Abstract
PURPOSE
Nonadherence to long-term treatments is often under-recognized by physicians and there is no gold standard for its assessment. In breast cancer, nonadherence to tamoxifen therapy after surgery constitutes a major obstacle to optimal outcomes. We sought to evaluate the rate of biochemical nonadherence to adjuvant tamoxifen using serum assessment and to examine its effects on short-term, distant disease-free survival (DDFS).
PATIENTS AND METHODS
We studied 1,177 premenopausal women enrolled in a large prospective study (CANTO/NCT01993498). Definition of biochemical nonadherence was based on a tamoxifen serum level < 60 ng/mL, assessed 1 year after prescription. Self-reported nonadherence to tamoxifen therapy was collected at the same time through semistructured interviews. Survival analyses were conducted using an inverse probability weighted Cox proportional hazards model, using a propensity score based on age, staging, surgery, chemotherapy, and center size.
RESULTS
Serum assessment of tamoxifen identified 16.0% of patients (n = 188) below the set adherence threshold. Patient-reported rate of nonadherence was lower (12.3%). Of 188 patients who did not adhere to the tamoxifen prescription, 55% self-reported adherence to tamoxifen. After a median follow-up of 24.2 months since tamoxifen serum assessment, patients who were biochemically nonadherent had significantly shorter DDFS (for distant recurrence or death, adjusted hazard ratio, 2.31; 95% CI, 1.05 to 5.06; P = .036), with 89.5% of patients alive without distant recurrence at 3 years in the nonadherent cohort versus 95.4% in the adherent cohort.
CONCLUSION
Therapeutic drug monitoring may be a useful method to promptly identify patients who do not take adjuvant tamoxifen as prescribed and are at risk for poorer outcomes. Targeted interventions facilitating patient adherence are needed and have the potential to improve short-term breast cancer outcomes.
INTRODUCTION
Previous studies suggested that 30% to 50% of patients with chronic conditions in developed countries are nonadherent to prescribed medications.1,2 Annually in the United States, nonadherence to chronic medications is responsible for increased mortality rate, hospitalizations and health care costs.1,3 Nonadherence also affects patient-physician relationships, possibly leading to breakdown in trust and communication.2,4 In addition, because health care systems are evolving into models where health care providers’ payments are tied to outcomes, nonadherence can also affect health care providers' reimbursement.5 Therefore, optimizing adherence may lead to dramatic improvements in health outcomes, patient satisfaction, and costs.
To design effective programs supporting adherence, it is first essential to better recognize when actual medication use differs from the prescribed regimen. There is no gold standard to identify nonadherence, with the prevalent use of indirect methods, commonly based on pharmacy prescription refills and patient-administered questionnaires, which, although informative, do not capture the actual medication intake. Particularly, it has been shown that patient self-report tends to overestimate adherence rates from two- to four-fold and pharmacy claims do not perfectly reflect medication intake, especially if out-of-pocket costs are low.6 Direct methods, such as measurement of the level of the drug or its metabolites in the blood or urine are less well studied and are not currently used in clinical practice.3,4,7,8 Furthermore, nonadherence is a complex phenomenon with a multitude of associated factors, including patient, health care provider, and disease-specific features, making it hard to identify and intervene on causes of nonadherence.1,3
Most (80%) breast cancer patients have hormone receptor–positive (HR+) disease and > 90% of these patients present with stage I to III disease, rendering them eligible for curative treatment.9 For patients with HR+ breast cancer receiving adjuvant endocrine therapy, previous studies suggested that nonadherence is a prevalent issue.10-12 Because 5 years of adjuvant endocrine therapy reduces disease recurrence rate by 50% throughout the first 10 years and mortality rate by a third throughout the first 15 years,9 and extending the duration of endocrine therapy beyond 5 years can also affect risk of recurrence by up to 40%, nonadherence constitutes a major obstacle to optimal disease and survival outcomes.9,13 In premenopausal patients with HR+ breast cancer, especially those younger than 40 years, nonadherence to adjuvant endocrine therapy seems to be a major issue, and evidence suggests poorer survival outcomes in this population compared with older ones, partly due to higher nonadherence rates.14
The Cancer Toxicities (CANTO) study (ClinicalTrials.gov identifier: NCT01993498) has prospectively collected detailed tumor, treatment, toxicities, health-related patient reported outcomes (HRPROs), and biologicical data on a cohort of 12,012 women with newly diagnosed early breast cancer.15 In this study, we evaluated the hypothesis that therapeutic drug monitoring may promptly identify patients who are nonadherent to breast cancer adjuvant endocrine therapy and at risk for a worse outcome. To do this, we examined nonadherence by serum assessment of tamoxifen among premenopausal patients of the CANTO cohort in the first year after the start of adjuvant endocrine treatment, and we studied its impact on short-term breast cancer survival outcomes.
PATIENTS AND METHODS
Study Design and Patient Selection
Data source.
The CANTO cohort enrolled patients across France from 2012 to 2018. Eligibility criteria included patients ≥ 18 years, with a primary diagnosis of invasive stage cT0-cT3, cN0-3 breast cancer and no previous treatments for current breast cancer. Patients were assessed at diagnosis and shortly after primary treatment (ie, primary surgery, chemotherapy, or radiotherapy, whichever came last), at the time of endocrine therapy prescription, if indicated, and then at years 1, 3, and 5 after the initial post-primary treatment evaluation. Data collection at each time point included clinical, treatment (including medication adherence assessed by a trained clinical research nurse [CRN]), toxicity data, HRPROs, and serum samples.15 The protocol is available in the Data Supplement.
Study oversight.
CANTO is coordinated by UNICANCER, the National Cooperative Group of French Cancer Centers. The study was approved by the national regulatory authorities and ethics committee (ID-RCB: 2011-A01095-36, 11-039). All patients enrolled in the study provided written informed consent, including consent for the biological data collection.
Variables Assessment
Assessment of nonadherence.
Nonadherence at year 1 after tamoxifen prescription was defined as nonpersistence (early discontinuation) and/or suboptimal medication implementation (eg, interruptions, skipped doses), in accordance with ESPACOMP Medication Adherence Reporting Guidelines at least 1 year after tamoxifen prescription.16 We focused on women who potentially initiated tamoxifen therapy and excluded those who were prescribed tamoxifen but did not agree to initiate the treatment.
Nonadherence at year 1 was determined using an objective and direct method, tamoxifen serum assessment (biochemical nonadherence; the primary outcome); and a subjective and indirect method, patient’s self-declaration (the secondary outcome).
Definition of primary outcome (biochemical nonadherence).
Blood samples were immediately stored at −80°C after collection (ET EXTRA Biological Resource Center, Gustave Roussy, NF 96-900 certified). Tamoxifen serum level was determined by liquid chromatography-tandem mass spectrometry on 200 to 400 µL of serum in the multiple reaction monitoring mode of a 6460 triple quadrupole mass spectrometer (Agilent Technologies, Waldbronn, Germany).17
We used a predefined threshold of 60 ng/mL for defining biochemical nonadherence to tamoxifen on the basis of previous pharmacological studies.17-20 Of note, tamoxifen serum concentration does not vary by CYP2D6 polymorphisms, unlike its main metabolite, endoxifen.21 Drug-drug interactions with CYP2D6 inhibitors (eg, paroxetine or fluoxetine) do not influence tamoxifen levels.22 There are a few drugs, such as rifampicin, aminogluthetimide, curcumin, and piperine, which may decrease tamoxifen serum levels.23-25
Tamoxifen metabolism and pharmacokinetic and cutoff definition of biochemical nonadherence are detailed in the Data Supplement.
Definition of secondary outcome.
Patients' self-declarations (defined in the Data Supplement) on adherence to tamoxifen were collected by trained CRNs through semistructured interviews at the same time as the blood collection for tamoxifen serum assessment. Patients were considered as having declared nonadherence if they mentioned one of the following conditions: no ongoing hormone therapy, treatment interruption, or treatment discontinuation during the year before assessment.
Assessment of survival outcomes.
For survival analyses, we focused primarily on distant disease-free survival (DDFS), given that locoregional recurrences are frequently amenable to definitive treatment, thus limiting results interpretation in a cohort with a relatively short follow-up and limited number of recurrences. DDFS was defined as time from serum assessment of tamoxifen to date of distant recurrence or death by any cause.26 Secondarily, we examined breast cancer–free interval (BCFI), which was defined as time from tamoxifen serum assessment to date of contralateral breast cancer; local, regional, or distant recurrence; or death resulting from breast cancer.26
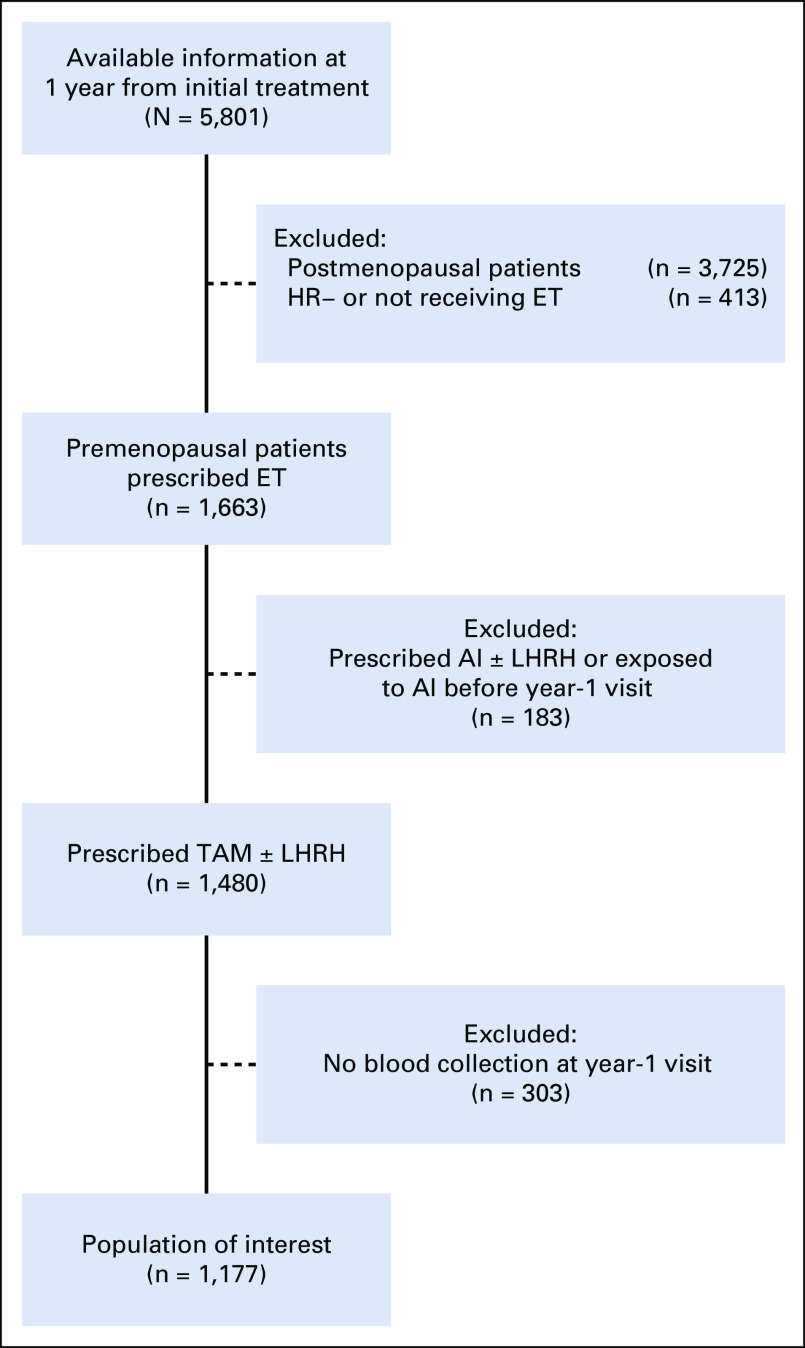
Because our focus was to assess the impact of nonadherence at year 1 after tamoxifen prescription, a landmark analysis was performed and, per the CONSORT diagram (Fig 1), all patients with a distant disease event before this time were excluded upfront from this study.
FIG 1.
CONSORT diagram of study participants. AI, aromatase inhibitor; ET, endocrine therapy; HR, hormone receptor; LHRH, luteinizing hormone-releasing hormone; TAM, tamoxifen.
Study covariates.
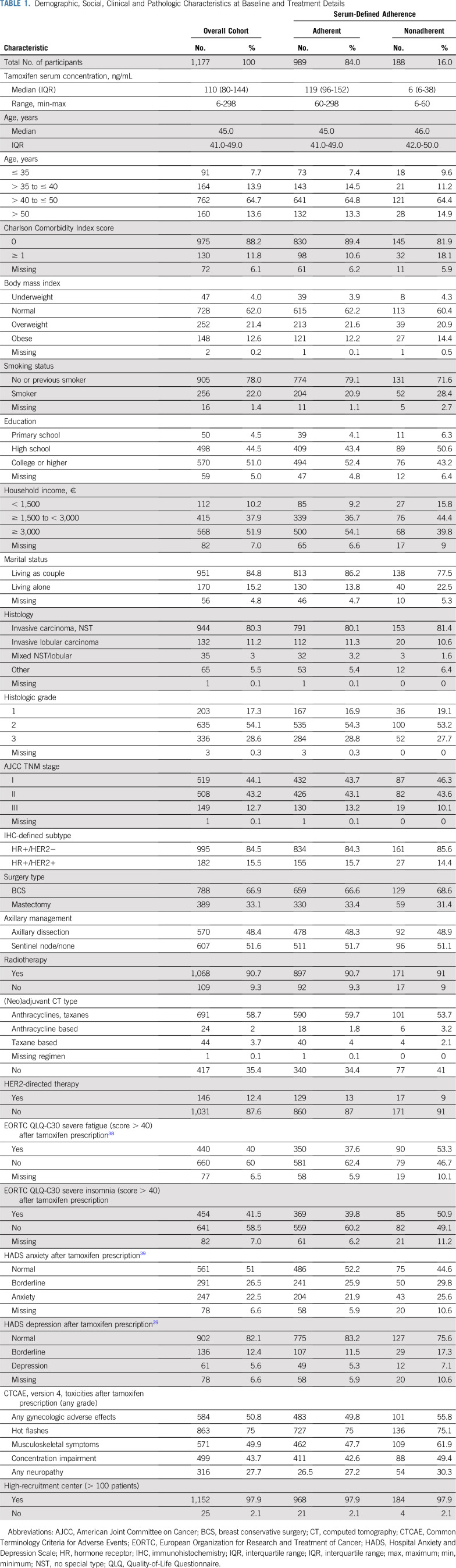
All study covariates were categorized as reported in Table 1, including baseline sociodemographic, clinical, and behavioral factors, treatment toxicities, and HRPROs shortly after treatment prescription.
TABLE 1.
Demographic, Social, Clinical and Pathologic Characteristics at Baseline and Treatment Details
Statistical Analysis
Concordance between serum assessment and patient’s self-report was determined by χ2 test, and estimated using the Cramer V coefficient. Multivariate logistic regression modeled the association of relevant covariates with nonadherence at year 1 after tamoxifen prescription. Several methods were used to examine the independent impact of biochemical nonadherence and patient-reported nonadherence on DDFS. Time-to-event outcomes were estimated and plotted using the Kaplan-Meier method. To deal with confounding, as a primary analysis, we used propensity score (PS) inverse probability treatment weighting (IPTW)27 in a Cox model. To assess robustness of results, a multivariable Cox proportional hazards model was also performed as a sensitivity analysis. Variables included in both the PS IPTW and Cox proportional hazards models were known breast cancer prognostic factors and included age at diagnosis, TNM staging, type of surgery, receipt of (neo)adjuvant chemotherapy, and center size. In secondary sensitivity analyses, PS IPTW was first weighted by Charlson Comorbidity Index score and then by marital status, education, body mass index, smoking habits, anxiety, depression, and symptoms at treatment initiation.
PS diagnostics were performed using a user-written package pstest (by E. Leuven and B. Sianesi, Boston College) for Stata (StataCorp, College Station, TX). Variance estimation was optimized by using a bootstrapped PS27 and, to deal with instability that can ensue from large weights, a stabilized IPTW was implemented.28 Because the year-1 visit did not occur exactly at the same time from diagnosis for all patients, description of time between scheduled visits in adherent and nonadherent patients was also performed. All time-to-event analyses met proportional hazards assumption as assessed by the Schoenfeld residuals. Given the low DDFS event rate, median follow-up was the median of the observed follow-up times using data from all patients.
There were low rates of missing variables, which were considered missing at random among adherent and nonadherent patients (Table 1), given balanced distribution between groups. Therefore, no multiple imputation was performed. Secondary analyses focused on BCFI were performed. All tests were two-sided and P ≤ .05 was considered statistically significant. No formal adjustment for multiplicity was performed, given the observational nature of the study. The analyses were performed using Stata, version 15.1 (StataCorp).
RESULTS
Study Cohort
From the 5,801 women enrolled in CANTO with available data, we first excluded those who were postmenopausal at cancer diagnosis (n = 3,725), those with HR− breast cancer or not receiving endocrine therapy (n = 413), and those prescribed aromatase inhibitors before the year-1 visit (n = 183). We then selected all women who were premenopausal at diagnosis and were prescribed and agreed to take adjuvant tamoxifen (n = 1,480). Finally, we selected women among whom tamoxifen serum assessment was performed at year 1 after tamoxifen prescription (n = 1,177; Fig 1). Characteristics of nonparticipant patients who were excluded due to absence of blood assessment (n = 303 of 1,480 [20.5%]) are reported in the Data Supplement. Nonparticipant patients had lower likelihood of belonging to a high-volume recruitment center; no other major differences emerged between groups.
Among the analytic cohort, median time from tamoxifen pre-scription to measurement of nonadherence was 16.2 months (interquartile range [IQR], 15.1-17.8 months). Median age was 45 years (IQR, 41-49 years). Patients’ characteristics at baseline and treatment details are reported in Table 1.
Nonadherence at Year 1 After Tamoxifen Prescription
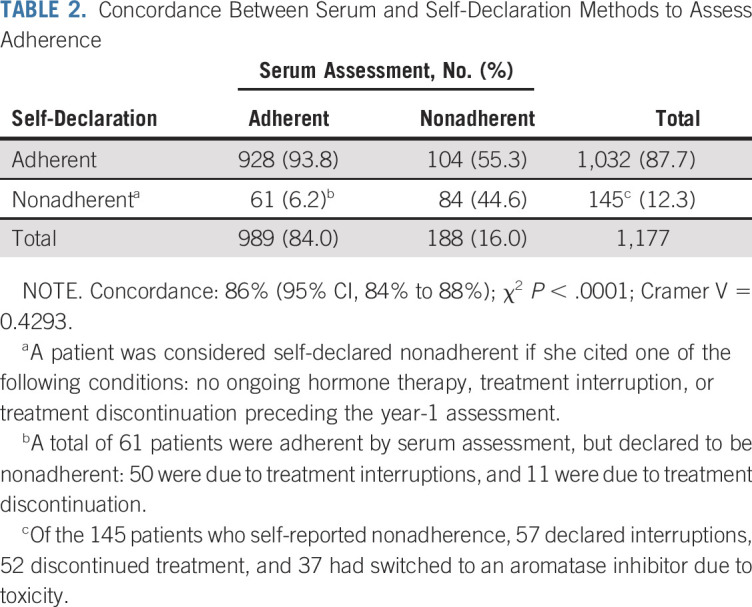
Tamoxifen serum concentrations at year 1 after prescription ranged between < 6 and 298 ng/mL, with a median of 110 ng/mL (Table 1; Data Supplement). Overall, 188 patients (16%) were below the set biochemical adherence threshold of tamoxifen at year 1; 145 patients (12.3%) self-declared to be nonadherent: 89 (7.6%) reported tamoxifen discontinuation and 56 (4.7%) reported temporary interruptions. Among the 145 patients declaring to be nonadherent, less than half (n = 67) provided a personal or medical reason for nonadherence. Among these, toxicity was mentioned by 57 patients. Of 188 patients who were biochemically nonadherent, 104 (55.3%) stated that they had been regularly taking tamoxifen over the past year. None of those with tamoxifen serum levels < 60 ng/mL was exposed to any of the drugs that may interfere with tamoxifen serum levels. Although biochemical and self-declared nonadherence were significantly associated (P < .0001), only moderate concordance between the two methods was found (concordance: 86%; 95% CI, 84% to 88%; Cramer V = 0.429; Table 2).
TABLE 2.
Concordance Between Serum and Self-Declaration Methods to Assess Adherence
Biochemical nonadherence was associated with multiple factors. Patients not living with a partner as a couple (v with a partner; adjusted odds ratio [aOR], 1.72; 95% CI, 1.02 to 2.89), those with more comorbidities (Charlson comorbidity score ≥ 1 v 0; aOR, 1.85 95% CI, 1.09 to 3.15), and patients who did not receive treatment with (neo)adjuvant chemotherapy (v those who received chemotherapy; aOR, 1.74; 95% CI, 1.04 to 2.91) had higher odds of biochemical nonadherence. In addition, symptoms after tamoxifen prescription (median time from prescription to assessment, 3.9 months [95% CI, 3.0 to 5.1 months]) including musculoskeletal symptoms (aOR, 1.58; 95% CI, 1.06 to 2.37) and severe fatigue (aOR, 1.65; 95% CI, 1.07 to 2.5) increased the risk of biochemical nonadherence (Fig 2). Factors associated with patient-reported nonadherence are described in the Data Supplement.
FIG 2.
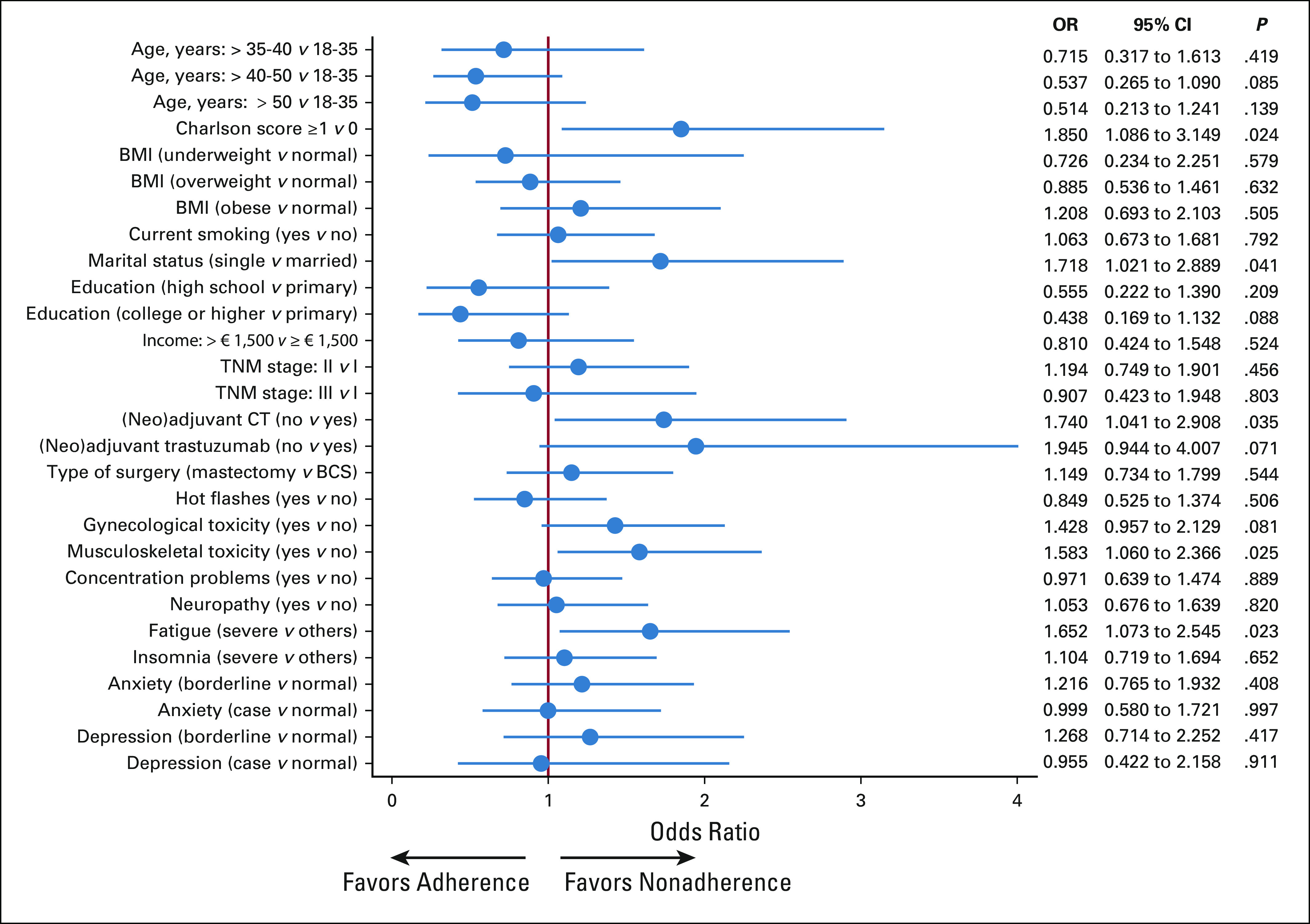
Multivariate estimates of variables associated with serum-defined adherence. Severe fatigue and insomnia were defined as the respective subscale European Organization for Research and Treatment of Cancer–C30 score > 40.38 Anxiety and depression were defined using the Hospital Anxiety and Depression Scale.39 BMI, body mass index; CT, chemotherapy; OR odds ratio.
Impact of Nonadherence at Year 1 After Tamoxifen Prescription on Survival Outcomes
After a median follow-up of 24.2 months from tamoxifen prescription (IQR, 22.8-27.0), 38 events were registered (Data Supplement) among 1,057 patients eligible for survival analysis. The median DDFS follow-up was balanced between adherent and nonadherent groups defined by serum assessment (median, 24.3 [IQR, 22.8-27.5] v 24.1 [IQR, 21.3-25.8] for nonadherence). In the PS IPTW, the proportion of patients alive and without distant recurrence at 3 years was 95.4% in the adherent cohort and 89.5% in the nonadherent cohort (Fig 3A). In the multivariate IPTW model, nonadherent patients had a 131% increase in the risk of death or disease recurrence (hazard ratio [HR], 2.31; 95% CI, 1.05 to 5.06).
FIG 3.
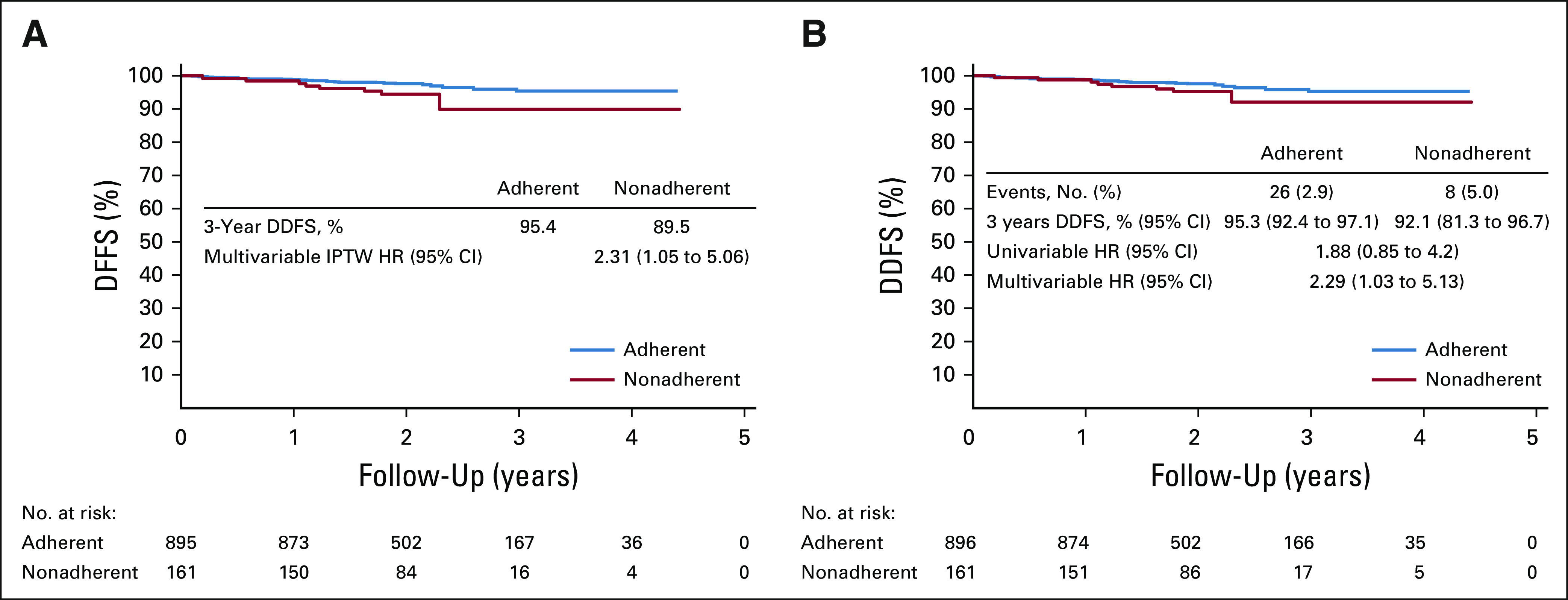
Distant disease-free survival (DDFS; distant recurrences and death) according to serum-defined adherence status in (A) the IPTW cohort and in the (B) non-IPTW cohort. Time 0 defines the time of the post-tamoxifen prescription visit and date of serum assessment of tamoxifen. HR, hazard ratio; IPTW, inverse probability treatment weighting.
Diagnoses of the models' performance are fully presented in the Data Supplement. Sensitivity and secondary analyses including BCFI demonstrated consistent results (Fig 3B; Data Supplement). Full univariable and multivariable models are presented in the Data Supplement. No difference in DDFS or BCFI outcomes was found between self-reported adherence and nonadherence (Data Supplement).
DISCUSSION
Nonadherence to adjuvant endocrine therapy for early breast cancer is often under-recognized partly because of the unavailability of a gold standard method for its detection and challenges in incorporating assessments of adherence into routine clinical practice. Our study emphasizes that the real-life prevalence of nonadherence to medications is still not well quantified: health care providers tend to overestimate to what extent patients take their prescribed, long-term, oral treatments, whereas patients tend to underreport treatment discontinuations or interruptions.29 Studies that tried to quantify the prevalence of nonadherence have yielded heterogeneous results, mostly reporting on indirect estimations obtained using patient self-report and prescription refill data.1,2,7 In breast cancer, previous studies based on indirect methods suggested that nonadherence to adjuvant endocrine therapy over 5 years ranges from 25% to 50%, with this proportion increasing over time.10-12,30 Only one study measured adherence to endocrine therapy by using an objective method based on drug serum assessment, although it did not provide correlations with breast cancer outcomes.8 In our study, serum assessment was able to identify a worryingly high proportion of patients, one in six, who were nonadherent to therapy at only 1 year after treatment prescription. Patient self-reports underestimated rates of nonadherence. Notably, 55% of patients who were nonadherent by serum assessment might not overtly acknowledge nonadherence.
Furthermore, nonadherence by serum assessment measured as early as year 1 after treatment prescription emerged as marker of poorer outcomes regardless of other main prognostic factors, suggesting that risk of recurrence increases as soon as the patients start to be nonadherent. Although it is unusual to see a significant impact on outcomes with such short-term follow-up among patients with HR+ breast cancer, results of prior research are consistent with our findings. Conflicting results were reported across different studies, suggesting the possibility that inadequate exposure to tamoxifen due to nonadherence may lead to a suboptimal concentration of its active metabolites.20,31 Prior retrospective analyses based on pharmacy claims data also suggested a negative impact of nonadherence on breast cancer outcomes but used an arbitrary cutoff of 80% medication possession ratio to define adequate adherence.32-34 However, pharmacy claims typically cannot be obtained in real time on an individual patient level and thus cannot be used to tailor treatment in the clinic.1,10,11
This study provides important insights on the complexity of nonadherence. We found that sicker, nonpartnered patients and those with higher symptom burden, including more severe fatigue and musculoskeletal symptoms, had a higher likelihood of being nonadherent to therapy. Most of these associations have also been observed in other chronic diseases, such as HIV, cardiac diseases, and diabetes, and are explained by several differences in social and clinical characteristics across patients. In addition to these previously known barriers, patients who did not receive adjuvant chemotherapy were also more likely not to be adherent to tamoxifen in our analysis. We hypothesize that these patients are less aware of the health risks related to their disease and misconceive the beneficial impact of adjuvant endocrine therapy on breast cancer outcomes.
CANTO offered an unparalleled opportunity to test the performance of therapeutic drug monitoring in adjuvant treatment of breast cancer. Nevertheless, we acknowledge some limitations. First, we used tamoxifen concentration thresholds that have not been previously validated to define biochemical nonadherence. However, we used a conservative approach based on previous pharmacological studies18-20 that focused on the 3-month steady-state tamoxifen concentration, which all our patients should have achieved. In addition, we did not assess active tamoxifen metabolites or CYP2D6 genotypes, although both might be associated with breast cancer outcomes and adverse effects from tamoxifen that ultimately influence treatment adherence.20,35 Second, the self-reported assessment of adherence and respective reasons were not based on validated scales, but they still reflect what is currently done in clinical practice. Although CRNs systematically asked for and collected the reasons for treatment interruption or discontinuation, only a small number of patients disclosed this information, limiting our ability to capture the complexity of factors affecting medication-taking behavior. Third, because of the low number of events and the lack of validation cohort, we cannot draw definitive conclusions on the generalizability of the negative impact of nonadherence on breast cancer outcomes. Nevertheless, our results are clinically plausible and the broad range of inclusion and exclusion criteria in CANTO suggest external validity of our results. Fourth, we are aware that it is hard to isolate the true impact of nonadherence to tamoxifen on outcomes because it is part of a multitude of health-related behaviors influencing prognosis.36 Indeed, because of the observational design, we cannot exclude unmeasured confounding affecting our survival analyses. Nevertheless, we used a PS weighting including relevant, known prognostic factors, aiming for a comprehensive adjustment in our analyses.27,28 Fifth, we cannot exclude the impact of awareness of being observed on adherence (the Hawthorne effect). Nevertheless, in our study, the long-term observation, assessment of multiple clinical and biologic data, and evaluation of adherence using indirect and direct methods may minimize this effect.37 Finally, our results may not be generalizable to other populations, because we restricted our analysis to the French premenopausal population with breast cancer.
This study adds to the understanding of the multifaceted and complex issue of nonadherence’ to chronic medications, suggesting that therapeutic drug monitoring may be an important tool to identify nonadherent patients who are at risk for a distant relapse event early in their adjuvant treatment trajectory. The impact of interventions to optimize adherence on a population level could thus be large. Targeted interventions managing adherence to adjuvant endocrine therapy are needed and have the potential to improve breast cancer outcomes.
Presented in part at European Society for Medical Oncology Congress 2018, Munich, Germany, October 22, 2018 (Ann Oncol 29: viii603-viii640, 2018 (suppl 8).
SUPPORT
Supported by Institut National Cancer-France (Grant No. SHS-E-SP 18-129 [B,P.]); grants from Gustave Roussy Foundation, Philanthropic Odyssea Gustave Roussy Program, and a Career Catalyst Research grant from Susan G. Komen (CCR17483507) to I.V.-L.; and National Research Agency (Grant No. ANR-10-COHO-0004 [A.F.]).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Barbara Pistilli, Angelo Paci, Arlindo R. Ferreira, Antonio Di Meglio, Agnes Dumas, Sarah Dauchy, Anne Lesur, Laurence Vanlemmens, Sibille Everhard, Patrick Arveux, Anne Laure Martin, Ann H. Partridge, Suzette Delaloge, Ines Vaz-Luis
Financial support: Barbara Pistilli, Fabrice André, Ines Vaz-Luis
Administrative support: Barbara Pistilli, Sibille Everhard, Anne Laure Martin, Ines Vaz-Luis
Provision of study material or patients: Barbara Pistilli, Paul H. Cottu, Florence Lerebours, Charles Coutant, Anne Lesur, Olivier Tredan, Patrick Soulie, Laurence Vanlemmens, Christelle Jouannaud, Christelle Levy, Sibille Everhard, Anne Laure Martin, Suzette Delaloge
Collection and assembly of data: Barbara Pistilli, Gwenn Menvielle, Agnes Dumas, Sarah Dauchy, Sandrine Pinto, Florence Lerebours, Charles Coutant, Anne Lesur, Olivier Tredan, Patrick Soulie, Laurence Vanlemmens, Christelle Jouannaud, Christelle Levy, Sibille Everhard, Patrick Arveux, Anne Laure Martin, Suzette Delaloge, Fabrice André, Ines Vaz-Luis
Data analysis and interpretation: Barbara Pistilli, Angelo Paci, Arlindo R. Ferreira, Antonio Di Meglio, Vianney Poinsignon, Aurelie Bardet, Agnes Dumas, Leonor Fasse, Paul H. Cottu, Charles Coutant, Anne Lesur, Laurence Vanlemmens, Christelle Levy, Patrick Arveux, Alexandra Dima, Nancy U. Lin, Ann H. Partridge, Suzette Delaloge, Stefan Michiels, Fabrice André, Ines Vaz-Luis
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Serum Detection of Nonadherence to Adjuvant Tamoxifen and Breast Cancer Recurrence Risk
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Barbara Pistilli
Consulting or Advisory Role: Puma Biotechnology, Pierre Fabre, Novartis, Myriad Genetics
Research Funding: Pfizer (Inst), Puma Biotechnology, Merus
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, MSD Oncology, Novartis
Angelo Paci
Honoraria: Fresenius Kabi, Pierre Fabre Médicament
Patents, Royalties, Other Intellectual Property: Research patents on drug delivery design
Travel, Accommodations, Expenses: Pierre Fabre Médicament
Arlindo R. Ferreira
Travel, Accommodations, Expenses: Roche (Inst), Novartis (Inst), Novartis
Aurelie Bardet
Employment: Roche SAS
Stock and Other Ownership Interests: Roche SAS
Consulting or Advisory Role: Roche SAS
Sarah Dauchy
Honoraria: Servier, Novartis, MSD Oncology, Bristol-Myers Squibb, Nutricia
Travel, Accommodations, Expenses: Servier
Leonor Fasse
Employment: GlaxoSmithKline
Research Funding: GlaxoSmithKline
Paul H. Cottu
Honoraria: Pfizer, Novartis (Inst), Roche, NanoString Technologies (Inst), Eli Lilly
Consulting or Advisory Role: Pfizer, Roche, Eli Lilly
Research Funding: Novartis (Inst), Pfizer (Inst)
Travel, Accommodations, Expenses: Roche, Pfizer
Olivier Tredan
Consulting or Advisory Role: Roche, Pfizer, Novartis, Eli Lilly, AstraZeneca, MSD Oncology, Sandoz
Research Funding: Roche (Inst), Bristol-Myers Squibb (Inst), MSD Oncology (Inst), AstraZeneca (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: Roche, Novartis, Pfizer, Eli Lilly, AstraZeneca
Patrick Soulie
Travel, Accommodations, Expenses: Pfizer, PharmaMar, Ipsen
Nancy U. Lin
Consulting or Advisory Role: Roche, Seattle Genetics, Puma Biotechnology, Novartis, Daiichi Sankyo
Research Funding: Genentech, Pfizer, Seattle Genetics, Merck
Patents, Royalties, Other Intellectual Property: Royalties for chapter in Up-to-Date regarding management of breast cancer brain metastases, royalties from Jones & Bartlett
Ann H. Partridge
Patents, Royalties, Other Intellectual Property: I receive small royalty payments for coauthoring the breast cancer survivorship section of UpToDate
Travel, Accommodations, Expenses: Novartis
Suzette Delaloge
Consulting or Advisory Role: AstraZeneca
Research Funding: AstraZeneca (Inst), Pfizer (Inst), Roche (Inst), Puma (Inst), Eli Lilly (Inst), Novartis (Inst), Sanofi (Inst)
Travel, Accommodations, Expenses: Pfizer, AstraZeneca, Roche
Stefan Michiels
Consulting or Advisory Role: IDDI, Hexal, Roche, QuintilesIMS, Sensorion, Biophytis, Janssen-Cilag France
Patents, Royalties, Other Intellectual Property: Co-inventor of patent WO2018002385A1 on prognostic gene score in early breast cancer
Fabrice André
Research Funding: AstraZeneca (Inst), Novartis (Inst), Pfizer (Inst), Eli Lilly (Inst), Roche (Inst), Daiichi (Inst)
Travel, Accommodations, Expenses: Novartis, Roche, GlaxoSmithKline, AstraZeneca
Ines Vaz-Luis
Honoraria: Novartis, Kephren, AstraZeneca, Amgen
Consulting or Advisory Role: Ipsen
No other potential conflicts of interest were reported.
REFERENCES
- 1.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 2. Sabaté E (ed): Adherence to Long-Term Therapies: Evidence for Action. Geneva, Switzerland, World Health Organization, 2003. [Google Scholar]
- 3.Kini V, Ho PM. Interventions to improve medication adherence: A review. JAMA. 2018;320:2461–2473. doi: 10.1001/jama.2018.19271. [DOI] [PubMed] [Google Scholar]
- 4.Waterhouse DM, Calzone KA, Mele C, et al. Adherence to oral tamoxifen: A comparison of patient self-report, pill counts, and microelectronic monitoring. J Clin Oncol. 1993;11:1189–1197. doi: 10.1200/JCO.1993.11.6.1189. [DOI] [PubMed] [Google Scholar]
- 5.Rosenbaum L, Shrank WH. Taking our medicine--Improving adherence in the accountability era. N Engl J Med. 2013;369:694–695. doi: 10.1056/NEJMp1307084. [DOI] [PubMed] [Google Scholar]
- 6.Lu CY, Zhang F, Wagner AK, et al. Impact of high-deductible insurance on adjuvant hormonal therapy use in breast cancer. Breast Cancer Res Treat. 2018;171:235–242. doi: 10.1007/s10549-018-4821-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garber MC, Nau DP, Erickson SR, et al. The concordance of self-report with other measures of medication adherence: A summary of the literature. Med Care. 2004;42:649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- 8.Oberguggenberger AS, Sztankay M, Beer B, et al. Adherence evaluation of endocrine treatment in breast cancer: Methodological aspects. BMC Cancer. 2012;12:474. doi: 10.1186/1471-2407-12-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davies C, Godwin J, Gray R, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet. 2011;378:771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partridge AH, Wang PS, Winer EP, et al. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 11.Hershman DL, Kushi LH, Shao T, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8,769 early-stage breast cancer patients. J Clin Oncol. 2010;28:4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320:479–484. doi: 10.1056/NEJM198902233200802. [DOI] [PubMed] [Google Scholar]
- 13.Burstein HJ, Lacchetti C, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2019;37:423–438. doi: 10.1200/JCO.18.01160. [DOI] [PubMed] [Google Scholar]
- 14.Partridge AH, Hughes ME, Warner ET, et al. Subtype-dependent relationship between young age at diagnosis and breast cancer survival. J Clin Oncol. 2016;34:3308–3314. doi: 10.1200/JCO.2015.65.8013. [DOI] [PubMed] [Google Scholar]
- 15.Vaz-Luis I, Cottu P, Mesleard C, et al. UNICANCER: French prospective cohort study of treatment-related chronic toxicity in women with localised breast cancer (CANTO) ESMO Open. 2019;4:e000562. doi: 10.1136/esmoopen-2019-000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Helmy R, Zullig LL, Dunbar-Jacob J, et al. ESPACOMP Medication Adherence Reporting Guidelines (EMERGE): A reactive-Delphi study protocol. BMJ Open. 2017;7:e013496. doi: 10.1136/bmjopen-2016-013496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teunissen SF, Jager NG, Rosing H, et al. Development and validation of a quantitative assay for the determination of tamoxifen and its five main phase I metabolites in human serum using liquid chromatography coupled with tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:1677–1685. doi: 10.1016/j.jchromb.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 18.MacCallum J, Cummings J, Dixon JM, et al. Concentrations of tamoxifen and its major metabolites in hormone responsive and resistant breast tumours. Br J Cancer. 2000;82:1629–1635. doi: 10.1054/bjoc.2000.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021807s005lbl.pdf Highlights of prescribing information. Soltamox (tamoxifen citrate)
- 20.Saladores P, Mürdter T, Eccles D, et al. Tamoxifen metabolism predicts drug concentrations and outcome in premenopausal patients with early breast cancer. Pharmacogenomics J. 2015;15:84–94. doi: 10.1038/tpj.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Madlensky L, Natarajan L, Tchu S, et al. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stearns V, Johnson MD, Rae JM, et al. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 23.Binkhorst L, van Gelder T, Loos WJ, et al. Effects of CYP induction by rifampicin on tamoxifen exposure. Clin Pharmacol Ther. 2012;92:62–67. doi: 10.1038/clpt.2011.372. [DOI] [PubMed] [Google Scholar]
- 24.Hussaarts KGAM, Hurkmans DP, Oomen-de Hoop E, et al. Impact of curcumin (with or without piperine) on the pharmacokinetics of tamoxifen. Cancers (Basel) 2019;11:403. doi: 10.3390/cancers11030403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lien EA, Anker G, Lønning PE, et al. Decreased serum concentrations of tamoxifen and its metabolites induced by aminoglutethimide. Cancer Res. 1990;50:5851–5857. [PubMed] [Google Scholar]
- 26.Gourgou-Bourgade S, Cameron D, Poortmans P, et al. Guidelines for time-to-event end point definitions in breast cancer trials: Results of the DATECAN initiative (Definition for the Assessment of Time-to-event Endpoints in CANcer trials) Ann Oncol. 2015;26:873–879. doi: 10.1093/annonc/mdv106. [DOI] [PubMed] [Google Scholar]
- 27.Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35:5642–5655. doi: 10.1002/sim.7084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: Reporting measures of effect similar to those used in randomized experiments. Stat Med. 2014;33:1242–1258. doi: 10.1002/sim.5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine AM, Richardson JL, Marks G, et al. Compliance with oral drug therapy in patients with hematologic malignancy. J Clin Oncol. 1987;5:1469–1476. doi: 10.1200/JCO.1987.5.9.1469. [DOI] [PubMed] [Google Scholar]
- 30.Partridge AH, LaFountain A, Mayer E, et al. Adherence to initial adjuvant anastrozole therapy among women with early-stage breast cancer. J Clin Oncol. 2008;26:556–562. doi: 10.1200/JCO.2007.11.5451. [DOI] [PubMed] [Google Scholar]
- 31.Sanchez-Spitman A, Dezentjé V, Swen J, et al. Tamoxifen pharmacogenetics and metabolism: Results from the prospective CYPTAM Study. J Clin Oncol. 2019;37:636–646. doi: 10.1200/JCO.18.00307. [DOI] [PubMed] [Google Scholar]
- 32.Hershman DL, Shao T, Kushi LH, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCowan C, Wang S, Thompson AM, et al. The value of high adherence to tamoxifen in women with breast cancer: A community-based cohort study. Br J Cancer. 2013;109:1172–1180. doi: 10.1038/bjc.2013.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barron TI, Cahir C, Sharp L, et al. A nested case-control study of adjuvant hormonal therapy persistence and compliance, and early breast cancer recurrence in women with stage I-III breast cancer. Br J Cancer. 2013;109:1513–1521. doi: 10.1038/bjc.2013.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rae JM, Sikora MJ, Henry NL, et al. Cytochrome P450 2D6 activity predicts discontinuation of tamoxifen therapy in breast cancer patients. Pharmacogenomics J. 2009;9:258–264. doi: 10.1038/tpj.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCorkle R, Ercolano E, Lazenby M, et al. Self-management: Enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin. 2011;61:50–62. doi: 10.3322/caac.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. J Clin Epidemiol. 2014;67:267–277. doi: 10.1016/j.jclinepi.2013.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abrahams HJ, Gielissen MF, Schmits IC, et al. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12 327 breast cancer survivors. Ann Oncol. 2016;27:965–974. doi: 10.1093/annonc/mdw099. [DOI] [PubMed] [Google Scholar]
- 39.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]