Abstract
Several neurobiological mechanisms are implicated in the formation of selective pair bonds in socially monogamous mammals, however much less is known about the mechanisms that underlie the long-term behavioral maintenance of these bonds. In prairie voles (Microtus ochrogaster), agonistic behavior that contributes to pair bond maintenance are regulated by dopamine activity at D1-like receptors (D1R) within the mesocorticolimbic system. Evidence suggests D1Rs similarly regulate the behavioral components of pair bond maintenance in socially monogamous titi monkeys (Callicebus cupreus); however, evaluation with behavioral pharmacology is necessary to evaluate this hypothesis. In the current study we evaluated the role of D1Rs in behavioral components of pair bond maintenance in captive male titi monkeys (N = 8). We administered two doses of a D1R selective antagonist, SCH23390, (0.1 mg/kg, 0.01 mg/kg) or saline vehicle to male titi monkeys and presented pairs with a simulated intruder monkey via the use of a mirror stimulus. The non-reflective back of the mirror stimulus was used for control sessions. We video recorded responses to the five-minute stimulus presentations and later scored for arousal and agonistic behaviors relevant to mate guarding as well as affiliative behavior between the pair mates. We also conducted a locomotor assessment to evaluate the potential side effect for SCH23390 of impaired locomotion. Finally, we collected blood samples at the end of each session to assay for plasma cortisol responses. We found evidence of locomotor impairment only with the high dose of SCH23390, and therefore analyses were conducted comparing only test sessions where low dose SCH23390 and saline were administered. With saline administration, males displayed more agonistic behavior via back arching and tail lashing as well as restraining their female partners when viewing the mirror compared to the back of the mirror. D1R antagonist treatment attenuated these agonistic behaviors indicative of mate guarding when males viewed the mirror. Results also indicated that this reduction in agonistic behavior occurred without evidence of overall behavioral blunting or generally reduced social interest. Likewise changes in agonistic behavior were not driven by differences in HPA activity across testing sessions. Mate-directed affiliative behavior, including lip smacks and approaches to female partners, were not altered by D1R antagonist treatment. Dyadic social contact was higher with D1R antagonist treatment, but this was due to a reduction in contact termination by the treated males, which was typically followed by an approach or arousal display to the simulated intruder. These results provide further evidence that D1R activity regulates mate guarding behaviors in titi monkeys and suggests that the dopamine system plays a similar role in the agonistic behavioral components of pair bond maintenance behavior in non-human primates and rodents.
Keywords: pair bond maintenance, mate guarding, dopamine, SCH23390, nonhuman primate, intruder paradigm
Graphical Abstract.
1. Introduction
One of the closest social relationships human adults experience is an attachment bond with a romantic partner. Attachment bonds were first used to describe bonds between mothers and infants (Bowlby, 1969; Ainsworth et al., 1978) but also characterize adult romantic relationships as partners similarly experience felt security from one another and have bidirectional regulatory influences on each other’s emotions and physiology (Hazan & Shaver, 1987; Butler & Randall, 2013). Our understanding of the neurobiology underlying adult attachments has been advanced with studies of mammals that have socially monogamous mating systems. Fewer than ten percent of non-human mammalian species are socially monogamous (Kleiman, 1977; Lukas & Clutton-Brock, 2013) and include rodents such as prairie voles (Microtus ochrogaster; Getz et al., 1981) and California mice (Peromyscus californicus; Kowalczyk et al., 2018), as well as many species in the Carnivora (Lukas & Clutton-Brock, 2013) and non-human primate orders (Opie et al., 2013) including titi monkeys (Callicebus cupreus, Mason & Mendoza, 1998; Bales et al., 2017). Even fewer mammals display pair bond relationships, broadly defined as a selective and enduring relationship between two non-kin adults (Mason & Mendoza, 1998; Fuentes, 2002). Pair bonding mammals display characteristics similar to human adult attachments including demonstrating a preference for the pair bonded partner over an opposite sex stranger (Williams et al., 1992; Carp et al., 2016), displaying behavioral and physiological distress upon involuntary separation from the partner (Mendoza & Mason, 1986; Sun et al., 2014), and experiencing buffering to stress when in the presence of the partner (Donovan et al., 2018; Hennessy et al., 1995; Mendoza et al., 2000).
Studies of pair bonding mammals point to two distinct phases of pair bonding, formation and maintenance, which can be identified behaviorally and have underlying neurobiological changes. The hypothalamic nonapeptides of oxytocin and vasopressin are involved pair bond formation and underlie the pairing of social salience with individual recognition (Carter et al., 1995; Walum & Young, 2018; Simmons et al., 2019). In laboratory-housed species, pair bond formation is widely considered to be complete once an individual displays a preference for the partner over an opposite sex stranger in a partner preference test (Williams et al., 1992; Winslow et al., 1993; Carp et al., 2016). At the onset of a maintenance phase, many pair bonded mammals display mate guarding behavior, including inter- and intra-sexual aggression toward strangers, to protect the integrity of the partnership (Getz et al., 1981; Resendez et al., 2016; Cubicciotti & Mason, 1978). The development of these agonistic components of pair bond maintenance are largely regulated by the mesolimbic dopamine (DA) system.
Research in prairie voles presents a clear example of changes in the mesolimbic DA system during the transition from pair bond formation to maintenance. The DA system is characterized by two classes of receptors: D1-like receptors (D1Rs) activate intracellular signaling and D2-like receptors (D2Rs) which inhibits intracellular signaling (Missale et al., 1998). Following initial pairing there is enhanced DA transmission in the nucleus accumbens (NAC) for both male and female prairie voles (Resendez et al., 2016). Administering a D2R agonist into the NAC expedites the development of a partner preference whereas administering a D2R antagonist in the NAC blocks the expression of partner preference behavior (Gingrich et al., 2000; Aragona et al., 2003). In contrast, agonism of D1Rs in the NAC eliminates partner preference formation (Aragona et al., 2006). During the onset of the pair bond maintenance phase, prairie voles begin to display mate guarding behavior, including inter- and intra-sexual aggression toward strangers (Getz et al., 1981). These new behaviors are accompanied by an increased abundance of D1R in the NAC of male prairie voles from established pair bonds compared to unpaired males. Importantly, blocking these upregulated D1Rs in pair bonded male prairie voles blocks inter-sexual aggression to a female intruder (Aragona et al., 2006). Together, these studies point to a role of D2Rs in pair bond formation behavior and D1Rs in the behavioral maintenance of the pair bond.
Evidence in titi monkeys suggests the dopamine system also underlies the changes from early to later stages of pair bonding in non-human primates. A cross-sectional study on the neurobiology of pair bonding in titi monkeys used positron emission tomography (PET) scans co-registered with structural magnetic resonance imaging (MRI) to look for differences in cerebral glucose metabolism before and after pairing in males (Bales et al., 2007). This revealed changes in regions of the mesolimbic dopamine system in the NAC and ventral pallidum (VP) such that males from long-term pair bonds had the lowest glucose uptake. Matched before and after comparisons showed that the decreased glucose uptake in these regions occurred within the first 48 hours after pairing. A follow up PET study utilizing a D1R PET ligand, SCH23390, found an increase in D1R binding between one and two months after pairing in the striatum (Hostetler et al., 2017). There was a site-specific increase in the lateral septum (Hostetler et al., 2017), which is a region that has been implicated in social memory and aggression (Clarke & File, 1982). These studies point to changes in the mesolimbic dopamine system during the process of forming a pair bond in titi monkeys, including specific changes in D1R abundance. Therefore, it can be hypothesized that the dopamine system may underlie the transition from pair bond formation to maintenance in titi monkeys similar to prairie voles. However, it is necessary to study the behavioral changes associated with pharmacological manipulation of dopamine receptors to appropriately assess this hypothesis.
The timing of the transition from pair bond formation to maintenance in titi monkeys is not yet established, however there is ample evidence that members of established pairs display agonistic behavior toward intruders including back arching and tail lashing, piloerection, vocalizing and grabbing or restraining the mate. Male and female pairs display more agonistic behavior toward a single stranger compared to a heterosexual pair of strangers (Anzenberger et al., 1986), when a single stranger is opposite-sex to the partner (Mendoza & Mason, 1986) and when strangers are located physically close to the pair-bonded partner (Cubicciotti & Mason, 1978). Male titi monkeys are also more reactive to same-sex stranger encounters than female titi monkeys such that males show more behavioral arousal in presence of strangers (Fernandez-Duque et al, 1997; Fernandez-Duque et al, 2000) and display increased HPA axis activity in the presence of an opposite-sex intruder whereas female titi monkeys did not (Mendoza & Mason, 1986). Finally, male titis maintain closer proximity with partners when opposite sex strangers are presented close to their mate compared to females (Cubicciotti & Mason, 1978). In all of these studies, the tenure of the titi pair bond was greater than one year or temporally undefined and described as an established pair bond. Therefore, it remains unclear when these agonistic behaviors relevant to mate guarding develop during pair bond formation.
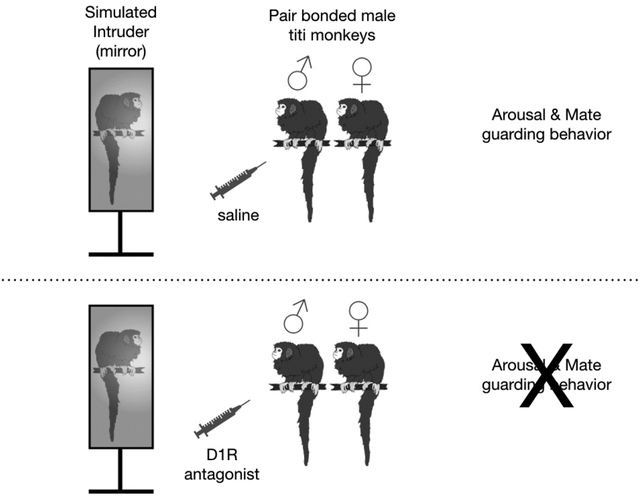
We assessed the role of D1R dopamine receptors in the behavioral components of pair bond maintenance in titi monkeys. We administered a D1R selective antagonist, SCH23390, or saline control to adult male titi monkeys in established pairs. We then presented the males and their female pair mates with a mirror stimulus, which has been used previously with titi monkeys to simulate an intruder monkey and elicit mate-guarding responses (Fisher-Phelps et al., 2016). We hypothesized that blocking D1Rs in titi monkeys would reduce the agonistic components of pair bond maintenance behaviors, including arousal and mate-guarding, displayed by males in the presence of the simulated intruder.
We also conducted a separate test of locomotion to assess the degree to which behavioral changes were influenced by sedative actions of SCH23390 on locomotor control (Löschmann et al., 1991). We assessed locomotor competence by presenting an appealing stimulus followed by an aversive stimulus to the titi monkeys and observing locomotor activity. Finally, we assessed HPA activation in response to test sessions by measuring plasma cortisol both prior to and during the experimental sessions.
2. Material and Methods
2.1. Subjects and Housing
This experiment utilized eight captive born male coppery titi monkeys (Callicebus cupreus) and their female mates. Mean (± SEM) male age was 9.22 (± 1.05) years (range 5.81 – 14.56 years) and mean female age was 10.64 (± 1.76) years (range 4.55 – 17.26 years). All pairs were considered to represent fully established bonds as they had been paired for at least one year (mean 4.16± 1.06 years, range 2.31 – 11.59 years) and had successfully raised offspring together. At the time of testing, six pairs had one offspring living in the home cage and the remaining two pairs had two offspring.
All animals were housed in indoor cages measuring 1.2 m × 1.2 m × 1.8 m at the California Primate Research Center (Davis, CA). Colony rooms were maintained at approximately 21 degrees Celsius on a 12:12 light dark cycle with lights on between 0600 and 1800. The monkeys were fed a diet including New World monkey chow, carrots, bananas, apples and rice cereal and water was available ad libitum. The University of California Davis Institutional Animal Care and Use Committee approved all procedures and all procedures complied with the National Institutes of Health guide for the care and use of Laboratory animals.
2.2. Pharmacological Administration
SCH23390 (Sigma Aldrich, www.sigmaaldrich.com) was used as a selective D1R antagonist. SCH23390 hydrochloride solid was dissolved sterile 0.9% NaCl saline and filtered through a 20 um filter before preparing two administration doses. A high dose of SCH23390 was prepared at 0.1 mg/kg and a low dose was prepared at 0.01 mg/kg. The doses were chosen based on behavioral pharmacology in squirrel monkeys, a nonhuman primate of similar body size to titi monkeys (Spealman et al., 1991). Stock solutions of both doses were stored in −20° C for no more than 30 days. SCH23390 can cross the blood brain barrier (Farde et al., 1987) and therefore we administered all injections intramuscularly into the males’ legs in a volume of 0.1 ml/kg.
2.3. Blood Sampling and Hormone Assessment
A baseline blood sample was collected prior to the beginning of the experiment. During the experiment, a blood sample was collected at the completion of each experimental session to measure plasma cortisol levels reflective of HPA responses during the mirror stimulus presentation and locomotor assessment (see below). For blood sample, males were removed from the home cage in a transfer cage (0.3 m × 0.3 m × 0.3 m), manually restrained and approximately 1 ml of blood was collected with a heparinized needle from the femoral vein. Mean (± SEM) time from cage entry to blood collection was 3.37 ± 0.24 minutes. Blood samples were placed on wet ice until centrifuged at 4° C for 15 minutes. Plasma was extracted and stored at – 80° C until assay. Hormone levels in plasma samples were estimated in duplicate for cortisol (CORT) using commercial radioimmunoassay kits (Siemens Healthcare, Malvern, PA). Plasma samples were diluted 1:4 in PBS gel buffer prior to assay and assay procedures were modified by adding 0.5 and 2.5 μg/dl concentration standards to the provided range of 1.0–50 μg/dl. These procedures have been validated for titi monkeys (Mendoza, unpublished data) and have been used reliably to measure levels of plasma cortisol in titi (Bales et al., 2007; Jarcho et al., 2011; Laugero et al., 2011). All samples were processed in a single assay with an intra-assay CV of 2.76%.
2.4. Mirror Stimulus
A mirror (36 × 22 cm) was utilized to simulate an intruder during the test sessions. In a previous study we found that titi monkeys do not recognize themselves in the mirror nor do they appear to habituate to their own reflection (Fisher-Phelps et al., 2016). Instead, titi monkeys reliably respond to the mirror with arousal and mate guarding behaviors, described in detail below. The non-reflective back side of the mirror stimulus served as a control stimulus.
2.5. Testing Procedure
A timeline of the testing procedure is available in Figure 1. At the beginning of the experiment animals were captured from the home cage with a transport cage, which all animals were trained to do prior to testing. We then administered an intra-muscular injection of the D1R antagonist (high or low dose) or a control saline injection. We then returned the male to the home cage with his mate and offspring until the D1R antagonist came to peak effect at forty minutes (Hietala et al., 1992). We then removed dependent offspring from the cage for the duration of the test. The mirror stimulus was attached to the top of a movable cart (82.6 cm in height) and was wheeled in front of the male’s home cage. The mirror was placed directly in front of the cage door so that an animal sitting on the small door perch would see the full image of itself sitting approximately 16.5 cm away. A visual cover was removed to reveal the reflective mirror or the non-reflective back of the mirror to signal the beginning of the experimental session. The mirror was presented for five minutes and the pair’s response was video recorded.
Figure 1.

Experimental Timeline. Time course of experimental sessions.
An assessment of locomotion was conducted following the mirror stimulus presentation. A common side effect of D1R antagonists is locomotor impairment (Löschmann et al., 1991); To determine the dose of SCH that does not impair locomotion, we presented appealing and aversive stimuli to the pair in the home cage to elicit locomotion to evaluate locomotor abilities. For an appealing stimulus we presented two peanuts on a forage board outside of the home cage that were easily accessible to the monkeys while sitting on the small door perch. The peanut trial lasted three minutes and we video recorded the males’ activity level, which was quantified as the frequency of locomotion during the presentation, as well as the latency to retrieve the peanut. For an aversive stimulus we utilized a modified Human Intruder Test whereby an experimenter stood directly in front of the home cage presenting a series of escalating threats. Specifically, the experimenter stared at the right side of the cage for 30 seconds, the left side of the cage for 30 seconds, directly at the male for 30 seconds, directly at the female for 30 seconds, displayed a leather catch glove on the right hand for 30 seconds and finally displayed a catch glove on the left hand for 30 seconds. The Human Intruder Test lasted for three minutes; again, we recorded the males’ activity level and latency to retreat from the threatening stimuli. Approximately 20 minutes later, a blood sample was collected so that plasma cortisol levels would reflect HPA activity in response to the mirror stimulus and locomotor assessment.
Experimental sessions lasted approximately 70 minutes for each pair. Each male participated in six experimental sessions, representing all possible combinations of pharmacological treatment (high dose, low dose, saline) and mirror stimulus presentations (mirror, control). At least one week separated sessions for the same male, and the order of conditions was quasi counter balanced so that each individual animal had a unique order of the six possible testing conditions during the experimental period.
2.6. Behavioral Data Collection
We video recorded the behavioral responses of the pairs to the mirror stimulus presentation and locomotor assessment and scored behaviors according to the ethogram in Table 1. All behavioral data collected from videos were scored by a single observer using the DVRecorder module of Behavior Tracker 1.5 (www.behaviortracker.com).
TABLE 1.
Ethogram
| Behaviors Relevant to Locomotor Competence | |
|---|---|
| Behavior | Definition |
| Locomotion Frequency | Frequency of bouts of movement where male moves entire body at least one body length in continuous motion until immobile for at least 1 second |
| Peanut Retrieval Latency | Total time (sec) for the male to move from the back of the home cage to pick up the peanut reward placed at the front of the cage |
| Catch Glove Retreat Latency | Total time (sec) for the male to move his body at least one body length further away from the catch glove presented outside the front of the home cage |
| Behaviors Relevant to Pair Bond Maintenance | |
| Behavior | Definition |
| Restrain Female Mate | Forcibly places hands on female’s shoulders, possibly also grabbing her fur |
| Back Arching and Tail Lashing | Arousal display that includes raising of the back, like a frightened cat, and/or repetitive thrashing of the tail from side to side Display may include one or both behaviors simultaneously |
| Movement Duration | Total time (sec) of bouts of movement where male moves entire body at least one body length in continuous motion until immobile for at least 1 second |
| Stimulus Approach Latency | Time (sec) from beginning of experimental session until male has come within approx. 6 inches of the mirror stimulus |
| Stimulus Approach Frequency | Frequency of moving the body within approx. 6 inches of the mirror stimulus |
| Lip Smack | Rapid and repeated opening and closing of the lips |
| Approach Female Mate | Moves body to within monkey arm’s length of female mate |
| Social Proximity | Total time (sec) pair is within one monkey arm’s length |
| Social Contact | Total time (sec) pair is in physical contact |
| Terminate Social Contact | Male moves his body so that he is no longer in physical contact with his female partner |
2.7. Data analysis for Locomotor Assessment
We assessed locomotor impairment using a General Linear Mixed Model (GLMM) (Littell et al., 1996) in SAS PROC GLM with a fixed effect for Drug Condition (saline, low dose D1R antagonist, high dose D1R antagonist) with a Fisher’s least-significant-difference posthoc test to evaluate differences across Drug Condition. We also included a random effect for individual animal in order to account for individual variability within the repeated measures design. We applied this model to the males’ behaviors according to the ethogram in Table 1.
2.8. Data analysis for Behavioral Responses
To evaluate the influence of D1R antagonist treatment on male behaviors related to pair bond maintenance we utilized a GLMM in SAS PROC GLM with fixed effects for Drug Condition (saline, D1R antagonist), Stimulus Presentation (mirror, back of mirror) and the interaction of Drug Condition*Stimulus Presentation with planned comparisons for mirror vs. back of mirror at each level of Drug Condition. We also included a random effect for individual animal in order to account for individual variability within the repeated measures design. We applied this GLMM to male and dyadic behaviors relevant to pair bond maintenance listed in Table 1. When the residuals for outcome variables were not normally distributed we performed a square root or quad root transformation on the variable. In cases where these transformations did not normalize the residuals we performed the GLMM on the original, untransformed variable. An F-test is recommended for non-normally distributed data (Feir-Walsh and Toothaker, 1974). For all statistical tests alpha was set at 0.05 and all tests were two-tailed. P-values for all non-significant results were greater than 0.06.
3. Results
3.1. Locomotor Assessment
Locomotor impairment was apparent during the high dose treatment (1.0 mg/kg) of the D1R antagonist. Male locomotion frequency was predicted by Drug Condition (Figure 2a) and males moved less frequently with the high dose treatment during the mirror presentation [F (2, 47) = 8.60, p = 0.0008], the peanut presentation [F (2,47) = 18.10, p < 0.0001] and the modified human intruder test [F (2, 47) = 5.75, p = 0.006]. Under administration of the high dose D1R antagonist males took significantly longer to retrieve the peanut after being administered the high dose D1R antagonist compared to saline administration (Figure 2b). Males also took longer to move in response to seeing the catch gloves during the modified human intruder test during high dose D1R antagonist treatment compared to low dose treatment (Figure 2c). These results indicate that the high dose of SCH23390 gave a sedative effect, therefore reducing locomotor competence and potentially altering behavioral responses. Therefore, remaining results compare responses to experimental sessions in which the low dose (0.01 mg/kg) treatment of the D1R antagonist or saline control were administered. We did not note any other movement-related side effects (i.e. rigidity, tremor, involuntary muscle movement, etc.) that can accompany dopamine antagonist treatment.
Figure 2.

Locomotor Assessment. a. The high dose of the D1R antagonist led to significantly reduced locomotion frequency during each component of the test session (i.e. mirror, peanut, human intruder presentations). b. High dose D1R antagonism led to longer latency to retrieve the appealing peanut stimulus. c. High dose D1R antagonism led to longer latency to retreat from the fearful catch glove stimulus. *p < .05. Error bars indicate standard error of the mean.
3.2. Male Pair Bond Maintenance Behavior
The frequency that males restrained their female mates was significantly predicted the interaction between Drug Condition and Stimulus Presentation [F (1, 31) = 5.22, p = 0.032; Figure 3a]. Specifically, males restrained their female mates significantly more when they were treated with saline and viewed the front of the mirror compared to all other test conditions. The frequency that males displayed behavioral arousal via back arching and tail lashing was significantly predicted by Stimulus Presentation [F (1, 31) = 6.28, p = 0.02] but was neither predicted by Drug Condition [F (1, 31) = 0.95, p = 0.34] nor the interaction of Drug Condition and Stimulus Presentation [F (1, 31) = 0.87, p = 0.36, Figure 3b]. A planned comparison for mirror vs. back of mirror at each level of Drug Condition revealed that males displayed significantly more arousal when viewing the front of the mirror compared to the back of the mirror stimulus when saline was administered, whereas males displayed similarly low levels of arousal behavior to both sides of the mirror stimulus following administration of the D1R antagonist.
Figure 3.

Mate Guarding and Arousal Behavior. a. With saline administration, males restrained their female mates more frequently when viewing the front of the mirror stimulus compared to the back. D1R antagonist administration attenuated the frequency at which males restrained their female mates when viewing the front of the mirror. b. Males displayed back arching and tail lashing more frequently when viewing the front of the mirror compared to the back with saline administration. With D1R antagonist administration males responded with similarly low levels of arousal behavior to both sides of the mirror stimulus. c. Male displayed longer movement durations when viewing the mirror compared to the back after saline was administered, whereas movement durations were similar across stimulus presentations following D1R antagonist administration. d. Males initially approached the stimulus with similar latencies regardless of test condition. e. With saline administration males approached the mirror more frequently compared to the back of the mirror stimulus. However, with D1R antagonism males approached both sides of the mirror stimulus with similarly low frequency. *p < .05; **p < .01. Error bars indicate standard error of the mean.
Male movement duration in the mirror stimulus presentation was significantly predicted by the interaction between Drug Condition and Stimulus Presentation [F (1, 31) = 9.89, p = 0.0049; Figure 3c]. Specifically, when males were treated with saline they displayed significantly more movement when viewing the mirror compared to the back of the stimulus. In contrast, when males were treated with the D1R antagonist, there was no difference between movement duration when viewing the mirror compared to the back of the stimulus.
The males’ frequency to approach the stimulus was significantly predicted by the interaction between Drug Condition and Stimulus Presentation [F (1, 31) = 9.64, p = 0.0054; Figure 3e]. Specifically, when males were treated with saline they approached the mirror significantly more than the back side of the mirror. When males were treated with the D1R antagonist they approached the stimulus at similar frequencies regardless of whether they were viewing the front or back of the mirror. Despite approaching the stimulus less frequently with D1R antagonist treatment, males’ latency to approach the stimulus was not predicted by Drug Condition, Stimulus Presentation or the interaction of Drug Condition and Stimulus Presentation, suggesting the males’ initial interest in the simulated intruder was not diminished by D1R antagonism (Figure 3d).
The frequency of male lip smacks to female mates was not predicted by Drug Condition, Stimulus Presentation or the interaction between Drug Condition and Stimulus Presentation (Figure 4a). Likewise, social proximity was not predicted by Drug Condition, Stimulus Presentation or the interaction between Drug Condition and Stimulus Presentation (Figure 4b), indicating that partners spent similar durations of time in proximity during test sessions regardless of the test conditions.
Figure 4.

Male Mate-directed Behavior. a. Males lip smacked to their female partners at similar frequencies regardless of test conditions. b. Social proximity between the males and their female partners was similar regardless of test condition. c. There was a trend for male and female partners to spend more time in social contact while viewing the mirror compared to the back after males had been administered the D1R antagonist. d. Male approaches to the female partners was similar across test conditions. e. Male terminated contact with their female partners significantly more while viewing the mirror after saline administration compared to all other test conditions. f. Following termination of contact with female partners, males engaged in one of three behaviors- locomotion only, approach stimulus or back arching and tail lashing. In the test condition where males terminated contact the most (mirror, saline), they also engaged in stimulus approach or back arching and tail lashing within 10 seconds of contact termination. *p < .05; **p < .01. Error bars indicate standard error of the mean.
Social contact between partners was significantly predicted by Stimulus Presentation [F (1, 31) = 4.64, p = 0.043] such that partners spent more time in social contact when viewing the mirror compared to the back of the mirror. Social contact duration was not significantly predicted by the interaction between Drug Condition and Stimulus Presentation [F (1, 31) = 0.13, p = 0.72; Figure 4c], but a planned comparison for mirror vs. back of mirror at each level of Drug Condition revealed a trend whereby partners spent more time in contact when viewing the front of the mirror compared to the back of the mirror stimulus when the D1R antagonist treatment was administered to the males [t = 1.98, p = 0.06]. A higher duration of social contact at the dyadic level could be due to initiating contact more frequently or terminating contact less frequently. Males approached their female mates at similar frequencies regardless of test conditions and approaches were not predicted by Drug Condition, Stimulus Presentation or the interaction between Drug Condition and Stimulus Presentation (Figure 4d). In contrast, terminating social contact by males was significantly predicted by the interaction between Drug Condition and Stimulus Presentation [F (1, 31) = 4.45, p = 0.047, Figure 4e]. Taken together, these results suggest that contact duration was higher during the mirror presentation with the D1R antagonist due to males terminating contact less frequently with their female partners when compared with viewing the mirror with saline administration. In order to better understand the context in which males were terminating contact less frequently with the D1 antagonist treatment, we investigated the behavior that males displayed within 10 seconds of terminating contact with their female mates. In all instances males displayed one of three behaviors: locomotion around the cage, approaching the stimulus or displaying arousal via back arching or tail lashing. It is apparent that when males are administered saline and view a simulated intruder (i.e. front of mirror), males terminated contact and then displayed arousal, approached the simulated intruder or displayed locomotion (Figure 4f).
3.3. Male Hormonal Responses
Hormone results reflect all but three plasma samples that were not able to be collected during the experiment. Male plasma cortisol levels were significantly predicted by Drug Condition with significantly higher cortisol levels during experimental sessions compared to the pre-experiment baseline measurement [F (3, 52) = 8.92, p = 0.0001; Figure 5]. Further, plasma cortisol levels were significantly higher for experimental sessions where males received the high dose of D1R antagonist compared to the low dose or saline. In experimental sessions, plasma cortisol was significantly predicted by drug dose [F (2, 44) = 6.65, p = 0.004] with higher plasma cortisol levels during high dose sessions compared to low dose and saline, which did not differ significantly from one another. In experimental sessions, plasma cortisol levels were predicted neither by Stimulus Presentation, nor by the interaction of Drug Condition and Stimulus Presentation.
Figure 5.

Male Plasma Cortisol. Plasma cortisol concentrations were significantly lower in the pre-experiment baseline samples compared to all test sessions. Plasma cortisol was highest in test sessions with the high dose of the D1R antagonist. a vs. b: p < .05; a vs. c: p < .001; b vs. c: p < .01. Error bars indicate standard error of the mean.
4. Discussion
We investigated the role of dopamine D1-like receptor (D1R) activity in pair bond maintenance behavior in male titi monkeys. As hypothesized, we found that blocking D1Rs diminished key agonistic and arousal behaviors displayed by pair bonded male titi monkeys in the presence of a simulated intruder. D1R antagonism diminished the frequency at which males restrained their female mates in the presence of the simulated intruder. Males also displayed less back arching and tail lashing in response to the simulated intruder with D1R antagonism, which are behavioral displays of arousal and territoriality in titi monkeys. Chasing behavior is also a common response to intruders in wild titi monkeys (Mason, 1966). Although our experimental paradigm did not permit the males to engage in chasing behavior, we did find that males diminished both their frequency of approaching the simulated intruder as well as their movement duration when viewing the mirror under the influence of the D1R antagonist. Therefore, it is possible that D1R antagonism would diminish chasing behavior and future studies could investigate this as an additional behavior in the suite of species-typical mate guarding behavior relevant to pair bond maintenance.
We found evidence of diminished interest in the simulated intruder with D1R antagonism. Specifically, the males approached the simulated intruder less frequently with D1R antagonism compared to control treatment. Moreover, males terminated social contact with their female partners less in the presence of the simulated intruder under D1R antagonist treatment. We interpret this as diminished interest in the simulated intruder because we found that, following contact termination, males typically engaged in stimulus-directed behavior such as approaching the simulated intruder or displaying tail lashing or back arching. Although males approached the simulated intruder less frequently with the D1R antagonist, we do not interpret these findings to represent broadly dampened social interest. Males’ initial approaches to the simulated intruder were fast regardless of drug treatment, and males approached their mates with similar frequencies while viewing the mirror, regardless of drug treatment. Therefore, we interpret our behavioral results to represent specific changes in response to a social threat to the pair bond.
Importantly, our results indicate these observed behavioral changes at the low dose of the D1R antagonist are not likely attributable to locomotor impairment or an excessive arousal response. Locomotor impairment was evident at the high dose of SCH23390, as males displayed diminished locomotion and impaired latency to quickly retrieve an appealing food reward and quickly retreat from a threatening stimulus. However, since these results were not found at the low dose of SCH23390, we conclude that animals retained locomotor competence at this dose. We found higher plasma cortisol levels for all experimental sessions compared to a pre-experiment baseline, indicating that overall test sessions elevated HPA activity. HPA activity in titi monkeys is sensitive to novelty and environmental change (Hennessy et al., 1995), and as our experimental sessions presented several disruptions not typical of non-experimental days including capture, intramuscular injection, presentation of the mirror stimulus and the locomotor assessment including the modified Human Intruder Test. The high dose of SCH23390 led to an even further increase in plasma cortisol, which could be due to the drug itself, as SCH23390 administration has been found to elevate plasma cortisol in dogs (Goiny et al., 1986). It is also possible that males experienced distress in response to the mobility deficits they experienced with the high dose of SCH23390. Together, we take these results to indicate that the changes in mate guarding and arousal behavior with the low dose D1R antagonist were not likely to be attributed to locomotor impairment or HPA elevation.
The behavioral changes observed with D1R antagonism were largely directed to the simulated intruder, rather than changes in interactions between pair mates. D1R antagonism did not alter the frequency with which males lip smacked to the female mate, the frequency of approaches to the female mate, nor the amount of time the partners spent in social proximity. Increased duration of social contact was found in the presence of the mirror but we believe this is not a representation of greater affiliation between partners but instead a consequence of males not terminating contact to approach or display toward the simulated intruder when under the influence of the D1R antagonist. Therefore, D1R activity appears to underlie a specific set of pair bond maintenance behaviors specifically related to mate guarding. This is consistent with literature from prairie voles where binding of dopamine at upregulated D1Rs in the striatum, specifically the rostral shell of the NAC, underlies inter and intra-sexual aggression (Aragona et al., 2003; Resendez et al., 2016).
A potential limitation was the use of a mirror to simulate an intruder rather than use a real intruder monkey, as a mirror stimulus poses concerns about whether or not a monkey can identify oneself in a mirror, or may respond differently to the mirror stimulus compared to a live monkey. However, previous use of the mirror stimulus found no evidence of self-directed behavior (indicative of self-recognition). The prior study also found that the rates of arousal and agonistic displays to the mirror were similar to published rates of displays to an actual intruder monkey (Fisher-Phelps et al., 2016). It is also important to note that the mirror stimulus relies on a test animal performing behavior to then view the “intruder” (aka himself) monkey responding similarly in the mirrored reflection. If an animal responds to the mirror with high arousal, he will see a “stranger” monkey responding with high arousal, thus creating a positive feedback loop of behavioral responding. In contrast if an animal responds with only mild arousal, he will see a “stranger” monkey responding with mild arousal. This phenomenon creates non-random variation across individuals in how individual animals respond to the stimulus. Despite this limitation, the positive feedback loop of behavioral responding greatly benefited this study, as we needed to maximize responses in order to properly evaluate whether behavioral responses were diminished via D1R antagonism.
These results bolster existing support for the hypothesis that the dopamine system underlies the transition from pair bond formation to maintenance in non-human primates (i.e. titi) monkeys as it does in rodents (i.e. prairie voles). Previous work with titi monkeys found increased glucose uptake throughout dopaminergic areas, as well as increased D1R specific binding in the lateral septum, during the early stages of pairing (Hostetler et al., 2017). Here we found that blocking D1Rs in males from established pair bonds blocks mate and territorial defense components of pair bond maintenance behavior. These findings with recent findings that marmosets from established pair relationships showed behavioral changes with D1R manipulation whereas marmosets from new relationships were more sensitive to D2R manipulation (Carp et al., 2018). These findings also fit with literature from socially monogamous California mice that D1R antagonism diminished chasing behavior in social contests (Becker & Marler, 2015). One limitation of the current study is that we cannot be certain where the peripherally administered D1R antagonist was acting in the brain. Future studies should combine D1R imaging with antagonist dosing to confirm these behavioral changes are a result of binding within the striatum, as is the case for prairie voles, or in the lateral septum. To further address the present hypothesis, future work should also evaluate the degree to which D2Rs are involved in pair bond formation in titi monkeys, as dopamine activity at this class of receptors regulates partner preference formation in prairie voles (Gingrich et al., 2000; Aragona et al., 2003). Support for similar roles of both classes of dopamine receptors across species would provide evidence that the highly conserved mesocorticolimbic pathway plays a key role in pair bonding widely across mammals.
Acknowledgments
We would like to thank Nicole Maninger, Becky Larke, Tamara Weinstein, Sarah Carp, Christian Constantz, Charlotte Blanz, Julie Ring, and Rachel Wu for their invaluable help during testing sessions. We would also like to thank Dr. Angela Colagross-Schouten and the veterinary staff for their excellent care of the animals.
Funding: This project was supported by the National Institutes of Health [HD053555, OD011107], the National Science Foundation [Graduate Research Fellowship], the Good Nature Institute and the American Society of Primatologists [Small Research Grant].
Footnotes
Declarations of interest: none.
References
- Ainsworth M, Blehar M, Waters E, & Wall S (1978). Patterns of attachment: Observations in the Strange Situation and at home. Hillsdale, N]: Erlbaum. [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, & Wang Z (2003). A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. Journal of Neuroscience, 23(8), 3483–3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, & Wang Z (2006). Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nature Neuroscience, 9(1), 133. [DOI] [PubMed] [Google Scholar]
- Anzenberger G, Mendoza SP, & Mason WA (1986). Comparative studies of social behavior in Callicebus and Saimiri: Behavioral and physiological responses of established pairs to unfamiliar pairs. American Journal of Primatology, 11(1), 37–51. [DOI] [PubMed] [Google Scholar]
- Bales KL, Mason WA, Catana C, Cherry SR, & Mendoza SP (2007). Neural correlates of pair-bonding in a monogamous primate. Brain Research, 1184, 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bales KL, del Razo RA, Conklin QA, Hartman S, Mayer HS, Rogers FD, … & Witczak LR (2017). Focus: comparative medicine: Titi monkeys as a novel non-human primate model for the neurobiology of pair bonding. The Yale Journal of Biology and Medicine, 90(3), 373. [PMC free article] [PubMed] [Google Scholar]
- Becker EA, & Marler CA (2015). Postcontest blockade of dopamine receptors inhibits development of the winner effect in the California mouse (Peromyscus californicus). Behavioral neuroscience, 129(2), 205. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK & Spealman RD. (1991). Behavioral effects of D1 and D2 dopamine receptors antagonists in squirrel monkeys. The Journal of Pharmacology and Experimental Therapeutics, 258, 910–917. [PubMed] [Google Scholar]
- Bowlby J (1969). Attachment and loss v. 3 (Vol. 1). Random House. Furman, W., & Buhrmester, D. (2009). Methods and measures: The network of relationships inventory: Behavioral systems version. International Journal of Behavioral Development, 33, 470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Spiegel LL, Inoue K, Murphy AZ, & Young LJ (2011). Activation of μ-opioid receptors in the dorsal striatum is necessary for adult social attachment in monogamous prairie voles. Neuropsychopharmacology, 36(11), 2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler EA, & Randall AK (2013). Emotional coregulation in close relationships. Emotion Review, 5(2), 202–210. [Google Scholar]
- Carp SB, Rothwell ES, Bourdon A, Freeman SM, Ferrer E, & Bales KL (2016). Development of a partner preference test that differentiates between established pair bonds and other relationships in socially monogamous titi monkeys (Callicebus cupreus). American Journal of Primatology, 78(3), 326–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carp SB, Taylor JH, Womack SL, & French JA (2018). Dopamine Modulation of Reunion Behavior in Short and Long Term Marmoset Pairs. Frontiers in Ecology & Evolution, 6(46). doi: 10.3389/fevo. [DOI] [Google Scholar]
- Clarke A, & File SE (1982). Selective neurotoxin lesions of the lateral septum: changes in social and aggressive behaviours. Pharmacology Biochemistry and Behavior, 17(4), 623–628. [DOI] [PubMed] [Google Scholar]
- Cubicciotti DD, & Mason WA (1978). Comparative studies of social behavior in Callicebus and Saimiri: heterosexual jealousy behavior. Behavioral Ecology and Sociobiology, 3(3), 311–322. [Google Scholar]
- Donovan M, Liu Y, & Wang Z (2018). Anxiety-like behavior and neuropeptide receptor expression in male and female prairie voles: The effects of stress and social buffering. Behavioural Brain Research, 342, 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farde L, Halldin C, Stone-Elander S, & Sedvall G (1987). PET analysis of human dopamine receptor subtypes using 11 C-SCH 23390 and 11 C-raclopride. Psychopharmacology, 92(3), 278–284. [DOI] [PubMed] [Google Scholar]
- Feir-Walsh BJ, & Toothaker LE (1974). An empirical comparison of the ANOVA F-test, normal scores test and Kruskal-Wallis test under violation of assumptions. Educational and Psychological Measurement, 34(4), 789–799. [Google Scholar]
- Fernandez‐Duque E, Mason WA, & Mendoza SP (1997). Effects of duration of separation on responses to mates and strangers in the monogamous titi monkey (Callicebus moloch). American Journal of Primatology, 43(3), 225–237. [DOI] [PubMed] [Google Scholar]
- Fernandez‐Duque E, Valeggia CR, & Mason WA (2000). Effects of pair‐bond and social context on male–female interactions in captive titi monkeys (Callicebus moloch, Primates: Cebidae). Ethology, 106(12), 1067–1082. [Google Scholar]
- Fisher‐Phelps ML, Mendoza SP, Serna S, Griffin LL, Schaefer TJ, Jarcho MR, Ragen BJ, Goetze LR & Bales KL (2016). Laboratory simulations of mate‐guarding as a component of the pair‐bond in male titi monkeys, Callicebus cupreus. American Journal of Primatology, 78(5), 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes A (2002). Patterns and trends in primate pair bonds. International Journal of Primatology, 23(5), 953–978. [Google Scholar]
- Getz LL, Carter CS, & Gavish L (1981). The mating system of the prairie vole, Microtus ochrogaster: field and laboratory evidence for pair-bonding. Behavioral Ecology and Sociobiology, 8(3), 189–194. [Google Scholar]
- Gingrich B, Liu Y, Cascio C, Wang Z, & Insel TR (2000). Dopamine D2 receptors in the nucleus accumbens are important for social attachment in female prairie voles (Microtus ochrogaster). Behavioral Neuroscience, 114(1), 173. [DOI] [PubMed] [Google Scholar]
- Goiny M, & Uvnäs-Moberg K (1986). Effects of dopaminomimetics on the secretion of VIP-like immunoreactivity in conscious dogs. Peptides, 7, 221–224. [DOI] [PubMed] [Google Scholar]
- Hazan C, & Shaver P (1987). Romantic love conceptualized as an attachment process. Journal of Personality and Social psychology, 52(3), 511. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Mendoza SP, Mason WA, & Moberg GP (1995). Endocrine sensitivity to novelty in squirrel monkeys and titi monkeys: species differences in characteristic modes of responding to the environment. Physiology & Behavior, 57(2), 331–338. [DOI] [PubMed] [Google Scholar]
- Hietala J, Seppälä T, Lappalainen J, & Syvälahti E (1992). Quantification of SCH 39166, a novel selective D1 dopamine receptor antagonist, in rat brain and blood. Psychopharmacology, 106(4), 455–458. [DOI] [PubMed] [Google Scholar]
- Hostetler CM, Hinde K, Maninger N, Mendoza SP, Mason WA, Rowland DJ, Wang GB, Kukis D, Cherry SR & Bales KL (2017). Effects of pair bonding on dopamine D1 receptors in monogamous male titi monkeys (Callicebus cupreus). American Journal of Primatology, 79(3), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho MR, Mendoza SP, Mason WA, Yang X, & Bales KL (2011). Intranasal vasopressin affects pair bonding and peripheral gene expression in male Callicebus cupreus. Genes, Brain and Behavior, 10(3), 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ZV, & Young LJ (2015). Neurobiological mechanisms of social attachment and pair bonding. Current opinion in behavioral sciences, 3, 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiman DG (1977). Monogamy in mammals. The Quarterly review of biology, 52(1), 39–69. [DOI] [PubMed] [Google Scholar]
- Kowalczyk AS, Davila RF, & Trainor BC (2018). Effects of social defeat on paternal behavior and pair bonding behavior in male California mice (Peromyscus californicus). Hormones and behavior, 98, 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laugero KD, Smilowitz JT, German JB, Jarcho MR, Mendoza SP, & Bales KL (2011). Plasma omega 3 polyunsaturated fatty acid status and monounsaturated fatty acids are altered by chronic social stress and predict endocrine responses to acute stress in titi monkeys. Prostaglandins, Leukotrienes and Essential Fatty Acids, 84(3–4), 71–78. [DOI] [PubMed] [Google Scholar]
- Lim MM, Liu Y, Ryabinin AE, Bai Y, Wang Z, & Young LJ (2007). CRF receptors in the nucleus accumbens modulate partner preference in prairie voles. Hormones and Behavior, 51(4), 508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, & Wolfinger RD (1996). SAS system for mixed models. Cary, North Carolina: SAS Institute. [Google Scholar]
- Löschmann PA, Lange KW, Kunow M, Rettig KJ, Jähnig P, Honore T, Turski L, Wachtel H, Jenner P & Marsden CD (1991). Synergism of the AMPA-antagonist NBQX and the NMDA-antagonist CPP with L-dopa in models of Parkinson’s disease. Journal of Neural Transmission-Parkinson’s Disease and Dementia Section, 3(3), 203–213. [DOI] [PubMed] [Google Scholar]
- Lukas D & Clutton-Brock TH (2013). “The evolution of social monogamy in mammals.” Science, 341 6145: 526–530. [DOI] [PubMed] [Google Scholar]
- Mason WA (1966). Social organization of the South American monkey, Callicebus moloch: A preliminary report. Tulane Studies in Zoology, 13(5). [Google Scholar]
- Mason WA, & Mendoza SP (1998). Generic aspects of primate attachments: parents, offspring and mates. Psychoneuroendocrinology, 23(8), 765–778. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, & Mason WA (1986). Contrasting responses to intruders and to involuntary separation by monogamous and polygynous New World monkeys. Physiology & behavior, 38(6), 795–801. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Capitanio JP, & Mason WA (2000). Chronic social stress: studies in non-human primates. Biology of animal stress: Basic principles and implications for animal welfare, 227–247. [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, & Caron MG (1998). Dopamine receptors: from structure to function. Physiological Reviews, 78(1), 189–225. [DOI] [PubMed] [Google Scholar]
- Opie C, Atkinson QD, Dunbar RI, & Shultz S (2013). Male infanticide leads to social monogamy in primates. Proceedings of the National Academy of Sciences, 110(33), 13328–13332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resendez SL, Keyes PC, Day JJ, Hambro C, Austin CJ, Maina FK, Edison LN, Porter-Stransky KA, Nevarez N, McLean JW, Kuhnmuench MA, Murphy AZ, Mathews TA & Aragona BJ (2016). Dopamine and opioid systems interact within the nucleus accumbens to maintain monogamous pair bonds. elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons TC, Rothwell ES, Savidge LE, del Razo RA, & Bales KL (2019). Neurobiology of Pair Bonding. Encyclopedia of Animal Behavior, 262–273. [Google Scholar]
- Sun P, Smith AS, Lei K, Liu Y, & Wang Z (2014). Breaking bonds in male prairie vole: long-term effects on emotional and social behavior, physiology, and neurochemistry. Behavioural Brain Research, 265, 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walum H, & Young LJ (2018). The neural mechanisms and circuitry of the pair bond. Nature Reviews Neuroscience, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Catania KC, & Carter CS (1992). Development of partner preferences in female prairie voles (Microtus ochrogaster): the role of social and sexual experience. Hormones and Behavior, 26(3), 339–349. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, & Insel TR (1993). A role for central vasopressin in pair bonding in monogamous prairie voles. Nature, 365(6446), 545. [DOI] [PubMed] [Google Scholar]


