Top-down approaches using inverse modeling have more widely been implemented to estimate fluxes (emissions) involving the balancing of sour ces and sinks while reproducing long-term atmospheric concentration records (Denzler et al., 2017; Denzler et al., 2019; Song et al., 2015). Initial steps were taken to validate gaseous elemental flux parameterizations commonly used in major chemical transport models (CTM) with long-term ecosystem level micrometeorological gaseous elemental Hg flux measurements (Khan et al., 2019).
2.3. Advances in understanding mercury methylation
Microbial mercury methylation is particularly susceptible to the changing environment, as the two key factors controlling methylmercury (MeHg) production from this natural process (i.e. microbial activity and Hg bioavailability) and both are strongly affected by environmental parameters (Hsu-Kim et al., 2018). As such, previously unexpected ‘hotspots’ of MeHg accumulation may emerge when the aquatic environments are subject to climate changes and anthropogenic alterations. In recent years, research progress has been made in experimental and analytical techniques to accommodate the needs of identifying and quantifying the potential occurrence of microbial MeHg production in aquatic settings. These collective research efforts have greatly advanced our mechanistic understanding in the heterogeneous distribution of MeHg as well as the disproportion between MeHg and total Hg under diverse and dynamic environmental conditions (Tang et al., 2020).
The discovery of the hgcAB genes provides the genetic basis for assessing Hg methylation from molecular biological perspectives (Parks et al., 2013). With the applications of clone libraries, Illumina sequencing of 16S rRNA amplicon and shotgun metagenomic analysis, the hgcAB gene clusters have thus far been proven effective biomarkers in examining complex matrices for the abundance of a diverse variety of Hg methylating communities (Bae et al., 2014; Bae et al., 2019; Bouchet et al., 2018; Gilmour et al., 2013; Johss et al., 2019; Podar et al., 2015). The microbial groups that possess the hgcAB genes are regarded as potential Hg methylators, predominantly sulfate reducing bacteria, iron reducing bacteria, methanogens and syntrophs, which are widespread in both natural and engineered aquatic systems (Gilmour et al., 2013; Liu et al., 2018; Parks et al., 2013; Podar et al., 2015). Despite the substantially different physical and chemical conditions among these microbial habitats, including extreme environments, none of the methylating taxa appear to be uniquely tied to any specific ecological niche. Given the prevalence and co-occurrence of these microbial taxa in aquatic environments, it is important to understand how the interplay among these Hg methylating microorganisms influence MeHg production, and this knowledge should be widely applicable.
Methods for quantitatively assessing the bioavailability of inorganic Hg for microbial methylation have recently evolved from chemical equilibrium modeling (Benoit et al., 1999) and selective extractions (Ticknor et al., 2015) to approaches using enriched stable isotope tracers (Jonsson et al., 2014), whole-cell biosensors (Chiasson-Gould et al., 2014) and diffusive gradient thin films (DGT) (Ndu et al., 2018). The simultaneous application of multiple stable Hg isotopes allows proper simulation of environmental matrices containing an array of geochemically relevant inorganic Hg species, such as dissolved complexes, nanoparticles and bulk minerals (Jiskra et al., 2014; Jonsson et al., 2014; Jonsson et al., 2012; Liem-Nguyen et al., 2016). These isotopically labeled Hg species have been utilized in experimental studies, ranging from bench-scale incubations to mesocosm and ecosystem investigations (Bridou et al., 2011; Hintelmann et al., 2002; Jonsson et al., 2014; Rodríguez Martín-Doimeadios et al., 2004). DGT can also be utilized in such matrices to evaluate the relative chemical lability of different Hg species (Merritt and Amirbahman, 2007; Ndu et al., 2018), particularly after the designs of the diffusion phase and binding phase of DGT are optimized to enhance Hg binding capacity and selectivity. Enriched Hg isotopes may be coupled with whole-cell biosensors to assess the cellular uptake and subcellular distribution of various Hg species during methylation (Butler et al., 2017). The application of whole-cell biosensors in Hg methylation research is currently limited by the lack of mechanistic information regarding the specific pathways of Hg uptake prior to methylation. Yet, recent findings that divalent metal transporters of methylating bacteria may be involved in Hg uptake (Lu et al., 2018; Schaefer et al., 2014) have identified the need for better construction of biosensors that well mimic microbial Hg methylation processes. Overall, these new techniques along with others, have assisted and developed ongoing research on mercury bioavailability, which has begun to recognize the essential roles of kinetically labile Hg species and interfacial reactions in dictating the methylation potential of ubiquitous inorganic Hg (Jiskra et al., 2014; Ndu et al., 2018; Stenzler et al., 2017; Zhang et al, 2012).
2.4. Advances in understanding atmosphere chemistry and inputs to ecosystems
Preconcentration on gold traps, followed by desorption into an atomic fluorescence detector, remains by far the most common method to measure elemental Hg, though other methods are sometimes used (Albuquerque et al., 2017; El-Feky et al., 2018; Hynes et al., 2017; Kalinchuk et al., 2018; Lian et al., 2018). As the bias in KCl denuder-based measurements of oxidized Hg has become well-known (Bu et al., 2018; Cheng and Zhang, 2017; Gustin et al., 2015; Lyman et al., 2016), several alternative methods have emerged (Bu et al., 2018; Gratz et al., 2015; Huang and Gustin, 2015a; Miller et al., 2019; Slemr et al., 2018; Urba et al., 2017). Of these, cation-exchange membrane methods are the most widely used. Cation-exchange membrane methods include (1) direct laboratory analysis of the amount of oxidized Hg collected on membranes (Huang and Gustin, 2015a; Huang et al., 2013; Miller et al., 2019) and (2) dual-channel systems that determine the oxidized Hg concentration as the difference between an air stream that passed elemental Hg through membrane and one passed air through a pyrolyzer to determine total Hg (Ambrose et al., 2013; Gratz et al., 2015; Gustin et al., 2019; Lyman and Jaffe, 2012). Methods to identify oxidized Hg species are under development. An indirect method that determines speciation based on thermal desorption profiles from nylon membranes has yielded interesting and defensible results (Huang and Gustin, 2015a; Luippold et al., 2020), and mass spectrometry-based methods are being explored (Jones et al., 2016; Khalizov et al., 2018).
Our understanding of the behavior of Hg in the atmosphere has advanced in several ways over the past few years. A growing body of computational research has determined likely mechanisms by which elemental Hg is oxidized and oxidized Hg is reduced (Dibble et al., 2012; Dibble and Schwid, 2016; Jiao and Dibble, 2017a, 2017b; Lam et al., 2019; Saiz-Lopez et al., 2019; Saiz-Lopez et al., 2018; Sitkiewicz et al., 2016; Sitkiewicz et al., 2019), and this research is narrowing down the field of probable Hg compounds in the atmosphere. Current computational work predicts that atmospheric oxidized Hg will be dominated by HgBrOH and HgBr2 (Saiz-Lopez et al., 2019). Indirect thermal desorption methods point to a more diverse array of atmospheric Hg compounds. Work using nylon membranes has demonstrated that there are multiple compounds in the atmosphere and these vary across space and time, and are dependent upon the oxidants present in the atmosphere (Luippold et al. submitted, Huang and Gustin, 2015b; Huang et al., 2017). This is important information, because each compound has unique chemistry that will influence, how it can be measured, and the deposition velocity and availability to ecosystems, as well as potential for methylation.
Recent experimental evidence showed that MeHg forms from inorganic Hg via abiotic processes within cloud and fog droplets (Li et al., 2018). Another area with recent advances, especially in China, is understanding of the properties of Hg bound to particulate matter. During extreme particle pollution events in Chinese cities, the ratio of Hg mass to total particle mass appears to increase (Chen et al., 2016), compounding health risks. Also, studies around the world have shown a bimodal distribution of particulate mercury, with a significant fraction occurring in the coarse mode (Chen et al., 2016; Fang et al., 2010; Feddersen et al., 2012; Wang et al., 2019).
Many studies show that ambient atmospheric Hg concentrations are declining (Martin et al., 2017; Marumoto et al., 2019; Navrátil et al., 2018; Zhang et al., 2016), though this appears not to be the case universally (Fu et al., 2015; Martin et al., 2017). Declining Hg will, in general, mean declining ecosystem impacts, but the timing and spatial aspects of those changes are uncertain because atmospheric impacts to the surface are complex. Several recent whole-ecosystem studies have investigated Hg concentrations in various environmental compartments and transfer rates of Hg between compartments (Du et al., 2018; Zhou et al., 2018), including stable isotope studies (Enrico et al., 2016; Zhu et al., 2016) that have elucidated the ultimate atmospheric sources of Hg in those compartments. These kinds of studies are improving understanding of the mechanisms by which Hg in the atmosphere ultimately leads to health effects for humans and other organisms.
2.5. Advances in understanding the marine mercury biogeochemical cycle
In this special issue, Bowman et al. (2020) highlight insights on Hg cycling gained from analysis of seawater sampled during cruises over the last three decades, especially those that cut large swaths across major ocean basins and biogeochemical features. These efforts have greatly increased the number of measurements of Hg speciation in the ocean, and therefore, the information available to explore and understand marine Hg biogeochemistry. The programs supporting some of these cruises, such as CLIVAR and GEOTRACES, also hosted scientists measuring many fundamental (temperature, salinity, oxygen, nutrients, pH, dissolved CO2) and more specialized (trace elements and isotopes) parameters, providing a rich and unprecedented palette within which Hg results can be more deeply interpreted than 20 to 30 years ago. The synthesis- and interpretation-phases of these recent interdisciplinary projects continue, and a few additional cruises are being proposed for coming years, but it is clear that some of the research community assumptions about how Hg is transformed in the ocean, and possibly other environs, may need to be re-examined. Our new understanding of Hg in the ocean required, and has resulted from, many analytical innovations to enable accurate and high-resolution sampling across all depths of the ocean. This included taking analytical equipment to sea and integrating continuous analyzers with underway water samplers and equilibrators (e.g., Andersson et al., 2008a; Andersson et al., 2008b; Andersson et al., 2011; Kuss et al., 2011; Mason et al., 2017; Soerensen et al., 2014). The analysis of methylated Hg species in seawater also was developed and streamlined for shipboard and high-throughput analysis (e.g., Baya et al., 2013; Bowman and Hammerschmidt, 2011; Cossa et al., 2011; Hammerschmidt and Bowman, 2012; Munson et al., 2014; Stoichev et al., 2002; Živković et al., 2017).
2.5.1. Mercury species and ocean nutrients
Previous vertical profile measurements of Hg species hinted at nutrient-like distributions (low at the surface, increasing with depth; e.g., Fitzgerald et al., 2007), and most recent cruises have confirmed this vertical segregation in detail (e.g., Bowman et al., 2015; Bowman et al., 2016; Coale et al., 2018; Cossa et al., 2018a; Munson et al., 2015; Sunderland et al., 2009). Indeed, total, elemental and dimethyl Hg concentrations are often distributed like nutrients. In the case of total Hg, this appears to be due to biological uptake and removal from surface water by sinking particles that contribute Hg to deeper waters upon remineralization. For elemental and dimethyl Hg, however, low concentrations in surface water are more likely due to in-situ loss processes, either evasion or destruction, and higher concentrations at depth imply that they are actively created under those conditions. This last point is an area of active research.
2.5.2. Mercury distribution in the Arctic Ocean
Mercury cycling in the Arctic is of concern and scrutiny, because Arctic marine organisms often contain anomalously high Hg concentrations compared to analogous animal species at temperate latitudes (e.g., AMAP, 2011). In recent years, the US, Canada, and Germany have sponsored GEOTRACES cruises that occupied oceanographic stations in different areas of the Arctic Ocean (Agather et al., 2019; Heimbürger et al., 2015; Petrova et al., 2015 in press; Tesán Onrubia et al., 2020; Wang et al., 2018), and Hg measurements from each cruise showed that Hg concentrations are higher in surface relative to deep water in the Arctic Ocean, in contrast to other ocean basins. Furthermore, similar surface-enriched profiles have been observed in the Southern Ocean (Cossa et al., 2011), and methylated Hg species were observed to have shallow maxima as well. These findings implicate an important role for sea ice and watershed runoff in Hg cycling, including ice preventing evasion and therefore retaining Hg in seawater (Fisher et al., 2012; Sonke et al., 2018) and bring elevated concentrations of the highly bioaccumulative methylated forms closer to or into the photic zone, promoting uptake by plankton. The sea-ice-land cycling of Hg in the Arctic is complex and almost certainly affected by climate change, and these dynamics will need further modeling to better understand now, and importantly test empirically, future implications for Hg cycling in the Arctic Ocean. This work is in the process of being assimilated into models, but highlights the difficulty of studying an ocean basin that is undergoing a high degree of temporal change and is therefore a moving target for modelers.
2.5.3. Mercury methylation and oxygen minimum zones
Much of the early Hg biogeochemical research was performed in freshwater and sediment, and observations there suggested that low- to no-oxygen conditions were required to make methylated Hg species, namely mono- and di-MeHg. While relatively high concentrations of MeHg (picomolar) are found in the mid-water oxygen minimum zones that are common in the ocean, recent cruises have documented relatively high concentrations of MeHg in shallower waters too (e.g., in surface waters and at the depth of the subsurface chlorophyll maximum), particularly when viewed as a percentage of total Hg (e.g., Hammerschmidt and Bowman, 2012). This observation challenges the view that MeHg can only be formed through the activity of obligate anaerobic microbes like sulfate- and iron-reducers. This also supports and extends the observation by Sunderland et al. (2009) that microbial activity, rather than low-oxygen, is a key variable predicting steady-state MeHg concentrations in the ocean. However, interiors of some marine particulates may have low-oxygen microzones within otherwise oxic water where anerobic processes can operate (Capo et al., 2020; Ortiz et al., 2015). Thus, the controlling variables for Hg methylation in much of the oceanic water column still need to be resolved. It is especially interesting to note that these shallow maxima are often associated with water columns that possess significant oxygen minima in deeper waters and could imply coupling of MeHg dynamics between surface and mid-waters in some way that we do not yet understand.
2.5.4. Marine mercury and genomics
Since the recent discovery of a pair of genes (hgcAB) required for Hg methylation in anaerobic prokaryotes (Parks et al., 2013), the hunt has been on for these genes in seawater (e.g., Bowman et al., 2020; Gionfriddo et al., 2016; Lin et al., in review; Podar et al., 2015; Villar et al., 2020). The data thus far indicate that the hgcAB gene sequences originally identified from cultured, obligate anaerobes are not found in the oceanic water column. However, there are some genes in the ocean that are similar (homologous) in their amino acid sequences in ways that are thought to be related to the Hg methylating ability coded by hgcAB. In some cases, the homologous sequence segments found in marine metagenomes can be tied back to specific taxa and implicate microbes that do not fit the previous view of the ecology of Hg methylators (Gionfriddo et al., 2016; Lin et al., in review; Villar et al., 2020). These efforts are just beginning and are not trivial (Christensen et al., 2019) and should be coupled to methylation assays (Munson et al., 2018; Wang et al., 2020) and done in collaboration with biogeochemical modeling (Archer and Blum, 2018; Semeniuk and Dastoor, 2017; Zhang et al., 2020). The synergistic combination of these efforts offers great potential for insight and an aid to experimental design and empirical evaluation.
2.6. Advances in understanding terrestrial Hg cycling
The review of advances in the understanding of terrestrial Hg cycling (Bishop et al., 2020) identified methodological developments as key factors in much of the recent progress. The advent of natural abundance isotope measurements has enabled the identification of Hg sources, how the inputs from different sources are transformed in the terrestrial environment (Yuan et al, 2019), and then how Hg is exported to food webs in surface waters as well as on land (Demers et al., 2018; Du et al., 2018). Microbial techniques have further contributed to the ability to resolve the biological dimension of the biogeochemical interactions that affect Hg cycling (Strickman et al., 2016, Liu et al., 2018). The other area of major advance is in micrometeorological techniques that document how changes in the terrestrial environment influence the bidirectional exchange of Hg between the atmosphere and terrestrial surfaces (Osterwalder et al., 2017, Sommar et al., 2020).
The most striking advance in the understanding of terrestrial Hg cycling is documentation of how climate warming is mobilizing the stores of Hg in Arctic and boreal soils due to both the thawing of permafrost (Schuster et al., 2018) and the increasing occurrence of wildfire (Kumar et al., 2018). This has raised the question as to whether these changes in the terrestrial environment might reverse the overall direction of global transport that previously moved Hg from warmer mid-latitudes to colder high-latitudes (Jiskra et al., 2018). The role of vegetation changes is thus increasingly recognized as a key terrestrial factor in global Hg cycling. Those vegetation changes can be both intentional due to management choices such as forestry, or unintended due to climate influences (greening or browning). Two human land use choices also are now recognized as affecting the exposure of wildlife as well as humans. One is through the cultivation of rice which bioaccumulates MeHg more effectively than other crops in contaminated landscapes (Abeysinghe et al., 2017). The other land use factor is artisanal gold mining which releases much more Hg than previously estimated (Obrist et al., 2018). Land use and climate will thus have a bearing on how effective Minamata-related reductions in the outputs of Hg will be in changing the exposure of people and wildlife to Hg (Bishop et al., 2020).
As societies around the world move forward with implementing the Minamata convention, the effectiveness of the measures in promoting recovery will remain a major question. While it has generally been believed that it will take generations of reduced Hg in the atmosphere to translate into reduced Hg export from terrestrial to aquatic ecosystems, there have been some intriguing indications that this aspect of recovery may occur on decadal scales (Gerson and Driscoll, 2016). This points to the value of continued and intensified monitoring of the fluxes of Hg within and between ecosystems using the improved methodological capabilities at our disposal. These include new micrometeorological techniques to resolve land-atmosphere exchange, as well as continuation and expansion of time-series measurements in soils, waters, and biota.
2.7. Mercury cycling in Freshwater systems
In their review of Hg biogeochemical cycling in freshwater systems, Branfireun et al. (2020) frame their examination of our current state of knowledge about the cycling of Hg in lakes in the context of the widely accepted conceptual model of Hg cycling in freshwater lakes that is practically accepted as common knowledge. The model is that gaseous elemental mercury (GEM) is emitted to the atmosphere from both anthropogenic and natural sources, oxidized to an ionic form of Hg in the atmosphere which falls in wet and dry deposition, transported to lakes in runoff, delivered to bottom waters and sediments via particle settling, methylated by sulphate-reducing bacteria (SRB), and then bioaccumulated and biomagnified up the food web from primary producers to top predators. This depiction is effective in conveying the complexity of the Hg cycle, however there is mounting evidence that the dominant processes that regulate inputs, transformations, and bioavailability of Hg in many lakes may be missing from this picture. They also contend that, despite numerous advances in the discipline, the fixation on the temperate dimictic lake archetype is impeding our exploration of understudied, but potentially important sources of MeHg to freshwater lakes. One only needs to consider the countless relatively shallow, well-mixed monomictic lakes at lower or higher latitudes to recognize that this conceptual model cannot be applied universally, despite the presence of elevated MeHg in the aquatic food webs of these systems.
Genetic approaches have revealed that methylating bacteria come from a diverse group of organisms, not just the SRB (Gilmour et al., 2018), however the complexity of redox transformations of Hg within the lake system itself challenges the simple view of passive uptake of bioavailable HgII and subsequent MeHg formation (Grégoire and Poulain, 2018). Moreover, the complex assemblage of microbes found in biofilms and periphyton (two vastly understudied important sources of Hg in many freshwater ecosystems) is an excellent illustration that microbial communities, not single strains, create the conditions that support HgII methylation (Bravo and Cosio, 2019).
Finally, dissolved organic matter (DOM) is proving to be a critical mediator in the freshwater Hg cycle from the cellular to catchment scale, yet its role is paradoxical in many instances. DOM can both enhance and inhibit HgII uptake, increase and decrease net methylation rates (Graham et al., 2013, Zhao et al., 2017), and increase and decrease the net amount of MeHg in lakes and biota (Isidorova et al., 2016, French et al., 2014). Although the export of DOM-associated MeHg catchments may be more important than in situ methylation (in lake sediments) in controlling sediment and water MeHg concentrations the relative importance of allochthonous versus authochthonous MeHg in bioaccumulation and biomagnification in food webs is still not quantified, and requires more attention. Branfireun et al. (2020) conclude that it is the molecular characteristics of DOM that regulate its interactions with Hg, not just absolute concentrations, and they call on the Hg research community to dig deeper into DOM biogeochemistry in natural ecosystems using either direct or proxy measures of DOM quality.
Many governing processes in the catchment Hg cycle remain poorly described - an impediment to conceptual and process-based modelling efforts that are required for predicting Hg concentrations in aquatic food webs over gradients of space and time. Branfireun et al. (2020) revised conceptual model of Hg cycling highlights these observations, and draws attention to what they consider to be the important scientific frontiers in research on the freshwater Hg biogeochemistry in the coming years.
2.8. Advances in the use of archives to evaluate temporal aspects of mercury pollution
Mercury research using natural archives, lake sediments and peat in particular, has become a well-established field, with consistent methodological (sampling and analytical) approaches. This research has contributed to the knowledge we have today on Hg cycling. Recently, additional advances have opened new possibilities and perspectives on past Hg cycling, by incorporating both new methodological applications and new types of archives. The most remarkable recent advancement is the inclusion of Hg isotopes. This technique has been applied to lake sediments, peat, marine sediments, ice cores, and tree rings. Mercury isotopic fractionation has revealed that lake sediments contain a mix of precipitation-derived and vegetation-bound Hg exported from the lake catchment (Cooke et al., 2013; Kurz et al., 2019; Chen et al., 2016). They have also allowed for discerning the dominant atmospheric (wet or dry) deposition mechanism in peatlands (Enrico et al., 2016), and record global-scale changes in the chemistry of the atmosphere. A global-scale shift in the mass independent fractionation of odd Hg isotopes has been noted in some studies (odd-MIF) (Cooke et al., 2013; Yin et al., 2016) that has been interpreted as a change in the photochemistry of the atmosphere (Kurz et al., 2019).
As for the natural archives, the beginning of the 21st century has witnessed the initiation and surge of studies on Hg in tree rings (Cooke et al., 2020 and references therein). Tree rings offer a time resolution that is hardly found in other archives, except for varved lake sediments, offering a new opportunity to study the past response of atmospheric Hg deposition to short-term changes in emissions. These studies demonstrate that uptake from air by foliage and translocation within the tree is the main pathway for Hg incorporation and, thus, tree ring Hg contents record gaseous elemental Hg concentrations in air. A second new archive worthy of mention is human bones (Rasmussen et al., 2008, 2015; Emslie et al., 2019; Walser et al., 2019), because this archive relates specifically to human health. Recent research has shown that human bones from individuals living in rural areas, far from mining and metallurgy centers, recorded preindustrial changes in atmospheric mercury pollution synchronous with other markers of atmospheric pollution (i.e., lead and lead isotopes) and with the chronology established from natural archives in the region (López-Costas et al., 2020).
Decades of research have helped to establish temporal trends in atmospheric Hg deposition at various time scales, but they have equally shown that all archives are affected by (integrate) a range of processes and reflect different aspects of the Hg cycle. Lake sediment records of mercury accumulation are controlled by atmospheric deposition, sediment focusing, fluxes from the catchment (runoff of eroded soil and subsurface discharge) and catchment size, morphology and land use; peat records are affected by atmospheric dust deposition, catchment fluxes (in minerogenic mires), biotic uptake, and internal long-term and short-term processes (including peat decomposition); ice cores record mercury scrubbed during precipitation; and tree rings record atmospheric mercury concentrations. The realization of the complexity of the mercury cycle, that no archive represents an absolute record of past mercury deposition, and that several processes influence mercury cycling to a different extent in each archive, has prompted a need for integrated studies using various archives and multiproxy data, so to obtain information of the main drivers and processes. In a few cases, the application of statistical modelling (PCA and PLS) on multiproxy data, enabled to determine the weight of the drivers affecting mercury content in lake sediments (Rydberg et al., 2015) and peat (Pérez-Rodríguez et al., 2015).
Despite this apparent constraint, natural archives provide a consistent picture of the variations in atmospheric mercury through time. They show that, in most cases, preindustrial pollution was restricted to areas downwind or downstream of cinnabar or precious metal mining centers. The earliest evidence, Copper age (3250 BCE), of mercury pollution was recorded in river sediments from SW Spain, resulting from runoff from the Iberian Pyrite Belt 60 km upstream (Leblanc et al., 2000). But the earliest evidence of atmospheric mercury pollution was found in lake sediments from the Peruvian Andes in South America. Cooke et al. (2009) found elevated mercury concentrations in sediments dating to c. 1400 BCE. Despite the intense mining and metallurgy activity occurred during the Roman period, as attested by lead research in natural archives, there is little evidence of atmospheric mercury pollution. One such evidence was provided by the recent study developed on human bones (López-Costas et al., 2020), which showed higher Hg content in individuals from the Roman period compared to those from Antiquity, even in a rural area far away from urban and mining centers and upwind from emission sources. In any case, legacy Hg from historical mining seems to be the main Hg source to lakes and marine coastal areas prior to the industrial period (Elbaz-Polichet et al., 2011; Bindler et al., 2012; Corella et al, 2017).
For the industrial period, most records show a significant and steady increase in Hg since the early 1800’s to peak between the 1970’s–1980’s. Flux ratios increased on average 3- to 5-fold compared to preindustrial values. Although lower ratios are estimated from tree rings studies and, in areas impacted by point emissions sources, Hg loads remain elevated. Regional differences in Hg accumulation are also illustrated by research in Arctic lakes, frequently showing unexpectedly high Hg fluxes (Cooke et al., 2012; Drevnick et al., 2012; Muir et al., 2009).
Future research oriented to combine multiproxy data with systematic application of statistical modeling (generalized linear models, GLM, generalized additive models GAM, or structural equation models, SEM) may help to deal with the many factors and complex interactions between the drivers involved in regional Hg cycling. In the same way, extensive application of Hg isotopes to natural archives may also contribute to shed light into the nature of preindustrial and industrial era Hg emissions to the atmosphere.
2.9. Methylmercury exposure in wildlife
Methylmercury biomagnifies in food webs, and sublethal toxicological effects on wildlife from elevated MeHg exposure are well documented (Evers, 2018; Schuhammer et al., 2012). Wildlife are widely used as biosentinels of ecosystem exposure to MeHg; however, complex ecological and physiological drivers can have large and variable influences on wildlife MeHg concentrations. In this special issue, Chételat and co-authors (2020) present a synthesis of theory and applied information for measuring and interpreting wildlife exposure to MeHg. The review integrates advances in both ecological and physiological research and the implications for interpreting wildlife MeHg concentrations.
Methylmercury concentrations in wildlife are the net result of ecological processes influencing dietary exposure combined with physiological processes in the body that regulate MeHg assimilation, transformation, and elimination. Ecological tracers can reveal often complex pathways of Hg exposure for wildlife that result from animal movement and migration, ontogenetic diet shifts, and cross-ecosystem feeding. Recent advances in the application of ecological tracers, such as fatty acids and compound-specific stable isotope analysis, are presented in the context of characterizing dietary MeHg exposure. Physiological research shows vertebrate species and tissues can differ markedly in their capacity to eliminate MeHg. Biological factors such as age, sex, life history, maternal transfer, and changes in body mass are also highly relevant. Important distinctions for the selection of tissues for sampling are discussed including whether they are inert or physiologically active, act as sites of storage, transformation or excretion, as well as the period of MeHg exposure they represent. Non-lethal external tissues such hair, toe nails, scales, and egg shells show promise as indicators of internal MeHg concentrations, although caveats and recommendations for further validation are identified. Wildlife are useful indicators of biological exposure to MeHg, and the advances synthesized in this paper provide guidance for effectively assessing future efforts to reduce Hg releases and MeHg bioaccumulation in the environment.
2.10. Advances in the assessment and remediation of contaminated sites
Much of the research on Hg pollution has focused on its role as a global pollutant. Specifically, its wide distribution and deposition from the atmosphere, and subsequent landscape/ecosystem-scale processes impacting watershed mobilization, methylation, and bioaccumulation. Mercury can also be a local pollutant, where contemporary or historical industrial activities have resulted in directly released Hg to the land or water. These areas typically have Hg concentrations several orders of magnitude higher than background areas and the Hg speciation/forms present at these sites are often different from what occurs in areas primarily impacted by atmospheric deposition. Common examples of industrial-scale Hg contaminated sites include abandoned mines (mercury, gold, or silver) and chemical production facilities.
Recent advances in the assessment and remediation of industrially Hg contaminated sites provide opportunities to reduce the impacts of Hg pollution at a local scale (Eckley et al., 2020). For example, improvements in the detection of Hg using portable X-ray Fluorescence Spectrometers (XRF) allow for near real-time measurements of soil Hg concentration in this field. The use of XRFs can greatly increase the number of samples collected, which improves our understanding of the spatial extent and heterogeneity of contamination (McComb et al., 2014; Miller et al., 2013). Another recent advancement in contaminated site assessments is the use of Hg stable isotope fractionation to identify the contribution from specific industrial sources and help differentiate it from Hg deposited from the atmospheric pool (Donovan et al., 2013; Foucher et al., 2013; Yin et al., 2013). Source-attribution is possible because Hg can be imprinted with distinct isotopic signatures from different industrial processes and can be detected using a multi-collector ICP-MS.
In addition to identifying sources of Hg pollution, it is also important to understand the speciation of the Hg present. X-ray absorption fine structure (XAFS) spectroscopy (which has recently improved detection limits for Hg) has been used to understand how Hg forms/speciation at Hg contaminated sites can change in response to soil properties and redox conditions in ways that impacts its mobility and bioavailability for methylation (Manceau et al., 2015; Poulin et al., 2016). There have also been other recent methodological advances specifically aimed at understanding the sub-fraction of Hg that is more available for methylation. An example is the use of diffuse gradient in thin-film samples (DGT) that are designed to identify bioavailable fractions of Hg (Ndu et al., 2018). These methods are particularly important at Hg contaminated sites where the total Hg concentrations may be high, but the vast majority may be present in recalcitrant forms that have low bioavailability (Eckley et al., 2017; Kim et al., 2000).
In addition to methodological advances, there has also been an increased understanding of the important role that meteorological and hydrological conditions play in determining the mobilization of Hg from contaminated sites into the surrounding landscape via fluxes to the water and to the air. For water fluxes, it has become well established that episodic/seasonal periods of elevated discharge are the primary driver of Hg transport at many sites and is highly correlated with suspended sediment dynamics (Eckley et al., 2020). The importance of surface-air fluxes from contaminated sites has also become more apparent due to recent studies focused on developing models to scale fluxes spatially and temporally (Eckley et al., 2011; Kocman and Horvat, 2011; Miller and Gustin, 2013).
Traditional remediation approaches at Hg contaminated sites have focused on lowering total Hg concentrations through removal/dredging or capping with low Hg content materials. These efforts are most feasible for relatively small and highly contaminated areas. Alternative approaches have recently been developed that do not focus on decreasing total Hg concentrations, but instead focus on reducing MeHg production. These approaches can be more cost-effective than dredging/capping and are applicable over broader landscape areas. An important recent advancement has been the use of in situ amendments such as biochar, activated carbon, thiol/sulfur modified materials, and iron. These amendments have been shown to reduce inorganic Hg availability for methylation by increasing sorption to the solid phase of sediment and reducing levels in pore water by up to 95% (Gilmour et al., 2018; Schwartz et al., 2019; Ting et al., 2018). However, site-specific sediment characteristics, such as the amount and quality of competing sorbents like dissolved organic matter, can have a large impact of the amendment effectiveness (Johs et al., 2019).
In addition to soil amendments, there are other remediation options that are aimed at reducing MeHg production. These can include the addition of oxygen or nitrogen to lake water to poise the redox conditions at levels above where methylation occurs (Matthews et al., 2013; McCord et al., 2016). In other scenarios, decreases in sulfate loading, carbon, loading, or reservoir water-level management can be utilized to decrease MeHg production (Eckley et al., 2017; Hsu-Kim et al., 2018; Wasik et al., 2012). Overall, while contaminated site assessment and remediation remains complex, recent methodological advances and novel remediation strategies have used this complexity to identify opportunities to decrease the mobility and bioavailability of Hg (Eckley et al., 2020).
3.0. Conclusions: Linking scientific advances with the Minamata Convention on Mercury
Recent scientific advances in Hg stable isotope chemistry and applications (Tsui et al., 2020) have important implications for the Minamata Convention especially in the context of source apportionment modeling studies near Hg hotspots (Kwon et al., 2020) and to identify ecosystems sensitive to and atmospheric deposition, Hg methylation, and efficient trophic transfer. These high-resolution analyses will also be critical for identifying natural and anthropogenic sources and the importance of different geochemical pools of Hg and MeHg in water, sediment, fish and subsequent MeHg exposure to humans. Temporal analyses of Hg stable isotopes can also be achieved by using archived samples (Lepak et al., 2019) to identify changes in Hg sources across spatiotemporal gradients. Furthermore, these approaches can be used within a global monitoring framework in support of the Minamata Convention effectiveness evaluation (Bank, 2020: Kwon et al., 2020).
Ecosystem sensitivity, as it relates to atmospheric deposition and subsequent methylation-demethylation dynamics and food web complexity, is an important theme that has been identified by the Conference of Parties in the context of the Minamata Convention effectiveness evaluation. Evaluating post-depositional processes (Wang et al., 2010) and sources simultaneously, in a more integrated manner, will be required since atmospheric loadings of Hg do not always translate into higher MeHg concentrations in local, terrestrial biotic communities (Bank et al., 2005; Shanley et al., 2019). Evaluating ecosystem change, post-depositional processes and food web characteristics will be essential for tracking changes of MeHg in biota over time (Braune et al., 2014; Braune et al., 2016; Wang et al., 2010; Wang et al., 2019; Chételat et al., 2020) which is an important aspect of the Minamata Convention effectiveness evaluation. Therefore, valid temporal assessments of Hg in biota need to evaluate concomitant changes in food webs and anthropogenic emissions and releases of Hg (Bank, 2020).
Since Hg modeling in different ecosystem compartments has a significant degree of uncertainty (Selin, 2014; Gustin et al., 2016), and because forecasting is currently either extremely limited or not possible, moving forward researchers will need to increase transparency and inform policy makers that these models have important limitations, rely on hypotheses and, at times, have completely unrealistic assumptions. Furthermore, the Minamata Convention on Mercury will benefit from treating Hg pollution as a seafood safety and food security issue (Bank, 2020) as this will engage an entire new discipline of researchers and policymakers to address this important environmental and public health problem.
Highlights.
This paper provides a brief summary of recent advances in Hg science.
Details are presented in 10 papers in a Virtual Special Issue of STOTEN.
Presented are updates in scientific knowledge regarding the fate and transport of Hg.
Advances in measurement methods are synthesized.
Discussion is provided as to how these fit within the Minamata Convention
Acknowledgements
We thank all the co-authors that contributed to this Special Issue of STOTEN, and importantly, the many Hg scientists that agreed to review these papers. We are also grateful to Dr. Elsie M. Sunderland for providing Figure 1, which is adapted from and re-drawn from Amos et al (2013). This work was supported, in part, by financing provided by the Norwegian Ministry of Trade, Industry and Fisheries to M.S.B. Any opinions expressed in this paper are those of the author(s) and do not, necessarily, reflect the official positions and policies of the USEPA. Any use of trade, product, or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. Thanks very much to Samantha Leftwich, undergraduate at UNR, who helped with the references.
Footnotes
Publisher's Disclaimer: This is a PDF file of an article that has undergone enhancements after acceptance, such as the addition of a cover page and metadata, and formatting for readability, but it is not yet the definitive version of record. This version will undergo additional copyediting, typesetting and review before it is published in its final form, but we are providing this version to give early visibility of the article. Please note that, during the production process, errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interest
There are no conflicts of interest.
References
- Abeysinghe KS, Qiu G, Goodale E, Anderson CWN, Bishop K, Evers DC, et al. , 2017. Mercury flow through an Asian rice-based food web. Environ. Pollut 229, 219–228. 10.1016/j.envpol.2017.05.067 [DOI] [PubMed] [Google Scholar]
- Albuquerque M, Coutinho M, Rodrigues J, Ginja J, Borrego C, 2017. Long-term monitoring of trace metals in PM10 and total gaseous mercury in the atmosphere of Porto, Portugal: Atmospheric Pollution Research; 8, 535–544. 10.1016/j.apr.2016.12.001 [DOI] [Google Scholar]
- AMAP Arctic Monitoring and Assessment Programme, 2011. AMAP Assessment 2011: Mercury in the Arctic Arctic Monitoring and Assessment Programme, Oslo, Norway, 193. [Google Scholar]
- Amos HM, Jacob DJ, Streets DG, Sunderland EM, 2013. Legacy impacts of all-time anthropogenic emissions on the global mercury cycle: GLOBAL IMPACTS OF LEGACY MERCURY. Global Biogeochem. Cycles 27, 410–421. 10.1002/gbc.20040 [DOI] [Google Scholar]
- Andersson ME, Gårdfeldt K, Wängberg I, 2008a. A description of an automatic continuous equilibrium system for the measurement of dissolved gaseous mercury. Anal. Bioanal. Chem 391, 2277–2282. 10.1007/s00216-008-2127-4 [DOI] [PubMed] [Google Scholar]
- Andersson ME, Sommar J, Gårdfeldt K, Lindqvist O, 2008b. Enhanced concentrations of dissolved gaseous mercury in the surface waters of the Arctic Ocean. Marine Chemistry 110, 190–194. 10.1016/j.marchem.2008.04.002 [DOI] [Google Scholar]
- Andersson ME, Sommar J, Gårdfeldt K, Jutterström S, 2011. Air-sea exchange of volatile mercury in the North Atlantic Ocean. Marine Chemistry 125, 1–7. 10.1016/j.marchem.2011.01.005 [DOI] [Google Scholar]
- Archer DE, Blum JD, 2018. A model of mercury cycling and isotopic fractionation in the ocean. Biogeosciences 15, 6297–6313. 10.5194/bg-15-6297-2018 [DOI] [Google Scholar]
- Arnold J, Gustin MS, Weisberg PJ, 2018. Evidence for Nonstomatal Uptake of Hg by Aspen and Translocation of Hg from Foliage to Tree Rings in Austrian Pine. Environ. Sci. Technol 52, 1174–1182. 10.1021/acs.est.7b04468 [DOI] [PubMed] [Google Scholar]
- Ariya PA, Amyot M, Dastoor A, Deeds D, Feinberg A, Kos G, et al. , 2015. Mercury Physicochemical and Biogeochemical Transformation in the Atmosphere and at Atmospheric Interfaces: A Review and Future Directions. Chem. Rev 115, 3760–3802. 10.1021/cr500667e [DOI] [PubMed] [Google Scholar]
- Bank MS, 2020. The mercury science-policy interface: History, evolution and progress of the Minamata Convention. Sci. Tot. Env 722, 137832 10.1016/j.scitotenv.2020.137832 [DOI] [PubMed] [Google Scholar]
- Bank MS, Loftin CS, Jung RE, 2005. Mercury bioaccumulation in northern two-lined salamanders from streams in the Northeastern United States. Ecotoxicology 14, 181–191. 10.1007/s10646-004-6268-8 [DOI] [PubMed] [Google Scholar]
- Baya PA, Hollinsworth JL, Hintelmann H, 2013. Evaluation and optimization of solid adsorbents for the sampling of gaseous methylated mercury species. Analytica Chimica Acta 786, 61–69. 10.1016/j.aca.2013.05.019 [DOI] [PubMed] [Google Scholar]
- Berquist B, 2018. Mercury Isotopes in The Encyclopedia of Geochemistry: A Comprehensive Reference Source on the Chemistry of the Earth, White WM Editor 1st; ed Springer International Publishing; 1557, 900–906. [Google Scholar]
- Bergquist BA, Blum JD, 2009. The odds and evens of mercury isotopes: Applications of mass-dependent and mass-independent isotope fractionation. Elements 5, 353–357. 10.2113/gselements.5.6.353 [DOI] [Google Scholar]
- Bishop K, Shanley JB, Riscassi A, de Wit HA, Eklöf K, Meng B, Mitchell C, Osterwalder S, Schuster PF, Webster J, Zhu W, 2020. Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. Science of The Total Environment, 721, 137647 10.1016/j.scitotenv.2020.137647 [DOI] [PubMed] [Google Scholar]
- Blum JD, 2011. Applications of stable mercury isotopes to biogeochemistry, in: Baskaran N (Ed.), Handbook of Environmental Isotope Geochemistry. Springer, Heidelberg, Germany: 229–245. 10.1007/978-3-642-10637-8 [DOI] [Google Scholar]
- Blum JD, Johnson MW, 2017. Recent developments in mercury stable isotope analysis. Rev. Mineral. Geochem 82, 733–757. 10.2138/rmg.2017.82.17 [DOI] [Google Scholar]
- Blum JD, Sherman LS, Johnson MW, 2014. Mercury isotopes in earth and environmental sciences. Annu. Rev. Earth Planet. Sci 42, 249–269. 10.1146/annurev-earth-050212-124107 [DOI] [Google Scholar]
- Bindler R, Yu R, Hansson S, Claßen N, Karlsson J, 2012. Mining, metallurgy and the historical origin of mercury pollution in lakes and watercourses in central Sweden. Environ. Sci. Technol 46, 7984–7991. 10.1021/es300789q [DOI] [PubMed] [Google Scholar]
- Bishop K, Shanley JB, Riscassi A, de Wit HA, Eklof K, Meng B, et al. , 2020. Recent advances in understanding and measurement of mercury in the environment: Terrestrial Hg cycling. The Science of The Total Environment. 721, 137647–137647. 10.1016/j.scitotenv.2020.137647 [DOI] [PubMed] [Google Scholar]
- Bowman KL, Hammerschmidt CR, 2011. Extraction of monomethylmercury from seawater for low-femtomolar determination: Extraction of seawater monomethylmercury. Limnol. Oceanogr. Methods 9, 121–128. 10.4319/lom.2011.9.121 [DOI] [Google Scholar]
- Bowman KL, Hammerschmidt CR, Lamborg CH, Swarr G, 2015. Mercury in the North Atlantic Ocean: The U.S. GEOTRACES zonal and meridional sections. Deep Sea Research Part II: Topical Studies in Oceanography 116, 251–261. 10.1016/j.dsr2.2014.07.004 [DOI] [Google Scholar]
- Bowman KL, Hammerschmidt CR, Lamborg CH, Swarr GJ, Agather AM, 2016. Distribution of mercury species across a zonal section of the eastern tropical South Pacific Ocean (U.S. GEOTRACES GP16). Marine Chemistry 186, 156–166. 10.1016/j.marchem.2016.09.005 [DOI] [Google Scholar]
- Bowman KL, Collins RE, Agather AM, Lamborg CH, Hammerschmidt CR, Kaul D, Dupont CL, Christensen GA, Elias DA, 2020. Distribution of mercury- cycling genes in the Arctic and equatorial Pacific Oceans and their relationship to mercury speciation. Limnol. Oceanogr 65 10.1002/lno.11310 [DOI] [Google Scholar]
- Bowman KL, Lamborg CL, Agather AM, 2020. A global perspective on mercury cycling in the ocean. Science of The Total Environment 710, 136166 10.1016/j.scitotenv.2019.136166 [DOI] [PubMed] [Google Scholar]
- Branfireun BA, Cosio C, Poulain A, Riise G, Bravo AG, 2020. Mercury Cycling in Freshwater Systems - An Updated Conceptual Model. Sci. Tot. Environ (this issue). [DOI] [PubMed] [Google Scholar]
- Bravo AG, Cosio C, 2019. Biotic formation of methylmercury: A bio-physic-chemical conundrum. Limnol. Oceanogr 65, 1010–1027. 10.1002/lno.11366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu X, Zhang H, Lv G, Lin H, Chen L, Yin X, Shen G, Yuan W, Zhang W, Wang X, Tong Y, 2018. Comparison of Reactive Gaseous Mercury Collection by Different Sampling Methods in a Laboratory Test and Field Monitoring. Environ. Sci. Technol. Lett 5, 600–607. 10.1021/acs.estlett.8b00439 [DOI] [Google Scholar]
- Buchachenko AL, 2018. Mercury isotopes in earth and environmental chemistry. Russ. J. Phys. Chem. B 12, 635–644. 10.1134/S1990793118040048 [DOI] [Google Scholar]
- Burger BJ, Estrada MV, Gustin MS, 2019What caused Earth’s largest mass extinction event? New evidence from the Permian-Triassic boundary in northeastern Utah. Global and Planetary Change 177, 81–100. 10.1016/j.gloplacha.2019.03.013 [DOI] [Google Scholar]
- Capo E, Bravo AG, Soerensen AL, Bertilsson S, Pinhassi J, Feng C, Andersson AF, Buck M, Björn E, 2020. Marine snow as a habitat for microbial mercury methylators in the Baltic Sea. (preprint). Microbiology. 10.1101/2020.03.04.975987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Hintelmann H, Zheng W, Feng X, Cai H, Wang Z, Yuan S, Wang Z, 2016. Isotopic evidence for distinct sources of mercury in lake waters and sediments. Chem. Geol 426, 33–44. 10.1016/j.chemgeo.2016.01.030 [DOI] [Google Scholar]
- Chen X, Balasubramanian R, Zhu Q, Behera SN, Bo D, Huang X, Xie H, Cheng J, 2016. Characteristics of atmospheric particulate mercury in size-fractionated particles during haze days in Shanghai. Atmos. Environ 131, 400–408. 10.1016/j.atmosenv.2016.02.019 [DOI] [Google Scholar]
- Cheng I, Zhang L, 2017. Uncertainty assessment of gaseous oxidized mercury measurements collected by Atmospheric Mercury Network. Environ. Sci. Technol 51, 855–862. 10.1021/acs.est.6b04926 [DOI] [PubMed] [Google Scholar]
- Chételat J, Ackerman JT, Eagles-Smith CA, Hebert CE, 2020. Methylmercury exposure in wildlife: A review of the ecological and physiological processes affecting contaminant concentrations and their interpretation. Science of The Total Environment 711, 135117 10.1016/j.scitotenv.2019.135117 [DOI] [PubMed] [Google Scholar]
- Christensen GA, Gionfriddo CM, King AJ, Moberly JG, Miller CL, Somenahally AC, et al. , 2019. Determining the Reliability of Measuring Mercury Cycling Gene Abundance with Correlations with Mercury and Methylmercury Concentrations. Environ. Sci. Technol 53, 8649–8663. 10.1021/acs.est.8b06389 [DOI] [PubMed] [Google Scholar]
- Coale KH, Heim WA, Negrey J, Weiss-Penzias P, Fernandez D, Olson A, Chiswell H, Byington A, Bonnema A, Martenuk S, Newman A, Beebe C, Till C, 2018. The distribution and speciation of mercury in the California current: Implications for mercury transport via fog to land. Deep Sea Research Part II: Topical Studies in Oceanography 151, 77–88. 10.1016/j.dsr2.2018.05.012 [DOI] [Google Scholar]
- Cooke CA, Hintelmann H, Ague JJ, Burger R, Biester H, Sachs JP, Engstrom DR, 2013. Use and Legacy of Mercury in the Andes. Environ. Sci. Technol 47, 4181–4188. 10.1021/es3048027 [DOI] [PubMed] [Google Scholar]
- Cooke CA, Martínez-Cortizas A, Bindler R 2020. Archives of atmospheric Hg deposition -A review. Sci. Total Environ 709, 134800 10.1016/j.scitotenv.2019.134800 [DOI] [PubMed] [Google Scholar]
- Cooke CA, Wolfe AP, Hobbs WO, 2009. Lake-sediment geochemistry reveals 1400 years of evolving extractive metallurgy at Cerro de Pasco, Peruvian Andes. Geology 37, 1019–1022. 10.1130/G30276A.1 [DOI] [Google Scholar]
- Cooke CA, Wolfe AP, Michelutti N, Balcom PH, Briner JP, 2012. A Holocene perspective on algal mercury scavenging to sediments of an Arctic lake. Environ. Sci. Technol 46, 7135–7141. 10.1021/es3003124 [DOI] [PubMed] [Google Scholar]
- Corella JP, Valero-Garcés BL, Wang F, Martínez-Cortizas A, Cuevas CA, Saiz-Lopez A, 2017. 700 years reconstruction of mercury and lead atmospheric deposition in the Pyrenees (NE Spain). Atmos. Environ 155, 970–1107. 10.1016/j.atmosenv.2017.02.018 [DOI] [Google Scholar]
- Cossa D, Heimbürger L-E, Lannuzel D, Rintoul SR, Butler ECV, Bowie AR, Averty B, Watson RJ, Remenyi T, 2011. Mercury in the Southern Ocean. Geochimica et Cosmochimica Acta 75, 4037–4052. 10.1016/j.gca.2011.05.001 [DOI] [Google Scholar]
- Cossa D, Heimbürger LE, Sonke JE, Planquette H, Lherminier P, García-Ibáñez MI, Pérez FF, Sarthou G, 2018a. Sources, cycling and transfer of mercury in the Labrador Sea (Geotraces-Geovide cruise). Marine Chemistry 198, 64–69. 10.1016/j.marchem.2017.11.006 [DOI] [Google Scholar]
- Cossa et al. , 2018b.
- Demers JD, Blum JD, Brooks SC, Donovan PM, Riscassi AL, Miller CL, et al. , 2018. Hg isotopes reveal in-stream processing and legacy inputs in East Fork Poplar Creek, Oak Ridge, Tennessee, USA. Environ. Sci.: Processes Impacts 20, 686–707. 10.1039/C7EM00538E [DOI] [PubMed] [Google Scholar]
- Denzler B, Bogdal C, Henne S, Obrist D, Steinbacher M, Hungerbühler K, 2017. Inversion approach to validate mercury emissions based on background air monitoring at the high altitude research station Jungfraujoch (3580 m). Environmental Science & Technology 51, 2846–2853. [DOI] [PubMed] [Google Scholar]
- Denzler B, Bogdal C, Kern C, Tobler A, Huo J, Hungerbühler K, 2019. Urban source term estimation for mercury using a boundary-layer budget method. Atmos. Chem. Phys 19, 3821–3831. [Google Scholar]
- Dibble T, Zelie M, Mao H, 2012. Thermodynamics of reactions of ClHg and BrHg radicals with atmospherically abundant free radicals. Atmos. Chem. Phys 12, 10271–10279. 10.5194/acp-12-10271-2012 [DOI] [Google Scholar]
- Dibble TS, Schwid AC, 2016. Thermodynamics limits the reactivity of BrHg radical with volatile organic compounds. Chem. Phys. Lett 659, 289–294. 10.1016/j.cplett.2016.07.065 [DOI] [Google Scholar]
- Donovan PM, Blum JD, Yee D, Gehrke GE, Singer MB, 2013. An isotopic record of mercury in San Francisco Bay sediment. Chemical Geology 349–350, 87–98. 10.1016/j.chemgeo.2013.04.017 [DOI] [Google Scholar]
- Drevnick PE, Yang H, Lamborg CH, Rose NL, 2012. Net atmospheric mercury deposition to Svalbard: estimates from lacustrine sediments. Atmos. Environ 59, 509–513. 10.1016/j.atmosenv.2012.05.048 [DOI] [Google Scholar]
- Du B, Feng X, Li P, Yin R, Yu B, Sonke JE, et al. , 2018. Use of mercury isotopes to quantify mercury exposure sources in inland populations, China. Environ. Sci. Technol 52, 5407–5416. 10.1021/acs.est.7b05638 [DOI] [PubMed] [Google Scholar]
- Du H, Ma M, Sun T, An S, Igarashi Y, Wang D, 2018. Methyl and total mercury in different media and associated fluxes in a watershed forest, Southwest China. Int. J. Environ. Res. Public Health 15, 2618 10.3390/ijerph15122618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckley CS, Gilmour CC, Janssen S, Luxton TP, Randall PM, Whalin L, Austin C, 2020. The assessment and remediation of mercury contaminated sites: A review of current approaches. Science of the Total Environment 707, 136031 10.1016/j.scitotenv.2019.136031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckley CS, Gustin M, Miller MB, Marsik F, 2011. Scaling Non-Point-Source Mercury Emissions from Two Active Industrial Gold Mines: Influential Variables and Annual Emission Estimates. Environ. Sci. Technol 45, 392–399. 10.1021/es101820q [DOI] [PubMed] [Google Scholar]
- Eckley CS, Luxton TP, Goetz J, McKernan J, 2017. Water-level fluctuations influence sediment porewater chemistry and methylmercury production in a flood-control reservoir. Environmental Pollution 222, 32–41. 10.1016/j.envpol.2017.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz-Poulichet F, Dezileau L, Freydier R, Cossa D, Sabatier P, 2011. A 3500-Year Record of Hg and Pb Contamination in a Mediterranean Sedimentary Archive (The Pierre Blanche Lagoon, France). Environ. Sci. Technol 45, 8642–8647. https://doi.org./10.1021/es20045999 [DOI] [PubMed] [Google Scholar]
- El-Feky AA, El-Azab W, Ebiad MA, Masod MB, Faramawy S, 2018. Monitoring of elemental mercury in ambient air around an Egyptian natural gas processing plant. J. Nat. Gas Sci. Eng 54, 189–201. 10.1016/j.jngse.2018.01.019 [DOI] [Google Scholar]
- Enrico M, Roux GL, Marusczak N, Heimbürger LE, Claustres A, Fu X, Sun R, Sonke JE, 2016. Atmospheric Mercury Transfer to Peat Bogs Dominated by Gaseous Elemental Mercury Dry Deposition. Environ. Sci. Technol 50, 2405–2412. 10.1021/acs.est.5b06058 [DOI] [PubMed] [Google Scholar]
- Emslie SD, Alderman A, McKenzie A, Brasso R, Taylor AR, Molina Moreno M, Cambra-Moo O, González Martín A, Silva AM, Valera A, García Sanjuán L, Vijande Vila E, 2019. Mercury in archaeological human bone: biogenic or diagenetic? Journal of Archaeological Science 108, 104969 10.1016/j.jas.2019.05.005 [DOI] [Google Scholar]
- Fang G-C, Lo C-T, Liu C-K, 2010. Seasonal Variations (Autumn, Winter, and Spring) of Atmospheric Particulate and Particulate-Bound Mercury Hg(p) at a Suburban/Coastal Area. Environmental Forensics 11, 300–308. 10.1080/15275922.2010.523757 [DOI] [Google Scholar]
- Feddersen DM, Talbot R, Mao H, Sive BC, 2012. Size distribution of particulate mercury in marine and coastal atmospheres. Atmos. Chem. Phys 12, 10899–10909. 10.5194/acp-12-10899-2012 [DOI] [Google Scholar]
- Fisher JA, Jacob DJ, Soerensen AL, Amos HM, Steffen A, Sunderland EM, 2012. Riverine source of Arctic Ocean mercury inferred from atmospheric observations. Nat. Geosci 5, 499–504. 10.1038/ngeo1478 [DOI] [Google Scholar]
- Fitzgerald WF, Lamborg CH, Hammerschmidt CR, 2007. Marine Biogeochemical Cycling of Mercury. Chem. Rev 107, 641–662. 10.1021/cr050353m [DOI] [PubMed] [Google Scholar]
- Foucher D, Hintelmann H, Al TA, MacQuarrie KT, 2013. Mercury isotope fractionation in waters and sediments of the Murray Brook mine watershed (New Brunswick, Canada): Tracing mercury contamination and transformation. Chemical Geology 336, 87–95. 10.1016/j.chemgeo.2012.04.014 [DOI] [Google Scholar]
- French TD, Houben AJ, Desforges JPW, Kimpe LE, Kokelj SV, Poulain AJ, Smol JP, Wang X, Blais JM, 2014. Dissolved organic carbon thresholds affect mercury bioaccumulation in Arctic lakes. Environ. Sci. Technol 48, 3162–3168. 10.1021/es403849d [DOI] [PubMed] [Google Scholar]
- Fu XW, Zhang H, Yu B, Wang X, Lin C-J, Feng XB, 2015. Observations of atmospheric mercury in China: a critical review. Atmos. Chem. Phys 15, 9455–9476. 10.5194/acp-15-9455-2015 [DOI] [Google Scholar]
- Gilmour CC, Bullock AL, Mcburney A, Podar M, 2018. Robust Mercury Methylation across Diverse Methanogenic Archaea 9, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour C, Bell T, Soren A, Riedel Georgia, Riedel Gerhardt, Kopec D, Bodaly D, Ghosh U, 2018. Activated carbon thin-layer placement as an in situ mercury remediation tool in a Penobscot River salt marsh. Science of The Total Environment 621, 839–848. 10.1016/j.scitotenv.2017.11.050 [DOI] [PubMed] [Google Scholar]
- Gilmour C, Podar M, Bullock AL, Graham AM, Brown SD, Somenahally AC, Johs A, Hurt RA, Bailey KL, Elias DA, 2013. Mercury methylation by novel microorganisms from new environments. Environ. Sci. Technol 47, 11810–11820. 10.1021/es403075t [DOI] [PubMed] [Google Scholar]
- Gionfriddo CM, Tate MT, Wick RR, Schultz MB, Zemla A, Thelen MP, Schofield R, Krabbenhoft DP, Holt KE, Moreau JW, 2016. Microbial mercury methylation in Antarctic sea ice. Nat. Microbiol 1, 16127 10.1038/nmicrobiol.2016.127 [DOI] [PubMed] [Google Scholar]
- Graham AM, Aiken GR, Gilmour CC, 2013. Effect of dissolved organic matter source and character on microbial Hg methylation in Hg-S-DOM solutions. Environ. Sci. Technol 47, 5746–5754. 10.1021/es400414a [DOI] [PubMed] [Google Scholar]
- Gerson JR, Driscoll CT, 2016. Is Mercury in a Remote Forested Watershed of the Adirondack Mountains Responding to Recent Decreases in Emissions? Environ. Sci. Technol 50, 10943–10950. 10.1021/acs.est.6b02127 [DOI] [PubMed] [Google Scholar]
- Gratz LE, Ambrose JL, Jaffe DA, Shah V, Jaeglé L, Stutz J, Festa J, Spolaor M, Tsai C, Selin NE, Song S, Zhou X, Weinheimer AJ, Knapp DJ, Montzka DD, Flocke FM, Campos TL, Apel E, Hornbrook R, Blake NJ, Hall S, Tyndall GS, Reeves M, Stechman D, Stell M, 2015. Oxidation of mercury by bromine in the subtropical Pacific free troposphere: FREE TROPOSPHERIC HG OXIDATION BY BR. Geophys. Res. Lett 42 10.1002/2015GL066645 [DOI] [Google Scholar]
- Grégoire DS, Poulain AJ, 2018. Shining light on recent advances in microbial mercury cycling. Facets 3, 858–879. 10.1139/facets-2018-0015 [DOI] [Google Scholar]
- Gustin MS, Evers DC, Bank MS, Hammerschmidt CR, Pierce A, Basu N, Blum J, Bustamante P, Chen C, Driscoll CT, Horvat M, Jaffe D, Pacyna J, Pirrone N, Selin N, 2016. Importance of integration and implementation of emerging and future mercury research into the Minamata convention. Environ. Sci. Technol 50, 2767–2770. 10.1021/acs.est.6b00573 [DOI] [PubMed] [Google Scholar]
- Gustin MS, Dunham-Cheatham SM, Zhang L, 2019. Comparison of four methods for measurement of reactive, gaseous oxidized, and particulate bound mercury. Environ. Sci. Technol. submitted 10.1021/acs.est.9b04648 [DOI] [PubMed] [Google Scholar]
- Gustin MS, Amos HM, Huang J, Miller MB, Heidecorn K, 2015. Measuring and modeling mercury in the atmosphere: a critical review. Atmos. Chem. Phys 15, 5697–5713. 10.5194/acp-15-5697-2015 [DOI] [Google Scholar]
- Gustin MS, Huang JY, Miller MB, Peterson C, Jaffe DA, Ambrose J, Finley BD, Lyman SN, Call K, Talbot R, Feddersen D, Mao HT, Lindberg SE, 2013. Do We Understand What the Mercury Speciation Instruments Are Actually Measuring? Results of RAMIX. Environmental Science & Technology 47, 7295–7306. [DOI] [PubMed] [Google Scholar]
- Gustin MS, Jaffe DA, 2013. Fast time resolution oxidized mercury measurements during the Reno Atmospheric Mercury Intercomparison Experiment (RAMIX). Environ. Sci. Technol 47, 7285–7294. 10.1021/es303916v [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Bowman KL, 2012. Vertical methylmercury distribution in the subtropical North Pacific Ocean. Marine Chemistry 132–133, 77–82. 10.1016/j.marchem.2012.02.005 [DOI] [Google Scholar]
- Heimbürger LE, Sonke JE, Cossa D, Point D, Lagane C, Laffont L, Galfond BT, Nicolaus M, Rabe B, van der Loeff MR, 2015. Shallow methylmercury production in the marginal sea ice zone of the central Arctic Ocean. Sci. Rep 5, 10318 10.1038/srep10318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu-Kim H, Eckley CS, Achá D, Feng X, Gilmour CC, Jonsson S, Mitchell CPJ, 2018. Challenges and opportunities for managing aquatic mercury pollution in altered landscapes. Ambio 47, 141–169. 10.1007/s13280-017-1006-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Gustin MS, 2015a. Uncertainties of Gaseous Oxidized Mercury Measurements Using KCl-Coated Denuders, Cation-Exchange Membranes, and Nylon Membranes: Humidity Influences. Environ. Sci. Technol 49, 6102–6108. 10.1021/acs.est.5b00098 [DOI] [PubMed] [Google Scholar]
- Huang J, Gustin MS, 2015b. Use of Passive Sampling Methods and Models to Understand Sources of Mercury Deposition to High Elevation Sites in the Western United States. Environ. Sci. Technol 49, 432–441. 10.1021/es502836w [DOI] [PubMed] [Google Scholar]
- Huang J, Miller MB, Edgerton E, Gustin MS, 2017. Deciphering potential chemical compounds of gaseous oxidized mercury in Florida, USA. Atmos. Chem. Phys 17, 1689–1698. 10.5194/acp-17-1689-2017 [DOI] [Google Scholar]
- Huang J, Miller MB, Weiss-Penzias P, Gustin MS, 2013. Comparison of Gaseous Oxidized Hg Measured by KCl-Coated Denuders, and Nylon and Cation Exchange Membranes. Environ. Sci. Technol 47, 7307–7316. 10.1021/es4012349 [DOI] [PubMed] [Google Scholar]
- Hynes AJ, Everhart S, Bauer D, Remeika J, Tatum Ernest C, 2017. In situ and denuder-based measurements of elemental and reactive gaseous mercury with analysis by laser-induced fluorescence - results from the Reno Atmospheric Mercury Intercomparison Experiment. Atmos. Chem. Phys 17, 465–483. 10.5194/acp-17-465-2017 [DOI] [Google Scholar]
- Isidorova A, Bravo AG, Riise G, Bouchet S, Björn E, Sobek S, 2016. The effect of lake browning and respiration mode on the burial and fate of carbon and mercury in the sediment of two boreal lakes: CARBON AND MERCURY BURIAL. J. Geophys. Res. Biogeosci 121, 233–245. 10.1002/2015JG003086 [DOI] [Google Scholar]
- deposition and re-emission pathways in boreal Forest soils investigated with Hg isotope signatures. Environmental Science & Technology 49, 7188–7196. [DOI] [PubMed] [Google Scholar]
- Jiskra M, Sonke JE, Obrist D, Bieser J, Ebinghaus R, Myhre CL, et al. , 2018. A vegetation control on seasonal variations in global atmospheric mercury concentrations. Nat. Geosci 11 (4), 244–250. 10.1038/s41561-018-0078-8 [DOI] [Google Scholar]
- Jiskra M, Marusczak N, Leung KH, Hawkins L, Prestbo E, Sonke JE, 2019a. Automated Stable Isotope Sampling of Gaseous Elemental Mercury (ISO-GEM): insights into GEM emissions from building surfaces. Environmental Science & Technology 53, 4346–4354. [DOI] [PubMed] [Google Scholar]
- Jiskra M, Sonke JE, Agnan Y, Helmig D, Obrist D, 2019b. Insights from mercury stable isotopes on terrestrial-atmosphere exchange of Hg0 in the Arctic tundra. Biogeosciences 16, 4051–4064. [Google Scholar]
- Johs A, Eller VA, Mehlhorn TL, Brooks SC, Harper DP, Mayes MA, Pierce EM, Peterson MJ, 2019. Dissolved organic matter reduces the effectiveness of sorbents for mercury removal. Science of The Total Environment 690, 410–416. 10.1016/j.scitotenv.2019.07.001 [DOI] [PubMed] [Google Scholar]
- Jones CP, Lyman SN, Jaffe DA, Allen T, O’Neil TL, 2016. Detection and quantification of gas-phase oxidized mercury compounds by GC/MS. Atmos. Meas. Tech 9, 2195–2205. 10.5194/amt-9-2195-2016 [DOI] [Google Scholar]
- Kalinchuk VV, Mishukov VF, Astakhov AS, 2018. Arctic source for elevated atmospheric mercury (Hg0) in the western Bering Sea in the summer of 2013. Journal of Environmental Sciences 68, 114–121. 10.1016/j.jes.2016.12.022 [DOI] [PubMed] [Google Scholar]
- Khalizov AF, Guzman F, Cooper MB, Antley J, Bozzelli J, 2018. Towards Direct Molecular Speciation of Atmospheric Gaseous Oxidized Mercury. AGU Fall Meeting Abstracts 33. [Google Scholar]
- Kim CS, Brown GE, Rytuba JJ, 2000. Characterization and speciation of mercury-bearing mine wastes using X-ray absorption spectroscopy. Science of the Total Environment 261, 157–168. 10.1016/S0048-9697(00)00640-9 [DOI] [PubMed] [Google Scholar]
- Kocman D, Horvat M, 2011. Non-point source mercury emission from the Idrija Hg-mine region: GIS mercury emission model. Journal of Environmental Management 92, 2038–2046. 10.1016/j.jenvman.2011.03.034 [DOI] [PubMed] [Google Scholar]
- Kritee K, Blum JD, Reinfelder JR, Barkay T, 2013. Microbial stable isotope fractionation of mercury: A synthesis of present understanding and future directions. Chem. Geol 336, 13–25. 10.1016/j.chemgeo.2012.08.017 [DOI] [Google Scholar]
- Kumar A, Wu S, Huang Y, Liao H, Kaplan JO, 2018. Mercury from wildfires: Global emission inventories and sensitivity to 2000–2050 global change. Atmospheric Environment 173, 6–15. 10.1016/j.atmosenv.2017.10.061 [DOI] [Google Scholar]
- Kurz AY, Blum JD, Washburn SJ, Baskaran M, 2019. Changes in the mercury isotopic composition of sediments from a remote alpine lake in Wyoming, USA. Sci. Total Environ 669, 973–982. 10.1016/j.scitotenv.2019.03.165 [DOI] [PubMed] [Google Scholar]
- Kuss J, Zülicke C, Pohl C, Schneider B, 2011. Atlantic mercury emission determined from continuous analysis of the elemental mercury sea-air concentration difference within transects between 50°N and 50°S. Global Biogeochemical Cycles 25 10.1029/2010GB003998 [DOI] [Google Scholar]
- Kwon SY, Blum JD, Yin R, Tsui MTK, Yang Y-H, Choi JW, 2020. Mercury stable isotopes for monitoring the effectiveness of the Minamata Convention on Mercury. Earth Sci. Rev 203, 103111 10.1016/j.earscirev.2020.103111 [DOI] [Google Scholar]
- Lam KT, Wilhelmsen CJ, Schwid AC, Jiao Y, Dibble TS, 2019. Computational Study on the Photolysis of BrHgONO and the Reactions of BrHgO • with CH4 , C2H6, NO, and NO2: Implications for Formation of Hg(II) Compounds in the Atmosphere. J. Phys. Chem. A 123, 1637–1647. 10.1021/acs.jpca.8b11216 [DOI] [PubMed] [Google Scholar]
- Leblanc M, Morales JA, Borrego J, Elbaz-Poulichet F, 2000. 4,500-year-old mining pollution in southwestern Spain: long-term implications for modern mining pollution. Geol. Econ 10.2113/gsecongeo.95.3.655 [DOI] [Google Scholar]
- Lepak RF, Hoffman JC, Janssen SE, Krabbenhoft DP, Ogorek JM, DeWild JF, Tate MT, Babiarz CL, Yin R, Murphy EW, Engstrom DR, Hurley JP, 2019. Mercury source changes and food web shifts alter contamination signatures of predatory fish from Lake Michigan. Proc. Natl. Acad. Sci 116, 23600–23608. 10.1073/pnas.1907484116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Wang Y, Mao H, Wang S, Talbot RW, Zhou Y, Wang Z, Nie X, Qie G, 2018. Insights on Chemistry of Mercury Species in Clouds over Northern China: Complexation and Adsorption. Environ. Sci. Technol 52, 5125–5134. 10.1021/acs.est.7b06669 [DOI] [PubMed] [Google Scholar]
- Lin C-J, Zhu W, Li X, Feng X, Sommar J, Shang L, 2012. Novel Dynamic Flux Chamber for Measuring Air-Surface Exchange of Hg0 from Soils. Environ. Sci. Technol 46, 8910–8920. 10.1021/es3012386 [DOI] [PubMed] [Google Scholar]
- Lin H, Ascher D, Myung Y, Lamborg CH, Hallam SJ, Gionfriddo CM, et al. , A novel and environmentally widespread Marinimicrobia clades methylates mercury in low-oxygen marine environments. in review.
- Liu YR, Johs A, Bi L, Lu X, Hu HW, Sun D, et al. , 2018. Unraveling microbial communities associated with methylmercury production in paddy soils. Environ. Sci. Technol 52, 13110–13118. 10.1021/acs.est.8b03052 [DOI] [PubMed] [Google Scholar]
- Lian M, Shang L, Duan Z, Li Y, Zhao G, Zhu S, Qiu G, Meng B, Sommar J, Feng X, Svanberg S, 2018. Lidar mapping of atmospheric atomic mercury in the Wanshan area, China. Environ. Pollut 240, 353–358. 10.1016/j.envpol.2018.04.104 [DOI] [PubMed] [Google Scholar]
- López-Costas O, Kylander M, Mattielli N, Álvarez-Fernández N, Pérez-Rodríguez M, Mighall T, Bindler R, Martínez Cortizas A, 2020. Human bones tell the story of atmospheric mercury and lead exposure at the edge of Roman World. Sci. Total Environ 710, 136319 10.1016/j.scitotenv.2019.136319 [DOI] [PubMed] [Google Scholar]
- Luippold A, Gustin MS, Dunham-Cheatham SM, Zhang L, 2020. Improvement of quantification and identification of atmospheric reactive mercury. Atmos. Environ 224, 117307 10.1016/j.atmosenv.2020.117307 [DOI] [Google Scholar]
- Luippold A, Gustin MS, Dunham-Cheatham SM, Castro M, Luke W, Lyman S, Zhang L, 2020. Use of multiple lines of evidence to understand reactive mercury concentrations and chemistry in Hawaii, Nevada, Maryland, and Utah, USA. Environmental Science and Technology, submitted. [DOI] [PubMed] [Google Scholar]
- Lyman SN, Cheng I, Gratz LE, Weiss-Penzias P, Zhang L, 2020. An updated review of atmospheric mercury. Science of The Total Environment 707, 135575 10.1016/j.scitotenv.2019.135575 [DOI] [PubMed] [Google Scholar]
- Lyman S, Jones C, O’Neil T, Allen T, Miller M, Gustin MS, Pierce AM, Luke W, Ren X, Kelley P, 2016. Automated Calibration of Atmospheric Oxidized Mercury Measurements. Environ. Sci. Technol 50, 12921–12927. 10.1021/acs.est.6b04211 [DOI] [PubMed] [Google Scholar]
- Lyman SN, Jaffe DA, 2012. Elemental and oxidized mercury in the upper troposphere and lower stratosphere. Nature Geosci. 5, 114–117. 10.1021/acs.est.6b04211 [DOI] [Google Scholar]
- Manceau A, Lemouchi C, Enescu M, Gaillot AC, Lanson M, Magnin V, et al. , 2015. Formation of Mercury Sulfide from Hg(II)-Thiolate Complexes in Natural Organic Matter. Environmental Science & Technology 49, 9787–9796. 10.1021/acs.est.5b02522 [DOI] [PubMed] [Google Scholar]
- Martin LG, Labuschagne C, Brunke EG, Weigelt A, Ebinghaus R, Slemr F, 2017. Trend of atmospheric mercury concentrations at Cape Point for 1995–2004 and since 2007. Atmos. Chem. Phys 17, 2393–2399. 10.5194/acp-17-2393-2017 [DOI] [Google Scholar]
- Marumoto K, Suzuki N, Shibata Y, Takeuchi A, Takami A, Fukuzaki N, Kawamoto K, Mizohata A, Kato S, Yamamoto T, 2019. Long-Term Observation of Atmospheric Speciated Mercury during 2007–2018 at Cape Hedo, Okinawa, Japan. Atmosphere 10, 362 10.3390/atmos10070362 [DOI] [Google Scholar]
- Mason RP, Hammerschmidt CR, Lamborg CH, Bowman KL, Swarr GJ, Shelley RU, 2017. The air-sea exchange of mercury in the low latitude Pacific and Atlantic Oceans. Deep Sea Research Part I: Oceanographic Research Papers 122, 17–28. 10.1016/j.dsr.2017.01.015 [DOI] [Google Scholar]
- Matthews DA, Babcock DB, Nolan JG, Prestigiacomo AR, Effler SW, Driscoll CT, Todorova SG, Kuhr KM, 2013. Whole-lake nitrate addition for control of methylmercury in mercury-contaminated Onondaga Lake, NY. Environmental Research 125, 52–60. 10.1016/j.envres.2013.03.011 [DOI] [PubMed] [Google Scholar]
- McComb JQ, Rogers C, Han FX, Tchounwou PB, 2014. Rapid Screening of Heavy Metals and Trace Elements in Environmental Samples Using Portable X-Ray Fluorescence Spectrometer, A Comparative Study. Water Air and Soil Pollution 225, 2169 10.1007/s11270-014-2169-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord SA, Beutel MW, Dent SR, Schladow SG, 2016. Evaluation of mercury cycling and hypolimnetic oxygenation in mercury-impacted seasonally stratified reservoirs in the Guadalupe River watershed, California: RESERVOIR MERCURY CYCLING AND HYPOLIMNETIC OXYGENATION. Water Resour. Res 52, 7726–7743. 10.1002/2016WR019061 [DOI] [Google Scholar]
- Miller CL, Watson DB, Lester BP, Lowe KA, Pierce EM, Liang L, 2013. Characterization of soils from an industrial complex contaminated with elemental mercury. Environmental Research 125, 20–29. 10.1016/j.envres.2013.03.013 [DOI] [PubMed] [Google Scholar]
- Miller MB, Dunham-Cheatham SM, Gustin MS, Edwards GC, 2019. Evaluation of cation exchange membrane performance under exposure to high Hg0 and HgBr2 concentrations. Atmos. Meas. Tech 12, 1207–1217. 10.5194/amt-12-1207-2019 [DOI] [Google Scholar]
- Miller MB, Gustin MS, Edwards GC, 2018. Reactive mercury flux measurements using cation exchange membranes. Atmos. Meas. Tech. Discuss, 10.5194/amt-2018-360 [DOI] [Google Scholar]
- Miller MB, Gustin MS, 2013. Testing and modeling the influence of reclamation and control methods for reducing nonpoint mercury emissions associated with industrial open pit gold mines. Journal of the Air & Waste Management Association 63, 681–693. 10.1080/10962247.2013.778221 [DOI] [PubMed] [Google Scholar]
- Minamataconvention.org. Minamata Convention on Mercury, n.d (accessed 4.1.20).
- Muir DCG, Wang X, Yang F, Nguyen N, Jackson TA, Evans MS, Douglas M, Köck G, Lamoureux S, Pienitz R, Smol JP, Vincent WF, Dastoor A, 2009. Spatial Trends and Historical Deposition of Mercury in Eastern and Northern Canada Inferred from Lake Sediment Cores. Environ. Sci. Technol 43, 4802–4809. 10.1021/es8035412 [DOI] [PubMed] [Google Scholar]
- Munson KM, Babi D, Lamborg CH, 2014. Determination of monomethylmercury from seawater with ascorbic acid-assisted direct ethylation: Enhanced monomethylmercury determination. Limnol. Oceanogr. Methods 12, 1–9. 10.4319/lom.2014.12.1 [DOI] [Google Scholar]
- Munson KM, Lamborg CH, Boiteau RM, Saito MA, 2018. Dynamic mercury methylation and demethylation in oligotrophic marine water (preprint). Biogeochemistry: Open Ocean. 10.5194/bg-2018-173 [DOI] [Google Scholar]
- Munson KM, Lamborg CH, Swarr GJ, Saito MA, 2015. Mercury species concentrations and fluxes in the Central Tropical Pacific Ocean: CENTRAL PACIFIC MERCURY. Global Biogeochem. Cycles 29, 656–676. 10.1002/2015GB005120 [DOI] [Google Scholar]
- Navrátil T, Nováková T, Shanley JB, Rohovec J, Matoušková Š, Vaňková M, Norton SA, 2018. Larch Tree Rings as a Tool for Reconstructing 20th Century Central European Atmospheric Mercury Trends. Environ. Sci. Technol 52, 11060–11068. 10.1021/acs.est.8b02117 [DOI] [PubMed] [Google Scholar]
- Ndu U, Christensen GA, Rivera NA, Gionfriddo CM, Deshusses MA, Elias DA, Hsu-Kim H, 2018. Quantification of Mercury Bioavailability for Methylation Using Diffusive Gradient in Thin-Film Samplers. Environ. Sci. Technol 52, 8521–8529. 10.1021/acs.est.8b00647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obrist D, Agnan Y, Jiskra M, Olson CL, Colegrove DP, Hueber J, et al. , 2017. Tundra uptake of atmospheric elemental mercury drives Arctic mercury pollution. Nature 547, 201–204. 10.1038/nature22997 [DOI] [PubMed] [Google Scholar]
- Obrist D, Kirk JL, Zhang L, Sunderland EM, Jiskra M, Selin NE, 2018. A review of global environmental mercury processes in response to human and natural perturbations: Changes of emissions, climate, and land use. Ambio 47, 116–140. 10.1007/s13280-017-1004-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz VL, Mason RP, Evan Ward J, 2015. An examination of the factors influencing mercury and methylmercury particulate distributions, methylation and demethylation rates in laboratory-generated marine snow. Marine Chemistry 177, 753–762. 10.1016/j.marchem.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder S, Fritsche J, Alewell C, Schmutz M, Nilsson MB, Jocher G, et al. , 2016. A dual-inlet, single detector relaxed eddy accumulation system for long-term measurement of mercury flux. Atmos. Meas. Tech 9, 509–524. 10.5194/amt-9-509-2016 [DOI] [Google Scholar]
- Osterwalder S, Bishop K, Alewell C, Fritsche J, Laudon H, Åkerblom S, et al. , 2017. Mercury evasion from a boreal peatland shortens the timeline for recovery from legacy pollution. Sci. Rep 7, 16022 10.1038/s41598-017-16141-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterwalder S, Sommar J, Åkerblom S, Jocher G, Fritsche J, Nilsson MB, et al. , 2018. Comparative study of elemental mercury flux measurement techniques over a Fennoscandian boreal peatland. Atmospheric Environment 172, 16–25. 10.1016/j.atmosenv.2017.10.025 [DOI] [Google Scholar]
- Osterwalder S, Eugster W, Feigenwinter I, Jiskra M, 2019. First eddy covariance flux measurements of gaseous elemental mercury (Hg0) over a grassland. Atmos. Meas. Tech. Discuss 1–27. [Google Scholar]
- Outridge PM, Mason RP, Wang F, Guerrero S, Heimbürger-Boavida LE, 2018. Updated Global and Oceanic Mercury Budgets for the United Nations Global Mercury Assessment 2018. Environ. Sci. Technol 52, 11466–11477. 10.1021/acs.est.8b01246 [DOI] [PubMed] [Google Scholar]
- Parks JM, Johs A, Podar M, Bridou R, Hurt RA, Smith SD, Tomanicek SJ, Qian Y, Brown SD, Brandt CC, Palumbo AV, Smith JC, Wall JD, Elias DA, Liang L, 2013. The Genetic Basis for Bacterial Mercury Methylation. Science 339, 1332–1335. 10.1126/science.1230667 [DOI] [PubMed] [Google Scholar]
- Pérez-Rodríguez M, Horák-Terra I, Rodríguez-Lado L, Aboal JR, Martínez Cortizas A, 2015. Long-Term (~57 ka) Controls on Mercury Accumulation in the Southern Hemisphere Reconstructed Using a Peat Record from Pinheiro Mire (Minas Gerais, Brazil). Environ. Sci. Technol 49, 1356–1364. 10.1021/es504826d [DOI] [PubMed] [Google Scholar]
- Petrova MV, Krisch S, Lodeiro P, Valk O, Dufour A, Rijkenberg MJA, et al. , 2015. Mercury species export from the Arctic to the Atlantic Ocean. Marine Chemistry in press, tba: tba. [Google Scholar]
- Pierce A, Moore CW, Wohlfahrt G, Hörtnagl L, Kljun N, Obrist D, 2015. Eddy Covariance Flux Measurements of Gaseous Elemental Mercury Using Cavity Ring-down Spectroscopy. Environ. Sci. Technol 49, 1559–1568. 10.1021/es505080z [DOI] [PubMed] [Google Scholar]
- Podar M, Gilmour CC, Brandt CC, Soren A, Brown SD, Crable BR, Palumbo AV, Somenahally AC, Elias DA, 2015. Global prevalence and distribution of genes and microorganisms involved in mercury methylation. Sci. Adv 1, e1500675 10.1126/sciadv.1500675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulin BA, Aiken GR, Nagy KL, Manceau A, Krabbenhoft DP, Ryan JN, 2016. Mercury transformation and release differs with depth and time in a contaminated riparian soil during simulated flooding. Geochimica et Cosmochimica Acta 176, 118–138. 10.1016/j.gca.2015.12.024 [DOI] [Google Scholar]
- Rasmussen KL, Boldsen JL, Kristensen HK, Skytte L, Hansen KL, Mølholm L, Grootes PM, Nadeau M-J, Flöche Eriksen KM, 2008. Mercury levels in Danish Medieval human bones. Journal of Archaeological Science 35, 2295–2306. 10.1016/j.jas.2008.03.003 [DOI] [Google Scholar]
- Rasmussen KL, Skytte L, Jensen AJ, Boldsen JL, 2015. Comparison of mercury and lead levels in the bones of rural and urban populations in Southern Denmark and Northern Germany during the Middle Ages. Journal of Archaeological Science: Reports 3, 358–370. 10.1016/j.jasrep.2015.06.021 [DOI] [Google Scholar]
- Rydberg J, Rösch M, Heinz E, Biester H, 2015. Influence of catchment vegetation on mercury accumulation in lake sediments from a long-term perspective. Total Environ. Sci 538, 896–904. 10.1016/j.scitotenv.2015.08.133 [DOI] [PubMed] [Google Scholar]
- Saiz-Lopez A, Acuña AU, Trabelsi T, Carmona-García J, Dávalos JZ, Rivero D, Cuevas CA, Kinnison DE, Sitkiewicz SP, Roca-Sanjuán D, Francisco JS, 2019. Gas-Phase Photolysis of Hg(I) Radical Species: A New Atmospheric Mercury Reduction Process. J. Am. Chem. Soc 141, 8698–8702. 10.1021/jacs.9b02890 [DOI] [PubMed] [Google Scholar]
- Saiz-Lopez A, Sitkiewicz SP, Roca-Sanjuán D, Oliva-Enrich JM, Dávalos JZ, Notario R, Jiskra M, Xu Y, Wang F, Thackray CP, Sunderland EM, Jacob DJ, Travnikov O, Cuevas CA, Acuña AU, Rivero D, Plane JMC, Kinnison DE, Sonke JE, 2018. Photoreduction of gaseous oxidized mercury changes global atmospheric mercury speciation, transport and deposition. Nat. Commun 9, 4796 10.1038/s41467-018-07075-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster PF, Schaefer KM, Aiken GR, Antweiler RC, Dewild JF, Gryziec JD, et al. , 2018. Permafrost stores a globally significant amount of mercury. Geophys. Res. Lett 45, 1463–1471. 10.1002/2017GL075571 [DOI] [Google Scholar]
- Schwartz GE, Sanders JP, McBurney AM, Brown SS, Ghosh U, Gilmour CC, 2019. Impact of dissolved organic matter on mercury and methylmercury sorption to activated carbon in soils: implications for remediation. Environ. Sci.: Process Impacts 21, 485–496. 10.1039/c8em00469b [DOI] [PubMed] [Google Scholar]
- Semeniuk K, Dastoor A, 2017. Development of a global ocean mercury model with a methylation cycle: outstanding issues: GLOBAL OCEAN MERCURY MODEL. Global Biogeochem. Cycles 10.1002/2016GB005452 [DOI] [Google Scholar]
- Sitkiewicz SP, Oliva JM, Dávalos JZ, Notario R, Saiz-Lopez A, Alcoba DR, Oña OB, Roca-Sanjuán D, 2016. Ab initio quantum-chemical computations of the electronic states in HgBr2 and IBr: Molecules of interest on the Earth’s atmosphere. The Journal of Chemical Physics 145, 244304 10.1063/1.4971856 [DOI] [PubMed] [Google Scholar]
- Sitkiewicz SP, Rivero D, Oliva-Enrich JM, Saiz-Lopez A, Roca-Sanjuán D, 2019. Ab initio quantum-chemical computations of the absorption cross sections of HgX2 and HgXY (X, Y= Cl, Br, and I): molecules of interest in the Earth’s atmosphere. Phys. Chem. Chem. Phys 21, 455–467. 10.1039/C8CP06160B [DOI] [PubMed] [Google Scholar]
- Shanley JB, Marvin-DiPasquale M Lane O, et al. , 2019. Resolving a paradox—high mercury deposition, but low bioaccumulation in northeastern Puerto Rico. Ecotoxicology. 10.1007/s10646-019-02108-z [DOI] [PubMed] [Google Scholar]
- Slemr F, Weigelt A, Ebinghaus R, Bieser J, Brenninkmeijer CAM, Rauthe-Schöch A, Hermann M, Martinsson BG, Van Velthoven P, Bönisch H, Neumaier M, Zahn A, Ziereis H, 2018. Mercury distribution in the upper troposphere and lowermost stratosphere according to measurements by the IAGOS-CARIBIC observatory: 2014–2016. Atmos. Chem. Phys 18, 12329–12343. 10.5194/acp-18-12329-2018 [DOI] [Google Scholar]
- Soerensen AL, Mason RP, Balcom PH, Jacob DJ, Zhang Y, Kuss J, Sunderland EM, 2014. Elemental Mercury Concentrations and Fluxes in the Tropical Atmosphere and Ocean. Environ. Sci. Technol 48, 11312–11319. 10.1021/es503109p [DOI] [PubMed] [Google Scholar]
- Sommar J, Osterwalder S, Zhu W, 2020. Recent advances in understanding and measurement of Hg in the environment: Surface-atmosphere exchange of gaseous elemental mercury (Hg0). Science of The Total Environment 721, 137648 10.1016/j.scitotenv.2020.137648 [DOI] [PubMed] [Google Scholar]
- Song S, Selin NE, Soerensen AL, Angot H, Artz R, Brooks S, et al. , 2015. Top-down constraints on atmospheric mercury emissions and implications for global biogeochemical cycling. Atmos. Chem. Phys 15, 7103–7125. 10.5194/acp-15-7103-2015 [DOI] [Google Scholar]
- Sonke JE, Teisserenc R, Heimbürger-Boavida L-E, Petrova MV, Marusczak N, Le Dantec T, Chupakov AV, Li C, Thackray CP, Sunderland EM, Tananaev N, Pokrovsky OS, 2018. Eurasian river spring flood observations support net Arctic Ocean mercury export to the atmosphere and Atlantic Ocean. Proc. Natl. Acad. Sci. USA 115, E11586–E11594. 10.1073/pnas.1811957115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoichev T, Martin-Doimeadios RCR, Amouroux D, Molenat N, Donard OFX, 2002. Application of cryofocusing hydride generation and atomic fluorescence detection for dissolved mercury species determination in natural water samples. J. Environ. Monitor 4, 517–521. 10.1039/b204233a [DOI] [PubMed] [Google Scholar]
- Strickman RJS, Fulthorpe RR, Coleman Wasik JK, Engstrom DR, Mitchell CPJ, 2016. Experimental sulfate amendment alters peatland bacterial community structure. Science of The Total Environment 566–567, 1289–1296. 10.1016/j.scitotenv.2016.05.189 [DOI] [PubMed] [Google Scholar]
- Sun R, Sonke JE, Liu G, 2016. Biogeochemical controls on mercury stable isotope compositions of world coal deposits: A review. Earth-Sci. Rev 152, 1–13. [Google Scholar]
- Sunderland EM, Krabbenhoft DP, Moreau JW, Strode SA, Landing WM, 2009. Mercury sources, distribution, and bioavailability in the North Pacific Ocean: Insights from data and models: MERCURY IN THE NORTH PACIFIC OCEAN. Global Biogeochem. Cycles 23, n/a-n/a. 10.1029/2008GB003425 [DOI] [Google Scholar]
- Tang W, Liu Y, Guan W, Zhong H, Qu X, Zhang T, 2020. Understanding mercury methylation in the changing environment: Recent advances in assessing microbial methylators and mercury bioavailability. Science of The Total Environment, 714, 136827 10.1016/j.scitotenv.2020.136827 [DOI] [PubMed] [Google Scholar]
- Tesán Onrubia JA, Petrova MV, Puigcorbé V, Black EE, Valk O, Dufour A, Hamelin B, Buesseler KO, Masqué P, Le Moigne FAC, Sonke JE, Rutgers van der Loeff M, Heimbürger-Boavida L-E, 2020. Mercury Export Flux in the Arctic Ocean Estimated from 234 Th/ 238 U Disequilibria. ACS Earth Space Chem. acsearthspacechem 0c00055. 10.1021/acsearthspacechem.0c00055 [DOI] [Google Scholar]
- Ting Y, Chen C, Ch’ng B-L, Wang Y-L, Hsi H-C, 2018. Using raw and sulfur-impregnated activated carbon as active cap for leaching inhibition of mercury and methylmercury from contaminated sediment. Journal of Hazardous Materials 354, 116–124. 10.1016/j.jhazmat.2018.04.074 [DOI] [PubMed] [Google Scholar]
- Tsui MT-K, Blum JD, Kwon SY, 2020. Review of stable mercury isotopes in ecology and biogeochemistry. Sci. Total Environ 716, 135386 10.1016/j.scitotenv.2019.135386 [DOI] [PubMed] [Google Scholar]
- Urba A, Valiulis D, Šarlauskas J, Kvietkus K, Šakalys J, Selskis A, 2017. A pilot study of different materials applied for active sampling of gaseous oxidized mercury in the atmospheric air. Atmos. Pollut. Res 8, 791–799. 10.1016/j.apr.2017.01.012 [DOI] [Google Scholar]
- U.N.E.P. Global Mercury Assessment, 2013. Global Mercury Assessment 2013: Sources, emissions, releases, and environmental transport [Google Scholar]
- Villar E, Cabrol L, Heimbürger- Boavida L, 2020. Widespread microbial mercury methylation genes in the global ocean. Environmental Microbiology Reports 12, 277–287. 10.1111/1758-2229.12829 [DOI] [PubMed] [Google Scholar]
- Walser JW, Kristjánsdóttir S, Gowland R, Desnica N, 2019. Volcanoes, medicine, and monasticism: Investigating mercury exposure in medieval Iceland. Int. J. Osteoarchaeol 29, 48–61. 10.1002/oa.2712 [DOI] [Google Scholar]
- Wang C, Wang Z, Hui F, Zhang X, 2019. Speciated atmospheric mercury and sea-air exchange of gaseous mercury in the South China Sea. Atmos. Chem. Phys 19, 10111–10127. 10.5194/acp-19-10111-2019 [DOI] [Google Scholar]
- Wang F, Macdonald RW, Stern GA, Outridge PM, 2010. When noise becomes the signal: Chemical contamination of aquatic ecosystems under a changing climate. Marine Pollution Bulletin 60, 1633–1635. 10.1016/j.marpolbul.2010.05.018 [DOI] [PubMed] [Google Scholar]
- Wang F, Outridge PM, Feng X, Meng B, Heimbürger-Boavida LE, Mason RP, 2019. How closely do mercury trends in fish and other aquatic wildlife track those in the atmosphere? - Implications for evaluating the effectiveness of the Minamata Convention. Sci. Total Environ 674, 58–70. 10.1016/j.scitotenv.2019.04.101 [DOI] [PubMed] [Google Scholar]
- Wang K, Munson KM, Armstrong DA, Macdonald RW, Wang F, 2020. Determining seawater mercury methylation and demethylation rates by the seawater incubation approach: A critique. Marine Chemistry 219, 103753 10.1016/j.marchem.2020.103753 [DOI] [Google Scholar]
- Wang K, Munson KM, Beaupré-Laperrière A, Mucci A, Macdonald RW, Wang F, 2018. Subsurface seawater methylmercury maximum explains biotic mercury concentrations in the Canadian Arctic. Sci Rep 8, 14465 10.1038/s41598-018-32760-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Bao Z, Lin C-J, Yuan W, Feng X, 2016. Assessment of global mercury deposition through litterfall. Environmental Science & Technology 50, 8548–8557. [DOI] [PubMed] [Google Scholar]
- Wasik JKC, Mitchell CPJ, Engstrom DR, Swain EB, Monson BA, Balogh SJ, Jeremiason JD, Branfireun BA, Eggert SL, Kolka RK, Almendinger JE, 2012. Methylmercury Declines in a Boreal Peatland When Experimental Sulfate Deposition Decreases. Environ. Sci. Technol 46, 6663–6671. 10.1021/es300865f [DOI] [PubMed] [Google Scholar]
- Webster JP, Kane TJ, Obrist D, Ryan JN, Aiken GR, 2016. Estimating mercury emissions resulting from wildfire in forests of the Western United States. Environ. Sci. Technol 568, 578–586. [DOI] [PubMed] [Google Scholar]
- Yin R, Feng X, Li X, Yu B, Du B, 2014. Trends and advances in mercury stable isotopes as a geochemical tracer. Trends Environ. Anal. Chem 2, 1–10. 10.1016/j.teac.2014.03.001 [DOI] [Google Scholar]
- Yin R, Feng X, Shi W, 2010. Application of the stable-isotope system to the study of sources and fate of Hg in the environment: A review. Appl. Geochem 25, 1467–1477. 10.1016/j.apgeochem.2010.07.007 [DOI] [Google Scholar]
- Yin R, Feng X, Wang J, Li P, Liu J, Zhang Y, Chen J, Zheng L, Hu T, 2013. Mercury speciation and mercury isotope fractionation during ore roasting process and their implication to source identification of downstream sediment in the Wanshan mercury mining area, SW China. Chemical Geology 336, 72–79. 10.1016/j.chemgeo.2012.04.030 [DOI] [Google Scholar]
- Yin R, Feng X, Hurley JP, Krabbenhoft DP, Lepak RF, Kang S, Yang H, Li X, 2016. Historical Records of Mercury Stable Isotopes in Sediments of Tibetan Lakes. Sci Rep 6, 23332 10.1038/srep23332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W, Sommar J, Lin C-J, Wang X, Li K, Liu Y, Zhang H, Lu Z, Wu C, Feng X, 2019. Stable Isotope Evidence Shows Re-emission of Elemental Mercury Vapor Occurring after Reductive Loss from Foliage. Environ. Sci. Technol 53, 651–660. 10.1021/acs.est.8b04865 [DOI] [PubMed] [Google Scholar]
- Zhao L, Chen H, Lu X, Lin H, Christensen GA, Pierce EM, Gu B, 2017. Contrasting Effects of Dissolved Organic Matter on Mercury Methylation by Geobacter sulfurreducens PCA and Desulfovibrio desulfuricans ND132. Environ. Sci. Technol 51, 10468–10475. 10.1021/acs.est.7b02518 [DOI] [PubMed] [Google Scholar]
- Zhang T, Kim B, Levard C, Reinsch BC, Lowry GV, Deshussus MA, Hsu-Kim H, 2012. Methylation of mercury by bacteria exposed to dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ. Sci. Technol 46, 6950–6958. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jacob DJ, Horowitz HM, Chen L, Amos HM, Krabbenhoft DP, Slemr F, St. Louis VL, Sunderland EM, 2016. Observed decrease in atmospheric mercury explained by global decline in anthropogenic emissions. Proc. Natl. Acad. Sci. USA 113, 526–531. 10.1073/pnas.1516312113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Soerensen AL, Schartup AT, Sunderland EM, 2020. A global model for methylmercury formation and uptake at the base of marine food webs. Global Biogeochem. Cycles 34 10.1029/2019GB006348 [DOI] [Google Scholar]
- Zhou J, Wang Z, Zhang X, 2018. Deposition and Fate of Mercury in Litterfall, Litter, and Soil in Coniferous and Broad-Leaved Forests. J. Geophys. Res. Biogeosci 123, 2590–2603. 10.1029/2018JG004415 [DOI] [Google Scholar]
- Zhu W, Lin C-J, Wang X, Sommar J, Fu X, Feng X, 2016. Global observations and modeling of atmosphere-surface exchange of elemental mercury: A critical review. Atmos. Chem. Phys 16, 4451–4480. 10.5194/acp-16-4451-2016 [DOI] [Google Scholar]
- Zhu W, Sommar J, Lin C-J, Feng X, 2015. Mercury vapor air-surface exchange measured by collocated micrometeorological and enclosure methods – Part I: Data comparability and method characteristics. Atmos. Chem. Phys 15, 685–702. 10.5194/acp-15-685-2015 [DOI] [Google Scholar]
- Živković I, Fajon V, Tulasi D, Obu Vazner K, Horvat M, 2017. Optimization and measurement uncertainty estimation of hydride generation-cryogenic trapping-gas chromatography-cold vapor atomic fluorescence spectrometry for the determination of methylmercury in seawater. Marine Chemistry 193, 3–7. 10.1016/j.marchem.2017.03.003 [DOI] [Google Scholar]


