Abstract
Air pollution is the second most important risk factor associated with noncommunicable diseases after smoking. The effects of pollution on health are commonly attributable to particulate matter (PM), a complex mixture of particles suspended in the air. PM can penetrate the lower respiratory tract and has harmful direct and indirect effects on different organs and tissues. Direct effects are caused by the ability of PM components to cross the respiratory membrane and enter the bloodstream; indirect effects are systemic consequences of the local airway response. Recent work suggests that PM is an independent risk factor for low bone mineral density and osteoporosis-related fractures. Osteoporosis is a common age-related disease closely linked to bone fractures, with severe clinical consequences affecting quality of life, morbidity, and mortality. In this review, we discuss potential mechanisms behind the association between outdoor air pollution, especially PM, and bone damage. The discussion features four main mechanisms: 1) several different atmospheric pollutants can induce low-grade systemic inflammation, which affects bone metabolism through a specific effect of cytokines such as TNFα, IL-1β, IL-6, and IL-17 on osteoblast and osteoclast differentiation and function; 2) some pollutants, particularly certain gas and metal compounds, can cause oxidative damage in the airway and bone cells; 3) different groups of pollutants can act as endocrine disruptors when binding to the receptors in bone cells, changing their functioning; and 4) air pollution can directly and indirectly cause vitamin D deficiency. Characterizing these mechanisms will better define the physiopathology of bone damage, and recognizing air pollution as a modifiable risk factor for osteoporosis will inform environmental policies. Such knowledge will also guide the prevention of fractures due to fragility and help reduce health-related costs.
Keywords: Air pollution, mechanisms, particulate matter, bone damage, fractures, osteoporosis
1. Introduction
1.1. Outdoor air pollution
Air pollution is a global health problem. According to data from the World Health Organization (WHO), 9 out of 10 people worldwide breathe polluted air (World Health Organization, 2018a). Further, outdoor air pollution was estimated to cause 4.2 million premature deaths worldwide in 2016, mostly in low- and middle-income countries. Of all premature deaths related to outdoor air pollution, 58% were due to cardiovascular disease, 18% to respiratory disease, and 6% to lung cancer (World Health Organization 2018b). Hence, air pollution has become the second most important risk factor for noncommunicable diseases worldwide, behind tobacco smoking (Prüss-Ustün, 2019). Growing evidence associates air pollution with damage to most body systems (Schraufnagel, 2019). This review focuses on potential mechanisms underlying the adverse effects of outdoor air pollution on bone health.
Particulate matter (PM) is a major contributor to the effects of pollution on health (World Health Organization, 2018b). PM is a complex mixture of solid and liquid particles suspended in the air. Common chemical components of PM include sulfates, nitrates, ammonium, other inorganic ions (sodium, potassium, calcium, magnesium, and chloride), organic and elemental carbon, crustal material, metals (including cadmium, copper, nickel, vanadium, and zinc), polycyclic aromatic hydrocarbons (PAHs), water, and biological components such as allergens and microbial compounds (World Health Organization, 2013). The most harmful effects of PM are related to small particle size, which has a greater ability to penetrate the lower respiratory tract and enter the bloodstream (Kim, 2015). PM is classified into PM10 (coarse) if its aerodynamic diameter is smaller than 10 μm; PM2.5 (fine) if it is smaller than 2.5 μm; and PM0.1 (ultrafine) if it is smaller than 0.1 μm (Kim, 2015). In 2016, 95% of the world population lived in areas where the environmental levels of PM2.5 were higher than the WHO air quality guidelines (Shaddick, 2018).
1.2. Osteoporosis and bone fragility
Bone strength is determined by its composition and structure. Bone is made of a highly organized protein matrix, mainly type I collagen, which provides sites for nucleation of hydroxyapatite crystals. The relative amounts of minerals, water, and organic material, as well as the quality and arrangement of these components, impart the necessary mechanical properties for bone to absorb the energy imposed during loading without structural failure (Currey, 2003). The purpose of bone modeling and remodeling throughout life is to adapt bone composition and structure to prevailing loads (Seeman & Delmas, 2006). Failure of bone adaptation results in bone fragility, a key cause of fractures and trauma (Cooper, 1993).
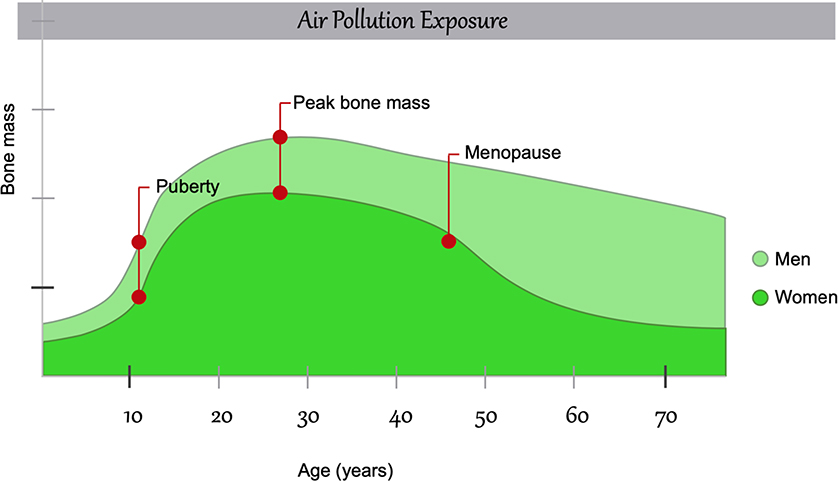
Bone mass increases exponentially during puberty and achieves its peak during young adulthood (around age 30); it declines nonlinearly after that, particularly during menopause (Cooper et al., 2008). The peak bone mass, influenced by lifestyle, genetic, and environmental factors, is an important determinant of the risk of osteoporosis during lifetime and bone fractures during late adulthood (Weaver et al., 2016) and it probably plays a key role in bone damage induced by air pollution (Figure 1).
Figure 1. Changes in bone composition over the lifetime and the influence of air pollution.
Air pollution effect is shown before birth, as during pregnancy may also induce molecular changes affecting potentially bone development.
The loss of bone mass can lead to osteoporosis, the most common chronic metabolic bone disease (Sözen, 2017). This bone damage is asymptomatic, so it is often called a “silent disease” (National Institutes of Health, 2018). However, osteoporotic fractures, which develop as a consequence of loss of bone strength, are a significant public health problem (Cooper, 1993) because they have devastating results in terms of decreased autonomy, morbidity, mortality, and health costs (Fonseca, 2014).
In the United States, more than 10.2 million people have osteoporosis, and 43.4 million have low bone density, called osteopenia (National Institutes of Health, 2018; Wright, 2014). By 2025, the annual incidence of osteoporosis-related fractures in the United States is estimated to be more than 3 million cases, with approximately $25.3 billion in treatment costs (Burge, 2007). The consequences of osteoporotic fractures are devastating: one year after a hip fracture, an estimated 40% of patients cannot walk independently, and the five-year mortality rate increases by 20% in patients who experience any bone fracture (Cooper, 1997).
Osteoporosis is diagnosed by measuring bone mineral density (BMD) with dual-energy X-ray absorptiometry (DXA). According to the WHO guidelines, osteoporosis is diagnosed when the femoral neck BMD is 2.5 or more standard deviations (SD) below the mean of the young and healthy population (T-score). Osteopenia is defined as a BMD value within 1 and 1.5 SD below the T-score (World Health Organization, 1994).
Although BMD measurements by DXA have demonstrated their usefulness in determining fracture risk and are the standard for the diagnosis of osteoporosis (Curtis et al., 2017), it is well known that some older individuals may have higher fracture risk without having a T-score below −2.5 SD (Samelson et al., 2019), which could explained because bone strength is determined by both bone mass and bone quality (Link & Heilmeier, 2016). Bone quality is a complex concept that involves aspects such as collagen properties, osteocyte density, and trabecular and cortical microarchitecture (Fonseca et al., 2014). New techniques may allow us to better assess bone quality (e.g., trabecular and cortical microarchitecture, which cannot be evaluated accurately by DXA). Other might include quantitative ultrasound and high-resolution peripheral quantitative computed tomography, bone biopsy, and quantitative computerized tomography (Link & Heilmeier, 2016). Further research on the effect of air pollution by using these technologies will be highly relevant in the field.
2. Air pollution and bone damage
In the 1980s, the first studies were published to indicate a higher incidence of hip fractures in urban areas compared with rural areas [in Norway (Falch, 1985; V. Finsen & Benum, 1987) and Sweden (Jarnlo, 1989; Larsson, 1989; Mannius, 1987; Sernbo, 1988)]. Subsequent studies corroborated this association in Norway and Sweden (Falch, 1993; Finsen, 2004; Jónsson, 1992; Kaastad, 1998) and in other countries such as the United States (Madhok, 1993; Melton, 1999), Australia (Cooley & Jones, 2002; Sanders, 2002) and Switzerland (Chevalley, 2002). This association between urban areas and hip fractures is age- and sex-specific; therefore, demographic differences were discarded from being responsible for these findings. Some researchers argued that differences in lifestyle (Jónsson, 1993) and body composition (Elmståhl, 1993) could explain the association between urban areas and hip fractures, but the results were inconclusive. Other studies found the same association regarding osteoporosis-related fractures in other areas of the body, such as the spine and distal forearm (Cooley & Jones, 2002; Melton, 1999; Omsland, 2011; Sanders, 2002; Søgaard, 2007). Two studies showed a higher incidence of fractures in rural Turkey (Dilsen, 1993) and Australia (Peach & Bath, 1999), and another study did not find differences in the incidence rates in Finland (Lüthje, 1995). However, later reports suggested these results were due to the higher proportion of bone fractures caused by high energy trauma in rural areas in the case of the Turkish study, the small sample size in the Australian study, and recently adopted urban lifestyles (many old people had spent most of their youth in rural areas) in the Finnish study (Brennan, 2010; Cooley & Jones, 2002). Even though the studies did not share a common definition of urban and rural areas, there was a clear tendency toward a higher incidence of fractures in urban areas. Several causal factors were examined, but the possibility that an environmental factor had a significant role in this association was not considered.
Moreover, several studies reported that bone mineral content (BMC) (Gärdsell, 1991; Specker, 2004) and BMD (Filip & Zagórski, 2001; Meyer, 2004; Omsland, 2011; Ringsberg, 2001; Rosengren, 2010, 2012; Specker, 2004; Sundberg, 1997) were lower in urban populations of countries such as Norway, Sweden, Poland, and the United States. Although this association is inconsistent with the mixed results from low- and middle-income countries (Gu, 2007; Li, 2005; Pongchaiyakul, 2006; Pongchaiyakul, 2005a; Pongchaiyakul, 2005b), a meta-analysis by Matsuzaki et al. (2015) suggested that this difference might be due to nutritional deficiencies in rural areas of developing countries. These findings indicated that reduction in bone quality was the main cause of the higher incidence of osteoporotic fractures in urban areas.
Later, Alvaer et al. (2007) observed a negative association between indicators of air pollution (PM2.5 and PM10) and total body BMD in older men. In another study, the same authors reported an association between PM2.5 and lower distal forearm BMD, with a higher prevalence of self-reported forearm fractures. However, the association was found in male smokers only (Alver, 2010). These studies were significant because they paved the way to considering air pollution as a risk factor for bone fragility and highlighted the need for additional research.
Considering this background, our research group gathered evidence of the association between air pollution and bone damage (Prada, 2017). After analyzing 9.2 million Medicare beneficiaries, we found that older men (>65 years old) from areas with the highest levels of PM2.5 had the highest rates of hospital admissions due to osteoporosis-related fractures, regardless of sex and other confounders. Also, during an 8-year follow-up of a cohort of 692 middle-aged men (mean age 47.5 years), we found that the levels of black carbon (BC), a PM component, were related to decreased BMD in several body parts over time, as well as a negative association between BC and parathyroid hormone (PTH) serum levels. These results were consistent with those previously published by Chang et al. (2015), which indicated a higher risk for osteoporosis in people exposed to higher levels of gaseous pollutants (CO and NO2) in Taiwan, and by Chen et al. (2015), who found that residential proximity to a motorway was related to lower BMD in Mexican Americans. Later, Mazzucchelli et al. (2018) found an association between levels of some gaseous pollutants (SO2, NO, and NO2) and incidence of hip fracture in older women from a region in Southern Europe. Likewise, a recent study in India (Ranzani et al., 2020) reported an association between PM2.5 levels and a lower BMC in the spine and hip in fully adjusted models.
Although no direct experimental evidence of the susceptibility of different bones to air pollution, Ranzani et al. (Ranzani et al., 2020) have suggested that trabecular bone is more affected than cortical bone. Further research about bone type susceptibility is warranted. On the other hand, although outdoor air pollution has been identified as an independent risk factor for osteoporosis and fractures due to bone fragility, little is known about the mechanisms underlying its association with bone damage. The chemical and physical heterogeneity of PM, which is made up of hundreds of different components, as well as the presence of other atmospheric pollutants not included in this classification (e.g., nitrogen oxides and volatile organic compounds), make it difficult to establish the molecular mechanisms involved in bone damage caused by air pollutants. In this review, we synthesize evidence of the role of different air pollutants in modifying bone homeostasis.
3. Physiopathology of osteoporosis
Bone remodeling depends on the joint action of osteoblasts, the bone-forming cells, and osteoclasts, the bone-resorbing cells. Although bones look inert, they are a highly dynamic organ; they are continuously reabsorbed and remodeled to adapt to mechanical use and repair microdamage (Florencio-Silva et al., 2015). Osteoporosis develops when the balance between bone formation and resorption shifts toward the latter (Kumar, 2014). Two cytokines produced by the osteoblasts and bone marrow stromal cells are essential for osteoclastogenesis: macrophage colony-stimulating factor (MCSF) and receptor activator for nuclear factor κ B ligand (RANKL) (Sipos, 2009). RANK, the receptor of RANKL, is expressed in osteoclast precursors and activates the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), a transcription factor inducing the expression of genes stimulating osteoclast formation, fusion, differentiation, function, and survival (Takahashi, 2011). Unlike M-CSF expression, which is constitutive, RANKL expression is inducible and thus has a functional role in the changes in bone metabolism (Takahashi, 2011). Osteoprotegerin (OPG), also produced by osteoblasts and stromal cells, is a natural bait receptor of RANKL, antagonizing its effect. The imbalance between OPG and RANKL production by osteoblasts and stromal cells is essential in the development of osteoporosis (Marie & Halbout, 2008). With increasing age, osteoblast replication and extracellular matrix synthesis decrease, as do the growth factors in the bone matrix, particularly insulin-like growth factor-1 (IGF-1) (Almeida & O’Brien, 2013; Kumar, 2014), which favors bone loss during aging.
Hormone factors also significantly influence the loss of bone mass. In postmenopausal women, decreased estrogen levels are associated with loss of cortical and trabecular bone (Kumar, 2014). Estrogen deficiency upregulates RANKL (Eghbali-Fatourechi, 2003), an effect mediated by the production of cytokines such as IL-1, IL-6, and TNF (Kumar, 2014). A decrease in estrogens also affects how the stimulation of osteoblast proliferation (Galea, 2013) and OPG production (Bord, 2003).
Furthermore, a sedentary lifestyle contributes to bone fragility. Osteoblasts and osteocytes work as mechanoreceptors by increasing connexin expression after application of mechanical stress, which triggers biochemical signals between both types of cells and increases extracellular matrix synthesis (Datta, 2008). Therefore, a sedentary lifestyle, which reduces osteoblast and osteocyte stimulation, is a significant risk factor in the development of osteopenia and osteoporosis (National Institutes of Health, 2018). To highlight, physical activity, particularly weight-bearing, contributes to bone health as it stimulates bone osteogenesis in osteoporotic patients (Benedetti et al., 2018).
4. Potential mechanisms of bone damage induced by air pollution
4.1. Air pollution promotes low-grade systemic inflammation
Substantial evidence demonstrates that exposure to air pollutants is associated with increased levels of proinflammatory mediators in the systemic circulation of both animals and humans (Araujo, 2010). Exposure to high PM concentrations significantly increases serum levels of monocytes, NK cells, and helper T cells (Pope, 2016), as well as proinflammatory cytokines such as TNFα, MCP-1, IL-8, MIP-1α, IL-6, IL-1β, and GM-CSF in in vitro and in vivo models (Alfaro-Moreno, 2009; Calderón-Garcidueñas, 2013; van Eeden, 2001). A recent high-resolution metabolomics (HRM) study (Liang, 2018) supports the hypothesis that systemic inflammation is an essential mediator of the health effects of air pollutants.
Inflammation induced by PM exposure is likely mediated in part by the response of alveolar macrophages (AM) and airway epithelial cells. Some PM components activate the AM and epithelial cells by signaling of toll-like receptors 2 and 4 (TLR2 and 4) and adaptor protein MyD88. This activation induces the expression of TNFα, IL-6, and IL-8 (Becker, 2005; Huang, 2003; Shoenfelt, 2009). PM2.5 components, such as metal pollutants with oxidative activity, polycyclic aromatic hydrocarbons, and bacterial or mycoplasma lipoproteins, are proposed to stimulate TLR2 activation, while the presence of lipopolysaccharides (LPS) in PM10 may explain TLR4 activation (Becker, 2005; Shoenfelt, 2009).
Ghio et al. proposed another mechanism by which air pollution exposure induces a systemic inflammatory response: disruption of iron homeostasis by humic-like substances (HULIS), a mixture of organic compounds in the particles of polluted air. HULIS are formed secondary to atmospheric oxidation of black carbon particles, which are PM2.5 components produced as a consequence of fossil fuel and biomass burning (Ghio, 2018). These compounds have a high number of functional groups containing oxygen (e.g., carboxylic and phenolic groups) and promote the formation of stable complexes with metals, particularly iron (Erdogan, 2007). Iron sequestration by HULIS induces oxidative stress, the release of proinflammatory mediators (IL-6 and IL-8), and, finally, apoptosis of airway epithelial cells (Ghio & Cohen, 2005; Ghio, 2016; Ghio, 2015). Ghio et al. (2015) observed that exposure of immortalized human bronchial epithelial cells to wood smoke particles increased the secretion of IL-6 and IL-8, which was related to iron sequestration by the particles. This effect was diminished by preventing the loss of mitochondrial iron with administration of ferric ammonium citrate.
It is likely that additional mechanisms induce systemic inflammation due to particle exposure, and that they are complementary. For example, there is evidence that the aryl hydrocarbon receptor (AhR) signaling pathways have a significant role in particle-induced inflammation (Lawal, 2017). AhR is a key receptor to which several air pollutants bind and will be fully described in the section on endocrine disruptors.
Bone loss, particularly in older people, may be related to low-grade systemic inflammation (Ding, 2008). In a rat model of chronic systemic inflammation induced by LPS, inflammation is associated with systemic bone loss, characterized by low BMD (Smith, 2006). Altered bone remodeling caused by systemic inflammation is characterized by an increase in bone resorption and deficient osteogenesis due to the effect of the inflammatory mediators over osteoclast and osteoblast differentiation and activity (Briot, 2017).
T-cells have an essential role in regulating bone resorption and formation by the activity of specialized cell lines such as Th17 and Treg cells (Briot, 2017). Immunological factors promoting osteoclast activity (and inhibiting osteoblasts) include MCSF, TNFα, IL-1, IL-6, and IL-17 (Briot, 2017). Besides the cytokine-mediated effects, activated T lymphocytes express the membrane marker semaphorin-4D (Sema4D). The binding of Sema4D to the receptor plexin-B1 on osteoblasts inhibits their differentiation by suppressing IGF-1 signaling (Negishi-Koga, 2011).
Cytokines affect bones and are significantly increased by PM exposure. TNFα promotes osteoclastogenesis by stimulating the differentiation of monocytes and macrophages into preosteoclasts (Lam, 2000). Likewise, TNFα increases RANK expression in osteoclast precursors in vivo, as well as MCSF and RANKL in stromal cells and synovial fibroblasts (Kitaura, 2013). Although the RANKL-mediated effect has been the most studied, there is evidence that TNFα induces osteoclastogenesis and bone erosion by RANKL-independent mechanisms (O’Brien, 2016). Finally, as a consequence of the multiple effects of TNFα on bone metabolism, a mouse model of rheumatoid arthritis (RA) revealed that BMD was increased by systemic administration of anti-TNFα antibodies (Saidenberg-Kermanac’h, 2004).
Furthermore, IL-1β directly activates RANK signaling and induces RANKL expression, which promotes osteoclastogenesis and increases bone resorption (Lee, 2010). Pacifici et al. (1991) conducted a study in premenopausal women who underwent oophorectomy and observed a sustained increase in IL-1β secretion by peripheral blood mononuclear cells, which was associated with a significant decrease in BMD. Systemic IL-1β plays a crucial role in bone loss in diseases such as RA because, even in cases with high levels of TNFα, the absence of IL-1β fully protects against systemic bone resorption (Polzer, 2010).
Although we have mentioned models of RA in animals, mainly to explain the effects of certain cytokines on bone health, it is interesting that RA is also associated with air pollution in humans (Sigaux et al., 2019). While murine models and clinical evidence have demonstrated that corticosteroid treatment in humans has a strong impact on bone health (Whittier & Saag, 2016), corticosteroids can cause additional injury in exposed individuals, including those with RA and other systemic inflammatory diseases.
Cytokines from the IL-6 family are reported to have dual antagonist roles: they sustain bone formation, but can also cause bone loss in several osteolytic pathologies (Blanchard, 2009). The mechanisms involved in osteoclastogenesis induced by IL-6, similar to TNFα, seem to be RANKL- dependent and independent (O’Brien, 2016). Likewise, recent evidence suggests that the variation in IL-6 levels in older adults may help to predict BMD loss (Ding, 2008). Moreover, Wells et al. (2017) found that continuous exposure to an organic dust extract (ODE) induces an increase in IL-6 concentration, with an adverse effect on bone homeostasis mediated by osteoclasts. The same authors found that ODE increases helper T cells with a marked polarization toward proinflammatory Th1 and Th17 phenotypes, which could be the source of systemic IL-6 (Poole, 2012). IL-17 is a cytokine mainly secreted by Th17 lymphocytes and has an important role in bone metabolism because it promotes the release of RANKL by osteoblasts and osteocytes. IL-17 also boosts the osteoclastogenic activity by regulating RANK expression, which increases the sensitivity to RANKL (Adamopoulos, 2010). Finally, parathyroid hormone (PTH) is reported to favor the differentiation of virgin T lymphocytes into Th17 cells. Additionally, this hormone increases the serum levels of IL-17 in both mice and humans (Li, 2015; Pacifici, 2016). Surprisingly, there is evidence that IL-17 increases the sensibility of osteocytes and osteoblasts to PTH (Li, 2019).
In 2,264 children aged 10 years, Liu et al. (Liu et al., 2015) found a positive and significant association between exposure to air pollutants (NO2, PM2.5–10 and PM10) and the levels of two biomarkers of bone turnover: serum osteocalcin (a bone formation marker that reflects osteoblastic activity) and C-terminal telopeptide of type I collagen (CTx, a bone resorption marker). These results are highly relevant because early alterations in bone homeostasis can potentially impair bone mass trajectories, an important contributor to bone resistance in adulthood (Cooper et al., 2008).
Although the association between the local response of the respiratory system, immunological activity, and bone metabolism has only begun to be studied, there is key evidence showing the mechanisms involved in bone damage due to air pollution. This section is an initial approach to understanding the role of the lung-periphery-bone axis in bone damage after exposure to environmental pollutants.
Finally, and in relation to potential effect of household air pollution, Saha et al. has reported that chronic exposure to biomass smoke increased membrane-bound and soluble RANKL and circulating osteoclast precursors but decreased OPG. These findings suggested an increased risk of bone resorption and consequent osteoporosis from household air pollution in women of childbearing age (Saha et al., 2016). More studies are needed to determine the contribution of indoors air pollution on bone mineral density and fracture risk.
4.2. Air pollutants and oxidative stress
Oxidative stress is characterized by the increase in free radicals [reactive oxygen species (ROS) and reactive nitrogen species (RNS)]. The most-studied pollutants concerning intracellular formation of free radicals are ozone (O3) (Yang & Omaye, 2009), nitrogen oxides (NO and NO2), and heavy metals (Solleiro-Villavicencio & Rivas-Arancibia, 2018). Reactive species and their damage to cells can modulate the inflammatory response.
These phenomena occur because oxygen reactive species modify signaling pathways; they can oxidize or reduce redox-sensitive amino acid residues (Corcoran & Cotter, 2013). Oxidative modifications change the structure or function of some signaling proteins and keep them active (phosphorylated) (Corcoran & Cotter, 2013), which in turn dysregulates the signaling pathways. Consequently, signal transductions stay active and lead to sustained expression of certain proteins that promote cell proliferation, apoptosis, and inflammatory responses (Solleiro-Villavicencio & Rivas-Arancibia, 2018). Furthermore, ROS can modify the patterns of gene expression because they act on certain transcription factors such as nuclear factor erythroid 2–related factor 2 (Nrf2), activator protein-1 (AP1), NF-kβ, HIF-1α, p53, and FOXO. Many of these transcription factors have oxidation-sensitive cysteine residues in their DNA binding sites. In a state of oxidative stress, reactive species overstimulate transcription factors and, consequently, create a deficit of antioxidant defenses and increase the formation of inflammatory and redox mediators (Farooqui, 2014). Ozone also activates transcription factor NF-κβ, which increases the expression of several proinflammatory cytokines (Rahman & MacNee, 1998).
In addition to the effects of oxidative stress on bone homeostasis mediated by the immune response, several pollutants can directly induce oxidative stress in bone tissues due to their capacity to cross cell membranes. In fact, cumulative oxidative damage may participate in several mechanisms of bone aging (Almeida & O’Brien, 2013). Evidence indicates that mesenchymal stem cells, which differentiate into osteoblasts, chondrocytes, and adipocytes, lose functionality with age due to cumulative oxidative stress (Stolzing & Scutt, 2006). Additionally, activating the peroxisome proliferator-activated receptor gamma (PPARγ) by ligands such as oxidized polyunsaturated fatty acids induces β-catenin degradation, inhibiting Wnt/β-catenin signaling, which is indispensable for osteoblastogenesis (Almeida, 2009; Sharma, 2004).
4.3. Bone damage due to environmental endocrine disruptors
Exogenous chemical substances called endocrine-disrupting chemicals (EDCs) affect hormone activity (Zoeller, 2012). Some EDCs are in polluted air as volatile (VOCs) or semi-volatile (SVOCs) organic compounds in gaseous form or are bound to particles (Darbre, 2018). Fossil fuel and residue burning, industrial activities, volatilization of synthetic chemicals, and other human activities release EDCs into the atmosphere, which produces adverse effects on health (Annamalai & Namasivayam, 2015). Major EDCs include phthalates, polychlorinated biphenyls, polycyclic aromatic hydrocarbons, dioxins, bisphenol A, perfluoroalkyl substances, and alkylphenol ethoxylates (Annamalai & Namasivayam, 2015).
Some EDCs, such as phthalates, are also associated with bone damage. Phthalates are phthalic acid esters that bind to plastics and modulate the properties of materials. Phthalates are used in the production of polyvinyl chloride (PVC) and building materials, among other products. In general, phthalates are not physically bound to polymers, which facilitates their dissemination in the environment (Annamalai & Namasivayam, 2015). Although phthalates are more common indoors, they also abound outdoors (Chen, 2018). Experimental studies link exposure to different phthalates with skeletal deformities and delay in ossification in mice (Ema, 1994; Ema, 1993; McKee, 2006; Saillenfait, 2009; Saillenfait, 2013). Additionally, higher urinary levels of phthalate metabolites are associated with low BMD and a higher risk of osteoporosis in postmenopausal women (DeFlorio-Barker & Turyk, 2016; Min & Min, 2014). In murine models, benzyl butyl phthalate (BBP) and dibutyl phthalate (DBP) can affect the nuclear translocation of fibroblast growth factor 2 (FGF2) in osteoblasts (Menghi, 2001), which is essential for osteoblast function and differentiation (Marie, 2003). Bhat et al. (2013) found that di-2-ethylhexyl phthalate (DEHP) affected in vitro osteoblast differentiation and matrix mineralization in mice. Finally, these compounds can modify the actin structure and induce expression of pro-apoptotic proteins in mouse osteoblasts (Agas, 2007; Marchetti, 2002; Sabbieti, 2009).
Polychlorinated biphenyls (PCBs) are synthetic chemicals used in several industrial and home products. PCBs exhibit high air concentrations in industrial areas and are considered persistent organic compounds (POC) (Kaya, 2012). Twelve PCB congeners (chemical constituents or related chemicals) that bind the aryl hydrocarbon receptor (AhR) are classified as dioxin-like PCBs (DL-PCBs) (Annamalai & Namasivayam, 2015). Serum concentrations of some DL-PCBs (105, 118, and 156) are associated with low stiffness index, a parameter of bone quality, in women (Paunescu, 2013). In another study, DL-PCB 118 was negatively associated with BMD in men (Hodgson, 2008). Evidence shows that PCB 169 (Brankovič, 2019, 2017) and 126 (Alvarez-Lloret, 2009; Lind, 2000) modify bone geometry, biomechanics, and mineral composition in mice. Furthermore, several POC, including many PCBs, are associated with low BMD in mammals (Daugaard-Petersen, 2018a; Daugaard-Petersen, 2018b). However, some studies did not find an association between these compounds and BMD and markers of bone metabolism, as in the case of PCB 153 (Glynn, 2000; Rignell-Hydbom, 2009; Wallin, 2005). Since 209 individual congeners exist, it is difficult to examine the specific effect of each compound on bones (Darbre, 2018). The effect of PCBs on bone is likely mediated by AhR, as we will discuss later.
Polycyclic aromatic hydrocarbons (PAHs), the common chemical components of PM, are a large group of organic compounds that also have potential effects on bone mineralization (Guo, 2018; Zhang, 2016). They are produced by the incomplete combustion of fossil fuels, wildfires, and cigarette smoke. Urinary levels of several metabolites of these compounds have been associated with low BMD and osteoporosis (Guo, 2018). Moreover, one of these compounds, benzo[a]pyrene (BaP), affects bone integrity in medaka fish (Oryzias latipes), a species sharing the basic mechanisms of bone formation with mammals. Fish offspring also exhibit these effects as a consequence of epigenetic changes that modify osteoblast differentiation (Seemann, 2017, 2015). This effect has also been observed in human mesenchymal stem cells exposed to BaP in vitro (Zhou, 2017). Likewise, BaP and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induce osteoclastogenesis in humans by activating the Cyp1 enzymes (Iqbal, 2013). In addition to this double effect in osteoblasts and osteoclasts, there is evidence that BaP enhances vitamin D3 catabolism, with potentially damaging effects on bone homeostasis (Matsunawa, 2009). The mechanisms involved in the biological effects of PAHs and dioxins remain unclear; however, the direct interaction between these compounds and AhR is one of the most studied pathways (Annamalai & Namasivayam, 2015; Machala, 2001; Zhang, 2016).
AhR is a receptor showing a high affinity for several compounds, many of which are found in PM (Mason, 1994). The prototypical AhR ligand, TCDD (DeGroot, 2011; Denison & Nagy, 2003), affects bone microarchitecture and biomechanical parameters in mice (Finnilä, 2010; Herlin, 2013, 2010; Jämsä, 2001; Miettinen, 2005). AhR activation inhibits osteoblast proliferation and differentiation in vitro in mice and humans (Gierthy, 1994; Korkalainen, 2009; Singh, 2000; Watson, 2019; H. Yu, 2014; Yun, 2018, 2017). Likewise, TCDD can inhibit bone healing in rat arthrodesis models (Hsu, 2015), affect the biomechanical properties of bone tissues in Rhesus monkeys (Macaca mulatta) (Hermsen, 2008), and interrupt skeletal development in zebrafish larvae (Danio rerio) (Burns, 2015). In mouse models, AhR agonists such as 3-methylcholanthrene (3MC) increase bone resorption. Moreover, systemic AhR knockout (AhRKO) mice show increased bone mass and are resistant to bone loss by 3MC due to altered osteoclastogenesis (T. Yu, 2014; T. Yu, 2015). Additionally, 3MC inhibits in vitro osteoblast proliferation and differentiation and in vivo ossification in mice (Naruse, 2002). Moreover, AhR expression in osteoblasts is upregulated due to lead exposure, which in turn increases the sensibility of bone cells to AhR ligands, which could partly explain why lead exposure causes bone damage (Ryan, 2007). Likewise, there is evidence that PAHs in PM stimulate both Th17 differentiation and IL-17 production independent of AhR activation (Castañeda, 2018; van Voorhis, 2013). As mentioned earlier, IL-17 is a crucial inductor of osteoclastogenesis (Adamopoulos, 2010). An inhibitory mechanism between the AhR signaling pathways and the estrogen receptor (ER) has been described (Safe & Wormke, 2003), which could cause the effects of AhR agonists on bone.
Another air pollutant, bisphenol A (BPA), is used to produce polycarbonates and epoxy resins. This compound is considered a xenoestrogen, a synthetic molecule imitating the effects of estrogen. BPA is ubiquitous in the atmosphere, but it reaches its highest concentrations in urban areas (Fu & Kawamura, 2010). This compound is known to bind to estrogen-related receptor gamma (ERRγ) (Matsushima, 2007), which acts as a negative regulator of RUNX2 (Jeong, 2009), an essential transcription factor in the differentiation of mesenchymal stem cells into osteoblasts. Jeong et al. (2009) found that ERRγ inhibition significantly increased osteoblast differentiation, osteocalcin expression, alkaline phosphatase (ALP) activity, and bone mineralization. Binding of BPA to ERRγ has an antiestrogen effect on bone cells, which affects bone metabolism via the RANKL pathway (Thent, 2018), induces osteoclast and osteoblast apoptosis (Hwang, 2013), and reduces the activation of the Wnt/β-catenin signaling pathway (Leem, 2017). Different skeletal effects are detected in animals exposed to BPA (Chin, 2018; Pelch et al, 2012; Toda et al, 2002). However, an association between BPA exposure and low BMD has not been identified in osteoporotic postmenopausal women (Kim, 2012), nor in healthy premenopausal women (Zhao, 2012). Comprehensive longitudinal studies are needed to verify the association between BPA and bone health in humans.
Polyfluoroalkyl substances (PFASs) are synthetic endocrine disruptors used industrially and widely spread in the atmosphere (Annamalai & Namasivayam, 2015). PFASs exposure can increase in vitro bone resorption (Koskela, 2017). Several PFASs have been associated with low BMD and osteoporosis in adults; however, most such associations have been described in women (Khalil, 2016).
Finally, alkylphenol ethoxylates (APEs), another type of EDC, are nonionic surfactants used in the production of plastics, detergents, and paints. Their main components are 4-tert-octylphenol (OP) and 4-nonylphenol (NP), which are both in the atmosphere (Agas, 2013; Annamalai & Namasivayam, 2015). There is evidence that these compounds inhibit osteoclast (Hagiwara, 2008) and osteoblast differentiation (Miyawaki, 2008) and induce osteoblast apoptosis in vitro (Sabbieti, 2011). However, there are no studies in humans that support the potential bone damage induced by these endocrine disruptors.
In summary, several air pollution components considered to be EDCs have proven to produce adverse effects on bone homeostasis in many species. However, the global effect of the mixture of these compounds is unknown. Therefore, another approach has been adopted by researchers such as Novák, who examined the effects of the mixture of these compounds from samples of polluted air (Novák, 2014). In their study, which included PM samples taken from different geographic locations, they found AhR-mediated activity in all samples, which was consistent with the PAH concentrations. When examining the estrogenic effect of different sample fractions, they found contradictory results: weak estrogenic effects, antiestrogenic effects, and no estrogenic effects. These mixed results agree with previous findings (Novák, 2009; Wenger, 2009), and could be explained by the site-specific composition of the mixture. However, as discussed earlier, AhR activation has an inhibitory effect on the estrogen signaling pathways. Thus, due to the presence of several AhR agonists in air pollution, the predominant effect of exposure to atmospheric pollutants could be antiestrogenic (Novák, 2014), with additional damaging effects on bone metabolism.
Some of the mechanisms involved in the antiestrogenic effect of tobacco could be studied regarding air pollutants because their components share similarities. It is thus possible that PM components have, as in tobacco, an antiestrogenic effect mediated by increased levels of sex hormone-binding globulin (Daniel, 1992) and increased hepatic metabolism of estrogens (Michnovicz, 1986), to name a few examples.
4.4. Mechanisms related to metals
Metals are considered as common components of PM (World Health Organization, 2013). The main sources of metals in the atmosphere include industrial and vehicular emissions (Suvarapu & Baek, 2017). While the introduction of unleaded gasoline has significantly reduced the concentration of lead in ambient air, the concentrations of purely anthropogenic heavy metals, such as cadmium (Cd), chromium (Cr), zinc (Zn), and mercury (Hg), are increasing (Suvarapu & Baek, 2017). (For evidence and reviews of the effect of metal exposure on bones, see Rodríguez, 2018). In this section we will focus on the effects of metals in the air.
Lead (Pb) accumulates in the bones due to its ability to replace divalent cations such as calcium, magnesium, and iron (Rodríguez, 2018). Several studies link Pb exposure to lower BMD in humans (Akbal et al., 2014; Campbell & Auinger, 2007; Dongre et al., 2013; Wong et al., 2015). Higher concentrations of metals (lead, cadmium, and cobalt) have been found in biopsies from the femoral head of osteoporotic subjects, compared with controls, particularly in trabecular bone tissue (Scimeca et al., 2017). Pb was present in 92% of the samples of osteoporotic patients, compared with 20% of controls, with significant differences when comparing the accumulation of two or more elements. Evidence suggests a cytotoxic effect of Pb on bone cells mediated by the induction of oxidative stress (Al-Ghafari et al., 2019).
Moreover, the outbreak of the ‘itai-itai disease’ in Japan due to the Cd poisoning of the Jinzu River revealed the harmful effects of this metal (Aoshima, 2017). Itai-itai disease was characterized, among other clinical findings, by frequent fractures and bone deformities. Recent epidemiological studies have shown an association between low-level Cd exposure and lower BMD and higher incidence of fractures (Engström et al., 2012; Wallin et al., 2016), which correlates with studies in experimental animals (Bhattacharyya, 2009). The Cd effect on bones seems to be mediated, as has been proposed for Pb, by the induction of oxidative stress (Al-Ghafari et al., 2019), increased bone resorption, and impaired calcium absorption (Reyes-Hinojosa et al., 2019). Likewise, an indirect effect due to renal involvement induced by Cd has been proposed (Chen et al., 2011).
Another metal in the atmosphere, mercury (Hg), is produced by industrial processes and coal combustion (Suvarapu & Baek, 2017). In fish, methylmercury exposure decreases the levels of tartrate-resistant acid phosphatase (TRAP) and alkaline phosphatase (ALP), which are, respectively, indicators of osteoclastic and osteoblastic activity (Suzuki et al., 2004; Yachiguchi et al., 2014). Methylmercury induces downregulation in ER and IGF-1 genes (Suzuki et al., 2004). A study in mice demonstrated adverse effects on bone formation parameters during embryonic development, which were counteracted by the administration of vitamin E that prevented lipid peroxidation (Abd El-Aziz et al., 2012). Therefore, bone damage caused by Hg could be associated with oxidative stress and the alteration of the estrogenic signaling and decreased activity of IGF-1, both essential for bone growth.
The main sources of chromium (Cr) are vehicle emissions and coal combustion (Suvarapu & Baek, 2017). Rats exposed to hexavalent chromium (Cr [VI]) showed a decrease in TRAP and ALP activity and an alteration in mandibular growth and dental eruption (De Lucca et al., 2009), similar to the effects of Hg. In vitro studies of osteoblasts derived from human osteosarcoma exposed to Co, Cr (III), and Cr (VI) revealed a synergistic effect (particularly between Co and Cr) that reduced osteoblast survival and function, determined by ALP activity and surface mineralization (Andrews et al., 2011; Shah et al., 2015).
Arsenic (As) is a metalloid found in industrial emissions and coal combustion (Suvarapu & Baek, 2017). At low concentrations, As inhibits endochondral ossification in rats due to an increase in cartilage thickness of the growth plate (Aybar Odstrcil et al., 2010). In addition, As affects osteoblastic differentiation and function in rats in in vitro and in vivo models, decreasing bone turnover markers (osteocalcin, procollagen type I N-terminal propeptide, and C-terminal cross-linking telopeptide) and BMD and altering bone microstructure (Hu et al., 2012; Wu et al., 2014). Arsenic has shown to induce apoptosis in bone marrow mesenchymal stem cells (BMSC) and rat osteoblasts (Cai et al., 2010; Tang et al., 2009); As is also associated with oxidative stress induction (Chiu et al., 2016).
While the main sources of nickel (Ni) are motor vehicles and coal and oil burning, cobalt (Co) comes mainly from coal burning (Suvarapu & Baek, 2017). Although there are no reports of the effects of these elements on human bones, Kanaji et al. 2014 showed that Ni and Co have a cytotoxic effect (apoptosis and necrosis, respectively) in in vitro mouse osteocytes at a concentration of 0.50 mM. More studies are needed to determine the potential effect of Ni and Co in humans (Kanaji et al., 2014).
In summary, various metals in PM have a proven effect on bones. Although it is difficult to determine their real impact, the concentration and the indirect effect of metals (e.g., through oxidative stress) in the bone matrix can potentially induce bone damage (Rodríguez & Mandalunis, 2018). In addition, the concentration of anthropogenic metals in the atmosphere is increasing, especially in developing countries (Suvarapu & Baek, 2017), and the risk of a potential synergistic effect of metals (Shah et al., 2015) has to be determined.
4.5. Deficient synthesis of vitamin D
Ozone (O3) is a gas in the stratosphere and troposphere (Leh, 1973). In the stratosphere, ozone protects against the damaging effects of the sun by absorbing UV radiation (Ehhalt, 1999), whereas in the troposphere, whose height ranges from 0 to 7 miles, ozone harms health by acting as an environmental pollutant (Chen, 2007). Tropospheric ozone production depends on the photochemical interaction of nitrogen oxides (NOx) (Monks, 2015) and VOCs (Duan, 2008). The anthropogenic sources of NOx and VOCs are fossil fuel and biomass burning, as well as solvent evaporation (Badr & Probert, 1993; Barletta, 2005). Since it absorbs UV radiation, ozone has been proposed by several studies to be responsible for low UVB exposure and deficient vitamin D synthesis. A cross-sectional study of a cohort of 85 postmenopausal Caucasian women in Belgium who lived in rural and urban areas revealed that women who lived in cities were exposed to three times as much ground-level ozone as those living in rural areas (Manicourt & Devogelaer, 2008). Likewise, women living in cities showed higher prevalence of having 25-hydroxyvitamin D levels below 75 nmol/L, even though they had a higher solar exposure index (SEI). This could be the result of low UVB exposure from high tropospheric ozone levels. It is well-documented that UV radiation is essential for the skin to synthesize vitamin D, and its deficiency affects calcium absorption, which in turn leads to bone density reduction and increased risk of osteoporosis-related fractures (Lips, 2001). Some studies show an association between air pollution and risk of vitamin D deficiency; however, these studies usually combine the values of PM2.5, NO2, and CO. Such studies do not examine the O3 levels separately (Feizabad, 2017) or do not mention how they measured air pollution levels (Agarwal, 2002; Hosseinpanah, 2010). Thus, studies are needed in which ozone levels are measured individually along with UVB levels and concentrations of serum vitamin D.
Because kidneys participate in vitamin D metabolism, kidney damage is another potential mechanism of bone damage associated with air pollution exposure. A recent review from Afsar et al. collected evidence about the role air pollution has in kidney damage (Afsar et al., 2019). Additionally, atmospheric metal (arsenic, cadmium, lead, mercury, and uranium) exposure is directly nephrotoxic. The effects of renal dysfunction on bones are evident in chronic kidney diseases (e.g., chronic kidney disease-mineral bone disorder [CKD-MBD], formerly called renal osteodystrophy). Although the main potential mechanism behind some air pollution-related effects on bones mediated by kidneys is through vitamin D metabolism and secondary hyperparathyroidism (K. A. Hruska & Teitelbaum, 1995), much more complex interactions may exist, including the presence of inhibitors of the Wnt pathway, such as activin A and Dickkopf 1, essential for osteoclastogenesis (Keith A. Hruska et al., 2017). Further research is needed about the potential interaction of kidney damage in the air pollution-mediated bone damage.
5. Conclusions
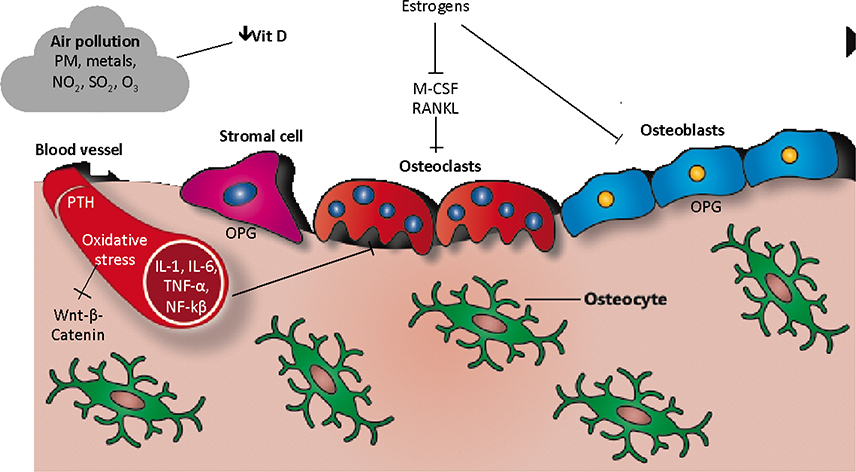
Air pollution predominates in urban areas of most parts of the world, particularly in densely populated and industrialized countries. Importantly, air pollution exposure is a risk factor for respiratory, cardiovascular, and bone diseases. Evidence indicates that exposure to air pollutants is associated with induction of a low-grade systemic inflammatory state and oxidative stress, endocrine disruption, and changes in vitamin D metabolism, with potential effects on bone homeostasis. As we have summarized, several components contribute to air pollution-induced bone damage (Figure 2). It is important to insist that, due to the effects of air pollution on various organs and tissues, particularly kidneys (Schraufnagel et al., 2019), other indirect mechanisms of bone damage related to air pollution could exist.
Figure 2. Cellular and molecular mechanisms involved in air pollution-induced bone damage.
PM: Particulate matter; NO2: Nitrogen dioxide; SO2: Sulphur dioxide; O3: Ozone; OPG: Osteoprotegerin, IL: Interleukin; M-CSF: Macrophage colony-stimulating factor; RANKL: Receptor activator of nuclear factor κ B; TNF-α: Tumor necrosis factor-alpha; NF-kβ: Nuclear factor kappa-light-chain-enhancer of activated B cells; PTH: Parathyroid hormone.
Knowing the mechanisms of bone damage induced by exposure to air pollution will contribute to improved understanding of the physiopathology of bone damage associated with environmental exposure, as well as to implementing new and better prevention strategies. Acknowledging air pollution as a modifiable risk factor for osteoporosis could improve environmental policies, which will have positive effects on bone health, prevent fractures, and reduce health-associated costs. Further research about mechanisms, mixtures and other air pollution components, as well as potential interventions to prevent air pollution-induced bone damage are guaranteed.
Funding:
This work was supported by National Institutes of Health [grants R01ES025225, P30ES009089 Baccarelli; R21ES027087 Prada & Baccarelli] and the Consejo Nacional de Ciencia y Tecnologia, CONACYT – Mexico [FOSISS 2017–289503, Prada; 2018 A3-S-48281, García-Cuellar].
Abbreviation and acronym index
- 3MC
3-methylcolatren
- AhR
Aryl hydrocarbon receptor
- ALP
Alkaline phosphatase
- AM
Alveolar macrophage
- AP1
Activator protein 1
- APEs
Alkylphenol ethoxylates
- BaP
Benzo[a]pyrene
- BBP
Butyl benzyl phthalate
- BMC
Bone mineral content
- BMD
Bone mineral density
- BPA
Bisphenol A
- CRP
C-reactive protein
- Cyp1
Cytochrome 450, family 1
- DBP
Dibutyl phthalate
- DEHP
Di-2-ethyl hexyl phthalate
- DL-PCBs
Dioxin-like polychlorinated biphenyls
- DXA
Dual-energy X-ray absorptiometry
- EDC
Endocrine disrupting chemicals
- ERRγ
Estrogen-related receptor γ
- FGF2
Fibroblast growth factor 2
- FOXO
Forkhead box protein O
- GM-CSF
Granulocyte and macrophage colony stimulating factor
- HIF1α
Hypoxia inducible factor 1α
- HULIS
Humic-like substances
- IGF-1
Insulin-like growth factor-1
- LPS
Lipopolysaccharide
- MCP-1
Monocyte chemoattractant protein 1
- MCSF
Macrophage colony stimulating factor
- MIP-1α
Macrophage inflammatory protein 1α
- MyD88
Myeloid differentiation primary response 88
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
Natural killer
- NO
Nitrogen monoxide
- NO2
Nitrogen dioxide
- NOX
Nitrogen oxides
- NP
4-nonylphenol
- Nrf2
Nuclear factor erythroid 2-related factor 2
- O3
Ozone
- OPE
Organic powder extract
- OP
4-tert-octylphenol
- OPG
Osteoprotegerin
- PAHs
Polycyclic aromatic hydrocarbons
- PCBs
Polychlorinated biphenyls
- PFASs
Perfluoroalkylated substances
- PM
Particulate matter
- POC
Persistent organochlorine compounds
- PPARγ
Peroxisome proliferator activated receptor γ
- PTH
Parathyroid hormone
- PVC
Polyvinyl chloride
- RANKL
Receptor activator of nuclear factor kappa-Β ligand
- RNS
Reactive nitrogen species
- ROS
Reactive oxygen species
- Runx2
Run-related transcription factor 2
- SEI
Sun exposure index
- Sema4D
Semaphorin 4D
- SO2,
Sulfur dioxide
- SVOC
Semi volatile organic compounds
- TCDD
Tetrachlorodibenzo-p-dioxin
- TLR
Toll-like receptor
- TNFα
Tumor necrosis factor α
- UVB
Ultraviolet radiation B
- VOC
Volatile organic compounds
Footnotes
Competing interests: None.
References
- Abd El-Aziz GS, El-Fark MMO, & Saleh HAM (2012). The prenatal toxic effect of methylmercury on the development of the appendicular skeleton of rat fetuses and the protective role of vitamin E. Anatomical Record (Hoboken, N.J.: 2007), 295(6), 939–949. 10.1002/ar.22485 [DOI] [PubMed] [Google Scholar]
- Adamopoulos IE, Chao C-C, Geissler R, Laface D, Blumenschein W, Iwakura Y, … Bowman EP (2010). Interleukin-17A upregulates receptor activator of NF-kappaB on osteoclast precursors. Arthritis Research & Therapy, 12(1), R29 10.1186/ar2936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afsar B, Elsurer Afsar R, Kanbay A, Covic A, Ortiz A, & Kanbay M (2019). Air pollution and kidney disease: Review of current evidence. Clinical Kidney Journal, 12(1), 19–32. 10.1093/ckj/sfy111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, & Puliyel JM (2002). The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Archives of Disease in Childhood, 87(2), 111–113. 10.1136/adc.87.2.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agas D, Sabbieti MG, Capacchietti M, Materazzi S, Menghi G, Materazzi G, … Marchetti L (2007). Benzyl butyl phthalate influences actin distribution and cell proliferation in rat Py1a osteoblasts. Journal of Cellular Biochemistry, 101(3), 543–551. 10.1002/jcb.21212 [DOI] [PubMed] [Google Scholar]
- Agas D, Sabbieti MG, & Marchetti L (2013). Endocrine disruptors and bone metabolism. Archives of Toxicology, 87(4), 735–751. 10.1007/s00204-012-0988-y [DOI] [PubMed] [Google Scholar]
- Akbal A, Tutkun E, & Yilmaz H (2014). Lead exposure is a risk for worsening bone mineral density in middle-aged male workers. The Aging Male: The Official Journal of the International Society for the Study of the Aging Male, 17(3), 189–193. 10.3109/13685538.2013.836482 [DOI] [PubMed] [Google Scholar]
- Al-Ghafari A, Elmorsy E, Fikry E, Alrowaili M, & Carter WG (2019). The heavy metals lead and cadmium are cytotoxic to human bone osteoblasts via induction of redox stress. PloS One, 14(11), e0225341 10.1371/journal.pone.0225341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Moreno E, Torres V, Miranda J, Martínez L, García-Cuellar C, Nawrot TS, … Osornio-Vargas AR (2009). Induction of IL-6 and inhibition of IL-8 secretion in the human airway cell line Calu-3 by urban particulate matter collected with a modified method of PM sampling. Environmental Research, 109(5), 528–535. 10.1016/j.envres.2009.02.010 [DOI] [PubMed] [Google Scholar]
- Almeida M, Ambrogini E, Han L, Manolagas SC, & Jilka RL (2009). Increased Lipid Oxidation Causes Oxidative Stress, Increased Peroxisome Proliferator-activated Receptor-γ Expression, and Diminished Pro-osteogenic Wnt Signaling in the Skeleton. The Journal of Biological Chemistry, 284(40), 27438–27448. 10.1074/jbc.M109.023572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida M, & O’Brien CA (2013). Basic Biology of Skeletal Aging: Role of Stress Response Pathways. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 68(10), 1197–1208. 10.1093/gerona/glt079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvaer K, Meyer HE, Falch JA, Nafstad P, & Søgaard AJ (2007). Outdoor air pollution and bone mineral density in elderly men—The Oslo Health Study. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 18(12), 1669–1674. 10.1007/s00198-007-0424-y [DOI] [PubMed] [Google Scholar]
- Alvarez-Lloret P, Lind PM, Nyberg I, Orberg J, & Rodríguez-Navarro AB (2009). Effects of 3,3’,4,4’,5-pentachlorobiphenyl (PCB126) on vertebral bone mineralization and on thyroxin and vitamin D levels in Sprague-Dawley rats. Toxicology Letters, 187(2), 63–68. 10.1016/j.toxlet.2009.01.030 [DOI] [PubMed] [Google Scholar]
- Alver K, Meyer HE, Falch JA, & Søgaard AJ (2010). Outdoor air pollution, bone density and self-reported forearm fracture: The Oslo Health Study. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 21(10), 1751–1760. 10.1007/s00198-009-1130-8 [DOI] [PubMed] [Google Scholar]
- Andrews RE, Shah KM, Wilkinson JM, & Gartland A (2011). Effects of cobalt and chromium ions at clinically equivalent concentrations after metal-on-metal hip replacement on human osteoblasts and osteoclasts: Implications for skeletal health. Bone, 49(4), 717–723. 10.1016/j.bone.2011.06.007 [DOI] [PubMed] [Google Scholar]
- Annamalai J, & Namasivayam V (2015). Endocrine disrupting chemicals in the atmosphere: Their effects on humans and wildlife. Environment International, 76, 78–97. 10.1016/j.envint.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Aoshima K (2017). Itai-itai disease: Lessons from the investigations of environmental epidemiology conducted in the 1970’s, with special reference to the studies of the Toyama Institute of Health. Nihon Eiseigaku Zasshi. Japanese Journal of Hygiene, 72(3), 149–158. 10.1265/jjh.72.149 [DOI] [PubMed] [Google Scholar]
- Araujo JA (2010). Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Quality, Atmosphere, & Health, 4(1), 79–93. 10.1007/s11869-010-0101-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aybar Odstrcil A del C, Carino SN, Ricci JCD, & Mandalunis PM (2010). Effect of arsenic in endochondral ossification of experimental animals. Experimental and Toxicologic Pathology: Official Journal of the Gesellschaft Fur Toxikologische Pathologie, 62(3), 243–249. 10.1016/j.etp.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Badr O, & Probert SD (1993). Oxides of nitrogen in the Earth’s atmosphere: Trends, sources, sinks and environmental impacts. Applied Energy, 46(1), 1–67. 10.1016/0306-2619(93)90076-2 [DOI] [Google Scholar]
- Barletta B, Meinardi S, Sherwood Rowland F, Chan C-Y, Wang X, Zou S, … Blake DR (2005). Volatile organic compounds in 43 Chinese cities. Atmospheric Environment, 39(32), 5979–5990. 10.1016/j.atmosenv.2005.06.029 [DOI] [Google Scholar]
- Becker S, Dailey L, Soukup JM, Silbajoris R, & Devlin RB (2005). TLR-2 is involved in airway epithelial cell response to air pollution particles. Toxicology and Applied Pharmacology, 203(1), 45–52. 10.1016/j.taap.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Benedetti MG, Furlini G, Zati A, & Letizia Mauro G (2018). The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. BioMed Research International, 2018, 4840531 10.1155/2018/4840531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat FA, Ramajayam G, Parameswari S, Vignesh RC, Karthikeyan S, Senthilkumar K, … Srinivasan N (2013). Di 2-ethyl hexyl phthalate affects differentiation and matrix mineralization of rat calvarial osteoblasts—In vitro. Toxicology in Vitro: An International Journal Published in Association with BIBRA, 27(1), 250–256. 10.1016/j.tiv.2012.09.003 [DOI] [PubMed] [Google Scholar]
- Bhattacharyya MH (2009). Cadmium osteotoxicity in experimental animals: Mechanisms and relationship to human exposures. Toxicology and Applied Pharmacology, 238(3), 258–265. 10.1016/j.taap.2009.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard F, Duplomb L, Baud’huin M, & Brounais B (2009). The dual role of IL-6-type cytokines on bone remodeling and bone tumors. Cytokine & Growth Factor Reviews, 20(1), 19–28. 10.1016/j.cytogfr.2008.11.004 [DOI] [PubMed] [Google Scholar]
- Bord S, Ireland DC, Beavan SR, & Compston JE (2003). The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone, 32(2), 136–141. [DOI] [PubMed] [Google Scholar]
- Brankovič J, Fazarinc G, Antanasova M, Jevnikar P, Jan J, Anders I, … Vrecl M (2019). Lactational exposure to dioxin-like polychlorinated biphenyl 169 and nondioxin-like polychlorinated biphenyl 155: Effects on rat femur growth, biomechanics and mineral composition. Ecotoxicology and Environmental Safety, 180, 106–113. 10.1016/j.ecoenv.2019.04.076 [DOI] [PubMed] [Google Scholar]
- Brankovič J, Jovanovski S, Jevnikar P, Hofmeister A, Reininger-Gutmann B, Jan J, … Vrecl M (2017). Alterations in geometry, biomechanics, and mineral composition of juvenile rat femur induced by nonplanar PCB-155 and/or planar PCB-169. Environmental Toxicology, 32(4), 1135–1146. 10.1002/tox.22309 [DOI] [PubMed] [Google Scholar]
- Brennan SL, Pasco JA, Urquhart DM, Oldenburg B, Hanna FS, & Wluka AE (2010). The association between urban or rural locality and hip fracture in community-based adults: A systematic review. Journal of Epidemiology and Community Health, 64(8), 656–665. 10.1136/jech.2008.085738 [DOI] [PubMed] [Google Scholar]
- Briot K, Geusens P, Em Bultink I, Lems WF, & Roux C (2017). Inflammatory diseases and bone fragility. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 28(12), 3301–3314. 10.1007/s00198-017-4189-7 [DOI] [PubMed] [Google Scholar]
- Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, & Tosteson A (2007). Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 22(3), 465–475. 10.1359/jbmr.061113 [DOI] [PubMed] [Google Scholar]
- Burns FR, Peterson RE, & Heideman W (2015). Dioxin disrupts cranial cartilage and dermal bone development in zebrafish larvae. Aquatic Toxicology (Amsterdam, Netherlands), 164, 52–60. 10.1016/j.aquatox.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai B-Z, Meng F-Y, Zhu S-L, Zhao J, Liu J-Q, Liu C-J, Chen N, Ye M-L, Li Z-Y, Ai J, Lu Y-J, & Yang B-F (2010). Arsenic trioxide induces the apoptosis in bone marrow mesenchymal stem cells by intracellular calcium signal and caspase-3 pathways. Toxicology Letters, 193(2), 173–178. 10.1016/j.toxlet.2010.01.001 [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Francolira M, Torres-Jardón R, Peña-Cruz B, Palacios-López C, … Frenk S (2013). Exposure to urban air pollution and bone health in clinically healthy six-year-old children. Arhiv Za Higijenu Rada I Toksikologiju, 64(1), 23–34. 10.2478/10004-1254-64-2013-2219 [DOI] [PubMed] [Google Scholar]
- Campbell JR, & Auinger P (2007). The association between blood lead levels and osteoporosis among adults—Results from the third national health and nutrition examination survey (NHANES III). Environmental Health Perspectives, 115(7), 1018–1022. 10.1289/ehp.9716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda AR, Pinkerton KE, Bein KJ, Magaña-Méndez A, Yang HT, Ashwood P, & Vogel CFA (2018). Ambient particulate matter activates the aryl hydrocarbon receptor in dendritic cells and enhances Th17 polarization. Toxicology Letters, 292, 85–96. 10.1016/j.toxlet.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K-H, Chang M-Y, Muo C-H, Wu T-N, Hwang B-F, Chen C-Y, … Kao C-H (2015). Exposure to air pollution increases the risk of osteoporosis: A nationwide longitudinal study. Medicine, 94(17), e733 10.1097/MD.0000000000000733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen T-M, Gokhale J, Shofer S, & Kuschner WG (2007). Outdoor air pollution: Ozone health effects. The American Journal of the Medical Sciences, 333(4), 244–248. 10.1097/MAJ.0b013e31803b8e8c [DOI] [PubMed] [Google Scholar]
- Chen X, Zhu G, Jin T, Lei L, & Liang Y (2011). Bone mineral density is related with previous renal dysfunction caused by cadmium exposure. Environmental Toxicology and Pharmacology, 32(1), 46–53. 10.1016/j.etap.2011.03.007 [DOI] [PubMed] [Google Scholar]
- Chen Y, Lv D, Li X, & Zhu T (2018). PM2.5-bound phthalates in indoor and outdoor air in Beijing: Seasonal distributions and human exposure via inhalation. Environmental Pollution (Barking, Essex: 1987), 241, 369–377. 10.1016/j.envpol.2018.05.081 [DOI] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Karim R, Toledo-Corral CM, Watanabe RM, Xiang AH, … Gilliland FD (2015). Living near a freeway is associated with lower bone mineral density among Mexican Americans. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 26(6), 1713–1721. 10.1007/s00198-015-3051-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalley T, Herrmann FR, Delmi M, Stern R, Hoffmeyer P, Rapin CH, & Rizzoli R (2002). Evaluation of the age-adjusted incidence of hip fractures between urban and rural areas: The difference is not related to the prevalence of institutions for the elderly. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 13(2), 113–118. 10.1007/s001980200002 [DOI] [PubMed] [Google Scholar]
- Chin K-Y, Pang K-L, & Mark-Lee WF (2018). A Review on the Effects of Bisphenol A and Its Derivatives on Skeletal Health. International Journal of Medical Sciences, 15(10), 1043–1050. 10.7150/ijms.25634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu P-R, Hu Y-C, Hsieh B-S, Huang T-C, Cheng H-L, Huang L-W, & Chang K-L (2016). Osteoblasts activate the Nrf2 signalling pathway in response to arsenic trioxide treatment. The International Journal of Biochemistry & Cell Biology, 79, 327–336. 10.1016/j.biocel.2016.08.036 [DOI] [PubMed] [Google Scholar]
- Cooley HM, & Jones G (2002). Symptomatic fracture incidence in southern Tasmania: Does living in the country reduce your fracture risk? Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 13(4), 317–322. 10.1007/s001980200032 [DOI] [PubMed] [Google Scholar]
- Cooper C (1993). The epidemiology of fragility fractures: Is there a role for bone quality? Calcified Tissue International, 53 Suppl 1, S23–26. [DOI] [PubMed] [Google Scholar]
- Cooper C (1997). The crippling consequences of fractures and their impact on quality of life. The American Journal of Medicine, 103(2A), 12S–17S; discussion 17S-19S. [DOI] [PubMed] [Google Scholar]
- Cooper C, Harvey N, Javaid K, Hanson M, & Dennison E (2008). Growth and bone development. Nestle Nutrition Workshop Series. Paediatric Programme, 61, 53–68. 10.1159/000113170 [DOI] [PubMed] [Google Scholar]
- Corcoran A, & Cotter TG (2013). Redox regulation of protein kinases. The FEBS Journal, 280(9), 1944–1965. 10.1111/febs.12224 [DOI] [PubMed] [Google Scholar]
- Currey JD (2003). Role of collagen and other organics in the mechanical properties of bone. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 14 Suppl 5, S29–36. 10.1007/s00198-003-1470-8 [DOI] [PubMed] [Google Scholar]
- Curtis EM, Moon RJ, Harvey NC, & Cooper C (2017). The impact of fragility fracture and approaches to osteoporosis risk assessment worldwide. Bone, 104, 29–38. 10.1016/j.bone.2017.01.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel M, Martin AD, & Drinkwater DT (1992). Cigarette smoking, steroid hormones, and bone mineral density in young women. Calcified Tissue International, 50(4), 300–305. 10.1007/bf00301626 [DOI] [PubMed] [Google Scholar]
- Darbre PD (2018). Overview of air pollution and endocrine disorders. International Journal of General Medicine, 11, 191–207. 10.2147/IJGM.S102230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta HK, Ng WF, Walker JA, Tuck SP, & Varanasi SS (2008). The cell biology of bone metabolism. Journal of Clinical Pathology, 61(5), 577–587. 10.1136/jcp.2007.048868 [DOI] [PubMed] [Google Scholar]
- Daugaard-Petersen T, Langebæk R, Rigét FF, Dyck M, Letcher RJ, Hyldstrup L, … Sonne C (2018a). Persistent organic pollutants and penile bone mineral density in East Greenland and Canadian polar bears (Ursus maritimus) during 1996–2015. Environment International, 114, 212–218. 10.1016/j.envint.2018.02.022 [DOI] [PubMed] [Google Scholar]
- Daugaard-Petersen T, Langebæk R, Rigét FF, Letcher RJ, Hyldstrup L, Jensen J-EB, … Sonne C (2018b). Persistent organic pollutants, skull size and bone density of polar bears (Ursus maritimus) from East Greenland 1892–2015 and Svalbard 1964–2004. Environmental Research, 162, 74–80. 10.1016/j.envres.2017.12.009 [DOI] [PubMed] [Google Scholar]
- DeFlorio-Barker SA, & Turyk ME (2016). Associations between bone mineral density and urinary phthalate metabolites among post-menopausal women: A cross-sectional study of NHANES data 2005–2010. International Journal of Environmental Health Research, 26(3), 326–345. 10.1080/09603123.2015.1111312 [DOI] [PubMed] [Google Scholar]
- DeGroot D, He G, Fraccalvieri D, Bonati L, Pandini A, & Denison MS (2011). AHR Ligands: Promiscuity in Binding and Diversity in Response. In The AH Receptor in Biology and Toxicology (pp. 63–79). 10.1002/9781118140574.ch4 [DOI] [Google Scholar]
- De Lucca RC, Dutrey PL, Villarino ME, & Ubios AM (2009). Effect of different doses of hexavalent chromium on mandibular growth and tooth eruption in juvenile Wistar rats. Experimental and Toxicologic Pathology: Official Journal of the Gesellschaft Fur Toxikologische Pathologie, 61(4), 347–352. 10.1016/j.etp.2008.10.001 [DOI] [PubMed] [Google Scholar]
- Denison MS, & Nagy SR (2003). Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annual Review of Pharmacology and Toxicology, 43, 309–334. 10.1146/annurev.pharmtox.43.100901.135828 [DOI] [PubMed] [Google Scholar]
- Dilsen G, Aydin R, Oral A, Sepici V, Alparsan B, Berker C, … Cinar A (1993). Regional differences in hip fracture risk in Turkey. Bone, 14 Suppl 1, S65–68. [DOI] [PubMed] [Google Scholar]
- Ding C, Parameswaran V, Udayan R, Burgess J, & Jones G (2008). Circulating levels of inflammatory markers predict change in bone mineral density and resorption in older adults: A longitudinal study. The Journal of Clinical Endocrinology and Metabolism, 93(5), 1952–1958. 10.1210/jc.2007-2325 [DOI] [PubMed] [Google Scholar]
- Dongre NN, Suryakar AN, Patil AJ, Hundekari IA, & Devarnavadagi BB (2013). Biochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral density. Indian Journal of Clinical Biochemistry: IJCB, 28(1), 65–70. 10.1007/s12291-012-0241-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan J, Tan J, Yang L, Wu S, & Hao J (2008). Concentration, sources and ozone formation potential of volatile organic compounds (VOCs) during ozone episode in Beijing. Atmospheric Research, 88(1), 25–35. 10.1016/j.atmosres.2007.09.004 [DOI] [Google Scholar]
- Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, & Riggs BL (2003). Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. The Journal of Clinical Investigation, 111(8), 1221–1230. 10.1172/JCI17215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehhalt DH (1999). Photooxidation of trace gases in the troposphere Plenary Lecture. Physical Chemistry Chemical Physics, 1(24), 5401–5408. 10.1039/A905097C [DOI] [Google Scholar]
- Elmståhl S, Gärdsell P, Ringsberg K, & Sernbo I (1993). Body composition and its relation to bone mass and fractures in an urban and a rural population. Aging (Milan, Italy), 5(1), 47–54. [DOI] [PubMed] [Google Scholar]
- Ema M, Amano H, & Ogawa Y (1994). Characterization of the developmental toxicity of di-n-butyl phthalate in rats. Toxicology, 86(3), 163–174. [DOI] [PubMed] [Google Scholar]
- Ema M, Itami T, & Kawasaki H (1993). Teratogenic phase specificity of butyl benzyl phthalate in rats. Toxicology, 79(1), 11–19. [DOI] [PubMed] [Google Scholar]
- Engström A, Michaëlsson K, Vahter M, Julin B, Wolk A, & Åkesson A (2012). Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone, 50(6), 1372–1378. 10.1016/j.bone.2012.03.018 [DOI] [PubMed] [Google Scholar]
- Erdogan S, Baysal A, Akba O, & Hamamci C (2007). Interaction of metals with humic acid isolated from oxidized coal (Vol. 16). [Google Scholar]
- Falch JA, Ilebekk A, & Slungaard U (1985). Epidemiology of hip fractures in Norway. Acta Orthopaedica Scandinavica, 56(1), 12–16. [DOI] [PubMed] [Google Scholar]
- Falch JA, Kaastad TS, Bøhler G, Espeland J, & Sundsvold OJ (1993). Secular increase and geographical differences in hip fracture incidence in Norway. Bone, 14(4), 643–645. [DOI] [PubMed] [Google Scholar]
- Farooqui AA (2014). Inflamación y estrés oxidativo en trastornos neurológicos, Texto original: Efecto del estilo de vida, los genes y la edad. Retrieved from https://www.springer.com/us/book/9783319041100
- Feizabad E, Hossein-Nezhad A, Maghbooli Z, Ramezani M, Hashemian R, & Moattari S (2017). Impact of air pollution on vitamin D deficiency and bone health in adolescents. Archives of Osteoporosis, 12(1), 34 10.1007/s11657-017-0323-6 [DOI] [PubMed] [Google Scholar]
- Filip RS, & Zagórski J (2001). Bone mineral density and osteoporosis in rural and urban women. Epidemiological study of the Lublin region (Eastern Poland). Annals of Agricultural and Environmental Medicine: AAEM, 8(2), 221–226. [PubMed] [Google Scholar]
- Finnilä MAJ, Zioupos P, Herlin M, Miettinen HM, Simanainen U, Håkansson H, … Jämsä T (2010). Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure on bone material properties. Journal of Biomechanics, 43(6), 1097–1103. 10.1016/j.jbiomech.2009.12.011 [DOI] [PubMed] [Google Scholar]
- Finsen V, & Benum P (1987). Changing incidence of hip fractures in rural and urban areas of central Norway. Clinical Orthopaedics and Related Research, (218), 104–110. [PubMed] [Google Scholar]
- Finsen V, Johnsen LG, Tranø G, Hansen B, & Sneve KS (2004). Hip fracture incidence in central norway: A followup study. Clinical Orthopaedics and Related Research, (419), 173–178. [DOI] [PubMed] [Google Scholar]
- Florencio-Silva R, Sasso GR da S, Sasso-Cerri E, Simões MJ, & Cerri PS (2015). Biology of Bone Tissue: Structure, Function, and Factors That Influence Bone Cells. BioMed Research International, 2015, 421746 10.1155/2015/421746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonseca H, Moreira-Gonçalves D, Coriolano H-JA, & Duarte JA (2014). Bone quality: The determinants of bone strength and fragility. Sports Medicine (Auckland, N.Z.), 44(1), 37–53. 10.1007/s40279-013-0100-7 [DOI] [PubMed] [Google Scholar]
- Fu P, & Kawamura K (2010). Ubiquity of bisphenol A in the atmosphere. Environmental Pollution (Barking, Essex: 1987), 158(10), 3138–3143. 10.1016/j.envpol.2010.06.040 [DOI] [PubMed] [Google Scholar]
- Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H, … Price JS (2013). Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. The Journal of Biological Chemistry, 288(13), 9035–9048. 10.1074/jbc.M112.405456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärdsell P, Johnell O, Nilsson BE, & Sernbo I (1991). Bone mass in an urban and a rural population: A comparative, population-based study in southern Sweden. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 6(1), 67–75. 10.1002/jbmr.5650060112 [DOI] [PubMed] [Google Scholar]
- Ghio AJ, & Cohen MD (2005). Disruption of iron homeostasis as a mechanism of biologic effect by ambient air pollution particles. Inhalation Toxicology, 17(13), 709–716. 10.1080/08958370500224482 [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Soukup JM, & Dailey LA (2016). Air pollution particles and iron homeostasis. Biochimica Et Biophysica Acta, 1860(12), 2816–2825. 10.1016/j.bbagen.2016.05.026 [DOI] [PubMed] [Google Scholar]
- Ghio AJ, Soukup JM, Dailey LA, Tong H, Kesic MJ, Budinger GRS, & Mutlu GM (2015). Wood Smoke Particle Sequesters Cell Iron to Impact a Biological Effect. Chemical Research in Toxicology, 28(11), 2104–2111. 10.1021/acs.chemrestox.5b00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Soukup JM, & Madden MC (2018). The toxicology of air pollution predicts its epidemiology. Inhalation Toxicology, 30(9–10), 327–334. 10.1080/08958378.2018.1530316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gierthy JF, Silkworth JB, Tassinari M, Stein GS, & Lian JB (1994). 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits differentiation of normal diploid rat osteoblasts in vitro. Journal of Cellular Biochemistry, 54(2), 231–238. 10.1002/jcb.240540211 [DOI] [PubMed] [Google Scholar]
- Glynn AW, Michaëlsson K, Lind PM, Wolk A, Aune M, Atuma S, … Mallmin H (2000). Organochlorines and bone mineral density in Swedish men from the general population. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 11(12), 1036–1042. 10.1007/s001980070025 [DOI] [PubMed] [Google Scholar]
- Gu W, Rennie KL, Lin X, Wang Y, & Yu Z (2007). Differences in bone mineral status between urban and rural Chinese men and women. Bone, 41(3), 393–399. 10.1016/j.bone.2007.05.010 [DOI] [PubMed] [Google Scholar]
- Guo J, Huang Y, Bian S, Zhao C, Jin Y, Yu D, … Chen G (2018). Associations of urinary polycyclic aromatic hydrocarbons with bone mass density and osteoporosis in U.S. adults, NHANES 2005–2010. Environmental Pollution (Barking, Essex: 1987), 240, 209–218. 10.1016/j.envpol.2018.04.108 [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Sugizaki T, Tsukamoto Y, Senoh E, Goto T, & Ishihara Y (2008). Effects of alkylphenols on bone metabolism in vivo and in vitro. Toxicology Letters, 181(1), 13–18. 10.1016/j.toxlet.2008.06.863 [DOI] [PubMed] [Google Scholar]
- Herlin M, Finnilä MAJ, Zioupos P, Aula A, Risteli J, Miettinen HM, … Viluksela M (2013). New insights to the role of aryl hydrocarbon receptor in bone phenotype and in dioxin-induced modulation of bone microarchitecture and material properties. Toxicology and Applied Pharmacology, 273(1), 219–226. 10.1016/j.taap.2013.09.002 [DOI] [PubMed] [Google Scholar]
- Herlin M, Kalantari F, Stern N, Sand S, Larsson S, Viluksela M, … Håkansson H (2010). Quantitative characterization of changes in bone geometry, mineral density and biomechanical properties in two rat strains with different Ah-receptor structures after long-term exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicology, 273(1–3), 1–11. 10.1016/j.tox.2010.04.006 [DOI] [PubMed] [Google Scholar]
- Hermsen SAB, Larsson S, Arima A, Muneoka A, Ihara T, Sumida H, … Lind PM (2008). In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) affects bone tissue in rhesus monkeys. Toxicology, 253(1–3), 147–152. 10.1016/j.tox.2008.09.005 [DOI] [PubMed] [Google Scholar]
- Hodgson S, Thomas L, Fattore E, Lind PM, Alfven T, Hellström L, … Jarup L (2008). Bone mineral density changes in relation to environmental PCB exposure. Environmental Health Perspectives, 116(9), 1162–1166. 10.1289/ehp.11107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinpanah F, Pour SH, Heibatollahi M, Moghbel N, Asefzade S, & Azizi F (2010). The effects of air pollution on vitamin D status in healthy women: A cross sectional study. BMC Public Health, 10, 519 10.1186/1471-2458-10-519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska Keith A., Sugatani T, Agapova O, & Fang Y (2017). The chronic kidney disease - Mineral bone disorder (CKD-MBD): Advances in pathophysiology. Bone, 100, 80–86. 10.1016/j.bone.2017.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruska KA, & Teitelbaum SL (1995). Renal Osteodystrophy. New England Journal of Medicine, 333(3), 166–175. doi: 10.1056/nejm199507203330307 [DOI] [PubMed] [Google Scholar]
- Hsu EL, Sonn K, Kannan A, Bellary S, Yun C, Hashmi S, … Hsu WK (2015). Dioxin Exposure Impairs BMP-2-Mediated Spinal Fusion in a Rat Arthrodesis Model. The Journal of Bone and Joint Surgery. American Volume, 97(12), 1003–1010. 10.2106/JBJS.N.01311 [DOI] [PubMed] [Google Scholar]
- Hu Y-C, Cheng H-L, Hsieh B-S, Huang L-W, Huang T-C, & Chang K-L (2012). Arsenic trioxide affects bone remodeling by effects on osteoblast differentiation and function. Bone, 50(6), 1406–1415. 10.1016/j.bone.2012.03.012 [DOI] [PubMed] [Google Scholar]
- Huang S-L, Hsu M-K, & Chan C-C (2003). Effects of submicrometer particle compositions on cytokine production and lipid peroxidation of human bronchial epithelial cells. Environmental Health Perspectives, 111(4), 478–482. 10.1289/ehp.5519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang JK, Min KH, Choi KH, Hwang YC, Jeong I-K, Ahn KJ, … Chang JS (2013). Bisphenol A reduces differentiation and stimulates apoptosis of osteoclasts and osteoblasts. Life Sciences, 93(9–11), 367–372. 10.1016/j.lfs.2013.07.020 [DOI] [PubMed] [Google Scholar]
- Iqbal J, Sun L, Cao J, Yuen T, Lu P, Bab I, … Avadhani NG (2013). Smoke carcinogens cause bone loss through the aryl hydrocarbon receptor and induction of Cyp1 enzymes. Proceedings of the National Academy of Sciences of the United States of America, 110(27), 11115–11120. 10.1073/pnas.1220919110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jämsä T, Viluksela M, Tuomisto JT, Tuomisto J, & Tuukkanen J (2001). Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on bone in two rat strains with different aryl hydrocarbon receptor structures. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 16(10), 1812–1820. 10.1359/jbmr.2001.16.10.1812 [DOI] [PubMed] [Google Scholar]
- Jarnlo GB, Jakobsson B, Ceder L, & Thorngren KG (1989). Hip fracture incidence in Lund, Sweden, 1966–1986. Acta Orthopaedica Scandinavica, 60(3), 278–282. [DOI] [PubMed] [Google Scholar]
- Jeong B-C, Lee Y-S, Park Y-Y, Bae I-H, Kim D-K, Koo S-H, … Choi H-S (2009). The orphan nuclear receptor estrogen receptor-related receptor gamma negatively regulates BMP2-induced osteoblast differentiation and bone formation. The Journal of Biological Chemistry, 284(21), 14211–14218. 10.1074/jbc.M808345200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jónsson B, Gärdsell P, Johnell O, Redlund-Johnell I, & Sernbo I (1992). Differences in fracture pattern between an urban and a rural population: A comparative population-based study in southern Sweden. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 2(6), 269–273. [DOI] [PubMed] [Google Scholar]
- Jónsson B, Gärdsell P, Johnell O, Sernbo I, & Gullberg B (1993). Life-style and different fracture prevalence: A cross-sectional comparative population-based study. Calcified Tissue International, 52(6), 425–433. [DOI] [PubMed] [Google Scholar]
- Kaastad TS, Meyer HE, & Falch JA (1998). Incidence of hip fracture in Oslo, Norway: Differences within the city. Bone, 22(2), 175–178. [DOI] [PubMed] [Google Scholar]
- Kanaji A, Orhue V, Caicedo MS, Virdi AS, Sumner DR, Hallab NJ, Yoshiaki T, & Sena K (2014). Cytotoxic effects of cobalt and nickel ions on osteocytes in vitro. Journal of Orthopaedic Surgery and Research, 9, 91 10.1186/s13018-014-0091-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya E, Dumanoglu Y, Kara M, Altiok H, Bayram A, Elbir T, & Odabasi M (2012). Spatial and temporal variation and air–soil exchange of atmospheric PAHs and PCBs in an industrial region. Atmospheric Pollution Research, 3(4), 435–449. 10.5094/APR.2012.050 [DOI] [Google Scholar]
- Khalil N, Chen A, Lee M, Czerwinski SA, Ebert JR, DeWitt JC, & Kannan K (2016). Association of Perfluoroalkyl Substances, Bone Mineral Density, and Osteoporosis in the U.S. Population in NHANES 2009–2010. Environmental Health Perspectives, 124(1), 81–87. 10.1289/ehp.1307909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Oh CH, Hwang Y-C, Jeong I-K, Ahn KJ, Chung H-Y, & Chang J-S (2012). Serum bisphenol a concentration in postmenopausal women with osteoporosis. Journal of Bone Metabolism, 19(2), 87–93. 10.11005/jbm.2012.19.2.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K-H, Kabir E, & Kabir S (2015). A review on the human health impact of airborne particulate matter. Environment International, 74, 136–143. 10.1016/j.envint.2014.10.005 [DOI] [PubMed] [Google Scholar]
- Kitaura H, Kimura K, Ishida M, Kohara H, Yoshimatsu M, & Takano-Yamamoto T (2013). Immunological reaction in TNF-α-mediated osteoclast formation and bone resorption in vitro and in vivo. Clinical & Developmental Immunology, 2013, 181849 10.1155/2013/181849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkalainen M, Kallio E, Olkku A, Nelo K, Ilvesaro J, Tuukkanen J, … Viluksela M (2009). Dioxins interfere with differentiation of osteoblasts and osteoclasts. Bone, 44(6), 1134–1142. 10.1016/j.bone.2009.02.019 [DOI] [PubMed] [Google Scholar]
- Koskela A, Koponen J, Lehenkari P, Viluksela M, Korkalainen M, & Tuukkanen J (2017). Perfluoroalkyl substances in human bone: Concentrations in bones and effects on bone cell differentiation. Scientific Reports, 7(1), 6841 10.1038/s41598-017-07359-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V, Abbas AK, & Aster JC (2014). Robbins & Cotran Pathologic Basis of Disease E-Book. Elsevier Health Sciences. [Google Scholar]
- Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, & Teitelbaum SL (2000). TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. The Journal of Clinical Investigation, 106(12), 1481–1488. 10.1172/JCI11176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson S, Eliasson P, & Hansson LI (1989). Hip fractures in northern Sweden 1973–1984. A comparison of rural and urban populations. Acta Orthopaedica Scandinavica, 60(5), 567–571. [DOI] [PubMed] [Google Scholar]
- Lawal AO (2017). Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicology Letters, 270, 88–95. 10.1016/j.toxlet.2017.01.017 [DOI] [PubMed] [Google Scholar]
- Lee Y-M, Fujikado N, Manaka H, Yasuda H, & Iwakura Y (2010). IL-1 plays an important role in the bone metabolism under physiological conditions. International Immunology, 22(10), 805–816. 10.1093/intimm/dxq431 [DOI] [PubMed] [Google Scholar]
- Leem Y-H, Oh S, Kang H-J, Kim J-H, Yoon J, & Chang J-S (2017). BPA-toxicity via superoxide anion overload and a deficit in β-catenin signaling in human bone mesenchymal stem cells. Environmental Toxicology, 32(1), 344–352. 10.1002/tox.22239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leh F (1973). Ozone. Properties, toxicity, and applications. Journal of Chemical Education, 50(6), 404–405. [DOI] [PubMed] [Google Scholar]
- Li J-Y, D’Amelio P, Robinson J, Walker LD, Vaccaro C, Luo T, … Pacifici R (2015). IL-17A Is Increased in Humans with Primary Hyperparathyroidism and Mediates PTH-Induced Bone Loss in Mice. Cell Metabolism, 22(5), 799–810. 10.1016/j.cmet.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J-Y, Yu M, Tyagi AM, Vaccaro C, Hsu E, Adams J, … Pacifici R (2019). IL-17 Receptor Signaling in Osteoblasts/Osteocytes Mediates PTH-Induced Bone Loss and Enhances Osteocytic RANKL Production. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 34(2), 349–360. 10.1002/jbmr.3600 [DOI] [PubMed] [Google Scholar]
- Li W, Tian Y, Song X, Zhang M, & Shen G (2005). Relationship between BMD and Zn, Cu, Ca levels in the hair and meal in elderly people. Journal of Huazhong University of Science and Technology. Medical Sciences = Hua Zhong Ke Ji Da Xue Xue Bao. Yi Xue Ying De Wen Ban = Huazhong Keji Daxue Xuebao. Yixue Yingdewen Ban, 25(1), 97–99. [DOI] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, … Sarnat JA (2018). Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environment International, 120, 145–154. 10.1016/j.envint.2018.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind PM, Larsson S, Oxlund H, Hâkansson H, Nyberg K, Eklund T, & Orberg J (2000). Change of bone tissue composition and impaired bone strength in rats exposed to 3,3’,4,4’,5-pentachlorobiphenyl (PCB126). Toxicology, 150(1–3), 41–51. [DOI] [PubMed] [Google Scholar]
- Link TM, & Heilmeier U (2016). Bone Quality-Beyond Bone Mineral Density. Seminars in Musculoskeletal Radiology, 20(3), 269–278. 10.1055/s-0036-1592365 [DOI] [PubMed] [Google Scholar]
- Lips P (2001). Vitamin D deficiency and secondary hyperparathyroidism in the elderly: Consequences for bone loss and fractures and therapeutic implications. Endocrine Reviews, 22(4), 477–501. 10.1210/edrv.22.4.0437 [DOI] [PubMed] [Google Scholar]
- Liu C, Fuertes E, Flexeder C, Hofbauer LC, Berdel D, Hoffmann B, Kratzsch J, von Berg A, Heinrich J, GINIplus Study Group, & LISAplus Study Group. (2015). Associations between ambient air pollution and bone turnover markers in 10-year old children: Results from the GINIplus and LISAplus studies. International Journal of Hygiene and Environmental Health, 218(1), 58–65. 10.1016/j.ijheh.2014.07.006 [DOI] [PubMed] [Google Scholar]
- Lüthje P, Peltonen A, Nurmi I, Kataja M, & Santavirta S (1995). No differences in the incidences of old people’s hip fractures between urban and rural populations—A comparative study in two Finnish health care regions in 1989. Gerontology, 41(1), 39–44. 10.1159/000213660 [DOI] [PubMed] [Google Scholar]
- Machala M, Vondrácek J, Bláha L, Ciganek M, & Neca JV (2001). Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutation Research, 497(1–2), 49–62. [DOI] [PubMed] [Google Scholar]
- Madhok R, Melton LJ, Atkinson EJ, O’Fallon WM, & Lewallen DG (1993). Urban vs rural increase in hip fracture incidence. Age and sex of 901 cases 1980–89 in Olmsted County, U.S.A. Acta Orthopaedica Scandinavica, 64(5), 543–548. [DOI] [PubMed] [Google Scholar]
- Manicourt D-H, & Devogelaer J-P (2008). Urban tropospheric ozone increases the prevalence of vitamin D deficiency among Belgian postmenopausal women with outdoor activities during summer. The Journal of Clinical Endocrinology and Metabolism, 93(10), 3893–3899. 10.1210/jc.2007-2663 [DOI] [PubMed] [Google Scholar]
- Mannius S, Mellström D, Odén A, Rundgren A, & Zetterberg C (1987). Incidence of hip fracture in western Sweden 1974–1982. Comparison of rural and urban populations. Acta Orthopaedica Scandinavica, 58(1), 38–42. [DOI] [PubMed] [Google Scholar]
- Marchetti L, Sabbieti MG, Menghi M, Materazzi S, Hurley MM, & Menghi G (2002). Effects of phthalate esters on actin cytoskeleton of Py1a rat osteoblasts. Histology and Histopathology, 17(4), 1061–1066. 10.14670/HH-17.1061 [DOI] [PubMed] [Google Scholar]
- Marie P, & Halbout P (2008). [OPG/RANKL: Role and therapeutic target in osteoporosis]. Medecine Sciences: M/S, 24(1), 105–110. 10.1051/medsci/2008241105 [DOI] [PubMed] [Google Scholar]
- Marie PJ (2003). Fibroblast growth factor signaling controlling osteoblast differentiation. Gene, 316, 23–32. [DOI] [PubMed] [Google Scholar]
- Mason GG (1994). Dioxin-receptor ligands in urban air and vehicle exhaust. Environmental Health Perspectives, 102 Suppl 4, 111–116. 10.1289/ehp.94102s4111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunawa M, Amano Y, Endo K, Uno S, Sakaki T, Yamada S, & Makishima M (2009). The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicological Sciences: An Official Journal of the Society of Toxicology, 109(1), 50–58. 10.1093/toxsci/kfp044 [DOI] [PubMed] [Google Scholar]
- Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, … Shimohigashi Y (2007). Structural evidence for endocrine disruptor bisphenol A binding to human nuclear receptor ERR gamma. Journal of Biochemistry, 142(4), 517–524. 10.1093/jb/mvm158 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Pant R, Kulkarni B, & Kinra S (2015). Comparison of Bone Mineral Density between Urban and Rural Areas: Systematic Review and Meta-Analysis. PloS One, 10(7), e0132239 10.1371/journal.pone.0132239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzucchelli R, Crespi Villarias N, Perez Fernandez E, Durban Reguera ML, Garcia-Vadillo A, Quiros FJ, … Gil de Miguel A (2018). Short-term association between outdoor air pollution and osteoporotic hip fracture. Osteoporosis International, 29(10), 2231–2241. 10.1007/s00198-018-4605-7 [DOI] [PubMed] [Google Scholar]
- McKee RH, Pavkov KL, Trimmer GW, Keller LH, & Stump DG (2006). An assessment of the potential developmental and reproductive toxicity of di-isoheptyl phthalate in rodents. Reproductive Toxicology (Elmsford, N.Y.), 21(3), 241–252. 10.1016/j.reprotox. 2005.09.002 [DOI] [PubMed] [Google Scholar]
- Melton LJ, Crowson CS, & O’Fallon WM (1999). Fracture incidence in Olmsted County, Minnesota: Comparison of urban with rural rates and changes in urban rates over time. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 9(1), 29–37. [DOI] [PubMed] [Google Scholar]
- Menghi G, Sabbieti MG, Marchetti L, Menghi M, Materazzi S, & Hurley MM (2001). Phthalate esters influence FGF-2 translocation in Py1a rat osteoblasts. European Journal of Morphology, 39(3), 155–162. [DOI] [PubMed] [Google Scholar]
- Meyer HE, Berntsen GKR, Søgaard AJ, Langhammer A, Schei B, Fønnebø V, … Norwegian Epidemiological Osteoporosis Studies (NOREPOS) Research Group. (2004). Higher bone mineral density in rural compared with urban dwellers: The NOREPOS study. American Journal of Epidemiology, 160(11), 1039–1046. 10.1093/aje/kwh337 [DOI] [PubMed] [Google Scholar]
- Michnovicz JJ, Hershcopf RJ, Naganuma H, Bradlow HL, & Fishman J (1986). Increased 2-hydroxylation of estradiol as a possible mechanism for the anti-estrogenic effect of cigarette smoking. The New England Journal of Medicine, 315(21), 1305–1309. 10.1056/NEJM198611203152101 [DOI] [PubMed] [Google Scholar]
- Miettinen HM, Pulkkinen P, Jämsä T, Koistinen J, Simanainen U, Tuomisto J, … Viluksela M (2005). Effects of in utero and lactational TCDD exposure on bone development in differentially sensitive rat lines. Toxicological Sciences: An Official Journal of the Society of Toxicology, 85(2), 1003–1012. 10.1093/toxsci/kfi136 [DOI] [PubMed] [Google Scholar]
- Min K, & Min J (2014). Urinary phthalate metabolites and the risk of low bone mineral density and osteoporosis in older women. The Journal of Clinical Endocrinology and Metabolism, 99(10), E1997–2003. 10.1210/jc.2014-2279 [DOI] [PubMed] [Google Scholar]
- Miyawaki J, Kamei S, Sakayama K, Yamamoto H, & Masuno H (2008). 4-tert-octylphenol regulates the differentiation of C3H10T1/2 cells into osteoblast and adipocyte lineages. Toxicological Sciences: An Official Journal of the Society of Toxicology, 102(1), 82–88. 10.1093/toxsci/kfm296 [DOI] [PubMed] [Google Scholar]
- Monks PS, Archibald AT, Colette A, Cooper O, Coyle M, Derwent R, … Williams ML (2015). Tropospheric ozone and its precursors from the urban to the global scale from air quality to short-lived climate forcer. Atmospheric Chemistry and Physics, 15(15), 8889–8973. 10.5194/acp-15-8889-2015 [DOI] [Google Scholar]
- Naruse M, Ishihara Y, Miyagawa-Tomita S, Koyama A, & Hagiwara H (2002). 3-Methylcholanthrene, which binds to the arylhydrocarbon receptor, inhibits proliferation and differentiation of osteoblasts in vitro and ossification in vivo. Endocrinology, 143(9), 3575–3581. 10.1210/en.2002-220003 [DOI] [PubMed] [Google Scholar]
- National Institutes of Health (2018). Osteoporosis Overview | NIH Osteoporosis and Related Bone Diseases National Resource Center; Retrieved October 7, 2019, from https://www.bones.nih.gov/health-info/bone/osteoporosis/overview [Google Scholar]
- Negishi-Koga T, Shinohara M, Komatsu N, Bito H, Kodama T, Friedel RH, & Takayanagi H (2011). Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nature Medicine, 17(11), 1473–1480. 10.1038/nm.2489 [DOI] [PubMed] [Google Scholar]
- Novák Jiří, Hilscherová K, Landlová L, Čupr P, Kohút L, Giesy JP, & Klánová J (2014). Composition and effects of inhalable size fractions of atmospheric aerosols in the polluted atmosphere. Part II. In vitro biological potencies. Environment International, 63, 64–70. 10.1016/j.envint.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Novák Jirí, Jálová V, Giesy JP, & Hilscherová K (2009). Pollutants in particulate and gaseous fractions of ambient air interfere with multiple signaling pathways in vitro. Environment International, 35(1), 43–49. 10.1016/j.envint.2008.06.006 [DOI] [PubMed] [Google Scholar]
- O’Brien W, Fissel BM, Maeda Y, Yan J, Ge X, Gravallese EM, … Charles JF (2016). Receptor activator of nuclear factor kappa-B (RANK) independent osteoclast formation and bone erosion in inflammatory arthritis. Arthritis & Rheumatology (Hoboken, N.J.), 68(12), 2889–2900. 10.1002/art.39837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omsland TK, Ahmed LA, Grønskag A, Schei B, Emaus N, Langhammer A, … Meyer HE (2011). More forearm fractures among urban than rural women: The NOREPOS study based on the Tromsø study and the HUNT study. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 26(4), 850–856. 10.1002/jbmr.280 [DOI] [PubMed] [Google Scholar]
- Pacifici R, Brown C, Puscheck E, Friedrich E, Slatopolsky E, Maggio D, … Avioli LV (1991). Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proceedings of the National Academy of Sciences of the United States of America, 88(12), 5134–5138. 10.1073/pnas.88.12.5134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici Roberto. (2016). The Role of IL-17 and TH17 Cells in the Bone Catabolic Activity of PTH. Frontiers in Immunology, 7, 57 10.3389/fimmu.2016.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu A-C, Dewailly E, Dodin S, Nieboer E, & Ayotte P (2013). Dioxin-like compounds and bone quality in Cree women of Eastern James Bay (Canada): A cross-sectional study. Environmental Health: A Global Access Science Source, 12(1), 54 10.1186/1476-069X-12-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peach HG, & Bath NE (1999). Hospital separation rates from osteoporotic and non-osteoporotic fractures in metropolitan and rural Australia. Injury, 30(2), 101–103. [DOI] [PubMed] [Google Scholar]
- Pelch KE, Carleton SM, Phillips CL, & Nagel SC (2012). Developmental exposure to xenoestrogens at low doses alters femur length and tensile strength in adult mice. Biology of Reproduction, 86(3), 69 10.1095/biolreprod.111.096545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzer K, Joosten L, Gasser J, Distler JH, Ruiz G, Baum W, … Zwerina J (2010). Interleukin-1 is essential for systemic inflammatory bone loss. Annals of the Rheumatic Diseases, 69(1), 284–290. 10.1136/ard.2008.104786 [DOI] [PubMed] [Google Scholar]
- Pongchaiyakul C, Apinyanurag C, Soontrapa S, Soontrapa S, Pongchaiyakul C, Nguyen TV, & Rajatanavin R (2006). Prevalence of osteoporosis in Thai men. Journal of the Medical Association of Thailand = Chotmaihet Thangphaet, 89(2), 160–169. [PubMed] [Google Scholar]
- Pongchaiyakul C, Nguyen TV, Kosulwat V, Rojroongwasinkul N, Charoenkiatkul S, Eisman JA, & Rajatanavin R (2005a). Contribution of lean tissue mass to the urban-rural difference in bone mineral density. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 16(12), 1761–1768. 10.1007/s00198-005-1921-5 [DOI] [PubMed] [Google Scholar]
- Pongchaiyakul C, Nguyen TV, Kosulwat V, Rojroongwasinkul N, Charoenkiatkul S, & Rajatanavin R (2005b). Effect of urbanization on bone mineral density: A Thai epidemiological study. BMC Musculoskeletal Disorders, 6, 5 10.1186/1471-2474-6-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole JA, Gleason AM, Bauer C, West WW, Alexis N, van Rooijen N, … Kielian TL (2012). CD11c(+)/CD11b(+) cells are critical for organic dust-elicited murine lung inflammation. American Journal of Respiratory Cell and Molecular Biology, 47(5), 652–659. 10.1165/rcmb.2012-0095OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope CA, Bhatnagar A, McCracken JP, Abplanalp W, Conklin DJ, & O’Tool T (2016). Exposure to Fine Particulate Air Pollution Is Associated with Endothelial Injury and Systemic Inflammation. Circulation Research, 119(11), 1204–1214. 10.1161/CIRCRESAHA.116.309279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada D, Zhong J, Colicino E, Zanobetti A, Schwartz J, Dagincourt N, … Baccarelli AA (2017). Association of air particulate pollution with bone loss over time and bone fracture risk: Analysis of data from two independent studies. The Lancet. Planetary Health, 1(8), e337–e347. 10.1016/S2542-5196(17)30136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prüss-Ustün A, van Deventer E, Mudu P, Campbell-Lendrum D, Vickers C, Ivanov I, … Neira M (2019). Environmental risks and non-communicable diseases. BMJ (Clinical Research Ed.), 364, l265 10.1136/bmj.l265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman I, & MacNee W (1998). Role of transcription factors in inflammatory lung diseases. Thorax, 53(7), 601–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzani OT, Milà C, Kulkarni B, Kinra S, & Tonne C (2020). Association of Ambient and Household Air Pollution With Bone Mineral Content Among Adults in Peri-urban South India. JAMA Network Open, 3(1), e1918504 10.1001/jamanetworkopen.2019.18504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Hinojosa D, Lozada-Pérez CA, Zamudio Cuevas Y, López-Reyes A, Martínez-Nava G, Fernández-Torres J, Olivos-Meza A, Landa-Solis C, Gutiérrez-Ruiz MC, Rojas Del Castillo E, & Martínez-Flores K (2019). Toxicity of cadmium in musculoskeletal diseases. Environmental Toxicology and Pharmacology, 72, 103219 10.1016/j.etap.2019.103219 [DOI] [PubMed] [Google Scholar]
- Rignell-Hydbom A, Skerfving S, Lundh T, Lindh CH, Elmståhl S, Bjellerup P, … Akesson A (2009). Exposure to cadmium and persistent organochlorine pollutants and its association with bone mineral density and markers of bone metabolism on postmenopausal women. Environmental Research, 109(8), 991–996. 10.1016/j.envres.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Ringsberg KA, Gärdsell P, Johnell O, Josefsson PO, & Obrant KJ (2001). The impact of long-term moderate physical activity on functional performance, bone mineral density and fracture incidence in elderly women. Gerontology, 47(1), 15–20. 10.1159/000052765 [DOI] [PubMed] [Google Scholar]
- Rodríguez J, & Mandalunis PM (2018). A Review of Metal Exposure and Its Effects on Bone Health. Journal of Toxicology, 2018, 4854152 10.1155/2018/4854152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren BE, Ahlborg HG, Gärdsell P, Sernbo I, Daly RM, Nilsson J-A, & Karlsson MK (2010). Bone mineral density and incidence of hip fracture in Swedish urban and rural women 1987–2002. Acta Orthopaedica, 81(4), 453–459. 10.3109/17453674.2010.492762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren BE, Ahlborg HG, Gärdsell P, Sernbo I, Nilsson J-Å, Daly RM, & Karlsson MK (2012). Forearm bone mineral density and incidence of hip fractures in Swedish urban and rural men 1987–2002. Scandinavian Journal of Public Health, 40(1), 102–108. 10.1177/1403494811425604 [DOI] [PubMed] [Google Scholar]
- Ryan EP, Holz JD, Mulcahey M, Sheu T-J, Gasiewicz TA, & Puzas JE (2007). Environmental toxicants may modulate osteoblast differentiation by a mechanism involving the aryl hydrocarbon receptor. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 22(10), 1571–1580. 10.1359/jbmr.070615 [DOI] [PubMed] [Google Scholar]
- Sabbieti MG, Agas D, Santoni G, Materazzi S, Menghi G, & Marchetti L (2009). Involvement of p53 in phthalate effects on mouse and rat osteoblasts. Journal of Cellular Biochemistry, 107(2), 316–327. 10.1002/jcb.22127 [DOI] [PubMed] [Google Scholar]
- Sabbieti MG, Agas D, Palermo F, Mosconi G, Santoni G, Amantini C, … Marchetti L (2011). 4-nonylphenol triggers apoptosis and affects 17-β-estradiol receptors in calvarial osteoblasts. Toxicology, 290(2–3), 334–341. 10.1016/j.tox.2011.10.014 [DOI] [PubMed] [Google Scholar]
- Safe S, & Wormke M (2003). Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chemical Research in Toxicology, 16(7), 807–816. 10.1021/tx034036r [DOI] [PubMed] [Google Scholar]
- Saha H, Mukherjee B, Bindhani B, & Ray MR (2016). Changes in RANKL and osteoprotegerin expression after chronic exposure to indoor air pollution as a result of cooking with biomass fuel. Journal of Applied Toxicology: JAT, 36(7), 969–976. 10.1002/jat.3275 [DOI] [PubMed] [Google Scholar]
- Saidenberg-Kermanac’h N, Corrado A, Lemeiter D, deVernejoul MC, Boissier MC, & Cohen-Solal ME (2004). TNF-alpha antibodies and osteoprotegerin decrease systemic bone loss associated with inflammation through distinct mechanisms in collagen-induced arthritis. Bone, 35(5), 1200–1207. 10.1016/j.bone.2004.07.004 [DOI] [PubMed] [Google Scholar]
- Saillenfait A-M, Gallissot F, & Sabaté J-P (2009). Differential developmental toxicities of di-n-hexyl phthalate and dicyclohexyl phthalate administered orally to rats. Journal of Applied Toxicology: JAT, 29(6), 510–521. 10.1002/jat.1436 [DOI] [PubMed] [Google Scholar]
- Saillenfait A-M, Sabaté J-P, Robert A, Cossec B, Roudot A-C, Denis F, & Burgart M (2013). Adverse effects of diisooctyl phthalate on the male rat reproductive development following prenatal exposure. Reproductive Toxicology (Elmsford, N.Y.), 42, 192–202. 10.1016/j.reprotox.2013.09.004 [DOI] [PubMed] [Google Scholar]
- Samelson EJ, Broe KE, Xu H, Yang L, Boyd S, Biver E, Szulc P, Adachi J, Amin S, Atkinson E, Berger C, Burt L, Chapurlat R, Chevalley T, Ferrari S, Goltzman D, Hanley DA, Hannan MT, Khosla S, … Bouxsein ML (2019). Cortical and trabecular bone microarchitecture as an independent predictor of incident fracture risk in older women and men in the Bone Microarchitecture International Consortium (BoMIC): A prospective study. The Lancet. Diabetes & Endocrinology, 7(1), 34–43. 10.1016/S2213-8587(18)30308-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM, Nicholson GC, Ugoni AM, Seeman E, Pasco JA, & Kotowicz MA (2002). Fracture rates lower in rural than urban communities: The Geelong Osteoporosis Study. Journal of Epidemiology and Community Health, 56(6), 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schraufnagel DE, Balmes JR, Cowl CT, Matteis SD, Jung S-H, Mortimer K, … Wuebbles DJ (2019). Air Pollution and Noncommunicable Diseases: A Review by the Forum of International Respiratory Societies’ Environmental Committee, Part 2: Air Pollution and Organ Systems. CHEST, 155(2), 417–426. 10.1016/j.chest.2018.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scimeca M, Feola M, Romano L, Rao C, Gasbarra E, Bonanno E, Brandi ML, & Tarantino U (2017). Heavy metals accumulation affects bone microarchitecture in osteoporotic patients. Environmental Toxicology, 32(4), 1333–1342. 10.1002/tox.22327 [DOI] [PubMed] [Google Scholar]
- Seeman E, & Delmas PD (2006). Bone quality—The material and structural basis of bone strength and fragility. The New England Journal of Medicine, 354(21), 2250–2261. 10.1056/NEJMra053077 [DOI] [PubMed] [Google Scholar]
- Seemann F, Jeong C-B, Zhang G, Wan MT, Guo B, Peterson DR, … Au DW-T (2017). Ancestral benzo[a]pyrene exposure affects bone integrity in F3 adult fish (Oryzias latipes). Aquatic Toxicology (Amsterdam, Netherlands), 183, 127–134. 10.1016/j.aquatox.2016.12.018 [DOI] [PubMed] [Google Scholar]
- Seemann F, Peterson DR, Witten PE, Guo B-S, Shanthanagouda AH, Ye RR, … Au DWT (2015). Insight into the transgenerational effect of benzo[a]pyrene on bone formation in a teleost fish (Oryzias latipes). Comparative Biochemistry and Physiology. Toxicology & Pharmacology: CBP, 178, 60–67. 10.1016/j.cbpc.2015.10.001 [DOI] [PubMed] [Google Scholar]
- Sernbo I, Johnell O, & Andersson T (1988). Differences in the incidence of hip fracture. Comparison of an urban and a rural population in southern Sweden. Acta Orthopaedica Scandinavica, 59(4), 382–385. [DOI] [PubMed] [Google Scholar]
- Shaddick G, Thomas ML, Amini H, Broday D, Cohen A, Frostad J, … Brauer M (2018). Data Integration for the Assessment of Population Exposure to Ambient Air Pollution for Global Burden of Disease Assessment. Environmental Science & Technology, 52(16), 9069–9078. 10.1021/acs.est.8b02864 [DOI] [PubMed] [Google Scholar]
- Shah KM, Wilkinson JM, & Gartland A (2015). Cobalt and chromium exposure affects osteoblast function and impairs the mineralization of prosthesis surfaces in vitro. Journal of Orthopaedic Research: Official Publication of the Orthopaedic Research Society, 33(11), 1663–1670. 10.1002/jor.22932 [DOI] [PubMed] [Google Scholar]
- Sharma C, Pradeep A, Wong L, Rana A, & Rana B (2004). Peroxisome proliferator-activated receptor gamma activation can regulate beta-catenin levels via a proteasome-mediated and adenomatous polyposis coli-independent pathway. The Journal of Biological Chemistry, 279(34), 35583–35594. 10.1074/jbc.M403143200 [DOI] [PubMed] [Google Scholar]
- Shoenfelt J, Mitkus RJ, Zeisler R, Spatz RO, Powell J, Fenton MJ, … Medvedev AE (2009). Involvement of TLR2 and TLR4 in inflammatory immune responses induced by fine and coarse ambient air particulate matter. Journal of Leukocyte Biology, 86(2), 303–312. 10.1189/jlb.1008587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigaux J, Biton J, André E, Semerano L, & Boissier M-C (2019). Air pollution as a determinant of rheumatoid arthritis. Joint Bone Spine, 86(1), 37–42. 10.1016/j.jbspin.2018.03.001 [DOI] [PubMed] [Google Scholar]
- Singh SU, Casper RF, Fritz PC, Sukhu B, Ganss B, Girard B, … Tenenbaum HC (2000). Inhibition of dioxin effects on bone formation in vitro by a newly described aryl hydrocarbon receptor antagonist, resveratrol. The Journal of Endocrinology, 167(1), 183–195. [DOI] [PubMed] [Google Scholar]
- Sipos W, Pietschmann P, Rauner M, Kerschan-Schindl K, & Patsch J (2009). Pathophysiology of osteoporosis. Wiener Medizinische Wochenschrift (1946), 159(9–10), 230–234. 10.1007/s10354-009-0647-y [DOI] [PubMed] [Google Scholar]
- Smith BJ, Lerner MR, Bu SY, Lucas EA, Hanas JS, Lightfoot SA, … Brackett DJ (2006). Systemic bone loss and induction of coronary vessel disease in a rat model of chronic inflammation. Bone, 38(3), 378–386. 10.1016/j.bone.2005.09.008 [DOI] [PubMed] [Google Scholar]
- Søgaard AJ, Gustad TK, Bjertness E, Tell GS, Schei B, Emaus N, … Norwegian Epidemiological Osteoporosis Studies (NOREPOS) Research Group. (2007). Urban-rural differences in distal forearm fractures: Cohort Norway. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 18(8), 1063–1072. 10.1007/s00198-007-0353-9 [DOI] [PubMed] [Google Scholar]
- Solleiro-Villavicencio H, & Rivas-Arancibia S (2018). Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4+T Cells in Neurodegenerative Diseases. Frontiers in Cellular Neuroscience, 12, 114 10.3389/fncel.2018.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sözen T, Özişik L, & Başaran NÇ (2017). An overview and management of osteoporosis. European Journal of Rheumatology, 4(1), 46–56. 10.5152/eurjrheum.2016.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specker B, Binkley T, & Fahrenwald N (2004). Rural versus nonrural differences in BMC, volumetric BMD, and bone size: A population-based cross-sectional study. Bone, 35(6), 1389–1398. 10.1016/j.bone.2004.09.005 [DOI] [PubMed] [Google Scholar]
- Stolzing A, & Scutt A (2006). Age-related impairment of mesenchymal progenitor cell function. Aging Cell, 5(3), 213–224. 10.1111/j.1474-9726.2006.00213.x [DOI] [PubMed] [Google Scholar]
- Sundberg M, Düppe H, Gärdsell P, Johnell O, Ornstein E, & Sernbo I (1997). Bone mineral density in adolescents. Higher values in a rural area—A population-based study of 246 subjects in southern Sweden. Acta Orthopaedica Scandinavica, 68(5), 456–460. [DOI] [PubMed] [Google Scholar]
- Suvarapu LN, & Baek S-O (2017). Determination of heavy metals in the ambient atmosphere. Toxicology and Industrial Health, 33(1), 79–96. 10.1177/0748233716654827 [DOI] [PubMed] [Google Scholar]
- Suzuki N, Yamamoto M, Watanabe K, Kambegawa A, & Hattori A (2004). Both mercury and cadmium directly influence calcium homeostasis resulting from the suppression of scale bone cells: The scale is a good model for the evaluation of heavy metals in bone metabolism. Journal of Bone and Mineral Metabolism, 22(5), 439–446. 10.1007/s00774-004-0505-3 [DOI] [PubMed] [Google Scholar]
- Takahashi N, Maeda K, Ishihara A, Uehara S, & Kobayashi Y (2011). Regulatory mechanism of osteoclastogenesis by RANKL and Wnt signals. Frontiers in Bioscience (Landmark Edition), 16, 21–30. [DOI] [PubMed] [Google Scholar]
- Tang C-H, Chiu Y-C, Huang C-F, Chen Y-W, & Chen P-C (2009). Arsenic induces cell apoptosis in cultured osteoblasts through endoplasmic reticulum stress. Toxicology and Applied Pharmacology, 241(2), 173–181. 10.1016/j.taap.2009.08.011 [DOI] [PubMed] [Google Scholar]
- Thent ZC, Froemming GRA, & Muid S (2018). Bisphenol A exposure disturbs the bone metabolism: An evolving interest towards an old culprit. Life Sciences, 198, 1–7. 10.1016/j.lfs.2018.02.013 [DOI] [PubMed] [Google Scholar]
- Toda K, Miyaura C, Okada T, & Shizuta Y (2002). Dietary bisphenol A prevents ovarian degeneration and bone loss in female mice lacking the aromatase gene (Cyp19 ). European Journal of Biochemistry, 269(8), 2214–2222. [DOI] [PubMed] [Google Scholar]
- van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, … Hogg JC (2001). Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). American Journal of Respiratory and Critical Care Medicine, 164(5), 826–830. 10.1164/ajrccm.164.5.2010160 [DOI] [PubMed] [Google Scholar]
- van Voorhis M, Knopp S, Julliard W, Fechner JH, Zhang X, Schauer JJ, & Mezrich JD (2013). Exposure to atmospheric particulate matter enhances Th17 polarization through the aryl hydrocarbon receptor. PloS One, 8(12), e82545 10.1371/journal.pone.0082545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin E, Rylander L, Jönssson BAG, Lundh T, Isaksson A, & Hagmar L (2005). Exposure to CB-153 and p,p’-DDE and bone mineral density and bone metabolism markers in middle-aged and elderly men and women. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 16(12), 2085–2094. 10.1007/s00198-005-2004-3 [DOI] [PubMed] [Google Scholar]
- Wallin M, Barregard L, Sallsten G, Lundh T, Karlsson MK, Lorentzon M, Ohlsson C, & Mellström D (2016). Low-Level Cadmium Exposure Is Associated With Decreased Bone Mineral Density and Increased Risk of Incident Fractures in Elderly Men: The MrOS Sweden Study. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 31(4), 732–741. 10.1002/jbmr.2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson ATD, Nordberg RC, Loboa EG, & Kullman SW (2019). Evidence for Aryl hydrocarbon Receptor-Mediated Inhibition of Osteoblast Differentiation in Human Mesenchymal Stem Cells. Toxicological Sciences: An Official Journal of the Society of Toxicology, 167(1), 145–156. 10.1093/toxsci/kfy225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R, O’Karma M, Wallace TC, & Zemel BS (2016). The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: A systematic review and implementation recommendations. Osteoporosis International: A Journal Established as Result of Cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA, 27(4), 1281–1386. 10.1007/s00198-015-3440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells A, Romberger DJ, Thiele GM, Wyatt TA, Staab E, Heires AJ, … Poole JA (2017). Systemic IL-6 Effector Response in Mediating Systemic Bone Loss Following Inhalation of Organic Dust. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research, 37(1), 9–19. 10.1089/jir.2016.0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger D, Gerecke AC, Heeb NV, Schmid P, Hueglin C, Naegeli H, & Zenobi R (2009). In vitro estrogenicity of ambient particulate matter: Contribution of hydroxylated polycyclic aromatic hydrocarbons. Journal of Applied Toxicology: JAT, 29(3), 223–232. 10.1002/jat.1400 [DOI] [PubMed] [Google Scholar]
- Whittier X, & Saag KG (2016). Glucocorticoid-induced Osteoporosis. Rheumatic Diseases Clinics of North America, 42(1), 177–189, x. 10.1016/j.rdc.2015.08.005 [DOI] [PubMed] [Google Scholar]
- Wong AKO, Beattie KA, Bhargava A, Cheung M, Webber CE, Chettle DR, Papaioannou A, Adachi JD, & Canadian Multicentre Osteoporosis Study (CaMos) Research Group. (2015). Bone lead (Pb) content at the tibia is associated with thinner distal tibia cortices and lower volumetric bone density in postmenopausal women. Bone, 79, 58–64. 10.1016/j.bone.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2018a). 9 out of 10 people worldwide breathe polluted air, but more countries are taking action. Retrieved October 7, 2019, from https://www.who.int/news-room/detail/02-05-2018-9-out-of-10-people-worldwide-breathe-polluted-air-but-more-countries-are-taking-action
- World Health Organization (2018b). Ambient (outdoor) air quality and health. Retrieved October 7, 2019, from https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health
- World Health Organization (1994). Assessment of fracture risk and its application to screening for postmenopausal osteoporosis Report of a WHO Study Group. World Health Organization Technical Report Series, 843, 1–129. [PubMed] [Google Scholar]
- World Health Organization (2013). Health effects of particulate matter. Policy implications for countries in eastern Europe, Caucasus and central Asia (2013). Retrieved October 7, 2019, from http://www.euro.who.int/en/health-topics/environment-and-health/air-quality/publications/2013/health-effects-of-particulate-matter.-policy-implications-for-countries-in-eastern-europe,-caucasus-and-central-asia-2013
- Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, & Dawson-Hughes B (2014). The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research, 29(11), 2520–2526. 10.1002/jbmr.2269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C-T, Lu T-Y, Chan D-C, Tsai K-S, Yang R-S, & Liu S-H (2014). Effects of arsenic on osteoblast differentiation in vitro and on bone mineral density and microstructure in rats. Environmental Health Perspectives, 122(6), 559–565. 10.1289/ehp.1307832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yachiguchi K, Sekiguchi T, Nakano M, Hattori A, Yamamoto M, Kitamura K, Maeda M, Tabuchi Y, Kondo T, Kamauchi H, Nakabayashi H, Srivastav AK, Hayakawa K, Sakamoto T, & Suzuki N (2014). Effects of inorganic mercury and methylmercury on osteoclasts and osteoblasts in the scales of the marine teleost as a model system of bone. Zoological Science, 31(5), 330–337. 10.2108/zs130265 [DOI] [PubMed] [Google Scholar]
- Yang W, & Omaye ST (2009). Air pollutants, oxidative stress and human health. Mutation Research, 674(1–2), 45–54. 10.1016/j.mrgentox.2008.10.005 [DOI] [PubMed] [Google Scholar]
- Yu H, Du Y, Zhang X, Sun Y, Li S, Dou Y, … Zhao W (2014). The aryl hydrocarbon receptor suppresses osteoblast proliferation and differentiation through the activation of the ERK signaling pathway. Toxicology and Applied Pharmacology, 280(3), 502–510. 10.1016/j.taap.2014.08.025 [DOI] [PubMed] [Google Scholar]
- Yu T, Kondo T, Matsumoto T, Fujii-Kuriyama Y, & Imai Y (2014). Aryl hydrocarbon receptor catabolic activity in bone metabolism is osteoclast dependent in vivo. Biochemical and Biophysical Research Communications, 450(1), 416–422. 10.1016/j.bbrc.2014.05.114 [DOI] [PubMed] [Google Scholar]
- Yu T, Pang W, & Yang G (2015). Aryl hydrocarbon receptors in osteoclast lineage cells are a negative regulator of bone mass. PloS One, 10(1), e0117112 10.1371/journal.pone.0117112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C, Katchko KM, Schallmo MS, Jeong S, Yun J, Chen CH, … Hsu EL (2018). Aryl Hydrocarbon Receptor Antagonists Mitigate the Effects of Dioxin on Critical Cellular Functions in Differentiating Human Osteoblast-Like Cells. International Journal of Molecular Sciences, 19(1). 10.3390/ijms19010225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun C, Weiner JA, Chun DS, Yun J, Cook RW, Schallmo MS, … Hsu EL (2017). Mechanistic insight into the effects of Aryl Hydrocarbon Receptor activation on osteogenic differentiation. Bone Reports, 6, 51–59. 10.1016/j.bonr.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Dong S, Wang H, Tao S, & Kiyama R (2016). Biological impact of environmental polycyclic aromatic hydrocarbons (ePAHs) as endocrine disruptors. Environmental Pollution (Barking, Essex: 1987), 213, 809–824. 10.1016/j.envpol.2016.03.050 [DOI] [PubMed] [Google Scholar]
- Zhao H, Bi Y, Ma L, Zhao L, Wang T, Zhang L, … Liu J (2012). The effects of bisphenol A (BPA) exposure on fat mass and serum leptin concentrations have no impact on bone mineral densities in non-obese premenopausal women. Clinical Biochemistry, 45(18), 1602–1606. 10.1016/j.clinbiochem.2012.08.024 [DOI] [PubMed] [Google Scholar]
- Zheng G, He K, Duan F, Cheng Y, & Ma Y (2013). Measurement of humic-like substances in aerosols: A review. Environmental Pollution (Barking, Essex: 1987), 181, 301–314. 10.1016/j.envpol.2013.05.055 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang R, An L, Wang H, Cheng S, Qiong S, & Weng Y (2017). Benzo[a]pyrene impedes self-renewal and differentiation of mesenchymal stem cells and influences fracture healing. The Science of the Total Environment, 587–588, 305–315. 10.1016/j.scitotenv.2017.02.152 [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, … Vom Saal FS (2012). Endocrine-disrupting chemicals and public health protection: A statement of principles from The Endocrine Society. Endocrinology, 153(9), 4097–4110. 10.1210/en.2012-1422 [DOI] [PMC free article] [PubMed] [Google Scholar]