Abstract
Purpose
After years of stable or declining HIV prevalence and declining incidence among people who inject drugs (PWID) in the United States, some rapidly emerging outbreaks have recently occurred in new areas (e.g., Scott County, Indiana). However, to our knowledge, trends over time in HIV prevalence among PWID in U.S. metropolitan statistical areas (MSAs) across all major regions of the country have not been systematically estimated beyond 2002, and the extent to which HIV prevalence may be increasing in other areas is largely unknown. The present paper estimates HIV prevalence among PWID in 89 of the most-populated U.S. MSAs, both overall and by geographic region, using more recent surveillance and HIV testing data.
Methods
We computed MSA-specific annual estimates of HIV prevalence (both diagnosed and undiagnosed infections) among PWID for these 89 MSAs, for 1992–2013, using several data series from the Centers for Disease Control and Prevention’s (CDC) National HIV Surveillance System and National HIV Prevention Monitoring and Evaluation data; Holmberg’s (1997) estimates of 1992 PWID population size and of HIV prevalence and incidence among PWID; and research estimates from published literature using 1992–2013 data. A mixed effects model, with time nested within MSAs, was used to regress the literature review estimates on all of the other data series. Multiple imputation was used to address missing data. Resulting estimates were validated using previous 1992–2002 estimates of HIV prevalence and data on ARV prescription volumes, and examined for patterns based on geographic region, numbers of people tested for HIV, and baseline HIV prevalence.
Results
Mean (across all MSAs) trends over time suggested decreases through 2002 (from approximately 11.4% in 1992 to 9.2% in 2002), followed by a period of stability, and steep increases after 2010 (to 10.6% in 2013). Validation analyses found a moderate positive correlation between our estimates and ARV prescription volumes (r = .45), and a very strong positive correlation (r = .94) between our estimates and previous estimates by Tempalski et al. (2009) for 1992–2002 (which used different methods). Analysis by region and baseline prevalence suggested that mean increases in later years were largely driven by MSAs in the Western US and by MSAs in the Midwest that had low baseline prevalence. Our estimates suggest that prevalence decreased across all years in the Eastern U.S. These trends were particularly clear when MSAs with very low numbers of people tested for HIV were removed from analyses to reduce unexplained variability in mean trajectories.
Conclusions
Our estimates suggest a fairly large degree of variation in 1992–2013 trajectories of PWID HIV prevalence among 89 U.S. MSAs, particularly by geographic region. They suggest that public health responses in many MSAs (particularly those with larger HIV prevalence among PWID in the early 1990s) were sufficient to decrease or maintain HIV prevalence over time. However, future research should investigate potential factors driving the estimated increase in prevalence after 2002 MSAs in the West and Midwest. These findings have potentially important implications for program and/or policy decisions, but estimates for MSAs with low HIV testing denominators should be interpreted with caution and verified locally before planning action.
Introduction
After years of declining numbers of new HIV infections attributed to injection drug use (IDU),1 and relatively stable or declining HIV prevalence among people who inject drugs (PWID) in cities with high HIV prevalence,2–4 HIV outbreaks may now be occurring among PWID in some areas of the United States. A 2015 outbreak in Scott County, Indiana demonstrated that HIV can spread rapidly upon introduction of a single HIV-positive individual into a tight PWID network, as 170 new HIV cases were reported among PWID within 7 months (in a relatively sparsely-populated area) and the estimated prevalence among PWID there reached 38%.5,6 A surge in new HIV cases among PWID (from 5 in 2016 to 18 in 2017) was recently reported for Northern Kentucky counties.7 In Massachusetts, the annual proportion of newly-diagnosed HIV infections attributed to IDU grew sharply from 4–8% in the past decade to 14% in 2017, with a vast majority of new cases registered in two neighboring cities.8,9
The United States is vulnerable to new outbreaks of HIV among PWID because of the large number of PWID in the country, the relatively high prevalence of HIV among PWID, low rates of viral suppression in this population, growing rates of young and new injectors,10 and poor syringe service access among PWID in many places (increasing the likelihood of syringe sharing). Per 2011 estimates, there were about 6.6 million people who reported IDU in their lifetime and 770 thousand who reported IDU in the past year (i.e., in 2010) in the United States.11 Also, the total number of PWID and of young PWID (aged 15–29) may be rising, as suggested by estimated increases in the population size of young PWID living in large U.S. metropolitan statistical areas (MSAs) between 1992 and 2007.10 According to the Centers for Disease Control and Prevention (CDC), more than 196 thousand PWID were living with HIV in 2014, and approximately 50% of them had not achieved viral suppression, thus serving as a large reservoir of the virus.12 Access to sterile syringes and drug treatment - effective HIV prevention tools when scaled appropriately - remains inadequate. In 2000, only 3 syringes were distributed on average per 100 injection events according to estimates for 35 metropolitan areas;13 and drug treatment coverage has been estimated to remain stably low (between 5% and 7%, on average) from 1993 to 2007 among U.S. MSAs.14 In 2015, CDC National HIV Behavioral Surveillance of PWID living in 20 US metropolitan areas found that just a quarter of current PWID reported obtaining all their syringes from sterile sources.15
Effective prevention of HIV among PWID should be based on reliable epidemiological data. HIV prevalence estimates among PWID are very challenging to create because of difficulties in accurately ascertaining both the numerator (i.e., the number of PWID living with HIV) and the denominator (i.e., the total number of PWID). The CDC’s surveillance systems (including its National HIV Surveillance System and its National HIV Prevention Monitoring and Evaluation – NHM&E – data) track HIV diagnoses, by key population (e.g., PWID, men who have sex with men, high-risk heterosexuals). However, our ability to use some of these data (specifically, NHM&E HIV testing data) to estimate HIV prevalence for specific geographic areas and specific years is limited by the fact that HIV-infected people are typically only tested and diagnosed once (and are therefore only counted in one year of testing data), and often move between geographic areas, which can lead to inaccuracy of prevalence estimates. The surveillance data only provide information about the number of PWID who have HIV (i.e., the numerator). Because they do not provide information about the size of the underlying PWID population (i.e., the denominator), calculating HIV prevalence among PWID with these data is challenging and requires creative strategies.
Without adequate estimates of HIV prevalence, if surveillance data show an increase in the number of PWID newly testing positive for HIV in a given area, we currently do not know if the increase reflects changes in that area in 1) PWID population size; 2) HIV incidence; 3) HIV testing rates; or 4) some combination of these three factors. Not knowing which of these factors is changing undermines our capacity to plan the best targeted intervention strategy and assess its impact. Improving our ability to estimate HIV prevalence could therefore improve our ability to mount appropriate responses to such increases and to prevent outbreaks from becoming catastrophic.
Several studies assessing HIV prevalence among PWID in the largest MSAs have been conducted over the past 30 years. These include cross-sectional assessments by Holmberg for 1992,16 and by Friedman et al. for 1998,17 as well as longitudinal estimates by Tempalski et al. for 1992–2002.18 These estimates of local HIV prevalence were useful for making informed decisions about policy reforms and resource allocations as well as for planning, implementing, and evaluating prevention and treatment interventions for MSAs within the United States. Data on HIV-related trends may help to forecast possible outbreaks,19 and longitudinal data are particularly valuable for examining the influence of sociostructural determinants of HIV and related outcomes among PWID and other populations.15,20, 21, 22 This information may be particularly useful in MSAs with established HIV epidemics among PWID, since it can enable distinguishing between prevalence fluctuations and serious outbreaks.
The last available estimates of HIV prevalence among PWID extend only through 200218. Since then, the United States has experienced several HIV outbreaks among PWID,5,8 as well as further acceleration of the opioid epidemic (including heroin use).23,24 We therefore need updated estimates of HIV prevalence among PWID in MSAs to guide national and local-level decision-making and to support research on sociostructural and other contextual determinants and outcomes of variation in HIV prevalence among PWID. The present paper produces and presents new annual HIV prevalence estimates among PWID in 89 of the largest U.S. MSAs, for 1992–2013. It also examines variation in MSA-level trajectories of HIV prevalence among PWID by geographic region and other key MSA characteristics.
Methods
Sample
The unit of analysis in this study is the MSA. The U.S. Census Bureau and Office of Management and Budget define an MSA as a set of contiguous counties that include one or more central cities of at least 50,000 people that collectively form a single, cohesive socioeconomic unit, defined by inter-county commuting patterns and socioeconomic integration (OMB, 2000). The MSA was selected as the unit of analysis because data were readily available at this geographic level and because MSAs are meaningful epidemiologic units with which to study PWID and services designated for them.25, 26 Injection-related epidemics extend from central cities to their surrounding suburbs, because PWID who live in the suburbs often buy drugs and perhaps receive drug-related social services in the central city.25, 26
The sample of MSAs included in the present study was obtained by selecting the universe of United States MSAs with populations greater than 500,000 in 1993 (N = 96). Although multiple imputation of missing data was conducted for tenable amounts of data missingness (see below), we were unable to compute estimates of HIV prevalence among PWID for 7 MSAs with insurmountably large amounts of missing data (i.e., MSAs with six or more consecutive years of data missing on at least one data series used for estimates creation – specifically, Birmingham, AL; Honolulu, HI; Little Rock, AR; Richmond, VA; San Juan, PR; Springfield, MA; and Wichita, KS). Estimates of HIV prevalence among PWID were computed for the resulting sample of 89 MSAs.
Measures
For the present study, estimates of HIV prevalence among PWID were created by using 1) MSA-level data on HIV among PWID from several data series from the CDC; 2) previous MSA-level estimates of PWID population size and of HIV prevalence and incidence among PWID for 1992, created by Holmberg16; and 3) available research estimates of HIV prevalence among PWID from relevant published literature. Table 1 provides details on each of these data sources. The rationale for including these specific data series is that the CDC data series are the best and most comprehensive data available on rates of HIV among PWID, but each alone is subject to various biases (e.g., reporting errors; undiagnosed HIV cases). By a) using multiple CDC data series together, b) anchoring trajectories by adjusting our model using a plausible and well-reasoned set of estimates of the characteristics of each MSA’s baseline (1992) HIV epidemic among PWID (i.e., the Holmberg estimates), and c) incorporating additional available data on HIV prevalence among PWID not captured by CDC (from the extant published literature), we aim to adjust the CDC surveillance data and reduce error in the available data to the highest degree possible.
Table 1.
Description of Data Sources Used to Compute Estimates of HIV Prevalence among People Who Inject Drugs (PWID)
| Database | Description and Characteristics |
|---|---|
| Counseling & Testing Services* | Individual reports of HIV testing events at CDC HIV testing sites (NHM&E) (1992–2013), plus HIV Expanded Testing Program Initiative (ETP) in 2008–2013. Data are a count of tests - not of unique individuals - and thus can count an individual more than once a year. Data reports are for PWID (anyone for whom drug injection was reported as a source of risk for HIV) ages 15–64. |
| Cumulative AIDS Deaths** | AIDS deaths data gives the number of individuals with diagnosed HIV infection ever classified as Stage 3, AIDS who have died, as reported to CDC by state and local health departments through the CDC’s National HIV Surveillance System. Deaths may have been due to any cause. These data report yearly number of AIDS deaths among PWID for ages 15–64 for years 1992–2013. We computed “cumulative” AIDS deaths by calculating, for each year, the sum of all AIDS deaths occurring that year and all previous years, starting in 1992. |
| Persons Living with AIDS** | Persons living with AIDS (PLWA) data gives the number of living individuals with HIV infection ever classified as AIDS as reported to CDC by state and local health departments through the CDC’s National HIV Surveillance System. These data report yearly number of PLWA among people reporting injection drug use, for ages 15–64 for years 1992–2013. |
| Holmberg Estimates | This series utilizes previously-published estimates by Holmberg from 1992 on HIV incidence and prevalence among PWID, ages 15–64. |
| Population Size | U.S. Census Bureau’s Population Estimates Program (PEP). Data for each MSAs are by race/ethnicity and ages 15–64 per 10,000 population for years 1990–2013. |
| Literature Estimates | Our research estimates were based on a review of published literature and conference abstracts, as well as web-based searches and inquiries of researchers to find HIV prevalence rate estimates among PWID in any of the 96 MSAs of interest. To be eligible, a study had to have been conducted during 1992–2013 and to have determined HIV serostatus through the testing of blood, urine, or saliva samples rather than through self-report. An additional inclusion criterion was that the study could not have been part of the CDC NHM&E HIV testing system (as we already captured NHM&E data directly and included it in the computation of our estimates). We identified eligible research-based estimates from 18 of 96 metropolitan areas totaling 132 data points over time. The annual number of research-based estimates were concentrated mainly within MSAs having substantial research institutions with an interest in drug using populations and tending to have highly populated central cities (i.e., New York City, Chicago, Baltimore, Los Angeles and San Francisco). The research studies were categorized by setting: (1) drug treatment centers and methadone maintenance treatment programs (MMTP); (2) syringe exchange programs (SEPs); (3) sexually transmitted disease (STD) clinics; (4) prisons; (5) street outreach/network; and (6) all other settings. |
| National HIV Behavioral Surveillance*** | Data were collected as part of the CDC’s National HIV Behavioral Surveillance (NHBS), a CDC-funded multi-city annual cross-sectional survey designed to characterize HIV prevalence, behavioral risks among high-risk populations and extent and nature of these populations’ contact with HIV related services. Surveillance is conducted in rotating, annual cycles in three different populations at increased risk for HIV: 1) MSM (cycle 2008; 2011); 2) PWID (cycle 2009; 2012); and 3) Heterosexuals (cycle 2007; 2010). |
Data were acquired through a special data request from CDC’s HIV Counseling and Testing in Publicly Funded Sites; and HIV Expanded Testing Program Initiative (ETP).
Data were acquired through a special data request to the CDC’s National HIV Surveillance System.
Data were acquired through a special data request to the CDC’s National HIV Behavioral Surveillance.
The present study defines PWID as people who reported having ever injected drugs. Most of our data sources defined PWID this way as well. However, not all of the published literature from which we obtained estimates of HIV prevalence among PWID defined PWID in this way; some published studies defined PWID as people who injected drugs within a specific time period (e.g., the previous 6 months or 1 year). We discuss potential implications of this variation in the Limitations section.
Computing Annual Estimates of HIV Prevalence among PWID, 1992–2013
Estimates of HIV prevalence among PWID were created using data on the following constructs, all of which are defined and described in detail in Table 1: 1) percent of PWID who were HIV positive in National HIV Prevention Monitoring and Evaluation (NHM&E) Testing data; 2) cumulative AIDS deathsa (from the CDC’s National HIV Surveillance System; NHSS), 3) people living with AIDS (PLWA) per 10k population (also from the CDC’s NHSS), 4) Holmberg’s 1992 estimate of HIV prevalence, 5) Holmberg’s 1992 estimate of HIV incidence, 6) Holmberg’s 1992 estimate of the population size of PWID, and 7) number of NHM&E testing events per 10k population as predictors of a “literature-based estimates” outcome. The “literature-based estimates” outcome was based on a literature review of all studies that used data collected for samples of PWID in our MSAs between 1992 and 2013 to measure HIV prevalence. This outcome variable included 2009 and 2012 HIV estimates for PWID from the multi-site NHBS study (see Table 1). Where there were two or more research estimates for a given MSA in a given year, the mean was taken. The following multilevel equation, with time nested within MSAs, summarizes the estimation model:
| Equation 1 |
with η0k : N(0, σ2η0), α0ijk : N(0, σ2α0), independent of one another, where j indexes MSAs, k indexes years, and c indexes all covariates (i.e., all seven predictor variables listed above); where η0k reflects variability across MSAs and α0jk reflects variability across years; where x indexes each of the independent variables listed above, and where Y = “literature-based estimates” as described above. In other words, all of the other data series (#s 1–7 above) were included as covariates in our multilevel model predicting the “literature-based estimates” outcome.
Multiple imputation was conducted in SAS to address missing data in all key variables. In addition to truly missing data, we deleted some cases from the NHM&E testing data because too few individuals were tested in a given MSA and year, and we observed during descriptive examination of these data that such denominators produced year-by-year NHM&E-computed percentages of PWID who tested positive that were highly unstable. Specifically, in the NHM&E data, if the number of individuals tested in a given MSA and year was less than 40%b of the number of individuals tested the previous year in that MSA, or if the number of individuals tested was less than 30 in a given MSA and year, the NHM&E data points for that MSA and year were deleted. However, we did not impute these low-denominator cases (N = 68), but instead left them missing, resulting in a slightly reduced overall sample size (N = 1,890).
To compute the HIV prevalence estimates, the multilevel, mixed effects equation described above was applied (using PROC MIXED in SAS) only to data from the subset of 18 MSAs that had a reasonable amount of data from research estimates (including NHBS estimates), where a “reasonable” amount was defined as having at least one research estimate from early years (before 1997), at least one research estimate between 1997 and 2003, and at least one research estimate in later years (after 2003). The coefficients from this model analyzing the subset of 18 MSAs were then entered into the equation and used to compute estimates for all 89 MSAs.
Validation and Sensitivity Analyses
We assessed convergent validity by computing bivariate correlations between these new 1992–2013 estimates and 1) our previously published estimates of HIV prevalence among PWID for years 1992–2002, which were produced using a different method18; 2) 2006–2013 data on volume of antiretroviral (ARV) prescriptions from each MSA; and CDC’s NHSS data on people living with HIV (PLWH), which were available from 2008–2013. We also conducted sensitivity analyses using slightly modified versions of Equation 1 (as described in detail in Results below), and then repeated the same validation analyses described above with these modified estimates.
Examination of MSA-level Variation in Estimates
After finalizing our annual MSA-level estimates of HIV prevalence among PWID, we observed considerable variation between the MSAs in terms of their 1992–2013 trajectories. In order to preliminarily understand which MSAs (and which characteristics of MSAs) might be driving the mean trajectory for HIV prevalence among PWID, we examined groups of MSAs based on characteristics that we hypothesized might contribute to variation in long-term trajectories of HIV prevalence among PWID. Specifically, we looked at groups of MSAs based on
1) geographic area;
2) baseline (1992) HIV prevalence;
3) MSA population size; and
4) NHM&E testing denominators in the last 5 years (2008–2013) of data.
We examined the fourth characteristic because changes in NHM&E testing procedures between 2007 and 2008 resulted in large drops in denominators for a sizeable proportion of MSAs between these two years, which persisted into later years. To examine these potential covariates and learn whether they were related to variation in trajectories of MSA-level HIV prevalence among PWID, we divided MSAs into groups based on quartiles for each of characteristics #2-#4, separately, and into groups based on the 9 U.S. Census-defined geographic divisions for #1, and then plotted and examined mean trajectories for each group to assess them for qualitatively different patterns. Table 2 lists quartile cutoffs for each of variables #2–4, and lists MSAs included in each group. We then created categorical groups of MSAs based on two variables at a time, and plotted trajectories of HIV prevalence for these groups and examined them for qualitatively different patterns.
Table 2.
Groupings of metropolitan statistical areas (MSAs) by Quartiles Based on Hypothesized Covariates of HIV Prevalence
| Baseline (1992) HIV Prevalence | NHM&E Testing Denominators (i.e., Average Number Tested, 2008–2013)** | Population Size | |
|---|---|---|---|
| Quartile 1 |
Less than 6.3% Akron, OH; Albuquerque, NM; Bakersfield, CA; Cincinnati, OH; Columbus, OH; Dayton, OH; El Paso, TX; Indianapolis, IN; Los Angeles, CA; Louisville, KY; Milwaukee, WI; Orange County, CA; Riverside, CA; Sacramento, CA; San Antonio, TX; San Jose, CA; Seattle, WA; St. Louis, MO; Tacoma, WA; Toledo, OH; Ventura, CA |
Less than 150 Akron, OH; Ann Arbor, MI; Charleston, SC; Denver, CO; El Paso, TX; Fresno, CA; Gary, IN; Grand Rapids, MI; Greenville, SC; Harrisburg, PA; Knoxville, TN; Louisville, KY; Middlesex, NJ; Norfolk, VA; Omaha, NE; Providence, RI; Stockton, CA; Tucson, AZ; Ventura, CA; Youngstown, OH |
Less than 863,268 Akron, OH; Albuquerque, NM; Allentown, PA; Ann Arbor, MI; Bakersfield, CA; Charleston, SC; El Paso, TX; Gary, IN; Harrisburg, PA; Jersey City, NJ; Knoxville, TN; Omaha, NE; Sarasota, FL; Scranton, PA; Stockton, CA; Syracuse, NY; Tacoma, WA; Toledo, OH; Tulsa, OK; Ventura, CA; Wilmington, DE; Youngstown, OH |
| Quartile 2 |
6.3 – 9.2% Cleveland, OH; Dallas, TX; Denver, CO; Fort Worth, TX; Fresno, CA; Gary, IN; Grand Rapids, MI; Harrisburg, PA; Houston, TX; Kansas City, MO; Knoxville, TN; Minneapolis, MN; Oakland, CA; Omaha, NE; Orlando, FL; Phoenix, AZ; Pittsburgh, PA; Portland, OR; Salt Lake City, UT; San Diego, CA; Sarasota, FL; Stockton, CA; Tucson, AZ; Tulsa, OK |
150–345 Albany, NY; Albuquerque, NM; Austin, TX; Columbus, OH; Greensboro, NC; Indianapolis, IN; Memphis, TN; Milwaukee, WI; Nashville, TN; Oklahoma City, OK; San Jose, CA; Scranton, PA; St. Louis, MO; Syracuse, NY; Tacoma, WA; Tulsa, OK |
863,268 – 1,387,243 Albany, NY; Austin, TX; Bergen, NJ; Buffalo, NY; Dayton, OH; Fresno, CA; Grand Rapids, MI; Greensboro, NC; Greenville, SC; Hartford, CT; Jacksonville, FL; Louisville, KY; Memphis, TN; Middlesex, NJ; Monmouth, NJ; Nashville, TN; New Orleans, LA; Oklahoma City, OK; Providence, RI; Raleigh, NC; Rochester, NY; Salt Lake City, UT; Tucson, AZ; West Palm Beach, FL |
| Quartile 3 |
9.3 – 13.9% Allentown, PA; Ann Arbor, MI; Austin, TX; Buffalo, NY; Charleston, SC; Charlotte, SC; Greensboro, NC; Greenville, SC; Las Vegas, NV; Memphis, TN; Nashville, TN; New Orleans, LA; Norfolk, VA; Oklahoma City, OK; Providence, RI; Raleigh, NC; Rochester, NY; Scranton, PA; Tampa, FL; Washington, DC; West Palm Beach, FL; Youngstown, OH |
346–624 Allentown, PA; Atlanta, GA; Bakersfield, CA; Bergen, NJ; Buffalo, NY; Charlotte, NC; Cincinnati, OH; Cleveland, OH; Dallas, TX; Dayton, OH; Fort Worth, TX; Hartford, CT; Houston, TX; Jacksonville, FL; Jersey City, NJ; Kansas City, MO; Las Vegas, NV; Minneapolis, MN; Monmouth, NJ; Nassau, NY; New Orleans, LA; Orange County, CA; Raleigh, NC; Riverside, CA; Rochester, NY; Sacramento, CA; San Antonio, TX; San Diego, CA; Seattle, WA; Toledo, OH; Wilmington, DE |
1,387,244 – 2,284,151 Charlotte, NC; Cincinnati, OH; Cleveland, OH; Columbus, OH; Denver, CO; Fort Lauderdale, FL; Fort Worth, TX; Indianapolis, IN; Kansas City, MO; Las Vegas, NV; Milwaukee, WI; New Haven, CT; Newark, NJ; Norfolk, CT; Orlando, FL; Portland, OR; Sacramento, CA; San Antonio, TX; San Francisco, CA; San Jose, CA |
| Quartile 4 |
14.0% or Greater Albany, NY; Atlanta, GA; Baltimore, MD; Bergen, NJ; Boston, MA; Chicago, IL; Detroit, MI; Fort Lauderdale, FL; Hartford, CT; Jacksonville, FL; Jersey City, NJ; Miami, FL; Middlesex, NJ; Monmouth, NJ; Nassau, NY; New Haven, CT; New York, NY; Newark, NJ; Philadelphia, PA; San Francisco, CA; Syracuse, NY; Wilmington, DE |
625 or Greater Baltimore, MD; Boston, MA; Chicago, IL; Detroit, MI; Fort Lauderdale, FL; Los Angeles, CA; Miami, FL; New Haven, CT; New York, NY; Newark, NJ; Oakland, CA; Orlando, FL; Philadelphia, PA; Phoenix, AZ; Pittsburgh, PA; Portland, OR; San Francisco, CA; Sarasota, FL; Tampa, FL; Washington, DC; West Palm Beach, FL |
2,284,152 or Greater Atlanta, GA; Baltimore, MD; Boston, MA; Chicago, IL; Dallas, TX; Detroit, MI; Houston, TX; Los Angeles, CA; Miami, FL; Minneapolis, MN; Nassau, NY; New York, NY; Oakland, CA; Orange County, CA; Philadelphia, PA; Phoenix, AZ; Pittsburgh, PA; Riverside, CA; San Diego, CA; Seattle, WA; St. Louis, MO; Tampa, FL; Washington, DC |
For NHM&E Testing Denominator variable, true quartiles were not used. Quartile ranges were created using 2008 values. Then, averages across 2008–2013 were computed, and MSAs were grouped according to how these averages mapped onto 2008-based quartiles. This primarily affected which MSAs were included in “Quartiles” 2–3.
In response to a large amount of unexplained variability around our fixed effects trajectories in the later years (2008–2013) of these trajectories, we removed MSAs with very low NHM&E testing denominators during these years, and plotted them again. First, we plotted the trajectories without the MSAs in the lowest quartile of NHM&E testing denominators, and then, we plotted them without the MSAs in the lowest two quartiles of NHM&E testing denominators. Both sets of plots produced similar trajectories, but with the unexplained variability reduced most optimally when we removed only the MSAs in the lowest quartile in terms of NHM&E testing denominators. (This was the most optimal set of plots in part because the Ns were not unduly reduced in any group.) This suggests that NHM&E testing denominators (i.e., the number of people tested for HIV) in the lowest quartile were, in many cases, so low as to produce dramatic fluctuation in our estimates and render them unreliable. We therefore dropped MSAs in the lowest quartile of NHM&E testing denominators for our subgroup analyses.
Results
Trends in HIV Prevalence among PWID, 1992–2013
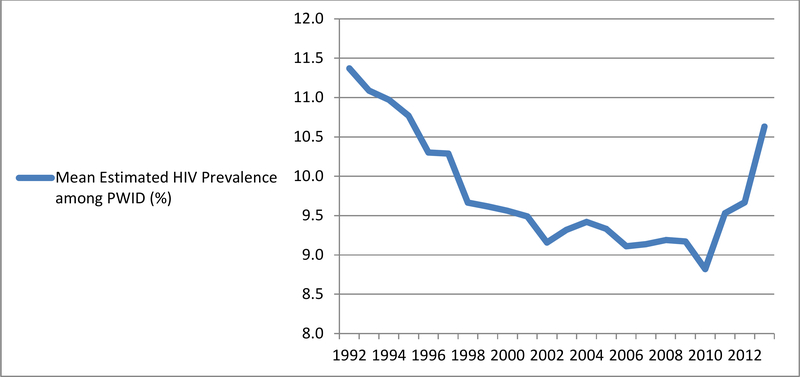
Figure 1 depicts the mean estimated annual percent HIV prevalence among PWID, across all 89 MSAs, from 1992–2013. (Plots of trajectories of estimated HIV prevalence among PWID for each MSA, individually, are available in the online supplement for this article, in Figure S1.) During the 1992–2013 time period, mean (across all 89 MSAs) estimated HIV prevalence among PWID was 9.82% (standard deviation = 5.09), with a range of 1.97–57.01%. From 1992–2010, mean (across 89 MSAs) HIV prevalence among PWID decreased, generally, from 11.37% in 1992 to 8.82% in 2010. It then increased rather steeply over the next three years, to 9.53% in 2011, to 9.67% in 2012, and to 10.63% in 2013, reflecting an estimated prevalence level produced by our most recent year of available data that is similar to the prevalence level estimated for the mid-1990s.
Figure 1.
Trajectories of Mean Estimated HIV Prevalence Among People Who Inject Drugs (PWID), Across 89 U.S. Metropolitan Statistical Areas, 1992–2013
Validation of Estimates and Sensitivity Analyses
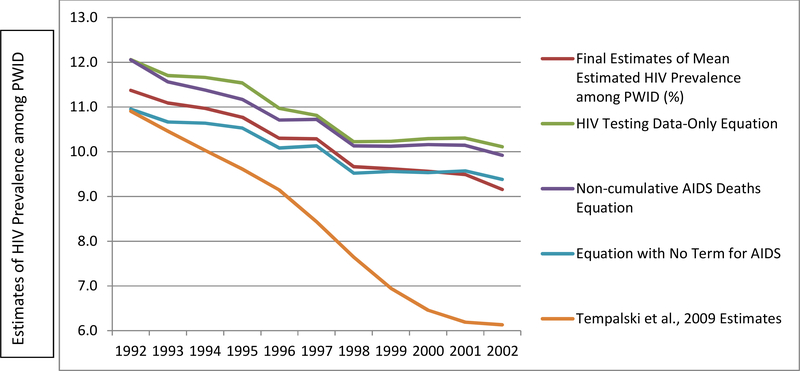
The new set of estimates was compared to our previous (1992–2002) estimates of HIV among PWID. While correlations between the two sets of estimates were very high (r = .94), on average, our new estimates were 1.8% higher than the previous Tempalski et al. estimates.18 To understand what might account for this difference, we conducted sensitivity analyses by rerunning the present model (which is a different model than was used to compute the old estimates) on only 1992–2002 data, and compared the coefficients. The coefficient that changed the most between these two models, by far, was the coefficient for cumulative AIDS deaths. Based on this finding, we created two additional versions of our estimate model, each with a change to this variable, in order to explore how it was affecting our estimates over time, and to see if using a different version of the variable would produce more stable results, or results that were more similar in magnitude to the previous18 set of 1992–2002 estimates. We tested the following modifications to Equation 1:
1) First, we tested a variation of our equation which simply excluded AIDS deaths.
2) We next tested a variation of our equation which replaced cumulative AIDS deaths with non-cumulative AIDS deaths (the number of AIDS deaths each year).
3) Finally, we tested a model which predicted our outcome variable from only time and percent of PWID who were HIV positive in NHM&E testing data.
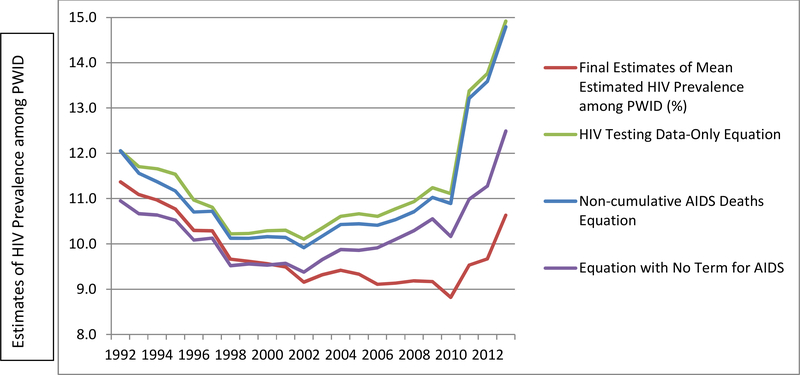
The graph in Figure 2 depicts the estimates produced from each of the four equations described above. Only the original equation maintained the consistently decreasing trend which we expected to see based on our knowledge of HIV trends among PWID in the United States. Each of the other three versions of the equation reflect an increase in HIV prevalence between 2002 and 2010, and unrealistically steep increases between 2010 and 2013. The graph in Figure 3 provides a comparison between the 1992–2002 PWID HIV prevalence estimates produced by Tempalski et al.18 and each of the four sets of estimates produced by the present study’s sensitivity analyses. The mean differences from the previous Tempalski et al. estimates18 were greater for all three of the revised versions than for the estimates produced from the original equation.
Figure 2.
Estimates of HIV prevalence among people who inject drugs (PWID) across 89 U.S. metropolitan statistical areas, created using four different equationsd
Figure 3.
Estimates of HIV prevalence among people who inject drugs (PWID) across 89 U.S. metropolitan statistical areas, previous estimates and the present study’s four equations,e 1992–2002
The new estimates were also validated using CDC’s NHSS data on people living with HIV (PLWH), which were available from 2008–2013. The estimates produced by Equation 1 were positively correlated with PLWH per 10k population (r = .57; p < .01). The new estimates were also validated using data from IMS Healthc on ARV prescription volumes, which were available from 2006–2013. All four versions of the estimates were correlated with MSA-level ARV prescription volumes per 10k population. The estimates resulting from the original equation which used cumulative AIDS deaths were most strongly positively correlated with ARV prescription volumes (r = .45) when all years of data were included. The estimates resulting from the equations which excluded AIDS deaths (r = .23), which included non-cumulative AIDS deaths (r = .33), and which excluded all variables except for time and percent of PWID who were HIV positive in NHM&E Testing data (r = .32) were relatively weakly correlated with ARV prescriptions. This same pattern held true when correlations were examined separately for each available year, with the version using cumulative AIDS maintaining the strongest correlation each year.
Final Estimates of HIV Prevalence among PWID, 1992–2013
Although higher than our previously-computed estimates18, the original equation (the version including cumulative AIDS deaths) produced estimates of HIV prevalence among PWID which were highly correlated with our previous estimates, and which, when compared to estimates derived from several other versions of the equation, were closest in magnitude to our previous 1992–2002 estimates.18 The estimates derived from the original model (including cumulative AIDS deaths) also were most highly correlated with ARV prescription volumes, our key variable for validation. We therefore determined that the estimates produced using this equation were the best estimates of HIV prevalence among PWID from 1992–2013, and heretofore refer to them as our “final” estimates of HIV prevalence among PWID for this period of time.
Examination of MSA-level Variation in Estimates
Upon visual examination of the trajectories of our estimates, we observed strikingly different trajectories among groups based on three variables: geographic area; NHM&E testing denominators; and baseline HIV prevalence. We did not, however, find any qualitatively interesting or meaningful variation between MSA population size-defined quartiles. Specifically, we found decreasing trajectories of HIV prevalence among PWID in the Northeast and Southeast (a subset of the Census-defined “South region”) regions, and increasing trajectories in the Western United States and Midwest. We also found increasing trajectories of HIV prevalence among PWID among MSAs in the first two quartiles for baseline HIV prevalence, and steeply decreasing trajectories among MSAs in the fourth quartile for baseline HIV prevalence. (Plots of these trajectories are available from the authors upon request.)
For further analyses, in order to retain qualitatively meaningful patterns of trajectories we observed in Census-defined geographic divisions, we used a data-driven approach to grouping MSAs from each division into larger geographic areas (rather than relying on the four Census-defined Regions). Based on the extreme similarity of trajectories among MSAs in the Northeast (both the New England and Middle Atlantic Divisions) and in the Southeast (South Atlantic Division and East South Central Division), we combined these four divisions, and now refer to the resulting geographic area as the “Eastern” United States. Based on the extreme similarity of trajectories among MSAs in the West (both the Mountain and Pacific Divisions) and in the “West South Central” division of the Census-defined South region, we combined these geographic clusters together as well, and refer to the resulting geographic area as the “Western United States.” Trajectories among MSAs in the East North Central and West North Central Divisions were similar. We therefore retained the Census-defined Region of “Midwest” for these divisions. Table 2 lists MSAs included in each geographic area.
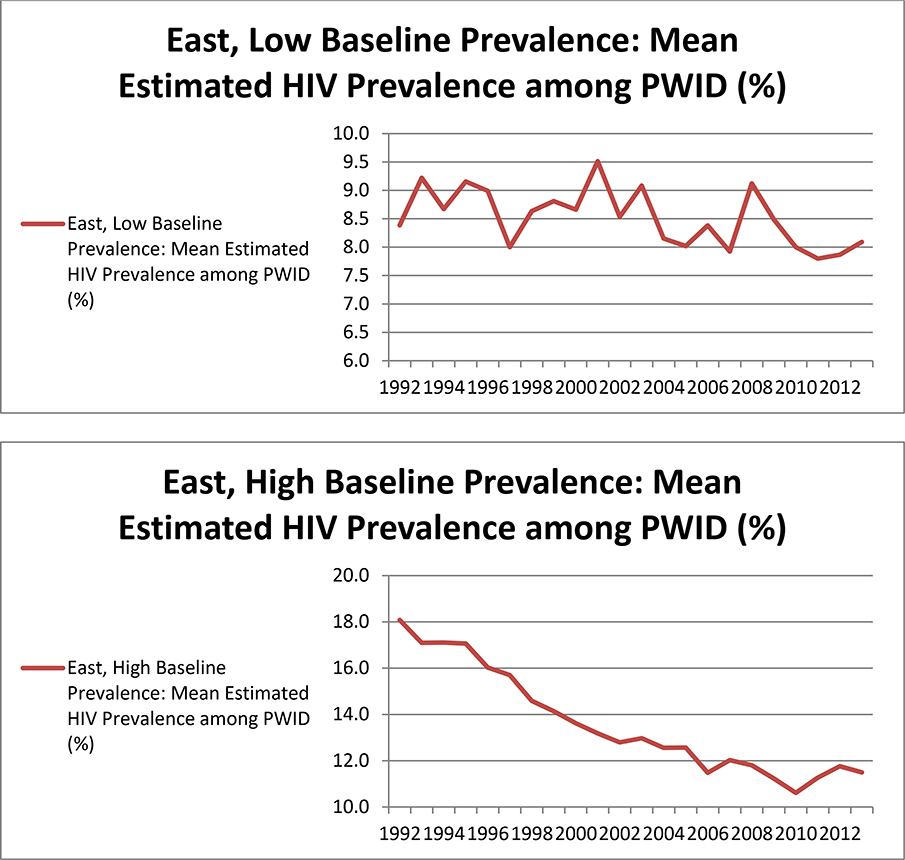
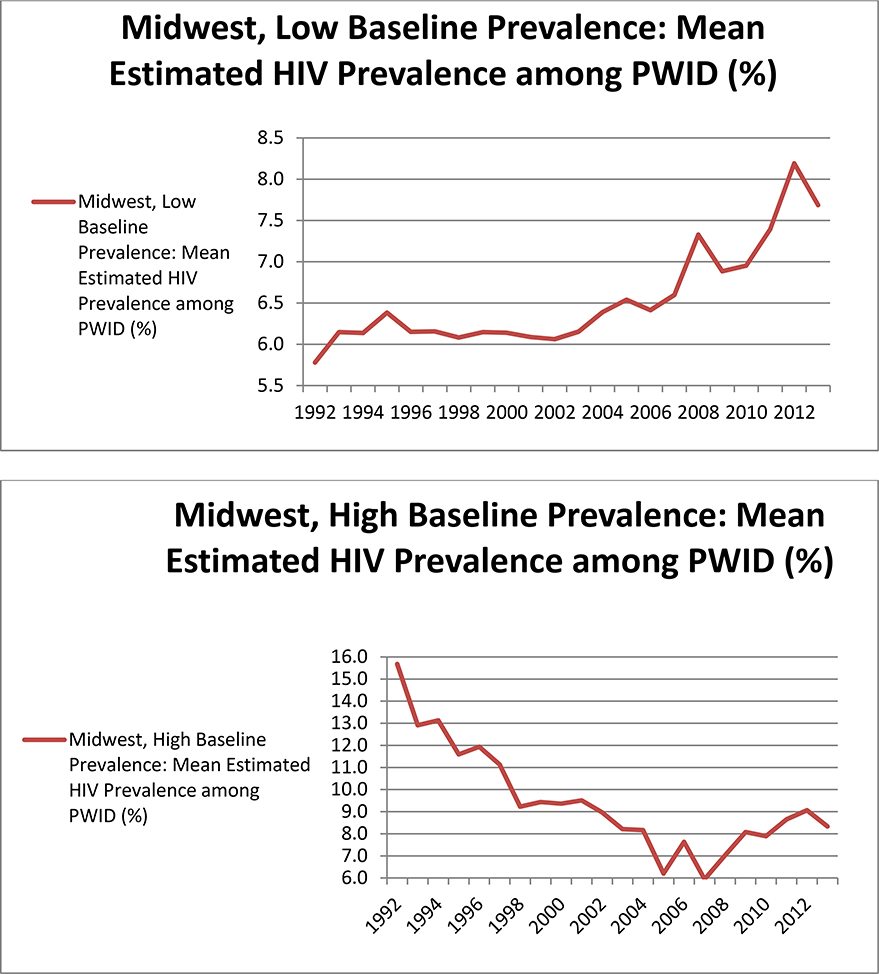
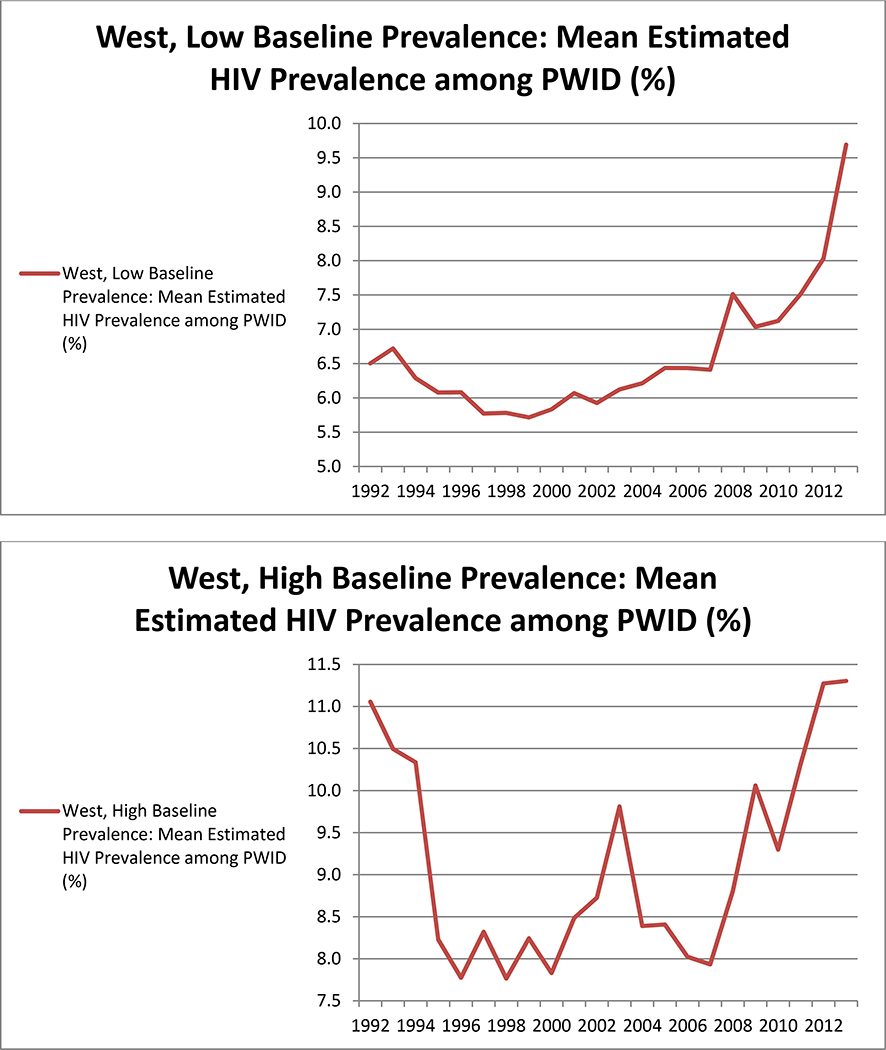
When examining trajectories of HIV prevalence among PWID by groups of MSAs based on two MSA characteristics at a time, we found that grouping MSAs by both baseline HIV prevalence and geographic area, simultaneously, produced the most meaningful and logical patterns in our HIV prevalence estimates. Table 2 lists MSAs included in each group. The plots in Figure 4 depict mean trajectories of HIV prevalence among PWID in these groups of MSAs, among the 68 MSAs in the highest 3 quartiles in terms of NHM&E testing denominators. The trajectories in these plots can be summarized as follows:
1) In the Eastern United states, HIV prevalence among PWID in MSAs with low (i.e., below the median) baseline HIV prevalence stayed relatively stable (within a range of 1.7% across all years). HIV prevalence among PWID in Eastern MSAs with high (i.e., above the median) baseline prevalence decreased steadily over time.
2) In the Western United States, MSAs with low baseline prevalence experienced increases in HIV prevalence among PWID over time. HIV prevalence among PWID in Western MSAs with high baseline prevalence declined initially, but increased again in later years. This U-shaped trajectory remained consistent regardless of whether outliers and/or MSAs with low NHM&E testing denominators were included or excluded during sensitivity analyses.
3) In the Midwest, HIV prevalence among PWID in MSAs with low baseline prevalence increased steadily over time, and HIV prevalence among PWID in MSAs with high baseline prevalence decreased steadily over time.
As an additional set of sensitivity analyses, these trajectories of HIV prevalence among PWID were plotted and examined again, using median values of HIV prevalence for each group of MSAs in each year, instead of mean values. The same pattern of results was found.
Figure 4.
Mean trajectories of HIV prevalence among people who inject drugs (PWID) for subgroups of metropolitan statistical areas (MSAs) based on geographic region and baseline6 HIV prevalence, among the 68 MSAs in the highest 3 quartiles in terms of denominators from HIV testing data
Discussion
Mean (across all 89 MSAs) trajectories plotted using our estimates revealed decreases in HIV prevalence among PWID through 2002, followed by a period of stabilization, and steep increases after 2010. We also found substantively interesting variation between groups of MSAs based on geographic region and baseline HIV prevalence among PWID. Examination of these groups of MSAs suggested that mean increases in HIV prevalence among PWID in later years were largely driven by MSAs in the Western United States (regardless of baseline HIV prevalence) and MSAs in the Midwest that had low baseline prevalence. Prevalence in the Eastern United States was estimated to decrease across all years.
Both HIV incidence and the survival of people living with HIV contribute to HIV prevalence. Before antiretroviral therapy (ART) became widely available, HIV prevalence rates among PWID were primarily a reflection of infection rates, and were modified by differences between the death rates of HIV-infected and uninfected PWID.27 However, in more recent years, increases in the extent to which HIV-positive PWID gain access to ART (given removal of pre-2012 CD4-based restrictions for ART eligibility)28 and are able to adhere to treatment regimens (which have become less onerous over time) have corresponded with steady decreases in HIV-related death rates among PWID.29, 30 Longer lives for PWID with HIV should have increased HIV prevalence. However, deaths due to competing causes (e.g., hepatitis C and overdose) remain high (or are rising) among PWID, and are disproportionately higher among PWID living with HIV.31,32 This, together with the roll-out of harm reduction to reduce HIV transmission in large Eastern and Midwestern MSAs with high prevalence, might explain why HIV prevalence declined, on average, among high-baseline HIV prevalence MSAs in the East and Midwest regions.
Both in the Western United States and the Midwest, MSAs with low baseline prevalence seem to have undergone modest increases in prevalence. We hypothesize that this pattern results from a) new infections that prevention efforts have not been able to prevent (perhaps due to changes in drug use behavior, to program underfunding, to political restrictions on syringe exchange programs, or to challenges related to program targeting), and b) the increasing-over-time availability of ART reducing HIV-related mortality.33 However, increases in our prevalence estimates were steeper in later years (after 2007). Additional research is therefore needed to establish whether or not new infection rates are high, and whether HIV-related mortality rates among the infected are also particularly low during these later years. One possible explanation for these increases in prevalence is that the Great Recession (which began in 2007) led to both government cutbacks for prevention programs and to socioeconomic difficulties (including homelessness) for many people. These factors, which affected the entire United States, but undoubtedly varied in degree and intensity among different MSAs and regions of the country, may have led to higher rates of unsafe injection behaviors and to increases in HIV transmission. Research in the United States has supported a link between homelessness and health risks among PWID;34, 35, 36 and research in Athens, Greece and elsewhere in Europe has described how factors related to the Great Recession led to HIV outbreaks among PWID those locations.37, 38, 39
Public health responses are needed to address these increases in HIV prevalence among PWID. Specifically, harm reduction programs for PWID (e.g., syringe exchange programs, drug treatment) and HIV treatment programs that support treatment linkage and adherence among PWID should be expanded and/or improved in MSAs where increases were estimated. Additionally, efforts to test PWID for HIV and to link those who test positive to care – which are vital not only to reduce new infections and thereby make progress towards the Ending HIV Epidemic (EHE) initiative goals,40 but also to help PWID with HIV infection live long and healthy lives – should also be optimized. Recently, some rural areas and MSAs experiencing HIV outbreaks (or potential outbreaks) among PWID, such as Scott County, Indiana and Louisville, KY, have begun syringe exchange programs and other prevention and harm reduction programs. The present findings underscore the importance of extending these programs to all areas where they are lacking.
Limitations
The estimates presented in this paper are limited by the multiple data sources that were used in our statistical models. Using many data sources collected by many entities over a period of 22 years might be expected to result in a degree of measurement error, in part because many data series modified operational definitions of constructs and/or changed other data collection and reporting methodologies during this period. Further measurement error was likely introduced by the fact that some of the data points we included in our model from our literature search-based estimates of HIV prevalence (which comprised our outcome variable for our analytic model) came from studies that defined PWID differently than we did (and differently than did most of our other data sources). Such studies which defined PWID as people who injected drugs only during a recent period of time would have underestimated the number of PWID identified based on our definition (having ever injected drugs). Although only true of a small number of data points used in our models of 1,958 cases (89 MSAs x 22 years), this could have nonetheless introduced bias into our HIV prevalence estimates. Along similar lines, our analyses combined (i.e., summed) any available data from the data series we used on men who have sex with men and inject drugs (MSMID) with data on other PWID. We were not able to separate these groups in our analyses or even to control for MSMID because of large amounts of missing data on MSMID in many of the smaller MSAs in our sample. This is a limitation because this group is at elevated risk for HIV. Additionally, as previously mentioned, HIV testing data is limited insofar as it cannot account for migration of PWID to other MSAs in years after they were tested for HIV. However, we do not believe that this limitation greatly impacted our estimation models, which combined HIV testing data with seven other data series in order to reduce the potential biases produced by any single data source.
Furthermore, some of our data sources contained missing data. Though we addressed data missingness with multiple imputation, this is an imperfect process that could have introduced bias to our estimates if, for example, our imputation model was poorly specified. As noted above, even when data were available, the reliability of NHM&E testing data on the percent of PWID who tested HIV-positive suffered in many cases from small denominators - a limitation that increased unexplained variability around our estimated trajectories and decreased data reliability. Based on this particular challenge, we suggest that the MSAs listed in the lowest NHM&E testing denominator-defined quartile, in particular, be used only with extreme caution, both analytically and for the purposes of informing programs and/or policy.
Additionally, our estimates are potentially limited insofar as they were created using only the secondary data that were available to us. We therefore may not have included all relevant/important covariates in our model and/or may have incorrectly specified our model (though the sensitivity analyses and validation analyses described above suggest that we specified the best model we could with the available data). One relevant covariate that we are aware we were unable to include is overdose mortality. Because HIV-positive PWID are at elevated risk for drug overdose,41 there is a substantial overlap between deaths among individuals ever diagnosed with AIDS (i.e., late-stage HIV) and overdose deaths that would be difficult to disentangle analytically. The former data series (i.e., deaths among individuals ever diagnosed with AIDS/late-stage HIV) is included in our models. Because we would not know what percentage of deaths among PWID with late-stage HIV were overdose deaths, and vice versa, for any given MSA and year, we therefore did not include the latter, but acknowledge that this is a potential limitation of our estimation model. Given the current opioid crisis and its growth in years since 2013, and associated changes in factors that may impact HIV prevalence, such as mode of opioid administration, future studies estimating HIV prevalence among PWID for these more recent years (when sufficient data for years after 2013 become available) should aim to include data on opioid misuse and/or overdose. Finally, our analyses of patterns of between-MSA variation in trajectories of HIV prevalence among PWID are preliminary. Additional research is needed to identify sociostructural and other correlates of these trajectories.
Conclusions
The estimates produced in this paper suggest a fairly large degree of variation in the 1992–2013 trajectories of HIV prevalence among PWID in the largest MSAs in the United States. Although our estimates suggest that public health responses were sufficient to decrease or maintain levels of HIV prevalence among PWID over time in most MSAs where prevalence was initially (in 1992) the highest, future research should investigate the potential hypothesized factors driving the estimated upward trajectories in other regions. Such research should focus in particular on MSAs in the Western United States and Midwest for which increases in prevalence among PWID were estimated to steepen after 2007. Also, public health responses (e.g., improvement and expansion of harm reduction programs) should be mounted to address these increases in the MSAs for which they are estimated.
Supplementary Material
Table 3.
Grouping of Metropolitan Statistical Areas (MSAs) by Geographic Region and Baseline HIV Prevalence
| Region | MSAs |
|---|---|
| Eastern United States/ Low Baseline** HIV Prevalence | Harrisburg, PA*; Knoxville, TN*; Louisville, KY*; Orlando, FL; Pittsburgh, PA; Sarasota, FL |
| Eastern United States/ High Baseline HIV Prevalence | Albany, NY; Allentown, PA; Atlanta, GA; Baltimore, MD; Bergen, NJ; Boston, MA; Buffalo, NY; Charleston, SC*; Charlotte, NC; Fort Lauderdale, FL; Greensboro, NC; Greenville, SC*; Hartford, CT; Jacksonville, FL; Jersey City, NJ; Memphis, TN; Miami, FL; Middlesex, NJ*; Monmouth, NJ; Nashville, TN; Nassau, NY; New Haven, CT; New York, NY; Newark, NJ; Norfolk, VA*; Philadelphia, PA; Providence, RI*; Raleigh, NC; Rochester, NY; Scranton, PA; Syracuse, NY; Tampa, FL; Washington, DC; West Palm Beach, FL; Wilmington, DE |
| Western United States/ Low Baseline HIV Prevalence | Albuquerque, NM; Bakersfield, CA; Dallas, TX; Denver, CO*; El Paso, TX*; Fort Worth, TX; Fresno, CA*; Houston, TX; Los Angeles, CA; Oakland, CA; Orange County, CA; Phoenix, AZ; Portland, OR; Riverside, CA; Sacramento, CA; Salt Lake City, UT*; San Antonio, TX; San Diego, CA; San Jose, CA; Seattle, WA; Stockton, CA*; Tacoma, WA; Tucson, AZ*; Tulsa, OK; Ventura, CA* |
| Western United States/ High Baseline HIV Prevalence | Austin, TX; Las Vegas, NV; New Orleans, LA; Oklahoma City, OK; San Francisco, CA |
| Midwest/ Low Baseline HIV Prevalence | Akron, OH*; Cincinnati, OH; Cleveland, OH; Columbus, OH; Dayton, OH; Gary, IN*; Grand Rapids, MI*; Indianapolis, IN; Kansas City, MO; Milwaukee, WI; Minneapolis, MN; Omaha, NE*; St. Louis, MO; Toledo, OH |
| Midwest/ High Baseline HIV Prevalence | Ann Arbor, MI*; Chicago, IL; Detroit, MI; Youngstown, OH* |
Deleted from mean trajectories depicted in Figure 4, due to small (Quartile 1) NHM&E testing denominators (i.e., number tested for HIV) between 2008–2013, which produced less reliable/ more ”noisy” estimates.
Baseline for the present study is 1992.
Acknowledgments
Research described in this manuscript was supported by the National Institute on Drug Abuse under award number R01DA037568 (Metropolitan Trajectories of HIV Epidemics and Responses in Key Populations) and award number P30DA011041.
Footnotes
The authors have no Conflicts of Interest to report.
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
“AIDS deaths” are deaths from any cause among persons with HIV ever classified as Stage 3, AIDS.
40% was chosen based on a series of sensitivity analyses to assess the feasibility and impact of using several other percentages (35%; 45%; 50%). The use of a 40% decrease in one year seemed to have the desired effect of removing cases in which extreme drops to very low numbers of people tested occurred, without necessitating the removal of an unduly large number of cases.
IMS Health is a healthcare information and technology company in the United States. “IMS” does not seem to be an abbreviation for a longer name (at least, not one used in recent history in public records). IMS Health recently merged with another company and is now part of the company “IQVIA.” However, as we obtained data from IMS Health before this occurred, we refer to these data using the IMS Health name.
“Final estimates…” equation refers to Equation 1 (Y = b0 + b1×1jk + Σbcxcjk + η0k + α0jk), as defined in detail in the text, in which Y refers to the literature review-based estimates from previous studies, and X refers to data series 1–7 defined in the text. “HIV Testing Data-Only Equation” refers to a model which predicted our outcome variable from only time and percent of PWID who were HIV positive in HIV testing data. “Non-cumulative AIDS Deaths Equation” refers to a variation of Equation 1 which replaced cumulative AIDS deaths with non-cumulative AIDS deaths (the number of AIDS deaths each year). “Equation with no term for AIDS” is a variation of Equation 1 which simply excluded AIDS deaths.
See Figure 2 and text for detailed descriptions of these equations.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Citations
- Centers for Disease Control and Prevention. National HIV Prevention Monitoring and Evaluation data (CDC NHM&E) 1992–2013. Division of HIV/AIDS Prevention. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. [Google Scholar]
- Centers for Disease Control and Prevention. Expanded Testing Program Initiative (ETP) data 2008–2013.Division of HIV/AIDS Prevention. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. [Google Scholar]
- Centers for Disease Control and Prevention. National HIV Surveillance System data (1992–2017). Division of HIV/AIDS Prevention. National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention. [Google Scholar]
- Centers for Disease Control and Prevention. National HIV Behavioral Surveillance data (2017). Division of HIV/AIDS Prevention, National Center for HIV/AIDS, Viral Hepatitis, Sexual Transmitted Diseases and Tuberculosis Prevention. [Google Scholar]
- Holmberg S The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. Am J Public Health 1996; 86: 642–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Commerce. Economics and Statistics Administration. U.S. CENSUS BUREAU; Census 1990, 2000, 2010 Census, and 2013 Population Estimates. [Google Scholar]
References
- 1.Centers for Disease Control and Prevention. HIV Surveillance Report, 2016; vol. 28 http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html Published November 2017.
- 2.Centers for Disease Control and Prevention. HIV infection and risk, prevention, and testing behaviors among injecting drug users—National HIV Behavioral Surveillance System, 20 US cities, 2009. 2014. 1546–0738. [PubMed]
- 3.Centers for Disease Control and Prevention. HIV Infection, Risk, Prevention, and Testing Behaviors among Persons Who Inject Drugs—National HIV Behavioral Surveillance: Injection Drug Use, 20 U.S. Cities, 2012. 2015.
- 4.Centers for Disease Control and Prevention. HIV Infection, Risk, Prevention, and Testing Behaviors among Persons Who Inject Drugs—National HIV Behavioral Surveillance: Injection Drug Use, 20 U.S. Cities, 2015. 2017.
- 5.Janowicz DM. HIV transmission and injection drug use: lessons from the Indiana outbreak. 2016. [PMC free article] [PubMed]
- 6.Peters PJ, Pontones P, Hoover KW, et al. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. New England Journal of Medicine. 2016;375(3):229–239. [DOI] [PubMed] [Google Scholar]
- 7.DeMio T CDC to help find roots of HIV ‘cluster’ tied to heroin, IV drugs in Campbell, Kenton counties. Cincinatti.com. The Enquirer. January 9, 2018, 2018. [Google Scholar]
- 8.Massachussets Department of Public Health. Clinical advisory. HIV transmission through injection drug use. 2017.
- 9.Schumaker E Opioid crisis fuels a spike in HIV infections, and experts fear there’s more to come. HuffPost. 04/18/2018, 2018. [Google Scholar]
- 10.Chatterjee S, Tempalski B, Pouget ER, Cooper HLF, Cleland CM, Friedman SR. Changes in the Prevalence of Injection Drug Use Among Adolescents and Young Adults in Large U.S. Metropolitan Areas. AIDS and Behavior. 2011;15(7):1570–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lansky A, Finlayson T, Johnson C, et al. Estimating the Number of Persons Who Inject Drugs in the United States by Meta-Analysis to Calculate National Rates of HIV and Hepatitis C Virus Infections. PLOS ONE. 2014;9(5):e97596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Fact sheet: HIV among people who inject drugs. 2018; https://www.cdc.gov/hiv/group/hiv-idu.html.
- 13.Tempalski B, Cooper HL, Friedman SR, Des Jarlais DC, Brady J, Gostnell K. Correlates of syringe coverage for heroin injection in 35 large metropolitan areas in the US in which heroin is the dominant injected drug. International Journal of Drug Policy. 2008;19:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tempalski B Cleland CM, Williams LD, Cooper HLF, Friedman SR (In press). Change in drug treatment coverage among people who inject drugs in 90 metropolitan areas in the USA, 1993–2007. Substance Abuse Treatment, Prevention, and Policy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. HIV and injection drug use. Syringe services programs for HIV prevention; 2016. https://www.cdc.gov/hiv/risk/ssps.html [Google Scholar]
- 16.Holmberg SD. The estimated prevalence and incidence of HIV in 96 large US metropolitan areas. American Journal of Public Health. 1996;86(5):642–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Friedman SR, Lieb S, Tempalski B, et al. HIV among injection drug users in large US metropolitan areas, 1998. Journal of Urban Health. 2005;82(3):434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tempalski B, Lieb S, Cleland CM, Cooper H, Brady JE, Friedman SR. HIV prevalence rates among injection drug users in 96 large US metropolitan areas, 1992–2002. Journal of Urban Health. 2009;86(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Handel MM, Rose CE, Hallisey EJ, et al. County-Level Vulnerability Assessment for Rapid Dissemination of HIV or HCV Infections Among Persons Who Inject Drugs, United States. Journal of acquired immune deficiency syndromes (1999). 2016;73(3):323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friedman SR, Cooper HL, Tempalski B, et al. Relationships of deterrence and law enforcement to drug-related harms among drug injectors in US metropolitan areas. Aids. 2006;20(1):93–99. [DOI] [PubMed] [Google Scholar]
- 21.Friedman SR, Tempalski B, Brady JE, et al. Income inequality, drug-related arrests, and the health of people who inject drugs: Reflections on seventeen years of research. International Journal of Drug Policy. 2016;32:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolopoulos GK, Fotiou A, Kanavou E, et al. National income inequality and declining GDP growth rates are associated with increases in HIV diagnoses among people who inject drugs in Europe: A panel data analysis. PLOS One. 2015; 10(4): e0122367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Substance Abuse and Mental Health Services Administration. Results from the 2013 National Survey on Drug Use and Health: summary of national findings. 2014. [PubMed]
- 24.<oj/>Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: Results from the 2016 National Survey on Drug Use and Health 2017.
- 25.Friedman SR, Tempalski B, Cooper H, Perlis T, Keem M, Friedman R, & Flom PL (2004). Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. Journal of Urban Health, 81(3), 377–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Friedman SR, Tempalski B, Brady JE, Friedman JJ, Cooper HL, Flom PL, ... & Des Jarlais DC (2007). Predictors of the degree of drug treatment coverage for injection drug users in 94 metropolitan areas in the United States of America. International Journal of Drug Policy, 18(6), 475–485. [DOI] [PubMed] [Google Scholar]
- 27.Friedman SR, Des Jarlais DC, Jose B, Neaigus B, Goldstein M. “Seroprevalence, Seroconversion, and the History of the HIV Epidemic among Drug Injectors” In: Nicolosi A (Ed.), HIV Epidemiology: Models and Methods (pp. 137–150). New York: Raven Press, 1994. [Google Scholar]
- 28.Bradley H, Hall HI, Wolitski RJ, et al. Vital Signs: HIV diagnosis, care, and treatment among persons living with HIV – United States, 2011. Morbidity and Mortality Weekly Report (MMWR). 2014; 63(47); 1113–1117. [PMC free article] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention - National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention - Division of HIV/AIDS Prevention. Mortality Slides Series through 2015. https://www.cdc.gov/hiv/pdf/library/slidesets/cdc-hiv-mortality.pdf. Accessed August 24, 2018.
- 30.Siddiqi A, Hu X, & Hall HI. Mortality among Blacks or African Americans with HIV infection – United States, 2008–2012. Morbidity and Mortality Weekly Report (MMWR). 2015; 64(04); 81–86. [PMC free article] [PubMed] [Google Scholar]
- 31.van Haastrecht HJ, Mientjes GH, van den Hoek A, & Coutinho RA (1994). Death from suicide and overdose among drug injectors after disclosure of first HIV test result. AIDS. [DOI] [PubMed] [Google Scholar]
- 32.Smit C, van den Berg C, Geskus R, Berkhout B, Coutinho R, & Prins M (2008). Risk of hepatitis-related mortality increased among hepatitis C virus/HIV-coinfected drug users compared with drug users infected only with hepatitis C virus: a 20-year prospective study. JAIDS Journal of Acquired Immune Deficiency Syndromes, 47(2), 221–225. [DOI] [PubMed] [Google Scholar]
- 33.Abbas UL, Anderson RM, & Mellors JW (2006). Potential impact of antiretroviral therapy on HIV-1 transmission and AIDS mortality in reource-limited settings. JAIDS, 41(5), 632–641. [DOI] [PubMed] [Google Scholar]
- 34.Aidala A, Cross JE, Stall R, Harre D, & Sumartojo E (2005). Housing status and HIV risk behaviors: implications for prevention and policy. AIDS and Behavior, 9(3), 251–265. [DOI] [PubMed] [Google Scholar]
- 35.Linton SL, Celentano DD, Kirk GD, & Mehta SH (2013). The longitudinal association between homelessness, injection drug use, and injection-related risk behavior among persons with a history of injection drug use in Baltimore, MD. Drug and alcohol dependence, 132(3), 457–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dickson-Gomez J, Hilario H, Convey M, Corbett AM, Weeks M, & Martinez M (2009). The relationship between housing status and HIV risk among active drug users: a qualitative analysis. Substance Use & Misuse, 44(2), 139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vana Sypsa; Paraskevis Dimitrios, Malliori Meni, Nikolopoulos Georgios K., Panopoulos Anastasios, Kantzanou Maria, Katsoulidou Antigoni, Psichogiou Mina, Fotiou Anastasios, Pharris Anastasia, Van De Laar Marita, Wiessing Lucas, Jarlais Don Des, Friedman Samuel R., and Hatzakis Angelos. 2014. Homelessness and Other Risk Factors for HIV Infection in the Current Outbreak Among Injection Drug Users in Athens, Greece. American Journal of Public Health. 0 0:0, e1–e9 doi: 10.2105/AJPH.2013.301656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nikolopoulos Georgios K., Fotiou Anastasios, Kanavou Eleftheria, Richardson Clive, Detsis Marios, Pharris Anastasia, Suk Jonathan E., Semenza Jan C., Claudia Costa-Storti Dimitrios Paraskevis, Sypsa Vana, Malliori Melpomeni-Minerva, Friedman Samuel R., Hatzakis Angelos. National Income Inequality and Declining GDP Growth Rates are Associated with Increases in HIV Diagnoses among People who Inject Drugs in Europe: A Panel Data Analysis. PLoS One. 2015; 10(4): e0122367 Published online 2015 Apr 15. doi: 10.1371/journal.pone.0122367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikolopoulos Georgios K., Sypsa Vana, Bonovas Stefanos, Paraskevis Dimitrios, Melpomeni Malliori-Minerva Angelos Hatzakis, Friedman Samuel R.. Big Events in Greece and HIV infection among People Who Inject Drugs. May 2015. Substance Use and Misuse. NIHMSID: 671922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.United States Department of Health and Human Services. (2020). What is ‘Ending the HIV Epidemic: A Plan for America?” https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview
- 41.Green TC, McGowan SK, Yokell MA, Pouget ER, & Rich JD (2012). HIV infection and risk of overdose: a systematic review and meta-analysis. AIDS, 26(4), 403–417. doi: 10.1097/QAD.0b013e32834f19b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.