Abstract
Background
Helminths and tuberculosis (TB) largely overlap at the population level. Whether helminth infections influence disease severity and bacterial burdens in TB is not well understood.
Methods
This study was conducted to examine the disease severity in a cohort of pulmonary TB (PTB) individuals with (Ss+) or without (Ss−) seropositivity for Strongyloides stercoralis infection.
Results
Ss+ was associated with increased risk of cavitation (odds ratio [OR], 4.54; 95% confidence interval [CI], 2.33–9.04; P < .0001) and bilateral lung involvement (OR, 5.97; 95% CI, 3.03–12.09; P < .0001) in PTB individuals. Ss+ was also associated with higher bacterial burdens (OR, 7.57; 95% CI, 4.18–14.05; P < .0001) in PTB individuals. After multivariate analysis adjusting for covariates, Ss+ was still associated with greater risk of cavitation (adjusted OR [aOR], 3.99; 95% CI, 1.73–9.19; P = .0014), bilateral lung involvement (aOR, 4.09; 95% CI, 1.78–9.41; P = .0011), and higher bacterial burden (aOR, 9.32; 95% CI, 6.30–13.96; P < .0001). Finally, Ss+ was also associated with higher plasma levels of matrix metalloproteinases ([MMP]-1, -2, -7, -8, and -9) in PTB individuals.
Conclusions
Therefore, our data demonstrate that coexistent Ss infection is associated with greater disease severity and higher bacterial burden in PTB. Our data also demonstrate enhanced plasma levels of MMPs in coinfected individuals, suggesting a plausible biological mechanism for these effects.
Keywords: disease severity, helminths, MMPs, tuberculosis
This study demonstrates that Strongyloides stercoralis coinfection is associated with increased disease severity and elevated mycobacterial burdens in adult pulmonary tuberculosis. This is also associated with heightened levels of MMPs in the coinfected individuals.
More than 2 billion people worldwide are infected with helminths, whereas one quarter of the world’s population may harbor Mycobacterium tuberculosis (Mtb) infection, often with a great degree of geographical overlap (with helminths being prevalent in 21 of 22 countries with the highest tuberculosis [TB] burden) [1, 2]. India accounted for 27% of the active TB cases worldwide, while also accounting for 24% of the world’s population for helminth infection [1, 2]. Understanding Mtb and helminth coinfection is critical because the immune response to helminths affect the ability to control Mtb and to respond to TB vaccines [3]. Epidemiological studies show that helminths are associated with TB pathogenesis [4], but the effect of helminths on TB disease pathogenesis and pathology is poorly understood.
On population levels, helminths and TB disease seem to interact. Thus, TB patients in Ethiopia were 4 times more likely to have a helminth infection than household contacts who had no evidence of TB disease, and the patients had a significantly higher prevalence of helminths compared with community controls [5, 6]. Likewise, TB patients in Brazil were 5 times as likely to have any helminth compared with matched controls [7]. However, a recent meta-analysis of 20 studies examining the risk of TB among intestinal helminth infections failed to show a significant association for helminths, with the exception of Strongyloides stercoralis (Ss) infection (TB patients had a 2.68-fold higher risk of Ss infection) [8]. Nevertheless, very few studies have examined TB disease severity or bacterial burden in helminth coinfections. Some studies have found no significant effects of helminth infection on TB severity [6, 9, 10], whereas one study demonstrated that TB-helminth coinfected individuals have been found to have more advanced clinical presentation [11].
Therefore, we have examined the association of Ss infection and pulmonary TB (PTB) in individuals from Chennai, South India. Our data reveal that Ss coinfection is strongly associated with TB disease severity as well as bacterial burdens in PTB. Our data also reveal an enhancement effect of Ss coinfection on the plasma levels of matrix metalloproteinases, which are typical drivers of lung pathology PTB [12], thereby offering a plausible biological mechanism for this interaction.
MATERIALS AND METHODS
Ethics Statement
All individuals were examined as part of a clinical research protocol (ClinicalTrials.gov: NCT01154959) approved by Institutional Review Board of the National Institute for Research in Tuberculosis, India, and informed written consent was obtained from all participants.
Study Population and Tuberculosis Evaluation
Newly diagnosed smear- and culture-positive PTB individuals were recruited from Chennai, India. A total of 200 culture-confirmed PTB individuals were recruited in this study. Individuals with PTB were diagnosed by positive solid cultures in Lowenstein-Jensen medium. Chest x-rays were used to determine cavitary disease as well as unilateral versus bilateral involvement. Chest x-rays were read by 2 independent radiologists. Smear grade was used to determine bacterial burdens and classified as 1+, 2+, and 3+. The laboratory investigators were blinded to the chest x-ray and bacteriology results. At the time of enrollment, all active TB cases had no record of prior TB disease or anti-TB treatment or any steroid use. All participants were Bacillus Calmette-Guérin vaccinated (identified by scar status) and human immunodeficiency virus (HIV) negative (using rapid test). Participants were excluded if they exhibited signs or symptoms of any associated lung or systemic disease and if they were found to be drug-resistant. Diabetic status was assessed using hemoglobin A1c (HbA1c) levels (HbA1c >6.5% was considered diabetic). Plasma samples were obtained from all 200 individuals at baseline (before TB treatment initiation) and stored until further use.
Strongyloides Serology
Strongyloides stercoralis infection was diagnosed by the presence of immunoglobulin G antibodies to the recombinant NIE antigen, as described previously [13, 14]. Filarial infection was excluded in all study participants by virtue of being negative in tests for circulating filarial antigen (TropBio Og4C3 assay).
Multiplex Assay
Matrix metalloproteinases (MMPs) were measured in a subset of Ss+ (n = 31) and Ss− (n = 62) individuals. Plasma levels of MMP-1, MMP-2, MMP-3, MMP-7, MMP-8, MMP-9, MMP-10, MMP-12, and MMP-13 were determined using a multiplex Luminex assay (Bio-Rad Laboratories, Inc.). The lowest detection limits were as follows: MMP-1, 115.8 pg/mL; MMP-2, 809 pg/mL; MMP-3, 199.2 pg/mL; MMP-7, 27.7 pg/mL; MMP-8, 31.7 pg/mL; MMP-9, 257.5 pg/mL; MMP-10, 78.4 pg/mL; MMP-12, 18.5 pg/mL; and MMP-13, 32.9 pg/mL.
Statistical Analysis
Geometric means (GMs) were used for measurements of central tendency. Comparisons between the Ss+ and Ss− groups used the Mann-Whitney test (for numeric variables), the central Fisher’s exact test (for binary variables), or a proportional odds model (for ordinal variables). Three separate analyses for each type of disease severity was performed, and within each a simple univariate test of association and a more complicated adjustment multivariate test that adjusts for covariates were performed. For the univariate tests, either a central Fisher’s exact test (for lung lesions and cavitation) or a Wilcoxon-Mann-Whitney test (for acid-fast bacilli [AFB] smear grade) was used. To compare with the multivariate test, a proportional odds model for the AFB smear grade was used. For the multivariate tests, propensity scores with inverse probability weighting for the models (logistic for lung lesions and cavitation, or proportional odds for AFB smear grade) were used. All analysis were prespecified. Conditional regression was used due to the case-control design of the study. Adjustments for variables used either weighted logistic regression (binary variables) or weighted proportional odds models (ordinal variables), using inverse probability weighting with the propensity scores estimated by the random Forest R package. Holm’s correction for multiple comparisons was used for the tests of Figure 1. Analyses were performed using Graph-Pad PRISM version 8.0 or R version 3.6.1. Proportional odds model used the MASS R package, and the central Fisher’s exact test used the exact 2x2 R package.
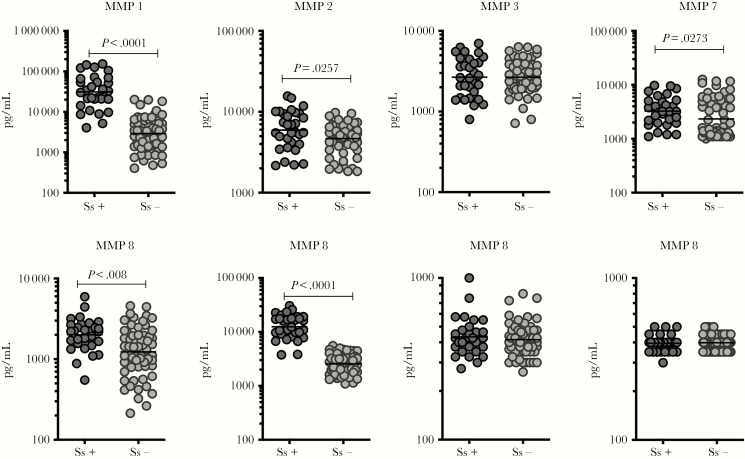
Figure 1.
Elevated plasma levels of matrix metalloproteinases (MMPs) in Ss+ individuals with pulmonary tuberculosis (PTB). The plasma levels of MMP-1, -2, -3, -7, -8, -9, -12, and -13 in Ss+ (n = 31) and Ss− (n = 62) PTB individuals were measured by multiplex assay. The data are represented as scatter plots with each circle representing a single individual. P values were calculated using the Mann-Whitney test with Holm’s correction for multiple comparisons.
RESULTS
Demographics of the Study Population
The total study population was 200 individuals with PTB, including 67 Ss+ and 133 Ss−. The median age was 42 (interquartile range [IQR], 18–60) years for Ss+ and 36 (IQR, 19–65) years for Ss− (P = .0613). There were no significant differences in gender, body mass index (BMI), smoking status, or alcohol use (Table 1). Hemoglobin A1c levels were significantly different between Ss+ (median, 5.6; IQR, 4.5–17.7) and Ss− (median, 6; IQR, 4.6–14.5) individuals.
Table 1.
Demographics and Clinical Characteristics of the Study Population
| Characteristic | Ss+ (n = 67 [34%]) | Ss− (n = 133 [66%]) | P Value |
|---|---|---|---|
| Age, years, median (IQR) | 42 (18–60) | 36 (19–65) | .0613 |
| Gender | |||
| Male | 55 [82%] | 104 [78%] | .6542 |
| Female | 12 [18%] | 29 [22%] | |
| BMI, kg/m2, median (IQR) | 18.9 (13.8–30.4) | 18.6 (12.8–29.5) | .2757 |
| HbA1c | 5.6 (4.5–17.7) | 6 (4.6–14.9) | .0219 |
| Duration of symptoms in days, median (range) | 45 (35–65) | 48 (30–61) | .8742 |
| Smoking | |||
| Yes, current smoker | 20 [30%] | 42 [31%] | .8549 |
| Yes, former smoker | 15 [22%] | 23 [17%] | |
| No, never | 32 [48%] | 68 [52%] | |
| Alcoholism | |||
| Yes | 27 [40%] | 47 [35%] | .5937 |
| Never | 40 [60%] | 86 [65%] |
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; IQR, interquartile range; Ss, Strongyloides stercoralis.
Ss+ Is Associated With Increased Risk of Bilateral Lung Lesions and Cavitation
To assess the association of Ss infection with disease severity and extent in TB disease, we performed a univariate and multivariate conditional regression analysis, with the latter correcting for age, gender, BMI, diabetic status (based on HbA1c levels), duration of symptoms, smoking status, or alcohol use (Table 2). Univariate analysis showed that Ss+ was associated with an increased risk of bilateral lung lesions (odds ratio [OR], 4.54; 95% confidence interval [CI], 2.33–9.04; P < .0001) and cavitation (OR, 5.97; 95% CI, 3.03–12.09; P < .001). Multivariate analysis showed that Ss+ was still associated with increased risk of bilateral lung lesions (adjusted OR [aOR], 3.99; 95% CI, 1.73–9.19; P = .0014) and cavitation (aOR, 4.09; 95% CI, 1.78–9.41; P = .0011), after correcting for confounding variables. Thus, for each of the 2 disease severity measures, Ss+ is associated with increased TB disease severity.
Table 2.
Univariate and Multivariate Analysis of Association of Ss Infection With Bilateral Lung Lesions, Cavitation and Mycobacterial Smear Grade
| Univariate Model | Multivariate Modela | |||
|---|---|---|---|---|
| OR (95% CI) | P Value | aORa (95% CI) | P Value | |
| Bilateral lung Lesions | 4.54 (2.33–9.04) | <.0001 | 3.99 (1.73–9.19) | .0014 |
| Cavitation | 5.97 (3.03–12.09) | <.0001 | 4.09 (1.78–9.41) | .0011 |
| Sputum smear grade | 7.57 (4.18–14.05) | <.0001 | 9.32 (6.30–13.96) | <.0001 |
Abbreviations: aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio; Ss, Strongyloides stercoralis.
aMultivariate logistic regression models are adjusted for age in years, gender, body mass index, diabetes status, duration of symptoms, smoking status, and alcohol use status.
Ss+ Is Associated With Increased Risk of Greater Bacterial Burdens
To assess the association of Ss infection with bacterial burdens in TB disease, we performed a univariate and multivariate conditional regression analysis, with the latter correcting for age, gender, BMI, diabetic status (based on HbA1c levels), duration of symptoms, smoking status, or alcohol use (Table 2). Univariate analysis showed that Ss+ was associated with increased risk of smear grade (OR, 7.57; 95% CI, 4.18–14.05; P < .0001). Multivariate analysis showed that Ss+ was still associated with increased risk of bilateral lung lesions (aOR, 9.32; 95% CI, 6.30–13.96; P < .0001), after correcting for confounding variables. Thus, Ss+ is associated with increased bacterial burdens in PTB.
Ss+ Is Associated With Increased Plasma Levels of Matrix Metalloproteinases
Because MMPs are linked with tissue damage/inflammation as well as matrix remodeling in TB [12, 15] and because certain MMPs are elevated in Ss infection [16], we wanted to examine the plasma levels of MMPs in Ss+ and Ss− individuals with PTB. To this end, we measured the plasma levels of MMP-1, -2, -3, -7, -8, -9, -10, -12, and -13 by multiplex assay in a subset of Ss+ (n = 31) and Ss− (n = 62) individuals (Figure 1). As shown in Figure 1, plasma levels of MMP-1 (GM of 31274 pg/mL in Ss+ versus 2907 pg/mL in Ss−), MMP-2 (GM of 5950 pg/mL in Ss+ versus 4668 pg/mL in Ss−), MMP-7 (GM of 3259 pg/mL in Ss+ versus 2342 pg/mL in Ss−), MMP-8 (GM of 2009 pg/mL in Ss+ versus 1233 pg/mL in Ss−), and MMP-9 (GM of 12446 pg/mL in Ss+ versus 2561 pg/mL in Ss−) were significantly higher in Ss+ compared with Ss− individuals. Thus, Ss+ is associated with heightened levels of MMPs in PTB.
DISCUSSION
Identifying predictors of TB disease severity is of critical public health importance because higher bacterial burdens are associated with increased transmission and poor unfavorable treatment outcomes (including failure and relapse) [17–20]. Likewise, cavitary disease in TB is associated with increased risk of transmission, increased rates of coprevalent disease in household contacts, and greater risk of failure and relapse [21–25]. Previous studies on the association of helminths and TB disease severity have suffered from various concerns, including small sample sizes, lack of recruiting-only microbiologically confirmed TB cases, etc [3]. A previous study had suggested that PTB individuals with helminths had more disease involved lung zones than those without [11]. Studies in Mycobacterium leprae demonstrated that helminth-infected individuals had more severe leprosy [26]. In contrast, another study revealed that coexistent helminth infection was associated with lower sputum smear grade [6]. Thus, there is conflicting literature and no clear cut evidence of link between helminth infections and TB disease severity or bacterial burdens.
Our study utilizes a well characterized group of PTB individuals diagnosed on the basis of sputum and culture (and therefore bacteriologically proven) positivity with thoroughly defined clinical and radiological parameters. Our study has strict exclusion criteria including HIV, drug-resistant TB, and retreatment cases. Other factors that have been known to influence disease severity and bacterial burdens including age, gender, BMI, diabetic status, smoking status, and alcohol use were controlled for in this study [27]. Because HbA1c levels differed between the Ss+ and Ss− groups, we have adjusted for its effect and demonstrated that the significant association of Ss infection with PTB still remains despite this difference. Therefore, with rigorous screening criteria, our study demonstrates an important association of Ss infection status with the increased risk of both bilateral lung lesions and cavitation. Our data further confirm an important association of Ss infection status with mycobacterial burdens as estimated by sputum smear grades in PTB. As mentioned before, the geographical level overlap in the prevalence of TB and helminth infections have major ramifications in terms of the clinical and pathological interactions between the 2 infections.
Tuberculosis disease severity is directly linked to the destruction of extracellular matrix of the lungs by host proteases, including MMPs [12, 15]. Matrix metalloproteinase levels have been shown to correlate with TB disease burden and severity. Moreover, helminth infections are known to be associated with elevated production of MMP-1, -2, -7, and -9 [16, 28, 29]. Thus, we examined the plasma levels of MMPs in PTB individuals with or without Ss infection on the hypothesis that Ss infection-mediated increase in lung pathology would be linked to enhanced expression of MMPs. Indeed, our data clearly reveal a significant association between Ss infection and increased systemic levels of MMP-1, -2, -7, -8, and -9. Thus, our study also reveals a plausible biological mechanism for the increased disease severity in PTB individuals with concomitant Ss infection.
CONCLUSIONS
Our study suffers from the limitations of having only a moderate sample size, not performing stool examinations for Ss and other intestinal helminths (and relying only on seropositivity as a diagnostic for Ss infection), being cross-sectional (without follow up of TB individuals with or without anthelmintic treatment), and being an associative study. Nevertheless, our data provide an important advance in the understanding of the effect of multiple infections within the same individual in terms of pathology and bacteriology. Our data also have important public health policy implications. This finding should incentivize programs to perform parasite screening and treatment for individuals at high risk for TB (because of comorbidities or large Mtb exposure in households). Future clinical trials with anthelmintic treatment administered to PTB individuals need to be conducted. Given the availability and simplicity of single-dose ivermectin and the absence of routine screening for parasitic infections, we would suggest that anthelmintic treatment with single-dose ivermectin for all PTB individuals would be a prudent value-added tool in the armamentarium against TB. Even a modest reduction in TB disease severity (and hence secondary transmission) from anthelmintic treatment could prevent large numbers of treatment failures and secondary cases among the millions of TB cases in Africa and Southeast Asia alone [30].
Notes
Acknowledgments. We thank the staff of Department of Clinical Research and Department of Social Work, NIRT, for valuable assistance in recruiting the patients for this study, the staff of Department of Bacteriology for cultures, and Prabbu Balakrishnan and Yukthi Bhootra of the National Institutes of Health (NIH)-International Centers for Excellence in Research for technical assistance.
Financial support. This work was funded in part by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. Global tuberculosis report 2018.2019. Available at: https://www.who.int/tb/publications/global_report/en/. Accessed 17 October 2019.
- 2. GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018; 392:1859–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Babu S, Nutman TB. Helminth-tuberculosis co-infection: an immunologic perspective. Trends Immunol 2016; 37:597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Donohue RE, Cross ZK, Michael E. The extent, nature, and pathogenic consequences of helminth polyparasitism in humans: a meta-analysis. PLoS Negl Trop Dis 2019; 13:e0007455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Elias D, Mengistu G, Akuffo H, Britton S. Are intestinal helminths risk factors for developing active tuberculosis? Trop Med Int Health 2006; 11:551–8. [DOI] [PubMed] [Google Scholar]
- 6. Abate E, Belayneh M, Idh J, et al. Asymptomatic helminth infection in active tuberculosis is associated with increased regulatory and Th-2 responses and a lower sputum smear positivity. PLoS Negl Trop Dis 2015; 9:e0003994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tristão-Sá R, Ribeiro-Rodrigues R, Johnson LT, Pereira FE, Dietze R. Intestinal nematodes and pulmonary tuberculosis. Rev Soc Bras Med Trop 2002; 35:533–5. [DOI] [PubMed] [Google Scholar]
- 8. Taghipour A, Mosadegh M, Kheirollahzadeh F, et al. Are intestinal helminths playing a positive role in tuberculosis risk? A systematic review and meta-analysis. PLoS One 2019; 14:e0223722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chatterjee S, Kolappan C, Subramani R, et al. Incidence of active pulmonary tuberculosis in patients with coincident filarial and/or intestinal helminth infections followed longitudinally in South India. PLoS One 2014; 9:e94603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Abate E, Elias D, Getachew A, et al. Effects of albendazole on the clinical outcome and immunological responses in helminth co-infected tuberculosis patients: a double blind randomised clinical trial. Int J Parasitol 2015; 45:133–40. [DOI] [PubMed] [Google Scholar]
- 11. Resende Co T, Hirsch CS, Toossi Z, Dietze R, Ribeiro-Rodrigues R. Intestinal helminth co-infection has a negative impact on both anti-Mycobacterium tuberculosis immunity and clinical response to tuberculosis therapy. Clin Exp Immunol 2007; 147:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Elkington PT, Ugarte-Gil CA, Friedland JS. Matrix metalloproteinases in tuberculosis. Eur Respir J 2011; 38:456–64. [DOI] [PubMed] [Google Scholar]
- 13. Buonfrate D, Sequi M, Mejia R, et al. Accuracy of five serologic tests for the follow up of Strongyloides stercoralis infection. PLoS Negl Trop Dis 2015; 9:e0003491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bisoffi Z, Buonfrate D, Sequi M, et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis 2014; 8:e2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stek C, Allwood B, Walker NF, Wilkinson RJ, Lynen L, Meintjes G. The immune mechanisms of lung parenchymal damage in tuberculosis and the role of host-directed therapy. Front Microbiol 2018; 9:2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rajamanickam A, Munisankar S, Bhootra Y, Dolla C, Nutman TB, Babu S. Microbial translocation associated with an acute phase response and elevations in MMP-1, HO-1 and pro-inflammatory cytokines in Strongyloides stercoralis infection. Infect Immun 2016; 85:e00772-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Shea MK, Koh GC, Munang M, Smith G, Banerjee A, Dedicoat M. Time-to-detection in culture predicts risk of Mycobacterium tuberculosis transmission: a cohort study. Clin Infect Dis 2014; 59:177–85. [DOI] [PubMed] [Google Scholar]
- 18. Epstein MD, Schluger NW, Davidow AL, Bonk S, Rom WN, Hanna B. Time to detection of Mycobacterium tuberculosis in sputum culture correlates with outcome in patients receiving treatment for pulmonary tuberculosis. Chest 1998; 113:379–86. [DOI] [PubMed] [Google Scholar]
- 19. Visser ME, Stead MC, Walzl G, et al. Baseline predictors of sputum culture conversion in pulmonary tuberculosis: importance of cavities, smoking, time to detection and W-Beijing genotype. PLoS One 2012; 7:e29588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hesseling AC, Walzl G, Enarson DA, et al. Baseline sputum time to detection predicts month two culture conversion and relapse in non-HIV-infected patients. Int J Tuberc Lung Dis 2010; 14:560–70. [PubMed] [Google Scholar]
- 21. Dholakia YN, D’souza DT, Tolani MP, Chatterjee A, Mistry NF. Chest X-rays and associated clinical parameters in pulmonary tuberculosis cases from the National Tuberculosis Programme, Mumbai. Infect Dis Rep 2012; 4:e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guwatudde D, Nakakeeto M, Jones-Lopez EC, et al. Tuberculosis in household contacts of infectious cases in Kampala, Uganda. Am J Epidemiol 2003; 158:887–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rodrigo T, Caylà JA, García de Olalla P, et al. Characteristics of tuberculosis patients who generate secondary cases. Int J Tuberc Lung Dis 1997; 1:352–7. [PubMed] [Google Scholar]
- 24. Benator D, Bhattacharya M, Bozeman L, et al. ; Tuberculosis Trials Consortium Rifapentine and isoniazid once a week versus rifampicin and isoniazid twice a week for treatment of drug-susceptible pulmonary tuberculosis in HIV-negative patients: a randomised clinical trial. Lancet 2002; 360:528–34. [DOI] [PubMed] [Google Scholar]
- 25. Singla R, Srinath D, Gupta S, et al. Risk factors for new pulmonary tuberculosis patients failing treatment under the Revised National Tuberculosis Control Programme, India. Int J Tuberc Lung Dis 2009; 13:521–6. [PubMed] [Google Scholar]
- 26. Prost A, Nebout M, Rougemont A. Lepromatous leprosy and onchocerciasis. Br Med J 1979; 1:589–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wallis RS, Wang C, Doherty TM, et al. Biomarkers for tuberculosis disease activity, cure, and relapse. Lancet Infect Dis 2010; 10:68–9. [DOI] [PubMed] [Google Scholar]
- 28. Verma A, Prasad KN, Nyati KK, et al. Association of MMP-2 and MMP-9 with clinical outcome of neurocysticercosis. Parasitology 2011; 138:1423–8. [DOI] [PubMed] [Google Scholar]
- 29. Gomez DE, De Lorenzo MS, Alonso DF, Andrade ZA. Expression of metalloproteinases (MMP-1, MMP-2, and MMP-9) and their inhibitors (TIMP-1 and TIMP-2) in schistosomal portal fibrosis. Am J Trop Med Hyg 1999; 61:9–13. [DOI] [PubMed] [Google Scholar]
- 30. Hotez PJ, Molyneux DH, Fenwick A, et al. Control of neglected tropical diseases. N Engl J Med 2007; 357:1018–27. [DOI] [PubMed] [Google Scholar]