Abstract
Aim
The aim of this study was to measure gender differences among COPD patients' quality of care (QOC) before and after two educational interventions in Southern Italy.
Methods
In this prospective cohort study, COPD patients were identified from primary care electronic medical records (EMRs). Twelve process indicators concerning diagnosis, preventative measures and therapeutic processes were developed as a measure of QOC. Educational interventions consisted of clinical seminars and audits on COPD QOC at baseline, and at 12 and 24 months. QOC indicators were stratified by gender: odds ratios (ORs) (males as reference group) of having a good QOC indicator were calculated at baseline, 12 and 24 months, with 95% confidence intervals (CIs) using hierarchical generalised linear models.
Results
Of 46 326 people registered in the EMRs, 1463 COPD patients (3.1%) were identified, of which 37% were women. QOC indicators reflecting best practice 24 months after the educational programme were generally not different to baseline, often favouring men. On the other hand, the composite global QOC indicator suggested that while a good overall QOC at baseline was significantly higher in men than women (OR: 0.74; 95% CI: 0.57–0.96), it became nonsignificant at 24 months (OR: 0.96; 95% CI: 0.72–1.29).
Conclusions
Specific QOC indicators among COPD patients often favoured men. However, several gender disparities seen at baseline disappeared at 24 months, suggesting that even general educational interventions which do not target gender can improve the gender disparity in QOC.
Short abstract
This observational study shows that quality of care (QOC) is better among male COPD patients in primary care. Planned educational interventions over 24 months abolished gender difference in global QOC, although not in all QOC items. https://bit.ly/3cfwPST
Introduction
COPD is a common disease involving airflow limitations and chronic respiratory symptoms [1] and is the third-leading cause of death worldwide [2]. However, COPD is often detected only after the appearance of serious symptoms. There is widespread misdiagnosis of COPD because of insufficient recourse to spirometry testing [3]. The impact of COPD in women is significantly understudied.
Historically, COPD was considered a predominantly male disease. The latest national epidemiology report prepared by the Italian Society of General Practitioners showed that the prevalence of COPD in Italy increased by 30% from 2006 to 2016, from 2.3% to 3.0%, with a higher prevalence in men than women (3.6% versus 2.5%, respectively) [4]. In contrast, recent studies suggest the worldwide prevalence of COPD has been increasing more rapidly among women compared to men [5–7]. The changing epidemiology of COPD may be due to the increasing use of cigarettes among women, a major cause of this disease [8]. Indeed, women seem to be more vulnerable to cigarette smoke [9], potentially because men and women differ significantly in terms of airway anatomy, genetic susceptibility to airway damage and possibly also in their lung microbiome [10].
Despite the known increase in the prevalence of COPD among females, healthcare practitioners remain more likely to diagnose COPD among males than females [3]. In fact, women tend to have a higher self-reported prevalence of COPD, whereas the opposite is seen using administrative healthcare databases, suggesting potential gender bias [11]. Gender bias in clinical practice is a phenomenon whereby a physician is overly influenced by the patient's gender. This can impact diagnosis, follow-up care and pharmacological management. Gender bias is known to occur in several diseases, notably osteoporosis [12], which is more common among women, but also in cardiovascular disease, likely due to the different symptomatic presentation [13]. Gender differences in COPD management may be due several reasons, including difficulty in distinguishing symptoms of COPD from those of asthma, of which the latter is known to be more common in women [14]. Gender differences in COPD diagnosis might also be related to the difference in clinical presentation of symptoms among women [3, 15]. Irrespective of the reason, COPD among women continues to be more commonly under-diagnosed or misdiagnosed in comparison to men, potentially also because women are less likely to have received spirometry tests [16]. Women are therefore less likely to be treated appropriately for COPD [17, 18]. It is currently not clear whether or how gender differences could influence the overall quality of care (QOC) among COPD patients. Other important aspects of the COPD management include the implementation of recommended preventative measures (e.g. noting and recording smoking habits, administration of vaccines) and recommendations on the therapeutic process (e.g. promoting high adherence to COPD medications). In general, data on whether women and men receive differential COPD care are somewhat limited [19]. It is also not clear how potential gender differences in clinical care among COPD patients affects clinical outcomes such as hospitalisation.
To date, various educational programmes for clinicians have been shown to be valid tools to improve the QOC of COPD patients in several countries [19–21]. In Italy, Ferrara et al. [22] evaluated the effectiveness of an educational programme promoting best practices for the management of COPD in primary care based on clinical audits and continuous remote education. However, this study did not shed light on potential gender differences in COPD management, including how educational intervention would impact any gender differences seen. Identifying gender disparities in COPD management is crucial to assess the need for tailored educational programmes that can improve QOC in primary care. The aim of the present study was therefore to evaluate gender differences in the management of COPD outpatients, in terms of QOC, and measure whether any such differences changed after the implementation of educational interventions in a Southern Italian general practice setting.
Methods
Study setting and data source
The study was conducted in a primary care setting. Electronic medical records (EMRs) containing anonymised patient data recorded by general practitioners (GPs) during routine clinical practice were used. Overall, 33 Sicilian GPs contributed data from 46 326 patients, including demographic information, diagnoses coded in International Classification of Diseases, 9th Edition, with clinical modifications (ICD-9 CM), drugs prescribed coded using the Anatomic Therapeutic and Chemical classification (ATC) coding system and lifestyle information such smoking habits. Other information, such as body mass index (BMI), administration of influenza vaccine and Streptococcus pneumoniae vaccine were also recorded.
Study design and participants
A prospective cohort study design was employed. Patients with COPD were identified from the EMRs between 2013 and 2015 using the following ICD-9 CM codes: 496 and subcodes (chronic airway obstruction, not elsewhere classified) and 491.2 and subcodes (obstructive chronic bronchitis). Suspected diagnoses of COPD were thereafter validated by GPs through clinical revaluation and, whenever possible, spirometry. The first date at which a COPD patient visited the GP during the study period was considered the cohort entry date (baseline).
Educational intervention
An educational intervention was addressed to all GPs who contributed data to the study [22]. The intervention consisted of planned continuing professional education seminars and continuous remote education on COPD diagnosis and management. Further information is provided in supplementary box 1.
COPD QOC indicators
Twelve indicators of good QOC reflecting best practices for COPD management were developed based on the COPD Global Initiative for Chronic Obstructive Lung Disease guidelines [23]. These QOC indicators were developed jointly by respiratory specialists, GPs and clinical pharmacologists. The indicators consisted of three macro-categories: COPD diagnostic processes, measures to prevent complications of COPD (hereafter referred to as preventative measures) and therapeutic processes. The full list of QOC indicators along with the rationale behind them is available in supplementary table 1. The 12 QOC indicators were considered singly to provide the greatest possible granular detail on COPD management, grouped together into a composite variable as a macro-category to provide an overview of the three general clinical aspects of COPD management and pooled altogether as a composite variable consisting of all three macro-categories to provide an overview of COPD management in the most general terms possible.
Covariates
The covariates included in the study are found in supplementary box 2 and supplementary tables 1 and 2.
Outcomes
Hospitalisation was identified as a clinical outcome, identified through patient-level deterministic linkage with hospital discharge records from Sicilian claims databases. Hospitalisation was categorised as all-cause hospitalisation, hospitalisation for COPD as a primary or secondary diagnosis and hospitalisation for COPD as a primary diagnosis (i.e. for an exacerbation of COPD). Italian claims records have been described in detail elsewhere [24]. Patient informed consent was specifically requested for data linkage.
Data analysis
Descriptive statistics were used to describe baseline patient characteristics. Among patients evaluated both at baseline and after 12 or 24 months, Hierarchical Generalised Linear Models (HGLMs) were fitted to estimate the frequencies of the 12 QOC indicators at such time points, in males compared to females. HGLMs account for clustering due to GPs (multilevel structure), assuming binomial distribution of data and the logistic link function, including follow-up time, gender and time-by-gender as categorical covariates, along with a random intercept. The odds ratios (ORs) of having a QOC indicator were derived from HGLMs (males as comparator group), along with 95% confidence intervals (CIs). A forest plot of the estimated ORs, stratified by their macro-categories, at baseline and over time was produced. Analyses were repeated for QOC macro-categories and for a composite indicator composed of all the QOC indicators. A subanalysis was performed, stratifying gender-specific analyses by smoking habits: current, former, never-smokers and those with unknown smoking status.
All analyses were performed using SAS Software, Release 9.4 (SAS Institute, Cary, NC, USA). All plots were created by R Core Team (2018). R: A language and environment for statistical computing (version 3.5.3).
Ethics statement
The Ethical Committee of Academic Hospital “G. Martino” of Messina approved the study (protocol no. 39-12, September 14, 2012). Informed consent to use patient data was obtained from all patients participating in the study.
Results
Cohort characteristics
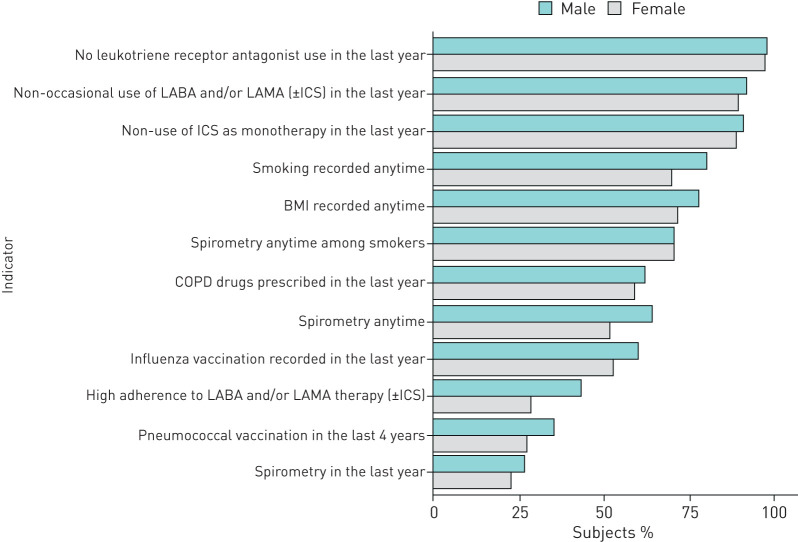
Of 46 326 persons registered in the practices of participating GPs, 1463 (3.1%) COPD patients were identified; of these, approximately a third (n=536) were women (table 1). The median age (1st to 3rd quartile) was similar between males and females, at 74 (66–81) versus 74 (64–82) years, respectively. Approximately 65.9% (n=612) and 31.2% (n=167) of males and females respectively were current or former smokers. Overall, smoking status was known for 80% of males (n=747) and 70% of females (n=375), respectively. In terms of burden of disease, males were slightly more likely to have a Charlson index score of 3 or more compared to females (46.9% males versus 40.8% females; p<0.05). Concerning the use of specific drugs, the most commonly used respiratory drugs among both sexes were long-acting beta-agonists (LABAs) and inhaled corticosteroids (ICSs) as a fixed combination. However, twice as many males were treated with LABAs, long-acting acting muscarinic agents (LAMAs) and ICSs concomitantly compared to females (14% versus 8%). Almost two-thirds of males or females were treated with antibiotics, with no significant differences between sexes. Among antibiotics, fluoroquinolones were most commonly prescribed in both sexes (41.0% in males versus 35.2% in females; p<0.05). The median number of distinct drugs as the presence of different ATC codes (1st to 3rd quartile) prescribed in the year prior to cohort entry was similar for both sexes: 9 (6–14) for males versus 10 (6–15) for females. GP clinical performance based on the QOC indicator frequencies was generally better among males (figure 1), remaining consistent at 12 and 24 months (supplementary figure 1).
TABLE 1.
Demographic, clinical and therapeutic characteristics at baseline of patients with a COPD diagnosis included in the cohort
| Baseline | p-value# | ||
| Males (n=927) | Females (n=536) | ||
| Age years | 74.0 (66.0–81.0) | 74.0 (64.0–82.0) | 0.39 |
| Age categories (years) | |||
| <45 | 19 (2.0) | 10 (1.9) | 0.80 |
| 45–54 | 39 (4.2) | 50 (9.3) | <0.05 |
| 55–64 | 141 (15.2) | 80 (14.9) | 0.88 |
| 65–74 | 274 (29.5) | 132 (24.6) | <0.05 |
| 75–84 | 332 (35.8) | 185 (34.5) | 0.61 |
| ≥85 | 124 (13.4) | 79 (14.7) | 0.46 |
| Last smoking status prior to cohort entry | |||
| Smoker | 286 (30.8) | 120 (22.4) | <0.05 |
| Former smoker¶ | 326 (35.1) | 47 (8.8) | <0.05 |
| Never-smoker | 135 (14.5) | 208 (38.8) | <0.05 |
| Unknown | 182 (19.6) | 161 (30.0) | <0.05 |
| Last BMI status prior to cohort entry | |||
| Underweight (<18.5) | 3 (0.3) | 6 (1.1) | 0.08 |
| Normal weight (18.5–24.9) | 96 (10.4) | 73 (13.6) | 0.06 |
| Overweight (25.0–29.9) | 282 (30.4) | 94 (17.5) | <0.05 |
| Obese (≥30.0) | 344 (37.1) | 212 (39.6) | 0.35 |
| Unknown | 202 (21.8) | 151 (28.2) | <0.05 |
| Hospital admissions for COPD exacerbation in the year prior to cohort entry | 25 (2.7) | 6 (1.1) | 0.04 |
| Comorbidities any time prior to cohort entry+ | |||
| Cardiovascular disorders | |||
| Hypertension | 666 (71.8) | 415 (77.4) | <0.05 |
| Heart failure | 133 (14.3) | 74 (13.8) | 0.77 |
| Arrhythmia | 41 (4.4) | 28 (5.2) | 0.62 |
| Atrial fibrillation | 96 (10.3) | 50 (9.3) | 0.52 |
| Ischaemic heart disease | 199 (21.5) | 66 (12.3) | <0.05 |
| Cerebrovascular disease | 264 (28.5) | 126 (23.5) | <0.05 |
| Metabolic diseases | |||
| Diabetes mellitus | 281 (30.3) | 138 (25.7) | 0.06 |
| Osteoporosis | 62 (6.7) | 207 (38.6) | <0.05 |
| Hyperlipidaemia | 386 (41.6) | 228 (42.5) | 0.73 |
| Thyroid disorders | 127 (13.7) | 188 (35.0) | <0.05 |
| Nervous system disorders | |||
| Anxiety | 63 (6.8) | 55 (10.3) | <0.05 |
| Schizophrenic disorder | 7 (0.7) | 5 (0.9) | 0.71 |
| Depression | 43 (4.6) | 57 (10.6) | <0.05 |
| Dementia | 39 (4.2) | 22 (4.1) | 0.22 |
| Parkinson's disease | 26 (2.8) | 14 (2.6) | 0.82 |
| Gastrointestinal disorders | |||
| GORD | 190 (20.5) | 131 (24.4) | 0.08 |
| History of peptic ulcer | 47 (5.1) | 19 (3.5) | 0.17 |
| Rheumatological diseases | |||
| Arthritis and arthrosis | 496 (53.5) | 414 (77.2) | <0.05 |
| Gout | 150 (16.2) | 82 (15.3) | 0.65 |
| Diseases affecting sense organs | |||
| Cataracts | 151 (16.3) | 86 (16.0) | 0.90 |
| Retinopathy | 70 (7.5) | 38 (7.1) | 0.74 |
| Other disorders | |||
| Liver disease | 131 (14.1) | 56 (10.4) | 0.04 |
| Chronic kidney disease | 175 (18.9) | 91 (16.9) | 0.36 |
| Charlson index score | |||
| 0 | 23 (2.4) | 10 (1.8) | 0.44 |
| 1–2 | 469 (50.6) | 307 (57.3) | <0.05 |
| ≥3 | 435 (46.9) | 219 (40.8) | <0.05 |
| Respiratory drugs used in the year prior to cohort entry+ | |||
| Single drugs with or without concomitant drugs | |||
| ICS | 260 (28.0) | 164 (30.6) | 0.30 |
| SABA | 65 (7.0) | 28 (5.2) | 0.17 |
| SAMA | 20 (2.1) | 6 (1.1) | 0.15 |
| LABA | 103 (11.1) | 42 (7.8) | 0.04 |
| LAMA | 284 (30.6) | 115 (21.4) | <0.05 |
| Xanthines | 70 (7.5) | 26 (4.8) | <0.05 |
| Leukotriene receptor antagonists | 19 (2.0) | 15 (2.8) | 0.35 |
| Chromones | 4 (0.4) | 4 (0.7) | 0.47 |
| Mucolytic agents | 154 (16.6) | 72 (13.4) | 0.10 |
| Specific combinations: fixed or nonfixed combinations | |||
| SABA+ICS | |||
| Concomitant use, nonfixed combination | 18 (1.9) | 8 (1.5) | 0.53 |
| LABA+ICS | |||
| Fixed combination | 287 (30.9) | 159 (29.6) | 0.60 |
| Concomitant use, nonfixed combination | 21 (2.3) | 10 (1.8) | 0.60 |
| LAMA+ICS nonfixed combination | 74 (7.9) | 27 (5.0) | <0.05 |
| LABA+LAMA | |||
| Concomitant use, nonfixed combination | 51 (5.5) | 19 (3.5) | 0.09 |
| LABA+LAMA+ICS | |||
| Concomitant use, nonfixed combination | 126 (13.6) | 45 (8.4) | <0.05 |
| Median number of respiratory drugs | 2.0 (1–3) | 2.0 (1–3) | 0.03 |
| Antibiotic use prior to cohort entry+ | |||
| Penicillins | 279 (30.1) | 156 (29.1) | 0.69 |
| Amoxicillin and β-lactamase inhibitor | 214 (23.0) | 120 (22.4) | 0.76 |
| Amoxicillin | 53 (5.7) | 27 (5.0) | 0.58 |
| Other penicillins | 12 (1.3) | 9 (1.7) | 0.55 |
| Cephalosporins (I, II, III and IV generations) | 239 (25.8) | 153 (28.5) | 0.25 |
| Ceftriaxone | 141 (15.2) | 94 (17.5) | 0.24 |
| Cefixime | 32 (3.4) | 20 (3.7) | 0.78 |
| Other cephalosporins | 66 (7.1) | 39 (7.2) | 0.91 |
| Macrolides | 140 (15.1) | 93 (17.3) | 0.25 |
| Clarithromycin | 77 (8.3) | 52 (9.7) | 0.36 |
| Azithromycin | 40 (4.3) | 27 (5.0) | 0.52 |
| Other macrolides | 23 (2.4) | 14 (2.6) | 0.88 |
| Fluoroquinolones | 380 (41.0) | 189 (35.2) | <0.05 |
| Levofloxacin | 244 (26.3) | 101 (18.8) | <0.05 |
| Ciprofloxacin | 95 (10.2) | 61 (11.3) | 0.50 |
| Other fluoroquinolones | 41 (4.4) | 27 (5.0) | 0.59 |
| Any antibiotic | 582 (62.8) | 325 (60.6) | 0.41 |
| Median number of different antibiotic drugs | 2.0 (1–2) | 1.0 (1–2) | 0.21 |
| Concomitant drug use in the year prior to cohort entry+ | |||
| Cardiovascular drugs | |||
| ACEIs | 327 (35.3) | 161 (30.0) | <0.05 |
| ARBs | 193 (20.8) | 104 (19.4) | 0.51 |
| Calcium channel blockers | 226 (24.4) | 130 (24.2) | 0.95 |
| Diuretics | 325 (35.0) | 209 (39.0) | 0.13 |
| Beta blockers | 208 (22.4) | 143 (26.7) | 0.06 |
| Other antihypertensives | 59 (6.3) | 50 (9.3) | <0.05 |
| Digitalis glycosides | 44 (4.7) | 21 (3.9) | 0.45 |
| Vasodilators | 91 (9.8) | 41 (7.6) | 0.16 |
| Anti-arrythmics of classes I and III | 40 (4.3) | 15 (2.8) | 0.14 |
| Anti-thrombotics | 520 (56.1) | 264 (49.2) | <0.05 |
| Drugs for metabolic disease | |||
| Antidiabetics | 255 (27.5) | 118 (22.0) | <0.05 |
| Antilipidaemic drugs | 369 (39.8) | 178 (33.2) | <0.05 |
| Anti-osteoporosis drugs | 11 (1.2) | 96 (17.9) | <0.05 |
| Drugs acting on the immune system | |||
| Systemic corticosteroids | 264 (28.5) | 179 (33.4) | <0.05 |
| Analgesics | |||
| NSAIDs | 385 (41.5) | 264 (49.2) | <0.05 |
| Opioids | 107 (11.5) | 100 (18.6) | <0.05 |
| Psychiatric drugs | |||
| Antidepressants | 114 (12.3) | 112 (20.9) | <0.05 |
| BDZs | 58 (6.2) | 53 (9.8) | <0.05 |
| Other drugs | |||
| Drugs for peptic ulcer and GORD | 609 (65.7) | 379 (70.7) | <0.05 |
| Median number of concomitant drugs | 9 (6–14) | 10 (6–15) | <0.05 |
Data are presented as n (%) or median (interquartile range), unless otherwise stated. BMI: body mass index; ICS: inhaled corticosteroids; SABA: short-acting β-agonist; SAMA: short-acting muscarinic antagonist; LABA: long-acting β-agonist; LAMA: long-acting muscarinic antagonist; ACEIs: angiotensin-converting enzyme inhibitors; ARBs: angiotensin receptor blockers; NSAIDs: nonsteroidal anti-inflammatory drugs; BDZs: benzodiazepines; GORD: gastro-oesophageal reflux disease. #: p-values were calculated using Chi-squared test or Fisher's exact test when the expected frequencies were <5. A Mann–Whitney U-test was used for median values. ¶: stopped smoking for at least 1 year. +: not mutually exclusive.
FIGURE 1.
Gender differences concerning quality of care indicators at baseline. BMI: body mass index; ICS: inhaled corticosteroid; LABA: long-acting β-agonist; LAMA: long-acting muscarinic agonist.
Changes of QOC indicators at baseline and over time
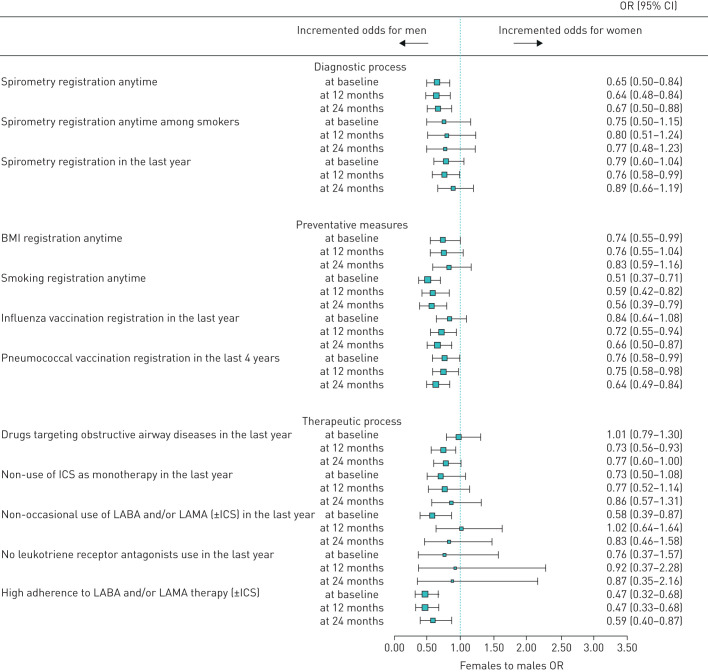
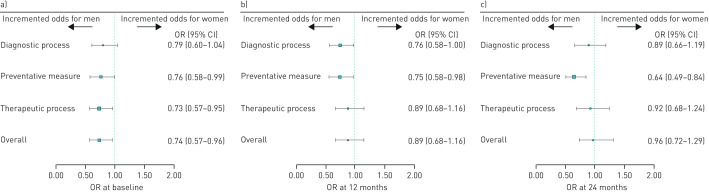
The odds of having an individual QOC indicator reflecting best practice 24 months after the educational programmes were generally not different to the baseline assessment, where men were generally more likely to have good QOC than women (figure 2). At baseline, the likelihood of being highly adherent to LABA and/or LAMA therapy (±ICSs), of having smoking status recorded and of non-occasional use of COPD medication was 53%, 49% and 42% lower among women, respectively. At 24 months, the gender disparity observed at baseline disappeared for two indicators of QOC at 24 months: having patient BMI recorded any time and the non-occasional use of COPD drugs. Worsening gender disparity was observed for two individual QOC indicators (i.e. the administration of the influenza vaccination (OR: 0.84; 95% CI: 0.64–1.08 at baseline versus OR: 0.66; 95% CI: 0.50–0.87 at 24 months) and use of any COPD drugs in the year prior to data collection (OR: 1.01; 95% CI: 0.79–1.30 at baseline versus OR: 0.77; 95% CI: 0.60–1.00 at 24 months)). Considering gender differences in the composite QOC indicators by macro-categories, there was a reduction in gender disparity from baseline to 24 months for diagnostic processes and therapeutic processes, but no improvement for preventative measures, with the latter favouring men more markedly at 24 months: OR: 0.76; 95% CI: 0.58–0.99 at baseline and OR: 0.64; 95% CI: 0.49–0.84 at 24 months (figure 3). Results from the comparison of the overall QOC indicator suggested that odds of receiving good QOC in general at baseline were significantly higher in men than women (OR: 0.74; 95% CI: 0.57–0.96) becoming nonsignificant at 24 months (OR: 0.96; 95% CI: 0.72–1.29). Concerning clinical outcomes among COPD patients, a decrease in the trend of hospitalisations over time was observed. The reduction in hospitalisations at 24 months compared to baseline was similar for men and women (table 2).
FIGURE 2.
Gender differences in individual quality of care indicators among COPD patients before and after the educational intervention. BMI: body mass index; ICS: inhaled corticosteroid; LABA: long-acting β-agonist; LAMA: long-acting muscarinic agonist.
FIGURE 3.
Gender differences in composite quality of care indicators by macro-categories, among COPD patients before and after the educational intervention. Data at a) baseline, b) 12 months and c) 24 months.
TABLE 2.
Gender-specific hospital admissions among COPD patients at baseline and at 24 months
| Type of hospital admission | Males | Females | ||||
| Baseline n (%) | 24 months n (%) | % difference | Baseline n (%) | 24 months n (%) | % difference | |
| Any diagnosis | 160 (25.4) | 128 (20.3) | 5.1 | 74 (21.6) | 56 (16.4) | 5.2 |
| COPD as primary or secondary diagnosis | 47 (7.5) | 30 (4.8) | 2.7 | 20 (5.8) | 9 (2.6) | 3.2 |
| COPD as primary diagnosis | 6 (1.0) | 0 (0) | 1.0 | 4 (1.2) | 1 (0.3) | 0.8 |
Subgroup analysis
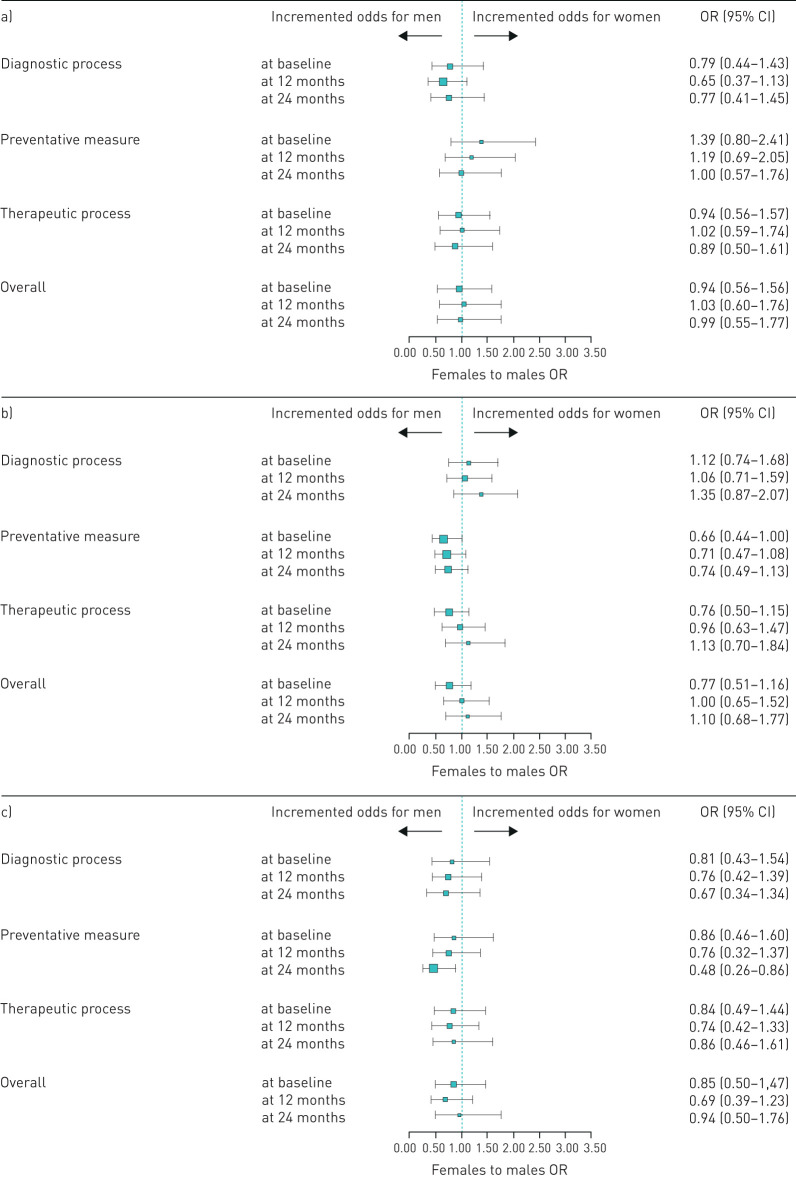
The statistically significant gender disparity in QOC observed at baseline in the whole cohort of COPD patients was generally not seen when restricted to different subgroups based on smoking habits, although a trend favouring men for most of macro-categories analysed was observed. Interestingly, the analysis on current and former smokers together showed a borderline significant trend favouring men for the preventative measure macro-category at baseline which become clearly nonsignificant at 24 months (figure 4). Considering the subgroup analysis on single QOC indicators, the only indicator indicating a statistically significant gender disparity at baseline was the non-occasional use of LABAs and/or LAMAs (±ICSs) among current smokers at baseline, which were more common among men; this became nonsignificant at 24 months (supplementary figures 2–5).
FIGURE 4.
Gender differences in individual quality of care indicators among COPD patients before and after the educational intervention, stratified by smoking status. Data for a) never-smokers, b) current and former smokers, and c) unknown smoking status.
Discussion
To our knowledge, the present study is the first to evaluate gender disparity in QOC among COPD patients in a primary care setting in Italy. There is very limited information on gender differences in COPD management in a primary setting. For example, a recently published study in Sweden described comorbidities and healthcare utilisation among males and females with COPD, as well the frequency of hospitalisation, which were generally similar to the present study [25]. However, there was no information on the QOC in terms of adherence to COPD therapy, administrations of vaccinations and use of spirometry tests. Our findings suggest that several gender differences exist in the QOC of COPD patients but that these can be partly mitigated through continuing professional education. Perhaps one of the most important findings of the present study is that the borderline gender disparity in the use of spirometry tests in the year prior to cohort entry became markedly nonsignificant at 24 months. This is important as spirometry is crucial to diagnose and monitor COPD [11]. In other words, on comparing the use of spirometry tests any time between males and females (i.e. a historical overview of gender bias using the earliest to the latest available EMRs), it emerged that the gender bias favouring men did not improve after the intervention. However, the effect of the educational programme could be observed in the QOC indicator indicative of recent behavioural changes in COPD diagnosis, showing that the borderline gender disparity in the use of spirometry tests at baseline improved, becoming more markedly nonsignificant at 24 months. Similarly, considering QOC indicators by diagnostic process macro-category, the overall gender bias favouring men which was borderline significant became nonsignificant at 24 months. Other important findings concerned gender disparity in QOC at baseline in terms of pharmacological therapy (i.e. non-occasional use of LABAs and/or LAMAs with or without ICSs and high adherence to LABA and/or LAMA therapy with or without ICSs). The gender disparity for the former indicator but not for high adherence to COPD medications disappeared after the implementation of the educational programme, where a persistence in gender disparity favouring men was observed 24 months after the intervention. Overall, considering all QOC indicators (i.e. a composite of the three macro-categories together), a gender disparity in QOC favouring males in the whole COPD cohort was seen at baseline but this disappeared after the educational programme at 24 months. The gender differences in QOC observed at baseline and beyond are unlikely to be explained by a different burden of treatment or disease of our cohort of COPD patients, as both men and women had a similar disease burden and intensity of pharmacological treatment at baseline. The driver behind the gender difference observed is therefore more likely to be physician attitudes and knowledge concerning COPD. Indeed, even when symptoms are similar, primary care clinicians still diagnose COPD more commonly in males [26]. The potentially subjective basis of COPD diagnoses was highlighted in a study conducted in Spain, which showed that the gender differences in diagnosing COPD disappeared after clinicians based their diagnosis on spirometry results, highlighting the importance of spirometry to improve COPD diagnosis [27]. Interestingly, the gender disparities in QOC at baseline and at 24 months did not co-occur with a gender difference in hospitalisations.
Our study has several strengths and limitations. We used data from EMRs, which are a rich source of real-world data. Indeed, in addition to shedding light on study-specific objectives, we were also able to describe patterns of drug utilisation in COPD patients that are not in line with GOLD guidelines [1]. For example, the use of ICS as monotherapy is not in line with the GOLD guidelines; similarly, the use of leukotriene receptor antagonists and chromones among COPD patients is not recommended, as these drugs are predominately used for the treatment of asthma. In a real-world setting, some degree of inappropriate medication use is to be expected, as indeed is actually seen. We consider that the value of observational studies such as the present study is precisely that we can closely examine drug use and other clinical details as they actually occur in clinical practice in order to identify areas for improvement. The main advantage of this data source in studying a disease such as COPD is the wealth of information on patient lifestyle and medical history such as smoking status, BMI and vaccinations. The longitudinal EMR data gave us 24 months of observation time, allowing us to measure the effect of the educational intervention in the short- and medium-term. The QOC indicators were developed to provide global overview of COPD management, encompassing various aspects of care. Analysing QOC at a very granular level and also using composite QOC indicators allowed us to identify potential clinical drivers of disparity in QOC, which can be potential targets to improve clinical management, without losing sight of the overall QOC.
However, our study also has some limitations. Primary care databases can have problems concerning COPD misclassification, for example misdiagnosing COPD as asthma. Whenever possible, validation of COPD diagnoses was carried out through spirometry tests. However, the validation of the COPD diagnosis was not as robust as it would have been in a clinical trial, but was limited to the level of diagnostic certainty that is available in a real-world primary care setting. The level of certainty underlying the COPD diagnoses in the present study is similar to that seen in similar EMR-based studies, such as those carried out using The Health Improvement Database [28], Health Search Database [29] and the Spanish Database for Pharmacoepidemiological Research in Primary Care [30]. The completeness of smoking data is contingent on the quality of GP data recording; indeed, 19.6% and 30.0% of males and females respectively had an unknown smoking status. For former smokers, we did not have information on the time elapsed since smoking cessation, although this is relevant information, especially concerning potentially preventable hospitalisation [31]. The higher number of female rather than male never-smokers was unexpected, but is in line with other studies [18, 32]. Female nonsmokers affected by other respiratory diseases could be theoretically be misclassified as COPD patients. However, almost two-thirds of females categorised as never-smokers had their COPD diagnosis confirmed by a spirometry test. The overall never-smoker proportion of 23% is in line with global estimates of never-smoker COPD patients, showing that 25–45% of COPD patients were never-smokers [33]. Regarding the preventative measure-related QOC, the use of vaccinations may also be under-reported in the EMRs, because GPs are already required to enter data in a vaccine registry, and may not re-enter the same data in the EMRs. It is therefore important to interpret these results with caution. Another limitation that could have negatively influenced the effect of the intervention on preventative measures is that the changes in clinical practice observed may be influenced by external factors that could not be adjusted for. One notable example is the excessive media attention in Italy given to some cases of very serious adverse drug reactions potentially attributable to the influenza vaccination during the study period [34]. It is possible that to improve QOC concerning preventative measures, the intervention may need to be longer and more intensive. Finally, results from the present study in a Southern Italian setting cannot be generalised to the management of COPD in Italy, as patient characteristics and clinical practice is known to vary from North to Southern Italy [4]. However, findings are likely to be generalisable to other Southern Italian settings.
Conclusion
Specific QOC indicators in a cohort of COPD patients in a Southern Italian general practice setting showed gender disparities favouring men in several aspects of COPD management, notably high adherence to COPD medications and non-occasional use of COPD medications. However, the global gender disparities seen at baseline disappeared at 24 months, suggesting that educational interventions often but not always reduced the gender disparity in QOC. Providing continuing professional education on gender difference in COPD may additionally reduce disparity in QOC.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00279-2020.supp (1.2MB, pdf)
Footnotes
This article has supplementary material available from openres.ersjournals.com
Conflict of interest: V. Isgrò has nothing to disclose.
Conflict of interest: J. Sultana has nothing to disclose.
Conflict of interest: A. Fontana has nothing to disclose.
Conflict of interest: V. Ientile has nothing to disclose.
Conflict of interest: U. Alecci has nothing to disclose.
Conflict of interest: R. Scoglio has nothing to disclose.
Conflict of interest: F. Magliozzo has nothing to disclose.
Conflict of interest: S. Scondotto has nothing to disclose.
Conflict of interest: G. Caramori has nothing to disclose.
Conflict of interest: M. Cazzola reports participation in courses sponsored by Almirall, AstraZeneca, Biofutura, Boehringer Ingelheim, Chiesi Farmaceutici, Cipla, Edmond Pharma, GlaxoSmithKline, Lallemand, Menarini Group, Mundipharma, Novartis, Pfizer, Teva, Verona Pharma and Zambon; and consultancy for ABC Farmaceutici, AstraZeneca, Chiesi Farmaceutici, Edmond Pharma, Lallemand, Novartis, Ockham Biotech, Verona Pharma and Zambon, all outside the submitted work.
Conflict of interest: G. Trifirò reports an unconditional grant from Novartis and a pharmacovigilance grant (Evaluating the appropriate use and safety of drugs used for chronic obstructive pulmonary disease in a Sicilian primary care setting) from the Italian Drug Agency/Sicilian Regional during the conduct of the study; participation on advisory boards for Sandoz, Hospira, Sanofi, Biogen, Ibsen and Shire, consultancy for Otsuka, acting as the principal investigator of studies funded by Amgen, AstraZeneca, Daiichi Sankyo and IBSA, and coordinator of a master's degree that received unconditional grants from several pharmaceutical companies, outside the submitted work.
Support statement: This study received unconditional funding from Novartis and from the Italian Drug Agency/Sicilian Region through the pharmacovigilance project Valutazione di appropriatezza prescrittiva e sicurezza dei farmaci per la broncopneumopatia cronica ostruttiva (BPCO) in medicina generale nella regione Siciliana (Evaluating the appropriate use and safety of drugs used for chronic obstructive pulmonary disease in a Sicilian primary care setting). The sponsors were not involved in any phase of the study design and execution. All the GPs participating in the study were members of the Italian College of General Practitioners (SIMG).
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2019 report) https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf Date last accessed: 4th April 2020. Date last updated: 2019.
- 2.McLean S, Hoogendoorn M, Hoogenveen RT, et al. Projecting the COPD population and costs in England and Scotland: 2011 to 2030. Sci Rep 2016; 6: 31893. doi: 10.1038/srep31893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romanelli AM, Raciti M, Protti MA, et al. How reliable are current data for assessing the actual prevalence of chronic obstructive pulmonary disease? PLoS ONE 2016; 11: e0149302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.XI Report Health Search. Research Institute of the Italian Society of General Practitioners https://report.healthsearch.it/Report_XI.pdf Date last accessed: 9 March 2020. Date last updated: 2018.
- 5.Akinbami LJ, Liu X. Chronic obstructive pulmonary disease among adults aged 18 and over in the United States, 1998–2009. NCHS Data Brief 2011; 63: 1–8. [PubMed] [Google Scholar]
- 6.Gershon AS, Wang C, Wilton AS, et al. Trends in chronic obstructive pulmonary disease prevalence, incidence, and mortality in Ontario, Canada, 1996 to 2007: a population-based study. Arch Intern Med 2010; 170: 560–565. doi: 10.1001/archinternmed.2010.17 [DOI] [PubMed] [Google Scholar]
- 7.Han MK, Postma D, Mannino DM, et al. Gender and chronic obstructive pulmonary disease: why it matters. Am J Respir Crit Care Med 2007; 176: 1179–1184. doi: 10.1164/rccm.200704-553CC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackay J, Amos A. Women and tobacco. Respirology 2003; 8: 123–130. doi: 10.1046/j.1440-1843.2003.00464.x [DOI] [PubMed] [Google Scholar]
- 9.Sørheim IC, Johannessen A, Gulsvik A, et al. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax 2010; 65: 480–485. doi: 10.1136/thx.2009.122002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nepal SK, Aryal S. Gender differences in chronic obstructive pulmonary disease – current knowledge and deficits In: Legato MJ, ed. Principles of Gender-Specific Medicine – Gender in the Genomic Era. 3rd Edn Cambridge, Academic Press, 2017; pp. 391–400. [Google Scholar]
- 11.Camp PG, Goring SM. Gender and the diagnosis, management, and surveillance of chronic obstructive pulmonary disease. Proc Am Thorac Soc 2007; 4: 686–691. doi: 10.1513/pats.200706-081SD [DOI] [PubMed] [Google Scholar]
- 12.Alswat KA. Gender disparities in osteoporosis. J Clin Med Res 2017; 9: 382–387. doi: 10.14740/jocmr2970w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liaudat C, Vaucher P, De Francesco T, et al. Sex/gender bias in the management of chest pain in ambulatory care. Womens Health (Lond) 2018; 14: 1745506518805641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Machado MC, Krishnan JA, Buist SA, et al. Sex differences in survival of oxygen-dependent patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2006; 174: 524–529. doi: 10.1164/rccm.200507-1057OC [DOI] [PubMed] [Google Scholar]
- 15.Roberts NJ, Patel IS, Partridge MR. The diagnosis of COPD in primary care; gender differences and the role of spirometry. Respir Med 2016; 111: 60–63. doi: 10.1016/j.rmed.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 16.Watson L, Vestbo J, Postma DS, et al. Gender differences in the management and experience of chronic obstructive pulmonary disease. Respir Med 2004; 98: 1207–1213. doi: 10.1016/j.rmed.2004.05.004 [DOI] [PubMed] [Google Scholar]
- 17.Ancochea J, Miravitlles M, García-Río F, et al. Underdiagnosis of chronic obstructive pulmonary disease in women: quantification of the problem, determinants and proposed actions. Arch Bronconeumol 2013; 49: 223–229. doi: 10.1016/j.arbres.2012.11.010 [DOI] [PubMed] [Google Scholar]
- 18.Raghavan D, Varkey A, Bartter T. Chronic obstructive pulmonary disease: the impact of gender. Curr Opin Pulm Med 2017; 23: 117–123. [DOI] [PubMed] [Google Scholar]
- 19.Steurer-Stey C, Dallalana K, Jungi M, et al. Management of chronic obstructive pulmonary disease in Swiss primary care: room for improvement. Qual Prim Care 2012; 20: 365–373. [PubMed] [Google Scholar]
- 20.Soler N, Ballester E, Martín A, et al. Changes in management of chronic obstructive pulmonary disease (COPD) in primary care: EMMEPOC study. Respir Med 2010; 104: 67–75. doi: 10.1016/j.rmed.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 21.Ulrik CS, Hansen EF, Jensen MS, et al. Management of COPD in general practice in Denmark—participating in an educational program substantially improves adherence to guidelines. Int J Chron Obstruct Pulmon Dis 2010; 5: 73–79. doi: 10.2147/COPD.S9102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrara R, Ientile V, Piccinni C, et al. Improvement in the management of chronic obstructive pulmonary disease following a clinical educational program: results from a prospective cohort study in the Sicilian general practice setting. NPJ Prim Care Respir Med 2018; 28: 10. doi: 10.1038/s41533-018-0077-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187: 347–365. doi: 10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 24.Trifirò G, Gini R, Barone-Adesi F, et al. The role of European healthcare databases for post-marketing drug effectiveness, safety and value evaluation: where does Italy stand? Drug Saf 2019; 42: 347–363. doi: 10.1007/s40264-018-0732-5 [DOI] [PubMed] [Google Scholar]
- 25.Lisspers K, Larsson K, Janson C, et al. Gender differences among Swedish COPD patients: results from the ARCTIC, a real-world retrospective cohort study. NPJ Prim Care Respir Med 2019; 29: 45. doi: 10.1038/s41533-019-0157-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chapman KR, Tashkin DP, Pye DJ. Gender bias in the diagnosis of COPD. Chest 2001; 119: 1691–1695. doi: 10.1378/chest.119.6.1691 [DOI] [PubMed] [Google Scholar]
- 27.Miravitlles M, de la Roza C, Naberan K, et al. Attitudes toward the diagnosis of chronic obstructive pulmonary disease in primary care. Arch Bronconeumol 2006; 42: 3–8. doi: 10.1157/13083272 [DOI] [PubMed] [Google Scholar]
- 28.Keene SJ, Adab P, de Vries F, et al. The stability of the ADO score among UK COPD patients from The Health Improvement Network. ERJ Open Res 2020; 6: 00196-2019. doi: 10.1183/23120541.00196-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vetrano DL, Zucchelli A, Bianchini E, et al. Triple inhaled therapy in COPD patients: determinants of prescription in primary care. Respir Med 2019; 154: 12–17. [DOI] [PubMed] [Google Scholar]
- 30.Aguilar-Shea AL, Bonis J. COPD from an everyday primary care point of view. J Family Med Prim Care 2019; 8: 2644–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tran B, Falster MO, Douglas K, et al. Smoking and potentially preventable hospitalisation: the benefit of smoking cessation in older ages. Drug Alcohol Depend 2015; 150: 85–91. doi: 10.1016/j.drugalcdep.2015.02.028 [DOI] [PubMed] [Google Scholar]
- 32.van Haren-Willems J, Heijdra Y. Increasing evidence for gender differences in chronic obstructive pulmonary disease. Womens Health (Lond) 2010; 6: 595–600. doi: 10.2217/WHE.10.37 [DOI] [PubMed] [Google Scholar]
- 33.Salvi SS, Barnes PJ. Chronic obstructive pulmonary disease in non-smokers. Lancet 2009; 374: 733–743. doi: 10.1016/S0140-6736(09)61303-9 [DOI] [PubMed] [Google Scholar]
- 34.Italian Medicine Agency 2014 http://www.agenziafarmaco.gov.it/content/aifa-divieto-d%E2%80%99utilizzo-lotti-fluad-solo-cautelativo-i-vaccini-sonorisorsa-preziosa-e-ins-0 Date last updated: 27th November 2014. Date last accessed 4th April 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00279-2020.supp (1.2MB, pdf)