Highlights
-
•
COVID-19 is largely localized in lungs.
-
•
SARS-CoV-2 binds to the heme groups in hemoglobin that leads to severe hypoxia.
-
•
Porphyrin-based photosensitizers (PS) act as a ‘decoy’ in which the SARS-CoV-2 virions would attach to PS molecules.
-
•
Photoactivation capable of destroying the bonded SARS-CoV-2 virions.
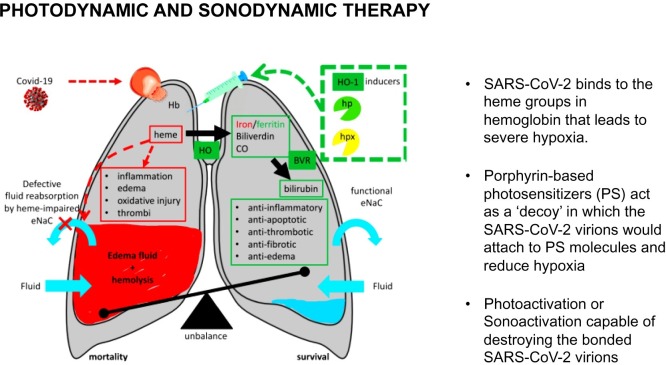
To the Editor −COVID-19 has infected more then 12 million people and claimed nearly 600,000 lives worldwide. Numerous case series reports and observational studies have described the clinical course and outcomes of patients who have contracted SARS-CoV-2 and were subsequently hospitalized. A majority of moderate-to-critically ill patients required high flow nasal cannula or mechanical ventilation. A proportion of those patients were treated with different off label or investigational drugs and convalescent plasma therapy [[1], [2], [3]]. However, only two pharmaceuticals demonstrated efficacy. Remdesivir has been shown to reduce the total amount of time in ICU required per patient [4], and dexamethasone had reduced deaths by 35 % in patients who needed treatment with invasive ventilation and by 20 % in those needing supplemental oxygen [5]. In this letter we propose the exploration of using photodynamic therapy (PDT) and Sonodynamic Therapy (SDT) to treat acute hypoxemic respiratory failure (AHRF) in patients with COVID-19 (Fig. 1 ).
Fig. 1.
Proposed pathway of SARS-CoV-2 targeting the heme groups of hemoglobin and the subsequent formation of hypoxic effects of COVID-19. Adapted from: Wagener and colleagues. Targeting the Heme-Heme Oxygenase System to Prevent Severe Complications Following COVID-19 Infections. Antioxidants 2020, 9, 540.
Several recent studies have argued that severe-critically ill COVID-19 patients develop acute hypoxemic respiratory failure (AHRF) and therefore cannot be successfully treated using established ICU protocol [6,7]. The unique challenges of COVID-19 were demonstrated in early radiological studies presenting bilateral interstitial lung inflammation and fibrosis, characterized by ground glass opacity, crazy-paving pattern, and consolidation in lung CT scans [8]. Beyond COVID-19 related complications, invasive ventilation can do more harm than good, including air leaks due to high ventilation pressures (barotrauma), pulmonary edema due to large tidal volumes (volutrauma), atelectasis due to the repetitive opening and closing of the lungs (atelectrauma), and oxytrauma from free oxygen radicals [9]. The damaging effects of intubation combined with the complications of severe COVID-19 may explain the large mortality rates in intubated COVID-19 patients. In an outcomes study examining over 2600 discharges/deaths in New York City area hospitals, among those who died, 88 % received mechanical ventilation [10]. This alarmingly high mortality rate may indicate a failure in established ICU protocols, though it could be confounded by the overreliance of intubation/ventilation therapy. However, there is a growing consensus that intubation should serve as a last resort for the most ill patients. Therefore, many hospitals are now transitioning to the use noninvasive ventilation and/or low-volume ventilation [6,7].
The challenges posed by COVID-19-induced AHRF had lead physicians to rethink the characterization of these effects to indicate a different mechanism of respiratory distress. [6,7,9,11], A recent study performed conserved domain analysis, homology modeling, and molecular docking on SARS-CoV-2 pathogensis, which proposed that SARS-CoV-2 binds to the heme groups in hemoglobin, leading to severe hypoxia. Porphyrins, a ring-like organic compound, make up Heme and attach to iron atoms. Iron atoms bind to oxygen as the blood travels between the lungs and the tissues. It was hypothesized that several nonstructural proteins (orf1ab, ORF10, and ORF3a) target the 1-beta chain of hemoglobin, which dissociates iron to form porphyrin (Fig. 1). This attack causes a disruption in the passage of hemoglobin-carrying-oxygen between the alveoli and capillaries [12]. While the study is in pre-print and some have criticized the study design, SARS-CoV-2-mediated attack on hemoglobin could explain the lack of efficacy with intubation therapy [13]. The attack on hemoglobin leads to desaturation, which coupled with the proliferation of pro-inflammatory cytokines cause the alveoli to become filled with fluid, white blood cells, mucus, and the detritus of destroyed lung cell. The attack on hemoglobin and the lung tissue can lead multi-organ failure.
Considering the unique challenges of AHRF in patients with COVID-19, we propose the use of Photodynamic Therapy (PDT). The earliest understanding that photonic energy in visible light could be harnessed through the presence of a photoreactive substance to promote a biological, photodynamic effect in an oxygenated tissue is attributed to the work of Professor von Tappeiner on xanthene derivatives, first published in 1900 [14]. Early photodynamic agents were naturally-derived porphyrins such as hematoporphyrin and typically were mixtures of many porphyrins leading to inconsistent biological results. Through many decades of purification, and ultimately the synthesis of new single-entity photoreactive agents with tissue-selective localization, together with advances in light sources and photonics, the versatility of PDT has been clinically validated in a wide range of pathologies from esophageal and certain lung cancers, to uses in treating age-related macular degeneration in the eye and in tumor margin identification during brain cancer surgery. Evidence for the antimicrobial effects of PDT have been shown (i.e. destruction of bacteria, viruses, protozoa, and fungi) [[15], [16], [17]]. Unlike antibiotics, there is little to no risk for the formation of resistance following repeated exposures to PDT, which could be useful to treat opportunistic infections in patients with COVID-19.
We propose to treat AHRF by the injection of porphyrin-based photosensitizers (PS) systemically or locally into the lungs either through the pulmonary artery and/or bronchial arteries using a Swan Ganz or micro catheters. Initially, and without the need for photoactivation, the PS molecules act as a ‘decoy’ in which the SARS-CoV-2 virions would attach to PS molecules instead of healthy lung tissue or hemoglobin. The therapy may improve clinical performance and increase oxygenation. However, to reduce viral load, illumination of the target lung tissue using fiberoptic catheter to deliver low-power light of a characteristic absorption wavelength for the photosensitizer (typical range 450−800 nm), causes photoactivation yielding a highly reactive oxygen species capable of destroying the bonded SARS-CoV-2 virions to the PS molecules through peroxidation. The predominant mechanism in PDT involves generation of singlet oxygen (1O2). The diffusion distance of 1O2 is around 0.01−0.02 μ before being quenched so the PS must be associated intimately with the target substrate for maximal impact. [18] Thus, the SARS-CoV-2 affinity for the heme structure of the PS, predicts high viral destruction titers limited to the zone of photoactivation. The application of nanoparticle technology may provide opportunities to enhance delivery, bioavailability, selectivity, and functionality of currently available PS while reducing side-effects. In the absence of specialized photonics or in resource-limited settings, some PS may be activated using transthoracic continuous wave ultrasound (sonodynamic therapy). In some clinical settings there may be incremental benefit in combining PDT and sonodynamic therapy (SDT). PDT does not preclude the use of conjunctive pharmacologic treatment of COVID-19 disease, in fact, PDT and/or SDT combined with an existing therapy may prove the most effective approach. However, pre-clinical and clinical studies are planned to examine early feasibility, safety, and efficacy of this approach.
Funding
The authors all equally contributed to the work in this manuscript. There is no funding source to report for this letter/commentary
Declaration of Competing Interest
The authors report no declarations of interest.
References
- 1.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young B.E., Ong S.W.X., Kalimuddin S. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020 doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020 doi: 10.1001/jama.2020.12839. Published online July 10. [DOI] [PubMed] [Google Scholar]
- 4.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C. Remdesivir for the treatment of Covid-19 - preliminary report. N. Engl. J. Med. 2020 May:22. doi: 10.1056/NEJMc2022236. [DOI] [PubMed] [Google Scholar]
- 5.Dexamethasone in hospitalized patients with Covid-19 — preliminary report the RECOVERY collaborative group*. NEJM. 2020 doi: 10.1056/NEJMoa2021436. July 17. [DOI] [Google Scholar]
- 6.Dondorp A.M., Hayat M., Aryal D., Beane A., Schultz M.J. Respiratory support in novel coronavirus disease (COVID-19) patients, with a focus on resource-limited settings. Am. J. Trop. Med. Hyg. 2020 doi: 10.4269/ajtmh.20-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel B.K., Kress J.P., Hall J.B. Alternatives to invasive ventilation in the COVID-19 pandemic. JAMA. 2020;324(1):43–44. doi: 10.1001/jama.2020.9611. [DOI] [PubMed] [Google Scholar]
- 8.Shi H., Han X., Jiang N. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect. Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Slutsky A.S., Ranieri V.M. Ventilator-induced lung injury. N. Engl. J. Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 10.Richardson S., Hirsch J.S., Narasimhan M. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area. JAMA. 2020 doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marini J.J., Gattinoni L. Management of COVID-19 respiratory distress. JAMA. 2020 doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 12.Liu W., Li H. COVID-19: Attacks the 1-Beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. Preprint. 2020 (ChemRxiv) [Google Scholar]
- 13.Read R.J. Flawed methods in“COVID-19: attacks the 1-Beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism”. Preprint. 2020 (ChemRxiv) [Google Scholar]
- 14.von Tappeiner H. On the action of fluorescent substances on infusoria according to the research of O. Raab. Münch. Med. Wochenschr. 1900;47:5–7. [Google Scholar]
- 15.Kharkwal G.B., Sharma S.K., Huang Y.Y., Dai T., Hamblin M.R. Photodynamic therapy for infections: clinical applications. Lasers Surg. Med. 2011;43:755–767. doi: 10.1002/lsm.21080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majiya H., Adeyemi O.O., Herod M., Stonehouse N.J., Millner P. Photodynamic inactivation of non-enveloped RNA viruses. J. Photochem. Photobiol. B. 2018;189:87–94. doi: 10.1016/j.jphotobiol.2018.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Wiehe A., O’Brien J.M., Senge M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019;18:2565–2612. doi: 10.1039/c9pp00211a. [DOI] [PubMed] [Google Scholar]
- 18.Moan J., Berg K. The photodegradation of porphyrins in cells can be used to estimate the lifetime of singlet oxygen. Photochem. Photobiol. 1991;53(April (4)):549–553. doi: 10.1111/j.1751-1097.1991.tb03669.x. [DOI] [PubMed] [Google Scholar]