Abstract
Background
Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnancy remain poorly understood. Identifying and understanding populations at a heightened risk of acquisition is essential to more effectively target outreach and prevention efforts.
Objective
This study aimed to compare sociodemographic and clinical characteristics of pregnant women with and without severe acute respiratory syndrome coronavirus 2 infection and, among those with severe acute respiratory syndrome coronavirus 2, to compare characteristics of those who reported coronavirus disease symptoms and those who were asymptomatic at diagnosis.
Study Design
This retrospective cohort study includes pregnant women who delivered or intended to deliver at Northwestern Memorial Hospital after initiation of a universal testing protocol on admission (April 8, 2020–May 31, 2020). Women were dichotomized by whether they had a positive test result for severe acute respiratory syndrome coronavirus 2. Among women with a positive test result, women were further dichotomized by whether they reported symptoms of coronavirus disease 2019. Bivariable analysis and parametric tests of trend were used for analyses. Logistic regression was used to control for potential confounders and to examine effect modification between race and ethnicity and any other identified risk factors.
Results
During the study period, 1418 women met inclusion criteria, of whom 101 (7.1%) had a positive test result for severe acute respiratory syndrome coronavirus 2. Of the 101 women who had a positive test result, 77 (76.2%) were symptomatic at the time of diagnosis. Compared with women who had a negative test result for severe acute respiratory syndrome coronavirus 2, those with a positive test result were younger and were more likely to have public insurance, to identify as black or African American or Latina, to be unmarried, to be obese, to have preexisting pulmonary disease, and to have living children. An increasing number of living children was associated with an increasing risk of severe acute respiratory syndrome coronavirus 2 infection, and this finding persisted after controlling for potential confounders. There was no effect modification between race or ethnicity and having living children with regard to the risk of infection. There were no significant differences identified between women who were symptomatic and asymptomatic.
Conclusion
Many risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnancy are similar to the social and structural determinants of health that have been reported in the general population. The observed association between severe acute respiratory syndrome coronavirus 2 infection and having children raises the possibility of children themselves being vectors of viral spread or behavior patterns of parents being mediators of acquisition.
Key words: COVID-19, health disparities, perinatal epidemiology, social determinants of health
AJOG MFM at a Glance.
Why was this study conducted?
Risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in pregnancy remain poorly understood. Identifying the populations at a heightened risk of acquisition is essential to effectively target outreach and prevention efforts.
Key findings
Compared with women who had a negative test result for SARS-CoV-2, women with a positive test result for SARS-CoV-2 were younger and were more likely to have public insurance, to identify as black or African American or Latina, to be unmarried, to be obese, to have preexisting pulmonary disease, and to have living children. An increasing number of living children was associated with an increasing risk of SARS-CoV-2 infection, and this finding persisted after controlling for potential confounders.
What does this add to what is known?
In addition to previously identified risk factors, having living children at home represents a significant risk factor for infection with SARS-CoV-2 among pregnant women.
Introduction
Since December 2019, coronavirus disease 2019 (COVID-19) has spread rapidly throughout the world. It has now caused more than 14 million infections worldwide, with more than 3 million infections in the United States.1 , 2 Emerging antibody surveillance data have suggested that many individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) do not manifest clinical symptoms. As such, many cases of the infection are thought to occur as a result of spread from asymptomatic individuals.3 , 4 Infection with SARS-CoV-2 in pregnancy has been associated, in some studies, with higher rates of miscarriage, preterm birth, and preeclampsia.5 The neonates born to women with SARS-CoV-2 have been found to have higher rates of perinatal mortality and admission to the neonatal intensive care unit.5 Accurately identifying pregnant women infected with SARS-CoV-2 is imperative for appropriate management and treatment. Their identification also allows frontline healthcare workers to improve their protection and take precautions to mitigate the spread of the virus.
Little research has been conducted on risk factors for SARS-CoV-2 infection specific to pregnant women. Whether observed associations in the general population apply to pregnant women, or whether unique risk factors can be identified specific to pregnant women, is unknown. For example, an overrepresentation of racial and ethnic minority groups in COVID-19 hospitalizations and deaths has been demonstrated in the general population.6 , 7 However, whether these disparities remain true among pregnant women, who may have different behaviors and exposures, has not been investigated. The American College of Obstetricians and Gynecologists (ACOG) recently called for health institutions to collect data on SARS-CoV-2 testing and outcomes that can recognize and evaluate the ways in which healthcare systems perpetuate racial inequalities in access to care and in health outcomes.8 Robust research on these factors can help institutions determine the most efficient way to distribute scarce resources to those women most in need.
Public health interventions, such as school closures, have been shown to decrease the risk of community viral spread on a population level.9 Epidemiologists have found that although children are less likely to exhibit SARS-CoV-2 symptoms compared with adults,10 , 11 they also have more subtle presentations11, 12, 13 and may spread disease to family members at home.14 , 15 On an individual level, these data suggest that families with children at home, particularly families who are not able to physically distance, may be at a higher risk of SARS-CoV-2 acquisition. Because many pregnant women in the United States have young children at home, they may be a particularly vulnerable population for SARS-CoV-2 acquisition, but this association has not been previously evaluated.
Universal SARS-CoV-2 testing among pregnant women represents an opportunity to better understand epidemiologic risk factors. As our hospital is a large volume center located in a high prevalence region of the United States, our objective was to leverage data ascertained from our testing policies to characterize the epidemiology of SARS-CoV-2 infection overall, as well as symptomatic infection, among pregnant women.
Materials and Methods
Study design
This retrospective cohort study includes pregnant women who were tested for SARS-CoV-2 at Northwestern Memorial Hospital or affiliated outpatient clinics between March 19, 2020, and May 31, 2020. Northwestern Memorial Hospital is a tertiary care referral center in which approximately 12,000 deliveries are performed annually. Routine care during the entire study period was to perform systematic screening using a comprehensive list of reported symptoms for COVID-19, including fever, shortness of breath, cough, sore throat, body aches, chills, new-onset vomiting, diarrhea, loss of taste or smell, or red or painful eyes.
Beginning on March 19, 2020, women who presented with clinical concern for COVID-19 underwent testing for SARS-CoV-2. Universal point-of-care testing for SARS-CoV-2 was performed for all women presenting for delivery or with pregnancy complications necessitating admission to the Labor & Delivery or Antepartum unit after April 8, 2020. During the period from March 19, 2020, to April 7, 2020, women who were symptomatic and had a positive test result for SARS-CoV-2 were included. During the period from April 8, 2020, to May 19, 2020, all women who were tested for SARS-CoV-2, including symptomatic and asymptomatic patients with a positive test result and patients with a negative test result, were included. During the period from May 20, 2020, to May 31, 2020, only women who had a positive test result for SARS-CoV-2, both symptomatic and asymptomatic, were included (Figure 1 ). Women with scheduled admissions were tested 12 to 36 hours before the admission at a designated drive-through testing center using an in-house polymerase chain reaction (PCR)–based platform with an 8-hour turnaround time. Women who presented in labor or with another unscheduled indication for admission were tested either in obstetrical triage or in the Labor & Delivery unit using a commercially available PCR-based platform with a 2- to 3-hour turnaround time. Women who had a negative test result for SARS-CoV-2 at admission but who developed possible symptoms of COVID-19 (eg, an intrapartum fever without an alternative diagnosis) were retested as clinically indicated.
Figure 1.

Timeline of study recruitment
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sakowicz et al. Severe acute respiratory syndrome coronavirus 2 risk factors in pregnancy. AJOG MFM 2020.
Testing was performed on nasopharyngeal specimens that were collected by registered nurses with special training in the proper collection and handling of the specimen.
Data collection
Electronic health records (EHRs) were reviewed for all pregnant women identified to have a SARS-CoV-2 test performed. Demographic and clinical data included maternal age, self-reported race or ethnicity, and insurance status. Medical history data included body mass index (BMI) at delivery, tobacco use, and any identified maternal preexisting disease (eg, diabetes, hypertension, pulmonary disease). Obstetrical data included parity (eg, term births, preterm births, and living children). The systematic symptom assessment was entered into the EHR in a form completed by the admitting nurse and was abstracted to the database. Details of the SARS-CoV-2 testing platform utilized and the test results were also abstracted. Data were entered into the research electronic data capture system (REDCap; Vanderbilt University),16 and missing or aberrant data were rereviewed by systematic assessment of the database.
Statistical analysis
Women were dichotomized by their SARS-CoV-2 test results. Women with a positive test result were further dichotomized by whether they exhibited any symptoms of COVID-19 on the systematic review. Bivariable analyses were used to compare the clinical characteristics associated with women who had a positive test result and those who had a negative test result for SARS-CoV-2. Mann-Whitney U tests were used for continuous variables, and chi-square or Fisher exact tests were used for categorical variables. A nonparametric test of trend was performed to identify whether an increasing number of children was associated with SARS-CoV-2 positivity.
Logistic regression was performed to control for potential confounders in the relationship between having children and SARS-CoV-2 infection. Race, ethnicity, public insurance, and marital status ultimately reflect overlapping constructs without direct biological mechanisms for SARS-CoV-2 acquisition. Accordingly, only insurance was included in the primary model because it seemed to best reflect social and structural determinants of health. This regression otherwise included variables associated with SARS-CoV-2 infection in bivariable analysis with P<.05. A sensitivity analysis was performed including all variables associated with SARS-CoV-2 infection (P<.05). Interaction terms were used to evaluate potential effect modification between race or ethnicity and having living children. Data were analyzed with Stata (version 15, StataCorp LLC, College Station, TX). This study was approved by the Northwestern University Institutional Review Board with a waiver of consent before its initiation.
Results
Patient characteristics
During the study period, 1510 SARS-CoV-2 tests were performed on 1418 unique pregnant women at Northwestern Memorial Hospital. Of these 1418 women, 101 (7.1%) had a positive test result for SARS-CoV-2. No patients declined SARS-CoV-2 testing during the study period.
Women with severe acute respiratory syndrome coronavirus 2
The demographic characteristics of the cohort are presented in Table 1 . Compared with women with a negative test result, women who had a positive test result for SARS-CoV-2 were younger and more likely to be publicly insured, to identify with a racial or ethnic minority group, and to be unmarried. In addition, women who had a positive test result for SARS-CoV-2 were more likely to be obese and to have a preexisting pulmonary disease. In terms of obstetrical characteristics, women who had a positive test result for SARS-CoV-2 were less likely to be nulliparous and, accordingly, were more likely to have living children. Furthermore, an increasing number of living children was associated with an increased prevalence of SARS-CoV-2 infection (Figure 2 , P<.001 for test of trend). Specifically, compared with women without any living children, women with more living children exhibited increasing odds of testing positive for SARS-CoV-2 (having 1 living child: odds ratio [OR], 2.5; 95% confidence interval [CI], 1.5–4.0; having 2 living children: OR, 2.1; 95% CI, 1.0–4.1; having 3 living children: OR, 4.1; 95% CI, 1.6–10.5; having at least 4 living children: OR, 7.0; 95% CI, 2.8–17.7).
Table 1.
Maternal characteristics stratified by SARS-CoV-2 infection status
| Maternal characteristic | SARS-CoV-2 status |
||
|---|---|---|---|
| SARS-CoV-2 negative (n=1317) | SARS-CoV-2 positive (n=101) | P value | |
| Maternal age (y) | 33.7 (30.9–36.3) | 30.6 (26.2–33.3) | <.001 |
| Public insurance (n=1408) | 218 (16.7) | 62 (62.0) | <.001 |
| Race (n=1417) | <.001 | ||
| Asian | 104 (7.9) | 3 (3.0) | |
| Black or African American | 141 (10.7) | 28 (28.0) | |
| White | 772 (58.6) | 23 (23.0) | |
| Other or unknown | 300 (22.8) | 46 (46.0) | |
| Latina ethnicity (n=1341) | 244 (19.7) | 53 (53.5) | <.001 |
| Married | 1027 (78.0) | 40 (39.6) | <.001 |
| BMI at delivery (kg/m2) (n=1307) | 29.8 (26.9–33.3) | 32.3 (28.9–34.6) | .002 |
| Obesity (n=1307) | 603 (48.0) | 35 (70.0) | .002 |
| Tobacco use (n=1413) | |||
| Never | 1181 (89.8) | 89 (90.8) | .50 |
| Past | 119 (9.1) | 7 (7.1) | |
| Current | 15 (1.1) | 2 (2.0) | |
| Any maternal chronic disease (n=1412) | 452 (34.3) | 42 (44.2) | .051 |
| Preexisting diabetes | 20 (1.5) | 1 (1.0) | >.99 |
| Hypertension | 56 (4.3) | 7 (6.9) | .21 |
| Pulmonary disease | 179 (13.6) | 22 (21.8) | .023 |
| Gestational diabetes (n=1314) | 87 (6.9) | 6 (12.8) | .12 |
| Nulliparous (n=1415) | 677 (51.5) | 30 (30.0) | <.001 |
| Any living children (n=1415) | 622 (47.3) | 70 (70.0) | <.001 |
| Number of living children | 0 (0–1) | 1 (0–1) | <.001 |
Data are presented as median (interquartile range) or number (percentage).
BMI, body mass index; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sakowicz et al. Severe acute respiratory syndrome coronavirus 2 risk factors in pregnancy. AJOG MFM 2020.
Figure 2.
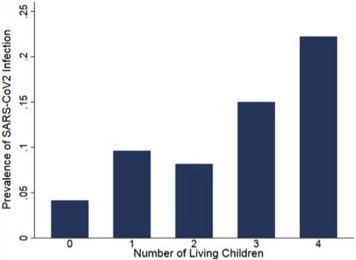
Prevalence of SARS-CoV-2 infection stratified by the number of living children
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sakowicz et al. Severe acute respiratory syndrome coronavirus 2 risk factors in pregnancy. AJOG MFM 2020.
In multivariable analyses of the relationship between having living children and SARS-CoV-2 infection (including maternal age, insurance, obesity, and pulmonary disease as potential confounders), having living children remained significantly associated with SARS-CoV-2 infection (Table 2 ). Inclusion of all variables in the model that were significantly associated with SARS-CoV-2 infection did not substantively change the association (adjusted OR, 2.29; 95% CI, 1.11–4.74). There was no significant effect modification between race or ethnicity and number of living children with respect to SARS-CoV-2 infection.
Table 2.
Multivariable analyses for the outcome of SARS-CoV-2 infection status
| Maternal characteristic | OR | 95% CI | Adjusted ORa | 95% CI |
|---|---|---|---|---|
| Maternal age (y) | 0.89 | 0.84–0.94 | 0.94 | 0.88–1.01 |
| Public insurance | 8.15 | 5.31–12.53 | 4.38 | 2.03–9.48 |
| Race | ||||
| Asian | 0.96 | 0.29–3.28 | — | — |
| Black or African American | 6.67 | 3.73–11.91 | — | — |
| White | ref | ref | — | — |
| Other or unknown | 5.15 | 3.07–8.64 | — | — |
| Latina ethnicity | 4.71 | 3.10–7.17 | — | — |
| Married | 0.18 | 0.12–0.28 | — | — |
| Obesity | 2.53 | 1.36–4.68 | 1.65 | 0.82–3.31 |
| Pulmonary disease | 1.77 | 1.08–2.91 | 1.58 | 0.78–3.23 |
| Any living children | 2.60 | 1.67–4.04 | 2.33 | 1.13–4.78 |
CI, confidence interval; OR, odds ratio; ref, referent.
Sakowicz et al. Severe acute respiratory syndrome coronavirus 2 risk factors in pregnancy. AJOG MFM 2020.
Model includes maternal age, insurance, obesity, pulmonary disease, and living children.
Demographics of asymptomatic women with severe acute respiratory syndrome coronavirus 2 infection
Among women diagnosed as having COVID-19, 77 (76.2%) were symptomatic on presentation (Table 3 ). Importantly, these data included epochs wherein only women with overt symptoms of COVID-19 could be tested for SARS-CoV-2. Accordingly, 76.2% is not reflective of the population-level symptom prevalence. No significant differences between women who presented with and without symptoms were found in terms of maternal age, use of public insurance, nulliparity, number of living children, race, ethnicity, marital status, BMI at delivery, rates of obesity, tobacco use, presence of any maternal chronic disease, rates of preexisting diabetes, rates of hypertension, rates of pulmonary disease, and rates of gestational diabetes.
Table 3.
Maternal characteristics by symptom presentation
| Maternal characteristic | Symptoms present |
||
|---|---|---|---|
| Asymptomatic (n=24) | Symptomatic (n=77) | P value | |
| Maternal age (y) | 31.0 (26.2–33.3) | 30.4 (25.9–35.6) | .84 |
| Public insurance | 17 (70.8) | 45 (59.2) | .31 |
| Nulliparous | 6 (25.0) | 24 (31.6) | .54 |
| Any living children | 18 (75.0) | 52 (68.4) | .54 |
| Number of living children | 1 (1–2) | 1 (0–1) | .41 |
| Race | .15 | ||
| Asian | 0 (0.0) | 3 (4.0) | |
| Black or African American | 11 (45.8) | 17 (22.4) | |
| White | 5 (20.8) | 18 (23.7) | |
| Other or unknown | 8 (33.3) | 38 (50.0) | |
| Latina ethnicity | 10 (41.7) | 43 (57.3) | .18 |
| Married | 6 (25.0) | 34 (44.2) | .09 |
| BMI at delivery (kg/m2) (n=50) | 31.2 (28.6–36.5) | 32.7 (28.9–34.5) | .96 |
| Obesity (n=50) | 16 (70.0) | 19 (70.4) | .95 |
| Tobacco use (n=98) | .81 | ||
| Never | 21 (91.3) | 68 (90.7) | |
| Past | 2 (8.7) | 5 (6.7) | |
| Current | 0 (0.0) | 2 (2.7) | |
| Any maternal chronic disease (n=95) | 11 (47.8) | 31 (43.1) | .69 |
| Preexisting diabetes | 1 (4.2) | 0 (0.0) | .24 |
| Hypertension | 0 (0.0) | 7 (9.1) | .19 |
| Pulmonary disease | 5 (20.8) | 17 (22.1) | .90 |
| Gestational diabetes (n=47) | 2 (8.7) | 4 (16.7) | .67 |
Data are presented as median (interquartile range) or number (percentage).
BMI, body mass index.
Sakowicz et al. Severe acute respiratory syndrome coronavirus 2 risk factors in pregnancy. AJOG MFM 2020.
Discussion
Principal findings
In this large observational cohort of pregnant women tested for SARS-CoV-2 in an epidemiologic epicenter within the United States, we identified several risk factors for SARS-CoV-2 infection including identifying with a racial or ethnic minority subgroup or having living children. SARS-CoV-2 has previously been documented to disproportionately affect racial and ethnic minorities,17 but this study specifically identifies these associations in pregnant women and further identifies having living children as a risk factor for SARS-CoV-2 infection.
Results and clinical implications
These data demonstrate that women with living children at home were more likely to be infected with SARS-CoV-2. Although children make up only 1% to 2% of all known SARS-CoV-2 cases,14 their presentation is often more subtle and may be missed, potentially allowing them to act as vectors of asymptomatic spread. Of children with SARS-CoV-2, 5% to 7% are asymptomatic, and 51% to 65% have only routine upper respiratory tract symptoms without cough or auscultatory abnormalities.10 , 18 Of children who are symptomatic, the presentation typically includes fever, but they are otherwise less visibly ill and their symptoms are often atypical.12 A recent clinical report describes 5 children in China who were originally admitted for nonrespiratory symptoms, but ultimately had a positive test result for SARS-CoV-2. In this report, 4 of the 5 children studied had gastrointestinal symptoms as the first manifestation of disease, raising the possibility that SARS-CoV-2 may not be identified in children at symptom onset.13 Ultimately, the average number of secondary infections transmitted within a family when a child is diagnosed as having COVID-19 is 2.4.12 These data become increasingly important in the context of discussions on school and daycare reopening across the United States. A recent study from the Republic of Korea demonstrated that young children with COVID-19 (younger than 10 years) were roughly half as likely to spread the infection to others, but older children (aged 10–19 years) were more likely to infect other household contacts compared with adults.15 We do not have the age of living children available in our data, and therefore, we are unable to assess whether the age of living children moderates the observed risk. In addition, we are unable to assess whether it is the number of children within the household itself that is a risk factor for SARS-CoV-2 acquisition, or whether the number of children at home is a surrogate marker for other structural determinants of health such as decreased capacity to physically distance within the home or increased exposures outside of the home to support the needs of the family. These findings suggest that having children at home may partially explain the increased rate of infection among women with living children. Although causal attribution cannot be made, the finding of an increasing prevalence of SARS-CoV-2 infection with increasing numbers of living children suggests that children may contribute to viral spread among pregnant women.
Other data have shown that the COVID-19 pandemic is disproportionately affecting individuals who identify as a racial or ethnic minority.19 This relationship has been demonstrated in other pandemics, including the 1918 and 2009 influenza pandemics.20 , 21 Individuals who identify as a minority race or ethnicity may have less of an opportunity to engage in public health prevention strategies owing to social and structural determinants of health. One example of this pertains to differences in occupations. According to the data from Bureau of Labor Statistics, racial or ethnic minority populations in the US workforce are overrepresented in essential industries. Nearly a quarter of employed Latino or Latina and black or African American workers are employed in service industry jobs as compared with 16% of non-Hispanic white workers.22 These workers may not be as readily able to practice risk-reducing social distancing behavior or work from home, increasing their likelihood of exposure to SARS-CoV-2. In addition, they may work within industries that are less likely to have benefits such as paid sick leave,22 a measure proven to mitigate contagion of viral respiratory illnesses.23 , 24 Alternatively, the number of living children may reflect a higher household density, independent of children themselves being a vector. This may inhibit ability of pregnant women to follow social distancing and isolate children infected with SARS-CoV-2. Finally, residential segregation by race or ethnicity may also contribute to disparities in SARS-CoV-2 prevalence.
These data also reinforce previous findings that SARS-CoV-2 infection cannot be reliably identified on the basis of symptomatic screening alone.25 , 26 Universal testing for pregnant women being admitted for labor should be considered in areas of high disease burden because symptomatic screening alone is insufficient to identify all women with SARS-CoV-2 infection.
Strengths and limitations
An important strength of this study is the large sample size with a relatively high prevalence of SARS-CoV-2 infection in our geographic region. However, this study is also subject to limitations. First, these data are limited to a single tertiary care center and may not be generalizable to other populations. Our data may differ from other institutions given the differences in patient populations between institutions. Future work in other settings may uncover other risk factors not observed in our cohort. Larger multicenter studies focused on pregnant women are an important next step in epidemiologic analyses. Second, SARS-CoV-2 PCR assays have a wide range of measured false-negative rates. A case report has been published that describes a negative nasopharyngeal SARS-CoV-2 reverse transcription (RT) PCR test followed by a positive SARS-CoV-2 RT-PCR using a bronchoalveolar lavage specimen in a pregnant woman.27 False-negative rates of 17% to 63% have been reported when using this test in the nonpregnant population.28 , 29 Although false-negative results would potentially reduce the order of magnitude of identified risk factors, they should not systematically bias our results. Next, this study uses the living children component of parity as a proxy for living children in the home and thus does not account for all social contexts, for example, women with children in foster care or children of other family members residing in the home. However, as these contexts are unlikely to systematically bias the associations observed and are epidemiologically uncommon, we do not think the use of this proxy substantially altered the true association. Finally, as symptoms were recorded in a designated form at the time of admission, the possibility remains that there are lapses in this recording system, and thus, women who are classified as asymptomatic did have atypical or mild symptoms or developed symptoms after their admission. Given the novel nature of the COVID-19 pandemic, not all information regarding the virus, disease presentation, or disease progression is known, and misclassification remains possible. This study spans a time frame of April and May 2020, a period of rapid dissemination of infection across Chicago7 and a time when school closures were common. Thus, these data may not necessarily be transposable to earlier or later epochs of the pandemic or in areas where other public health strategies were implemented.
Research implications
The identified association between having living children and SARS-CoV-2 infection augments growing concern that asymptomatic or mildly symptomatic children may contribute to disease spread. As pregnant women are a population with a disproportionate exposure to young children at home, future research should corroborate this association and evaluate interventions targeted for multiparous women, such as augmented public health messaging about hand washing and the utilization of masks to prevent airborne transmission.
Conclusions
This study reinforces the significant racial and ethnic disparities that exist in SARS-CoV-2 infections among pregnant women and the critical need for public health interventions to combat them. Currently, Chicago’s Racial Equity Rapid Response Team (RERRT) strives to address COVID-19–related disparities with targeted interventions.30 RERRT aims to increase testing in Southside Chicago, host virtual town halls in underserved neighborhoods, and overall lessen the burden that this unprecedented public health crisis has created for Chicago’s racial and ethnic minority groups. Similar community efforts focused on health equity will be important to attempt to mitigate the observed disparities.
In addition to recognizing the racial and ethnic disparities in identified SARS-CoV-2 infections, obstetrical clinicians must consider how changes in obstetrical care delivery for women diagnosed as having COVID-19 may disproportionately affect socially vulnerable or disadvantaged women.31 Awareness of the epidemiologic factors associated with SARS-CoV-2 infection in pregnancy and the corresponding disparities that exist is the requisite first step to improving health equity. The onus is on us to ensure it is not the only step.
Footnotes
This paper is part of a supplement that represents a collection of COVID-related articles selected for publication by the editors of AJOG MFM without additional financial support.
The authors report no conflict of interest.
Research reported in this publication was supported, in part, by the National Institutes of Health’s National Center for Advancing Translational Sciences (grant number UL1TR001422). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Cite this article as: Sakowicz A, Ayala AE, Ukeje CC, et al. Risk factors for severe acute respiratory syndrome coronavirus 2 infection in pregnant women. Am J Obstet Gynecol MFM 2020;2:100198.
References
- 1.World Health Organization Coronavirus disease (COVID-19) weekly epidemiological update and weekly operational update. 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports Available at:
- 2.Centers for Disease Control and Prevention Coronavirus Disease 2019 (COVID-19): Cases in the U.S. 2020. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html Available at:
- 3.Arons M.M., Hatfield K.M., Reddy S.C., et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/NEJMoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.New York State Office of Governor Andrew M. Cuomo. Amid ongoing COVID-19 pandemic, Governor Cuomo announces results of completed antibody testing study of 15,000 people showing 12.3 percent of population has COVID-19 antibodies [press release] https://www.governor.ny.gov/news/amid-ongoing-covid-19-pandemic-governor-cuomo-announces-results-completed-antibody-testing Available at:
- 5.Di Mascio D., Khalil A., Saccone G., et al. Outcome of coronavirus spectrum infections (SARS, MERS, COVID-19) during pregnancy: a systematic review and meta-analysis. Am J Obstet Gynecol MFM. 2020;2:100107. doi: 10.1016/j.ajogmf.2020.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.APM Research Lab The color of coronavirus: COVID-19 deaths by race and ethnicity in the U.S. https://www.apmresearchlab.org/covid/deaths-by-race Available at:
- 7.Illinois Department of Public Health COVID-19 statistics. 2020. https://www.dph.illinois.gov/covid19/covid19-statistics Available at:
- 8.American College of Obstetricians and Gynecologists Addressing health equity during the COVID-19 pandemic. 2020. https://www.acog.org/en/Clinical%20Information/Policy%20and%20Position%20Statements/Position%20Statements/2020/Addressing%20Health%20Equity%20During%20the%20COVID-19%20Pandemic Available at:
- 9.Ferguson N.M., Cummings D.A., Fraser C., Cajka J.C., Cooley P.C., Burke D.S. Strategies for mitigating an influenza pandemic. Nature. 2006;442:448–452. doi: 10.1038/nature04795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 11.Di Nardo M, van Leeuwen G, Loreti A, et al. A literature review of 2019 novel coronavirus (SARS-CoV2) infection in neonates and children. Pediatr Res [Epub ahead of print]. [DOI] [PubMed]
- 12.Xu Y., Li X., Zhu B., et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502–505. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai X., Ma Y., Li S., Chen Y., Rong Z., Li W. Clinical characteristics of 5 COVID-19 cases with non-respiratory symptoms as the first manifestation in children. Front Pediatr. 2020;8:258. doi: 10.3389/fped.2020.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.She J., Liu L., Liu W. COVID-19 epidemic: disease characteristics in children. J Med Virol. 2020;92:747–754. doi: 10.1002/jmv.25807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park Y.J., Choe Y.J., Park O., et al. Contact tracing during coronavirus disease outbreak, South Korea, 2020. Emerg Infect Dis. 2020 doi: 10.3201/eid2610.201315. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laurencin C.T., McClinton A. The COVID-19 pandemic: a call to action to identify and address racial and ethnic disparities. J Racial Ethn Health Disparities. 2020;7:398–402. doi: 10.1007/s40615-020-00756-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan J.F., Yuan S., Kok K.H., et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention Coronavirus disease 2019 (COVID-19). Health equity considerations and racial and ethnic minority groups. https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/racial-ethnic-minorities.html Available at:
- 20.Grantz K.H., Rane M.S., Salje H., Glass G.E., Schachterle S.E., Cummings D.A. Disparities in influenza mortality and transmission related to sociodemographic factors within Chicago in the pandemic of 1918. Proc Natl Acad Sci USA. 2016;113:13839–13844. doi: 10.1073/pnas.1612838113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention 2009 Pandemic influenza A (H1N1) virus infections --- Chicago, Illinois, April--July 2009. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5833a1.htm Available at: [PubMed]
- 22.U.S. Bureau of Labor Statistics Labor force characteristics by race and ethnicity. 2018. https://www.bls.gov/opub/reports/race-and-ethnicity/2018/home.htm Available at:
- 23.Heymann J., Raub A., Waisath W., et al. Protecting health during COVID-19 and beyond: a global examination of paid sick leave design in 193 countries. Glob Public Health. 2020;15:925–934. doi: 10.1080/17441692.2020.1764076. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S., Grefenstette J.J., Galloway D., Albert S.M., Burke D.S. Policies to reduce influenza in the workplace: impact assessments using an agent-based model. Am J Public Health. 2013;103:1406–1411. doi: 10.2105/AJPH.2013.301269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller E.S., Grobman W.A., Sakowicz A., Rosati J., Peaceman A.M. Clinical implications of universal severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) testing in pregnancy. Obstet Gynecol. 2020;136:232–234. doi: 10.1097/AOG.0000000000003983. [DOI] [PubMed] [Google Scholar]
- 26.Lokken E.M., Walker C.L., Delaney S., et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2020 doi: 10.1016/j.ajog.2020.05.031. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly J.C., Dombrowksi M., O’neil-Callahan M., Kernberg A.S., Frolova A.I., Stout M.J. False-negative COVID-19 testing: considerations in obstetrical care. Am J Obstet Gynecol MFM. 2020 doi: 10.1016/j.ajogmf.2020.100130. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Long C., Xu H., Shen Q., et al. Diagnosis of the coronavirus disease (COVID-19): rRT-PCR or CT? Eur J Radiol. 2020;126:108961. doi: 10.1016/j.ejrad.2020.108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li Y., Yao L., Li J., et al. Stability issues of RT-PCR testing of SARS-CoV-2 for hospitalized patients clinically diagnosed with COVID-19. J Med Virol. 2020;92:903–908. doi: 10.1002/jmv.25786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chicago. Office of the Mayor Mayor Lightfoot and the Racial Equity Rapid Response Team announce latest efforts to address racial and health disparities among minority communities. 2020. https://www.chicago.gov/content/city/en/depts/mayor/press_room/press_releases/2020/april/RERRTUpdate.html Available at:
- 31.Onwuzurike C., Meadows A.R., Nour N.M. Examining inequities associated with changes in obstetric and gynecologic care delivery during the coronavirus disease 2019 (COVID-19) pandemic. Obstet Gynecol. 2020;136:37–41. doi: 10.1097/AOG.0000000000003933. [DOI] [PubMed] [Google Scholar]


