Abstract
Lipids have been recognized as key players in cell signaling and disease. Information on their location and distribution within a biological system, under varying conditions, is necessary to understand the contributions of different lipid species to an altered phenotype. Imaging mass spectrometry techniques, such as time of flight - secondary ion mass spectrometry (ToF-SIMS) and matrix-assisted laser desorption/ionization (MALDI) are capable of revealing global lipid distributions in tissues in an untargeted fashion. However, to confidently identify the species present in a sample, orthogonal analyses like tandem MS (MS/MS) are often required. This can be accomplished by bulk sample analysis with liquid chromatography (LC)-MS/MS, which can provide confident lipid identifications, at the expense of losing location-specific information. Here, using planarian flatworms as a model system, we demonstrate that imaging gas cluster ion beam (GCIB)-ToF-SIMS has the unique capability to simultaneously detect, identify, and image lipid species with sub-cellular resolution in tissue sections. The parallel detection of both, intact lipids and their respective fragments, allows for unique identification of some species without the need of performing an additional orthogonal MS/MS analysis. This was accomplished by correlating intact lipid and associated fragment SIMS-images. The lipid assignments, respective fragment identities, and locations gathered from ToF-SIMS data were confirmed via LC-MS/MS on lipid extracts and ultra-high mass resolution MALDI-MS imaging. Together, these data show that the semi-destructive nature of ToF-SIMS can be utilized advantageously to enable both confident molecular annotations and to determine the locations of species within a biological sample.
Graphical Abstract

Introduction
Lipids are a highly diverse group of molecules involved in signal transduction, energy storage and forming structural components of cell membranes in all living organisms. Lipids are of growing research interest due to their role as second messengers, capability of rapidly reacting to stress/changes,1–2 and their involvement in various diseases (e.g., cancer3 and Alzheimers4). The numerous species of lipids, distinguished mainly by their head-group and fatty acid (ester lipids) or fatty acyl (ether lipids, indicated with prefix P-/O-) tail combinations have different chemical properties and are unevenly distributed in cells and organisms.5 For example, phosphatidylserine (PS) is mainly present in the inner leaflet of the cell membrane,6 and phosphatidylglycerol (PG) is rarely found in mammalian cells, except in the lung surfactant.7 The general lack of research methods capable of gathering cellular resolution location and single lipid specific information simultaneously limits the ability to draw conclusions about lipid and fatty acid involvement in any given biological processes such as cancer progression.8–9 Imaging mass spectrometry (IMS) is capable of studying the distribution of single lipid species in tissues in a multiplexed fashion, but most techniques (e.g., secondary ion mass spectrometry (SIMS) and matrix-assisted laser desorption/ionization (MALDI)) typically distinguish lipids using the parent ion exact mass information. This makes exact lipid identification impossible , even for ultrahigh mass resolution analyzers like Fourier transform ion cyclotron resonance (FTICR) mass spectrometers (mass resolutions > 1M FWHM.)10, as different classes of lipids can be isomers.
Adding tandem MS (MS/MS) capability to an instrument can isolate and fragment lipid species in a sample to help identify the lipid head-group and associated chains, such as fatty acids. This is often sufficient to determine the lipid species. Additional knowledge on the biological processes during lipid synthesis (e.g., double bond introducing enzymes present in an organism) aids in determining the lipids’ molecular structures. Tandem MS use in MALDI-MSI is well established in tissues analysis,11–12 where automated data-dependent approaches that structurally identify the majority of the lipids detected in the sample is now possible.13 One drawback of these approaches is the limited spatial resolution achievable with MALDI. A laser spot size of about <1 μm is technically possible for some MALDI instruments,14 but gathering sufficient molecular ions for imaging or to perform tandem MS within a ~1 μm area remains challenging. Instead, MALDI-ToF approaches have resorted to pixel sizes that use the combined signal from multiple cells in order to attain sufficient signal for MS/MS.15 Similarly, MALDI-FTICR-IMS for lipid/metabolite mapping is commonly performed using ~50 μm/pixel.16
Tandem-MS in ToF-SIMS imaging is a more recent development.17–20 A significant benefit of ToF-SIMS imaging is the ability to regularly attain higher imaging resolution, where < 2-3 μm/pixel for single lipid species is common21–22. However, with current SIMS instrumentation, MS/MS can only be performed on relatively highly abundant species and must be performed in a serial fashion (i.e., one species at a time). Additionally, despite the constant effort to decrease fragmentation and increase the yield of molecular secondary ions in ToF-SIMS imaging,23 ToF-SIMS remains a destructive technique that produces a notable amount of fragments from the parent lipids present in a sample. Although fragmentation reduces the number of molecular ions detected, here we demonstrate how to utilize ToF-SIMS fragmentation to our advantage to attain both confident lipid identifications and localizations within our tissue samples.
Lipid fragmentation with common MS/MS techniques has been thoroughly studied.24–26 However, to our knowledge there are very few reports on fragmentation of lipids due to the primary ion impact on the sample27. Previously, Adams et al28. and Anderton el al29. looked at fragments in lipid standards, but mainly focused on head-groups, fatty acid tails and their fragments. When studying a highly complex sample such as a tissue section, looking only at fatty acids and lipid head-groups is insufficient for the identification of single, specific lipid species since many lipids have common fragments. Ideally, for unique identification of a lipid species, a set of fragment species including [M–H]−, [M–snl]−, [M–sn2]−, and [M–head-group]− should be identified, where the fatty acid tails are designated as snl and sn2 according to IUPAC-IUBMB.30 We propose that if those fragments are present in the spectra, and co-localize with the molecular ion in the image, the structure of the lipid species can be annotated with greater confidence, although lipid isomers present in one sample can be an issue.12. However, the complete absence of those fragment species means the assignment should be doubted unless the absence can be explained, for example, for ether lipids the fragment [M–ether fatty acid]− is not observed31 and PS lipids only produce fragments without their head-group.32
ToF-SIMS data (spectra) usually contain head-group and fatty acid species/fragments (m/z~ 100-350), various lipid fragments like pseudo-lysolipids (m/z~ 350-650), and intact parent species (m/z~ 650-950+). While most research has focus on using the mass spectral regions of intact fatty acid and/or lipids and ignore the lipid fragment region, we exploit the wealth of information contained within the entire spectrum. As such, we demonstrate both the small and large mass lipid fragments are invaluable in structurally identifying lipids present in every single pixel.
Using data gathered on tissue sections of our model biological system, the flatworm Phagocata gracilis,33 we show that GCIB-ToF-SIMS data (imaging at 3-4 μm/pixel) contains quasi-MS/MS signals, which includes all the fragment species needed to uniquely identify a lipid species without the need of an additional analysis. Ultrahigh mass resolution MALDI-FTICR-IMS for this system was used to support the ToF-SIMS peak identification of the intact molecular species. The validity of this approach was further confirmed by with LC-MS/MS of flatworm homogenates. While fragments in ToF-SIMS spectra can originate from multiple different lipid species, using the location specific information from images of both the lipids and respective fragments serves as evidence for their common origin.
Methods
Flatworm handling
Imaging GCIB-ToF-SIMS, LC-MS/MS, and MALDI-FTICR-IMS was performed on planarian flatworm Phagocata gracilis. Live black Planaria (Ward’s Science, NY), 5-8 mm in size were kept in aged water (stored at room temperature for several days to allow dissipation of chlorine) and starved one week prior to analysis to avoid interference from nonspecific signals derived from food products within the intestines.
Bulk lipid analysis: LC-MS/MS
Five P. gracilis worms were lysed separately by bead beating in a Bullet Blender (Next Advance, Averill Park, NY) with 0.1-mm-diameter zirconia beads at speed 8 for 3 min at 4°C. The resulting lysate was spun into a Falcon tube at 2,000 × g for 10 min at 4 °C. The lipid extraction procedure was adapted from Nakayasu et al., 34 but the monophasic step was not performed, as all solvents were simultaneously added.
Lipid samples were then reconstituted in 50 μL of methanol, and analyzed by LC-MS/MS as previously described.35 LC-MS/MS raw data files were imported into LIQUID for confident identification of individual lipid species,35 where the identifications were assigned by examining tandem mass spectra for diagnostic ion fragments along with associated chain fragment information. In addition, the isotopic profile, extracted ion chromatogram, mass error of measured precursor and fragment ions, and retention time were examined to assist in identifications.
Imaging mass spectrometry
For ToF-SIMS and MALDI-FTICR-MS longitudinal worm tissue sections were prepared in a similar fashion. Sample preparation for ToF-SIMS analysis was described earlier.33 Briefly, worms were sedated by being submerged in a 6% ethanol water solution for 15 minutes, embedded in into cryosection-media (optimum cutting temperature, Tissue-Tek® O.C.T. Compound, Sakura® Finetek), frozen on dry ice and cryo-section using a Leica CM1580 cryostat at −20 °C with a section thickness of 15 μm. Sections were thaw mounted onto indium tin oxide (ITO)-coated glass slides (Delta Technologies), kept on dry ice until 1 hour prior to analysis and analyzed at room temperature. For MALDI-FTICR-MS the sedated worms were gently pressed to the bottom of a cryo-mold (Tissue-Tek, 25 ×20 ×5 mm), left to freeze for 5 min on a bed of dry ice, and then embedded in 2.5% carboxymethyl cellulose (CMC). The embedded samples were left an additional 30 min on a bed of dry ice before cryosectioning. Embedded worms were cryosectioned (CryoStar NX 70, Thermo Scientific) where temperature of sample chuck and cutting blade were maintained at −20 °C. Sections of 20 μm thickness were thaw-mounted onto ITO-coated glass slides (Bruker Daltonics) and kept frozen at −80 °C until further MALDI sample preparation.
ToF-SIMS imaging was described in detail elsewhere.36 Briefly, the ToF-SIMS instrument used in this study was a J105-3D chemical imager (Ionoptika Ltd, UK) mounted with a 40 keV gas cluster ion beam (GCIB), where the beam was composed of 15% CO2 in Argon (Matheson, USA) with a nominal cluster size of 4000. Worm sections were analyzed in negative and positive ion mode at 3 and 12 μm per pixel respectively using a total ion dose of 1 × 1013/5 × 1012 ions/cm2. Spectra were recorded over a mass range of 70-1000 Da with a mass resolution of 11,500 @ m/z 885.54 (FWHM). Here, two sections are shown analyzed in negative ion mode and one section in positive ion mode.
Prior to MALDI matrix application, frozen ITO slides with worm sections were quickly transferred to a vacuum desiccator and dried for 20 min. Application of MALDI matrix was performed using a TM-Sprayer (HTX Technologies), where 2,5-dihydroxybenzoic acid (DHB) and norharmane were used for positive and negative ion mode analysis, respectively. For DHB, 40 mg/mL in 50% MeOH was sprayed with 12 passes at 50 μL/min at 80 °C with spray spacing of 3 mm. For norharmane, 7 mg/mL in CHChMeOH (2:1) was used, and seven passes were sprayed at 120 μL/min and 30 °C, with a spray spacing of 2 mm. A spray pressure of 10 psi (N2), a spray velocity of 1200 mm/min, and a sprayer nozzle distance from the sample of 40 mm was maintained for all samples.
MALDI-FTICR-IMS was performed on a 15 Tesla MALDI-FTICR-MS (Solarix, Bruker Daltonics) equipped with a SmartBeam II laser source (355 nm, 2 kHz). Data were collected in both positive and negative polarity mode over range of m/z 300-1200, using a 680 ms transient, which resulted in a mass resolution of ~250,000 at 400 m/z. External calibration of instrument was performed using TuneMix (Agilent), resulting in mass measurement accuracy within 1 ppm across the entire m/z range. The laser was stepped across the sample in 50 μm increments (accumulating 100 laser shots per step at 2 kHz). Image data was acquired using Fleximaging (v 4.1, Bruker Daltonics). Image processing (i.e., peak alignment, segmentation, determining colocalized m/z values, and calculation of Pearson correlation coefficients) and visualization were performed using SCiLS Lab (GmbH, Bremen, Germany). Negative and positive ion mode data was generated using consecutive planarian tissue sections. Imaging data was uploaded to METASPACE (https://metaspace2020.eu) to generate putative molecular identifications, and can be found by searching ‘20180418_dv_worm_p1_50um’ and ‘n2_50um_min’ for positive and negative ion mode analysis, respectively.
Correlation Plots
Pearson correlation coefficient (R) analysis was performed of ToF-SIMS images to quantitatively compare images from various ToF-SIMS peaks. Correlation plots and images were generated using the ImageJ plugin Image Correlation! lo.37 Prior to correlation analysis, the signal in each image pixel was averaged over seven pixels to reduce the noise in ToF-SIMS images without sacrificing area specificity.
Results
Location specific lipid identification using complementary techniques
ToF-SIMS imaging of tissue sections of the flatworm species Phagocata gracilis reported previously,33 revealed key lipid peaks and their localization within the worm. For example, m/z 718.50 was detected solely in the outer epithelial lining of the pharynx. Line scans show that this signal is restricted to a ~ 12 μm broad ring corresponding to the width of one cell. Additionally, imaging PCA identifies a discrete set of signals that colocalized with m/z 718.50 (Line scan and PCA in Figure SI). Previously, clear assignments of this lipid peak (and others) could only be made based on the combination of ToF-SIMS and LC-MS/MS.33 However, the PCA results suggested there was significance to those colocalizing signals that might expand the utility of ToF-SIMS data.
Figure 1 shows the related data for m/z 718.50 in the ToF-SIMS, MALDI-FTICR-IMS and LC MS/MS (negative ion mode) from this sample. The exact masses, identities, and ppm values of the molecular ion and fragments detected with all three techniques are listed in Table S1. The signals identified by PCA of the ToF-SIMS data (Figure S1d) are the same as the fragments listed in Table S1. Additionally, the MALDI-FTICR-MS image of m/z 718.5027 (Figure 1a) shows localization corresponding with the pharynx area and outer skin, verifying that the position and molecular ion identified by ToF-SIMS was accurate. METASPACE analysis of the MALDI-FTICR-MS data identified this species as one or a combination of five possible phosphatidylserine (PS) ether lipid isomers. The combination of co-localized fragments in the ToF-SIMS data (Figure 1b (2)-(6)) correspond to the species PS(P-16:0/16:0). It is noted that, in Figure 1b, the fragment peak distributions are not always an exact match to the parent ion.
Figure 1.

Identification and localization of PS(P-16:0/16:0) within P. gracilis using three different MS methods, a) Distribution of [M–H]− species detected with MALDI-FTICR-MSI in a flatworm tissue section, b) distribution of [M–H]− species and its fragments detected with ToF-SIMS imaging in flatworm tissue section, c) molecular structure of PS(P-16:0/16:0) and the predicted fragments, d) LC-MS/MS spectrum of PS(P-16:0/16:0) (average of six spectra) extracted from worm lipid extracts with the predicted fragments present indicated by the numbers on top/to the right of the detected mass. Detected masses and ppm errors for all three techniques, exact masses, and fragment identities 1-9 are listed in Table S1.
The most obvious is fragment m/z 255.2, assigned to palmitic acid (FA 16:0), which shows a broad distribution across the entire section. This difference in distribution can be explained as palmitic acid being a ubiquitous fatty acid species which is a component of many different lipids, therefore, this fragment species alone cannot be used solely as a proxy for PS(P-16:0/16:0). Similarly, Figure S2 shows an example for a fragment at m/z 375.23 which originates from at least 2 lipids but co-localization can still be used to identify both parent ions.
Figure 1c shows the fragmentation mechanism and structures for all species that PS(P-16:0/16:0) should produce according to simulated LipidMaps data.38 The LC-MS/MS data confirms this assignment by showing that these same fragments are generated from the m/z 718.50 parent ion. A similar analysis of m/z 746.60 using all three techniques in positive ion mode, resulted in the m/z 746.60 species being assigned to ether lipid PC(O-18:0/16:1) (data shown in Figure S3), verifying that the co-localization of multiple peaks in ToF-SIMS data can be used to uniquely identify lipid species without further MS/MS.
Correlation analysis: Parent vs. Fragment ion ToF-SIMS images
While a visual comparison of peak localization is useful, it would be preferable to better quantify the co-localization of molecular peaks and their potential fragments. In order to determine the similarity between parent ion and fragment images computationally, we performed Pearson correlation analysis on ToF-SIMS images, where the correlation of two images is expressed as a numerical value from 1 (100% correlated) to −1(100% anticorrelated).39 (Further explanation can be found in the supplementary information.) The value for correlated ion species in ToF-SIMS images can be diminished due to two issues. First, the distribution of lipid fragment ions in ToF-SIMS images may not always correlate exclusively with one parent ion (as noted in Figure S2). Second, the mass resolution of the ToF-based mass analyzer may be insufficient to resolve species with similar exact mass (m/∆m ~10 000), so an image generated from a single peak might show the merged distribution of two or more unresolved species.
Figure 2 shows a Pearson correlation coefficient (R) analysis of ToF-SIMS images for parent ion PS(P-16:0/16:0) at m/z 718.50 and its fragment at m/z 631.47 (Figures 2a and 2b, respectively). Distributions and intensities of parent and fragment ions show a predominately strong (R between 0.95 – 0.88) correlation (Figure 2c). While the fragment ion of PS (P-16:0/16:0) at m/z 631.47 (Figure 2b) shows intense signals in all areas where the parent ion is present, it also has additional signals in the penis bulb (red arrow). This is due to an overlap with parent ion of species PE-Cer (d32:l) at m/z 631.48. This outlying area can be identified in the correlation plot (Figure 2c) as a separate population of data points (red circle). While most data points in this plot follow the line of best fit, these outlying data points indicate that they do not correspond with the parent ion. It can be concluded that these data points correspond with signal in the penis bulb from the overlapping PE-Cer(d32:l) signal. The MALDI-FTICR-IMS data shows one peak at m/z 631.4820 (PE-Cer(d32:1)) exclusively in the penis bulb and one at m/z 631.4708 (PS(P-16:0/16:0)-serine) colocalizing with m/z 718.50 (data shown in METASPACE) confirming that the differences in distributions (parent vs. fragment ion) are due to these overlapping peaks of very similar mass.
Figure 2.

Pearson correlation coefficient analysis for ToF-SIMS images, a) Source image of parent ion PS(P-16:0/16:0) at m/z 718.50 b) Target image of fragment at m/z 631.47 and c) correlation scatter plot of m/z 718.50 vs m/z 631.47 image including the Pearson’s coefficient R and Coefficient of Determination R2; a red circle indicates data points where the fragment and parent ion do not correlate, d) Target image of lipid at m/z 911.56 and e) correlation scatter plot of m/z 718.50 vs m/z 911.56 images including the Pearson’s coefficient R and Coefficient of Determination R2. Signal intensity in MS-images 0-100%.
Unlike the fragment ion feature, the area of the correlation plot that refers to signals exclusively from the parent ion image does not contain any outlying data points (grey triangle area in Figure 2c). This means there are no pixels in the image where the parent is present and the fragment ion is not, indicating the m/z 718.50 ToF-SIMS peak corresponds to a single lipid species. The same is true for the correlation plots using the other fragment ions (Figure S4c–e). It should be noted that the correlation analysis works best if a peak is composed of a single lipid species (such as m/z 718.50). If lipid isomers are present with different distributions in the sample, there could be pixels in the image containing the parent but not the fragment ions for one of the species.
To verify that the correlation plots are truly tracking correlation between images, this method was challenged by comparing images for PS(P-16:0/16:0) (Figure 2a) and PI( 18:0/22:5) at m/z 911.57 (Figure 2d), two lipid species that show different spatial distributions. As expected, these peaks show a low correlation coefficient (R=−0.1) and a negative slope for the line of best fit.
Identifying lipids using only ToF-SIMS
In order to determine if lipid species could be unambiguously identified using only ToF-SIMS data we tested this approach with the species at m/z 849.59. A database (Lipidmaps) search based on the ToF-SIMS data, m/z 849.5864 (mass accuracy: 0.87 ppm) produces six possible assignments which are all PI-isomers. There are no other known lipid species within 5 ppm of this mass. Figure 3a shows ToF-SIMS images of the m/z 849.59 molecular ion as well as those for the fragment ions for potential isomer species of PI(O-18:0/18:1) (Figure 3b) and PI(P-18:0/18:0) (Figure 3c). The nominal masses and image correlation coefficients are included in the images. The fragments for PI(O-18:0/18:1) show the most similar distributions to the molecular ion with correlation coefficients ranging from R=0.92-0.79. In contrast, the correlation for the PI(P-18:0/18:0) fragments is poor with correlation coefficients in the range of R=0.81 – 0.10) All other potential isomers also showed less correlation to their fragment ions than those for PI(O-18:0/18:1) (Figure S5). Therefore, the ToF-SIMS data demonstrates that PI(O-18:0/18:1) is the only logical assignment for the detected species at m/z 849.5862. We propose that the majority of peaks in the spectrum can be identified unambiguously in the same way.
Figure 3.
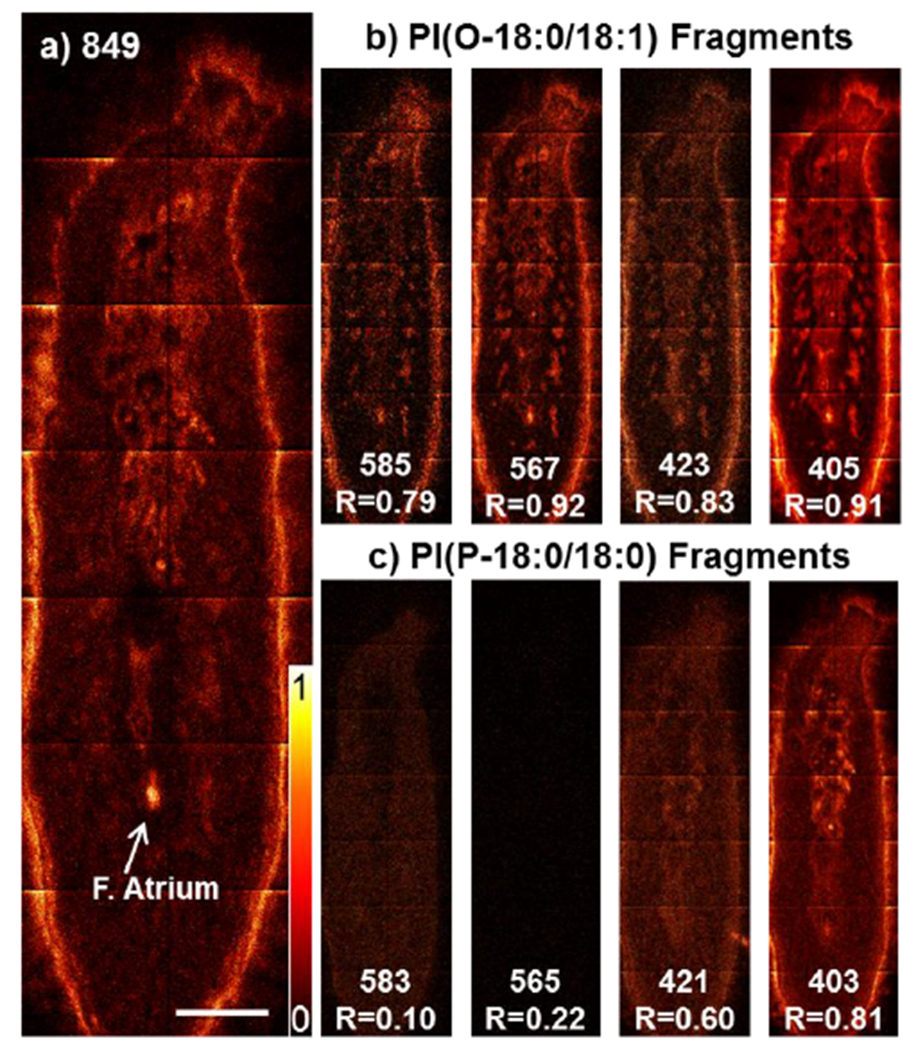
Assigned lipid species PI(O-18:0/18:1) based on fragments in ToF-SIMS data, a) Distribution of the molecular ion m/z 849.59 in planarian flatworm sections. Scalebar 500 μm. b) Distribution of PI(O-18:0/18:l) and c) PI(P-18:0/18:0) specific fragments with nominal mass and correlation coefficient (R).
Even without the images, one can conclude that PI(O-18:0/18:1) is the most likelv assignment for m/z 849.59 just from ToF-SIMS spectrum. In the ToF-SIMS spectrum (Figure 4 blue) extracted from the pixels covering the female atrium region, the peaks for the fragments for PI(O-18:0/18:1) (highlighted in black) are very prevalent. Key fragments which would support an isomer other than PI(O-18:0/18:1) are of low intensity. A comparison between the highlighted fragments in black and the LC-MS/MS spectrum of m/z 849.6 in red shows that the same fragments are produced. Identities for each fragment peak are listed in Figure S5c.
Figure 4.

ToF-SIMS and LC-MS/MS spectra comparison for PI(O-18:0/18:1). GCIB-ToF-SIMS spectra: total ion (grey), female atrium (blue) where PI(O-18:0/18:1) and its fragments peaks are highlighted in black. Mirroring the ToF-SIMS spectra is a partial LC-MS/MS spectra of PI(O-18:0/18:1) extracted from worm 1 (average of 6 spectra) (red). ToF-SIMS and LC spectra were normalized to m/z 405.28.
Identifying overlapping peaks
While the previous sections demonstrate that lipid species can be uniquely identified in ToF-SIMS imaging data without performing MS/MS experiments, there remain some challenges to this approach. As mentioned earlier, lower mass fragments are often produced by numerous lipid species so their overall distribution can diverge from the parent ion which can complicate lipid identification. A more difficult challenge is the current limited mass resolution in ToF-SIMS spectral data. In typical ToF-SIMS analysis, species with similar masses can produce overlapping peaks in the spectrum. This results in poor mass accuracy for either species, making an accurate mass assignment difficult.
As an example, Figure 5 shows ToF-SIMS, and MALDI-FTICR-MS of overlapping lipid species, PS(P-16:0/18:1) at m/z 744.52 and PE(18:1/18:0) at m/z 744.55 (as well as the C13 isotopologue at m/z 744.61 of a Ceramide species at m/z 743.60). Figure 5a shows a MALDI-FTICR-MS spectrum where, due to the higher mass resolution of this technique, those species are detected as separate signals with different distributions in the MALDI-FTICR images in Figure 5c. In contrast, the average ToF-SIMS spectrum (Σ ToF-SIMS) for entire worm in Figure 5b shows only one oddly shaped peak. The corresponding ToF-SIMS image in Figure 5d (Σ 744) resembles a merged version of the MALDI images in Figure 5c. However, when region of interest (ROI) spectra are extracted from Area 1 and 2 (A1, A2), as indicated in ToF-SIMS image Σ 744 in Figure 5d, the resulting spectra show 2 separate peaks around m/z 744.5 (Figure 5b: A1 (PS) and A2 (PE)). The ToF-SIMS peak at 744.52 corresponds to PS(P-16:0/18:1) as the fragments corresponding with this species are fully resolved in the spectrum and co-localize with the parent ion (shown in Figure S2). However, the ToF-SIMS peak and the corresponding image at m/z 744.60 are likely a mixture of PE(18:1/18:0) and the ceramide isotopologue. The low abundance of the PE species hinders us from further distinguishing it from other signals.
Figure 5.

Distinguishing lipid species with similar mass, a) MALDI-FTICR mass spectrum, labelled species PS(P-16:0/18:1) at m/z 744.5185, PE(18:1/18:0) at m/z 744.5549 and the C13 isotopologue at m/z 744.6105 of ceramide species at m/z 743.6, b) ToF-SIMS spectra of entire tissue section (Σ ToF-SIMS) elevated by +0.5 for clarity, and ROI spectra from Area 1 (At (PS)) and 2 (A2 (PE)) as highlighted in ToF—SIMS image (Σ 744) in d), spectra normalized to max intensity at m/z 744.5±0.5amu. c) MALDI-FTICR images of labelled peaks in a), d), c) ToF-SIMS images of peak at m/z 744.5±0.05amu, m/z 744.5±0.5amu (Σ 744) and 744.60±0.05amu.
Discussion
The examples discussed here show that correlating parent and fragment ions spatially in the sample can be used to more confidently identify lipids using ToF-SIMS. The fragmentation due to primary ion impact during GCIB-ToF-SIMS imaging can be utilized as an “inherent/on-sample MS/MS” capability. This approach enables us to even assign lipid species of relatively low abundance, a task that would be difficult using currently available SIMS-MS/MS devices. The additional MALDI-FTICR-IMS and LC-MS/MS data assist in confirming the lipid assignments and validate this approach. We demonstrated that ToF-SIMS produces many of the same fragments for lipids as have been reported using other techniques, which means that databases currently used for analyzing MS/MS spectra could also be used to assist in identifying lipids in ToF-SIMS data. The excellent spatial resolution provided by ToF-SIMS enables us not only to identify lipids found in very limited regions (e.g. present in features the width of a single cell layer), but also to determine the exact tissue feature/organ this lipid is in. An example was shown for PS(P-16:0/16:0), which is present only in the single cell, epithelial layer coating of the pharynx, female atrium, and skin of the worm. While the same information could be obtained by using a combination of MALDI-FTICR-MS and LC-MS/MS the combination of the improved spatial resolution and the fragmentation data within a single ToF-SIMS experiment makes it potentially more desirable than the multi-technique approach. In addition, ToF-SIMS does not require complex sample preparation often needed for these other methods. This could be a benefit, especially for precious and unique samples. Knowing the exact species and location of lipids could aid immensely in generating biologically relevant conclusions, understanding the underlying mechanisms leading to the exact localized lipid profile, as well as understanding the role a lipid has to play in a given location.
The mass resolution for ToF-SIMS is generally sufficient to resolve many lipid species, but this can be highly dependent on the analyzed sample. The spectra generated from the planarian worm contained a great number of species of similar mass that were only resolved in the MALDI-FTICR mass spectra. One solution we presented here is to resolve the overlapping peaks by analyzing spectra from different regions of interest in the sample and look for resolved fragments, but this approach only works if the overlapping species do not co-localize. If fragments from overlapping peaks do co-localize, FTICR-MS data might be necessary to identify the signal. While the limits in mass resolution of ToF-SIMS could be resolved by exchanging the ToF mass analyzer for one with higher mass resolving power (e.g. Orbitrap-SIMS22). However, these high resolution approaches can significantly increases the analysis time.
Lipid isomers present in one sample can pose a challenge for this approach. However, it can still be possible to identify isomers in the sample by looking at multiple colocalizing fragments. Often one possible assignment will be supported by co-localization of the fragment species pair [M–sn1]− and [M–sn2]− with the parent ion while other possible assignments will only show co-localization with one or no associated fragment. If two or more fragment pairs supporting different assignments are present, colocalize with each other, and partially colocalize with the parent ion, then multiple lipid isomers are present. In this situation, using additional methods to confirm isomer identification is advisable.
For the purpose of this study, ToF-SIMS data was analyzed manually, peak by peak creating lists for each chemically different area of the worm and indicating if the peak is present. We previously reported on a comprehensive list of peaks and co-localizing fragments,33 and this list was used to associate fragments with parent ions using the “Glycerophospholipid MS/MS Prediction” tool in LipidMaps, starting from the highest mass. Using this method, nearly all signals present in the ToF-SIMS spectrum were assigned. However, this assignment method is work intensive and introduces a lot of human bias. To the best of our knowledge there are currently no assignment programs specifically for imaging ToF-SIMS data, which presents a huge drawback.
Conclusion
Lipidomics is an important field of biomedical research. Information on the molecular species of a lipid and its spatial origin are equally important to determine a lipid’s function, the significance of its localization, and its involvement in disease. Here we demonstrate that for some lipids imaging GCIB-ToF-SIMS has the capability to simultaneously detect, identify, and localize lipid species with sub-cellular resolution in Phagocata gracilis planarian flatworm tissue sections. The wealth of information contained in GCIB-ToF-SIMS imaging data enables us to make specific lipid assignments. The validity of our approach was confirmed by MALDI-FTICR-IMS and LC-MS/MS. However, it should be noted that for some lipids, such as multiple isomers present in one sample, multi-technique experiments may still be necessary. These new insights dramatically increase the significance and reliability of ToF-SIMS data for biological studies, and provide evidence that it is a viable option for lipid analysis of biological samples.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are thankful for the funding received from the NIH for funding this work (No. NESACBIO NIH P41 EB002027). Part of this work was conducted at the Molecular Analysis Facility, a National Nanotechnology Coordinated Infrastructure site at the University of Washington which is supported in part by the National Science Foundation (grant NNCI-1542101), the University of Washington, the Molecular Engineering & Sciences Institute, and the Clean Energy Institute. Another portion of this work was performed at EMSL, a U.S. Department of Energy (DOE) User Facility located on the campus of Pacific Northwest National Laboratory, which is sponsored by the Office of Biological and Enviromnental Research (BER) within the US-DOE.
Footnotes
Supporting Information
The supporting information contains information on LC-MS/MS, lipid data, details for correlation analysis and additional figures and descriptions.
The authors declare no competing financial interest
References
- 1.Fernandis AZ; Wenk MR, Membrane lipids as signaling molecules. Curr Opin Lipidol, 2007,18 (2), 121–128. [DOI] [PubMed] [Google Scholar]
- 2.Nishikawa M; Horning M; Ueda M; Shibata T, Excitable Signal Transduction Induces Both Spontaneous and Directional Cell Asymmetries in the Phosphatidylinositol Lipid Signaling System for Eukaryotic Chemotaxis. Biophys J 2014,106 (3), 723–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Röhrig F; Schulze A, The multifaceted roles of fatty acid synthesis in cancer. Nature Reviews Cancer 2016, 7(5(11), 732. [DOI] [PubMed] [Google Scholar]
- 4.Di Paolo G; Kim T-W, Linking lipids to Alzheimer's disease: cholesterol and beyond. Nature Reviews Neuroscience 2011, 72, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harayama T; Riezman H, Understanding the diversity of membrane lipid composition. Nat Rev Mol Cell Bio 2018,19 (5), 281–296. [DOI] [PubMed] [Google Scholar]
- 6.Sune A; Bette-Bobillo P; Bienvenue A; Fellmann P; Devaux PF, Selective outside-inside translocation of aminophospholipids in human platelets. Biochemistry 1987, 26 (11), 2972–2978. [DOI] [PubMed] [Google Scholar]
- 7.King RJ; MacBETH MC, Interaction of the lipid and protein components of pulmonary surfactant Role of phosphatidylglycerol and calcium. Biochimica et Biophysica Acta (BBA)-Biomemhranes 1981, 647 (2), 159–168. [DOI] [PubMed] [Google Scholar]
- 8.Angerer TB; Magnusson Y; Landberg G; Fletcher JS, Lipid Heterogeneity Resulting from Fatty Acid Processing in the Human Breast Cancer Microenvironment Identified by GCIB-ToF-SIMS Imaging. Anal Chem 2016, 88 (23), 11946–11954. [DOI] [PubMed] [Google Scholar]
- 9.Bluestein BM; Morrish F; Graham DJ; Huang L; Hockenbery D; Gamble LJ, Analysis of the Myc-induced pancreatic β cell islet tumor microenvironment using imaging ToF-SIMS. Biointerphases 2018,13 (6), 06D402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stopka SA; Samarah LZ; Shaw JB; Liyu AV; Velickovic D; Agtuca BJ; Kukolj C; Koppenaal DW; Stacey G; Pasa-Tolic L; Anderton CR; Vertes A, Ambient Metabolic Profiling and Imaging of Biological Samples with Ultrahigh Molecular Resolution Using Laser Ablation Electrospray Ionization 21 Tesla FTICR Mass Spectrometry. Anal Chem 2019, 91 (8), 5028–5035. [DOI] [PubMed] [Google Scholar]
- 11.Jackson SN; Wang H-YJ; Woods AS, In situ structural characterization of phosphatidylcholines in brain tissue using MALDI-MS/MS. J Am Soc Mass Spectr 2005, 16 (12), 2052–2056. [DOI] [PubMed] [Google Scholar]
- 12.Cerruti CD; Benabdellah F; Laprevote O; Touboul D; Brunelle A, MALDI Imaging and Structural Analysis of Rat Brain Lipid Negative Ions with 9-Aminoacridine Matrix. Anal Chem 2012, 84 (5), 2164–2171. [DOI] [PubMed] [Google Scholar]
- 13.Ellis SR; Paine MRL; Eijkel GB; Pauling JK; Husen P; Jervelund MW; Hermansson M; Ejsing CS; Heeren RMA, Automated, parallel mass spectrometry imaging and structural identification of lipids. Nature methods 2018. [DOI] [PubMed] [Google Scholar]
- 14.Spengler B; Hubert M, Scanning microprobe matrix-assisted laser desorption ionization (SMALDI) mass spectrometry: instrumentation for sub-micrometer resolved LDI and MALDI surface analysis. Journal of the American Society for Mass Spectrometry 2002, 13 (6), 735–748. [DOI] [PubMed] [Google Scholar]
- 15.Kaya I; Brinet D; Michno W; Syvänen S; Sehlin D; Zetterberg H; Blennow K; Hanrieder J, Delineating Amyloid Plaque Associated Neuronal Sphingolipids in Transgenic Alzheimer’s Disease Mice (tgArcSwe) Using MALDI Imaging Mass Spectrometry. ACS Chemical Neuroscience 2017, 8 (2), 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veličković D; Agtuca BJ; Stopka SA; Vertes A; Koppenaal DW; Paša-Tolić L; Stacey G; Anderton CR, Observed metabolic asymmetry within soybean root nodules reflects unexpected complexity in rhizobacteria-legume metabolite exchange. The ISME Journal 2018,12 (9), 2335–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher GL; Hammond JS; Larson PE; Bryan SR; Heeren RMA, Parallel imaging MS/MS TOF-SIMS instrument. J Vac Sci Technol B 2016, 34 (3), 03H126. [Google Scholar]
- 18.Phan NTN; Munem M; Ewing AG; Fletcher JS, MS/MS analysis and imaging of lipids across Drosophila brain using secondary ion mass spectrometry. Analytical and Bioanalytical Chemistry 2017, 409 (16), 3923–3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu T; Touboul D; Della-Negra S; Houel E; Amusant N; Duplais C; Fisher GL; Brunelle A, Tandem Mass Spectrometry Imaging and in Situ Characterization of Bioactive Wood Metabolites in Amazonian Tree Species Sextonia rubra. Anal Chem 2018, 90 (12), 7535– 7543. [DOI] [PubMed] [Google Scholar]
- 20.Smith DF; Robinson EW; Tolmachev AV; Heeren RMA; Paša-Tolić L, C60 Secondary Ion Fourier Transform Ion Cyclotron Resonance Mass Spectrometry. Anal Chem 2011,83 (24), 9552–9556. [DOI] [PubMed] [Google Scholar]
- 21.Angerer TB; Pour MD; Malmberg P; Fletcher JS, Improved Molecular Imaging in Rodent Brain with Time-of-Flight-Secondary Ion Mass Spectrometry Using Gas Cluster Ion Beams and Reactive Vapor Exposure. Anal Chem 2015, 87 (8), 4305–4313. [DOI] [PubMed] [Google Scholar]
- 22.Passarelli ΜK; Pirkl A; Moellers R; Grinfeld D; Kollmer F; Havelund R; Newman CF; Marshall PS; Arlinghaus H; Alexander MR; West A; Horning S; Niehuis E; Makarov A; Dollery CT; Gilmore IS, The 3D OrbiSIMS-label-free metabolic imaging with subcellular lateral resolution and high mass-resolving power. Nat Methods 2017, 14 (12), 1175-+. [DOI] [PubMed] [Google Scholar]
- 23.Sheraz née Rabbani S; Berrueta Razo I; Kohn T; Lockyer NP; Vickerman JC, Enhancing Ion Yields in Time-of-Flight-Secondary Ion Mass Spectrometry: A Comparative Study of Argon and Water Cluster Primary Beams. Anal Chem 2015, 87 (4), 2367–2374. [DOI] [PubMed] [Google Scholar]
- 24.Hartler J; Triebl A; Ziegl A; Trötzmüller M; Rechberger GN; Zeleznik OA; Zierler KA; Torta F; Cazenave-Gassiot A; Wenk MR; Fauland A; Wheelock CE; Armando AM; Quehenberger O; Zhang Q; Wakelam MJO; Haemmerle G; Spener F; Köfeler HC; Thallinger GG, Deciphering lipid structures based on platform-independent decision rules. Nat Methods 2017, 14 (12), 1171–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cajka T; Fiehn O, Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. Trac-TrendAnal Chem 2014, 61, 192–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Köfeler HC; Fauland A; Rechberger GN; Trötzmüller M, Mass spectrometry based lipidomics: an overview of technological platforms. Metabolites 2012, 2 (1), 19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor MJ; Zhang KY; Graham DJ; Gamble LJ, Fatty acid and lipid reference spectra. Surface Science Spectra 2018, 25 (2), 025001. [Google Scholar]
- 28.Adams KJ; DeBord JD; Fernandez-Lima F, Lipid specific molecular ion emission as a function of the primary ion characteristics in TOF-SIMS. J Vac Sci Technol B 2016, 34 (5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderton CR; Vaezian B; Lou K; Frisz JF; Kraft ML, Identification of a lipid-related peak set to enhance the interpretation of TOF-SIMS data from model and cellular membranes. Surf Interface Anal 2012, 44 (3), 322–333. [Google Scholar]
- 30.Fahy E; Cotter D; Sud M; Subramaniam S, Lipid classification, structures and tools. Biochim Biophys Acta 2011, 1811 (11), 637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu FF; Turk J, Differentiation of 1-O-alk-1 ‘-eny1-2-acyl and 1-O-alkyl-2-acyl glycerophospholipids by multiple-stage linear ion-trap mass spectrometry with electrospray ionization. J Am Soc Mass Spectr 2007, 18 (11), 2065–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu FF; Turk J, Studies on phosphatidylserine by tandem quadrupole and multiple stage quadrupole ion-trap mass spectrometry with electrospray ionization:Structural characterization and the fragmentation processes. J Am Soc Mass Spectr 2005, 16 (9), 1510–1522. [DOI] [PubMed] [Google Scholar]
- 33.Angerer TB; Chakravarty N; Taylor MJ; Nicora CD; Graham DJ; Anderton CR; Chudler EH; Gamble LJ, Insights into the histology of planarian flatworm Phagocata gracilis based on location specific, intact lipid information provided by GCIB-ToF-SIMS imaging. Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids 2019, 1864 (5), 733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayasu ES; Nicora CD; Sims AC; Burnum-Johnson KE; Kim Y-M; Kyle JE; Matzke MM; Shukla AK; Chu RK; Schepmoes AA; Jacobs JM; Baric RS; Webb-Robertson B-J; Smith RD; Metz TO, MPLEx: a Robust and Universal Protocol for Single-Sample Integrative Proteomic, Metabolomic, and Lipidomic Analyses. mSystems 2016, 1 (3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kyle JE; Crowell KL; Casey CP; Fujimoto GM; Kim S; Dautel SE; Smith RD; Payne SH; Metz TO, LIQUID: an-open source software for identifying lipids in LC-MS/MS-based lipidomics data. Bioinformatics 2017, 33 (11), 1744–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fletcher JS; Rabbani S; Henderson A; Blenkinsopp P; Thompson SP; Lockyer NP; Vickerman JC, A New Dynamic in Mass Spectral Imaging of Single Biological Cells. Anal Chem 2008, 80 (23), 9058–9064. [DOI] [PubMed] [Google Scholar]
- 37.Chinga G; Syverud K, Quantification of paper mass distributions within local picking areas. Nord Pulp Pap Res J 2007, 22 (4), 441–446. [Google Scholar]
- 38.Fahy E; Sud M; Cotter D; Subramaniam S, LIPID MAPS online tools for lipid research. Nucleic Acids Res 2007, 35, W606–W612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson K; Galton F, VII. Note on regression and inheritance in the case of two parents. Proceedings of the Royal Society of London 1895, 58 (347–352), 240–242. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


