Abstract
Background
Human papillomavirus (HPV) vaccination of girls with very high (>90%) coverage has the potential to eradicate oncogenic HPVs, but such high coverage is hard to achieve. However, the herd effect (HE) depends both on the HPV type and the vaccination strategy.
Methods
We randomized 33 Finnish communities into gender-neutral HPV16/18 vaccination, girls-only HPV16/18 vaccination, and hepatitis B virus vaccination arms. In 2007–2010, 11 662 of 20 513 of 40 852 of 39 420 resident boys/girls from 1992 to 1995 birth cohorts consented. In 2010–2014, cervicovaginal samples from vaccinated and unvaccinated girls at age 18.5 years were typed for HPV6/11/16/18/31/33/35/39/45/51/52/56/58/59/66/68. Vaccine efficacy for vaccinated girls, HE for unvaccinated girls, and the protective effectiveness (PE) for all girls were estimated. We extended the community-randomized trial results about vaccination strategy with mathematical modeling to assess HPV eradication.
Results
The HE and PE estimates in the 1995 birth cohort for HPV18/31/33 were significant in the gender-neutral arm and 150% and 40% stronger than in the girls-only arm. Concordantly, HPV18/31/33 eradication was already predicted in adolescents/young adults in 20 years with 75% coverage of gender-neutral vaccination. With the 75% coverage, eventual HPV16 eradication was also predicted, but only with the gender-neutral strategy.
Conclusions
Gender-neutral vaccination is superior for eradication of oncogenic HPVs.
Keywords: elimination, eradication, gender-neutral vaccination, herd effects, human papillomavirus
Results from a community-randomized trial about girls-only and gender-neutral HPV vaccination strategies are presented and extended with mathematical modeling to show that gender-neutral vaccination with moderate 75% coverage will eradicate oncogenic HPV types from young adults in 20–30 years.
( See the Editorial Commentary by Sanjose and Bruni, on pages 888–9.)
The first prophylactic vaccines against oncogenic human papillomaviruses (HPVs) with high vaccine efficacy (VE) were licensed more than 10 years ago [1, 2]. However, implementation of these safe vaccines, which protect against invasive HPV-associated cancers [3], has not been very successful, particularly in affluent countries. Girls-only HPV vaccination coverage varies from low in opportunistic settings (25%–40%; France and the United States) to moderate in most organized programs (70%–80%; Finland and Sweden). In Europe, very high coverage (>90%) has been achieved only in Scotland [4–7]. Gender-neutral vaccination is being launched in some affluent countries to also protect boys from HPV diseases.
Population studies have observed first order herd protection (females to males) and second order herd protection (females via males to females), associated with girls-only HPV vaccination, from the occurrence of genital warts in both females and males [8–10]. This suggests that low-risk HPV types with fast clearance rates can be controlled by girls-only HPV vaccination and the associated herd effects (HEs) [11]. The multiple high-risk (hr)HPV types included in the vaccine [12] or vaccine-induced cross-protection [13, 14] are pivotal for the generation of protection and HEs against the wide variety of HPV types [15, 16]. However, eradication of HPV16/18 with girls-only vaccination requires the generation of strong second order HE against HPV16/18 among unvaccinated girls, from vaccinated girls via the first order HE-protected unvaccinated boys, which requires the exceptionally high (>90%) vaccination coverage [7, 11]. The current girls-only HPV vaccination achieving low to moderate coverage fails to deliver the necessary HE [4–7, 11, 17]. Gender-neutral vaccination not only protects boys, but it also provides a chance to strengthen herd protection.
Our community-randomized trial (CRT) compares the overall impacts of girls-only and gender-neutral vaccination strategies on HPV prevalence reduction in an originally HPV vaccination-naive adolescent population in Finland [15, 16, 18]. The overall impact, ie, protective effectiveness (PE), comprises VE for vaccinated females and HE for unvaccinated females for the entire adolescent female population. In the CRT, however, the randomization did not fully succeed to control background HPV prevalence heterogeneity between communities, which was tackled with advanced statistical methods [15, 16]. In addition, however, communities had variable vaccination coverage, unevenly between intervention arms, which may have impacted the published estimates.
In this work, we present final, outlier-free results with respect to vaccination coverage from the CRT. This outlier-free analysis, which was planned already in the study protocol before launching our CRT in 2007, tackles uneven distribution of vaccination coverage by excluding communities with an exceptional coverage of HPV vaccination from the analysis (Appendix 1). Starting with the trial vaccination coverage, we further present model-based timelines for eradication of oncogenic HPV types [19] with different assumptions of vaccination strategy, coverage, and VE.
METHODS
To evaluate the impact of girls-only versus gender-neutral HPV vaccination strategies, Finland conducted a CRT in 2007–2014 with the AS04-adjuvanted HPV16/18 (Cervarix) vaccine and hepatitis B virus (HBV) (Engerix) control vaccine [18]. The trial was started immediately after licensure of HPV vaccines and conducted in early adolescents aged 12 to 15 years. We briefly repeat the trial procedures, which are described elsewhere with more details, including characteristics for study groups [15, 16, 18].
Procedures
The 33 trial communities were randomized into 3 arms. In 11 Arm A communities with gender-neutral HPV vaccination, 90% of participating girls and boys received the HPV16/18 vaccine, and 10% received the HBV vaccine. In the 11 Arm B communities with girls-only HPV vaccination, 90% of participating girls received the HPV16/18 vaccine, and 10% of girls and all participating boys received the HBV vaccine. Finally, in the remaining 11 control Arm C communities, all participating girls and boys received the HBV vaccine [18].
All trial communities had more than 35 000 inhabitants and were located outside the Helsinki metropolitan area, at ≥35-km distance from each other. Background HPV16/18 exposure was estimated using HPV16/18 seroprevalence measured in a random sample of 50 women per community under age 23. The 33 communities were stratified into low (<20.5%, 12 communities), intermediate (20.5%–24%, 9 communities), and high (>24%, 12 communities) HPV16/18 seroprevalence categories and randomized based on these categories to reduce the variation across arms [18].
The HPV-040 study (Eudra-CT-2007-001731-55, NCT00534638) and parallel ancillary studies on Chlamydia trachomatis screening (Dnro 111/2009) and HPV screening (ETL-code 13149, NCT02149030) were approved by Finnish ethical committees of the Pirkanmaa and Pohjois-Pohjanmaa hospital districts in 2007, 2009, and 2013, respectively [15, 16, 18]. The primary HPV-040 study provided a beneficial vaccination in the control arm and cross-vaccination at the study end. The latter two studies provided cervicovaginal self-samples for C trachomatis and HPV analyses.
The Finnish Population Register was used (1) to identify all 80 272 residents of study communities born in 1992–1995 and (2) to follow their residential history throughout the study. In 2007–2009, invitation letters and consent forms were sent to their parents or legal guardians, resulting in 11 662/20 513 participants of 40 852/39 420 resident boys/girls. Three vaccine doses (at months 0, 1, and 6) were given to 99.4% of participants at schools: girls and boys in Arm A and girls in Arm B were blinded until age 18.5 years [15, 16].
All female residents of the study communities born in 1992–1995, including initial nonparticipants, were invited to attend follow-up visits at the age 18.5 years during 2010–2014. Self-collected cervicovaginal samples for HPV and C trachomatis testing for the ancillary studies were typed for HPV6, 11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68 (HPV deoxyribonucleic acid [DNA] positivity by polymerase chain reaction [PCR]). All female attendees consented to participate in a C trachomatis screening trial and filled in a questionnaire on demographic information, life-style factors, and sexual health. Finally, the attendees were offered the vaccine they had not received at baseline [15, 16].
Statistical Analyses
To determine the HE, 1 - ratio of risks of HPV infection (HPV DNA positivity) was estimated in non-HPV-vaccinated women for Arm A versus Arm C and Arm B versus Arm C. Likewise, the VE was estimated in HPV- versus HBV-vaccinated women for Arm A versus Arm C and Arm B versus Arm C [16]. The overall PE of HPV vaccination for all females born in 1992–1995, in Arm A and Arm B communities, was determined as a weighted combination of the VE in HPV-vaccinated women and the HE in non-HPV-vaccinated women. The VE was weighted by the coverage of HPV vaccination, and the HE was weighted by 1—coverage [15, 16].
Both VE and HE were estimated by the generalized estimating equation (GEE) method (Appendix 2) using SAS 9.4 (SAS Institute Inc., Cary, NC) [15, 16, 20, 21]. Binomial response and logit link was applied. An exchangeable correlation structure between responses of women from the same community and independence between responses of women from different communities were assumed. The 95% confidence intervals (CIs) were based on the profile likelihood [15]. To compare Arm A and B estimates for HE and PE, the probabilities P(A>B) were calculated from the log-normal approximated likelihoods.
Herd effect estimates were corrected for exceptional coverage of vaccination (outlier communities) and for the different proportion of HBV-vaccinated women among follow-up attendees in Arm C compared with Arms A and B. The outlier communities were defined by a cutoff level of 20% relative difference from the mean vaccination coverage of girls and boys in Arm A, and of girls in Arm B, in the remaining communities. To correct the unbalance in HBV-vaccinated/unvaccinated ratio, Arm C attendees were represented by all unvaccinated attendees and a stratified random sample from each 44 community-birth year strata of HBV-vaccinated attendees using the sampling ratio 0.125 in each stratum. For such Arm C representation, the HBV-vaccinated/unvaccinated ratio was similar to Arms A and B, and the GEE method was applied. The sampling procedure was repeated 21 times, and the HE estimates are means of the random-sample-specific GEE estimates. The 95% CIs were estimated using the entire non-HPV-vaccinated cohort [15, 16].
Two separate dynamic transmission models [19, 22] were used to emulate the trial in advance. Both models predicted delays in the HE so that HPV prevalence in the 1994–1995 birth cohorts was lower than in the 1992–1993 birth cohorts, both among nonvaccinated and vaccinated women [18]. Indeed, HPV prevalence peaks between 18 and 22 years of age, above the ages at vaccination of trial birth cohorts, and, therefore, the older vaccinated trial birth cohorts can be expected to block HPV transmission into the younger ones (Figure 1). Thus, both the VE and the HE estimates were also calculated separately for the different birth cohorts. All comparisons were against combined Arm C (1992–1995) birth cohorts to decrease the impact of natural variation in the control Arm C prevalence rates on the estimates.
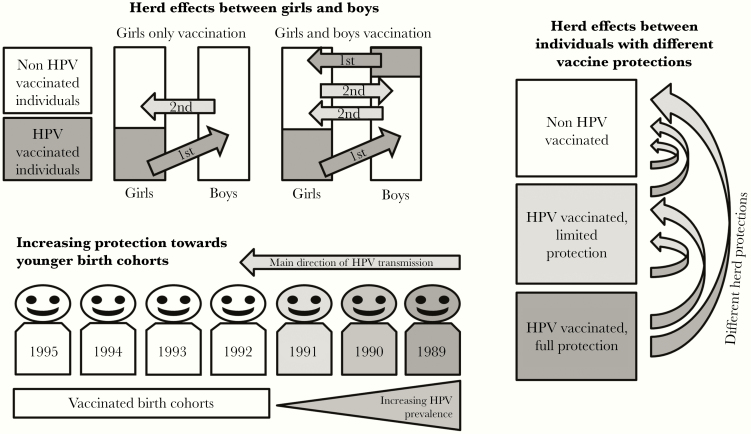
Figure 1.
Herd effects (HEs) induced by human papillomavirus (HPV) vaccination. The first order HE from vaccinated girls to unvaccinated boys (girls-only vaccination), and from both vaccinated girls and boys to unvaccinated boys and girls (gender-neutral vaccination), as well as the second order HE effects from unvaccinated but herd-protected individuals are visualized (top panel). Herd effects take place towards younger HPV-vaccinated birth cohorts by increasing the number of vaccinated birth cohorts from HPV-infected ones (bottom panel). Herd effects between differentially protected parts of population are visualized (right panel).
Vaccine efficacy and HE estimates were adjusted at the individual level for C trachomatis positivity, used as a surrogate to control for heterogeneity in behavior between the communities, and/or different sexual behavior between vaccinated and unvaccinated women, respectively. Adjustment at the community level was also performed for prevalence of smoking (<25%/≥25% current active smokers at age 18.5), as a surrogate of risky health behavior, and for HPV vaccination coverage of girls (reference <50%, and 50%–55%/55+% or 50+% if the model did not converge). Mobility was adjusted for by a variable consisting of both individual and community level aspects (semiurban vs urban) at study baseline and at the time of the follow-up visit [15, 16].
Mathematical Modeling
The mathematical transmission model, used for predictions about elimination, is described elsewhere in detail [19]. In short, the deterministic and dynamic transmission model is based on Finnish contact structure of sexual partners, and it produces age-specific prevalence of HPV by type under a given vaccination scenario. For the current work, the infection duration-dependent clearance rates were re-estimated (1) for HPV16 and HPV18 separately and (2) for two classes HPV31/33/45/52/58 (moderate clearance) and the remaining other oncogenic HPV types (fast clearance). The duration of vaccine-induced protection was assumed to remain for 20 years and to be lost at the rate of 0.05 1/year thereafter [23].
The vaccination-strategy-specific immunity threshold for each HPV type, ie, the effective coverage of vaccination needed to eradicate the type, was determined numerically by increasing the vaccination coverage of a theoretic 100% VE vaccine until the eradication occurred in the new steady state. The basic reproduction number (R0) was then calculated as the inverse of the immunity threshold. For girls-only vaccination, the R0 corresponds to from girls to girls (via boys) transmission, and for gender-neutral vaccination R0 is for transmission between both genders. The critical coverage of vaccination for VE of 95%, 80%, or 50% was then obtained by dividing the immunity threshold by VE.
To illustrate the potential eradication following different vaccination strategies, the relative reduction of type-specific prevalence among women under 25 was computed with time since the start of vaccination using different vaccination scenarios and coverages. In addition to a high 95% VE, lower 80% and 50% VEs were also used for moderate and fast clearance types, reflecting different levels of cross-protection.
RESULTS
We identified and excluded 4 Arm A and 1 Arm B outlier communities (Supplementary Figures 1–2). The resulting outlier-free coverage of HPV vaccination was 49% (47% with all communities) for girls and 21% (19%) for boys in Arm A and 47% (44%) for girls in Arm B. The elimination of outlier communities reduced the variation of community-specific vaccination coverage from 33%–60% to 45%–55% for girls and from 12%–29% to 13%–27% for boys in Arm A and from 34%–52% to 39%–52% for girls in Arm B. The HBV vaccination coverage in the Arm C was 53% and 31% for girls and boys, respectively.
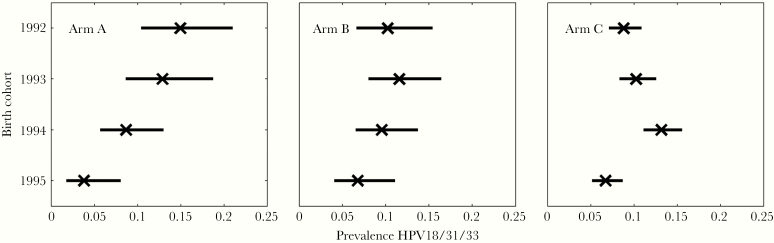
The HE in non-HPV-vaccinated 18.5-year-old females was estimated as a reduction of HPV prevalence for HPV-vaccine covered types 16/18/31/33/35/45 (Table 1). The combined HPV18/31/33 prevalence among these females had a clear decreasing trend over time in the gender-neutral vaccination Arm A only (Figure 2). Accordingly, the HPV18/31/33 HE estimate in the youngest 1995 birth cohort was significant only in Arm A (59%), and it was higher than in Arm B (24%) with high probability P(HEA > HEB) = 91.6% (Table 1). The current outlier-free HE estimates agreed substantially with the earlier estimates including all study communities [16] (Supplementary Figure 3). Type-specific HPV18, HPV31, and HPV33 HE estimates were all materially equal and approximately 150% higher in the gender-neutral arm than in the girls-only arm. The numbers for HPV45 alone were too low for reliable estimates. No HE against HPV16 was observed (Table 1).
Table 1.
Herd Effect (With 95% Confidence Interval) Against Genital Human Papillomavirus (HPV) Infection and Type-Specific HPV Prevalence (%) by HPV Vaccination Strategya in Non-HPV-Vaccinated 18.5-Year-Old Females by Trial Arm and Birth Cohort
| Arm A (Gender-Neutral) | Arm B (Girls-Only) | Arm C (Control) | ||||
|---|---|---|---|---|---|---|
| HPV Type | Birth Cohorts | Prevalence (95%CI) | HEc (95% CI) | Prevalence (95% CI) | HEc (95% CI) | Prevalencef (95% CI) |
| HPV16 | 1992–1995 | 7.5 (5.8–9.6) | 14.9 (−13.2 to 36.0) | 7.6 (6.0–9.6) | −1.3 (−29.4 to 20.7) | 8.3 (7.4–9.2) |
| 1995 | 9.5 (5.8–15.1) | −19.9 (−92.9 to 25.5) | 8.7 (5.6–13.3) | 19.2 (−81.8 to 21.9) | 8.1 (6.4–10.2) | |
| HPV18 | 1992–1995 | 5.0 (3.7–6.9) | −16.3 (−60.8 to 15.9) | 3.9 (2.8–5.4) | 15.1 (−19.8 to 39.8) | 5.0 (4.3–5.8) |
| 1995 | 1.9 (0.6–5.4) | 58.3 (−13.5 to 84.7) | 2.9 (1.3–6.2) | 27.0 (−45.8 to 63.5) | 2.7 (1.8–4.1) | |
| HPV31 | 1992–1995 | 3.5 (2.4–5.1) | 6.6 (−40.5 to 37.9) | 3.8 (2.7–5.2) | −5.9 (−51.8 to 26.2) | 3.8 (3.2–4.5) |
| 1995 | 0.6 (0.1–3.5) | 83.9 (21.2–96.7)e | 2.9 (1.3–6.2) | 34.9 (−49.0 to 71.5) | 3.4 (2.3–4.9) | |
| HPV33 | 1992–1995 | 2.9 (1.9–4.3) | −41.4 (−125 to 11.2) | 3.0 (2.0–4.3) | −86.2 (−176 to −25.5)e | 2.1 (1.7–2.7) |
| 1995 | 1.3 (0.3–4.5) | 64.9 (−57.5 to 92.2) | 1.4 (0.5–4.2) | 24.8 (−108 to 72.8) | 1.4 (0.8–2.5) | |
| HPV35 | 1992–1995 | 1.1 (0.6–2.1) | 46.1 (−17.6 to 75.3) | 1.1 (0.6–2.1) | 50.7 (−4.5 to 76.7) | 1.6 (1.3–2.1) |
| 1995 | 1.3 (0.3–4.5) | 3.4 (−203 to 69.2) | 1.4 (0.5–4.2) | 4.2 (−168 to 65.8) | 2.1 (1.3–3.3) | |
| HPV45 | 1992–1995 | 3.0 (2.0–4.5) | −36.2 (−110 to 11.8) | 2.2 (1.4–3.3) | −4.5 (−63.9 to 33.4) | 2.5 (2.0–3.1) |
| 1995 | 2.5 (1.0–6.3) | −14.5d (−178 to 52.7) | 1.0 (0.3–3.5) | 52.1d (−49.8 to 84.7) | 2.1 (1.3–3.4) | |
| HPV18/31/33 | 1992–1995 | 10.1 (8.1–12.5) | −39.6 (−71.9 to −13.4)e | 9.5 (7.8–11.7) | −6.4 (−32.4 to 14.4) | 9.8 (8.9–10.9) |
| 1995 | 3.8 (1.8–8.0)b | 59.1 (14.3–80.5)e | 6.8 (4.1–11.0) | 23.9 (−23.2 to 53.0) | 6.7 (5.2–8.7) |
Abbreviations: CI, confidence interval, HE, herd effect.
aArm A, gender-neutral (49% girls, 23% boys HPV-vaccinated); Arm B, girls-only (47% girls HPV-vaccinated); Arm C, hepatitis B virus (HBV) vaccination.
bSignificantly different.
cGeneralized estimating equation (GEE) estimates adjusted for Chlamydia trachomatis/mobility/smoking/vaccination coverage, balanced for equal proportions of HBV vaccinated and nonvaccinated. Herd effect (1-ratio of risks, GEE model, HBV-vaccinated/unvaccinated-ratio balanced between arms using mean GEE estimates from 21 balanced materials).
dNot adjusted for mobility.
eSignificant.
fThe prevalence in Arm C is mean of prevalence estimates from 21 balanced materials. For the estimation of pertinent 95% CI, a material with the mean prevalence and actual size of Arm C was used.
Figure 2.
Prevalence reduction of human papillomavirus (HPV) types 18/31/33 in unvaccinated 18-year-old females by birth cohorts (1992–1995) and vaccination strategy: (Arm A) gender-neutral (49% girls and 23% boys HPV vaccinated [P for trend .0005]); (Arm B) girls-only (47% girls vaccinated [P for trend .092]); (Arm C) control, hepatitis B virus vaccination (P for trend .447) at the age of 12–15 years in 2007–2010. The trend of reduction by birth cohorts in the gender-neutral Arm A was stronger than that of the girls-only Arm B (P = .0556) or control Arm C (P = .0015).
The overall PE of the vaccination strategy for all 18.5-year-old females (Table 2) was a weighted combination of the strategy-specific HE among non-HPV-vaccinated girls (Table 1) and the VE among HPV-vaccinated girls [16] (Supplementary Table 1). The HPV16 PE estimates (36% and 33%, respectively) were significant for the 1995 birth cohort both in the gender-neutral and girls-only arms. In the gender-neutral Arm A, the combined HPV18/31/33 PE estimate (66%) for the 1995 birth cohort was 40% higher than the corresponding girls-only Arm B estimate (47%): P(PEA > PEB) = 92.2%.
Table 2.
Overall Protective Effectiveness (With 95% Confidence Interval) Against Genital Human Papillomavirus (HPV) Infection and Type-Specific HPV Prevalence (%) by HPV Vaccination Strategya and Birth Cohort
| Arm A (Gender-Neutral) | Arm B (Girls-Only) | Arm C (Control) | ||||
|---|---|---|---|---|---|---|
| Birth Cohorts | Prevalence (95%CI) | PEb (95% CI) | Prevalence (95% CI) | PEb (95% CI) | Prevalence (95% CI) | |
| HPV Type | ||||||
| HPV16 | 1992–1995 | 4.1 (3.2–5.2) | 53.3 (40.1–64.9)c | 4.3 (3.5–5.4) | 42.0 (27.9–54.4)c | 8.1 (7.3–9.1) |
| 1995 | 5.0 (3.2–8.0) | 35.5 (2.5–61.1)c | 5.0 (3.3–7.5) | 32.4 (2.6–56.6)c | 7.7 (6.1–9.8) | |
| HPV18 | 1992–1995 | 2.6 (1.9–3.6) | 38.5 (17.6–56.1)c | 2.2 (1.6–3.0) | 51.5 (34.4–65.9)c | 5.0 (4.3–5.8) |
| 1995 | 1.1 (0.4–2.9) | 76.5 (47.6–92.6)c | 1.6 (0.7–3.4) | 57.9 (25.1–80.4)c | 2.7 (1.8–4.1) | |
| HPV31 | 1992–1995 | 2.2 (1.6–3.0) | 40.5 (17.9–58.6)c | 2.4 (1.8–3.2) | 32.0 (8.9–51.1)c | 3.8 (3.2–4.5) |
| 1995 | 0.7 (0.3–1.8) | 79.9 (55.6–90.6)c | 1.9 (1.0–3.7) | 53.8 (16.6–77.4)c | 3.4 (2.3–4.9) | |
| HPV33 | 1992–1995 | 2.2 (1.7–3.0) | −4.0 (−45.1 to 28.4) | 2.3 (1.7–3.0) | −25.9 (−70.9 to 10.9) | 2.1 (1.7–2.7) |
| 1995 | 1.0 (0.4–2.5) | 50.1 (0.9–73.9)c | 1.1 (0.5–2.3) | 33.4 (−24.4 to 66.4) | 1.4 (0.8–2.5) | |
| HPV35 | 1992–1995 | 0.9 (0.5–1.3) | 45.2 (11.1–68.6)c | 1.1 (0.8–1.6) | 32.3 (−1.9 to 56.9) | 1.6 (1.3–2.1) |
| 1995 | 1.0 (0.4–2.6) | 23.8 (−61.4 to 68.1) | 1.1 (0.5–2.3) | 7.5 (−69.9 to 52.6) | 2.1 (1.3–3.3) | |
| HPV45 | 1992–1995 | 1.7 (1.2–2.5) | 19.8 (−15.1 to 47.4) | 1.4 (0.9–2.0) | 35.1 (5.9–57.9)c | 2.5 (2.0–3.1) |
| 1995 | 1.4 (0.6–3.3) | 37.7 (−35.9 to 78.1) | 0.7 (0.2–1.8) | 67.6 (20.4–90.4)c | 2.1 (1.3–3.4) | |
| HPV18/31/33 | 1992–1995 | 6.4 (5.3–7.6) | 15.3 (−0.6 to 29.9) | 6.2 (5.2–7.3) | 30.6 (17.4–42.9)c | 10.9 (9.9–12.0) |
| 1995 | 2.8 (1.6–4.8) | 65.6 (46.1–78.9)c | 4.3 (2.8–6.6) | 46.8 (24.4–64.4)c | 8.4 (6.6–10.5) |
Abbreviations: CI, confidence interval; PE, protective effectiveness.
aArm A, gender-neutral (49% girls, 23% boys HPV-vaccinated); Arm B, girls-only (47% girls HPV-vaccinated); Arm C, hepatitis B-virus vaccination.
bPE = overall protective effectiveness, a combination of vaccine efficacy and herd effects weighted by the proportions of HPV16/18-vaccinated and non-HPV16/18-vaccinated women, respectively.
cSignificant.
The dynamics of HEs established in our heterosexual transmission model (Figure 1) agreed with the CRT findings (Table 1). When only girls are vaccinated, the strongest (first order) HE is the protection exerted from vaccinated girls onto unvaccinated boys (Figure 1, top panel). The protection of unvaccinated boys is subsequently reflected in unvaccinated girls but gets weaker at each reflection (second order HE, third order HE, etc). Generating the first order HE directly onto the unvaccinated girls by vaccinating 23% of the boys in our CRT more than doubled the HPV18/31/33 prevalence reduction among unvaccinated girls in 4 years (Table 1). The model predicted strengthening HEs toward the younger trial birth cohorts (Figure 1) were observed in our trial as the strongest HEs, and prevalence reductions were found among the 1995 birth cohort with the gender-neutral vaccination strategy (Table 1, Figure 2). It is remarkable that when the cross-protective vaccine efficacies (HPV31/33 VE) were not optimal, the profit from gender-neutral vaccination strategy also in the HPV-vaccinated individuals due to the increased herd protection become visible as significant PE estimates in the youngest 1995 birth cohort (Figure 1, right panel; Table 2).
The model-predicted immunity thresholds for specific HPV types, and the corresponding VE-specific critical coverages of vaccination, varied remarkably according to type-specific HPV clearance rates (Table 3). To eradicate HPV 16 with a vaccine with a high 95% VE, even 95% girls-only coverage is required, whereas for the gender-neutral strategy 74% coverage is sufficient. For HPV18 and other, faster clearing HPV types, the critical coverage with the 95% VE vaccine were lower, accordingly, with the gender-neutral strategy still remaining beneficial. On the other hand, even with lower VE (80%/50%), the gender-neutral vaccination still provides achievable critical coverages for moderate and fast clearance HPV types (Table 3).
Table 3.
Model-Based Reproduction Numbers, Immunity Thresholds for Eradication of Vaccine-Covered Oncogenic Human Papillomaviruses (HPVs), and Corresponding Critical Coverage of Vaccination by Vaccine Efficacy for Gender-Neutral (Girls and Boys) and Girls-Only (Girls) Vaccination Strategies
| Reproduction Numbera | Immunity Threshold | Critical Coverage of Vaccination | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| VE 95% | VE 80% | VE 50% | ||||||||
| HPV Type | Girls and Boys | Girls | Girls and Boys | Girls | Girls and Boys | Girls | Girls and Boys | Girls | Girls and Boys | Girls |
| HPV16 | 3.3 | 10 | 70% | 90% | 74% | 95% | 88% | NEb | NEb | NEb |
| HPV18 | 2.2 | 4.5 | 55% | 78% | 58% | 82% | 69% | 98% | NEb | NEb |
| HPV31/33 | 1.7 | 2.9 | 40% | 65% | 42% | 68% | 50% | 81% | 80% | NEb |
| HPV45 | 1.7 | 2.9 | 40% | 65% | 42% | 68% | 50% | 81% | 80% | NEb |
| HPV35 | 1.3 | 1.5 | 20% | 35% | 21% | 37% | 25% | 44% | 40% | 70% |
Abbreviations: NE, no eradication; VE, vaccine efficacy.
aReproduction number (R0) is calculated by determining the immunity threshold using the transmission model with a computational vaccine with 100% efficacy. In the gender-neutral vaccination strategy (girls and boys), R0 is from girls/boys to boys/girls. In the girls-only vaccination strategy (girls) R0 is from girls to girls (via boys, heterosexual model).
bNE = no eradication even with a 100% coverage of vaccination.
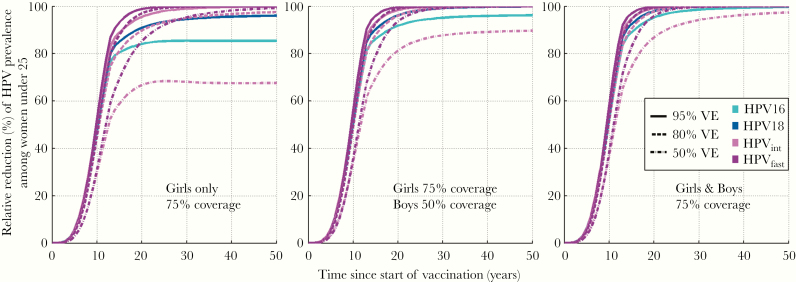
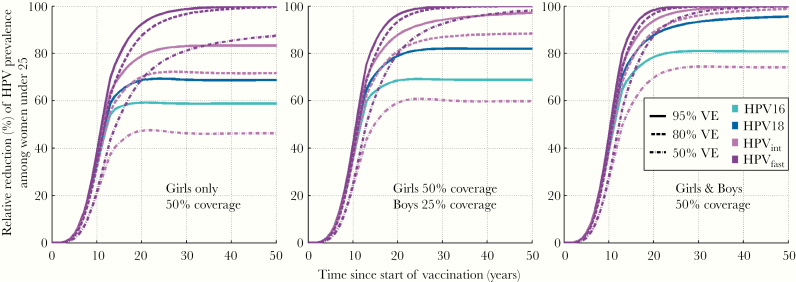
With 75% gender-neutral vaccination coverage, just above the critical coverage, our model predicted the HPV16 eradication to take place among young adults in 30 years and other HPV types being eradicated in approximately 20 years (Figure 3). The 75% girls-only vaccination, remaining below the corresponding critical coverage, did not eradicate HPV16 or HPV18. Modeled gender-neutral vaccination with 25% boys and 50% girls coverage eventually ends up to approximately 70% relative reduction for HPV16 prevalence, clearly better than that of the 50% girls-only coverage (Figure 4). Compared with HPV16, the HEs are much higher for HPV18 and moderate clearance types, even with a suboptimal VE (Figures 3–4).
Figure 3.
Modeled eradication of human papillomavirus (HPV) types 16 [■], 18 [■], and oncogenic HPV types with moderate (31 of 33) [■] or fast (35) [■] clearance rates by vaccine efficacy ([VE] 95%/80%/50%), with 75% girls-only vaccination coverage, with 50% boys and 75% girls vaccination coverage, and with 75% gender-neutral vaccination coverage.
Figure 4.
Modeled eradication of human papillomavirus (HPV) types 16 [■], 18 [■], and oncogenic HPV types with moderate (31 of 33) [■] or fast (35) [■] clearance rates by vaccine efficacy ([VE] 95%/80%/50%), with 50% girls-only vaccination coverage, with 25% boys and 50% girls vaccination coverage, and with 50% gender-neutral vaccination coverage.
DISCUSSION
Based on unique CRT data and associated modeling, we show in an originally HPV-vaccination-free population that vaccination with moderate coverage will rapidly eradicate hrHPVs from young adults, except HPV16, if a gender-neutral strategy is applied.
At the outset, the moderate to fast clearance rates characteristic of HPV18/31/33 infection made them permissive to gender-neutral vaccination-derived herd protection and prevalence reduction even with low to moderate vaccination coverage. The HEs from vaccinated boys and girls to unvaccinated girls and boys rose rapidly when low-to-moderate coverage, gender-neutral vaccination was applied. Successive modeling revealed that eradication of HPV18/31/33 and eventually HPV16 from the young adult population can occur respectively within 20 and 30 years with a feasible 75% vaccination coverage when applying existing cross-reactive or multitype hrHPV vaccines and gender-neutral vaccination strategy.
The variable vaccination coverage was accounted for with the a priori designed outlier-free analysis. Arm A had a higher number of outlier communities, reflecting uneven distribution of vaccination coverage between the study arms. However, differences between current outlier-free estimates and earlier estimates [15, 16] were small, which warrant that our earlier observations about HEs were not materially biased by the variable coverage of vaccination.
Due to the assortative sexual behavior, the HE induced by gender-neutral HPV vaccination limits circulating oncogenic genital HPV types in the general population [24]. Eradication of an infectious agent from a population by means of vaccination is related to the agent’s basic R0, which depends on its clearance rate and pertinent contact structure of the population [25]. The R0 determines immunity thresholds for the eradication of a given pathogen from a population. After country-wise eradication, imported infections due to contacts to other societies are possible, but they are unlikely to cause major outbreaks. The combined evidence from our CRT and mathematical modeling clearly indicates that for the genital hrHPV types, the immunity threshold, ie, the critical vaccination coverage for the eradication of hrHPV types, are within reach when exploiting gender-neutral vaccination derived HEs.
HPV16 is notorious for its ability to persist, is widely circulated, and has a uniquely high R0 [26, 27]. This explains why gender-neutral vaccination with approximately 25% male and 50% female vaccination coverage failed to induce HE against HPV16 observable with transient PCR positivity. However, in a recent work [28], we report on observed HPV16 HE from the low to moderate coverage gender-neutral vaccination based on HPV16 pseudovirion-serology, a measure of persistent infection rather than all (transient and persistent) HPV infections by PCR. The transmission model predicted even 90+% critical coverage of vaccination for HPV16 elimination with the girls-only strategy. In addition, in real-life, the reduction of HPV16 PCR prevalence among nonvaccinated females with girls-only vaccination has required exceptionally high (90%) coverage [7]. In contrast, with a feasible 75% coverage of the gender-neutral vaccination strategy, which is just above HPV16 critical coverage of vaccination, HPV16 eradication is predicted in 30 years among young adults.
The critical girls-only vaccination coverage for HPV18 was 82% according to our model, but HPV18 eradication with this strategy would take several decades. On the contrary, with vaccination coverage of 75% among girls and 50%–75% among boys, HPV18 is already predicted to be eradicated in 20–25 years. For HPV types with lower R0 such as HPV31, HPV33 or HPV45, the critical vaccination coverage would naturally be lower for a high efficacy vaccine. Even when cross-protective vaccine efficacies are suboptimal, the gender-neutral vaccination with 75% coverage is predicted to result in the eradication of HPV31, HPV33, or HPV45 also in 20 years. It is very important to note that these model predictions for the HEs under vaccination with moderate coverage were in line with our CRT results.
The main strength of this study is its unique trial setting of applying vaccination strategy in a vaccination-naive population, with a large number of study communities, covering practically the entire country. Taking into account that the study was a population-based randomized trial, the achieved vaccination coverage of 47% to 49% among females was at least moderate. The achieved coverage of 23% among boys was not quite satisfactory, and it resulted in only partial herd protection, which, however, revealed differences between HPV types. Another strength is combination of CRT with modeling. The models suggested that we consider the youngest birth cohorts separately when analyzing the trial outcomes for HEs. These proved to be in accordance with the model predictions. Naturally, different model parameters (eg, sexual behavior) would potentially imply differences in the predicted timelines. Analyses of model variation are left out from the current work, but they can be found for eventual eradication, including our model described in [11].
To achieve high HPV vaccination coverage, the proportion of control-vaccinated individuals among study participants in intervention arms was allocated to 10% only, which was going to lead to high statistical uncertainty in analyzing HEs. To increase the number of non-HPV-vaccinated participants, initial nonparticipants were also invited to the follow-up visits. As a result, statistically significant HE estimates were observed for groups of HPV types.
Current girls-only vaccination programs vary by country and their coverage is often low. However, even moderate (50%–75%) girls-only vaccination coverage would not result in HPV eradication, and later HPV-disease elimination. Imminent suboptimal girls-only vaccination coverage and HE threaten to leave marginalized females unjustly unprotected [29, 30]. Moreover, compared to gender-neutral vaccination, girls-only vaccination may fail to provide a resilient HE able to withstand temporary changes in the vaccination coverage [31].
CONCLUSIONS
In conclusion, the ultimate gains of gender-neutral prophylactic HPV vaccination are realistic. Country-wise eradication of oncogenic HPVs and HPV-disease elimination are within reach already in the foreseeable future by applying the gender-neutral HPV vaccination strategy, even with only moderate coverage.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Presented in part: EUROGIN, 15–18 June 2016, Salzburg, Austria; EUROGIN, 8–11 October 2017, Amsterdam, the Netherlands; EUROGIN, 2–5 December 2018, Lisbon, Portugal; IPV Conference, 28 February – 2 March 2017, Cape Town, South Africa.
Acknowledgments. We thank the steering committee of the HPV-040 trial—Allan Donner, Eduardo Franco, Pauli Leinikki, Achim Schneider, and Margaret Stanley—for scientific advice and support. We thank Dr. Saara Kares (Fimlab) for providing extracted deoxyribonucleic acids (DNAs) for human papillomavirus (HPV) DNA analyses.
Author contributions. M. L., G. D., and G. G. designed the study. M. L., G. G., I. B., E. P., T. L., V. N. P., and S. V. participated in planning the statistical analysis. T. L. and S. V. performed the statistical analyses. S. V. performed the mathematical modeling. D. A., P. N., and J. P. were clinical study doctors. T. E. and K. N. coordinated the study. A. S.-S. and J. D. were responsible for the laboratory analyses. M. L. and S. V. wrote the first draft of the manuscript. All of the coauthors contributed to the writing of important intellectual content by revisions and comments.
Disclaimer. Where authors are identified as personnel of the International Agency for Research on Cancer/World Health Organization, the authors alone are responsible for the views expressed in this article and they do not necessarily represent the decisions, policy or views of the International Agency for Research on Cancer/World Health Organization. GlaxoSmithKline Biologicals SA was provided the opportunity to review this manuscript for accuracy of primary HPV-040 data, but the authors are solely responsible for final content and interpretation.
Financial support. This ancillary study was partially funded by Academy of Finland, Finnish Cancer Organizations, and EU FP7 networks PREHDICT (242061) and CoheaHR (F3-2013-603019). GlaxoSmithKline Biologicals SA funded the primary study HPV-040 (NCT00534638; data to be published in a separate manuscript) but was not involved in the conduct of this ancillary study. V. N. P. was supported by a personal grant from the Ministry of Health, Government of Catalonia (PERIS SLT002/16/00496).
Potential conflicts of interest. D. A., J. D., G. G., M. L., and J. P. have received grants from Merck & Co. Inc. and/or the GSK group of companies through their employers (Family Federation Finland [D. A.], Karolinska Institute [J. D., M. L.], Universities of Tampere [M. L.] or Helsinki [J. P.], or Imperial College London [G. G.]) for HPV vaccination studies. G. D. is currently a full-time employee of Takeda Vaccines but was working for GSK Biologicals until 2015. He holds patents in the HPV field that have been assigned to the GSK groups of companies and has stock shares in both GSK and Takeda. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. European Medicines Agency. Human medicine European public assessment report (EPAR): Gardasil. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/gardasil. Accessed 14 October 2019. [Google Scholar]
- 2. European Medicines Agency. Human medicine European public assessment report (EPAR): Cervarix. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/cervarix. Accessed 14 October 2019. [Google Scholar]
- 3. Luostarinen T, Apter D, Dillner J, et al. Vaccination protects against invasive HPV-associated cancer. Int J Cancer 2017; 42:2186–7. [DOI] [PubMed] [Google Scholar]
- 4. Elfström KM, Dillner J, Arnheim-Dahlström L. Organization and quality of HPV vaccination programs in Europe. Vaccine 2015; 33:1673–81. [DOI] [PubMed] [Google Scholar]
- 5. Williams WW, Lu PJ, O’Halloran A, et al. Surveillance of vaccination coverage among adult populations - United States, 2015. MMWR Surveill Summ 2017; 66:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Skufca J, Ollgren J, Artama M, Ruokokoski E, Nohynek H, Palmu AA. The association of adverse events with bivalent human papilloma virus vaccination: a nationwide register-based cohort study in Finland. Vaccine 2018; 36:5926–33. [DOI] [PubMed] [Google Scholar]
- 7. Kavanagh K, Pollock KG, Cuschieri K, et al. Changes in the prevalence of human papillomavirus following a national bivalent human papillomavirus vaccination programme in Scotland: a 7-year cross-sectional study. Lancet Infect Dis 2017; 17:1293–302. [DOI] [PubMed] [Google Scholar]
- 8. Sinka K, Kavanagh K, Gordon R, et al. HPV vaccine in Scotland. J Epidemiol Commun Health 2014; 68:57–63. [DOI] [PubMed] [Google Scholar]
- 9. Brotherton JM, Fridman M, May CL, Chappell G, Saville AM, Gertig DM. Early effect of the HPV vaccination programme on cervical abnormalities in Victoria, Australia: an ecological study. Lancet 2011; 377:2085–92. [DOI] [PubMed] [Google Scholar]
- 10. Howell-Jones R, Soldan K, Wetten S, et al. Declining genital warts in young women in england associated with HPV 16/18 vaccination: an ecological study. J Infect Dis 2013; 208:1397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brisson M, Bénard É, Drolet M, et al. Population-level impact, herd immunity, and elimination after human papillomavirus vaccination: a systematic review and meta-analysis of predictions from transmission-dynamic models. Lancet Public Health 2016; 1:e8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Joura EA, Giuliano AR, Iversen OE, et al. ; Broad Spectrum HPV Vaccine Study A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med 2015; 372:711–23. [DOI] [PubMed] [Google Scholar]
- 13. Draper E, Bissett SL, Howell-Jones R, et al. A randomized, observer-blinded immunogenicity trial of Cervarix(®) and Gardasil(®) human papillomavirus vaccines in 12-15 year old girls. PLoS One 2013; 8:e61825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Faust H, Toft L, Sehr P, et al. Human papillomavirus neutralizing and cross-reactive antibodies induced in HIV-positive subjects after vaccination with quadrivalent and bivalent HPV vaccines. Vaccine 2016; 34:1559–65. [DOI] [PubMed] [Google Scholar]
- 15. Lehtinen M, Söderlund-Strand A, Vänskä S, et al. Impact of gender-neutral or girls-only vaccination against human papillomavirus-results of a community-randomized clinical trial (I). Int J Cancer 2018; 142:949–58. [DOI] [PubMed] [Google Scholar]
- 16. Lehtinen M, Luostarinen T, Vänskä S, et al. Gender-neutral vaccination provides improved control of human papillomavirus types 18/31/33/35 through herd immunity: results of a community randomized trial (III). Int J Cancer 2018; 143:2299–310. [DOI] [PubMed] [Google Scholar]
- 17. Gallagher KE, Howard N, Kabakama S, et al. Human papillomavirus (HPV) vaccine coverage achievements in low and middle-income countries 2007–2016. Papillomavirus Res 2017; 4:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lehtinen M, Apter D, Baussano I, et al. Characteristics of a cluster-randomized phase IV human papillomavirus vaccination effectiveness trial. Vaccine 2015; 33:1284–90. [DOI] [PubMed] [Google Scholar]
- 19. Vänskä S, Auranen K, Leino T, et al. Impact of vaccination on 14 high-risk HPV type infections: a mathematical modelling approach. PLoS One 2013; 8:e72088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donner A, Klar N. Confidence interval construction for effect measures arising from cluster randomization trials. J Clin Epidemiol 1993; 46:123–31. [DOI] [PubMed] [Google Scholar]
- 21. Donner A. Some aspects of the design and analysis of cluster randomized trials. Appl Stat 1998; 47:95–113. [Google Scholar]
- 22. Baussano I, Elfström KM, Lazzarato F, et al. Type-specific human papillomavirus biological features: validated model-based estimates. PLoS One 2013; 8:e81171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Artemchuk H, Eriksson T, Poljak M, et al. Long-term antibody response to human papillomavirus vaccines: up to 12 years of follow-up in the Finnish maternity cohort. J Infect Dis 2019; 219:582–9. [DOI] [PubMed] [Google Scholar]
- 24. Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis 2005; 191(Suppl 1):S97–106. [DOI] [PubMed] [Google Scholar]
- 25. Brouwer AF, Meza R, Eisenberg MC. Transmission heterogeneity and autoinoculation in a multisite infection model of HPV. Math Biosci 2015; 270:115–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lehtinen M, Dillner J. Clinical HPV vaccine trials and beyond. Nature Rev Clin Oncol 2013; 109:400–10. [DOI] [PubMed] [Google Scholar]
- 27. Baussano I, Lazzarato F, Ronco G, et al. How human papillomavirus 16 becomes harder to eliminate than other types: a modelling study. J Infect Dis 2017; 216:366–74.28666374 [Google Scholar]
- 28. Gray P, Kann H, Pimenoff VN, et al. Population-based HPV serosurvey among unvaccinated females reveals HPV16 herd effect postgender-neutral vaccination with moderate vaccination coverage: Follow-up of a community randomised trial. EUROGIN, 4 December 2019, Monaco. [Google Scholar]
- 29. Malmqvist E, Helgesson G, Lehtinen J, Natunen K, Lehtinen M. The ethics of implementing human papillomavirus vaccination in developed countries. Med Health Care Philos 2011; 14:19–27. [DOI] [PubMed] [Google Scholar]
- 30. Hall MT, Simms KT, Lew JB, et al. The projected timeframe until cervical cancer elimination in Australia: a modelling study. Lancet Public Health 2019; 4:e19–27. [DOI] [PubMed] [Google Scholar]
- 31. Elfström KM, Lazzarato F, Franceschi S, Dillner J, Baussano I. Human papillomavirus vaccination of boys and extended catch-up vaccination: effects on the resilience of programs. J Infect Dis 2016; 213:199–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.