Abstract
Background
Sepsis is a life-threatening systemic disease with severe microvascular dysfunction. Pericytes preserve vascular homeostasis. To our knowledge, the potential roles of microRNAs in sepsis-induced pericyte dysfunction have not been explored.
Methods
We determined lung pericyte expression of miR-145a in cecal ligation and puncture (CLP)–induced sepsis. Mouse lung pericytes were isolated and transfected with a miR-145a mimic, followed by stimulation with lipopolysaccharide (LPS). We measured inflammatory cytokine levels. To assess the functions of miR-145a in vivo, we generated a pericyte-specific miR-145a–knockout mouse and determined sepsis-induced organ injury, lung and renal vascular leakage, and mouse survival rates. We used RNA sequencing and Western blotting to analyze the signaling pathways regulated by miR-145a.
Results
CLP led to decreased miR-145a expression in lung pericytes. The miR-145a mimic inhibited LPS-induced increases in cytokines. In CLP-induced sepsis, pericytes lacking miR-145a exhibited increased lung and kidney vascular leakage and reduced survival rates. We found that miR-145a could suppress LPS-induced NF-κB activation. In addition, we confirmed that the transcription factor Friend leukemia virus integration 1 (Fli-1) is a target of miR-145a and that Fli-1 activates NF-κB signaling.
Conclusion
Our results demonstrated that pericyte miR-145a mediates sepsis-associated microvascular dysfunction, potentially by means of Fli-1–mediated modulation of NF-κB signaling.
Keywords: miR-145a, Fli-1, NF-κB, pericytes, sepsis
In the murine cecal ligation and puncture model, miR-145a−/− mice exhibited decreased survival and increased organ injury through the promotion of vascular leakage and inflammation, and miR-145a exerted protective effects through its target Friend leukemia virus integration 1.
Sepsis is a complex disorder that develops as a dysregulated host response to an infection and is associated with acute organ dysfunction and a high risk of death [1]. Current therapeutic approaches are confined to early antibiotic administration and supportive care, owing to the absence of therapies specific to microvascular dysfunction [2]. Microvascular dysfunction during sepsis causes important microenvironmental changes that have deleterious effects not only locally, but also systemically, and contribute to multiorgan dysfunction [3].
Pericytes are vascular smooth muscle lineage cells embedded in the basement membrane of the microvasculature that enwrap the adjacent endothelial cells [4]. Pericytes are involved in the preservation of vascular rheology and homeostasis, including regulation of blood flow, angiogenesis, structural stabilization of the vasculature, and vascular permeability [5]. Despite their critical role in microvascular function, the underlying mechanisms that regulate pericytes and microvascular dysfunction in sepsis remain largely unknown.
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression by binding to complementary target messenger RNAs (mRNAs), thereby inhibiting translation. They are expressed in all cell lineages and play critical roles in nearly all biological processes, including cell differentiation, development, and metabolism; thus, they are frequently associated with complex diseases [6]. Studies have indicated that miRNAs may play important roles in sepsis [7]. They are released both passively and actively into the circulation and specific circulating miRNAs have been associated with the presence of infection, and sepsis [8]. By using array- and single polymerase chain reaction (PCR)–based methods, a variety of dysregulated miRNAs, including, miR-126, miR-146a, miR-150, and miR-223, have all been associated with sepsis [8–11]. Although these associations between sepsis and miRNAs are provocative, their roles in the biology of sepsis and its mechanistic underpinnings have not been fully explored.
Friend leukemia virus integration 1 (Fli-1), an E26 transformation–specific transcription factor, was isolated a quarter century ago through a retrovirus mutagenesis screen [12]. Fli-1 has been recognized to play critical roles in normal development and homeostasis. For example, it transcriptionally regulates genes that drive normal hematopoiesis and vasculogenesis [13]. Our previous work suggested that Fli-1–mediated lung pericyte loss and vascular dysfunction via regulating expression levels of essential pyroptosis markers including caspase 1 and interleukin 18 in mice with cecal ligation and puncture (CLP)–induced sepsis [14]. We further illustrated that Fli-1 regulates caspase-1 gene expression by directly binding and activating the caspase-1 promoter in lung pericytes [15]. Using in silico analysis we found that Fli-1 mRNA has complementarity with miR-145a. miR-145 is abundantly expressed in smooth muscle cells and pericytes [16]. However, the role of miR-145a in pericyte biology during sepsis is unknown. In the present study, we aimed to investigate the role and mechanisms of miR-145a in the pathogenesis of murine sepsis. We hypothesized that miR-145a will play a critical role in microvascular homeostasis through regulation of Fli-1 signaling.
METHODS
Mouse Lung Pericyte Isolation, Culture, and Stimulation
Mouse lung pericytes were isolated as described elsewhere [14]. Pericytes (up to 3 passages) were seeded in 12-well plates and incubated with lipopolysaccharide (LPS; 100 ng/mL) for 24 hours. mRNA levels of miR-145a and interleukin 6 (IL-6) were determined with real-time PCR. Pericytes were transfected with miR-145a-5p mimic (40 nmol/L) or the negative control (40 nmol/L) using Lipofectamine 2000 Transfection Reagent (Invitrogen) for 24 hours, followed by stimulation with LPS (100 ng/mL) for another 24 hours. Total RNA and supernatant were collected for further analyses. Pericytes were transfected with Fli-1–specific small interfering RNA (siRNA) or scrambled siRNA (both 50 nmol/L) by HiPerFect Transfection Reagent (Qiagen) for 24 hours, followed by stimulation with LPS (100 ng/mL) for another 24 hours. Total protein was collected for further analyses.
Generation of Pericyte-Specific miR-145a–Knockout Mice
PDGFRβ-positive pericyte miR-145a–knockout mice were generated by crossing PDGFRβ tm13(pdgfrβ)Sor/J Cre mice (kind gift from Lynn M. Schnapp, Medical University of South Carolina) with miR-143/145flox/flox mice (stock no. 028503; The Jackson Laboratory).
CLP-Induced Sepsis and Survival Study
Mice (7–8 weeks old) were housed in a pathogen-free environment. All procedures complied with the standards for care and use of animal subjects, as stated in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Resources, National Academy of Sciences). The protocol for all animal studies was approved by the Institutional Animal Care and Use Committee at the Medical University of South Carolina. All surgery was performed under anesthesia. CLP was performed as described elsewhere [14], and sham operations were performed in the same way as CLP but without ligation and puncture of the cecum.
Measurement of Organ Function and Cytokine/Chemokine levels
Whole blood was collected from mice of each group at 24 hours after surgery and was transferred to a tube containing ethylenediaminetetraacetic acid. Plasma was separated by centrifugation at 10 000 rpm for 30 minutes and stored at–80°C for future analysis. The plasma levels of alanine aminotransferase, serum urea nitrogen, and creatinine were used as indicators for liver and kidney function, respectively, and were measured using enzyme-linked immunosorbent assay kits (BioAssay). The plasma levels of IL-6, interleukin 10, tumor necrosis factor α, interferon γ, and MCP-1 were determined by means of mouse cytokine and chemokine array proinflammatory focused 10-plex, which was performed and analyzed by Eve Technologies.
RNA Sequencing
Total RNA was extracted from pericytes with the RNeasy Plus Mini Kit (Qiagen). Complementary CNA (cDNA) was synthesized with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). RNA sequencing was performed using an Illumina HiSeq 2500 instrument at the MUSC Genomic Sequencing Core Facility. Total RNA was extracted from sham group and CLP group mice. Three micrograms of RNA per sample was used for RNA sample preparations. The data analysis was performed with the QIAseq RNA quantification platform (Qiagen) using unique molecular index counts according to the manufacturer’s instructions. Gene networks were generated based on gene connectivity and aligned against the Kyoto Encyclopedia of Genes and Genomes (KEGG) database (https://www.kegg.jp/). Genes with adjusted P values of .05 and log fold change of >1.5-fold were recorded.
Western Blot Analysis
Lung and kidney tissues or isolated lung pericytes were lysed with ice-cold RIPA lysis buffer (Cell Signaling). Western blotting assay was performed using the protein collected from the lung pericytes. The cell lysates were separated on 12% sodium dodecyl sulfate–polyacrylamide gels and transferred onto polyvinylidene fluoride membranes (Bio-Rad). Primary antibodies, including p65 (1:1000), total p65 (1:1000), and α-tubulin (1:2000) were used. The Western blot results were further analyzed using the Bio-Rad ChemiDoc XRS+ system (Bio-Rad).
Immunofluorescence
Pericytes were seeded on cover glass and transfected with control or miR-145a mimic for 24 hours, followed by stimulation with LPS for another 24 hours. The cells were stained with primary antibody against p65 (1:200; Cell Signaling) followed by Alexa Fluor 549 goat anti-rabbit immunoglobulin G (H chain and L chain) secondary antibody and 4’,6-diamidino-2-phenylindole staining reagent (ThermoFisher). Image capture and processing were performed using fluorescence microscopy. The fluorescence density of p65 in nucleus was analyzed with National Institutes of Health ImageJ 1.47V software.
Lung and Kidney Vascular Leakage and Lung Wet/Dry Ratio Measurement
Vascular leakage was quantified using the Evans blue dye assay in lung and kidney tissue, as described elsewhere [17]. The concentration of Evans blue dye in the supernatant was quantified by measuring absorbance at 620 nm and calculated from a standard curve using a plate reader. For lung water content, the left lung was harvested, weighed, and then dried in an oven at 60°C for 48 hours and reweighed to determine the dry weight. The lung water content was calculated as the ratio of wet weight to dry weight.
Statistical Analysis
The in vitro experiments were performed ≥3 times independently. Data were analyzed using GraphPad Prism 7.01 software and are represented as means with standard errors. Log-rank tests were used for comparisons in the survival study, and Student t test or analysis of variance with Fisher probable least-squares difference test for other comparisons. Differences were considered statistically significant at P < .05.
RESULTS
miR-145a Regulation of LPS-Induced Inflammatory Response in Lung Pericytes
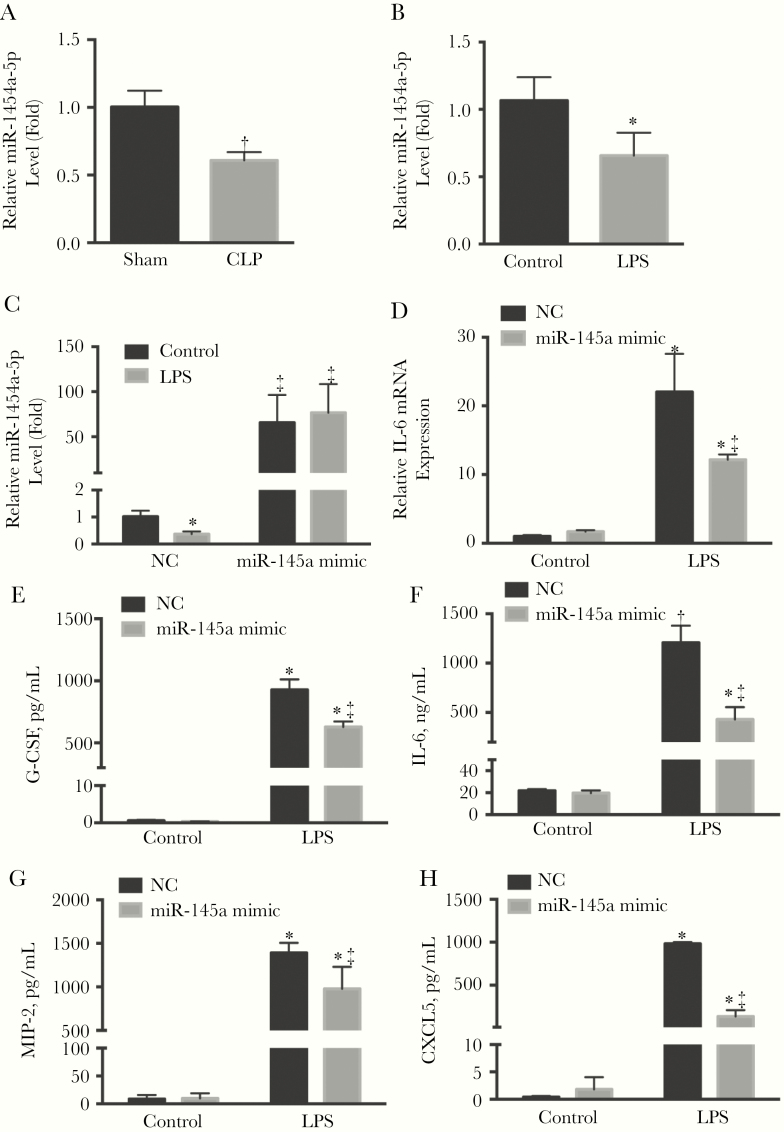
To determine its role in pericyte dysfunction, we measured miR-145a levels in pericytes from control and septic mice. The miR-145a levels were decreased by 40% (P < .05) at 24 hours after CLP surgery (Figure 1A). In addition, LPS also significantly decreased miR-145a expression in cultured mouse lung pericytes (Figure 1B). To investigate the function of miR-145a in vitro, lung pericytes were transfected with the miR-145a mimic, which caused a 70-fold increase in levels compared with the control (Figure 1C). Exposure of lung pericytes to LPS significantly increased mRNA levels of IL-6, whereas overexpression of miR-145a mitigated LPS-induced IL-6 transcripts (Figure 1D). In addition, we determined whether overexpression of miR-145a had an effect on cytokine secretion in LPS-stimulated lung pericytes. LPS significantly increased the release of proinflammatory cytokines including granulocyte colony-stimulating factor, IL-6, macrophage inflammatory protein 2, and C-X-C motif chemokine 5 (CXCL5), whereas overexpression of miR-145a significantly attenuated these increases (P < .05; Figure 1E–1H).
Figure 1.
miR-145a levels were decreased in lung pericytes in sepsis, and miR-145a regulates lipopolysaccharide (LPS)–induced inflammatory response in pericytes. A, C57BL/6J mice were subjected to sham or cecal ligation and puncture (CLP)–induced severe sepsis. Lung pericyte miR-145a-5p levels were determined (n = 3–6 mice per group). B, Lung pericytes were isolated from C57BL/6J mice and stimulated with LPS (100 ng/mL) for 24 hours. Relative miR-145a-5p expression levels were determined (n = 3). Lung pericytes transfected with miR-145a-5p mimic (40 nmol/L) or negative control (NC; 40 nmol/L) were stimulated with LPS for 24 hours. C, D, The relative expressions of miR-145a-5p (C) and interleukin 6 (IL-6) messenger RNA (mRNA) (D) were measured using quantitative reverse-transcription polymerase chain reaction. E–H, Protein levels of granulocyte colony-stimulating factor (G-CSF), IL-6, macrophage inflammatory protein (MIP) 2, and CXCL5 in the supernatant were also determined (n = 3 independent experiments); data are expressed as means with standard errors. *P < .05 and †P < .01 (comparison with control group); ‡P < .05 (comparison with NC LPS group).
Effect of miR-145a Knockout on CLP-Induced Deaths
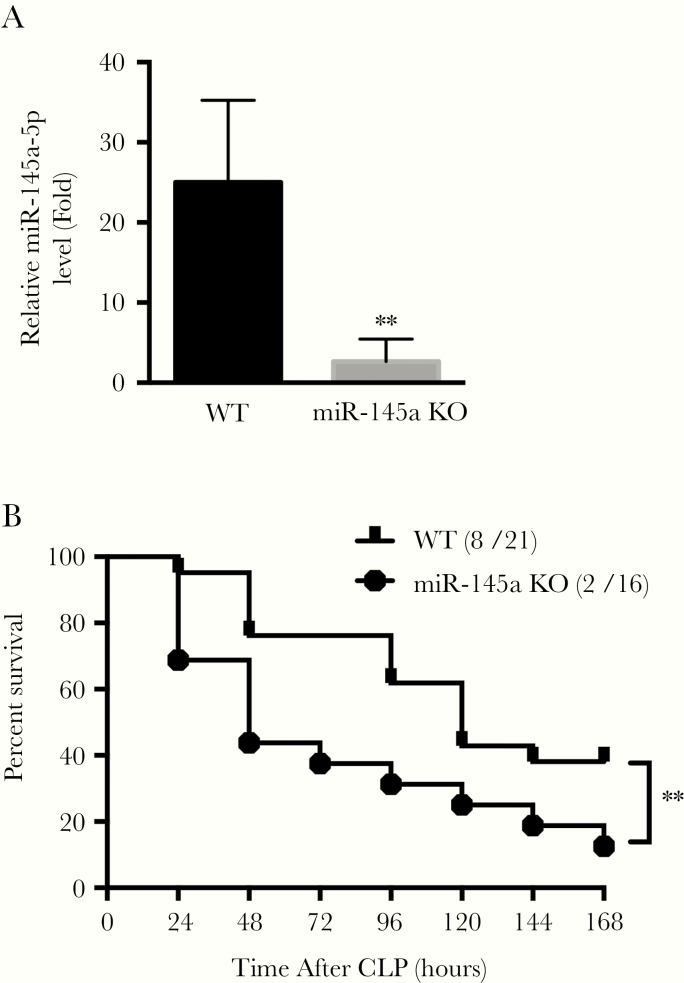
To further understand the role of miR-145a in sepsis, we generated a pericyte-specific miR-145a–knockout mouse model in which miR-145a was specifically deleted in pericytes (miR-145a−/−). Quantitative reverse-transcription PCR confirmed that miR-145a expression was suppressed by 92% in lung pericytes from miR-145a−/− mice (P < .01; Figure 2A). To explore whether knockout of miR-145a is deleterious in sepsis, we compared the survival rate in wild-type (WT) and miR-145a−/− mice after CLP surgery. Mouse survival was monitored for 7 days (168 hours). We demonstrated that the mortality rate was increased in miR-145a−/− mice compared with WT controls (P < .01; Figure 2B).
Figure 2.
Knockout of miR-145a increased cecal ligation and puncture (CLP)–induced deaths. A, Transgenic mice knockout of miR-145a in pericytes was confirmed in lung pericytes by means of quantitative reverse-transcription polymerase chain reaction. Results are represented as means with standard errors (n = 4–5 mice per group). B, Wild-type (WT) and miR-145a–knockout mice (KO) were subjected to CLP. Survival was monitored for 168 hours. Number of mice in each group were labeled as survived/total mice. *P < .01 (comparison with WT group).
Effect of miR-145a Knockout on Organ Injury and Plasma Cytokine Levels in CLP-Induced Sepsis
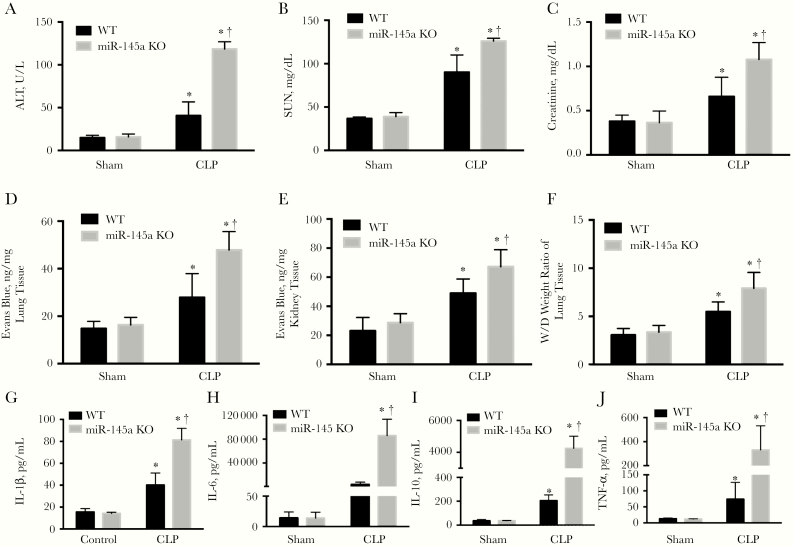
Multiorgan dysfunction is a major cause of death from sepsis. We thus determined the effect of pericyte miR-145a knockout on organ dysfunction in septic mice. Sepsis induced both liver and renal injury as evidenced by the increased alanine aminotransferase, serum urea nitrogen, and creatinine levels in the plasma of septic mice (P < .05; Figures 3A–3C). However, in the miR-145a−/− mice, organ injury was more severe than in septic WT mice (P < .05; Figures 3A–3C). We further investigated the effect of miR-145a on vascular leakage and lung edema. Mice in the CLP group exhibited a marked increase in lung and kidney vascular leakage, as assessed by means of Evans blue dye tissue concentrations. This leakage was significantly greater in septic miR-145a−/− mice (P < .05; Figures 3D and 3E) than in septic WT mice. Knockout of miR-145a also significantly increased lung water content compared with septic WT mice (Figure 3F).
Figure 3.
miR-145a–knockout (KO) mice exhibited increased lung, liver, and kidney injury and plasma cytokine levels in cecal ligation and puncture (CLP)–induced sepsis. A–C, Plasma levels of alanine aminotransferase (ALT) (A), serum urea nitrogen (SUN), (B) and creatinine (C) were measured at 24 hours after CLP (n = 3–4 mice per group). D, E, Vascular leakage in lung (D) and kidney (E) were measured by injecting Evans blue dye at 24 hours after CLP (n = 3–7 mice per group). F, Lung water content was determined by wet-dry (W/D) lung tissue weight ratio (n = 3–7 mice per group). G–J, Plasma cytokine interleukin 1β, 6, and 10 (IL-1β, IL-6, and IL-10) and tumor necrosis factor (TNF) α levels were determined at 24 hours after CLP (n = 3–5 mice per group). *P < .05 (comparison with sham group); †P < .05 (comparison with wild-type [WT] CLP group). Results are represented as means with standard errors.
Sepsis is associated with a systemic inflammatory response driven, in part, by cytokines and chemokines. CLP-induced sepsis significantly increased the circulating proinflammatory cytokines including IL-6, interleukin 10 and 1β, and tumor necrosis factor α (P < .05; Figure 3G–3J), which were further increased in the miR-145a−/− mice (P < .05; Figure 3G–3J). Collectively, our data indicate that knockout of miR-145a promoted organ injury and led to an increased inflammatory response during sepsis.
miR-145a Regulation of NF-κB Signaling Pathway
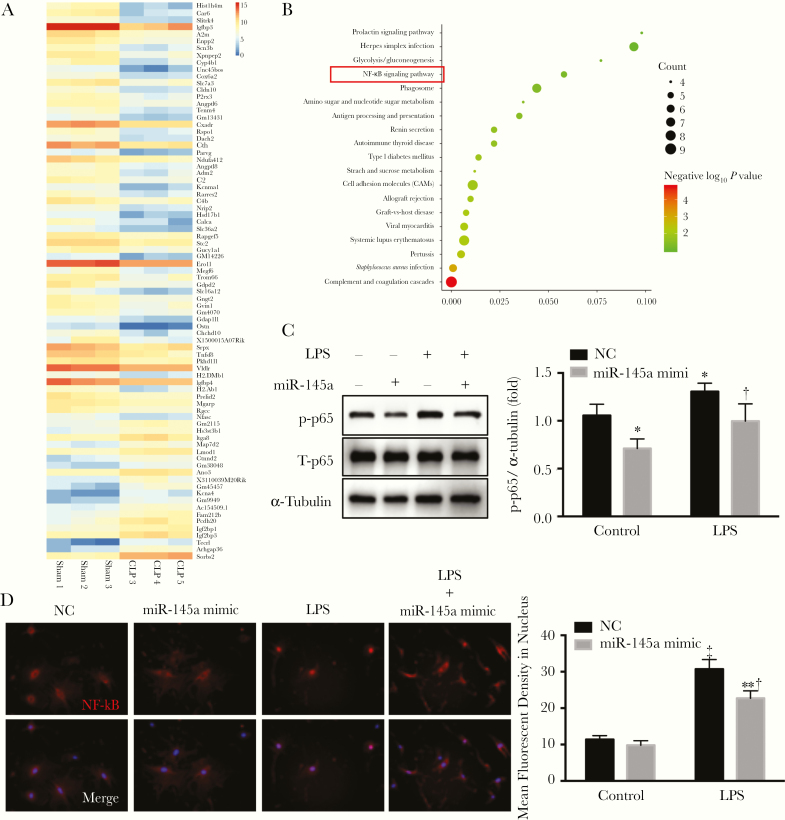
To gain insight into the mechanistic pathways altered by sepsis and miR-145a, we used RNA sequencing to analyze the differences in gene expression of lung pericytes from mice in sham and CLP group. Eighty genes were differentially expressed, as shown by the bioinformatics analysis (Figure 4A). KEGG pathway analysis demonstrated that the differentially expressed genes were highly involved in the NF-κB signaling pathway (Figure 4B). Thus, we determined whether miR-145a affects the NF-κB signaling pathway in lung pericytes using Western blot analysis. Overexpression of miR-145a inhibited the expression of phosphorylated NF-κB (phosphorylated p65) but also reduced phosphorylated NF-κB levels in lung pericytes treated with LPS (Figure 4C). Immunofluorescence demonstrated that LPS induced the translocation of NF-κB unit p65 from the cytoplasm to the nucleus; however, miR-145a overexpression inhibited this process (Figure 4D).
Figure 4.
miR-145a suppressed lipopolysaccharide (LPS)–induced nuclear factor kappa B (NF-κB) activation. A, RNA sequence data were analyzed by means of bioinformatics. B, Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis was used to determine the different signaling pathways. C, D, Lung pericytes were transfected with miR-145a-5p mimic and negative control. Pericytes were stimulated with LPS (100 ng/mL) for 2 hours. C, LPS-induced NF-κB phosphorylation was determined using Western blot analysis (n = 3). *P < .05 (comparison with negative control [NC] group); †P < .05 (comparison with LPS control group). D, LPS-induced nuclear translocation of p65 was determined with immunofluorescence staining. The first row shows NF-αB staining, and the second row, NF-κB staining merged with DAPI staining. Fluorescent densities of activated NF-κB in nucleus were quantified (n = 3). ‡P < .01 (comparison with control group). †P < .05 (comparison with LPS control group). 4′,6-diamidino-2-phenylindole (DAPI) staining is shown in blue; p65 in red.
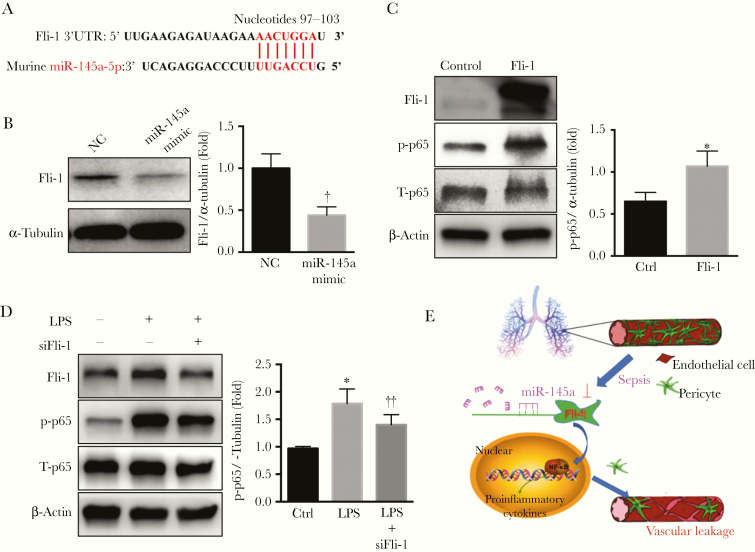
Fli-1 as a Target of miR-145a and in Regulating the NF-κB Signaling Pathway in Lung Pericytes
Our group previously found that Fli-1 regulates lung pericyte loss and contributes to the vascular dysfunction of sepsis [14]. Using in silico analysis, we found that Fli-1 mRNA has complementarity with miR-145a, with a predicted binding site at nucleotide positions 97 to 103 (Figure 5A). We transfected miR-145a mimic into lung pericytes and found that Fli-1 protein levels were significantly decreased, by 50% (Figure 5B). Previous results showed that miR-145a suppressed the NF-κB signaling pathway, raising the question of whether Fli-1 contributes to pericyte function by regulating NF-κB signaling. To evaluate this possibility, we first assessed the expression of total and phosphorylated NF-κB (p65) in lung pericytes after overexpression of Fli-1. As shown in Figure 5C, overexpression of Fli-1 significantly increased phosphorylated p65 protein level. In lung pericytes treated with LPS, the phosphorylated p65 levels decreased after treatment with the Fli-1 siRNA, suggesting that Fli-1 is a mediator of NF-κB activation (Figure 5D).
Figure 5.
Friend leukemia virus integration 1 (Fli-1) is a target of miR-145a and regulates NF-κB activation. A, Fli-1 3’ untranslated region (UTR) sequences containing miR-145a-5p–binding sites. B, Lung pericytes transfected with miR-145a-5p mimic (40 nmol/L) or negative control (NC; 40 nmol/L) for 48 hours. Expression levels of Fli-1 were determined (n = 3). †P < .05 (comparison with NC group). C, Lung pericytes were transfected with a control vector or constructs overexpressing Fli-1. The protein levels of the total and phosphorylated- NF-κB were detected with Western blot analysis (n = 3). *P < .05 (comparison with control group). D, The pericytes were transfected with Fli-1 small interfering RNA (siRNA) (50 nmol/L) or scrambled siRNA (50 nmol/L) and stimulated with lipopolysaccharide (LPS; 100 ng/mL) for 6 hours. The lysates were processed to measure total and phosphorylated p65 with Western blot analysis (n = 4) *P < .05 (comparison with control group); ‡P < .05 (comparison with LPS control group). E, Graphic depicting how miR-145a regulates lung vascular leakage by targeting NF-κB signaling via Fli-1.
DISCUSSION
In the present study, we demonstrated that miR-145a plays a critical role in sepsis-induced microvascular dysfunction. Specifically, we discovered that both sepsis and LPS stimulation decreased the expression of miR-145a in lung pericytes and that miR-145a negatively regulates the sepsis-induced inflammatory response. This occurs, in part, through the direct targeting of Fli-1, which modulates NF-κB signaling. These observations were supported by our findings that loss of miR-145a in pericytes results in worsened vascular leakage, organ injury, and mortality rate in sepsis.
Sepsis results in a marked decrease in capillary blood flow [18] and disruption of the endothelial vascular barrier, resulting in microvascular leakage and multiple organ failure [19, 20]. Findings of studies involving mouse mutants with reduced pericyte coverage of blood vessels indicate that pericytes have multiple effects on the vasculature [21]. Disruption of the blood-brain barrier is associated with detachment of pericytes from endothelial cells [22], supporting their potential role in microvasculature integrity. Furthermore, loss of pericytes leads to an increase in microvascular permeability in heart and lung [21, 23]. Thus, it is important to examine whether pericyte function and loss are associated with the microvascular dysfunction of sepsis.
Studies have indicated that miRNAs play critical roles in sepsis [24]. A group of host cellular miRNAs (miR-34a-5p, miR-122-5p, miR-145-5p, miR-146a-5p, and miR-210-3p) are released into the blood during sepsis, some of which are capable of inducing complement activation, cytokine production, and leukocyte migration [25]. In particular, miR-145 has been shown to ameliorate lung injury by inhibiting TGFBR2 signaling in LPS-induced sepsis [26, 27]. However, ours is the first in vivo study demonstrating the protective effects of miR-145a on lung pericytes in sepsis. We observed that CLP-induced sepsis led to decreased miR-145a expression in lung pericytes, whereas overexpression of miR-145a inhibited LPS-induced increases in inflammatory cytokine levels. These findings are consistent with those of Li et al [28], who indicated that miR-145 is a candidate for anti-inflammatory treatment for metabolic diseases. We also demonstrated the relevance of miR-145a to sepsis, through the generation of a pericyte-specific miR-145a–knockout mouse, which demonstrated a more severe phenotype.
miRNAs regulate gene expression by binding to the 3’-untranslated regions of specific mRNAs to control mRNA stability and the efficiency of translation [29, 30]. They can target several different genes through this mechanism and can, therefore, impart pleiotropic effects on cellular pathways. Our group’s previous research found that Fli-1 was increased in lung pericytes during CLP-induced murine sepsis and Fli-1 pericyte deletion attenuated lung pericyte loss [14]. Fli-1 belongs to the E26 transformation–specific transcription factor family and regulates a variety of cellular processes, including the inflammatory response [31]. Sepsis is also characterized by an overwhelming inflammatory response, which is, in part, driven by up-regulated cytokines and chemokines production [32]. Furthermore, prior investigation has shown that Fli-1 is a novel target of miR-145 in Ewing sarcoma, and its expression seems to be associated with biologically more aggressive tumors [33]. Likewise, functional inhibition of Fli-1 by diterpenoidlike compounds triggered its further down-regulation through miR-145, whose promoter is normally repressed by Fli-1 [34]. This report uncovers the importance of Fli-1 in leukemogenesis, a Fli-1–miR-145 autoregulatory loop and new anti-Fli-1 diterpenoid agents for the treatment of diverse hematological cancers overexpressing this transcription factor.
Our current data suggest that Fli-1 potentiates NF-κB signaling and is inhibited by miR-145a. Management of overwhelming inflammatory response is an important goal in the treatment of sepsis [35]. NF-κB is an inducible transcription factor with key roles in regulating the development and homeostasis of the immune system and the coordination of the inflammatory response [36]. In our experiments, miR-145a−/− mice expressed significantly more NF-κB–mediated proinflammatory cytokines and had more severe vascular leakage and organ injury than control mice. These data indicate that modulation of miR-145a expression in pericytes could be a potential therapeutic strategy that warrants further investigation.
Our study had several limitations. First, the expression of miR-145 was concomitantly affected by the loss of miR-143, because both miR-143 and miR-145 originate from the same transcriptional unit and the same primary miRNA [37]. This raises the possibility that some effects of the miR-145 knockout could be due to the simultaneous knockout of miR-143. This clearly deserves further investigation. Second, although we observed that miR-145a−/− pericytes mediate lung vascular leakage, we were unable to quantify pericyte cells. Further study is needed to explore whether miR-145a knockout reduces the number of pericytes through programmed cell death. Although miR-145a is abundantly expressed in pericytes, it may also affect endothelial cell and macrophage inflammatory response [38, 39].
Taken together, our composite findings provide critical evidence that the effects of miR-145a are correlated with its target, Fli-1, during sepsis. In the murine CLP model, miR-145a−/− mice exhibited decreased survival and increased organ injury through the promotion of vascular leakage and inflammation.
Notes
Financial support. This work was supported by the National Institute of General Medical Sciences, National Heart, Lung, and Blood Institute, and National center for advancing translational sciences (grants 1K23HL135263-01A1 to A. J. G., UL1TR001450 to P. V. H., 1R01GM113995 and R01GM130653 to H.F., UL1TR001450, and TL1TR001451).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet 2018; 392:75–87. [DOI] [PubMed] [Google Scholar]
- 2. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med 2017; 43:304–77. [DOI] [PubMed] [Google Scholar]
- 3. Pool R, Gomez H, Kellum JA. Mechanisms of organ dysfunction in sepsis. Crit Care Clin 2018; 34:63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res 2005; 97:512–23. [DOI] [PubMed] [Google Scholar]
- 5. Ferland-McCollough D, Slater S, Richard J, Reni C, Mangialardi G. Pericytes, an overlooked player in vascular pathobiology. Pharmacol Ther 2017; 171:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Carthew RW, Sontheimer EJ. Origins and mechanisms of miRNAs and siRNAs. Cell 2009; 136:642–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kingsley SMK, Bhat BV. Role of microRNAs in sepsis. Inflamm Res 2017; 66:553–69. [DOI] [PubMed] [Google Scholar]
- 8. Benz F, Roy S, Trautwein C, Roderburg C, Luedde T. Circulating microRNAs as biomarkers for sepsis. Int J Mol Sci 2016; 17:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang HJ, Zhang PJ, Chen WJ, Feng D, Jia YH, Xie LX. Four serum microRNAs identified as diagnostic biomarkers of sepsis. J Trauma Acute Care Surg 2012; 73:850–4. [DOI] [PubMed] [Google Scholar]
- 10. An R, Feng J, Xi C, Xu J, Sun L. miR-146a attenuates sepsis-induced myocardial dysfunction by suppressing IRAK1 and TRAF6 via targeting ErbB4 expression. Oxid Med Cell Longev 2018; 2018:7163057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhou J, Chaudhry H, Zhong Y, et al. Dysregulation in microRNA expression in peripheral blood mononuclear cells of sepsis patients is associated with immunopathology. Cytokine 2015; 71:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Akamata K, Asano Y, Yamashita T, et al. Endothelin receptor blockade ameliorates vascular fragility in endothelial cell-specific Fli-1-knockout mice by increasing Fli-1 DNA binding ability. Arthritis Rheumatol 2015; 67:1335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Luo H, Liu T, Zacksenhaus E, Ben-David Y. The ETS transcription factor Fli-1 in development, cancer and disease. Oncogene 2015; 34:2022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li P, Zhou Y, Goodwin AJ, et al. Fli-1 governs pericyte dysfunction in a murine model of sepsis. J Infect Dis 2018; 218:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li P, Goodwin AJ, Cook JA, Halushka PV, Zhang XK, Fan H. Fli-1 transcription factor regulates the expression of caspase-1 in lung pericytes. Mol Immunol 2019; 108:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cheng Y, Liu X, Yang J, et al. MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ Res 2009; 105:158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan H, Goodwin AJ, Chang E, et al. Endothelial progenitor cells and a stromal cell-derived factor-1α analogue synergistically improve survival in sepsis. Am J Respir Crit Care Med 2014; 189:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wafa K, Lehmann C, Wagner L, Drzymulski I, Wegner A, Pavlovic D. Desmopressin improves intestinal functional capillary density and decreases leukocyte activation in experimental endotoxemia. Microvasc Res 2015; 97:98–104. [DOI] [PubMed] [Google Scholar]
- 19. Goldenberg NM, Steinberg BE, Slutsky AS, Lee WL. Broken barriers: a new take on sepsis pathogenesis. Sci Transl Med 2011; 3:88ps25. [DOI] [PubMed] [Google Scholar]
- 20. Reséndiz-Martínez J, Asbun-Bojalil J, Huerta-Yepez S, Vega M. Correlation of the expression of YY1 and Fas cell surface death receptor with apoptosis of peripheral blood mononuclear cells, and the development of multiple organ dysfunction in children with sepsis. Mol Med Rep 2017; 15:2433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zeng H, He X, Tuo QH, Liao DF, Zhang GQ, Chen JX. LPS causes pericyte loss and microvascular dysfunction via disruption of Sirt3/angiopoietins/Tie-2 and HIF-2α/Notch3 pathways. Sci Rep 2016; 6:20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Nishioku T, Dohgu S, Takata F, et al. Detachment of brain pericytes from the basal lamina is involved in disruption of the blood-brain barrier caused by lipopolysaccharide-induced sepsis in mice. Cell Mol Neurobiol 2009; 29:309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chintalgattu V, Rees ML, Culver JC, et al. Coronary microvascular pericytes are the cellular target of sunitinib malate-induced cardiotoxicity. Sci Transl Med 2013; 5:187ra69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve the outcome of a murine model of sepsis. Mol Ther 2018; 26:1375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu J, Feng Y, Jeyaram A, Jay SM, Zou L, Chao W. Circulating plasma extracellular vesicles from septic mice induce inflammation via microRNA- and TLR7-dependent mechanisms. J Immunol 2018; 201:3392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma F, Li Z, Cao J, Kong X, Gong G. A TGFBR2/SMAD2/DNMT1/miR-145 negative regulatory loop is responsible for LPS-induced sepsis. Biomed Pharmacother 2019; 112:108626. [DOI] [PubMed] [Google Scholar]
- 27. Cao X, Zhang C, Zhang X, Chen Y, Zhang H. miR-145 negatively regulates TGFBR2 signaling responsible for sepsis-induced acute lung injury. Biomed Pharmacother 2019; 111:852–8. [DOI] [PubMed] [Google Scholar]
- 28. Li R, Shen Q, Wu N, et al. miR-145 improves macrophage-mediated inflammation through targeting Arf6. Endocrine 2018; 60:73–82. [DOI] [PubMed] [Google Scholar]
- 29. Zhang Y, Sun X, Icli B, Feinberg MW. Emerging roles for microRNAs in diabetic microvascular disease: novel targets for therapy. Endocr Rev 2017; 38:145–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rogobete AF, Sandesc D, Bedreag OH, et al. MicroRNA expression is associated with sepsis disorders in critically Ill polytrauma patients. Cells 2018; 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sato S, Zhang XK. The Friend leukaemia virus integration 1 (Fli-1) transcription factor affects lupus nephritis development by regulating inflammatory cell infiltration into the kidney. Clin Exp Immunol 2014; 177:102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Goodwin AJ, Guo C, Cook JA, Wolf B, Halushka PV, Fan H. Plasma levels of microRNA are altered with the development of shock in human sepsis: an observational study. Crit Care 2015; 19:440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang J, Guo H, Zhang H, et al. Putative tumor suppressor miR-145 inhibits colon cancer cell growth by targeting oncogene Friend leukemia virus integration 1 gene. Cancer 2011; 117:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu T, Xia L, Yao Y, et al. Identification of diterpenoid compounds that interfere with Fli-1 DNA binding to suppress leukemogenesis. Cell Death Dis 2019; 10:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu X, Wen M, Li X, et al. β1 receptor blocker decreases the myocardial inflammation in the sepsis adult rats through inhibition of TLR4/NF-ΚB signaling pathway [in Chinese]. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2019; 31:193–7. [DOI] [PubMed] [Google Scholar]
- 36.Wullaert A. Role of NF-kappaB activation in intestinal immune homeostasis. Int J Med Microbiol 2010; 300:49–56. [DOI] [PubMed] [Google Scholar]
- 37. Cordes KR, Sheehy NT, White MP, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 2009; 460:705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. He M, Wu N, Leong MC, et al. miR-145 improves metabolic inflammatory disease through multiple pathways. J Mol Cell Biol 2020; 12:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hui Y, Yin Y. MicroRNA-145 attenuates high glucose-induced oxidative stress and inflammation in retinal endothelial cells through regulating TLR4/NF-κB signaling. Life Sci 2018; 207:212–8. [DOI] [PubMed] [Google Scholar]