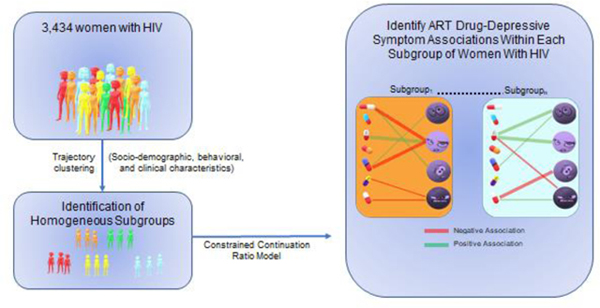
Abstract
Antiretroviral therapy (ART) is inconsistently associated with depression. These associations may depend on factors such as biological sex, age, and health status. Identifying such factors may help optimize treatment of HIV and depression. We implemented a novel approach to examine interindividual variability in the association between ART agents and depressive symptoms. 3,434 women living with HIV (WLWH) from the Women’s Interagency HIV Study (WIHS) were computationally divided into subgroups based on sociodemographic (e.g., age) and longitudinal (from 1995 to 2016) behavioral and clinical profiles (e.g., substance use, HIV RNA, CD4 counts). Five subgroups (n’s ranged from 482 to 802) were identified and characterized as those with: controlled HIV/vascular comorbidities; profound HIV legacy effects; younger women [<45 years of age] with hepatitis C; primarily 35–55 year olds; and poorly controlled HIV/substance use. Within each subgroup, we examined associations between ART agents used over the past 6 months and item-level depressive symptoms on the Center for Epidemiologic Studies Depression Scale. Tenofovir (4 of 5 subgroups) followed by efavirenz, emtricitabine, stavudine, lopinavir, etravirine, nelfinavir, ritonavir, and maraviroc were the most common agents associated with depressive symptoms, although the pattern and directionality varied by subgroup. For example, lopinavir was associated with fewer symptoms among the subgroup with a legacy HIV effect but more symptoms among the subgroup with well-controlled HIV/vascular comorbidities. Unexpectedly, dolutegravir and raltegravir were not associated with depressive symptoms among any subgroup. Findings underscore marked interindividual variability in ART agents on depression in WLWH. Sociodemographic, clinical, and behavioral factors are important determinants of the relationship between ART agents and depressive symptoms in WLWH.
Keywords: HIV, women, depression, heterogeneity, antiretrovirals
Graphical Abstract
Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders among people living with HIV (PLWH), who show higher rates of MDD compared to the general population (Ciesla and Roberts 2001; Cook 2008; Do et al. 2014). The prevalence of depression is twice as high in women as men in the general population (Kessler et al. 1993), a sex difference often (Semple et al. 1996; Rabkin and Rabkin 1997; Robertson et al. 2014; Aljassem et al. 2016), but not always, found among PLWH (Rubin et al. 2019). Depression is a syndrome, and individuals diagnosed with depression may exhibit markedly different profiles of emotional, cognitive, somatic, and interpersonal symptoms. The determinants of depression after HIV infection are multifactorial. They may be attributed in part to HIV itself, viral proteins (e.g., Tat, gp120), HIV-associated CNS inflammation and alterations in brain white matter, psychosocial factors including stigma, occupational disability, history of psychiatric illness (Rabkin 2008; Sherr and Cluver 2017), and antiretroviral therapy (ART)-related neurotoxicity (Robertson et al. 2012; Underwood et al. 2015; Shah et al. 2016). ART is a potentially modifiable risk factor for depression. When evaluating the association between ART and depression, it is important to consider biological sex, as the efficacy, mechanisms of action, and adverse side effects for many ART drugs may differ by sex (Feinberg 1993; Gandhi et al. 2004; Mangoni and Jackson 2004; Lee et al. 2014).
The ART agents most commonly associated with somatic (e.g., sleep disturbances) and non-somatic depressive symptoms (e.g., feelings of depression and sadness, suicidal ideation) include the non-nucleoside reverse transcriptase inhibitor [NNRTI] efavirenz (EFV) (Mollan et al. 2014; Bengtson et al. 2017; Arenas-Pinto et al. 2018) and the integrase inhibitors (IIs) dolutegravir (DTG) (de Boer et al. 2016; Borghetti et al. 2017; Elzi et al. 2017; Fettiplace et al. 2017; Hoffmann et al. 2017; Menard et al. 2017; Penafiel et al. 2017; Borghetti et al. 2018; Revuelta-Herrero et al. 2018) and raltegravir (RAL) (Madeddu et al. 2012; Hoffmann et al. 2017; Penafiel et al. 2017). Little is known about the effects of other ART agents on depressive symptoms, particularly in WLWH. Possible mechanisms for the negative effects of ART drugs on depressive symptoms include direct effects on neuronal function (e.g., neuronal shrinkage, dendritic pruning), mitochondrial function (e.g., depletion of mitochondrial DNA), blood brain barrier permeability and indirect effects on cerebral blood flow, interference with neurotransmitters, inhibition of microglial innate immune responses, and astrocyte metabolism (Schweinsburg et al. 2005; Ellis et al. 2007; Kohler and Lewis 2007; Liner et al. 2010; Giunta et al. 2011; Manda et al. 2011; Underwood et al. 2015; Shah et al. 2016; Cohen et al. 2017).
In the present analysis, we focus on ART-related depressive symptomatology among WLWH, a group that may be more vulnerable to mood related effects of ART agents (Hoffmann et al. 2017). To accomplish our aim, we leveraged advances in computational modeling and bioinformatics to examine the associations between ART agents and depressive symptomatology among subgroups of WLWH with similar socio-demographic and longitudinal behavioral and clinical characteristics. This subgrouping is important as not only sex but other inter-individual difference factors, including age, body mass index, and compliance influence the effects of ART drugs (Feinberg 1993; Gandhi et al. 2004; Mangoni and Jackson 2004; Lee et al. 2014). We hypothesized that the pattern of associations between ART agents and item-level depressive symptomatology (negative affect, i.e., feelings of depression and sadness, and somatic symptoms i.e., sleep) would depend on socio-demographic, clinical, and behavioral characteristics of WLWH. Additionally, we expected that EFV and the IIs (DTG, RAL) would be most strongly associated with greater item-level depressive symptomatology in WLWH.
Methods
Study Population
The WIHS is a multi-center, longitudinal, study of the natural and treated history of women with HIV. The first three waves of study enrollment occurred between October 1994 and November 1995, October 2001 and September 2002, and January 2011 and January 2013 from six sites (Brooklyn, Bronx, Chicago, DC, Los Angeles, and San Francisco). A more recent wave of enrollment occurred at sites in the southern US (Chapel Hill, Atlanta, Miami, Birmingham, and Jackson) between October 2013 and September 2015. Study methodology, including recruitment procedures and eligibility criteria, training, and quality assurance procedures have been previously published (Barkan et al. 1998; Bacon et al. 2005; Adimora et al. 2018). In brief, WIHS participants complete “core” visits every 6 months and at each of these visits they undergo clinical examination, extensive medical interview (including the collection of ART medications), questionnaires (including the CES-D), and a blood draw. This analysis was restricted to data collected at all WIHS study visits where ART and CES-D scores were collected. Participants were excluded from analysis if ART use “at study visit” and “since last study visit” (~ past 6 months) were discordant as we wanted to ensure stability on ART drugs for the previous 6 months. After excluding 6,943 observations (out of 54,320, 12.79%), 47,377 observations from 3,434 participants remained for analysis with not all women contributing the same number of visits (mean number of visits/participant= 13.8; range 1 to 44). Longitudinal data (sociodemographic, clinical, and behavioral characteristics) was used to divide participants into subgroups so that the clustering is based on each participant’s entire history. Within subgroups, cross-sectional data was used to examine associations between ART agents and depressive symptoms among each subgroup.
Depressive symptomatology
The CES-D is a 20-item self-administered questionnaire measuring how often (0=“rarely” to 3=“most of the time”) participants experience depressive symptomatology which can include emotional, somatic, and interpersonal symptoms in the past two weeks (Radloff 1977). Emotional symptoms on the CES-D include the lack of positive affect (or anhedonia) and the presence of negative affect. Positive affect reflects positive emotions, such as hopefulness and feelings that life is enjoyable, whereas negative affect refers to the experience of feeling negative emotions, including fearfulness, loneliness, sadness, and failure. Somatic symptoms on the CES-D include states of depressive mood that comprise unpleasant or worrisome bodily sensations, including sleep, appetite, and concentration (Kapfhammer 2006). Finally, interpersonal symptoms on the CES-D reflect interpersonal challenges (“people were unfriendly”, “I felt people disliked me”). The CES-D has excellent reliability, validity, and factor structure (Radloff 1977) and is commonly used in HIV studies often using a clinical cutoff score of 16 to indicate depression (Moore et al. 1999; Ickovics et al. 2001; Cook et al. 2002; Cook et al. 2007; Rubin et al. 2011; Maki et al. 2012). The primary outcomes used for analysis were the item-level responses which are discussed in terms consistent with previous factor analyses where CES-D items often cluster into negative, lack of positive, somatic, and interpersonal symptoms (Kim et al. 2011). Importantly, the four items reflecting positive affect were reversed scored so that higher values on each item reflected more symptoms.
Covariates
Covariates were selected based on prior studies demonstrating links between the following list of covariates and depressive symptomatology in WLWH (Cook et al. 2002; Cook et al. 2007; Rubin et al. 2011; Maki et al. 2012). Covariates (see Table 1) included clinic site (11 sites), enrollment wave (4 waves), sociodemographic, behavioral, and clinical factors. Sociodemographic factors (self-reported) included age, race/ethnicity, years of education, employment status, average annual household income, and marital status. Behavioral factors (self-reported) included current smoking status, recent alcohol use, marijuana, and crack, cocaine, and/or heroin use. Clinical factors included Hepatitis C antibody positive, as well as metabolic and cardiovascular factors, including body mass index (BMI), hypertension (systolic blood pressure ≥140, diastolic blood pressure ≥90, self-report or use of anti-hypertensive medications), and diabetes (self-reported anti-diabetic medication or any of fasting glucose ≥126 or HgbA1C >6.5% or self-reported diabetes is confirmed). HIV-related clinical factors included HIV RNA (copies/ml), CD4 count (current and nadir; cells per mm3), and self-reported previous AIDS diagnosis.
Table 1.
Demographic, behavioral, and clinical characteristics at the initial WIHS visit among the overall sample and among subgroups of women living with HIV.
| Variable | Overall (n=3434) n (%) | Subgroup |
P-value | ||||
|---|---|---|---|---|---|---|---|
| 1 (n=658) n (%) | 2 (n=802) n (%) | 3 (n=482) n (%) | 4 (n=762) n (%) | 5 (n=730) n (%) | |||
| Site | <0.001 | ||||||
| Brooklyn, NY | 455 | 51 | 125 | 90 | 98 | 91 | |
| Bronx, NY | 544 | 95 | 138 | 65 | 104 | 142 | |
| Chicago, IL | 415 | 64 | 107 | 58 | 99 | 87 | |
| Los Angeles, CA | 532 | 88 | 109 | 100 | 119 | 116 | |
| San Francisco, CA | 459 | 72 | 102 | 72 | 103 | 110 | |
| Washington, DC | 412 | 59 | 113 | 71 | 67 | 102 | |
| Chapel Hill, NC | 148 | 55 | 19 | 12 | 43 | 19 | |
| Atlanta, GA | 188 | 81 | 27 | 3 | 50 | 27 | |
| Miami, FL | 109 | 34 | 31 | 8 | 22 | 14 | |
| Birmingham, AL | 87 | 31 | 11 | 2 | 34 | 9 | |
| Jackson, MS | 85 | 28 | 20 | 1 | 23 | 13 | |
| Enrollment Wave | <0.001 | ||||||
| 1994–1995 | 1815 | 255 | 461 | 282 | 347 | 470 | |
| 2001–2002 | 735 | 88 | 196 | 155 | 174 | 122 | |
| 2011–2013 | 277 | 86 | 40 | 22 | 72 | 57 | |
| 2013–2015 | 607 | 229 | 105 | 23 | 169 | 81 | |
| Age | <0.001 | ||||||
| <25 | 219 (6) | 7 (1) | 7 (<1) | 161 (33) | 39 (5) | 5 (1) | |
| 26–35 | 1222 (36) | 164 (25) | 614 (76) | 172 (36) | 174 (23) | 98 (13) | |
| 36–45 | 1295 (38) | 43 (7) | 156 (19) | 102 (21) | 378 (49) | 616 (84) | |
| 45–55 | 603 (17) | 383 (58) | 21 (3) | 45 (9) | 143 (19) | 11 (2) | |
| >55 | 95 (3) | 61 (9) | 4 (<1) | 2 (<1) | 28 (4) | 0 (0) | |
| Years of education | 0.06 | ||||||
| Less than high school | 1261 (37) | 250 (38) | 291 (37) | 183 (38) | 244 (32) | 293 (40) | |
| High school | 1051 (31) | 193 (29) | 255 (32) | 141 (29) | 241 (32) | 221 (31) | |
| College or above | 1116 (32) | 215 (33) | 251 (31) | 158 (33) | 277 (36) | 215 (29) | |
| Race/ethnicity | <0.001 | ||||||
| White, non-Hispanic | 452 (13) | 94 (14) | 107 (13) | 37 (8) | 130 (17) | 84 (11) | |
| White, Hispanic | 242 (7) | 72 (11) | 32 (4) | 26 (5) | 39 (5) | 73 (10) | |
| Black, non-Hispanic | 2140 (62) | 406 (62) | 525 (65) | 271 (56) | 510 (67) | 428 (59) | |
| Black, Hispanic | 71 (2) | 5 (<1) | 24 (3) | 11 (2) | 18 (2) | 13 (2) | |
| Other, Hispanic | 424 (12) | 68 (10) | 88 (11) | 114 (24) | 51 (7) | 103 (14) | |
| Asian or Pacific Islander | 35 (1) | 3 (<1) | 12 (1) | 10 (2) | 2 (<1) | 8 (1) | |
| Native American or Alaskan | 23 (<1) | 3 (<1) | 2 (<1) | 1 (<1) | 6 (<1) | 11 (1) | |
| Other | 47 (1) | 7 (1) | 12 (1) | 12 (2) | 6 (<1) | 10 (1) | |
| Average annual household income | 0.05 | ||||||
| <$6000 | 881 (26) | 154 (23) | 212 (27) | 122 (25) | 191 (25) | 202 (28) | |
| $6001–12000 | 117 (35) | 254 (39) | 246 (31) | 160 (33) | 261 (34) | 258 (36) | |
| $12001–18000 | 438 (13) | 69 (10) | 114 (14) | 72 (15) | 95 (12) | 88 (12) | |
| $18001–24000 | 285 (8) | 67 (10) | 65 (8) | 40 (8) | 67 (9) | 46 (6) | |
| $24001–30000 | 189 (5) | 26 (4) | 52 (6) | 32 (7) | 38 (5) | 41 (6) | |
| $30001–36000 | 150 (4) | 33 (5) | 41 (5) | 19 (4) | 31 (4) | 26 (4) | |
| $36001–75000 | 216 (6) | 41 (6) | 52 (6) | 29 (6) | 48 (6) | 46 (6) | |
| >$75000 | 68 (1) | 10 (1) | 14 (2) | 4 (<1) | 24 (3) | 16 (2) | |
| Currently employed | 930 (27) | 151 (23) | 226 (28) | 144 (30) | 219 (29) | 190 (26) | 0.04 |
| Married | 1212 (35) | 221 (34) | 307 (38) | 168 (35) | 263 (35) | 253 (35) | 0.36 |
| Currently smoking | 1697 (49) | 299 (45) | 401 (50) | 220 (46) | 364 (48) | 413 (56) | <0.001 |
| Recent use | |||||||
| Alcohol | 0.008 | ||||||
| Abstainer | 1886 (55) | 370 (56) | 441 (22) | 255 (53) | 426 (56) | 394 (54) | |
| 0–7 drinks/wk | 1183 (34) | 220 (33) | 284 (35) | 191 (40) | 252 (33) | 236 (32) | |
| 7–12 drinks/wk | 101 (3) | 23 (3) | 23 (3) | 9 (2) | 27 (3) | 19 (3) | |
| >12 drinks/wk | 261 (8) | 45 (7) | 52 (6) | 26 (5) | 57 (7) | 81 (11) | |
| Marijuana | 707 (20) | 120 (18) | 157 (19) | 107 (22) | 173 (23) | 150 (20) | 0.23 |
| Crack, cocaine, and/or heroin | 539 (16) | 103 (16) | 106 (13) | 58 (12) | 119 (16) | 153 (21) | <0.001 |
| Hepatitis C RNA positive | 855 (25) | 93 (14) | 158 (20) | 150 (31) | 193 (25) | 261 (36) | <0.001 |
| Body mass index (kg/m2) | 0.002 | ||||||
| <18.5 | 114 (3) | 15 (2) | 26 (3) | 17 (3) | 31 (4) | 25 (3) | |
| 18.5–24.9 | 1119 (33) | 194 (30) | 253 (32) | 180 (38) | 218 (29) | 274 (38) | |
| 25–29.9 | 954 (28) | 187 (29) | 217 (27) | 138 (29) | 203 (27) | 209 (29) | |
| 30–34.9 | 568 (17) | 114 (17) | 142 (18) | 69 (14) | 144 (19) | 99 (14) | |
| 35–39.9 | 304 (9) | 73 (11) | 65 (8) | 34 (7) | 75 (10) | 57 (8) | |
| ≥40 | 337 (10) | 65 (10) | 92 (11) | 38 (8) | 84 (11) | 58 (8) | |
| Hypertension | 993 (29) | 303 (46) | 152 (19) | 88 (18) | 257 (34) | 193 (26) | <0.001 |
| Diabetes | 201 (6) | 73 (11) | 28 (3) | 15 (3) | 51 (7) | 34 (5) | <0.001 |
| CD4 count, median (IQR) | <0.001 | ||||||
| Current | |||||||
| <250 | 906 (26) | 154 (23) | 223 (28) | 109 (23) | 163 (21) | 257 (35) | |
| 251–500 | 1193 (35) | 240 (36) | 277 (35) | 160 (33) | 258 (34) | 258 (35) | |
| 501–1000 | 1126 (33) | 221 (33) | 246 (31) | 177 (37) | 285 (37) | 197 (27) | |
| >1001 | 205 (6) | 43 (6) | 53 (7) | 36 (7) | 55 (7) | 18 (2) | |
| Nadir | <0.001 | ||||||
| <250 | 1905 (55) | 291 (44) | 490 (61) | 251 (52) | 380 (50) | 493 (67) | |
| 251–500 | 1018 (30) | 223 (34) | 220 (28) | 162 (34) | 233 (31) | 180 (25) | |
| 501–1000 | 469 (14) | 133 (20) | 84 (10) | 59 (12) | 137 (18) | 56 (8) | |
| >1001 | 40 (1) | 11 (2) | 6 (<1) | 10 (2) | 12 (1) | 1 (<1) | |
| HIV RNA (copies/mL) | <0.001 | ||||||
| Undetectable | 884 (26) | 243 (37) | 172 (22) | 100 (20) | 238 (31) | 131 (18) | |
| <500 | 366 (10) | 82 (12) | 73 (9) | 46 (10) | 84 (11) | 81 (11) | |
| 501–5000 | 549 (16) | 89 (13) | 148 (18) | 86 (18) | 118 (15) | 108 (5) | |
| 5001–50000 | 921 (27) | 141 (21) | 230 (29) | 153 (32) | 191 (25) | 206 (28) | |
| >50001 | 710 (21) | 103 (16) | 177 (22) | 97 (20) | 129 (17) | 204 (28) | |
| Prior AIDS diagnosis | 800 (23) | 130 (20) | 194 (24) | 104 (21) | 151 (20) | 221 (30) | <0.001 |
| Median number of visits (IQR) | 10 (20) | 6 (10) | 14 (22) | 15 (19) | 10 (18) | 10 (20) | 0.01 |
Note. Current, refers to within the past week; recent, refers to within 6 months of the most recent WIHS visit. Variables reported as n (%) were analyzed with Chi-square tests. IQR=interquartile range
Statistical Analyses
To study ART-associated effects on depressive symptomatology among similar groups of WLWH, we initially clustered 3,434 WIHS women based on their longitudinal covariates, such as BMI, CD4 count, age, etc. using a semi-parametric latent class trajectory model called k-means for longitudinal data with covariates using the R package kmlcov, version 1.0.1 (Mickaël et al. 2013), which clusters longitudinal data using likelihood as a the distance metric. Selection for the optimal number of clusters was based on model fit statistics including the Akaike information criterion and the Bayesian information criterion. The advantage of clustering based on the longitudinal data compared to cross-sectional data is that we can take into account the entire history of a participant rather than treating each visit of one participant independently. Essentially, clustering based on cross-sectional data ignores the intrinsic link among the visits associated with the same corresponding participant. Clustering based on cross-sectional data often results in information loss as well as increases the difficulty of interpreting each cluster as participants could be classified into multiple clusters. For each subgroup, we then fitted a constrained continuation ratio (CCR) model via penalized maximum likelihood using the ART use information as independent variables (X) as well as other covariates (e.g., age, BMI) and each CES-D items as the dependent variable (Y). Data fit and precision were optimized using the lasso penalty and cross-validation. For robustness of the inference on ART drug and item-level depressive symptom associations, we employed a bootstrap aggregation procedure to generate 100 bootstrapping datasets by randomly sampling half of the number of observations without replacement. For robustness of the inference on ART drug and item-level depressive symptom associations and adjustment of multiple comparisons, we employed a bootstrap aggregation procedure to control the false discovery rate (FDR)(Benjamini and Hochberg 1995). Specifically, we generated 100 bootstrapping datasets by randomly sampling half of the number of observations without replacement. We then applied the CCR model to the 100 datasets separately and obtained significant drug-depression associations for each of the datasets. The association of a specific drug-depression item pair was designated as significant if that drug was selected as an important predictor for that depression item in at least 90% of the bootstrapped datasets.
Results
Overall Study Population Characteristics
Our study population included 3,434 WLWH who contributed 47,377 visits in WIHS from April 1995 to September 2017. Based on the initial study visit, 74% of WLWH ranged in age from 26–46 years, and 37% had less than a high school education (Table 1). Minority representation was high, with 62% identifying as Black, non-Hispanic and 21% identifying as Hispanic. The average annual household income was low, with 61% being below $12,000. Sixteen percent reported crack, cocaine, and/or heroin use and 20% reported marijuana use since the previous study visit 6 months earlier. With respect to HIV-related clinical characteristics, 39% had a current CD4+ lymphocyte count greater than 500 cells/μL and plasma HIV RNA was not detected in 26%. Twenty-three percent had a previous diagnosis of AIDS.
Overall, the most commonly used nucleoside reverse-transcriptase inhibitors (NRTI) across all visits were tenofovir disoproxil fumarate (TDF; 60%), lamivudine (3TC; 59%), and emtricitabine (FTC; 54%). The most common NNRTIs were efavirenz (EFV; 29%) and nevirapine (NVP; 18%). The most common protease inhibitors (PIs) were ritonavir (RTV; 36%) and atazanavir (ATV; 24%) (Table 2; see Supplemental Table 1 for number of visits that ART drugs were being used). IIs were less common, with raltegravir (RAL; 12%) being the most common. Only 1% were on an entry inhibitor (maraviroc, MVC). With respect to depressive symptom scores, 35% of women had CES-D scores greater than 16, and the average CES-D score in the sample was 13.9 (SD=12.4). The most common symptoms endorsed (item mean >0.80) were effort, restlessness, lack of happiness, feeling depressed, and lack of feeling hopeful about the future (Table 4).
Table 2.
Number of specific antiretroviral drugs that were being used in the overall sample and by each subgroup of women living with HIV at any study visit.
| Subgroup |
||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | Drug Class | Overall (N=3434) | 1 (n=658) n (%) | 2 (n=802) n (%) | 3 (n=482) n (%) | 4 (n=762) n (%) | 5 (n=730) n (%) | P-value |
| Tenofovir disoproxil fumarate (TDF) | NRTI | 2070 (60) | 405 (62) | 505 (63) | 278 (58) | 492 (65) | 390 (53) | <0.001 |
| Lamivudine (3TC) | NRTI | 2038 (59) | 309 (47) | 513 (64) | 330 (68) | 430 (56) | 456 (62) | <0.001 |
| Emtricitabine (FTC) | NRTI | 1842 (54) | 377 (57) | 444 (55) | 247 (51) | 435 (57) | 339 (46) | <0.001 |
| Zidovudine (ZDV/AZT) | NRTI | 1504 (44) | 190 (29) | 402 (50) | 263 (55) | 315 (41) | 334 (46) | <0.001 |
| Stavudine (d4T) | NRTI | 1101 (32) | 138 (21) | 300 (37) | 174 (36) | 219 (29) | 270 (37) | <0.001 |
| Abacavir (ABC) | NRTI | 984 (29) | 166 (25) | 238 (30) | 143 (30) | 229 (30) | 208 (28) | 0.27 |
| Didanosine (DDI) | NRTI | 680 (20) | 80 (12) | 175 (22) | 102 (21) | 137 (18) | 186 (25) | <0.001 |
| Zalcitabine (DDC) | NRTI | 195 (6) | 29 (4) | 48 (6) | 30 (6) | 35 (5) | 53 (7) | 0.11 |
| Tenofovir alafenamide (TAF) | NRTI | 34 (<1) | 6 (<1) | 7 (<1) | 8 (2) | 8 (1) | 5 (<1) | 0.54 |
| Efavirenz (EFV) | NNRTI | 998 (29) | 185 (28) | 234 (29) | 130 (27) | 236 (31) | 213 (29) | 0.62 |
| Nevirapine (NVP) | NNRTI | 607 (18) | 82 (12) | 157 (20) | 100 (21) | 131 (17) | 137 (19) | 0.001 |
| Rilpivirine (RPV) | NNRTI | 251 (7) | 52 (8) | 69 (9) | 46 (10) | 53 (7) | 31 (4) | 0.003 |
| Etravirine (ETR) | NNRTI | 133 (4) | 25 (4) | 35 (4) | 20 (4) | 30 (4) | 23 (3) | 0.80 |
| Delavirdine (DLV) | NNRTI | 27 (<1) | 2 (<1) | 5 (<1) | 2 (<1) | 10 (1) | 8 (1) | 0.15 |
| Ritonavir (RTV) | PI | 1244 (36) | 216 (33) | 305 (38) | 189 (39) | 277 (36) | 257 (35) | 0.16 |
| Atazanavir (ATV) | PI | 821 (24) | 130 (20) | 215 (27) | 148 (31) | 172 (23) | 156 (21) | <0.001 |
| Nelfinavir (NFV) | PI | 691 (20) | 80 (12) | 185 (23) | 127 (26) | 134 (18) | 165 (22) | <0.001 |
| Lopinavir (LPV) | PI | 534 (15) | 77 (12) | 138 (17) | 86 (18) | 111 (15) | 122 (17) | 0.01 |
| Darunavir (DRV) | PI | 473 (14) | 93 (14) | 107 (13) | 65 (13) | 110 (14) | 98 (13) | 0.04 |
| Indinavir (IDV) | PI | 491 (14) | 65 (10) | 118 (15) | 65 (13) | 109 (14) | 134 (18) | <0.001 |
| Saquinavir (SQV) | PI | 353 (10) | 52 (8) | 98 (12) | 54 (11) | 72 (9) | 77 (11) | 0.08 |
| Fosamprenavir (FPV) | PI | 139 (4) | 19 (3) | 38 (5) | 20 (4) | 32 (4) | 30 (4) | 0.50 |
| Amprenavir (APV) | PI | 68 (2) | 6 (<1) | 23 (3) | 6 (1) | 13 (2) | 20 (2) | 0.03 |
| Tipranavir (TPV) | PI | 11 (<1) | 1 (<1) | 3 (<1) | 0 (0) | 3 (<1) | 4 (<1) | 0.48 |
| Raltegravir (RAL) | II | 425 (12) | 89 (14) | 109 (14) | 65 (13) | 86 (11) | 76 (10) | 0.21 |
| Dolutegravir (DTG) | II | 317 (9) | 63 (10) | 74 (9) | 44 (9) | 77 (10) | 59 (8) | 0.75 |
| Elvitegravir (EVG) | II | 221 (6) | 54 (8) | 45 (6) | 30 (6) | 61 (8) | 31 (4) | 0.01 |
| Maraviroc | EI | 38 (1) | 4 (<1) | 11 (1) | 5 (1) | 7 (<1) | 11 (2) | 0.50 |
NRTI= nucleoside reverse-transcriptase inhibitors; NNRTI= non-nucleoside reverse-transcriptase inhibitor; II= integrase inhibitor; PI= protease inhibitor; EI=entry inhibitor
Table 4.
Item level and total score on the CES-D averaged over all visits for the overall sample and for each subgroup of women living with HIV.
| Subgroup |
||||||||
|---|---|---|---|---|---|---|---|---|
| # | Items Description | Overall M (SD) | 1 (n=658) M (SD) | 2 (n=802) M (SD) | 3 (n=482) M (SD) | 4 (n=762) M (SD) | 5 (n=730) M (SD) | P-value |
| 1 | Bothered | 0.73 (0.97) | 0.68 (0.93) | 0.78 (0.99) | 0.69 (0.95) | 0.70 (0.96) | 0.78 (0.97) | 0.04 |
| 2 | Appetite | 0.62 (0.94) | 0.60 (0.93) | 0.62 (0.94) | 0.59 (0.93) | 0.58 (0.92) | 0.68 (0.98) | <0.001 |
| 3 | Blues | 0.65 (0.96) | 0.61 (0.94) | 0.68 (0.97) | 0.59 (0.92) | 0.62 (0.94) | 0.71 (0.98) | <0.001 |
| 4† | Good as others | 0.66 (1.05) | 0.60 (1.02) | 0.72 (1.07) | 0.59 (1.01) | 0.63 (1.05) | 0.69 (1.06) | 0.15 |
| 5 | Concentration | 0.73 (0.96) | 0.72 (0.94) | 0.74 (0.96) | 0.67 (0.93) | 0.70 (0.94) | 0.80 (0.99) | <0.001 |
| 6 | Depressed | 0.82 (1.02) | 0.77 (0.99) | 0.84 (1.05) | 0.74 (0.98) | 0.80 (1.02) | 0.88 (1.04) | <0.001 |
| 7 | Effort | 1.18 (1.22) | 1.12 (1.20) | 1.27 (1.23) | 1.17 (1.24) | 1.09 (1.20) | 1.21 (1.22) | 0.15 |
| 8† | Hopeful of future | 0.80 (1.07) | 0.80 (1.09) | 0.83 (1.07) | 0.71 (1.04) | 0.76 (1.06) | 0.85 (1.09) | 0.32 |
| 9 | Failure | 0.46 (0.85) | 0.45 (0.82) | 0.50 (0.87) | 0.41 (0.81) | 0.42 (0.82) | 0.52 (0.89) | 0.07 |
| 10 | Fearful | 0.52 (0.86) | 0.47 (0.82) | 0.57 (0.88) | 0.47 (0.84) | 0.48 (0.85) | 0.56 (0.89) | 0.08 |
| 11 | Restless | 1.06 (1.12) | 1.09 (1.13) | 1.04 (1.12) | 0.96 (1.09) | 1.05 (1.13) | 1.14 (1.14) | <0.001 |
| 12† | Happy | 0.85 (1.04) | 0.81 (1.03) | 0.88 (1.03) | 0.80 (1.02) | 0.81 (1.03) | 0.92 (1.07) | <0.001 |
| 13 | Talked less | 0.68 (0.96) | 0.67 (0.94) | 0.70 (0.97) | 0.64 (0.96) | 0.66 (0.95) | 0.74 (0.98) | <0.001 |
| 14 | Lonely | 0.72 (1.00) | 0.70 (0.97) | 0.74 (1.01) | 0.64 (0.95) | 0.67 (0.98) | 0.79 (1.03) | <0.001 |
| 15 | People unfriendly | 0.42 (0.81) | 0.43 (0.82) | 0.42 (0.80) | 0.36 (0.76) | 0.41 (0.80) | 0.46 (0.84) | 0.06 |
| 16† | Enjoyed life | 0.68 (0.99) | 0.63 (0.98) | 0.71 (1.00) | 0.62 (0.96) | 0.65 (0.98) | 0.73 (1.02) | <0.001 |
| 17 | Crying spells | 0.59 (0.93) | 0.55 (0.90) | 0.64 (0.96) | 0.54 (0.89) | 0.58 (0.92) | 0.61 (0.94) | 0.28 |
| 18 | Sadness | 0.76 (0.96) | 0.72 (0.93) | 0.81 (0.99) | 0.69 (0.92) | 0.74 (0.95) | 0.80 (0.98) | 0.16 |
| 19 | People disliked me | 0.37 (0.77) | 0.36 (0.76) | 0.39 (0.79) | 0.31 (0.71) | 0.35 (0.76) | 0.41 (0.81) | 0.03 |
| 20 | Energy | 0.65 (0.93) | 0.67 (0.95) | 0.66 (0.94) | 0.55 (0.88) | 0.62 (0.92) | 0.71 (0.96) | 0.26 |
| Total CES-D score | 13.93(12.40) | 13.44 (11.98) | 14.56 (12.60) | 12.75 (11.92) | 13.32 (12.19) | 14.98 (12.84) | <0.001 | |
| CES-D ≥16 | 0.35 (0.48) | 0.34 (0.47) | 0.38 (0.48) | 0.31 (0.46) | 0.33 (0.47) | 0.38 (0.49) | <0.001 | |
| # of CES-D ≥16, n (%) | 16645 (35) | 2301 (34) | 4779 (38) | 2402 (31) | 3359 (33) | 3804 (38) | ||
Reversed scored item
Identification of Similar Subgroups of WLWH
Using longitudinal covariates (see Methods), the semi-parametric latent class trajectory model identified five subgroups of WLWH that differed on socio-demographic, behavioral, and clinical factors (Table 1; Supplemental Tables 2 and 3). Factors best distinguishing subgroups (P’s<0.001) included age, race/ethnicity, current smoking, crack, cocaine, and/or heroin use, hepatitis C RNA positive, hypertension, diabetes, CD4 current and nadir, HIV RNA, and a prior AIDS diagnosis (Table 3; Supplemental Figures 1 and 2). Women in Subgroup 1 (controlled HIV/vascular comorbidities) had the highest frequency of vascular and metabolic comorbidities (46% hypertension, 11% diabetes) and the highest percentage of undetectable HIV RNA (<500 cp/mL, 49%). Women in Subgroup 2 (profound HIV legacy effects) were primarily 26–45 year olds (95%) with a high frequency of CD4 nadir <250 cells/μL(61%). Women in Subgroup 3 (younger women/hepatitis C) were primarily ≤45 years of age (90%) and a high prevalence of Hepatitis C (31%). Age was the only identifying factor for Subgroup 4 (primarily 35–55 year olds), where 68% were between 36–55 years of age. Subgroup 5 (poorly controlled HIV/substance use) was primarily comprised of 36–45 year old women (84%), with a higher percentage of current smokers (56%) and crack, cocaine, and/or heroin use (21%). This subgroup also had the worst HIV-related clinical characteristics (30% prior AIDS diagnosis, 67% CD4 nadir <250, 70% current CD4 count <250 cells/μL, and 56% HIV RNA >5000 cp/mL).
Table 3.
Socio-demographic, behavioral, and clinical factors that distinguish five subgroups of women living with HIV through the use of a semi-parametric latent class trajectory model.
| Subgroup Number | Subgroup Name | Subgroup Characteristics (%) |
|---|---|---|
| 1 (n=658) | Controlled HIV with Vascular Comorbidities | >45 years (67%), 26–35 years (25%) |
| Hypertension (46%), Diabetes (11%) | ||
| Undetectable HIV RNA (49%) | ||
| 2 (n=802) | HIV Legacy Effects | 26–45 years (95%) |
| CD4 nadir <250 cells/μL (61%) | ||
| 3 (n=482) | Young with Hepatitis C | <45 years (90%) |
| Hispanic ethnicity (31%) | ||
| Hepatitis C RNA (31%) | ||
| 4 (n=762) | Young to Middle Aged | 36–55 years (68%) |
| 5 (n=730) | Substance Abuse and Poorly Controlled HIV | 36–45 years (84%) |
| Smokers (56%), crack, cocaine, and/or heroin use (21%) | ||
| Prior AIDS diagnosis (30%) | ||
| CD4 Nadir <250 cells/μL (67%) | ||
| Current CD4 <250 (70%) | ||
| HIV RNA >5000 cp/mL (56%) | ||
Associations between Individual ART Drugs and Item-level Depressive Symptoms in Subgroups of WLWH
Figure 1 provides the results of the association between ART drug and item-level depressive symptoms in subgroups of WLWH. Blue lines indicate that the ART drug is associated with less symptomatology and red lines indicate that the ART drug is associated with more symptomatology. The weight of the line indicates the magnitude of the association. Table 5 provides the magnitude of the association (or edge weight) between ART drug and item-level depressive symptoms (ordinal scale 0 to 3) in subgroups of WLWH, whereby a negative edge weight is associated with less symptomatology and a positive edge indicates that the ART drug is associated with more symptomatology.
Figure 1.
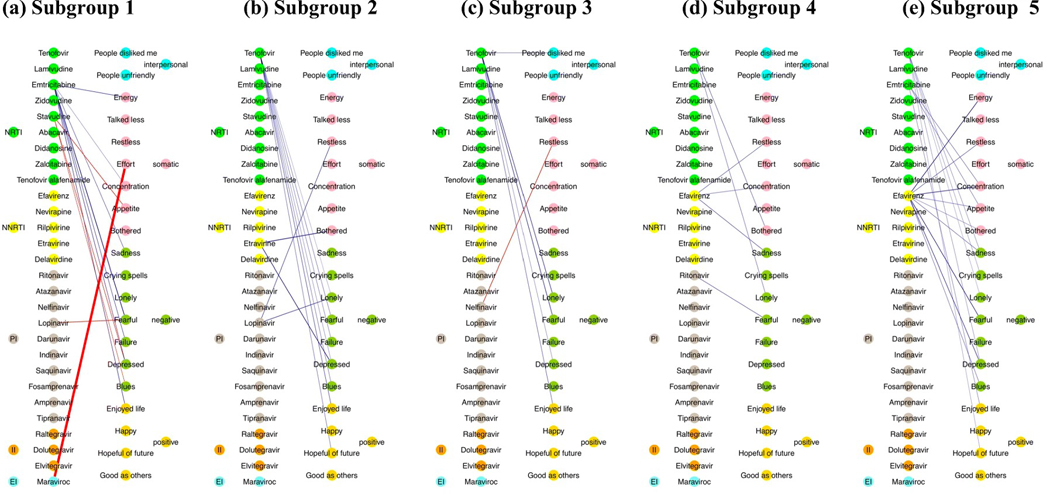
Results of the association between ART drugs and item-level depressive symptoms in each subgroup of women living with HIV, whereby blue lines indicate that the ART drug is associated with less symptomatology and red lines indicate that the ART drug is associated with more symptomatology. The weight of the line indicates the magnitude of the association. The circle colors reflect the ART agent type (e.g., integrase inhibitor, etc) and depression symptom type (e.g., somatic and non-somatic symptoms). NRTI= nucleoside reverse-transcriptase inhibitors; NNRTI= non-nucleoside reverse-transcriptase inhibitor; II= integrase inhibitor; PI= protease inhibitor; EI=entry inhibitor.
Table 5.
Edge weights (ART drug-depressive symptoms) for the data depicted in Figure 1 for each subgroup of women living with HIV.
| Drug Class | Edge | Subgroup | CES-D Category | CES-D Item | Weight | |
|---|---|---|---|---|---|---|
| NRTI | Tenofovir (TDF) | 2 | Positive | Enjoy life | −0.14 | |
| Positive | Hopeful | −0.11 | ||||
| Negative | Fearful | −0.11 | ||||
| Negative | Blues | −0.11 | ||||
| Negative | Failure | −0.10 | ||||
| Negative | Depressed | −0.08 | ||||
| Negative | Lonely | −0.06 | ||||
| 3 | Negative | Fearful | −0.20 | |||
| Negative | Lonely | −0.19 | ||||
| Positive | Enjoyed life | −0.13 | ||||
| Interpersonal | Disliked | −0.12 | ||||
| Negative | Depressed | −0.09 | ||||
| 4 | Somatic | Bothered | −0.13 | |||
| Negative | Lonely | −0.11 | ||||
| 5 | Negative | Depressed | −0.08 | |||
| Negative | Sadness | −0.08 | ||||
| Negative | Crying spells | −0.07 | ||||
| Negative | Lonely | −0.06 | ||||
| Positive | Hopeful | −0.07 | ||||
| Emtricitabine (FTC) | 1 | Negative | Fearful | −0.27 | ||
| Positive | Enjoyed Life | −0.17 | ||||
| Somatic | Energy | −0.17 | ||||
| Negative | Depressed | −0.16 | ||||
| Negative | Failure | −0.15 | ||||
| Negative | Sadness | −0.13 | ||||
| Somatic | Concentration | −0.08 | ||||
| 5 | Somatic | Concentration | −0.11 | |||
| Somatic | Appetite | −0.10 | ||||
| Positive | Enjoy life | −0.10 | ||||
| Stavudine (d4T) | 1 | Somatic | Appetite | 0.21 | ||
| Negative | Depressed | 0.17 | ||||
| Negative | Blues | 0.14 | ||||
| NNRTI | Efavirenz (EFV) | 4 | Negative | Sadness | −0.14 | |
| Somatic | Restless | −0.13 | ||||
| Somatic | Concentration | −0.12 | ||||
| 5 | Somatic | Energy | −0.24 | |||
| Negative | Fearful | −0.22 | ||||
| Somatic | Concentration | −0.22 | ||||
| Negative | Depressed | −0.19 | ||||
| Somatic | Restless | −0.13 | ||||
| Negative | Sadness | −0.12 | ||||
| Negative | Blues | −0.11 | ||||
| Somatic | Appetite | −0.11 | ||||
| Somatic | Bothered | −0.09 | ||||
| Etravirine (ETR) | 2 | Somatic | Bothered | −0.27 | ||
| PI | Lopinavir (LPV) | 1 | Negative | Fearful | 0.21 | |
| 2 | Negative | Lonely | −0.18 | |||
| Somatic | Restless | −0.16 | ||||
| Nelfinavir (NFV) | 3 | Somatic | Restless | 0.22 | ||
| Ritonavir (RTV) | 4 | Negative | Fearful | −0.14 | ||
| EI | Maraviroc | 1 | Somatic | Effort | 1.08 | |
NRTI= nucleoside reverse-transcriptase inhibitors; NNRTI= non-nucleoside reverse-transcriptase inhibitor; II= integrase inhibitor; PI= protease inhibitor; EI=entry inhibitor; CES-D=Center for Epidemiologic Studies Depression Scale; Negative weights indicates that the ART drug is associated with less symptomatology and positive weights indicate that the ART drug is associated with more symptomatology.
The most commonly used NRTI in the sample, TDF (60% of women at 36% of visits), was associated with less severe item-level depressive symptoms in all subgroups (4 out of 5), except the group of women with primarily controlled HIV with vascular comorbidities (Subgroup 1). Of these subgroups where TDF was linked to fewer symptoms, the most common indicated less negative affective symptoms (seen in all 4 subgroups), followed by less positive affective symptoms (seen in 3 out of 4 subgroups). Among one of the 4 subgroups, use of TDF was associated with less somatic and less interpersonal symptoms. Specifically, in the subgroup termed HIV legacy effects (Subgroup 2), use of TDF was associated with less negative and a lack of positive symptoms. In the subgroup primarily <45 years of age with high rates of hepatitis C (Subgroup 3), use of TDF was associated with less negative, a lack of positive, and interpersonal symptoms. In the group of WLWH primarily between 36–55 years of age (Subgroup 4), use of TDF was associated with less negative and somatic symptoms. In the subgroup with highest rates of substance abuse and poorly controlled HIV (Subgroup 5), use of TDF was associated with less negative and less positive symptoms.
FTC, the third most commonly used NRTI in the overall sample (54% of women at 29% of visits), was also associated with less severe item-level depressive symptoms. Links were seen among two of the five subgroups (Subgroups 1 and 5), with the most common symptoms being lack of positive and somatic symptoms. Specifically, in Subgroup 1, use of FTC was associated with less negative, positive, and somatic symptoms. In Subgroup 2, use of FTC was associated with less positive and somatic symptoms.
The only other NRTI that was associated with item-level depressive symptoms was stavudine (D4T) which was used by 32% of women at 10% of visits. In contrast to TDF and FTC, use of D4T was associated with increased item-level depressive symptoms, including negative and somatic symptoms for women with primarily controlled HIV with vascular comorbidities (Subgroup 1). There were no associations between D4T and item-level depressive symptomology for women in the other subgroups.
EFV was the most commonly used NNRTI among women (29% at 15% of visits), and was associated with item-level depressive symptoms in 2 of 5 subgroups (Subgroups 4 and 5). Use of EFV was commonly linked to less negative and somatic symptoms. Specifically, in primarily young-to-middle-aged women (Subgroup 4), use of EFV was associated with less sadness (negative symptoms), better concentration, and less restless sleep (both somatic symptoms). In the subgroup with highest rates of substance abuse and poorly controlled HIV (Subgroup 5), use of EFV was associated with less blues, sadness, feeling depressed and fearful (negative symptoms) and less somatic items (bothered, appetite, concentration, restless sleep, energy). The only other NNRTI linked to item-level depressive symptoms was etravirine (ETR; 4% of women at 1% of visits) in Subgroup 2. Use of ETR was only associated with less feeling of being bothered, a somatic item.
Among the PIs, lopinavir (LPV) was the most common ART drug associated with item-level depressive symptoms. Used by 15% of women at 7% of visits, LPV was associated with greater feelings of fearfulness (negative symptom) in the subgroup with primarily controlled HIV with vascular comorbidities (Subgroup 1), whereas use of LPV was associated with less feelings of restlessness (somatic symptom) and less loneliness (negative symptom) among the subgroup termed HIV legacy effects (Subgroup 2). The only other two PIs linked to item-level depressive symptoms, specifically negative symptoms, were RTV (36% of women used at 18% of visits) and nelfinavir (NFV; 20% of women used at 5% of visits). In the subgroup of young women with high rates of hepatitis C (Subgroup 3), use of NFV was associated with greater feelings of restlessness (somatic symptom), whereas in primarily young-to-middle-aged women (Subgroup 4), use of RTV was associated with less feelings of fearfulness (negative symptom).
Unexpectedly, none of the IIs, including DTG, were associated with any item-level depressive symptoms across subgroups. MVC, an entry inhibitor used by 1% of women at <1% of visits was associated with less feeling that everything was an effort (somatic item) in the subgroup with primarily controlled HIV with vascular comorbidities.
Discussion
We performed a prospective study to evaluate the effects of ART on item-level depressive symptomatology among five computationally driven subgroups of WLWH with respect to socio-demographic, behavioral, and clinical characteristics. Consistent with our first hypothesis, our primary finding was that the prospective associations (including directionality), or lack thereof, between specific ART agents and depressive symptoms were dependent on subgroup membership. Our second hypothesis was not supported as we expected that EFV and the IIs would be among the ART drugs most frequently associated with greater depressive symptoms. Rather, EFV was only associated with less negative and somatic symptoms for women in only 2/5 subgroups (Subgroups 4 and 5) and IIs were not associated with depressive symptoms. Unexpectedly, TDF was the ART drug that was most frequently associated with depressive symptoms, which was linked to less item-level depressive symptomatology (less negative and more positive affective symptoms) in 4/5 subgroups of women. These findings highlight the importance of evaluating the heterogeneous effects of ART on depressive symptoms in WLWH.
Among the different types of depressive symptoms examined, we determined that most ART drugs were associated with negative and positive affective symptoms. There were stronger associations between ART drugs and negative affect with 27 medications associated with less negative affect symptoms and six medications associated with less positive affect. Specifically, many of the ART drugs were associated with less negative affective symptoms. ART was also associated with somatic symptoms (17 associations) across all WLWH subgroups. The most common symptoms were feelings of restlessness, being bothered, lack of appetite, and concentration. Unlike that which occurred for negative affect, the associations with somatic symptoms differed in directionality across the ART drugs. Many ART drugs were associated with less somatic symptoms (i.e. FTC, ETR, LPV TDF, and EFV). However, some ART drugs that were associated with more somatic symptoms (D4T, LPV, NFV, and MVC) were highly dependent on the subgroup analyzed. These findings demonstrate that individual ART drugs have differing effects on the direction (i.e. a less or more symptoms) and category of depressive symptoms (i.e. positive or negative affect vs. somatic) that are unique to differing groups of WLWH. This reflects what has been shown historically in the clinical psychiatry literature and practice for the treatment of depression in the absence of HIV. The indication for use of specific antidepressants for treatment of major depressive disorder can be symptom-dependent (Cleare et al. 2015). Our data suggest that likewise this consideration should be made when treating depression in PLWH. Furthermore, these findings caution against oversimplifying depression analyses in association with ART, despite the common primary indication of all ART drugs to treat HIV infection by lowering HIV viral load, a peripheral (vs. central) phenomenon. Despite having a common end goal of inhibiting HIV replication, ART drugs have differing mechanisms of action, pharmacokinetic properties, and potential CNS toxicities that must be taken into consideration when evaluating associations with psychiatric manifestations among PLWH.
In contrast to our hypothesis, none of the IIs were associated with depressive symptoms for WLWH in any of the subgroups. IIs have been linked previously to psychiatric phenomenon, such as depression, anxiety, and psychosis, and have been reported by multiple groups as a primary reasons for stopping II treatment (Cohen et al. 2011; Madeddu et al. 2012; Abers et al. 2014; de Boer et al. 2016; Fettiplace et al. 2017). Associations between II and increased depressive symptoms have been reported previously in WLWH. However, in this study, II were not evaluated specifically and were instead analyzed collectively with other ART drugs (Todd et al. 2017). This categorical classification approach of assigning ART treatment into a binary status (yes/no) may have contributed to the discrepant results between this study evaluating II and depressive symptoms in WLWH and our study. This suggests that our approach of evaluating the associations between depressive symptoms and individual ART drugs provides results that are highly sensitive and less likely to bias, as compared to assigning groups based on ART class or the yes/no binary of ART status. Alternative explanations for the lack of association between any II drugs and depressive symptoms could also be related to the combinations in which the ART drugs were given, that other studies investigated the ART initiation, or the relative low sample size for II among the women in the study.
Our findings indicated that, unexpectedly, TDF was the ART drug most associated with depressive symptomatology in 4/5 subgroups of WLWH. We found that TDF had a neuropsychiatric benefit as it was associated with reduced item-level depressive symptomatology (less negative and more positive affective symptoms). It is unclear why TDF was so frequently associated with less depressive symptomatology among the WLWH in our study, with the exception of those in Subgroup 1 for whom no associations with TDF occurred. However, it is not completely surprising as TDF is considered to have a minimal potential for neurotoxicity, as compared to other ART drugs, because of its low CNS penetrance due to a high polarity and low lipid solubility (Anthonypillai et al. 2006; Letendre et al. 2008; Best et al. 2012). With a restricted CNS penetrance, TDF is unlikely to result in neurotoxic CNS effects and clinical trials demonstrate that TDF had limited neuropsychiatric effects (Gallant et al. 2006; Cassetti et al. 2007).
Our findings provide preliminary insight into the underlying mechanisms by which ART promotes CNS toxicity. Neural circuits related to emotional behavior are involved in the development of negative, and the lack of positive, affective symptoms and involve limbic-cortical-striatal-pallidal-thalamic circuits, formed by connections between the orbital and medial prefrontal cortex, amygdala, hippocampal subiculum, ventromedial striatum, mediodorsal and midline thalamic nuclei, and ventral pallidum (Drevets et al. 2008; Price and Drevets 2010). The neural circuitry for somatic symptomatology involves striatum, anterior cingulate cortex, insula, amygdala, and hippocampus (Perez et al. 2015), as well as motor cortex, midfrontal gyrus, anterior cingulate cortex, insula, and posterior cingulate cortex (Boeckle et al. 2016). Therefore, our findings indicate that these brain regions may be sensitive to ART neurotoxicity in the context of depressive symptoms. Indeed, the ART drugs that we evaluated may promote low-level neurotoxicity through dendritic spine injury, dysregulated mitochondrial function, oxidative and endoplasmic reticulum stress, and impaired neurite growth in in vitro settings (Robertson et al. 2012; Shah et al. 2016). Additionally, ART can promote both peripheral and central neurotoxicity in PLWH as evidenced by peripheral neuropathy, neuropsychiatric symptoms, lethal toxicity in patients with AIDS, and improved cognition upon ART discontinuation (Shah et al. 2016), although the literature is mixed with other studies suggesting ART has beneficial or stabilizing effects for cognition (Robertson et al. 2004). We identified only one association between ART and more interpersonal symptoms, which occurred for TDF in primarily young women with high rates of hepatitis C (Subgroup 3). This suggests a specificity for ART neurotoxicity that affects the neural circuits specific for affective and somatic symptoms, but not interpersonal domains, that underlie depression.
The present study has a number of limitations including the fact that we only examined the cross-sectional associations of ART drugs on depressive symptoms rather than the longer-term effects of ART or ART drug switches on item-level depressive symptoms longitudinally. For this, new analytic methods need to be developed and we are currently in the process of addressing this challenge. Here, our analyses focused on each ART drug in relation to depressive symptoms. We acknowledge that ART drugs are typically given in combination and as such we are also working to develop methodologies to handle drug combinations, both HIV- and non-HIV-related, and their link to CNS function. Our large sample size; however, allows for a preliminary look at individual drugs. Additional limitations to the present study include the availability of certain ART agents over the longitudinal course of the study which confines the clinical applicability of the findings. For example, given the epochs of the study, not all WLWH at all visits had the opportunity to be evaluated on all of the ART agents. This concern is somewhat mitigated as the distribution of enrollment epochs and follow-up time/dropout was not substantially different between the identified cluster groups. In addition, WLWH who are the most ill might be those that access some of the less commonly prescribed ART medications and thus may confound the results. Our findings are also only generalizable to WLWH and the pattern of associations may not be the same among men living with HIV (MLWH). We plan to extend our analytics to MLWH so that we can compare the pattern of associations between men and women. We also did not include non-ART drugs that have known CNS effects in WLWH (e.g., common drugs with anticholinergic burden (Rubin et al. 2018), the influence of genetic polymorphisms of drug metabolism (e.g., cytochrome P450 (CYP)3A4, CYP2B6) (Rathbun and Liedtke 2011), and other factors, including menopausal stage as covariates. Menopause stage may be an important factor distinguishing Subgroup 4 (mostly 36 to 55 year olds); however, staging of menopause began in 2005 in the WIHS and is ongoing work, future studies will examine these factors as well as drug-drug interactions in smaller subsets of WIHS women.
In summary, we identified heterogeneity in ART-related effects on item-level depressive symptoms by evaluating the associations of individual ART drugs across WLWH in five subgroups that differed based on sociodemographic, behavioral, and clinical factors. We determined that the effects of ART on depressive symptoms varied according to subgroup, where some effects occurred uniquely in one group of WLWH, but not others. Additionally, we identified associations between other ART drugs, specifically TDF, that occurred more broadly and were associated with lower depressive symptoms among almost all subgroups. These findings provide insight into the heterogeneous effects of ART on brain events that may lead to depressive symptoms among homogeneous subgroups of WLWH. More importantly, our findings provide potential clinical utility. Specifically, in the context of ART, physicians have the option of prescribing from a host of potentially effective medications to achieve virologic suppression. Our findings suggest that the patients’ background, including her socio-demographic, clinical, and behavioral characteristics, should be taken into account so the physician can prescribe the combination of ART drugs that is least likely to be associated with specific depression-related symptomatology.
Supplementary Material
Acknowledgement
This work was supported by the Johns Hopkins University NIMH Center for novel therapeutics for HIV-associated cognitive disorders (P30MH075773) 2018 pilot award to Dr. Rubin. Dr. Williams effort was supported by R00DA044838. Data in this manuscript were collected by the Women’s Interagency HIV Study (WIHS). The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). WIHS (Principal Investigators): UAB-MS WIHS (Mirjam-Colette Kempf and Deborah Konkle-Parker), U01-AI-103401; Atlanta WIHS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-AI-103408; Bronx WIHS (Kathryn Anastos and Anjali Sharma), U01-AI-035004; Brooklyn WIHS (Deborah Gustafson and Tracey Wilson), U01-AI-031834; Chicago WIHS (Mardge Cohen and Audrey French), U01-AI-034993; Metropolitan Washington WIHS (Seble Kassaye and Daniel Merenstein), U01-AI-034994; Miami WIHS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-AI-103397; UNC WIHS (Adaora Adimora), U01-AI-103390; Connie Wofsy Women’s HIV Study, Northern California (Bradley Aouizerat and Phyllis Tien), U01-AI-034989; WIHS Data Management and Analysis Center (Stephen Gange and Elizabeth Golub), U01-AI-042590; Southern California WIHS (Joel Milam), U01-HD-032632 (WIHS I – WIHS IV). The WIHS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute on Mental Health (NIMH). Targeted supplemental funding for specific projects is also provided by the National Institute of Dental and Craniofacial Research (NIDCR), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), the National Institute on Deafness and other Communication Disorders (NIDCD), and the NIH Office of Research on Women’s Health. WIHS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR000454 (Atlanta CTSA), P30-AI-050410 (UNC CFAR), and P30-AI-027767 (UAB CFAR).
Footnotes
Financial Disclosures
All authors have nothing to disclose.
References
- Abers MS, Shandera WX, Kass JS (2014) Neurological and psychiatric adverse effects of antiretroviral drugs. CNS drugs 28:131–145. [DOI] [PubMed] [Google Scholar]
- Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf MC, Tien PC, Kassaye SG, Anastos K, Cohen M, Minkoff H, Wingood G, Ofotokun I, Fischl MA, Gange S (2018) Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol 47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljassem K, Raboud JM, Hart TA, Benoit A, Su D, Margolese SL, Rourke SB, Rueda S, Burchell A, Cairney J, Shuper P, Loutfy MR, Team OCSR (2016) Gender Differences in Severity and Correlates of Depression Symptoms in People Living with HIV in Ontario, Canada. Journal of the International Association of Providers of AIDS Care 15:23–35. [DOI] [PubMed] [Google Scholar]
- Anthonypillai C, Gibbs JE, Thomas SA (2006) The distribution of the anti-HIV drug, tenofovir (PMPA), into the brain, CSF and choroid plexuses. Cerebrospinal Fluid Res 3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Pinto A, Grund B, Sharma S, Martinez E, Cummins N, Fox J, Klingman KL, Sedlacek D, Collins S, Flynn PM, Chasanov WM, Kedem E, Katlama C, Sierra-Madero J, Afonso C, Brouwers P, Cooper DA, group ISs (2018) Risk of Suicidal Behavior With Use of Efavirenz: Results from the Strategic Timing of Antiretroviral Treatment Trial. Clin Infect Dis 67:420–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, Gange S, Barranday Y, Holman S, Weber K, Young MA (2005) The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, Young M, Greenblatt R, Sacks H, Feldman J (1998) The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 9:117–125. [PubMed] [Google Scholar]
- Bengtson AM, Pence BW, Mollan KR, Edwards JK, Moore RD, O’Cleirigh C, Eaton EF, Eron JJ, Kitahata MM, Mathews WC, Crane H, Mugavero MJ (2017) The Relationship Between Efavirenz as Initial Antiretroviral Therapy and Suicidal Thoughts Among HIV-Infected Adults in Routine Care. J Acquir Immune Defic Syndr 76:402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B 57:125–133. [Google Scholar]
- Best BM, Letendre SL, Koopmans P, Rossi SS, Clifford DB, Collier AC, Gelman BB, Marra CM, McArthur JC, McCutchan JA, Morgello S, Simpson DM, Capparelli EV, Ellis RJ, Grant I, Group CS (2012) Low cerebrospinal fluid concentrations of the nucleotide HIV reverse transcriptase inhibitor, tenofovir. J Acquir Immune Defic Syndr 59:376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckle M, Schrimpf M, Liegl G, Pieh C (2016) Neural correlates of somatoform disorders from a meta-analytic perspective on neuroimaging studies. NeuroImage Clinical 11:606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghetti A, Baldin G, Lombardi F, Ciccullo A, Capetti A, Rusconi S, Sterrantino G, Latini A, Cossu MV, Gagliardini R, De Luca A, Di Giambenedetto S (2018) Efficacy and tolerability of lamivudine plus dolutegravir as a switch strategy in a multicentre cohort of patients with suppressed HIV-1 replication. HIV Med. [DOI] [PubMed] [Google Scholar]
- Borghetti A, Baldin G, Capetti A, Sterrantino G, Rusconi S, Latini A, Giacometti A, Madeddu G, Picarelli C, De Marco R, Cossu MV, Lagi F, Cauda R, De Luca A, Di Giambenedetto S, Odoacre Study G (2017) Efficacy and tolerability of dolutegravir and two nucleos(t)ide reverse transcriptase inhibitors in HIV-1-positive, virologically suppressed patients. AIDS 31:457–459. [DOI] [PubMed] [Google Scholar]
- Cassetti I, Madruga JV, Suleiman JM, Etzel A, Zhong L, Cheng AK, Enejosa J, Study ET (2007) The safety and efficacy of tenofovir DF in combination with lamivudine and efavirenz through 6 years in antiretroviral-naive HIV-1-infected patients. HIV Clin Trials 8:164–172. [DOI] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE (2001) Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 158:725–730. [DOI] [PubMed] [Google Scholar]
- Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, Dickens C, Ferrier IN, Geddes J, Gilbody S, Haddad PM, Katona C, Lewis G, Malizia A, McAllister-Williams RH, Ramchandani P, Scott J, Taylor D, Uher R, Members of the Consensus M (2015) Evidence-based guidelines for treating depressive disorders with antidepressants: A revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol 29:459–525. [DOI] [PubMed] [Google Scholar]
- Cohen C, Elion R, Ruane P, Shamblaw D, DeJesus E, Rashbaum B, Chuck SL, Yale K, Liu HC, Warren DR, Ramanathan S, Kearney BP (2011) Randomized, phase 2 evaluation of two single-tablet regimens elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate versus efavirenz/emtricitabine/tenofovir disoproxil fumarate for the initial treatment of HIV infection. AIDS 25:F7–12. [DOI] [PubMed] [Google Scholar]
- Cohen J, D’Agostino L, Wilson J, Tuzer F, Torres C (2017) Astrocyte Senescence and Metabolic Changes in Response to HIV Antiretroviral Therapy Drugs. Front Aging Neurosci 9:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JA (2008) The role of psychiatric and substance use comorbidity in HIV treatment and clinical outcomes for women. In. [Google Scholar]
- Cook JA, Cohen MH, Burke J, Grey D, Anastos K, Kirstein L, Palacio H, Richardson J, Wilson T, Young M (2002) Effects of depressive symptoms and mental health quality of life on use of highly active antiretroviral therapy among HIV-seropositive women. J Acquir Immune Defic Syndr 30:401–409. [DOI] [PubMed] [Google Scholar]
- Cook JA, Grey DD, Burke-Miller JK, Cohen MH, Vlahov D, Kapadia F, Wilson TE, Cook R, Schwartz RM, Golub ET, Anastos K, Ponath C, Goparaju L, Levine AM (2007) Illicit drug use, depression and their association with highly active antiretroviral therapy in HIV-positive women. Drug Alcohol Depend 89:74–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Boer MG, van den Berk GE, van Holten N, Oryszcyn JE, Dorama W, Moha DA, Brinkman K (2016) Intolerance of dolutegravir-containing combination antiretroviral therapy regimens in real-life clinical practice. AIDS 30:2831–2834. [DOI] [PubMed] [Google Scholar]
- Do AN, Rosenberg ES, Sullivan PS, Beer L, Strine TW, Schulden JD, Fagan JL, Freedman MS, Skarbinski J (2014) Excess burden of depression among HIV-infected persons receiving medical care in the united states: data from the medical monitoring project and the behavioral risk factor surveillance system. PloS one 9:e92842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML (2008) Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain structure & function 213:93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E (2007) HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci 8:33–44. [DOI] [PubMed] [Google Scholar]
- Elzi L, Erb S, Furrer H, Cavassini M, Calmy A, Vernazza P, Gunthard H, Bernasconi E, Battegay M, Swiss HIVCSG (2017) Adverse events of raltegravir and dolutegravir. AIDS 31:1853–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg M (1993) The problems of anticholinergic adverse effects in older patients. Drugs Aging 3:335–348. [DOI] [PubMed] [Google Scholar]
- Fettiplace A, Stainsby C, Winston A, Givens N, Puccini S, Vannappagari V, Hsu R, Fusco J, Quercia R, Aboud M, Curtis L (2017) Psychiatric Symptoms in Patients Receiving Dolutegravir. J Acquir Immune Defic Syndr 74:423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, Lu B, McColl D, Chuck S, Enejosa J, Toole JJ, Cheng AK, Study G (2006) Tenofovir DF, emtricitabine, and efavirenz vs. zidovudine, lamivudine, and efavirenz for HIV. N Engl J Med 354:251–260. [DOI] [PubMed] [Google Scholar]
- Gandhi M, Aweeka F, Greenblatt RM, Blaschke TF (2004) Sex differences in pharmacokinetics and pharmacodynamics. Annu Rev Pharmacol Toxicol 44:499–523. [DOI] [PubMed] [Google Scholar]
- Giunta B, Ehrhart J, Obregon DF, Lam L, Le L, Jin J, Fernandez F, Tan J, Shytle RD (2011) Antiretroviral medications disrupt microglial phagocytosis of beta-amyloid and increase its production by neurons: implications for HIV-associated neurocognitive disorders. Mol Brain 4:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M, Larsen G, Montaner JS (2008) Exacerbation of depression associated with starting raltegravir: a report of four cases. AIDS 22:1890–1892. [DOI] [PubMed] [Google Scholar]
- Hoffmann C, Welz T, Sabranski M, Kolb M, Wolf E, Stellbrink HJ, Wyen C (2017) Higher rates of neuropsychiatric adverse events leading to dolutegravir discontinuation in women and older patients. HIV Med 18:56–63. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, Moore J, Group HIVERS (2001) Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: longitudinal analysis from the HIV Epidemiology Research Study. JAMA 285:1466–1474. [DOI] [PubMed] [Google Scholar]
- Kapfhammer HP (2006) Somatic symptoms in depression. Dialogues Clin Neurosci 8:227–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB (1993) Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord 29:85–96. [DOI] [PubMed] [Google Scholar]
- Kheloufi F, Allemand J, Mokhtari S, Default A (2015) Psychiatric disorders after starting dolutegravir: report of four cases. AIDS 29:1723–1725. [DOI] [PubMed] [Google Scholar]
- Kim G, Decoster J, Huang CH, Chiriboga DA (2011) Race/ethnicity and the factor structure of the Center for Epidemiologic Studies Depression Scale: a meta-analysis. Cultur Divers Ethnic Minor Psychol 17:381–396. [DOI] [PubMed] [Google Scholar]
- Kohler JJ, Lewis W (2007) A brief overview of mechanisms of mitochondrial toxicity from NRTIs. Environmental and molecular mutagenesis 48:166–172. [DOI] [PubMed] [Google Scholar]
- Lee JW, Aminkeng F, Bhavsar AP, Shaw K, Carleton BC, Hayden MR, Ross CJ (2014) The emerging era of pharmacogenomics: current successes, future potential, and challenges. Clin Genet 86:21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre S, Marquie-Beck J, Capparelli E, Best B, Clifford D, Collier AC, Gelman BB, McArthur JC, McCutchan JA, Morgello S, Simpson D, Grant I, Ellis RJ (2008) Validation of the CNS Penetration-Effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 65:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liner KJ 2nd, Ro MJ, Robertson KR (2010) HIV, antiretroviral therapies, and the brain. Curr HIV/AIDS Rep 7:85–91. [DOI] [PubMed] [Google Scholar]
- Madeddu G, Menzaghi B, Ricci E, Carenzi L, Martinelli C, di Biagio A, Parruti G, Orofino G, Mura MS, Bonfanti P, Group CISAI (2012) Raltegravir central nervous system tolerability in clinical practice: results from a multicenter observational study. AIDS 26:2412–2415. [DOI] [PubMed] [Google Scholar]
- Maki PM, Rubin LH, Cohen M, Golub ET, Greenblatt RM, Young M, Schwartz RM, Anastos K, Cook JA (2012) Depressive symptoms are increased in the early perimenopausal stage in ethnically diverse human immunodeficiency virus-infected and human immunodeficiency virus-uninfected women. Menopause 19:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manda KR, Banerjee A, Banks WA, Ercal N (2011) Highly active antiretroviral therapy drug combination induces oxidative stress and mitochondrial dysfunction in immortalized human blood-brain barrier endothelial cells. Free Radic Biol Med 50:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangoni AA, Jackson SH (2004) Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. Br J Clin Pharmacol 57:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard A, Montagnac C, Solas C, Meddeb L, Dhiver C, Tomei C, Ravaux I, Tissot-Dupont H, Mokhtari S, Colson P, Stein A (2017) Neuropsychiatric adverse effects on dolutegravir: an emerging concern in Europe. AIDS 31:1201–1203. [DOI] [PubMed] [Google Scholar]
- Mickaël C, Genolini C, Ecochard R (2013) KmLcov: k-means for longitudinal data with covariates. In. [Google Scholar]
- Mollan KR, Smurzynski M, Eron JJ, Daar ES, Campbell TB, Sax PE, Gulick RM, Na L, O’Keefe L, Robertson KR, Tierney C (2014) Association between efavirenz as initial therapy for HIV-1 infection and increased risk for suicidal ideation or attempted or completed suicide: an analysis of trial data. Ann Intern Med 161:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J, Schuman P, Schoenbaum E, Boland B, Solomon L, Smith D (1999) Severe adverse life events and depressive symptoms among women with, or at risk for, HIV infection in four cities in the United States of America. Aids 13:2459–2468. [DOI] [PubMed] [Google Scholar]
- Penafiel J, de Lazzari E, Padilla M, Rojas J, Gonzalez-Cordon A, Blanco JL, Blanch J, Marcos MA, Lonca M, Martinez-Rebollar M, Laguno M, Tricas A, Rodriguez A, Mallolas J, Gatell JM, Martinez E (2017) Tolerability of integrase inhibitors in a real-life setting. The Journal of antimicrobial chemotherapy 72:1752–1759. [DOI] [PubMed] [Google Scholar]
- Perez DL, Barsky AJ, Vago DR, Baslet G, Silbersweig DA (2015) A neural circuit framework for somatosensory amplification in somatoform disorders. J Neuropsychiatry Clin Neurosci 27:e40–50. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC (2010) Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin J, Rabkin R (1997) [Depression and HIV]. Sidahora:19–22. [PubMed] [Google Scholar]
- Rabkin JG (2008) HIV and depression: 2008 review and update. Curr HIV/AIDS Rep 5:163–171. [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977) The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1:385–401. [Google Scholar]
- Rathbun RC, Liedtke MD (2011) Antiretroviral drug interactions: overview of interactions involving new and investigational agents and the role of therapeutic drug monitoring for management. Pharmaceutics 3:745–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revuelta-Herrero JL, Chamorro-de-Vega E, Rodriguez-Gonzalez CG, Alonso R, Herranz-Alonso A, Sanjurjo-Saez M (2018) Effectiveness, Safety, and Costs of a Treatment Switch to Dolutegravir Plus Rilpivirine Dual Therapy in Treatment-Experienced HIV Patients. Ann Pharmacother 52:11–18. [DOI] [PubMed] [Google Scholar]
- Robertson K, Liner J, Meeker RB (2012) Antiretroviral neurotoxicity. J Neurovirol 18:388–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson K, Bayon C, Molina JM, McNamara P, Resch C, Munoz-Moreno JA, Kulasegaram R, Schewe K, Burgos-Ramirez A, De Alvaro C, Cabrero E, Guion M, Norton M, van Wyk J (2014) Screening for neurocognitive impairment, depression, and anxiety in HIV-infected patients in Western Europe and Canada. AIDS Care 26:1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Robertson WT, Ford S, Watson D, Fiscus S, Harp AG, Hall CD (2004) Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr 36:562–566. [DOI] [PubMed] [Google Scholar]
- Rubin LH, Cook JA, Grey DD, Weber K, Wells C, Golub ET, Wright RL, Schwartz RM, Goparaju L, Cohan D, Wilson ML, Maki PM (2011) Perinatal depressive symptoms in HIV-infected versus HIV-uninfected women: a prospective study from preconception to postpartum. J Womens Health (Larchmt) 20:1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Springer G, Martin EM, Seaberg EC, Sacktor NC, Levine A, Valcour VG, Young MA, Becker JT, Maki PM, Neuropsychology Working Groups of the Women’s InterAgency HIVS, the Multicenter ACS (2019) Elevated depressive symptoms are a stronger predictor of executive dysfunction in HIV-infected women than men. J Acquir Immune Defic Syndr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin LH, Radtke KK, Eum S, Tamraz B, Kumanan KN, Springer G, Maki PM, Anastos K, Merenstein D, Karim R, Weber KM, Gustafson D, Greenblatt RM, Bishop JR (2018) Cognitive burden of common non-antiretroviral medications in HIV-infected women. J Acquir Immune Defic Syndr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg BC, Taylor MJ, Alhassoon OM, Gonzalez R, Brown GG, Ellis RJ, Letendre S, Videen JS, McCutchan JA, Patterson TL, Grant I, Group H (2005) Brain mitochondrial injury in human immunodeficiency virus-seropositive (HIV+) individuals taking nucleoside reverse transcriptase inhibitors. J Neurovirol 11:356–364. [DOI] [PubMed] [Google Scholar]
- Semple SJ, Patterson TL, Straits-Troster K, Atkinson JH, McCutchan JA, Grant I (1996) Social and psychological characteristics of HIV-infected women and gay men. HIV Neurobehavioral Research Center (HNRC) Group. Women Health 24:17–41. [DOI] [PubMed] [Google Scholar]
- Shah A, Gangwani MR, Chaudhari NS, Glazyrin A, Bhat HK, Kumar A (2016) Neurotoxicity in the Post-HAART Era: Caution for the Antiretroviral Therapeutics. Neurotoxicity research 30:677–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr L, Cluver L (2017) World Health Day focus on HIV and depression - a comorbidity with specific challenges. Journal of the International AIDS Society 20:21956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd JV, Cole SR, Pence BW, Lesko CR, Bacchetti P, Cohen MH, Feaster DJ, Gange S, Griswold ME, Mack W, Rubtsova A, Wang C, Weedon J, Anastos K, Adimora AA (2017) Effects of Antiretroviral Therapy and Depressive Symptoms on All-Cause Mortality Among HIV-Infected Women. Am J Epidemiol 185:869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood J, Robertson KR, Winston A (2015) Could antiretroviral neurotoxicity play a role in the pathogenesis of cognitive impairment in treated HIV disease? AIDS 29:253–261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


