The World Health Organization (WHO) considers symptomatic sickle cell disease (SCD) the most deadly genetic disease worldwide.1 Recently, the European Hematology Association (EHA) reported that due to migration, an increasing number of patients suffering from SCD are living in almost every European country.2 Often combined with blood transfusions, approved drugs are currently used to reduce the incidence and severity of pain crises, hemolysis and vaso-occlusive events (VOC). The main biological effect of the standard-of-care treatment hydroxyurea is induction of fetal hemoglobin (HbF) synthesis.3 Several new compounds with different modes of action have been evaluated and licensed recently including L-Glutamine, that decreases red blood cell (RBC) oxidative injury4; Voxelotor, that elevates the oxygen affinity to Hb, thus stabilizing the RBC in the oxygenated state, thereby preventing HbS fiber formation5 and Crizanlizumab, an anti-P-selectin antibody targeting adhesion of sickled RBCs to endothelium and activation of platelets in VOCs.6
Earlier we reported that intracellular Ca2+ concentration in human RBCs depends on the abundance and activity of N-methyl D-aspartate receptors (NMDARs) that are present on the RBC's membrane.7 These non-selective cation channels are activated when stimulated upon exposure to glutamate (homocysteine, homocysteic acid) and glycine as well as by mechanical stimuli in a variety of cell types including RBCs. Their activation results in a transient excess of cellular Ca2+ uptake.7,8 NMDARs are essential for cytoprotection and during maturation, of erythroid precursors the number or receptors drops from hundreds of thousands in a single proerythroblast to as little as 30 in young healthy RBCs.9,10 Inhibition of NMDARs using the channel inhibitor memantine (an approved anti-Alzheimer drug) resulted in an acute decrease in intracellular free Ca2+ levels in RBCs of healthy volunteers and SCD patients.7,9 Cells of SCD patients responded to NMDAR inhibition with rehydration, sickling reduction upon deoxygenation and with amelioration of oxidative stress. Based on these ex vivo findings, we hypothesized that NMDARs in the RBCs of SCD patients are a potential target for pharmacological intervention. Memantine represents a second generation NMDAR inhibitors combining safety and low price and is used to treat dementia, autism and other psychiatric conditions in adults, adolescent and children.11,12
In this proof-of-principle clinical phase 2 study termed MemSID, 6 SCD patients (Fig. 1A) were enrolled and four treated for up to 44 weeks with 5 to 20 mg memantine (clinicaltrials.gov: NCT02615847). The primary objective was to test for safety and tolerability of the drug by SCD patients. The impact of the therapy on quality of life (QoL) and major hematological parameters were secondary objectives. The study was divided into 5 periods (Fig. 1C): (i) screening (up to 4 weeks), (ii) up-titration (4 weeks), (iii) treatment with 20 mg memantine (41–44 weeks), (iv) down-titration (3 weeks) and (v) follow-up period (8 weeks). The planned total duration of the study was 56 to 59 weeks. For patient eligibility and study design see Supplementary Data. From the nine intended patients, 2 could not be enrolled because of recent transfusions and one withdrew before treated started (Fig. S1). All 6 patients had a HbSS phenotype. For further information concerning demographics, characteristics and co-medication with hydroxyurea (see Fig. 1A). Two of 6 patients discontinued the study due to an adverse event (AE; dizziness/vertigo, P6) and a severe AE (SAE; psychosis, P5, see below and Fig. 1D). Memantine intake was monitored by determining plasma levels (Fig. 1B). After up-titration memantine levels were in a range of 30 to 90 ng/ml reflecting overall good compliance by the patients with three exceptions that are mentioned below.
Figure 1.
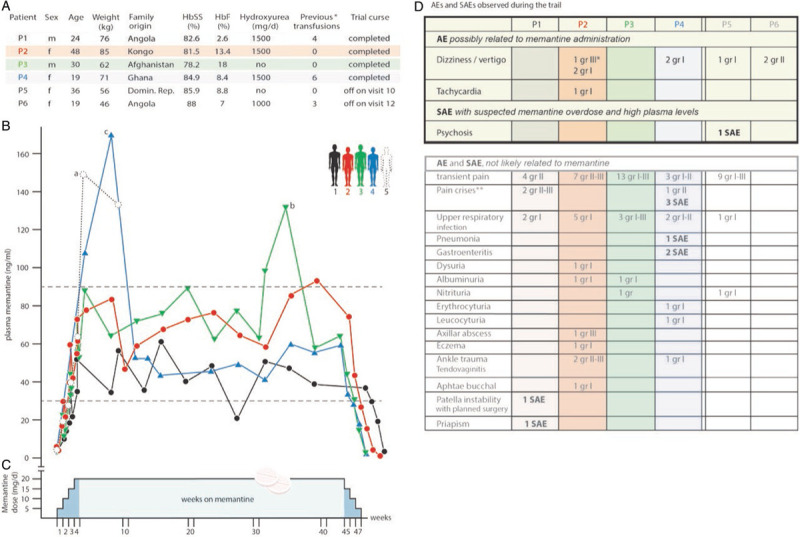
Study design, information on the patients and plasma memantine pharmacokinetics. A. Demographics of the SCD patients and characteristics at enrolment. Sex, age, weight and family origin as well as the corresponding percentage of HbS and HbF measured during the screening period of patients P1-6 are shown. All patients had the HbSS phenotype. ∗number of previous transfusions that applied 9 to 12 months before enrolment. B. Plasma memantine pharmacokinetics during the study periods. The analysis of the memantine concentration in the plasma of the patients revealed a good compliance during the trial with the following exceptions: overdosing by the patient P5 (a) led to psychosis (SAE) and the trial was terminated. Overdosing by P3 (b) and P4 (c) caused reversible increase in plasma memantine levels without discontinuation. Note that the memantine levels found in P6 who stopped the trail at week 5 was within the normal range (not shown). C. Schematic representation of the trial periods. The study was divided into five periods as described in the Supplementary Data. D. Adverse Effects (AE) and Severe Adverse Effects (SAE). The upper part of the table shows the AE and SAE that were attributed to memantine intake, while the lower part reflects the AE and SAE that were considered as not likely related to memantine. ∗Dizziness/vertigo led to a transient dose reduction (10 mg per day for 10 days) that was increased again to 20 mg after cessation of this symptom. ∗∗A pain crisis was defined as ongoing pain lasting for longer than 4 hours (shorter pain periods are defined as “transient pain”) and requiring the application of opiates or treatment at the out patients clinic or emergency department for less than 12 hours.
Figure 1D shows all AEs and SAEs of all 6 patients that started the trial. Dizziness/vertigo and tachycardia were judged as AEs possibly related to memantine, whereas dizziness/vertigo was found to be the most common symptom in 4 out of 6 patients (Fig. 1D, top). Accordingly, memantine dose was adjusted in 2 patients. One adjustment (P2 with grade III symptoms of dizziness/vertigo) was transient, having no further consequence on the patient's participation in the trial, while P6 (grade II dizziness/vertigo) withdrew from the study. Of note, P6 retrospectively reported suffering from dizziness even before the trial. The only observed SAE possibly related to memantine was an acute psychosis that occurred in P5 and consequently, memantine therapy was stopped immediately. Post-study analysis of plasma memantine revealed that this symptom was timely associated with very high plasma memantine levels reaching values of 155 and 137 ng/ml at week 5 and 9, respectively (Fig. 1B, marked a), before withdrawing from the trial at week 11. This patient recovered completely without rebound effects. We observed 2 additional elevations in plasma memantine levels that did not lead to psychological changes. P3 increased drug intake on his own during a pain crisis (Fig. 1B, marked b) and P4 reached similar high memantine plasma levels by accidentally taking double doses at the beginning of the study (Fig. 1B, marked c).
More AEs and SAEs were recorded but rated as not likely related to treatment (Fig. 1D, bottom). Of these AEs some were disease-related like transient pain, pain crises for over 4 hours, as well as urine analysis abnormalities and also infectious complications. More non memantine-related SAEs were patellar instability (with planned surgery) and disease-related priapism in P1 as well as severe pain crises, pneumonia and gastroenteritis in P4. Again, only one SAE (psychosis due to suspected overdose) was likely related to memantine. Two transfusion episodes were required during the study, one in the case of patient P1 before planned surgery and the second (2 units) in the case of P4 during pain crisis (1 unit). Knee surgery in P1 was associated with interruption of blood supply in the extremity, but the following hypoxemia did not lead to pain crises.
Patients’ QoL was continuously scored throughout the trial and the total score is summarized for each individual patient in Figure S2. Pain crisis and infections were found to be the most important factors that influenced the scoring of the QoL. Three out of 4 patients showed a tendency of improvement in total QoL score: patient P1 and P2 (the latter upon reaching the full memantine dose) reported steady improvement during the trail. P3 started with a good QoL score and suffered from pain crises at week 33 (upon which he increased his memantine dose). Finally, P4 suffered from repetitive gastrointestinal infections and pneumonia that triggered hospitalizations (e.g., SAEs) after intercontinental flights (week 20 and 25).
RBC count remained stable during all phases (Fig. 2, Table S2). Regression analysis (considering blood transfusion and infection as control variables) showed no significant changes (Table S1). Hb concentrations were more variable and showed a minor reduction between screening and follow-up phase with −5.85 g/l (95%-CI: −11.5, −0.202 g/l; p value: 0.043). In P1 reticulocyte number decreased, while in P2 and P4 it remained stable, and in P3 it decreased when entering the follow-up phase (Fig. 2). LDH activity decreased during the treatment period but the overall level still remained elevated. Only P1 showed a major reduction of bilirubin.
Figure 2.
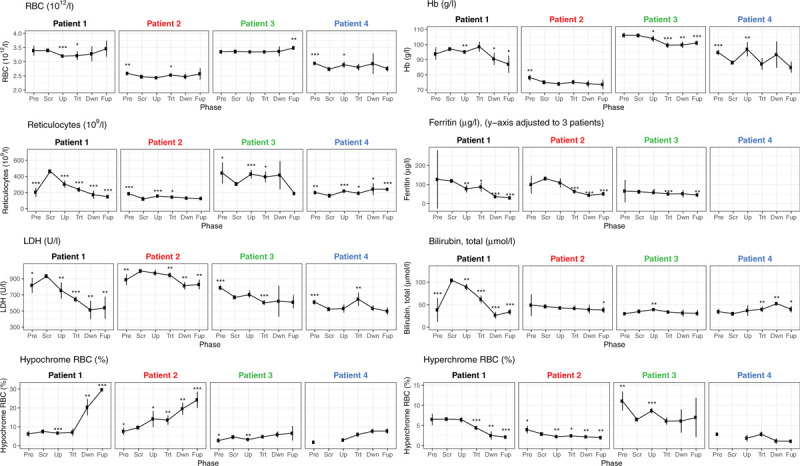
The impact of memantine on some RBC-related parameters. Means and 95%-CIs of RBC count, Hb, reticulocyte count, lactate dehydrogenase (LDH) activity, ferritin and total bilirubin in plasma as well as percentage of hypochromic and hyperchromic RBC isolated from patients 1 to 4 before enrolment and during the trial are shown. Note that the ferritin values for P4 reach levels around 1500 μg/l and thus are not shown here but appear in Table S2. Screening has no 95%-CI since there is only one measurement. Symbols (dot, ∗,∗∗,∗∗∗ meaning p < 0.1, p < 0.05, p < 0.01, p < 0.001, respectively; values are compared with screening value using Welch t test, not adjusted.). Periods: Pre = pre-screening, Scr = screening, Up = up-titration, Trt = treatment, Dwn = down-titration, Fup = follow-up.
Plasma ferritin levels of patients P1, P2, and P3 were within the normal range, for example, 62–131 μg/l (Fig. 2, Table S2), whereas patient P4 started already with elevated iron storage and plasma ferritin level >1500 mcg/l (not shown in Fig. 2 because out of scale but reported in Table S2). Patients P1, P2, and P3 responded to memantine treatment with a decrease in plasma ferritin (from regression analysis: screening phase 98.9 μg/l to a fraction of 42% (95%-CI: 29%–61%; p value < 0.001) of screening phase estimate) (Table S1: P1, P2, and P3).
In agreement with ex vivo findings on rehydration of RBC upon memantine treatment9 we observed an increase in hypochrome and a decrease in hyperchrome RBC abundance in all four patients (Fig. 2). P1 showed an increase of hypochrome RBC from 7.5% to 29.7% (95%-CI: 29.0, 30.3%) and P2, P3 and P4 from 9.6, 4.6 and 2.0% to 24.3% (95%-CI: 20.1, 28.5%), 6.6% (95%-CI: 2.8, 10.4%) and 7.8% (95%-CI: 6.5, 9.1%), respectively (Fig. 2 and Table S2). Dehydration of RBCs of SCD patients facilitates formation of HbS fibers that damage the cell membrane and compromise deformability promoting hemolysis and VOCs. Earlier, application of the Gardos channel blocker Senicapoc that interferes with RBC's water balance by retention of intracellular K+ was not found to reduce pain and VOCs.13 Memantine, acting upstream from the inhibition of Ca2+-dependent Gardos channels, might have the potential to reduce VOCs.
In conclusion, our study on memantine in SCD patients demonstrated comparable safety profile as described before for memantine in Alzheimer‘s disease.11 All side effects were transient and manageable and we observed a tendency to improve QoL. More in depth analysis of memantine's impact on RBC characteristics is published elsewhere.14 Our study is not powered to make any final conclusions on efficacy but the obtained results on four patients that completed the study for one year served as a precedent to conduct a larger trial including young adults and adolescents that is now ongoing. Assuming that memantine reduces SCD symptoms, this drug – in combination with other already approved compounds – has a very cost effective potential.
Sources of Funding
The present work has been partially supported by the following foundations: Baugarten Zürich Genossenschaft und Stiftung, the Ernst Goehner Stiftung, the René und Susanna Braginsky Stiftung, the Stiftung Symphasis and the Botnar Foundation. We are also grateful to the Foundation for Clinical Research Hematology for supporting the clinical trail at the Division of Hematology, University Hospital Zurich.
Disclosures
The authors have no conflicts of interest to disclose. The University of Zurich holds the patent to use memantine against sickle cell disease.
Supplementary Material
Footnotes
Citation: Hegemann I, Sasselli C, Valeri F, Makhro A, Müller R, Bogdanova A, Manz MG, Gassmann M, Goede JS. MEMSID: Results from a Phase 2 Pilot Study on Memantine Treatment for Sickle Cell Disease. HemaSphere, 2020;4:4(e452). http://dx.doi.org/10.1097/HS9.0000000000000452
Max Gassmann and Jeroen S. Goede are senior authors and contributed equally.
Clinical trial to study the safety and tolerability of Memantin Mepha® in Sickle Cell Disease Patients (MemSID, NCT02615847).
References
- 1.Piel FB, Patil AP, Howes RE, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381:142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iolascon A, De Franceschi L, Muckenthaler M, et al. EHA research roadmap on hemoglobinopathies and thalassemia: an update. HemaSphere. 2019;3:e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McGann PT, Ware RE. Hydroxyurea therapy for sickle cell anemia. Expert Opin Drug Saf. 2015;14:1749–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niihara Y, Miller ST, Kanter J, et al. A phase 3 trial of l-glutamine in sickle cell disease. N Engl J Med. 2018;379:226–235. [DOI] [PubMed] [Google Scholar]
- 5.Vichinsky E, Hoppe CC, Ataga KI, et al. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381:509–519. [DOI] [PubMed] [Google Scholar]
- 6.Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevetntion of pain crises in sickle cell disease. N Engl J Med. 2017;376:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makhro A, Hanggi P, Goede JS, et al. N-methyl D-aspartate (NMDA) receptors in human erythroid precursor cells and in circulating red blood cells contribute to the intracellular calcium regulation. Am J Physiol Cell Physiol. 2013;305:C1123–C1138. [DOI] [PubMed] [Google Scholar]
- 8.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. [DOI] [PubMed] [Google Scholar]
- 9.Hanggi P, Makhro A, Gassmann M, et al. Red blood cells of sickle cell disease patients exhibit abnormally high abundance of N-methyl D-aspartate receptors mediating excessive calcium uptake. Br J Haematol. 2014;167:252–264. [DOI] [PubMed] [Google Scholar]
- 10.Hanggi P, Telezhkin V, Kemp PJ, et al. Functional plasticity of the N-methyl-d-aspartate receptor in differentiating human erythroid precursor cells. Am J Physiol Cell Physiol. 2015;308:C993–C1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kavirajan H. Memantine: a comprehensive review of safety and efficacy. Expert Opin Drug Saf. 2009;8:89–109. [DOI] [PubMed] [Google Scholar]
- 12.Rossignol DA, Frye RE. The use of medications approved for Alzheimer's disease in autism spectrum disorder: a systematic review. Front Pediatr. 2014;2:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ataga KI, Reid M, Ballas SK, et al. Improvements in haemolysis and indicators of erythrocyte survival do not correlate with acute vaso-occlusive crises in patients with sickle cell disease: a phase III randomized, placebo-controlled, double-blind study of the Gardos channel blocker senicapoc (ICA-17043). Br J Haematol. 2011;153:92–104. [DOI] [PubMed] [Google Scholar]
- 14.Makhro A, Hegemann I, Seiler E., et al. A pilot clinical phase II trial MemSID: Acute and durable changes of red blood cells of sickle cell disease patients on memantine treatment. eJHaem. 2020;190:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


