Abstract
In late 2019, an outbreak of a novel human coronavirus causing respiratory disease was identified in Wuhan, China. The virus spread rapidly worldwide, reaching pandemic status. Chest computed tomography scans of patients with coronavirus disease-2019 (COVID-19) have revealed different stages of respiratory involvement, with extremely variable lung presentations, which require individualized ventilatory strategies in those who become critically ill. Chest physiotherapy has proven to be effective for improving long-term respiratory physical function among ICU survivors. The ARIR recently reported the role of chest physiotherapy in the acute phase of COVID-19, pointing out limitation of some procedures due to the limited experience with this disease in the ICU setting. Evidence on the efficacy of chest physiotherapy in COVID-19 is still lacking. In this line, the current review discusses the important role of chest physiotherapy in critically ill mechanically ventilated patients with COVID-19, around the weaning process, and how it can be safely applied with careful organization, including the training of healthcare staff and the appropriate use of personal protective equipment to minimize the risk of viral exposure.
Keywords: Chest physiotherapy, COVID-19, SARS-CoV-2, Mechanical ventilation, Extubation
1. Introduction
In late 2019, an outbreak of respiratory disease caused by a novel human coronavirus (SARS-CoV-2) was identified in Wuhan, China. The infection, now known as coronavirus disease 2019 (COVID-19), spread rapidly worldwide, and on March 11, 2020, was characterized as a pandemic by the World Health Organization (Wu et al., 2020). The classical routes of infection for SARS-CoV-2 are through respiratory droplets and by human-to-human contact. Within a few days of infection, a mild febrile illness appears, with dry cough and moderate to severe respiratory distress. At intensive care unit (ICU) admission, most COVID-19 patients present with hypoxemic respiratory failure (Wu et al., 2020), as first step of an awful systemic disease (MODS-CoV-2) (C. Robba et al., 2020b). Chest CT scans have revealed different stages of respiratory involvement in COVID-19, making lung presentations extremely variable (Li and Xia, 2020). Thus, different ventilatory strategies may be required for different patients, including early chest physiotherapy (CPT) and rehabilitation. CPT maneuvers are considered essential in patient management during ICU stay in general (Thomas et al., 2020). This also applies to COVID-19 patients, as suggested in a recent paper by the Italian Association of Respiratory Physiotherapists (ARIR) (Lazzeri et al., 2020). Early mobilization and rehabilitation may help prevent or mitigate sequelae related to bed rest, thus improving physical function and outcomes and reducing length of stay by increasing ventilator free-days (Kayambu et al., 2013). As this is an extremely new topic, the role of CPT in critically ill patients with SARS-CoV-2 infection requires investigation (Thomas et al., 2020). Recent manuscripts on respiratory physiotherapy in COVID-19 patients provided general recommendations but did not focus on critically ill COVID-19 cases (Lazzeri et al., 2020; Thomas et al., 2020). The role of respiratory rehabilitation was reported in three groups of COVID-19 patients (Vitacca et al., 2020): 1) acute phase, presenting with critical respiratory impairment (emergency department, first aid, ICU, stepdown unit); 2) acute phase, with severe respiratory impairment (internal medicine, respiratory, infectious disease, or other wards); and 3) post-acute phase (other units, intermediate care facilities, subacute wards). The current review discussed the data regarding the important role of chest physiotherapy in critically ill patients with COVID-19, during mechanical ventilation and after weaning process, and how it can be safely applied with careful organization, including the training of healthcare staff and the appropriate use of personal protective equipment to minimize the risk of exposure to SARS-CoV-2.
2. Respiratory characteristics of COVID-19 and its management
2.1. Characteristics
Respiratory characteristics of severe COVID-19 include hypoxemia and acute respiratory failure. COVID-19 is associated with peculiar characteristics in terms of respiratory mechanics, with relatively well preserved, high or low respiratory system compliance(Li and Xia, 2020). Additionally, chest CT scans of COVID-19 patients have revealed distinct patterns of pulmonary involvement: 1) a multifocal, overperfused ground-glass phenotype, with centrilobular nodules, patchy consolidation, and intra-bronchial air bronchogram; 2) dilatation and congestion of septal capillaries, followed by exudation into the alveolar space with interstitial edema; 3) vascular exudation in the interstitium, with consolidations filled by air bronchogram; 4) fibrous exudation with multiple consolidations; and 5) thickening of bronchial walls, the interlobular septum, and patchy consolidations (Li and Xia, 2020). This explains why COVID-19 patients present with an extremely variable clinical course, and why individualized ventilatory strategies are required (C. Robba et al., 2020a). Accordingly, distinct phenotypes based on both clinical and CT characteristics have recently been identified, as follows: 1) Phenotype-1/L-type: high or normal lung compliance associated with severe hypoxemia. This phenotype is characterized by multiple focal, overperfused ground-glass opacities. Treatment should include tidal volume of 6–8 mL/kg predicted body weight (PBW) and low to moderate positive end-expiratory pressure (PEEP) to redistribute pulmonary blood flow and shunt; 2) Phenotype-2/L-type: predominantly characterized by inhomogeneously distributed atelectasis, as well as peribronchial opacities, with hyperperfused ground glass areas. Treatment should include tidal volumes of 6 mL/kg PBW and moderate-to-high PEEP, as well as lateral or prone positioning; 3) Phenotype-3/H-type: patchy acute respiratory distress syndrome (ARDS)-like appearance, characterized by alveolar edema and low compliance. Treatment should follow standard ARDS guidelines. Steroids, prone positioning, and extracorporeal membrane oxygenation (ECMO) can be considered for the most severe cases (C. Robba et al., 2020a; Gattinoni et al., 2020).
In summary, conventional ARDS is characterized by diffuse alveolar capillary membrane damage, with edema and atelectasis in the dependent lung regions. Application of PEEP or prone position, recruits collapsed lung regions associated with improvement in respiratory mechanics and gas-exchange, while no major changes in redistribution of regional pulmonary perfusion. On the contrary in COVID-19, lesions are compartmentalized with less edema and pneumolysis, alveolar cell infiltration and necrosis. Application of PEEP or prone position result in redistribution of perfusion, but not alveolar recruitment. In this sense, COVID-19 pneumonia represents a “true” primary ARDS, as defined previously (Rocco and Pelosi, 2008).
2.2. Ventilatory management
At the beginning of the pandemic, management of COVID-19 was based on classification of the respiratory involvement as ARDS-like (C. Robba et al., 2020a), thus consisting of low tidal volume (VT; 6 mL/kg PBW) and plateau pressure (<30 cmH2O), with high PEEP (Fan et al., 2017). Initial guidelines for the management of COVID-19 patients (Alhazzani et al., 2020) corroborated this strategy, recommending low-VT ventilation (4−8 mL/kg PBW) with PEEP levels titrated according to peripheral oxygen saturation (SpO2). However, this should only be applied to patients with ARDS-like COVID-19 (C. Robba et al., 2020a; Gattinoni et al., 2020). Continuous positive-pressure ventilation (CPAP) or non-invasive ventilation (NIV) with vigorous breathing effort may be detrimental in COVID-19, as it could increase the risk of patient self-inflicted lung injury (P-SILI) (Telias et al., 2020). In fact, as suggested by Gattinoni et al. (Gattinoni et al., 2020), longer periods with non-invasive ventilatory supports should be avoided and intubation prioritized in order to prevent the development of P-SILI, which may worsen lung damage. Additionally, in non-intubated patients, an esophageal balloon should be inserted to maintain the pressure below 15 cmH2O, thus limiting the risk of P-SILI. On the other hand, caution should be adopted with early intubation since endotracheal intubation and mechanical ventilation may also lead to lung damage. Therefore, whether to choose early intubation or not should be carefully weighed (Tobin et al., 2020). The cardiac abnormalities reported in COVID-19 might be distinguished based on patient’s phenotype. In the phenotype-1/L-type, the right heart impairment is estimated to be less evident than in the phenotype-3/ H-type due to the expected lower ventilatory pressures and tidal volumes delivered to the lungs (Guarracino et al., 2020). Following increased respiratory effort, phenotype-1/ l-type may reduce stroke volume as a result of ventricular interdependence with consequent diastolic ventricular septal shift. Additionally, high pressures and tidal volumes applied on a poorly recruitable lung, such as observed in this phenotype, may yield haemodynamic instability and fluid retention (Gattinoni et al., 2020). On the other hand, when positive-pressure ventilation is applied on a phenotype-3/H-type, ventricular dilatation, tricuspid insufficiency, reduced right-heart systolic function, and left-heart compression may occur, determining the so called “ventilator-induced heart dysfunction” (Guarracino et al., 2020).
The need for recruitment maneuvers (RMs) should be individualized on the basis of each patient’s phenotype, since several cases of impaired shunt fraction or poor lung recruitability have been identified (C. Robba et al., 2020a). Traditional RMs are preferred instead of an incremental PEEP strategy (Alhazzani et al., 2020). Prone positioning, which redistributes pulmonary blood flow and alveolar ventilation to improve gas exchange, may be considered in the management of mechanically ventilated critically ill COVID-19 patients (Guérin et al., 2013). Evidence of the efficacy of prone positioning in COVID-19 is still lacking, although clinical knowledge suggests reserving this strategy only for those subtypes of patients which should benefit based on chest CT findings (Li and Xia, 2020) (C. Robba et al., 2020a). However, performing a CT-scan in each patient became unfeasible due to the high turnout of patients (up to 60–80 affected patients every day) during the peak of pandemic, limiting the specific phenotype diagnosis and subsequent therapeutic choices. Therefore, clinical analysis, chest-X-ray and lung ultrasound (LUS) are regarded as better options to assess COVID-19 phenotypes at bedside (Cosentini, 2020). The sensitivity and specificity of LUS in COVID-19 patients remain to be determined. Four basic patterns at LUS have been identified: 1) normal pattern: A-lines and <3 B-lines; 2) mild disease: ≥3 B-lines with some confluents and thickened pleura (phenotype 1–2 and l-type); 3) B-lines with broken pleural line; 4) typical ARDS pattern with subpleural consolidation (phenotype 3 and H-type). LUS cannot be considered as a substitute of CT-scan but can be a valid option when CT-scan is difficult to be done (Denault et al., 2020).
Prone positioning has also been used in small cohorts of awake COVID-19 patients during spontaneous or assisted breathing. Among 24 patients in one study, 15 tolerated the prone position for more than 1 h, of whom only six showed increased oxygen saturation, and half of them returned to baseline levels after supine positioning (Elharrar et al., 2020). Larger randomized controlled trials are underway to elucidate whether prone positioning during spontaneous and assisted breathing can be used to reduce the intubation rate (Antonelli, 2020; Al-Hazzani, 2020). If beneficial, it may be further considered as a novel respiratory physiotherapy strategy for awake patients with COVID-19 and ARDS. Finally, a substantial number of COVID-19 patients are able to start the weaning process. Traditional criteria for extubation are considered suitable for COVID-19 patients. Patients who might be eligible for a spontaneous breathing trial should receive CPT before and after extubation, since improved outcomes have been observed in patients who underwent respiratory physiotherapy around extubation time (Lazzeri et al., 2020; Thomas et al., 2020). NIV, CPAP, and high-flow nasal oxygen (HFNO) should also be considered for short periods after extubation, until complete respiratory autonomy is reached (Lazzeri et al., 2020).
3. Chest physiotherapy for mechanically ventilated COVID-19 patients
Physiotherapy has proven effective for improving long-term physical function among ICU survivors (Calvo-Ayala et al., 2013). However, the true benefit of chest physiotherapy in ICU remains controversial, especially in those patients with already established alveolar damage (Lazzeri et al., 2020; Thomas et al., 2020). The ARIR recently published a position paper concerning the role of chest physiotherapy in COVID-19 patients (Lazzeri et al., 2020), suggesting limitation of some procedures—such as diaphragmatic breathing, bronchial hygiene, lung re-expansion techniques, manual mobilization, respiratory muscle training, nasal washing, and exercise training—in the acute phase of the illness. The literature suggests that physiotherapy maneuvers result in significant changes in respiratory function (Cerqueira-Neto et al., 2013), as well as in changes of cardiovascular and cerebral hemodynamic (Cerqueira-Neto et al., 2013), which could lead to potentially harmful effects.
Physiotherapy for critically ill patients in general, in critical and post-critical illness, is based on a multisystem approach which comprises not only chest physiotherapy but also musculoskeletal rehabilitation, in order to reduce the incidence of complications, encourage weaning from mechanical ventilation, and facilitate recovery of functional autonomy (Thomas et al., 2020). Few literature is available on physiotherapy during COVID-19 pandemic, especially regarding chest physiotherapy in ICU patients (Lazzeri et al., 2020) (Simonelli et al., 2020)
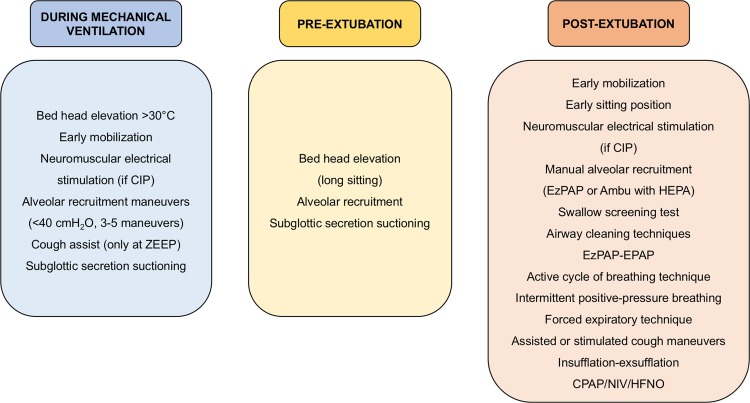
Conventional chest physiotherapy maneuvers for critically ill patients in general include airway clearance techniques, lung re-expansion through RMs, patient-ventilator interactions, inhalational therapies, humidification, and tracheostomy and bronchial aspiration (Yang et al., 2013). Other equally efficient methods, which can replace these techniques, have been recently identified and introduced in clinical practice. Since the severe pulmonary illness associated with COVID-19 can lead to long-term mechanical ventilation with a high ICU mortality rate, we believe that early physiotherapy and mobilization may be essential for improving outcomes. In the following paragraphs, we describe the chest physiotherapy maneuvers applied in COVID-19 patients in our ICU and their rationale. Fig. 1 summarizes the physiotherapy techniques currently applied in our ICU in COVID-19 patients.
Fig. 1.
Genoa−COVID-19 algorithm for respiratory physiotherapy.
Chest physiotherapy techniques commonly used in our COVID-19 unit during mechanical ventilation, before and after extubation. CIP, critical illness polyneuropathy; ZEEP, zero PEEP; PEEP, positive end-expiratory pressure; HEPA, exhalation/expiratory filter; EPAP, expiratory positive airway pressure; CPAP, continuous positive airway pressure; HFNO, high flow nasal oxygen; NIV, non-invasive ventilation.
3.1. Chest physiotherapy during mechanical ventilation
Early physiotherapy, i.e., started during mechanical ventilation, is considered feasible and safe to improve patient performance and long-term quality of life (Kayambu et al., 2013), although this has not yet been proven in COVID-19. Among chest physiotherapy strategies during mechanical ventilation, mucus clearance and alveolar RMs are very commonly applied in clinical practice. Sputum production was reported in about 34 % of COVID-19 patients (Guan et al., 2020), thus suggesting that, by promoting mucus clearance during mechanical ventilation, early physiotherapy interventions (such as subglottic secretion drainage, postural hygiene, and ventilator hyperinflation) may produce beneficial effects in this new critically ill population (Thomas et al., 2020). Before starting chest physiotherapy, we recommend the use of adequate personal protective equipment, limiting healthcare workers in the room to one physician and one physiotherapist, as well as choosing a negative-pressure chamber if available (Lazzeri et al., 2020; Thomas et al., 2020).
3.1.1. Alveolar recruitment
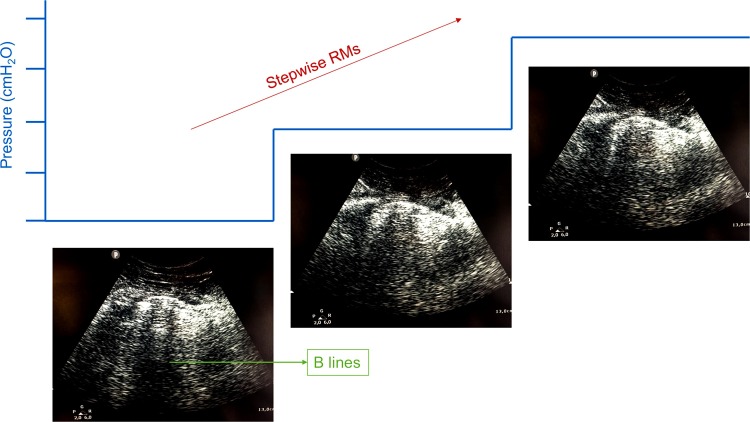
RMs are transient increases in transpulmonary pressure that may open non-aerated or poorly aerated areas of the lung, while concomitantly increasing the risk of endothelial-cell damage and increased capillary permeability (Silva et al., 2016). Although alveolar recruitment can be obtained through a variety of techniques during mechanical ventilation in critically ill patients in general, whether alveolar RMs should be used at all has been widely debated. In experimental ARDS, “slow” RMs showed a more homogeneous inflation of the lung and led to functional impairment with less ventilator-induced lung injury (VILI) as compared to “fast” RMs (Silva et al., 2013). In a large, multicenter, randomized controlled trial of ARDS patients, a strategy based on lung RMs and PEEP titration according to the best respiratory system compliance resulted in increased 28-day all-cause mortality than a low-PEEP strategy (Cavalcanti et al., 2017), thus suggesting this type of recruitment is best avoided. Different respiratory phenotypes of COVID-19 have been identified (C. Robba et al., 2020a; Gattinoni et al., 2020). As suggested above, not all phenotypes can benefit from RMs (C. Robba et al., 2020a). We suggest the use of LUS as well as monitoring of the partial pressure of oxygen during RMs to identify COVID-19 patients who are responsive to alveolar recruitment, as suggested in the literature in critically ill patients in general (Tusman et al., 2016). Fig. 2 shows the use of lung ultrasound to evaluate RM in a COVID-19 patient.
Fig. 2.
Stepwise recruitment manoeuvres and lung ultrasound.
Results of chest physiotherapy evaluated by lung ultrasound. The figure represents a stepwise recruitment manoeuvres (RM) at different positive end-expiratory pressure (PEEP) levels that allowed to recruit atelectatic areas of a COVID-19 patient.
3.1.2. Drainage of subglottic secretions
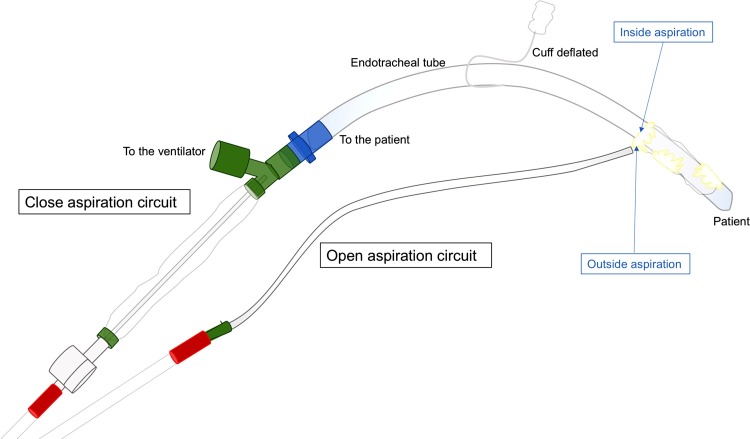
In intubated and mechanically ventilated critically ill patients in general, the tracheal tube completely bypasses the larynx, facilitating passage of microbes to the lower respiratory tract, which can lead to nosocomial infections such as ventilator-associated pneumonia (VAP) and tracheobronchitis (Li Bassi et al., 2018). To date, one of the most fearsome complications of SARS-CoV-2 infection is secondary bacterial infection. In a retrospective single-center study, bacterial infections were found in 43 % of elderly patients infected with SARS-CoV-2, and were a strong predictor of overall risk of death (Wang et al., 2020). Similar findings were reported in a case series of COVID-19 patients (Dong et al., 2020). Subglottic secretion drainage (SSD) has been proposed in critically ill patients in general to reduce the risk of VAP (Lacherade et al., 2018). According to a recent meta-analysis of 20 randomized controlled trials (Mao et al., 2016), SSD reduced VAP incidence and shortened the duration of mechanical ventilation in four clinical trials, whereas no differences were found for ICU length of stay or for in-hospital and ICU mortality. Moreover, the use of endotracheal tubes with an incorporated polyurethane cuff and SSD maneuvers helped reduce the risk of early- and late-onset VAP as compared to traditional care (Lorente et al., 2007). The safety of SSD also remains controversial. Although SSD reduced VAP incidence, a recent meta-analysis of 17 randomized controlled trials found no benefits in terms of duration of mechanical ventilation, ICU length of stay, ventilator-associated events, or antibiotic use (Caroff et al., 2016), raising further controversy as to the use of this technique. Although the literature is not conclusive concerning the real clinical benefits of SSD in COVID-19, we believe that a strategy based on early physiotherapy (including SSD) may reduce the risk of secondary pulmonary infections. Nevertheless, the only available paper on CPT in COVID-19 does not suggest that this technique should be started too early, and explains that SSD should be performed only under a closed aspiration circuit in order to limit droplet dispersion and avoid PEEP loss (Lazzeri et al., 2020). Based on our direct experience with respiratory physiotherapy in our ICU, we propose a novel method to assess this maneuver and reduce the risk of aerosol dispersion. In brief, we perform SSD by reducing the endotracheal cuff pressure, thus providing subglottic aspiration with a closed-aspiration circuit, while simultaneously aspirating the oral cavity with another circuit. In our experience, this technique limits airborne dispersion and ensures complete SSD. This technique is depicted in Fig. 3 .
Fig. 3.
Genoa−COVID-19 subglottic secretion drainage novel technique.
Genoa−COVID-19 subglottic secretion drainage technique using a mixture of closed aspiration circuit and open aspiration circuit to minimize airborne dispersion.
3.1.3. Postural drainage
While postural drainage has been abandoned because it requires a considerable time investment and provides only minor clinical benefit, patient positioning is still considered an optimal and quick technique to mobilize secretions and increase lung volumes, perfusion, and oxygenation (Li Bassi et al., 2017a). Critically ill patients are at high risk of nosocomial infections, and the aspiration of mucus from the endotracheal tube cuff to the lower respiratory tract is the main mechanism for the development of ventilator-associated infections (Li Bassi et al., 2017a). Patient positioning has been identified as a major contribution to nosocomial infections. In a large, multicenter randomized controlled trial in critically ill patients in general, the incidence of VAP was 0.5 % in the lateral Trendelenburg position and 4% in the semi-recumbent position (head of bed elevated to 30−45° above horizontal plane), whereas no differences were found in terms of 28-day mortality and other secondary outcomes (Li Bassi et al., 2017b). Although no differences in outcome were found between the two groups in this study, the semi-recumbent position may increase the hydrostatic pressure exerted by bacteria around the endotracheal cuff, thus facilitating gravitational pulmonary aspiration (Li Bassi et al., 2017a). Another study which compared the semi-recumbent position and supine positions did not find any differences in outcome (Van Nieuwenhoven et al., 2006). Finally, a recent meta-analysis of 10 randomized controlled trials compared the semi-recumbent and supine positions, concluding that a higher head position (30−60°) reduces the risk of VAP (Wang et al., 2016). As in ARDS, some COVID-19 patients require prone positioning to homogenize lung perfusion and improve ventilation/perfusion mismatch (Gattinoni et al., 2020). Prone positioning may reduce the risk of VAP by a still-unclear mechanism, which may involve prevention of lung translocation of oropharyngeal pathogens and easier drainage of respiratory secretions (Li Bassi et al., 2017a). In a meta-analysis of 1066 ARDS patients, prone positioning resulted in lower VAP incidence. Conversely, the most recent study which assessed the use of prone position in ARDS patients found a higher VAP rate in the prone group than in the supine group, and VAP occurrence in the prone position group was associated with higher mortality (Ayzac et al., 2016). The ARIR position paper on CPT in COVID-19 suggests early implementation of postural changes, although no conclusive data are available for COVID-19. In summary, the above-mentioned positioning maneuvers may represent an important strategy to reduce the risk of secondary respiratory bacterial infections in mechanically ventilated COVID-19 patients, facilitating mucus clearance and mobilizing secretions, thereby improving lung volumes, perfusion, and oxygenation.
3.1.4. Ventilator hyperinflation
Ventilator hyperinflation is a technique commonly applied by physiotherapists to promote airway clearance in mechanically ventilated ICU patients (Thomas, 2015). Ventilator hyperinflation requires the use of a ventilator generating an expiratory flow rate bias when the peak inspiratory flow rate is less than 90 % of the peak expiratory flow rate, with a minimal difference of 17 L/min and an expiratory flow rate of 40 L/min (Volpe et al., 2008). Recently, Ribeiro et al. compared six models of ventilator hyperinflation. Volume-controlled ventilation and pressure support ventilation achieved the best effectiveness score (p < 0.05), with less patient-ventilator asynchronies in pressure support mode (Ribeiro et al., 2019). However, it is still uncertain whether ventilator asynchronies are associated with a worse outcome (Bruni et al., 2019). The effectiveness of manual versus ventilator hyperinflation has been compared both in clinical and pre-clinical settings. In an experimental study in pigs, neither manual nor ventilator hyperinflation modified pulmonary parameters. Rather, both maneuvers significantly decreased inspiratory flow and increased peak expiratory flow up to 44 L/min (Li Bassi et al., 2019). In summary, the ventilator hyperinflation technique may be considered for severe COVID-19 patients to promote airway clearance, although its actual beneficial effects have yet to be proven.
3.1.5. Neuromuscular rehabilitation
ICU-acquired weakness is a very common global muscle weakness that affects around 50 % of ICU patients mechanically ventilated for more than 48 h (Hodgson et al., 2015). The risk factors include bed rest, sepsis and multiorgan failure, hyperglycemia, and use of corticosteroids and neuromuscular blockers (Shang et al., 2020). The literature published to date about critically ill COVID-19 patients has confirmed a need for long-term mechanical ventilation, high doses of neuromuscular blocking agents, and prolonged bed rest (C. Robba et al., 2020a; Gattinoni et al., 2020). Moreover, one of the key therapeutic strategies for these patients has been the early use of corticosteroids (Battaglini et al., 2020), which is another important risk factor implicated in ICU-acquired weakness (Shang et al., 2020). The ARIR position paper (Lazzeri et al., 2020) suggests careful planning of protocols for early mobilization in COVID-19 patients, rather than random application of these techniques. Inspiratory muscle training, electrical muscle stimulation, and early mobilization could be considered as key strategies in the prevention of ICU-acquired weakness (Shang et al., 2020; Nakamura et al., 2020), and should be used rationally to help ensure rapid recovery of those who can benefit. A systematic review and meta-analysis concluded that inspiratory muscle training is able to improve maximal inspiratory pressure and weaning success (Elkins and Dentice, 2015). However, improved muscle function and strength have not translated into improved ICU outcomes. One randomized controlled trial reported improved quality of life within 2 weeks of interventions, but further studies are needed to confirm these findings (Bissett et al., 2016). While evidence for these strategies is still limited, early mobilization of critically ill patients is feasible, safe, and proven to reduce ICU length of stay (Stiller, 2013), as recently confirmed by the rehabilitation strategies applied in COVID-19 patients at San Raffaele Hospital, Milan, Italy. These were based on a multidisciplinary strategy (Iannaccone et al., 2020). Therefore, it should be considered and implemented early in the course of ICU stay in patients with severe COVID-19 (Lazzeri et al., 2020).
3.2. Pre-extubation chest physiotherapy
As for intubation, the extubation process in critically ill COVID-19 patients should be carefully organized, considering the high risk of aerosol generation (Thomas et al., 2020). Health care workers should begin the process only after donning appropriate personal protective equipment and, if possible, should organize the procedure in a negative-pressure room with an antechamber to minimize exposure (Thomas et al., 2020). Before extubation, an air leak test is recommended. Endotracheal suctioning should also be performed, although during cuff deflation and extubation it produces leakage. The application of a CPAP of 15 cmH2O or pressure support ventilation (PSV) at 15/10 or 20/5 cmH2O can also result in lower leakage during the extubation phase (Andreu et al., 2014). A recent study in critically ill patients demonstrated that using positive-pressure ventilation before extubation reduced the incidence of major complications (Andreu et al., 2019). Alveolar RMs, when feasible and necessary, may be considered before extubation to reduce alveolar derecruitment (Silva et al., 2016).
Criteria for extubation of COVID-19 patients are the same as for other critically ill patients. A daily awakening trial followed by a spontaneous breathing trial (SBT) is suggested to improve outcomes in critically ill mechanically ventilated patients (Girard et al., 2008). In 2017, Sklar et al. conduced a meta-analysis of 16 randomized controlled trials to evaluate which SBT test determines higher breath effort. Pressure support ventilation resulted in lower breath effort when compared to use of a T-piece, while a continuous positive airway pressure of 0 cmH2O and T-piece more accurately reflected the post-extubation physiologic condition (Sklar et al., 2017).
It should be noted that SBTs can be exhausting, thus reconnecting patients to the ventilator for 1 h after the SBT before extubation is recommended (Fernandez et al., 2017). Finally, a recent paper proposed a novel technique to limit aerosol generation during extubation of COVID-19 patients. The authors suggested the so-called “mask over tube” method, which uses a second airway filter to avoid staff exposure (D’Silva et al., 2020).
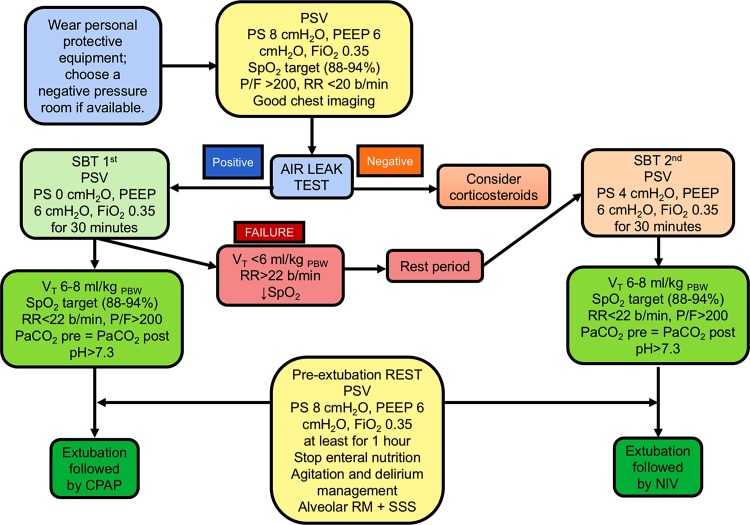
In Fig. 4 we propose a comprehensive algorithm of physiotherapy maneuvers for extubation of COVID-19 patients (Sklar et al., 2017):
-
•
Use adequate personal protective equipment, limit healthcare workers in the room to one physician and one physiotherapist, choose a negative-pressure chamber if available (Lazzeri et al., 2020; Thomas et al., 2020);
-
•
Bed head elevation and prolonged sitting, recruitment maneuvers (Lazzeri et al., 2020; Thomas et al., 2020);
-
•
Air leak test (Schnell et al., 2019);
-
•
SBT with 0 cmH2O pressure support, PEEP = 6 cmH2O, FiO2 = 0.35 for 30 min; if the patient passes the SBT (P/F > 200, VT = 6 mL/kg PBW, SpO2 target, RR < 22 breaths/min) (Cabello et al., 2010), set a final cycle of pressure support ventilation with 8 cmH2O of pressure support and PEEP = 6 cmH2O for at least 1 h to rest muscles (Fernandez et al., 2017);
-
•
Suction subglottic secretions using a closed-aspiration circuit (Lazzeri et al., 2020; Thomas et al., 2020);
-
•
Choose the next ventilatory support modality (NIV, CPAP, HFNO);
-
•
Set PEEP and pressure support < 30 cmH2O (to recruit and prevent VILI) immediately before extubation (Gattinoni et al., 2020);
-
•
Extubate while aspirating secretions via a closed circuit (Lazzeri et al., 2020; Thomas et al., 2020);
-
•
Be prepared for a rapid, skilled re-intubation if necessary.
Fig. 4.
Genoa−COVID-19 algorithm for extubation and weaning.
Algorithm for weaning and extubation of COVID-19 patients routinely used in our intensive care unit. PS, pressure support; PEEP, positive end-expiratory pressure; FiO2, fraction of inspired oxygen; SpO2, peripheral saturation of oxygen; P/F, partial pressure of oxygen/FiO2 ratio; RR, respiratory rate; Vt, tidal volume; SBT, spontaneous breathing trial; PSV, pressure support ventilation; PaCO2, partial pressure of carbon dioxide; PBW, predicted body weight; NIV, non-invasive ventilation; CPAP, continuous positive airway pressure.
3.3. Post-extubation chest physiotherapy
Recent studies have confirmed that CPT in critically ill patients is able to improve respiratory function immediately after extubation (Papadopoulos and Kyprianou, 2002; Wang et al., 2018). As suggested by the ARIR position paper (Lazzeri et al., 2020), CPT may be considered in all COVID-19 patients who require mechanical ventilation, as well as during and after the extubation process. The most common techniques applied after extubation include neuromuscular electrical stimulation, early sitting, airway suctioning, swallow screening, manual hyperinflation, airway cleaning techniques, early mobilization, positive expiratory pressure with an EzPAP device, positive expiratory pressure, active cycle of breathing techniques (ACBT), intermittent positive pressure breathing, forced expiratory technique, assisted or stimulated cough maneuvers, insufflation-exsufflation, CPAP, NIV, and HFNO (Lazzeri et al., 2020). Moreover, we recommend a water swallow test (WST) (Brodsky et al., 2016) to evaluate patients at risk for dysphagia-associated aspiration.
The following section provides a brief overview of techniques that could be applied to critically ill COVID-19 patients in the post-extubation phase. These techniques pose a high risk of aerosol generation, which hinders their use (Thomas et al., 2020). Nevertheless, based on our direct experience with COVID-19 patients, we believe that with proper personal protective equipment and airborne precautions, all of these techniques can be safely applied—including those not recommended or even recommended against elsewhere in the literature (Lazzeri et al., 2020; Thomas et al., 2020).
3.3.1. Active cycle of breathing techniques
ACBTs promote airway clearance, thus avoiding sputum retention and inflammation. ACBTs include the forced expiration technique (FET) and chest expansion exercises (Lewis et al., 2012). The FET consists of one or two forced expirations followed by relaxed breathing. In a meta-analysis of 24 randomized controlled trials, ACBTs were associated with higher sputum clearance, vital capacity, and forced expiratory volume in respect to conventional physiotherapy (Lewis et al., 2012). A meta-analysis of 14 studies concluded that participants prefer autogenic drainage over ACBTs, which in turn are preferred over airway oscillating devices. No differences were found in term of lung function, disease exacerbations, sputum weight, oxygen saturation, or exercise tolerance, casting doubt as to the real efficacy of ACBTs (Mckoy et al., 2016). No data are available specifically for COVID-19 patients. These maneuvers should only be performed while wearing personal protective equipment in a negative-pressure room (Thomas et al., 2020).
3.3.2. Manual hyperinflation
Manual hyperinflation is a technique that delivers a high tidal volume up to a Peak pressure of 40 cmH2O. It starts with a slow inspiration, followed by a 2- or 3-second inspiratory hold, followed by a rapid expiration (similar to forced expiration). Some techniques include the use of a manual hyperinflation bag with a PEEP valve, which allows maintenance of PEEP and thus reduces derecruitment and atelectrauma. The advantage of using manual hyperinflation over ventilator hyperinflation is the proprioceptive feedback from the bag to the operator, while the advantage of ventilator hyperinflation is the safe maintenance of PEEP and standardization and reproducibility of the technique. In awake patients, use of the manual hyperinflation technique is considered simpler than a ventilator hyperinflation manoeuvre (Pathmanathan et al., 2015). This technique has not yet been studied in COVID-19. As for the other techniques mentioned, personnel protective equipment must be worn, and the procedure performed in a negative-pressure room if available.
3.3.3. EzPAP
The EzPAP is a positive expiratory pressure device that delivers a continuous expiratory pressure through the mouth using airflow delivered from a flowmeter to treat and prevent atelectasis. In a randomized controlled trial of 210 postoperative patients randomly allocated to EzPAP or control, SpO2 did not differ between the two groups, whereas the EzPAP group restarted oxygen therapy less frequently and had a reduced incidence of postoperative complications. In patients at risk of hypoxemia, the EzPAP improved pulmonary oxygenation (Rieg et al., 2012). Another trial compared incentive spirometry to EzPAP in 112 postoperative patients, and found no differences between the two strategies in terms of lung expansion or postoperative pulmonary complications (Rowley et al., 2019). As for other techniques, data are limited in COVID-19. Although the risk for health care workers is higher with such devices, they have proven beneficial in critically ill patients. Thus, using personal protective equipment, disposable circuits, airborne precautions, and placing a filter over the machine and patient is strongly recommended (Thomas et al., 2020).
3.3.4. Mechanical insufflation/exsufflation
Mechanical insufflation/exsufflation is a device that promotes maximal lung inflation, followed by a negative pressure, in order to simulate cough. This technique is used when the patient is unable to cough or coughs ineffectively. It is particularly efficient when provided in conjunction with assisted cough techniques or thoraco-abdominal trust (Pathmanathan et al., 2015). No evidence in COVID-19 is available; however, the same recommendations described above may be applied. In our unit, we use a face mask and oral aspiration during the procedure to reduce aerosol dispersal.
3.3.5. Sputum induction
Although sputum induction has not been recommended because of the high risk of aerosol generation (Thomas et al., 2020), critically ill patients (included those with COVID-19) frequently develop neuromuscular weakness and swallowing dysfunction, thus often requiring sputum induction. Based on our experience and on clinical evidence, we believe that sputum should be incentivized in COVID-19 patients to reduce the rate of reintubation, but only in case personal protective equipment are guaranteed.
3.3.6. Neuromuscular mobilization
As proposed above, early neuromuscular mobilization should be considered to facilitate recovery, particularly in the post-extubation phase, as around 50 % of mechanically ventilated patients develop ICU-acquired weakness (Hodgson et al., 2015).
3.3.7. NIV, CPAP, HFNO
Post-extubation respiratory support may be required to reduce the risk of reintubation. In the specific setting of COVID-19, the risk of extubation also includes health personnel. As suggested by the ARIR position statement (Lazzeri et al., 2020), conventional oxygen therapy (such as a nasal cannula) should be avoided in order to reduce droplet dispersion. A face mask with an oxygen flow up to 5 L/min, a reservoir mask up to 10 L/min, or a Venturi mask with 0.6 FiO2 may be preferred, and a surgical mask should be placed over the oxygen mask to further reduce dispersion. For patients who require HFNO, flows up to 50 L/min and FiO2 up to 0.6 should be adopted, again covering the patient’s mouth and nose with a surgical mask (Thomas et al., 2020). The ARIR suggests that, for patients not admitted to the ICU, CPAP and NIV can be employed for no longer than 1 h, followed by reintubation if no improvement is observed (Lazzeri et al., 2020). In our ICU experience, 2- to 3 -h cycles of NIV can be beneficial for COVID-19 patients. Among the available interfaces, the helmet is considered the safer choice to minimize risk to health care workers, as it inherently limits droplet dispersion (Lazzeri et al., 2020); a viral filter should be placed on the expiratory valve to limit aerosol dispersion. A protocol for a randomized controlled trial comparing early post-extubation respiratory support versus standard care was recently proposed, and the trial is ongoing (Casey et al., 2019). Finally, when considering post-extubation respiratory support strategies, it is worth noting that the peripheral oxygenation target for COVID-19 patients who present with hypoxemic respiratory failure is an SpO2 of 96 % (Thomas et al., 2020).
4. Conclusions
COVID-19 is a new disease process that has not been completely characterized. Although there is still no evidence of the efficacy of chest physiotherapy in the specific setting of COVID-19, several established physiotherapy techniques can be safely applied in this subgroup of patients to reduce atelectasis and improve outcomes. All physiotherapy interventions should be carefully organized, and personnel must always wear appropriate personal protective equipment to minimize exposure. Further studies are warranted to confirm the efficacy of CPT techniques in this new critically ill population.
Authors’ contribution
DB, literature search, study design, manuscript preparation. CR, SC, LB, IB, ML, DRG, AV, MB, NP, AT, review of the manuscript. PRMR, PP, study design, review of the manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors
Declaration of Competing Interest
All authors declare they have no conflict of interests.
Acknowledgments
None.
References
- Alhazzani W., Hylander Møller M., Arabi Y.M., Loeb M., Ng Gong M., Fan E., Oczkowski S., Levy M.M., Maitland K., Alshamsi F., Belley-Cote E., Greco M., Laundy M., Morgan J.S., Kesecioglu J., McGeer A., Mermel L., Mammen M.J., Alexander P.E., Arrington A., Centofanti J., Citerio G., Baw B., Memish Z.A., Hammond N., Hayden F.G., Evans L., Rhodes A. Surviving Sepsis Campaign: Guidelines on the Management of Critically Ill Adults with Coronavirus Disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hazzani W. 2020. Awake Prone Position in Hypoxemic Patients With Coronavirus Disease 19 (COVI-PRONE): A Randomized Clinical Trial (COVI-PRONE) [WWW Document]. ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04350723 (accessed 6.21.20). [Google Scholar]
- Andreu M.F., Salvati I.G., Donnianni M.C., Ibañez B., Cotignola M., Bezzi M. Effect of applying positive pressure with or without endotracheal suctioning during extubation: a laboratory study. Resp Care. 2014;59:1905–1911. doi: 10.4187/respcare.03121. [DOI] [PubMed] [Google Scholar]
- Andreu M.F., Dotta M.E., Bezzi M.G., Borello S., Cardoso G.P., Dib P.C., Schustereder S.L.G., Galloli A.M., Castro D.R., Di Giorgio V.L., Villalba F.J., Bertozzi M.N., Carballo J.M., Martín M.C., Brovia C.C., Pita M.C., Pedace M.P., De Benedetto M.F., Carpini J.D., Aguirre P., Montero G. Safety of positive pressure extubation technique. Resp Care. 2019;64:899–907. doi: 10.4187/respcare.06541. [DOI] [PubMed] [Google Scholar]
- Antonelli M. 2020. Prone Positioning During High Flow Oxygen Therapy in Acute Hypoxemic Respiratory Failure (Optiprone) [WWW Document]. ClinicalTrials.gov. URL https://clinicaltrials.gov/ct2/show/NCT03095300 (accessed 6.21.20). [Google Scholar]
- Ayzac L., Girard R., Baboi L., Beuret P., Rabilloud M., Richard J.C., Guérin C. Ventilator-associated pneumonia in ARDS patients: the impact of prone positioning. A secondary analysis of the PROSEVA trial. Intensive Care Med. 2016;42:871–878. doi: 10.1007/s00134-015-4167-5. [DOI] [PubMed] [Google Scholar]
- Battaglini D., Robba C., Ball L., Cruz F.F., Silva P.L., Pelosi P., Rocco P.R.M. Emerging therapies for COVID-19 pneumonia. Expert Opin. Investig. Drugs. 2020:1–5. doi: 10.1080/13543784.2020.1771694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett B.M., Leditschke I.A., Neeman T., Boots R., Paratz J. Inspiratory muscle training to enhance recovery from mechanical ventilation: a randomised trial. Thorax. 2016;71:812–819. doi: 10.1136/thoraxjnl-2016-208279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky M.B., Suiter D.M., González-Fernández M., Michtalik H.J., Frymark T.B., Venediktov R., Schooling T. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest. 2016;150:148–163. doi: 10.1016/j.chest.2016.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruni A., Garofalo E., Pelaia C., Messina A., Cammarota G., Murabito P., Corrado S., Vetrugno L., Longhini F., Navalesi P. Patient-ventilator asynchrony in adult critically ill patients. Minerva Anestesiol. 2019;85:676–688. doi: 10.23736/S0375-9393.19.13436-0. [DOI] [PubMed] [Google Scholar]
- Cabello B., Thille A.W., Roche-Campo F., Brochard L., Gómez F.J., Mancebo J. Physiological comparison of three spontaneous breathing trials in difficult-to-wean patients. Intensive Care Med. 2010;36:1171–1179. doi: 10.1007/s00134-010-1870-0. [DOI] [PubMed] [Google Scholar]
- Calvo-Ayala E., Khan B.A., Farber M.O., Wesley Ely E., Boustani M.A. Interventions to improve the physical function of ICU survivors: a systematic review. Chest. 2013;144:1469–1480. doi: 10.1378/chest.13-0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caroff D.A., Li L., Muscedere J., Klompas M. Subglottic secretion drainage and objective outcomes: a systematic review and meta-analysis. Crit. Care Med. 2016;44:830–840. doi: 10.1097/CCM.0000000000001414. [DOI] [PubMed] [Google Scholar]
- Casey J.D., Vaughan E.R., Lloyd B.D., Bilas P.A., Hall E.J., Toporek A.H., Buell K.G., Brown R.M., Richardson R.K., Rooks J.C., Wang L., Lindsell C.J., Ely E.W., Self W.H., Bernard G.R., Rice T.W., Semler M.W. Protocolized post-extubation respiratory support to prevent reintubation: protocol and statistical analysis plan for a clinical trial. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalcanti A.B., Suzumura E.A., Laranjeira L.N., De Moraes Paisani D., Damiani L.P., Guimarães H.P., Romano E.R., De Moraes Regenga M., Taniguchi L.N.T., Teixeira C., De Oliveira R.P., Machado F.R., Diaz-Quijano F.A., De Alencar Filho M.S., Maia I.S., Caser E.B., De Oliveira Filho W., De Carvalho Borges M., De Aquino Martins P., Matsui M., Ospina-Tascón G.A., Giancursi T.S., Giraldo-Ramirez N.D., Vieira S.R.R., De Lima Assef M.P., Hasan M.S., Szczeklik W., Rios F., Amato M.B.P., Berwanger O., De Carvalho C.R.R. Effect of lung recruitment and titrated Positive End-Expiratory Pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome - A randomized clinical trial. JAMA. 2017;318:1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira-Neto M., Moura A., Cerqueira T., Aquim E., Rea-Neto A., Oliveira M., Silva-Junior W., Santana-Filho V., Scola R. Acute effects of physiotherapeutic respiratory maneuvers in critically ill patients with craniocerebral trauma. Clinics. 2013;68:1210–1214. doi: 10.6061/clinics/2013(09)06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentini R. 2020. È Come Un Terremoto. [WWW Document]. SIMEU. URL https://www.simeu.it/w/articoli/leggiArticolo/3977/leggi. [Google Scholar]
- D’Silva D.F., McCulloch T.J., Lim J.S., Smith S.S., Carayannis D. Extubation of patients with COVID-19. Br. J. Anaesth. 2020;125:e192–e195. doi: 10.1016/j.bja.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denault A.Y., Delisle S., Canty D., Royse A., Royse C., Serra X.C., Gebhard C.E., Couture E.J., Girard M., Cavayas Y.A., Peschanski N., Langevin S., Ouellet P. A proposed lung ultrasound and phenotypic algorithm for the care of COVID-19 patients with acute respiratory failure. Can J Anesth. 2020;21:1–12. doi: 10.1007/s12630-020-01704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X., Cao Y., Lu X., Zhang J., Du H., Yan Y., Akdis C.A., Gao Y. Eleven faces of coronavirus disease 2019. Allergy. 2020;75:1699–1709. doi: 10.1111/all.14289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elharrar X., Trigui Y., Dols A.M., Touchon F., Martinez S., Prud’Homme E., Papazian L. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323:2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins M., Dentice R. Inspiratory muscle training facilitates weaning from mechanical ventilation among patients in the intensive care unit: a systematic review. J. Physiother. 2015;61:125–134. doi: 10.1016/j.jphys.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Fan E., Del Sorbo L., Goligher E.C., Hodgson C.L., Munshi L., Walkey A.J., Adhikari N.K.J., Amato M., Branson R., Brower R.G., Ferguson N.D., Gajic O., Gattinoni L., Hess D., Mancebo J., Meade M.O., McAuley D.F., Pesenti A., Ranieri M., Rubenfeld G.D., Rubin E., Seckel M., Slutsky A.S., Talmor D., Thompson B.T., Wunsch H., Uleryk E., Brozek J., Brochard L.J., American Thoracic Society, European Society of Intensive Care Medicine, S. of C.C.M An official american thoracic Society/European society of intensive care Medicine/Society of critical care medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2017;195:1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- Fernandez M.M., González-Castro A., Magret M., Bouza M.T., Ibañez M., García C., Balerdi B., Mas A., Arauzo V., Añón J.M., Ruiz F., Ferreres J., Tomás R., Alabert M., Tizón A.I., Altaba S., Llamas N., Fernandez R. Reconnection to mechanical ventilation for 1 h after a successful spontaneous breathing trial reduces reintubation in critically ill patients: a multicenter randomized controlled trial. Intensive Care Med. 2017;43:1660–1667. doi: 10.1007/s00134-017-4911-0. [DOI] [PubMed] [Google Scholar]
- Gattinoni L., Coppola S., Cressoni M., Busana M., Chiumello D. Covid-19 does not lead to a “Typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020;201:1299–1300. doi: 10.1164/rccm.202003-0817LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard T.D., Kress J.P., Fuchs B.D., Thomason J.W., Schweickert W.D., Pun B.T., Taichman D.B., Dunn J.G., Pohlman A.S., Kinniry P.A., Jackson J.C., Canonico A.E., Light R.W., Shintani A.K., Thompson J.L., Gordon S.M., Hall J.B., Dittus R.S., Bernard G.R., Ely E.W. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- Guan W., Ni Z., Hu Yu, Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang Jin-lin, Liang Z., Peng Y., Wei L., Liu Y., Hu Ya-hua, Peng P., Wang Jian-ming, Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarracino F., Vetrugno L., Forfori F., Corradi F., Orso D., Bertini P., Ortalda A., Federici N., Copetti R., Bove T. Lung, heart, vascular, and diaphragm ultrasound examination of COVID-19 patients: a comprehensive approach. J Cardiothorac Vasc Anesth S. 2020;1053-0770(20) doi: 10.1053/j.jvca.2020.06.013. 30519-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin C., Reignier J., Richard J.C., Beuret P., Gacouin A., Boulain T., Mercier E., Badet M., Mercat A., Baudin O., Clavel M., Chatellier D., Jaber S., Rosselli S., Mancebo J., Sirodot M., Hilbert G., Bengler C., Richecoeur J., Gainnier M., Bayle F., Bourdin G., Leray V., Girard R., Baboi L., Ayzac L. Prone positioning in severe acute respiratory distress syndrome. N. Engl. J. Med. 2013;368:2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- Hodgson C., Bellomo R., Berney S., Bailey M., Buhr H., Denehy L., Harrold M., Higgins A., Presneill J., Saxena M., Skinner E., Young P., Webb S. Early mobilization and recovery in mechanically ventilated patients in the ICU: a bi-national, multi-centre, prospective cohort study. Crit Care. 2015:19. doi: 10.1186/s13054-015-0765-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannaccone S., Castellazzi P., Tettamanti A., Houdayer E., Brugliera L., de Blasio F., Cimino P., Ripa M., Meloni C., Alemanno F., Scarpellini P. Role of Rehabilitation Department for Adult Individuals With COVID-19: The Experience of the San Raffaele Hospital of Milan. Arch Phys Med Rehab. 2020;S0003-9993:30365–30368. doi: 10.1016/j.apmr.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayambu G., Boots R., Paratz J. Physical therapy for the critically ill in the ICU: a systematic review and meta-analysis. Crit. Care Med. 2013;41:1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- Lacherade J.-C., Azais M.-A., Pouplet C., Colin G. Subglottic secretion drainage for ventilator-associated pneumonia prevention: an underused efficient measure. Ann. Transl. Med. 2018;6:422. doi: 10.21037/atm.2018.10.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzeri M., Lanza A., Bellini R., Bellofiore A., Cecchetto S., Colombo A., D’Abrosca F., Del Monaco C., Gaudellio G., Paneroni M., Privitera E., Retucci M., Rossi V., Santambrogio M., Sommariva M., Frigerio P. Respiratory physiotherapy in patients with COVID-19 infection in acute setting: a Position Paper of the Italian Association of Respiratory Physiotherapists (ARIR) Monaldi Arch. Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1285. [DOI] [PubMed] [Google Scholar]
- Lewis L.K., Williams M.T., Olds T.S. The active cycle of breathing technique: a systematic review and meta-analysis. Respir. Med. 2012;106:155–172. doi: 10.1016/j.rmed.2011.10.014. [DOI] [PubMed] [Google Scholar]
- Li Y., Xia L. Coronavirus disease 2019 (COVID-19): role of chest CT in diagnosis and management. AJR Am. J. Roentgenol. 2020;214:1280–1286. doi: 10.2214/AJR.20.22954. [DOI] [PubMed] [Google Scholar]
- Li Bassi G., Aguilera-Xiol E., Pagliara F., Hua Y., Torres A. Body position and ventilator-associated pneumonia prevention. Semin. Respir. Crit. Care Med. 2017;38:371–380. doi: 10.1055/s-0037-1603111. [DOI] [PubMed] [Google Scholar]
- Li Bassi G., Panigada M., Ranzani O.T., Zanella A., Berra L., Cressoni M., Parrini V., Kandil H., Salati G., Selvaggi P., Amatu A., Sanz-Moncosi M., Biagioni E., Tagliaferri F., Furia M., Mercurio G., Costa A., Manca T., Lindau S., Babel J., Cavana M., Chiurazzi C., Marti J.D., Consonni D., Gattinoni L., Pesenti A., Wiener-Kronish J., Bruschi C., Ballotta A., Salsi P., Livigni S., Iotti G., Fernandez J., Girardis M., Barbagallo M., Moise G., Antonelli M., Caspani M.L., Vezzani A., Meybohm P., Gasparovic V., Geat E., Amato M., Niederman M., Kolobow T., Torres A. Randomized, multicenter trial of lateral Trendelenburg versus semirecumbent body position for the prevention of ventilator-associated pneumonia. Intensive Care Med. 2017;43:1572–1584. doi: 10.1007/s00134-017-4858-1. [DOI] [PubMed] [Google Scholar]
- Li Bassi G., Chiurazzi C., Aguilera E., Travierso C., Battaglini D., Yang M., Motos A., Yang H., Meli A., Marti D., Ranzani O.T., Blasi F., Pelosi P., Chiumello D., Torres A. In-vitro analysis of a novel ‘add-on’ silicone cuff to improve sealing properties of tracheal tubes. Anaesthesia. 2018;73:1372–1381. doi: 10.1111/anae.14413. [DOI] [PubMed] [Google Scholar]
- Li Bassi G., Martí J.D., Comaru T., Aguilera-Xiol E., Rigol M., Ntoumenopoulos G., Terraneo S., De Rosa F., Rinaudo M., Fernandez-Barat L., Battaglini D., Meli A., Ferrer M., Pelosi P., Chiumello D., Torres A. Short-term appraisal of the effects and safety of manual versus ventilator hyperinflation in an animal model of severe pneumonia. Respir. Care. 2019;64:760–770. doi: 10.4187/respcare.06487. [DOI] [PubMed] [Google Scholar]
- Lorente L., Lecuona M., Jiménez A., Mora M.L., Sierra A. Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia. Am. J. Respir. Crit. Care Med. 2007;176:1079–1083. doi: 10.1164/rccm.200705-761OC. [DOI] [PubMed] [Google Scholar]
- Mao Z., Gao L., Wang G., Liu C., Zhao Y., Gu W., Kang H., Zhou F. Subglottic secretion suction for preventing ventilator-associated pneumonia: an updated meta-analysis and trial sequential analysis. Crit Care. 2016;20:353. doi: 10.1186/s13054-016-1527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mckoy N.A., Wilson L.M., Saldanha I.J., Odelola O.A., Robinson K.A. Active cycle of breathing technique for cystic fibrosis. Cochrane Database Syst. Rev. 2016;7 doi: 10.1002/14651858.CD007862.pub4. CD007862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Nakano H., Naraba H., Mochizuki M., Hashimoto H. Early rehabilitation with dedicated use of belt-type electrical muscle stimulation for severe COVID-19 patients. Crit. Care. 2020;24:342. doi: 10.1186/s13054-020-03080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos M., Kyprianou T. Chest physiotherapy after extubation affects vital capacity and inspiratory muscles’ strength. Intensive Care Med. 2002;28:S198. [Google Scholar]
- Pathmanathan N., Beaumont N., Gratrix A. Respiratory physiotherapy in the critical care unit. BJA Edu. 2015;15:20–25. [Google Scholar]
- Ribeiro B.S., Lopes A.J., Menezes S.L.S., Guimarães F.S. Selecting the best ventilator hyperinflation technique based on physiologic markers: a randomized controlled crossover study. Hear. Lung. 2019;48:39–45. doi: 10.1016/j.hrtlng.2018.09.006. [DOI] [PubMed] [Google Scholar]
- Rieg A.D., Stoppe C., Rossaint R., Coburn M., Hein M., Schälte G. EzPAP® therapy of postoperative hypoxemia in the recovery room : experiences with the new compact system of end-expiratory positive airway pressure. Anaesthesist. 2012;61:867–874. doi: 10.1007/s00101-012-2083-4. [DOI] [PubMed] [Google Scholar]
- Robba C., Battaglini D., Ball L., Patroniti N., Loconte M., Brunetti I., Vena A., Giacobbe D., Bassetti M., Rocco P.R.M., Pelosi P. Distinct phenotypes require distinct respiratory management strategies in severe COVID-19. Respir. Physiol. Neurobiol. 2020;279 doi: 10.1016/j.resp.2020.103455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robba C., Battaglini D., Pelosi P., Rocco R.M.P. Multiple organ dysfunction in SARS-CoV-2: MODS-CoV-2. Exp Rev Respir Med. 2020:1–4. doi: 10.1080/17476348.2020.1778470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco P.R.M., Pelosi P. Pulmonary and extrapulmonary acute respiratory distress syndrome: Myth or reality? Curr. Opin. Crit. Care. 2008;14:50–55. doi: 10.1097/MCC.0b013e3282f2405b. [DOI] [PubMed] [Google Scholar]
- Rowley D.D., Malinowski T.P., Di Peppe J.L., Sharkey R.M., Gochenour D.U., Enfield K.B. A randomized controlled trial comparing two lung expansion therapies after upper abdominal surgery. Resp Care. 2019;64:1181–1192. doi: 10.4187/respcare.06812. [DOI] [PubMed] [Google Scholar]
- Schnell D., Planquette B., Berger A., Merceron S., Mayaux J., Strasbach L., Legriel S., Valade S., Darmon M., Meziani F. Cuff leak test for the diagnosis of post-extubation stridor: a multicenter evaluation study. J. Intensive Care Med. 2019;34:391–396. doi: 10.1177/0885066617700095. [DOI] [PubMed] [Google Scholar]
- Shang L., Zhao J., Hu Y., Du R., Cao B. On the use of corticosteroids for 2019-nCoV pneumonia. Lancet. 2020;395:683–684. doi: 10.1016/S0140-6736(20)30361-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva P.L., Moraes L., Santos R.S., Samary C., Ramos M.B.A., Santos C.L., Morales M.M., Capelozzi V.L., Garcia C.S.N.B., De Abreu M.G., Pelosi P., Marini J.J., Rocco P.R.M. Recruitment maneuvers modulate epithelial and endothelial cell response according to acute lung injury etiology. Crit. Care Med. 2013;41:e256–65. doi: 10.1097/CCM.0b013e31828a3c13. [DOI] [PubMed] [Google Scholar]
- Silva P.L., Pelosi P., Rocco P.R.M. Recruitment maneuvers for acute respiratory distress syndrome: the panorama in 2016. Rev. Bras. Ter. Intensiva. 2016;28:104–106. doi: 10.5935/0103-507X.20160023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonelli C., Paneroni M., Fokom A.G., Saleri M., Speltoni I., Favero I., Garofali F., Scalvini S., Vitacca M. How the COVID-19 infection tsunami revolutionized the work of respiratory physiotherapists: an experience from Northern Italy. Monaldi Arch. Chest Dis. 2020;90 doi: 10.4081/monaldi.2020.1085. [DOI] [PubMed] [Google Scholar]
- Sklar M.C., Burns K., Rittayamai N., Lanys A., Rauseo M., Chen L., Dres M., Chen G.Q., Goligher E.C., Adhikari N.K.J., Brochard L., Friedrich J.O. Effort to breathe with various spontaneous breathing trial techniques. Am. J. Respir. Crit. Care Med. 2017;195:1477–1485. doi: 10.1164/rccm.201607-1338OC. [DOI] [PubMed] [Google Scholar]
- Stiller K. Physiotherapy in intensive care: an updated systematic review. Chest. 2013;144:825–847. doi: 10.1378/chest.12-2930. [DOI] [PubMed] [Google Scholar]
- Telias I., Katira B.H., Brochard L. Is the Prone Position Helpful during Spontaneous Breathing in Patients with COVID-19? JAMA. 2020 doi: 10.1001/jama.2020.8539. [DOI] [PubMed] [Google Scholar]
- Thomas P.J. The effect of mechanical ventilator settings during ventilator hyperinflation techniques: a bench-top analysis. Anaesth. Intensive Care. 2015;43:81–87. doi: 10.1177/0310057X1504300112. [DOI] [PubMed] [Google Scholar]
- Thomas P., Baldwin C., Bissett B., Boden I., Gosselink R., Granger C.L., Hodgson C., Ym A., Michelle J., Kho E., Moses R., Ntoumenopoulos G., Parry S.M., Patman S., Van Der Lee L. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J. Physiother. 2020;66:73–82. doi: 10.1016/j.jphys.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin M.J., Laghi F., Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann. Intensive Care. 2020;10:78. doi: 10.1186/s13613-020-00692-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusman G., Acosta C.M., Costantini M. Ultrasonography for the assessment of lung recruitment maneuvers. Crit. Ultrasound J. 2016;8:8. doi: 10.1186/s13089-016-0045-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nieuwenhoven C.A., Vandenbroucke-Grauls C., Van Tiel F.H., Joore H.C.A., Strack Van Schijndel R.J.M., Van Der Tweel I., Ramsay G., Bonten M.J.M. Feasibility and effects of the semirecumbent position to prevent ventilator-associated pneumonia: a randomized study. Crit. Care Med. 2006;34:396–402. doi: 10.1097/01.ccm.0000198529.76602.5e. [DOI] [PubMed] [Google Scholar]
- Vitacca M., Carone M., Clini E.M., Paneroni M., Lazzeri M., Lanza A., Privitera E., Pasqua F., Gigliotti F., Castellana G., Banfi P., Guffanti E., Santus P., Ambrosino N. Joint statement on the role of respiratory rehabilitation in the COVID-19 crisis: the italian position paper. Respiration. 2020;99:493–499. doi: 10.1159/000508399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe M.S., Adams A.B., Amato M.P.B., Marini J.J. Ventilation patterns influence airway secretion movement. Respir. Care. 2008;53:1287–1294. [PubMed] [Google Scholar]
- Wang L., Li X., Yang Z., Tang X., Yuan Q., Deng L., Sun X. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst. Rev. 2016;2016 doi: 10.1002/14651858.CD009946.pub2. CD009946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T.H., Wu C.P., Wang L.Y. Chest physiotherapy with early mobilization may improve extubation outcome in critically ill patients in the intensive care units. Clin. Respir. J. 2018;12:2613–2621. doi: 10.1111/crj.12965. [DOI] [PubMed] [Google Scholar]
- Wang L., He W., Yu X., Hu D., Bao M., Liu H., Zhou J., Jiang H. Coronavirus Disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J. Infect. 2020;80:639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:1–11. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Yan Y., Yin X., Wang B.Y., Wu T., Liu G.J., Dong B.R. Chest physiotherapy for pneumonia in adults. Cochrane Database Syst. Rev. 2013;2013 doi: 10.1002/14651858.CD006338.pub3. CD006338. [DOI] [PubMed] [Google Scholar]