Abstract
Background
In human blood, mucosal-associated invariant T (MAIT) cells are abundant T cells that recognize antigens presented on non-polymorphic major histocompatibility complex-related 1 (MR1) molecules. The MAIT cells are activated by mycobacteria, and prior human studies indicate that blood frequencies of MAIT cells, defined by cell surface markers, decline during tuberculosis (TB) disease, consistent with redistribution to the lungs.
Methods
We tested whether frequencies of blood MAIT cells were altered in patients with TB disease relative to healthy Mycobacterium tuberculosis-exposed controls from Peru and South Africa. We quantified their frequencies using MR1 tetramers loaded with 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil.
Results
Unlike findings from prior studies, frequencies of blood MAIT cells were similar among patients with TB disease and latent and uninfected controls. In both cohorts, frequencies of MAIT cells defined by MR1-tetramer staining and coexpression of CD161 and the T-cell receptor alpha variable gene TRAV1-2 were strongly correlated. Disease severity captured by body mass index or TB disease transcriptional signatures did not correlate with MAIT cell frequencies in patients with TB.
Conclusions
Major histocompatibility complex (MHC)-related 1-restrictied MAIT cells are detected at similar levels with tetramers or surface markers. Unlike MHC-restricted T cells, blood frequencies of MAIT cells are poor correlates of TB disease but may play a role in pathophysiology.
Keywords: household contacts, MAIT, MR1, tetramer, tuberculosis
Mycobacterium tuberculosis (Mtb) is the leading cause of death from infectious disease globally, with one quarter of the world’s population estimated to be infected with Mtb [1]. The commonly used interferon-γ release assay (IGRA) measures major histocompatibility complex (MHC)-restricted αβ T-cell responses to Mtb antigens as a reliable diagnostic test for infection [2]. Thus, it is broadly accepted that expansion of antigen-specific, MHC-restricted T cells in blood is the usual human response to Mtb infection.
Recent studies showed that non-MHC encoded, antigen-presenting molecules can present mycobacterial antigens to activate αβ T-cell responses in experimental Mtb infections [3, 4]. Prominent among these T-cell types are mucosal-associated invariant T (MAIT) cells, which recognize MHC-related 1 (MR1) and are particularly abundant, comprising ~0.1% to 10% of circulating T cells in healthy individuals [5]. Mucosal-associated invariant T-cell antigens include riboflavin derivatives and other metabolites [6–8]. Unlike highly polymorphic MHC genes, MR1 is almost monomorphic in humans [4]. Thus, MAIT cells can recognize antigens presented by antigen-presenting cells from any human, and they are hence known as donor-unrestricted T cells [4].
The abundance of MAIT cells and reactivity towards mycobacterial antigens [9] raises the possibility that MAIT cells could play a role in controlling natural Mtb infection. Unlike conventional T cells, MAIT cell frequencies were reported to decline in the blood of tuberculosis (TB) patients, relative to Mtb-unexposed controls [10–14], or after Mtb infection of animals [13, 15]. This outcome is consistent with their suspected relocation to the lungs or other sites of infection in vivo. Consistent with this prediction, a recent study reported that MAIT cell frequencies were enriched in the bronchoalveolar lavage of TB patients [16]. However, these studies are small and detect MAIT cells by activation through MR1 [14] or expression of the TRAV1-2 variable region of the T-cell receptor alpha (TCRα), which is frequently rearranged with the TCRα joining region TRAJ33 in MAIT cells [17, 18]. However, there are examples of TRAV1-2– T cells that recognize MR1 [19, 20, 21]. In contrast, TRAV1-2+ TCRs can recognize antigens presented by MHC or CD1b proteins [3]. Thus, TCR sequence-independent methods to unequivocally identify MR1-binding T cells are important. Major histocompatibility complex-related 1 tetramers loaded with the vitamin B-like metabolite 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU) directly identify MAIT cells based on binding specificity to MR1 and the antigenic ligand [5, 6, 18, 21].
A third definition of MAIT cells relies on expression of cell surface markers. Mucosal-associated invariant T cells are predominantly CD8+ or CD4–CD8– T cells, with a small CD4+ fraction [5, 22], and coexpress the C-type lectin CD161 [23] and CD26 ectopeptidase [5, 24, 25]. Thus, clinical studies have tracked cells coexpressing CD3 and CD161 [11, 10] or CD26 [14], usually in combination with TRAV1-2 [5]. These “phenotypic” MAIT cells emerged mainly from human studies in which functional responses to MR1-ligand complex were not feasible, or before development of MR1-tetramers.
The emergence of parallel TCR-, tetramer-, and phenotype-based criteria raises basic questions about the best MAIT cell definition and the concordance of these measurements in humans. Major histocompatibility complex-related 1 tetramers are now well validated to identify and characterize subpopulations of MAIT cells [5] or characterize MAIT cell functions in vitro [19]. However, MR1 tetramers have not been applied to large cohorts of TB patients. Because prior studies of MAIT cells in TB patients and controls relied on expression of TRAV1-2 and CD161 [10, 11, 12], we undertook a study of patients with TB disease and healthy Mtb-exposed participants from 2 distinct populations to measure peripheral blood MAIT cells using either TRAV1-2 and CD161 coexpression or 5-OP-RU-loaded MR1 tetramers to test whether blood MAIT cell frequencies were altered in TB disease.
MATERIALS AND METHODS
Participants were enrolled in 2 cross-sectional studies in Peru and South Africa. Adults and parents or legal guardians of minors provided informed consent, whereas minors provided assent.
Peruvian Household Contacts Cohort
Bacillus Calmette-Guérin (BCG)-vaccinated human immunodeficiency virus (HIV)-uninfected participants were recruited through Socios En Salud, around Lima, Peru [26]. Participants included adults with recently diagnosed sputum culture-positive, drug-sensitive pulmonary TB disease (TB disease, n = 50) and asymptomatic household contacts assessed within 2 weeks of diagnosing the index case (n = 100). Contacts were excluded if clinical symptoms of TB disease were present. Healthy household contacts were assessed for Mtb infection using the QuantiFERON TB-Gold In-Tube assay (QIAGEN) and considered latently Mtb-infected if their interferon (IFN)γ levels were ≥0.35 international units (IU)/mL (n = 50) and uninfected if <0.35 IU/mL (n = 50). Peripheral blood mononuclear cells (PBMCs) were isolated from 50 mL venous blood using ficoll, then cryopreserved and shipped to Boston for storage and analysis. The Institutional Review Board of the Harvard Faculty of Medicine and Partners Healthcare (protocol number IRB16-1173) and the Institutional Committee of Ethics in Research of the Peruvian Institutes of Health approved this study protocol.
South African Cohort
We recruited HIV-negative adults who received BCG vaccination at birth from communities in the Worcester town near Cape Town, South Africa into the previously described Cross-sectional TB Cohort (CTBC) [27]. Individuals with newly diagnosed sputum Xpert MTB/RIF-positive TB disease (TB disease, n = 19) and asymptomatic, QuantiFERON TB-Gold In-Tube-positive latently Mtb-infected (latent, n = 19) adults were enrolled. We did not recruit any uninfected participants because high TB prevalence rates limit recruitment of reliably uninfected participants. Peripheral blood mononuclear cells samples were processed from blood collected in Vacutainer CPT tubes (BD) and cryopreserved for flow cytometry analysis in Cape Town. The CTBC study protocol was approved by the University of Cape Town Human Research Ethics Committee (HREC 761/2015).
Flow Cytometry Analysis
For Peruvian samples, MR1 monomers loaded with 5-OP-RU, or 6-formylpterin (6-FP) as a negative control, were produced at The University of Melbourne as described previously [6, 18]. To generate MR1 tetramers, 1 µg of MR1 protein was tetramerized using 6 aliquots of 1 µL Streptavidin-PerCP-Cy5.5 (BioLegend) diluted 1:4 in phosphate-buffered saline (PBS). Cryopreserved samples from Peru were thawed at 37°C, and 3 × 106 cells were stained with fixable blue viability stain (Thermo Fisher Scientific), followed by MR1 tetramers in staining media (5% bovine serum albumin and 0.01% sodium azide in PBS) for 10 minutes at room temperature in the dark, followed by cell surface antibodies (Supplementary Table 1A) for 5 minutes. Subsequently, cells were treated with unconjugated OKT-3 antibody and incubated for 5 minutes at room temperature then 10 minutes at 4°C. Cells were fixed in 2% paraformaldehyde in PBS for 20 minutes. For South African samples, MR1 tetramers were from the National Institutes of Health Tetramer Core Facility and used to stain PBMC samples at a 1:200 dilution in 50 μL at room temperature for 45 minutes, followed by antibodies at 4°C for 30 minutes (Supplementary Table 1B).
Ribonucleic Acid Processing and Tuberculosis Risk Score Analysis
Peruvian ribonucleic (RNA) samples were extracted from 106 PBMCs using RNeasy kit (QIAGEN), and aliquots were shipped to the University of Cape Town. RNA samples from South Africa were extracted from 2.5 mL of blood was drawn into PAXgene blood RNA tubes and frozen. Ribonucleic acid was extracted using the PreAnalytiX PAXgene Blood RNA extraction kit (QIAGEN). All RNA samples were reverse transcribed to complementary deoxyribonucleic acid using EpiScript RNase H-Reverse Transcriptase (Lucigen), preamplified with a master mix of TaqMan primer probes (Supplementary Table 2) in 2× PCR Master Mix (Thermo Fisher Scientific), and analyzed by microfluidic real-time polymerase chain reaction (PCR) using the Biomark 192.24 gene expression integrated fluidic circuit (IFC) system (Fluidigm) as previously described [28].
Data Analysis
Flow cytometry data were analyzed in FlowJo v10.4.2. Transcriptomic signature scores from quantitative reverse-transcription PCR cycle threshold values and generalized linear regression models were analyzed in R v3.5.1-3.6 for Mac. Other analyses were performed in GraphPad Prism versions 7 and 8.
RESULTS
From 145 Peruvian enrollees, 135 PBMC samples passed quality control for yield, viability, and sterility: 48 patients with TB disease, 48 asymptomatic uninfected, and 49 latently Mtb-infected (latent) adults (Table 1A and Supplementary Table 3A). Participants with latent TB were older than either uninfected participants (Mann-Whitney, P = .022) or patients with TB disease (Mann-Whitney, P = .028). As expected from higher TB disease prevalence in adult males [1], the proportion of males in TB disease patients was higher than in other groups (Table 1A).
Table 1.
Summary of Demographic Characteristics of Participants in the Peruvian Cohort of Index TB Cases and Household Contacts and South African CTBC Cohort
| Peruvian Cohort | Variable | Uninfected (n = 48) | Latent (n = 49) | TB Disease (n = 48) | P Value |
|---|---|---|---|---|---|
| Median age, years (interquartile range) | 28.5 (21.5–41) | 38 (25–52) | 28.5 (19–40.8) | Kruskal-Wallis .035 | |
| Gender, n (%) Male | 23 (47.9) | 19 (38.8) | 31 (64.6) | χ 2 .036 | |
| South African Cohort | Variable | Latent (n = 19) | TB Disease (n = 19) | P Value | |
| Median age, years (interquartile range) | 41 (37–46) | 32 (27–38) | Mann-Whitney .025 | ||
| Gender, n (%) Male | 6 (31.6) | 13 (68.4) | χ 2 .023 |
Abbreviations: CTBC, Cross-sectional TB Cohort; TB, tuberculosis.
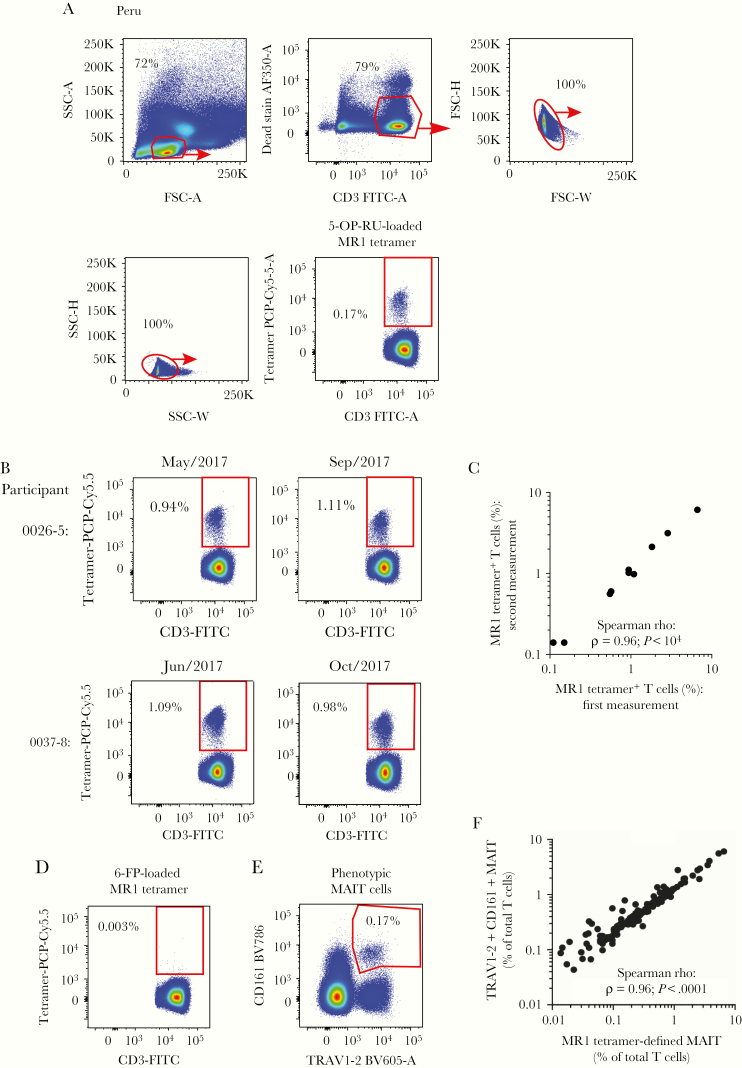
Tetramer-based MAIT cell detection requires a multistep process in which 5-OP-RU-loaded MR1 monomers are assembled with labeled streptavidin for staining and flow cytometry. To our knowledge, large TB disease-focused human studies with MR1-5-OP-RU tetramers had not been carried out previously, so we designed quality controls to assess tetramer-staining reproducibility. After pregating to exclude dead lymphocytes and doublets (Figure 1A), CD3+ tetramer+ cells clearly separated from CD3+ tetramer– cells (Figure 1A). Tetramer-based MAIT cell frequencies were defined as the proportion of CD3+ tetramer+ cells among all CD3+ lymphocytes. From 135 analyzable Peruvian samples, median MAIT cell frequency was 0.43% (interquartile range, 0.19%–0.9%) (Supplementary Table 3A). To assess tetramer staining reproducibility, we repeated analyses of the same PBMC samples from 10 participants using different tetramer batches assembled on different days measured an average of 117 days apart (Supplementary Table 3A). Absolute frequencies of MAIT cells were highly reproducible (Spearman rho = 0.96) ( Figure 1B and C). As a negative control, we stained the samples with MR1 tetramers loaded with the inhibitory MR1 ligand 6-FP [29], which showed very low false-positive staining (Figure 1D).
Figure 1.
Frequencies of mucosal-associated invariant T (MAIT) cells defined by major histocompatibility complex-related 1 (MR1) tetramers are reproducible and correlate with CD161 and TRAV1-2 coexpression in Peruvian samples. (A) A gating strategy defines 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil (5-OP-RU) loaded MR1-tetramer-binding MAIT cells in peripheral blood mononuclear cells (PMBCs) from Peruvian samples. (B) Two examples of flow cytometry plots of 5-OP-RU-loaded MR1 tetramer staining in T cells in the same PBMC samples from 2 Peruvian participants were acquired on the indicated dates. (C) The frequencies of 5-OP-RU-loaded MR1 tetramer+ cells among all CD3+ lymphocytes from 10 PBMC samples were measured twice by flow cytometry on different dates. The correlation coefficient rho (ρ) and P value are calculated using a 2-tailed Spearman correlation test. (D) Flow cytometry plot of 6-FP-loaded MR1 tetramer staining in T cells is shown from the same sample indicated in (A). (E) A flow cytometry gating strategy defines CD3+TRAV1-2+CD161+ phenotypically defined MAIT cells from on the sample depicted in (A). (F) Spearman correlation is shown between MR1-tetramer+ and phenotypically defined TRAV1-2+CD161+ MAIT cells as a proportion of total CD3+ T cells in Peruvian PBMC samples analyzed. 5-OP-RU, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil; FITC, fluorescein isothiocyanate; FSC, forward scatter; SSC, side scatter.
Before MR1-5-OP-RU tetramer-based studies, MAIT cells were defined by coexpression of TRAV1-2+ TCRs, cell surface CD161 and CD26, or combinations of these criteria [5, 22, 24, 25]. Because several key reports used CD161 and TRAV1-2 coexpression instead of MR1 tetramers to define MAIT cells in the blood of TB patients [10–12], we sought to determine the concordance of these 2 measurements. All samples were concurrently stained with anti-TRAV1-2 and CD161 antibodies and 5-OP-RU-loaded MR1 tetramers (Figure 1E). Frequencies of MAIT cells defined as TRAV1-2+CD161+ or MR1-tetramer+ were highly correlated, and samples with marked deviations in these 2 measures were not observed (Figure 1F).
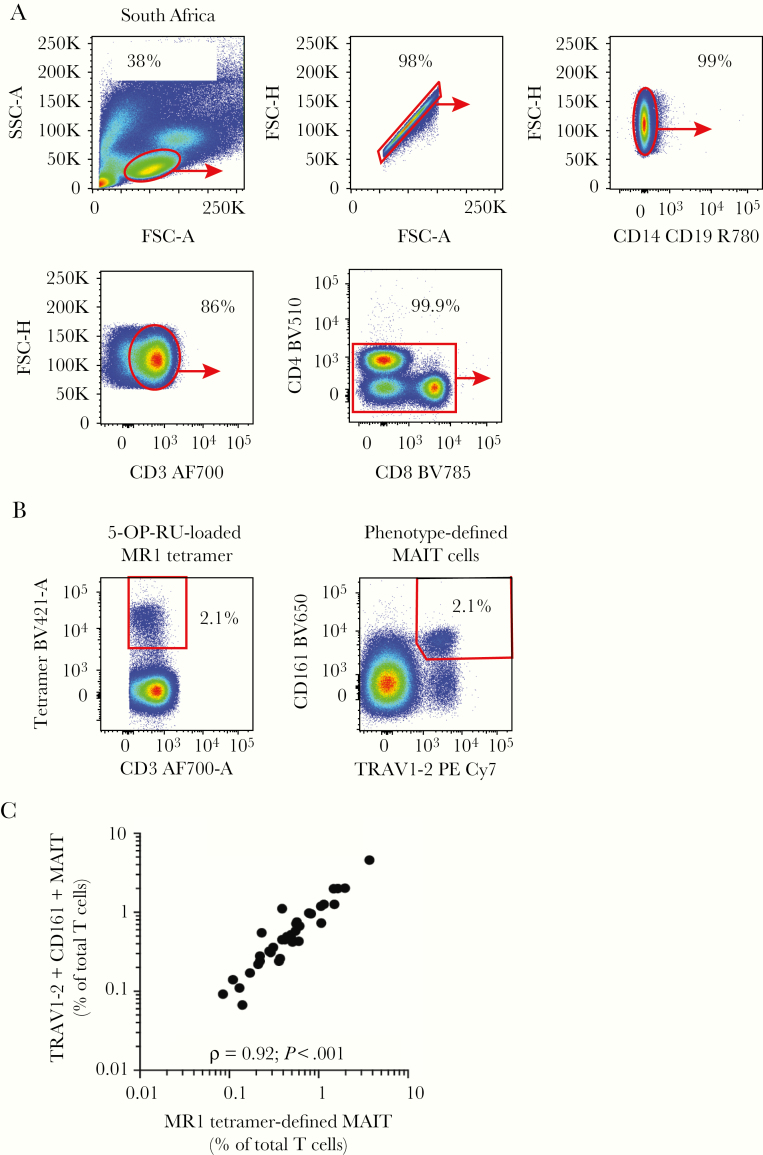
To test the association between MAIT cell frequencies and TB disease status in another population, we analyzed MAIT cell frequencies in independently recruited South African participants with recently diagnosed TB disease (n = 19) or latent infection (n = 19) (Table 1A and Figure 2A). Patients with TB disease were younger and more likely to be male than latent counterparts (Table 1A and Supplementary Table 3B). Frequencies of blood MAIT cells defined by MR1-tetramer-binding or TRAV1-2/CD161 coexpression were also significantly correlated (Figure 2B and C).
Figure 2.
Frequencies of mucosal-associated invariant T (MAIT) cells defined by major histocompatibility complex-related 1 (MR1) tetramers and CD161 and TRAV1-2 coexpression are highly correlated in South African samples. (A) Gating strategy defines MAIT cells in South African peripheral blood mononuclear cell (PMBC) samples. (B) Flow cytometry plots show MR1-tetramer-binding MAIT cells and CD3+TRAV1-2+CD161+ cells (phenotypic MAIT cells) among all CD3+ lymphocytes. (C) Spearman correlation between frequencies of MR1-tetramer+ and phenotypic MAIT cells (TRAV1-2+CD161+) as a proportion of total CD3+ T cells in 39 South African PBMC samples. FSC, forward scatter; SSC, side scatter.
Next, we asked whether blood MAIT cell frequencies were different in TB patients compared with healthy controls. Unlike prior studies [10–12], we did not detect significant differences between frequencies of blood MR1-tetramer+ MAIT cells in Peruvian TB patients versus either uninfected or latent contacts (Kruskal-Wallis, P = .14) ( Figure 3A). Likewise, the frequencies of blood MAIT cells in PBMC samples from South African participants with TB disease or latent Mtb infection were almost identical (Figure 3A). We also could not detect an association between MAIT cell frequencies and TB disease status after adjustment for age and gender (Supplementary Table 4A–C). Furthermore, despite geographic and genetic differences between the 2 populations, which were analyzed in 2 different laboratories with MR1 tetramers from 2 different sources, median frequencies among participants from Peru and South Africa were highly similar (Mann-Whitney, P = .63).
Figure 3.
Mucosal-associated invariant T (MAIT) cell frequencies do not distinguish tuberculosis (TB) disease state among Peruvian or South African participants. (A) Proportions of major histocompatibility complex-related 1 (MR1)-tetramer-binding MAIT cells among CD3+ T cells in peripheral blood mononuclear cell samples are shown with error bars denoting medians and interquartile ranges. P values are calculated using Mann-Whitney U test. (B) Plots shown as in (A) give proportions of CD3+TRAV1-2+CD161+ cells out of all T cells. (C) Comparison of frequencies of MR1-tetramer-binding cells are shown by gender in the 2 populations. Unadjusted P values correspond to Mann-Whitney U test. Error bars denote medians and interquartile ranges. 5-OP-RU, 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil.
As predicted by the correlation between MAIT cell frequencies defined as CD3+tetramer+ or CD3+CD161+TRAV1-2+ cells (Figures 1F and 2C), frequencies of CD3+CD161+TRAV1-2+ MAIT cells were also not significantly different between TB patients and healthy participants from Peru (Kruskal-Wallis, P = .11) or South Africa (Mann-Whitney, P = .86) (Figure 3B). In addition, we included anti-CD26 surface staining to more accurately capture MR1-tetramer+ MAIT cells in South African samples, as reported previously [5, 24, 25] (Supplementary Figure 1A). Likewise, peripheral blood MAIT cell frequencies defined as CD3+CD26+MR1-tetramer+ cells from latent participants and TB disease patients were not significantly different (Supplementary Figure 1B). Thus, our inability to detect associations between tetramer-defined MAIT cell frequencies and TB status did not change after defining MAIT cells by widely used surrogate surface markers.
Because our results differed from previous studies, which reported lower frequencies of blood MAIT cells in TB patients compared with controls [10–12], we asked whether demographic factors confounded the association between MAIT cells and TB status. In previous studies, healthy control samples were randomly selected from community blood bank donors, and hence they were not necessarily exposed to Mtb [10, 11]. Thus, we hypothesized that MAIT cell frequencies in healthy controls might depend on the extent of Mtb infection [2]. We used Mtb-specific IFNγ release as a surrogate for extent of Mtb infection [2], but we did not observe a correlation between MAIT cell frequencies and magnitude of Mtb-specific IFNγ release (Supplementary Figure 2A and Supplementary Table 4A). Age was weakly positively correlated with Mtb-specific IFNγ release (Supplementary Figure 2B) and negatively associated with MAIT cell frequencies in healthy Peruvian participants (Supplementary Figure 2C). Frequencies of MAIT cells were lower in male TB patients than female counterparts (P = .0074) (Figure 3C). Overall, age and gender confounded the association between MAIT cell frequencies and TB status (Supplementary Table 4A–C).
We considered that pooling patients of ranging degrees of TB disease severity could have weakened any association between MAIT cell frequencies and TB status. We hypothesized that stratification by disease severity might reveal an association, as reported in Korean TB patients [11]. Weight loss is a clinical indicator of TB disease severity [30], but frequencies of MR1-tetramer+ MAIT cells did not correlate with body mass index in TB patients (Figure 4A and Supplementary Table 3). Blood transcriptional profiling of TB patients and controls had previously identified transcriptional signatures of TB diagnosis and disease severity [31], presence of progressive incipient TB disease in healthy Mtb-exposed individuals [28], and treatment outcome [32]. We hypothesized that patients with high scores for host transcriptional TB signatures [33–35] (Supplementary Tables 2 and 5) as surrogates for disease severity [31] would show reduced frequencies of MAIT cells in the blood. DIAG3, a PCR-adapted TB disease signature, known to detect subclinical and TB disease and to correlate with TB disease severity [33–36], did not correlate with MAIT cell frequencies in patients with TB disease (Figure 4B). Likewise, RISK6, a 6-transcript signature that can detect incipient disease in Mtb-infected individuals as well as TB disease (under review [37]), did not correlate with MAIT cell frequencies in TB patients from either cohort (Figure 4C). Additional parsimonious signatures of incipient TB (RISK4) [33] or TB disease (DIAG4) [34, 35] also did not correlate with MAIT cell frequencies (Supplementary Figure 3 and Supplementary Table 5). Our data, taken together, did not support that variable disease severities among TB patients explains the poor association between blood MAIT cell frequencies and TB status.
Figure 4.
Association between disease severity and mucosal-associated invariant T (MAIT) cell frequencies. Correlation between frequencies of MAIT cells and body mass index (A) or transcriptional signatures of tuberculosis (TB) including DIAG3 scores (B) and RISK6 (C) in Peruvian (left) or South African (right) patients with TB disease. Correlation coefficient rho (ρ) and P values are calculated using Spearman non-parametric test.
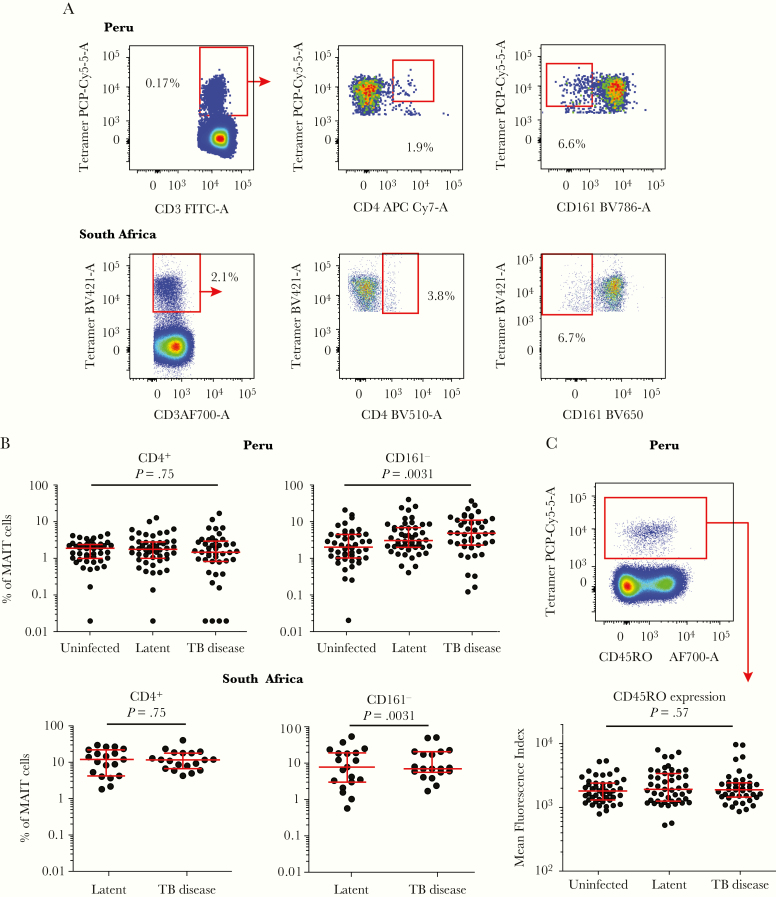
Although most MAIT cells are CD4–, CD161+, and CD45RO+, recent studies have highlighted subpopulations of “atypical” MAIT cells that lack some of these features, with possible distinct functions [5, 19]. Therefore, we tested whether frequencies of CD4+ and CD161– MR1-tetramer+ T cells were altered in patients with TB (Figure 5A). Frequencies of CD4+ MR1-tetramer+ MAIT cells in the blood were not different in TB patients and controls in either group (Figure 5B). We obtained similar results when MAIT cells were defined as CD26+ MR1-tetramer+ MAIT cells in South African samples (Supplementary Figure 4). Proportions of CD161– MR1-tetramer+ T cells were significantly lower in the blood of Peruvian uninfected individuals compared with either latent (P = .014) or TB disease patient (P = .0014) counterparts (Figure 5B).
Figure 5.
Mucosal-associated invariant T (MAIT) cells with atypical phenotypes are not associated with tuberculosis (TB) status. (A) Flow cytometric gating strategy for MAIT cells with atypical phenotypes shows T cells positively staining with the 5-(2-oxopropylideneamino)-6-D-ribitylaminouracil-loaded major histocompatibility complex-related 1 (MR1) tetramer, then gated to identify proportions of TRAV1-2–, CD4+, and CD161– MAIT cells. (B) Proportions of MAIT cells showing a CD4+ or CD161– phenotype are shown with error bars to denote medians and interquartile ranges. P values are calculated using the Kruskal-Wallis test among the 3 Peruvian groups or a Mann-Whitney U test between South African latent and TB disease samples. (C) Top, Flow cytometry plot depicting expression of the CD45RO memory marker in MR1 tetramer+ T cells in a Peruvian sample compared with tetramer– T cells. Bottom, Mean fluorescence index of PerCPCy5.5-conjugated anti-CD45RO antibody staining in MR1 tetramer+ T cells in the 3 Peruvian groups. Error bars denote median and interquartile range. The P value is calculated using the Kruskal-Wallis test. FITC, fluorescein isothiocyanate.
Mucosal-associated invariant T cells have been reported to virtually universally express the memory marker CD45RO in blood, as part of their preprogrammed nature as innate T cells with effector functions [38], rather than infection-driven conversion to memory cells observed in classical MHC-restricted T cells. In consistent tests, CD45RO expression on MAIT cells among Peruvian blood samples was uniformly high and similar among TB patients, latent and uninfected controls (Figure 5C). The MR1-tetramer– T cells showed a bimodal distribution of CD45RO that marks memory and nonmemory T cells in MHC-restricted T cells. In contrast, MAIT cells showed a unimodal distribution and intermediate CD45RO expression levels compared with CD45RO+ MR1-tetramer– T cells (Figure 5C). These data, taken together, suggest that frequencies of blood MAIT cells with atypical cell surface markers were not associated with TB status.
DISCUSSION
We report a large study profiling MR1-tetramer-binding MAIT cells in TB disease in 2 genetically and geographically distinct populations. Using samples from 183 donors, we did not detect differences between MR1-tetramer+ MAIT cell frequencies in the blood of TB patients and healthy Mtb-exposed controls. Measuring MAIT cell frequencies in a large-scale clinical study with tetramers, which identify all MR1-5-OP-RU-reactive cells [5, 6, 18, 39], was feasible and highly reproducible. More importantly, frequencies of MAIT cells, defined as MR1 tetramer+ or CD161+TRAV1-2+ T cells, as in prior studies [10], showed virtually identical outcomes. Therefore, the discordance between our finding and previous clinical studies reporting a decline in MAIT cell frequencies in the blood of TB patients relative to healthy controls was unlikely due to the method of MAIT cell detection.
Several factors may underlie the difference between our study and previous studies of TB disease and MAIT cells [10–12]. First, in prior studies, blood bank donors were recruited in nonendemic areas—France, Korea, and China [10–12]—and thus Mtb exposure was likely lower than controls in our study. In our study, Peruvian controls were recently exposed to Mtb in the household, and South African controls were IGRA positive and from a high Mtb transmission area [27, 40]. We did not recruit IGRA-negative South African participants, due to the extremely high prevalence of latency in communities from which participants were recruited, which both limits available participants and the reliability with which they can be assigned as truly uninfected [41]. Mycobacterium tuberculosis-infected household contacts of TB patients were reported to have inflammatory profiles that more closely resemble ones observed in active TB patients than latently Mtb-infected individuals [30]. Therefore, the high Mtb exposure rates in our control groups [42] might explain the lack of difference in MAIT cell frequencies between TB patients and healthy controls in this study, which we also observed in CD1b-reactive T cells [26]. We consistently detected lower median frequencies of blood MAIT cells in Peruvians (0.43%) and South Africans (0.47%), compared with healthy Australians (2.6%) [5], which may have resulted from the infection history of participants in our study. We and others [43] hypothesized that blood MAIT cell frequencies would decrease with increasing exposure to Mtb, or clinical severity of disease due to MAIT cell recruitment to the infection site [16]. Our data did not detect associations between MAIT cell frequencies and Mtb infection, estimated by IFNγ release, or disease severity, inferred through transcriptional risk scores, originally defined in whole blood RNA samples [28, 33]. Although risk scores in Peruvians were derived from PBMC samples, they accurately predicted TB disease [37, 44], yet they still did not influence MAIT cell frequencies. Consistent with this finding, MAIT cell frequencies were reported to decline in blood samples from patients with non-TB mycobacterial lung disease [11] or bacterial pneumonia [10]. This suggests that inflammation caused by infection with any lung pathogen may drive recruitment of MAIT cells to the lung from the blood, regardless of clinical progression to TB disease. However, TB status could still induce nuanced changes in MAIT cell functions, such as higher TCR-mediated responses in infected individuals in Haiti than uninfected counterparts [45] and changes in TCR clonotypes [46]. Hence, systematic evaluation of MAIT cell functions using unbiased profiling approaches may yield insight.
Age and gender had detectable effects on MAIT cell frequencies in our study. Similar to cohorts from Australia [5] and China [47], blood MAIT cell frequencies in Peruvians peaked in the third decade of life, then they declined steadily. It is interesting to note that blood MAIT cell frequencies were reported to be lower in males than females only during reproductive years [48]. Because we detected lower MAIT cell frequencies in Peruvian male TB patients only, the combined age and gender effect might have been missed in the study of Korean TB patients, because participants were mostly elderly [11]. The higher TB disease severity and lower MAIT cell frequencies in males than females [48] may suggest a role for MAIT cells in controlling Mtb infection. However, larger prospective studies would evaluate these interactions more reliably.
Several lines of evidence support the hypothesis that MAIT cells relocate from the blood to the lungs in individuals infected with Mtb. Oligoclonal expansion of lung-resident MAIT cells detected in bronchoalveolar lavage samples of TB patients was recently reported [16], consistent with a potential role for MAIT cells in controlling early Mtb infection. However, frequencies of MR1-tetramer+ MAIT cells in both peripheral blood and bronchoalveolar lavage samples were lower in children with TB disease compared with latent counterparts [49], arguing against MAIT cell migration to the alveolar space to explain their reported decline in the blood of TB patients. In addition, 2 recent reports in nonhuman primates reported that MAIT cell accumulation in the lung after Mtb infection was transient [50] and did not correlate with disease outcome [15]. Major histocompatibility complex-related 1-deficient Mtb-infected mice showed higher lung bacterial burdens than MR1-sufficient counterparts, suggesting a host protective role. Therefore, MAIT cell roles in the lung after infection warrant additional investigation.
CONCLUSIONS
Our study of MR1-tetramer-detected MAIT cells, analyzed separately in South American and African cohorts, alludes to generalizable MAIT cell features. We show that unlike MHC-restricted T cells in IFNγ-release assays, peripheral MAIT cell frequencies are not reliable correlates of Mtb infection, TB disease, or severity in high Mtb transmission settings. However, considering their diverse roles in microbial infections [43], MAIT cells could still play a role in TB pathophysiology, particularly in the lung, the major disease site in pulmonary TB. Future studies should focus on their functional profiles in TB, and tissue-specific roles, in high- and low-endemic settings.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. This study was funded by the National Institutes of Health (NIH) TB Research Unit Network (Grant U19 AI111224-01), the National Institute of Allergy and Infectious Diseases (R01 AI049313), the Strategic Health Innovation Partnerships Unit of the South African Medical Research Council with funds received from the South African Department of Science and Technology and a research grant from Aeras. A. J. C. is supported by a Future Fellowship (FT160100083) from the Australian Research Council. S. B. G. E. is supported by a DECRA Fellowship (DE170100407) from the Australian Research Council. S. C. M. received training in research that was supported by the Fogarty International Centre of the NIH under Award Number D43 TW010559.
Disclaimer. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Potential conflicts of interest. L. K.-N., S. B. G. E., A. J. C., J. M., and J. R. are named coinventors on patents describing major histocompatibility complex-related 1 (MR1) tetramers. For experiments using the NIH-supplied MR1 tetramer, the MR1 tetramer technology was developed jointly by J. M., J. R., and Dr. David Fairlie, and the material was produced by the NIH Tetramer Core Facility as permitted to be distributed by the University of Melbourne. S. S. and T. J. S. are named coinventors on a filed provisional patent application for biosignature for prediction of progression to tuberculosis. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
The South African Tuberculosis Vaccine Initiative (SATVI) Clinical Immunology Team
Sindile Matiwane, Lungisa Jaxa, Noncedo Xoyana, Constance Schreuder, Janelle Botes, Hadn Africa, Lebohang Makhethe, Marcia Steyn, Onke Nombida, Rodney Raphela, and Mzwandile Erasmus (South African Tuberculosis Vaccine Initiative, Institute of Infectious Disease and Molecular Medicine and Division of Immunology, Department of Pathology, University of Cape Town, Cape Town, South Africa).
References
- 1. Dye C, Glaziou P, Floyd K, Raviglione M. Prospects for tuberculosis elimination. Annu Rev Public Health 2013; 34:271–86. [DOI] [PubMed] [Google Scholar]
- 2. Pai M, Denkinger CM, Kik SV, et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 2014; 27:3–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Rhijn I, Kasmar A, de Jong A, et al. A conserved human T cell population targets mycobacterial antigens presented by CD1b. Nat Immunol 2013; 14:706–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Rhijn I, Moody DB. Donor unrestricted T cells: a shared human T cell response. J Immunol 2015; 195:1927–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gherardin NA, Souter MN, Koay HF, et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol Cell Biol 2018; 96:507–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Corbett AJ, Eckle SB, Birkinshaw RW, et al. T-cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 2014; 509:361–5. [DOI] [PubMed] [Google Scholar]
- 7. Kjer-Nielsen L, Corbett AJ, Chen Z, et al. An overview on the identification of MAIT cell antigens. Immunol Cell Biol 2018; 96:573–87. [DOI] [PubMed] [Google Scholar]
- 8. Harriff MJ, McMurtrey C, Froyd CA, et al. MR1 displays the microbial metabolome driving selective MR1-restricted T cell receptor usage. Sci Immunol 2018; 3:eaao2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kjer-Nielsen L, Patel O, Corbett AJ, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 2012; 491:717–23. [DOI] [PubMed] [Google Scholar]
- 10. Le Bourhis L, Martin E, Péguillet I, et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat Immunol 2010; 11:701–8. [DOI] [PubMed] [Google Scholar]
- 11. Kwon YS, Cho YN, Kim MJ, et al. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb) 2015; 95:267–74. [DOI] [PubMed] [Google Scholar]
- 12. Jiang J, Yang B, An H, et al. Mucosal-associated invariant T cells from patients with tuberculosis exhibit impaired immune response. J Infect 2016; 72:338–52. [DOI] [PubMed] [Google Scholar]
- 13. Chua WJ, Truscott SM, Eickhoff CS, Blazevic A, Hoft DF, Hansen TH. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect Immun 2012; 80:3256–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gold MC, Cerri S, Smyk-Pearson S, et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLoS Biol 2010; 8:e1000407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bucsan AN, Rout N, Foreman TW, Khader SA, Rengarajan J, Kaushal D. Mucosal-activated invariant T cells do not exhibit significant lung recruitment and proliferation profiles in macaques in response to infection with Mycobacterium tuberculosis CDC1551. Tuberculosis (Edinb) 2019; 116S:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wong EB, Gold MC, Meermeier EW, et al. TRAV1-2+ CD8+ T-cells including oligoconal expansions of MAIT cells are enriched in the airways in human tuberculosis. Commun Biol 2019; 2:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med 1993; 178:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reantragoon R, Corbett AJ, Sakala IG, et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J Exp Med 2013; 210:2305–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Meermeier EW, Laugel BF, Sewell AK, et al. Human TRAV1-2-negative MR1-restricted T cells detect S. pyogenes and alternatives to MAIT riboflavin-based antigens. Nat Commun 2016; 7:12506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gherardin NA, Keller AN, Woolley RE, et al. Diversity of T cells restricted by the MHC class I-related molecule MR1 facilitates differential antigen recognition. Immunity 2016; 44:32–45. [DOI] [PubMed] [Google Scholar]
- 21. Koay HF, Gherardin NA, Xu C, et al. Diverse MR1-restricted T cells in mice and humans. Nat Commun 2019; 10:2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tilloy F, Treiner E, Park SH, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med 1999; 189:1907–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker LJ, Kang YH, Smith MO, et al. Human MAIT and CD8αα cells develop from a pool of type-17 precommitted CD8+ T cells. Blood 2012; 119:422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sharma PK, Wong EB, Napier RJ, et al. High expression of CD26 accurately identifies human bacteria-reactive MR1-restricted MAIT cells. Immunology 2015; 145:443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Suliman S, Murphy M, Musvosvi M, et al. MR1-independent activation of human mucosal-associated invariant T cells by mycobacteria. J Immunol 2019; 203:2917–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lopez K, Iwany SK, Suliman S, et al. CD1b tetramers broadly detect T cells that correlate with mycobacterial exposure but not tuberculosis disease state. Front Immunol 2020; 11:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Darboe F, Mbandi SK, Naidoo K, et al. ; SATVI Clinical Immunology Team Detection of tuberculosis recurrence, diagnosis and treatment response by a blood transcriptomic risk signature in HIV-infected persons on antiretroviral therapy. Front Microbiol 2019; 10:1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zak DE, Penn-Nicholson A, Scriba TJ, et al. ; ACS and GC6-74 cohort study groups A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 2016; 387:2312–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Eckle SB, Birkinshaw RW, Kostenko L, et al. A molecular basis underpinning the T cell receptor heterogeneity of mucosal-associated invariant T cells. J Exp Med 2014; 211:1585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wallgren A. The time-table of tuberculosis. Tubercle 1948; 29:245–51. [DOI] [PubMed] [Google Scholar]
- 31. Berry MP, Graham CM, McNab FW, et al. An interferon-inducible neutrophil-driven blood transcriptional signature in human tuberculosis. Nature 2010; 466:973–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thompson EG, Du Y, Malherbe ST, et al. ; Catalysis TB–Biomarker Consortium Host blood RNA signatures predict the outcome of tuberculosis treatment. Tuberculosis (Edinb) 2017; 107:48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Suliman S, Thompson E, Sutherland J, et al. Four-gene Pan-African blood signature predicts progression to tuberculosis. Am J Respir Crit Care Med 2018; 197. doi: 10.1164/rccm.201711-2340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sweeney TE, Braviak L, Tato CM, Khatri P. Genome-wide expression for diagnosis of pulmonary tuberculosis: a multicohort analysis. Lancet Respir Med 2016; 4:213–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Maertzdorf J, McEwen G, Weiner J 3rd, et al. Concise gene signature for point-of-care classification of tuberculosis. EMBO Mol Med 2016; 8:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Warsinske HC, Rao AM, Moreira FMF, et al. Assessment of validity of a blood-based 3-gene signature score for progression and diagnosis of tuberculosis, disease severity, and treatment response. JAMA Netw Open 2018; 1:e183779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Penn-Nicholson A, Mbandi SK, Thompson E, et al. RISK6, a universal 6-gene transcriptomic signature of TB disease risk, diagnosis and treatment response. medRxiv 2019. doi: 10.1101/19006197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gutierrez-Arcelus M, Teslovich N, Mola AR, et al. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat Commun 2019; 10:687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gherardin NA, McCluskey J, Rossjohn J, Godfrey DI. The diverse family of MR1-restricted T cells. J Immunol 2018; 201:2862–71. [DOI] [PubMed] [Google Scholar]
- 40. Blaser N, Zahnd C, Hermans S, et al. Tuberculosis in Cape Town: an age-structured transmission model. Epidemics 2016; 14:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mahomed H, Hawkridge T, Verver S, et al. The tuberculin skin test versus QuantiFERON TB Gold® in predicting tuberculosis disease in an adolescent cohort study in South Africa. PLoS One 2011; 6:e17984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yates TA, Khan PY, Knight GM, et al. The transmission of Mycobacterium tuberculosis in high burden settings. Lancet Infect Dis 2016; 16:227–38. [DOI] [PubMed] [Google Scholar]
- 43. Meermeier EW, Harriff MJ, Karamooz E, Lewinsohn DM. MAIT cells and microbial immunity. Immunol Cell Biol 2018; 96:607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Darboe F, Mbandi SK, Thompson EG, et al. ; SATVI Clinical Immunology Team Diagnostic performance of an optimized transcriptomic signature of risk of tuberculosis in cryopreserved peripheral blood mononuclear cells. Tuberculosis (Edinb) 2018; 108:124–6. [DOI] [PubMed] [Google Scholar]
- 45. Vorkas CK, Wipperman MF, Li K, et al. Mucosal-associated invariant and gammadelta T cell subsets respond to initial Mycobacterium tuberculosis infection. JCI Insight 2018; 3:e121899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ogongo P, Steyn AJ, Karim F, et al. Differential skewing of donor-unrestricted and γδ T cell repertoires in tuberculosis-infected human lungs. J Clin Invest 2020; 130:214–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chen P, Deng W, Li D, et al. Circulating mucosal-associated invariant T cells in a large cohort of healthy Chinese individuals from newborn to elderly. Front Immunol 2019; 10:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Novak J, Dobrovolny J, Novakova L, Kozak T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand J Immunol 2014; 80:271–5. [DOI] [PubMed] [Google Scholar]
- 49. Malka-Ruimy C, Ben Youssef G, Lambert M, et al. Mucosal-associated invariant T cell levels are reduced in the peripheral blood and lungs of children with active pulmonary tuberculosis. Front Immunol 2019; 10:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kauffman KD, Sallin MA, Hoft SG, et al. Limited pulmonary mucosal-associated invariant T cell accumulation and activation during Mycobacterium tuberculosis infection in rhesus macaques. Infect Immun 2018; 86:e00431–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.