Abstract
Reactive sulfur species, including hydrogen sulfide (H2S), are important biological mediators and play key roles in different pathophysiological conditions. Small molecules that release H2S on demand, often referred to as ‘H2S donors’, constitute a key investigative tool for H2S-related research. A significant challenge, however, is correlating the rate of H2S release from such donors in complex systems with biological outcomes, because release rates are commonly perturbed by different biological environments. In this chapter, we outline an approach to utilize H2S donors that provide a fluorescent response upon H2S release. These compounds leverage the intermediate release of carbonyl sulfide (COS), which is quickly converted to H2S by the endogenous enzyme carbonic anhydrase (CA), to provide triggerably donors with an optical response. The described donors are activated by biological thiols and provide a fluorescence response that correlates directly with H2S delivery, which allows for delivered H2S levels to be measured in real time by fluorescence techniques.
Keywords: hydrogen sulfide, H2S, fluorescence, donors, reactive sulfur species
1. Introduction
Reactive sulfur species (RSS) have gained significant interest in the last decade due to their roles in different biological processes and systems. Of such species, hydrogen sulfide (H2S) has garnered significant interest because it is produced endogenously from both enzymatic and non-enzymatic pathways and functions as an important signalling molecule akin to nitric oxide (NO) and carbon monoxide (CO).(Wang, 2002, 2012) As brief examples of this activity, misregulation of endogenous H2S has been implicated in different (patho)physiological processes, including angiogenesis, wound healing, carcinogenesis, protection against ischemia/reperfusion injury, as well as other activities.(Cortese-Krott, Fernandez, Kelm, Butler, & Feelisch, 2015; Filipovic, Zivanovic, Alvarez, & Banerjee, 2018; Kolluru, Shen, Bir, & Kevil, 2013; Szabo et al., 2014; Yuan, Shen, & Kevil, 2017) Complementing the expansion of biological activities associated with H2S and related RSS, small molecule chemical tools for H2S detection and delivery have emerged as key tools for investigate complex RSS in diverse environments.(Levinn, Cerda, & Pluth, 2020; Li, Yin, & Huo, 2015; Lin, Chen, Xian, & Chang, 2015; Powell, Dillon, & Matson, 2018; Szabo & Papapetropoulos, 2017)
Of such tools, small molecules that release H2S on demand form a cornerstone of our ability to manipulate biological levels of H2S.(Levinn et al., 2020; Powell et al., 2018; Szabo & Papapetropoulos, 2017) Simple sources of H2S, such as Na2S or NaSH, are commonly used in biological investigations, yet lack physiological relevance due to the rapid release and subsequent oxidation of H2S from these salts. Building from the need for slow-releasing H2S donors, researchers have made significant advances in the development of passive releasing donors, often activated by hydrolysis or reaction with common biological nucleophiles, as well as activatable donors, often triggered by specific biological or biorthogonal stimuli. One approach that has enabled different donors to be tuned for activation by different stimuli is the development of systems that release carbonyl sulfide (COS) as a precursor for H2S delivery. Under biological conditions, COS is quickly converted to H2S by the endogenous enzyme carbonic anhydrase (CA).
One significant challenge in using small molecule donors for H2S or other biologically-relevant species, is relating measured release rates in buffer with H2S-related activities in more complex systems. Release in buffer, however, typically do not match rates of release in more complex systems, such as cell culture or animal models. For example, even the presence of simple proteins, such as bovine serum albumin (BSA), has been demonstrated to significantly change the rate of H2S release from certain donors.(Zhao, Steiger, & Pluth, 2018a) H2S release rates from donors can be readily measured in buffer or in vitro using H2S-responsive electrodes, or analytical methods including the methylene blue (MB) or monobromobimane (mBB) methods.(Hartle & Pluth, 2016) All of these methods, however, are destructive and consume H2S and also often require significant sample preparation. One approach to addressing this challenge is to use H2S donors that couple an optical signal with H2S releasing, such that H2S delivery can be monitored in real time by common spectroscopic methods. In this chapter, we describe the procedure for preparing and using the fluorescent H2S donors FLD-1 and FLD-3, which enable direct real-time monitoring of H2S release using fluorescence spectroscopy and microscopy.(Zhao, Cerda, & Pluth, 2019)
2. Preparation and Properties of Fluorescent H2S Donors
2.1. General Design Principles
To access H2S donors that release H2S slowly under physiological conditions with a concomitant fluorescence readout, we developed a system that could be readily activated by biological thiols to release both H2S and fluorescein. By using the thiol-mediate reduction of sulfenyl thiocarbonates, reaction with biological thiols including reduced glutathione (GSH) or cysteine (Cys) results in disulfide exchange and the intermediate release carbonyl sulfide (COS) (Scheme 1). The released COS is quickly converted to H2S by CA. This strategy for leveraging intermediate COS release to enable H2S delivery is now a broadly-used approach to develop H2S donor compounds,(Levinn, Cerda, & Pluth, 2019) and has been used to develop donors activated by cellular nucleophiles,(Powell, Foster, Okyere, Theus, & Matson, 2016; Zhao, Steiger, & Pluth, 2018b, 2019) reactive oxygen species,(Chauhan, Jos, & Chakrapani, 2018; Hu et al., 2019; Zhao, Henthorn, & Pluth, 2017; Zhao & Pluth, 2016) enzymes,(Chauhan, Bora, Ravikumar, Jos, & Chakrapani, 2017; Levinn, Steiger, & Pluth, 2019; Steiger, Marcatti, Szabo, Szczesny, & Pluth, 2017) light,(Sharma et al., 2017; Stacko, Muchova, Vitek, & Klan, 2018; Zhao, Bolton, & Pluth, 2017) and other stimuli (Gilbert, Zhao, Otteson, & Pluth, 2019; Steiger, Yang, Royzen, & Pluth, 2017).
Scheme 1.
Activation and response mechanism for the sulfenyl thiocarbonate donors. Reaction with cellular thiols results in disulfide reduction and COS release, which is quickly converted to H2S by carbonic anhydrase (CA).
On the basis of these design principles, two different fluorescein-based H2S donors (FLD-1 and FLD-3) can be readily prepared. Treatment with either fluorescein or 3-O-methylfluorescein with ((benzyl)dithio)carbonyl chloride yields the fluorescent donors FLD-1 and FLD-3, respectively, in moderate yield (Scheme 2). Both compounds are readily purified by SiO2 column chromatography and are stable at room temperature, but should be stored in a freezer over extended periods of time. These donors provide systems with a turn-on fluorescence response that corresponds to H2S donation. The chromophores used for FLD-1 (λex = 490 nm; λem = 500 – 650 nm) and FLD-3 (λex = 454 nm; λem = 500 – 650 nm) correspond to commonly used FITC/GFP green channels and filter cubes used for fluorescence microscopy. Fluorescein is commercially available from many sources, and ((benzyl)dithio)carbonyl chloride and 3-O-methylfluorescein are readily available through simple synthetic procedures.(Mugherli, Burchak, Chatelain, & Balakirev, 2006)
Scheme 2.
Synthesis of FLD-1 and FLD-2.
2.2. Materials, Equipment, and Reagents
Fluorescein (CAS# 2321–07-5) or 3-O-methylfluorescein (CAS# 70672–05-8)
(Benzyl(dithio))carbonyl chloride (CAS# 31331–36-9)
Chloroform
Ethyl acetate
Hexanes
Silica gel
Diisopropyl ethylamine (DIPEA)
Magnesium sulfate (MgSO4)
Brine
Round bottom flask
Separatory funnel
Column for chromatography
NMR instrument to verify compound identity and purity
HPLC to verify compound purity
2.3. Preparation procedures
General Procedure for preparation of FLD-1 and FLD-3
Add 1.0 equiv. of fluorescein and 3.0 equiv. of ((benzyl)dithio)carbonyl chloride to CHCl3 (~5–10 mL) under nitrogen.
Cool the reaction mixture to 0 °C for 5 minutes in an ice bath, and then slowly add 3.0 equiv. of diisopropyl ethylamine (DIPEA).
Remove the ice bath and stir the reaction mixture for 2 hours, or until the reaction is complete as indicated by thin layer chromatography.
Quench the reaction mixture by adding 25 mL of brine.
Extract the aqueous solution with ethyl acetate (3 × 15 mL).
Combine the organic layers and dry over MgSO4.
Filter off the MgSO4 and remove the solvent under vacuum.
Purify the crude product using SiO2 column chromatography using ethyl acetate/hexanes as the eluent.
The product can be isolated as a yellow solid in approximately 40–55% yield.
Reaction scales and product identification for FLD-1 and FLD-3
Reaction scale for FLD-1: fluorescein (332 mg, 1.00 mmol), ((benzyl)dithio)carbonyl chloride (654 mg, 3.00 mmol), DIPEA (390 mg, 3.00 mmol); SiO2 purification with 1:3 ethyl acetate:hexanes (v/v), Rf = 0.31. Characterization data for FLD-1: 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.09 (d, J = 5.0 Hz, 1H), 7.85 (t, J = 5.0 Hz, 1H), 7.79 (t, J = 5.0 Hz, 1H), 7.38 (m, 13H), 7.01 (d, J = 10.0 Hz, 2H), 6.95 (d, J = 10.0 Hz, 2H), 4.18 (s, 4H). 13C{1H} NMR (125 MHz, DMSO-d6) δ (ppm): 168.8, 167.8, 152.6, 151.2, 136.5, 131.1, 130.1, 130.0, 129.0, 128.2, 125.7, 125.6, 124.6, 118.4, 117.5, 110.6, 81.1, 42.4. IR (cm−1): 2981, 1744, 1608, 1408, 1420, 1237, 1143, 1107, 1060, 988, 881, 751. HRMS m/z [M + H]+ calcd. For [C36H25O7S4]+ 697.0483; found 697.0474.
Reaction scale for FLD-3: 3-O-methylfluorescein (69.0 mg, 0.207 mmol), ((benzyl)dithio)carbonyl chloride (136 mg, 0.623 mmol), DIPEA (81.0 mg, 0.623 mmol); SiO2 purification with 1:1 ethyl acetate:hexanes (v/v) Rf = 0.64. Characterization data for FLD-3: 1H NMR (500 MHz, DMSO-d6) δ (ppm): 8.06 (d, J = 10.0 Hz, 1H), 7.83 (t, J = 5.0 Hz, 1H), 7.77 (t, J = 5.0 Hz, 1H), 7.36 (m, 7H), 6.98 (d, J = 10.0 Hz, 2H), 6.91 (d, J = 10.0 Hz, 2H), 6.77 (d, J = 10.0 Hz, 1H), 6.73 (d, J = 10.0 Hz, 1H), 4.18 (s, 2H), 3.84 (s, 3H). 13C{1H} NMR (125 MHz, DMSO-d6) δ (ppm): 168.9, 167.8, 161.7, 152.7, 152.4, 151.9, 151.5, 136.5, 13 6.4, 130.9, 130.1, 130.0, 129.5, 129.0, 128.2, 126.1, 125.4, 124.5, 118.0, 117.8, 113.0, 110.9, 110.4, 101.3, 81.9, 56.2, 42.4. IR (cm−1): 2981, 1747, 1607, 1491, 1420, 1241, 1220, 1144, 1103, 1060, 986, 874. HRMS m/z [M + H]+ calcd. For [C29H21O6S2]+ 529.0780; found 529.0779.
2.4. Donor usage in vitro
Both FLD donors react with thiols to release COS, which is subsequently converted to H2S by CA. For simplicity, Cys can be used to verify donor activity due to the higher solubility of Cys in buffer than GSH. As an example of the reactivity of FLD-1, incubation of 10 μM of FLD-1 with 100 μM Cys in PBS buffer (pH 7.4, 10 mM) containing physiologically-relevant concentrations of CA (25 μg/mL) results in a significant fluorescent response as shown in Figure 1a. Over the course of 2 hours under these reaction conditions, a ~500-fold increase in fluorescence intensity is observed for FLD-1. The release rate from the FLD compounds is dependent on both the donor and thiol concentration. As an example of this dependence, Figure 1b shows the Cys concentration dependence of the fluorescence response from FLD-1. Using conditions of 200 μM Cys and 10 μM FLD-1, the activation of the donor is complete within 20 minutes.
Figure 1.
(a) Fluorescence response of FLD-1 (10 μM) in PBS (pH 7.4, 10 mM) containing Cys (100 μM) and CA (25 μg/mL). (b) Cys-dependent (0 – 200 μM) fluorescence turn on of FLD-1 (10 μM) in PBS. General fluorescence acquisition parameters: λex = 490 nm, λem = 500 – 650 nm. The data shown is the average of three replicates and errors are shown as mean ± SD (n = 3).
Both FLD-1 and FLD-3 become fluorescent when activated by thiols to release COS/H2S, but the magnitude of this fluorescence response is different. FLD-1, which releases 2 equivalents of COS/H2S, shows a more gradual fluorescence response because the intermediate in which 1 equivalent of COS/H2S has been released is moderately fluorescent. By contrast, FLD-3 shows a faster fluorescence response, although a smaller dynamic range (Figure 2).
Figure 2.
Fluorescence turn on of FLD-1 (grey) and FLD-3 (blue). General conditions: 10 μM FLD in PBS (pH 7.4, 10 mM) with Cys (100 μM). λex = 490 nm for FLD-1, λex = 454 nm for FLD-3, λem = 500 – 650 nm. The data shown is the average of three replicates and errors are shown as mean ± SD (n = 3).
2.5. Correlating fluorescence signal to H2S release
The H2S release from the fluorescent FLD compounds can be readily measured and quantified using the methylene blue (MB) assay. This method allows for trapping of released H2S to form the methylene blue dye, which can then be quantified by UV-vis spectroscopy. Incubation of FLD-3 (10 μM) with Cys (100 μM) resulted in a rapid fluorescence response with 96% of the expected H2S release measured by the MB assay. In the absence of CA, negligible H2S was detected, which is consistent with the intermediate formation of COS, which requires CA to be converted to H2S (Figure 3a). Using FLD-3, the fluorescence response can be monitored and plotted against H2S measurements from the MB assay under the same conditions (Figure 3b). These experiments show a linear correlation between the fluorescence response and quantified H2S, which allows for the fluorescence response to be correlated directly with H2S concentrations. Note that because all fluorimeters will have different sensitivities, this calibration curve should be repeated for each instrument system used to ensure accurate correlations from fluorescence to H2S concentrations.
Figure 3.
Fluorescence response (red) and H2S release (blue) after treatment of FLD-3 (10 μM) in PBS (pH 7.4, 10 mM) with Cys (100 μM) and CA (25 μg/mL). No H2S was detected in the absence of CA (black). λex = 454 nm, λem = 500 – 650 nm. (b) Correlation between quantified H2S released and fluorescence response. The data shown is the average of three replicates and errors are shown as mean ± SD (n = 3).
2.6. Materials, Equipment, and Reagents
Carbonic anhydrase (from bovine erythrocytes, CAS# 9001–03-0; EC# 4.2.1.1)
Quartz fluorescent cuvette
Disposable plastic cuvettes
Fluorimeter
UV-vis spectrophotometer
Cysteine
Zinc acetate (Zn(OAc)2 dihydrate; CAS# 5970–45-6)
Iron trichloride (FeCl3 hexahydrate; CAS# 10025–77-1)
Hydrochloric acid (HCl)
N,N-dimethyl-p-phenylene diamine (HCl salt, CAS# 536–46-9)
Sodium hydrosulfide (NaSH, CAS# XXX)
Dimethyl sulfoxide (DMSO)
PBS buffer (pH 7.4, 10 mM PBS)
Measurement of Fluorescence Intensity of FLD Donors
Prepare a 10.0 mM stock solution of the desired FLD donor in DMSO
Add 3.00 μL of the stock solution to 3.00 mL of PBS (pH 7.4, 10 mM) containing carbonic anhydrase (25.0 μg/mL) in a quartz cuvette.
Prepare a 10.0 mM stock solution of cysteine in PBS buffer.
Measure the background fluorescence spectrum of the FLD donor (λex = 454 or 490 nm depending on the donor; λscan = 500 −650 nm)
Add 30 μL of the cysteine stock solution to the cuvette.
Repeat fluorescence measurements of the sample after the addition of cysteine until the reaction is complete (~120 minutes)
Integrate the fluorescence signal from 500 – 650 nm and plot as a function of time.
Measurement of H2S Release from FLD Donors
Prepare a 10 mM stock solution of the FLD donor in DMSO and add 20.0 μL of the stock solution to 20.0 mL of PBS (pH 7.40, 10.0 mM) containing CA (25.0 μg/mL) in a 20 mL scintillation vial.
Prepare a 100 mM cysteine stock solution in PBS buffer.
Prepare stock solutions for the methylene blue (MB) analysis. These stock solutions include 1.00% w/v Zn(OAc)2, 30.0 mM FeCl3 in 1.20 M HCl, and 20.0 mM N,N-dimethyl-p-phenylene diamine in 7.20 M HCl.
Remove a 300 μL aliquot from the FLD solution as a time = 0 point, and add this to a disposable plastic UV-vis cuvette containing 300 μL of MB cocktail (60.0 μL Zn(OAc)2 (1.00% w/v), 120 μL FeCl3 (30.0 mM in 1.20 M HCl), and 120 μL N,N-dimethyl-p-phenylene diamine (20.0 mM in 7.20 M HCl)).
Add 20.0 μL of the cysteine stock solution to the FLD donor solution in the scintillation vial.
Remove a 300 μL aliquot from the reaction mixture and add it to a disposable plastic UV-vis cuvette containing 300 μL of MB cocktail (60.0 μL Zn(OAc)2 (1.00% w/v), 120 μL FeCl3 (30.0 mM in 1.20 M HCl), and 120 μL N,N-dimethyl-p-phenylene diamine (20.0 mM in 7.20 M HCl)) at different time points.
Allow each aliquot to equilibrate for 1 hour, and then measure the absorbance value at 670 nm
Plot the absorbance values at 670 nm as a function of time. To convert this data to quantitative H2S levels, prepare an H2S calibration curve (described below).
Prepare an H2S calibration curve
Add 500 μL of the MB cocktail (100 μL Zn(OAc)2 (1.00% w/v), 200 μL FeCl3 (30.0 mM in 1.20 M HCl), and 200 μL N,N-dimethyl-p-phenylene diamine (20.0 mM in 7.20 M HCl)) and 500 μL of PBS (pH 7.4, 10 mM) to a 1.5 mL disposable cuvettes.
Prepare a 1.00 mM NaSH stock solution in PBS buffer.
Add aliquots of the 1.00 mM NaSH stock solution to the cuvettes containing the MB cocktail to result in final concentration of 1, 3, 5, 10, 15, and 20 μM sulfide.
Incubate the cuvettes for 1 hour prior to measuring the absorbance at 670 nm.
Plot the absorbance as a function of sulfide concentration. The resultant plot should produce a straight line, which can be used as a calibration curve for quantitation experiments.
3. Probe usage and application in cell models
The FLD donor compounds can also be used to deliver H2S in live cell environments after activation by endogenous thiols. As an example of this activity, treatment of HeLa cells with FLD-1 results in donor internalization and release of COS/H2S by endogenous thiols and CA. As shown in Figure 4, donor activation results in an increase in fluorescence in the green channel due to liberation of fluorescein after donor activation. The released H2S can be visualized by using a reaction-based H2S responsive probe that does not overlap with the FLD-1 emission spectrum. Co-incubation with the commercially-available, H2S-responsive 7-azido-4-methylcoumarin (C7-Az) fluorescent probe results in C7-Az activation, confirming H2S release from FLD-1 (Figure 4). In the absence of FLD-1, minimal background fluorescence from C7-Az is observed.
Figure 4.
H2S delivery from FLD-1 in HeLa cells. HeLa cells were treated with the H2S-responsive probe C7-Az (50 μM) in DMEM only (top row) or DMEM containing FLD-1 (50 μM) (bottom row) for 30 min. Cells were then washed with PBS and cell images were taken in PBS using a fluorescent microscope. Bar scale: 50 μm
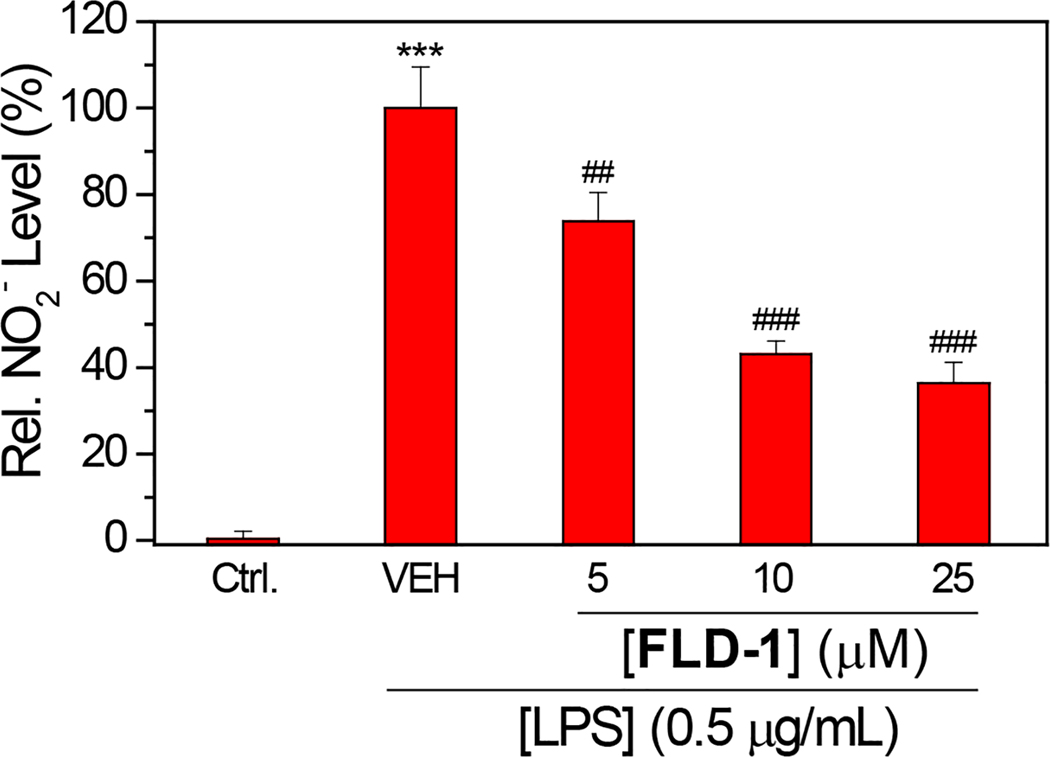
A second example of cell-based applications of the FLD system is blocking NO formation in RAW 264.7 macrophage cells treated with lipopolysaccharide (LPS). Prior reports have demonstrated that H2S can reduce inflammatory responses and decrease NO levels in LPS-stimulated macrophage cells, and this assay has been used previously to investigate anti-inflammatory response of H2S-releasing compounds (Whiteman et al., 2010). To measure the impacts of FLD-1 on NO formation, cultured Raw 264.7 macrophage cells were treated with FLD-1 (0 – 25 μM) for 2 hours followed by LPS (0.5 μg/mL) administration and further incubation 24 hours. After this incubation period, accumulated nitrate (NO2–), which is the downstream oxidation product of NO, was measured using a commercially-available Griess assay kit. From these experiments, a dose-dependent decrease in NO2– formation was observed, which is consistent with both H2S release from FLD-1 under these experimental conditions and also anti-inflammatory activity of the FLD donor (Figure 5).
Figure 5.
Effects of FLD-1 on LPS-induced NO2– accumulation. RAW 264.7 cells were pretreated with FLD-1 (0 – 25 μM) for 2 h, followed by a 24-h treatment of LPS (0.5 μg/mL). Results are expressed as mean ± SD (n = 4). *** p < 0.001 vs the control group; ## p < 0.01 vs vehicle-treated group; ### p < 0.001 vs vehicle-treated group.
Materials
HeLa cells (ATCC: CCL-2)
Raw 264.7 cells (ATCC: TIB-71)
Dulbecco’s modified Eagle’s medium
Fetal bovine serum (FBS)
Penicillin
Streptomycin
Cell incubator with 5% CO2
Poly-D-lysine coated plates (MatTek)
7-Azido-4-methylcoumarin (C7-Az); commercially available from Santa Cruz Biotech. or readily prepared by previously published methods.(Thorson, Majtan, Kraus, & Barrios, 2013)
Inverted fluorescence microscope with filter cubes for blue (DAPI) and green (GPF, FITC) channels
Lipopolysaccharide (LPS)
Griess nitrite assay kit for nitrite quantification (ThermoFisher, G7921)
Procedure
Cell culture
Culture HeLa cells (ATCC) in high glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C under 5% CO2.
Plate HeLa cells in poly-D-lysine coated plates (MatTek) containing 2.00 mL of DMEM and incubated at 37 °C under 5% CO2 for 24 hours.
Wash the cells with PBS and then add C7-Az (50.0 μM) and FLD (50.0 μM) and incubate for 30 min.
Wash the cells with PBS and bathe in 2.0 mL of PBS for imaging
Collect fluorescence images for using an inverted fluorescence microscope. The excitation and emission profiles for C7-Az (λex = 365 nm; λem = 400 – 550 nm)) and FLD-1 (λex = 490 nm; λem = 500 – 650 nm) match common filter sets used for blue/DAPI and green/GFP, respectively.
Anti-Inflammatory Activities of FLD-1 and Control Compounds
Seed Raw 264.7 macrophage cells (ATTC) in a 24-well plate (5 × 105 cells/well) containing 500 μL of DMEM and incubate at 37 °C under 5% CO2 for 24 h.
Wash the cells with PBS and incubate with the FLD donor (0 – 25.0 μM) at 37 °C for 2 hours.
Wash the cells with PBS to remove excess FLD donor.
Incubate the FLD-treated cells with FBS-free DMEM containing LPS (0.500 μg/mL) for 24 hours.
Measure NO2– levels were measured by using a Griess Reagent Kit.
4. Concluding Remarks
The delivery of small molecules from donor platforms provides a key tool for perturbing levels of biological analytes in complex systems. By being able to monitor the release in real time using non-invasive spectroscopic, researchers now have an additional methods monitor experiments under different conditions. In addition to the FLD-1 and FLD-3 donors described in this chapter, additional optically-responsive H2S-releasing donors have recently been reported. Select examples include colorimetric donors(Zhao et al., 2018a), photoactivatable fluorescent donors,(Venkatesh et al., 2018) and other fluorescent donor motifs.(Hu et al., 2019; Kim et al., 2019) In addition, fluorescent donors are also available for NO donors (Ravikumar, Bagheri, Saini, & Chakrapani, 2017) (Hibbard & Reynolds, 2019) and CO donors (De La Cruz et al., 2018; Popova, Soboleva, Ayad, Benninghoff, & Berreau, 2018), which provide a diverse palette for monitoring the release of these important small gaseous signalling molecules in complex environments.
Acknowledgement
This work was supported by the NIH (R01GM113030) and Dreyfus Foundation. NMR, fluorescence microscopy, and MS instrumentation in the UO CAMCOR facility that enabled this work is supported by the NSF (CHE-1427987, CHE-1531189, and CHE-1625529). Figures in this manuscript were modified from (Zhao, Cerda, et al., 2019) with permission from the RSC.
References
- Chauhan P, Bora P, Ravikumar G, Jos S, & Chakrapani H (2017). Esterase activated carbonyl sulfide/hydrogen sulfide (H2S) donors. Organic Letters, 19(1), 62–65. [DOI] [PubMed] [Google Scholar]
- Chauhan P, Jos S, & Chakrapani H (2018). Reactive oxygen species-triggered tunable hydrogen sulfide release. Organic Letters, 20(13), 3766–3770. [DOI] [PubMed] [Google Scholar]
- Cortese-Krott MM, Fernandez BO, Kelm M, Butler AR, & Feelisch M (2015). On the chemical biology of the nitrite/sulfide interaction. Nitric Oxide-Biology and Chemistry, 46, 14–24. [DOI] [PubMed] [Google Scholar]
- De La Cruz LKC, Benoit SL, Pan Z, Yu B, Maier RJ, Ji X, & Wang B (2018). Click, release, and fluoresce: A chemical strategy for a cascade prodrug system for codelivery of carbon monoxide, a drug payload, and a fluorescent reporter. Organic Letters, 20(4), 897–900. [DOI] [PubMed] [Google Scholar]
- Filipovic MR, Zivanovic J, Alvarez B, & Banerjee R (2018). Chemical biology of H2S signaling through persulfidation. Chemical Reviews, 118(3), 377–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert AK, Zhao Y, Otteson CE, & Pluth MD (2019). Development of acid-mediated H2S/COS donors that respond to a specific pH window. Journal of Organic Chemistry, 84(22), 14469–14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartle MD, & Pluth MD (2016). A practical guide to working with H2S at the interface of chemistry and biology. Chemical Society Reviews, 45(22), 6108–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbard HAJ, & Reynolds MM (2019). Fluorescent nitric oxide donor for the detection and killing of pseudomonas aeruginosa. Journal of Materials Chemistry B, 7(12), 2009–2018. [DOI] [PubMed] [Google Scholar]
- Hu YM, Li XY, Fang Y, Shi W, Li XH, Chen W, . . . Ma HM (2019). Reactive oxygen species-triggered off-on fluorescence donor for imaging hydrogen sulfide delivery in living cells. Chemical Science, 10(33), 7690–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NH, Moon H, Kim JH, Huh Y, Kim YJ, Kim BM, & Kim D (2019). A benzothioate native chemical ligation-based cysteine-selective fluorescent probe. Dyes and Pigments, 171, 107764. [Google Scholar]
- Kolluru GK, Shen XG, Bir SC, & Kevil CG (2013). Hydrogen sulfide chemical biology: Pathophysiological roles and detection. Nitric Oxide-Biology and Chemistry, 35, 5–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinn CM, Cerda MM, & Pluth MD (2019). Development and application of carbonyl sulfide-based donors for H2S delivery. Accounts of Chemical Research, 52(9), 2723–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinn CM, Cerda MM, & Pluth MD (2020). Activatable small-molecule hydrogen sulfide donors. Antioxidants & Redox Signaling, 32(2), 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinn CM, Steiger AK, & Pluth MD (2019). Esterase-triggered self-immolative thiocarbamates provide insights into COS cytotoxicity. ACS Chemical Biology, 14(2), 170–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JF, Yin CX, & Huo FJ (2015). Chromogenic and fluorogenic chemosensors for hydrogen sulfide: Review of detection mechanisms since the year 2009. Rsc Advances, 5(3), 2191–2206. [Google Scholar]
- Lin VS, Chen W, Xian M, & Chang CJ (2015). Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chemical Society Reviews, 44(14), 4596–4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mugherli L, Burchak ON, Chatelain F, & Balakirev MY (2006). Fluorogenic ester substrates to assess proteolytic activity. Bioorganic & Medicinal Chemistry Letters, 16(17), 4488–4491. [DOI] [PubMed] [Google Scholar]
- Popova M, Soboleva T, Ayad S, Benninghoff AD, & Berreau LM (2018). Visible-light-activated quinolone carbon-monoxide-releasing molecule: Prodrug and albumin-assisted delivery enables anticancer and potent anti-inflammatory effects. Journal of the American Chemical Society, 140(30), 9721–9729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CR, Dillon KM, & Matson JB (2018). A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochemical Pharmacology, 149, 110–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell CR, Foster JC, Okyere B, Theus MH, & Matson JB (2016). Therapeutic delivery of H2S via COS: Small molecule and polymeric donors with benign byproducts. Journal of the American Chemical Society, 138(41), 13477–13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravikumar G, Bagheri M, Saini DK, & Chakrapani H (2017). FLUORO/NO: A nitric oxide donor with a fluorescence reporter. ChemBioChem, 18(15), 1529–1534. [DOI] [PubMed] [Google Scholar]
- Sharma AK, Nair M, Chauhan P, Gupta K, Saini DK, & Chakrapani H (2017). Visible-light-triggered uncaging of carbonyl sulfide for hydrogen sulfide (H2S) release. Organic Letters, 19(18), 4822–4825. [DOI] [PubMed] [Google Scholar]
- Stacko P, Muchova L, Vitek L, & Klan P (2018). Visible to nir light photoactivation of hydrogen sulfide for biological targeting. Organic Letters, 20(16), 4907–4911. [DOI] [PubMed] [Google Scholar]
- Steiger AK, Marcatti M, Szabo C, Szczesny B, & Pluth MD (2017). Inhibition of mitochondrial bioenergetics by esterase-triggered COS/H2S donors. ACS Chemical Biology, 12(8), 2117–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger AK, Yang Y, Royzen M, & Pluth MD (2017). Bio-orthogonal “click-and-release” donation of caged carbonyl sulfide (COS) and hydrogen sulfide (H2S). Chemical Communications, 53(8), 1378–1380. [DOI] [PubMed] [Google Scholar]
- Szabo C, & Papapetropoulos A (2017). International union of basic and clinical pharmacology. CII: Pharmacological modulation of H2S levels: H2S donors and H2S biosynthesis inhibitors. Pharmacological Reviews, 69(4), 497–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C, Ransy C, Modis K, Andriamihaja M, Murghes B, Coletta C, . . . Bouillaud F (2014). Regulation of mitochondrial bioenergetic function by hydrogen sulfide. Part I. Biochemical and physiological mechanisms. British Journal of Pharmacology, 171(8), 2099–2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson MK, Majtan T, Kraus JP, & Barrios AM (2013). Identification of cystathionine -synthase inhibitors using a hydrogen sulfide selective probe. Angewandte Chemie-International Edition, 52(17), 4641–4644. [DOI] [PubMed] [Google Scholar]
- Venkatesh Y, Das J, Chaudhuri A, Karmakar A, Maiti TK, & Singh NDP (2018). Light triggered uncaging of hydrogen sulfide (H2S) with real-time monitoring. Chemical Communications, 54(25), 3106–3109. [DOI] [PubMed] [Google Scholar]
- Wang R (2002). Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? Faseb Journal, 16(13), 1792–1798. [DOI] [PubMed] [Google Scholar]
- Wang R (2012). Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiological Reviews, 92(2), 791–896. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Li L, Rose P, Tan CH, Parkinson DB, & Moore PK (2010). The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxidants & Redox Signaling, 12(10), 1147–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Shen XG, & Kevil CG (2017). Beyond a gasotransmitter: Hydrogen sulfide and polysulfide in cardiovascular health and immune response. Antioxidants & Redox Signaling, 27(10), 634–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Bolton SG, & Pluth MD (2017). Light-activated COS/H2S donation from photocaged thiocarbamates. Organic Letters, 19(9), 2278–2281. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Cerda MM, & Pluth MD (2019). Fluorogenic hydrogen sulfide (H2S) donors based on sulfenyl thiocarbonates enable H2S tracking and quantification. Chemical Science, 10(6), 1873–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Henthorn HA, & Pluth MD (2017). Kinetic insights into hydrogen sulfide delivery from caged-carbonyl sulfide isomeric donor platforms. Journal of the American Chemical Society, 139(45), 16365–16376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, & Pluth MD (2016). Hydrogen sulfide donors activated by reactive oxygen species. Angewandte Chemie-International Edition, 55(47), 14638–14642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Steiger AK, & Pluth MD (2018a). Colorimetric carbonyl sulfide (COS)/hydrogen sulfide (H2S) donation from gamma-ketothiocarbamate donor motifs. Angewandte Chemie-International Edition, 57(40), 13101–13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Steiger AK, & Pluth MD (2018b). Cysteine-activated hydrogen sulfide (H2S) delivery through caged carbonyl sulfide (COS) donor motifs. Chemical Communications, 54(39), 4951–4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Steiger AK, & Pluth MD (2019). Cyclic sulfenyl thiocarbamates release carbonyl sulfide and hydrogen sulfide independently in thiol-promoted pathways. Journal of the American Chemical Society, 141(34), 13610–13618. [DOI] [PMC free article] [PubMed] [Google Scholar]