Abstract
Introduction
A great deal of literature has recently discussed the evaluation and management of the coronavirus disease of 2019 (COVID-19) patient in the emergency department (ED) setting, but there remains a dearth of literature providing guidance on cardiac arrest management in this population.
Objective
This narrative review outlines the underlying pathophysiology of patients with COVID-19 and discusses approaches to cardiac arrest management in the ED based on the current literature as well as extrapolations from experience with other pathogens.
Discussion
Patients with COVID-19 may experience cardiovascular manifestations that place them at risk for acute myocardial injury, arrhythmias, and cardiac arrest. The mortality for these critically ill patients is high and increases with age and comorbidities. While providing resuscitative interventions and performing procedures on these patients, healthcare providers must adhere to strict infection control measures and prioritize their own safety through the appropriate use of personal protective equipment. A novel approach must be implemented in combination with national guidelines. The changes in these guidelines emphasize early placement of an advanced airway to limit nosocomial viral transmission and encourage healthcare providers to determine the effectiveness of their efforts prior to placing staff at risk for exposure.
Conclusions
While treatment priorities and goals are identical to pre-pandemic approaches, the management of COVID-19 patients in cardiac arrest has distinct differences from cardiac arrest patients without COVID-19. We provide a review of the current literature on the changes in cardiac arrest management as well as details outlining team composition.
Keywords: COVID-19, SARS-CoV-2, Cardiac arrest, Emergency medicine, Critical care, Intensive care
1. Introduction
In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), originating in Wuhan, Hubei, China, was identified as the cause of a pandemic, resulting in millions of cases and hundreds of thousands of deaths worldwide [1]. The number of patients requiring hospital admission and more specifically intensive care unit (ICU) level care continues to rise at an exponential rate; 10–20% of hospitalized coronavirus disease of 2019 (COVID-19) patients require admission to the ICU [2]. Although COVID-19 primarily affects the respiratory tract, cardiovascular manifestations have been described in the literature, most notably myocardial injury and myocarditis [[3], [4], [5], [6]]. The impairment of myocardial function may lead to both tachy- and brady-arrhythmias; this myocardial dysfunction significantly contributes to clinical deterioration and eventual cardiac arrest, particularly when occurring simultaneously with acute respiratory failure and related hypoxia [3,7]. Not surprisingly, critically ill COVID-19 patients with myocardial injury demonstrate a higher risk of in-hospital mortality [3,5,6,8].
As the numbers of critically ill patients infected with COVID-19 continue to rise, this narrative aims to review the current literature, to discuss the composition of the healthcare team required to safely and adequately resuscitate a critically COVID-19 patient, and finally to highlight changes to national guidelines on cardiopulmonary resuscitation (CPR) and the management of COVID-19 patients in cardiac arrest.
2. Methods
This narrative review provides a focused overview of cardiac arrest in patients with suspected or confirmed COVID-19 for emergency clinicians. The authors searched PubMed and Google Scholar for articles using keywords “COVID19”, “Coronavirus”, “Cardiac” or “Cardiac Arrest”. The search was conducted from database inception to June 1st, 2020. PubMed identified 431 articles, and Google Scholar yielded 1454 articles. Authors evaluated case reports and series, retrospective and prospective studies, systematic reviews and meta-analyses, and other narrative reviews. Authors also reviewed guidelines, supporting citations of included articles, and peer-reviewed Free Open Access Medical Education literature. The literature search was restricted to studies published in English, with focus on the emergency medicine and critical care literature. Authors decided which studies to include for the review by consensus. When available, systematic reviews and meta-analyses were preferentially selected. These were followed sequentially by randomized controlled trials, prospective studies, retrospective studies, case reports, and other narrative reviews when alternate data were not available. A total of 100 resources were selected for inclusion in this narrative review.
3. Discussion
3.1. Preparedness: Planning and preparation
3.1.1. Environmental considerations
Both symptomatic and asymptomatic patients are a source of COVID-19 spread; healthcare providers caring for these patients are at the greatest risk of contracting the virus [[9], [10], [11], [12], [13], [14], [15]]. Patients with suspected or unknown COVID-19 status should immediately be placed in a negative pressure room, if possible [16,17]. If not available, a private room with a closed door is recommended. During the resuscitation of a critically ill patient, healthcare providers may be required to perform aerosol generating procedures (AGPs) such as nebulization, non-invasive positive pressure ventilation (NIPPV), bag-mask ventilation, airway suctioning, endotracheal intubation (ETI), and CPR [[18], [19], [20], [21], [22]]. Performing AGPs in negative pressure rooms, where air-exchange occurs more than 12 times an hour, reduces cross-contamination among staff and patients outside the room [23]. Success in preventing cross-contamination when using these rooms was found during the SARS epidemic in 2002 [[24], [25], [26]]. Importantly, these negative pressure rooms may prevent viral particles from traveling outside of the room, but they do not reduce the viral load or risk of contamination inside the room itself [27]. Many healthcare facilities have a limited number of negative pressure rooms, making this setting a somewhat scare resource; furthermore, in some instances, it is difficult to predict the likelihood of cardiac arrest occurring in a subset of these patients, making pre-placement in a negative pressure room another challenge.
3.1.2. Personal protective equipment
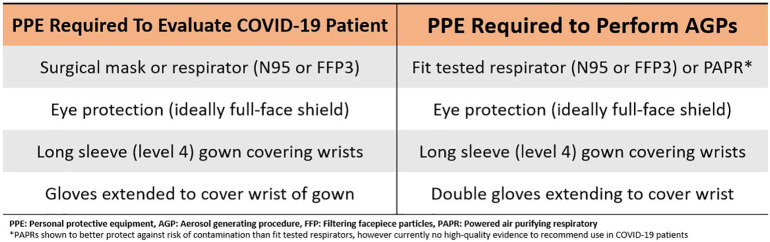
Multiple organizations have recommended that adequate personal protective equipment (PPE) and staff safety is the number one priority before assessing any patient in cardiac arrest [15,19,20,28]. PPE protocols have been proposed by several clinical societies, but these recommendations are based on low-quality evidence [29]. Fig. 1 outlines the minimum PPE needed to assess a patient infected with COVID-19 and the minimum PPE needed to perform AGPs as recommended by the Centers for Disease Control and Prevention (CDC) and the American Heart Association's (AHA) updated Advanced Cardiac Life Support (ACLS) guidelines [15,23,30]. Although there is currently no high-quality evidence recommending the use of powered air purifying respirators (PAPRs) for AGPs, they have been shown to protect against the risk of contamination better than an N95 mask alone [29].
Fig. 1.
Minimum recommended personal protective equipment required to evaluate COVID-19 patients and perform aerosol generating procedures.
3.1.3. Team composition
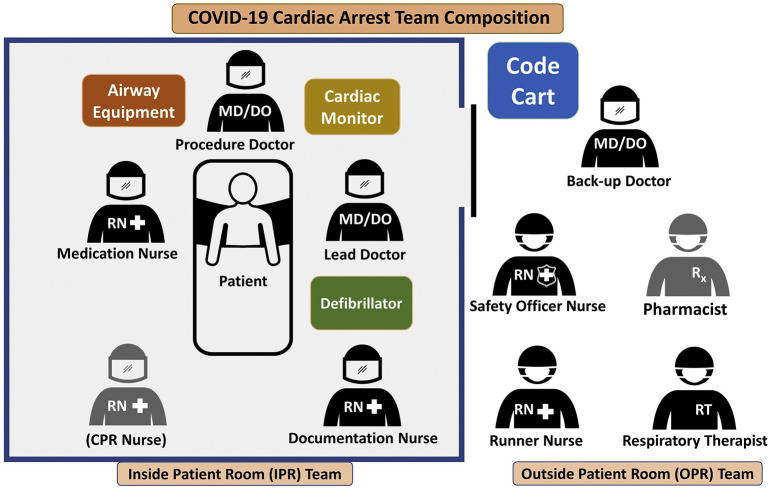
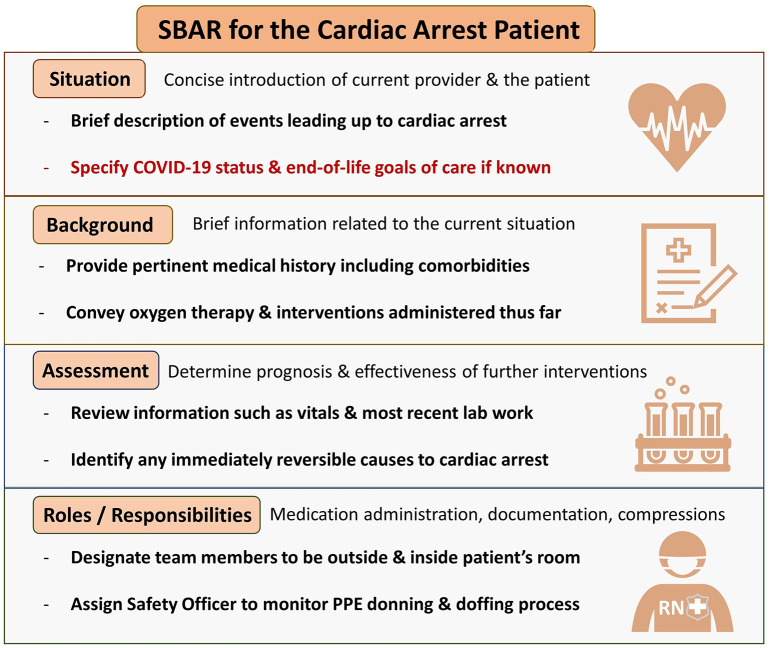
To further minimize the risk of COVID transmission, only essential personnel should enter the room of a COVID-infected patient to perform CPR [15]. Communication challenges can arise among staff in healthcare settings where rapid and effective interventions are required for patient care [[31], [32], [33], [34]]. Therefore, it is recommended to use a structured communication strategy, such as the situation, background, assessment, and recommendation (SBAR) technique to improve information delivery and patient safety in critical situations [[35], [36], [37], [38]]. Prior to responding to a cardiac arrest, appropriate planning and team huddles should be performed to identify roles, responsibilities, equipment, and medications. Team composition of healthcare providers responding to a patient in cardiac arrest should be divided into those inside patient's room (IPR) and those outside patient's room (OPR). In addition to “recommendations” on the SBAR communication instrument, the assembled team should review the roles and responsibilities of each member (Fig. 2 and Fig. 3 ).
Fig. 2.
Team composition displaying in-patient room (IPR) and outside-patient room (OPR) members.
Fig. 3.
SBAR communication tool specific for cardiac arrest patients with known or suspected COVID-19.
When assigning roles, one of the most important roles is the designated safety officer. This OPR team member is responsible for coaching all donning and doffing of PPE and serving as the door monitor to keep record of staff entering and leaving the room. IPR providers should strictly adhere to the PPE protocols mentioned above, and OPR members should quickly obtain all the necessary and needed equipment if not already immediately available, as delays in the initiation of compressions will already be expected. The designated safety officer, among many important roles, should stop the entry of any personnel lacking appropriate PPE, regardless of the patient condition, acuity, or event, including new onset cardiac arrest.
IPR healthcare providers should consist of the following members:
-
-
One physician overseeing the cardiac arrest
-
-
One physician focusing on procedures (which include AGPs and ultrasound)
-
-
One nurse dedicated to administering medications
-
-
A second nurse dedicated to documentation and additional oversight
-
-
A third nurse or medical technician dedicated to CPR (if mechanical CPR device unavailable)
IPR teams should consist of a total of four members if a mechanical chest compression device is available to provide CPR, otherwise a fifth member should be added to perform the task of compressions [23]. If a mechanical chest compression device is unavailable, provider fatigue when performing compressions should be prevented by having the nurses on the IPR team rotate their roles during each 2-minute cycle of CPR. To avoid fatigue, providers may consider rotating through the role of compressions every minute instead [39]. This rotation should occur simultaneously while the physician overseeing the arrest interprets the rhythm and next course of action per advanced life support guidelines. When changing compressors, regardless of the reasons for stopping compressions (i.e., rhythm interpretation, defibrillation, etc.), interruptions must be minimized. To conserve PPE and resource allocation, it is not recommended that OPR team members wear full PPE unless they plan to enter the room. They should maintain droplet contact precautions and be ready to don full PPE, however, should they be needed to change out with IPR team members, assist with a difficult procedure, or contribute to patient care in some other fashion.
OPR healthcare providers should consist of the following members:
-
-
One backup physician ready to don PPE and assist where needed
-
-
One nurse designated as the safety officer
-
-
One nurse designated as the “runner” to rapidly acquire any additional unanticipated equipment or medications
-
-
One pharmacist if available
-
-
One respiratory therapist
3.1.4. Equipment considerations
Any equipment or personnel that do not require immediate entry into the patient room should remain in the hallway for prompt access. For example, the code cart with appropriate medications and supplies can remain in the hallway immediately adjacent to the doorway. The safety officer or other assigned personnel can provide rapid access to any requested medications, supplies, or equipment. With this approach, the code cart and its contents do not need to be discarded afterwards due to contamination. In addition to the code cart remaining outside the room, if the hospital's code team has a pharmacist, this person can also remain immediately outside the patient's room, allowing for rapid consultation as needed. The defibrillator device, typically paired with the code cart, should also remain outside of the patient's room. Procedure trays such as central line and arterial line kits should be quickly obtained and brought to the patient's room by the Runner Nurse if they are not already present. Additional equipment allowing for real-time communication from the patient room, such as baby monitors, walkie-talkies, a small microphone, or dry erase boards, has been recommended by some to facilitate communication between the IPR and OPR teams [40].
Institutions should create a mobile endotracheal intubation pack that is decontaminated after each intubation. This pack should contain single-use equipment brought into the patient's room during intubation. Additional airway equipment may be stored in the hallway as necessary. All essential medications should be present before the procedure, including appropriate post-intubation analgesia, sedation, and paralyzing medications. Push-dose vasopressors or a norepinephrine infusion should also be available.
3.2. Cardiac arrest management in patients with known or suspected COVID-19
In addition to the changing team dynamics and equipment allocation, management of cardiac arrest in patients with known or suspected COVID-19 raises considerations in airway management, circulatory support, and medication administration. The important principles of cardio-cerebral perfusion, via high-quality chest compressions with minimal interruptions, and management of shockable dysrhythmias via automated external defibrillators (AEDs) or manual defibrillators, remain; these priorities have not changed in the COVID-19 patient.
3.3. Considerations in airway management
3.3.1. Preoxygenation and ventilation
Although not the focus of this review, oxygenation strategies limiting aerosolization risk, such as application of a non-rebreather or face mask with high-flow oxygen and insertion of an oral airway, should be performed immediately following the donning of appropriate PPE. If a provider donned in PPE is alone with an immediately unresponsive patient, he/she should immediately call for assistance, apply a non-rebreather with oral airway, and then begin chest compressions. Once additional help arrives, the non-rebreather can be replaced with a bag valve mask (BVM) attached to a high-efficiency particulate absorbing (HEPA) filter, and tight seal of the face mask should be utilized [15]. A two-person technique is recommended when using the BVM to improve the seal between the patient's face and mask [20,[41], [42], [43], [44]]. The “V” and “E” hand position ensures a tight mask seal and is recommended (Fig. 4 ) [45].
Fig. 4.
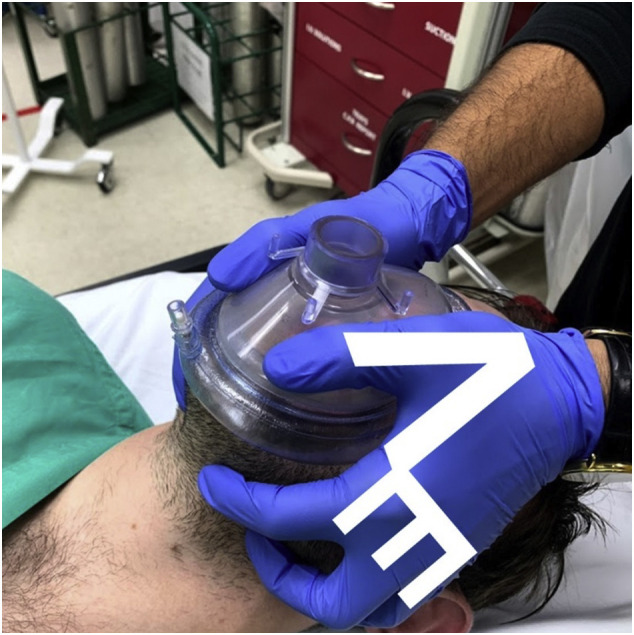
Recommended “V” and “E” hand position to ensure a tight mask and seal when using a BVM.
The placement of an appropriately sized advanced airway must be prioritized as the chest compressions performed during CPR may be aerosol producing [41,42,46,47]. There is a consensus agreement in the literature stating airway management of a COVID-19 patient in cardiac arrest should begin with opening the airway and placement of an advanced airway [15,19,20,28,42]. Additionally, a supraglottic airway (SGA) device with an attached viral filter may be used for airway protection [20,21,27,35,36,38].
3.3.2. Endotracheal intubation
Should intubation be required, several guidelines recommend the use of video laryngoscopy over direct laryngoscopy to allow further distance between the provider and patient [15,45,[47], [48],49]. The placement of a clear drape/tarp or box has been suggested to limit viral aerosolization; however, it is unclear how this influences first pass success [50]. The airway box is cumbersome and, when added to the already compromising PPE, can be a significant challenge to successful airway management. One study evaluating the use of aerosol boxes to protect healthcare providers found reduced first pass success, longer time to intubation, and decreased laryngoscopic grade [51]. Other studies evaluating their efficacy concluded they should not be used outside the operating room or in situations where a surgical airway may be needed [52,53]. Similar to the placement of an SGA, the most experienced healthcare provider should perform the intubation procedure [15,19,20,28,49]. The AHA recommends that chest compressions should be paused during intubation to reduce risk of disease transmission and to ensure first-pass intubation success [15]. It must be noted that this recommendation is a significant deviation from pre-pandemic management yet is warranted for the stated reasons; an approximate 10-second compression pause is considered appropriate in this setting. In the event of a difficult airway, placement of an SGA is reasonable to secure the airway and focus on chest compressions. Following intubation, the patient should be connected to a ventilator with a HEPA filter attached when possible to further limit the risk of viral aerosolization. Extra caution should be taken to maximize a closed circuit between the patient and the ventilator, as this carries a lower risk of aerosolization compared to other forms of positive pressure ventilation [54].
3.3.3. Proned patients
Proning awake COVID-19 patients in the ED has been shown to improve oxygen saturations and respiratory symptoms [55]. For proned patients in cardiac arrest without an advanced airway, it is recommended that they be placed back in the supine position prior to any continued resuscitation [15]. However, proned patients with an advanced airway should avoid being turned to prevent the risk of equipment disconnections. CPR should be provided to these proned patients with hands in the standard position over T7–10 [15].
3.4. Considerations in circulatory support
3.4.1. Mechanical CPR devices
Providers should consider the use of a mechanical CPR device, if available, to decrease the number of staff required in the room and increase cognitive offloading [15,39,56]. Several simulation studies have demonstrated the quality of CPR and success of other life-saving procedures performed by healthcare providers and emergency medical services (EMS) personnel decrease when wearing PPE [[57], [58], [59]].
3.4.2. Vascular access
There is a significant amount of literature suggesting hypercoagulability is an indicator of severe COVID-19 [7,[60], [61], [62], [63], [64], [65], [66]]. One study found a 31% incidence of thrombotic complications among intensive care unit (ICU) patients with COVID-19, and among them the most common was pulmonary embolism (PE) [64]. Healthcare providers should have a lower threshold to obtain additional vascular access, as this hypercoagulable state may result in the clotting of pre-existing lines. If not already obtained, intravascular (IV) or intraosseous access (IO) should also be rapidly obtained. An analysis of several randomized control trials suggest that the success of placing a peripheral IV is significantly reduced when healthcare providers have donned PPE [[67], [68], [69]]. In a cardiac arrest situation, the placement of either a peripheral or central venous catheter usually requires a significant amount of time and distraction of team members, compared to the rapid and reliable placement of an IO device. Therefore, it is recommended that medical providers have a low threshold for initiating IO access if IV access is unsuccessful [70]. In fact, many authorities recommend use of an IO device as the initial vascular access intervention unless pre-existing device exists [83].
3.4.3. Defibrillation
Evidence extrapolated from the 2012 SARS outbreak has shown that healthcare providers who performed defibrillation on critically ill patients did not become infected with SARS [71]. Additionally, studies looking at defibrillation as a potential exposing procedure in this population did not find a clinically significant increase in risk [72,73]. In patients with COVID-19, defibrillation is considered a non-aerosolizing procedure, and its use is recommended by the ACLS guidelines [15,74]. In their Consensus on Science with Treatment Recommendations, The International Liaison Committee on Resuscitation suggests that healthcare providers consider defibrillation before donning full AGP PPE in situations where the benefits may exceed the risks [74]; this recommendation is contrary to the recommending of donning full PPE prior to patient intervention and must be considered with extreme caution. COVID-19 patients with myocarditis or other cardiac complications leading to cardiac arrest have benefited from early defibrillation [75,76]. Treating shockable rhythms early may prevent the need for further resuscitative measures.
As per current ACLS guidelines, algorithms should be closely followed, including identifying and treating any reversible causes (e.g., hypoxia, electrolyte abnormalities, etc.) prior to termination of resuscitative efforts [20,41]. Transthoracic echocardiography (TTE), if available, should be considered to guide resuscitative efforts [77]. However, it is important to follow proper guidelines to avoid contamination of the ultrasound transducer [78].
3.4.4. Medications
While the administration of tissue plasminogen activator (tPA) has demonstrated improvement in the P/F ratio of COVID-19 patients with acute respiratory distress syndrome (ARDS), there is limited evidence regarding its role in cardiac arrest [62]. Thrombolysis should be considered if PE is the cause of cardiac arrest. Administration of other standard ACLS medications such as epinephrine in the COVID-19 cardiac arrest patient is no different as compared to pre-pandemic guidelines.
3.5. Considerations in underlying etiology
Significantly elevated D-dimer levels, low anti-thrombin levels, and pulmonary congestion with microvascular thrombosis increase the risk of PE, which may be the cause for sudden cardiac arrest in some patients with COVID-19 [63,64,[79], [80], [81]]. A systematic overview of 80 autopsies found several cases of pneumonia combined with pulmonary artery emboli, and 40% of the total cases had deep vein thromboses [82]. The injury to endothelial cells and facilitation of viral infiltration in the setting of a systemic hypercoagulable state may contribute to the development of PE [83].
The use of transesophageal echocardiography (TEE) during cardiac arrest may optimize the quality of chest compressions and provide additional diagnostic information regarding the cause of the arrest [84]. The ability to perform TEE in proned patients and its low risk of viral aerosolization in already intubated patients has led to a consensus statement by a multidisciplinary group of experts in point-of-care echocardiography to recommend its use in COVID-19 patients [84].
3.6. Post-cardiac arrest care
3.6.1. Role of the cardiac catherization lab
Given the high mortality rate of COVID-19 patients who survive an initial cardiac arrest, there is an understandable lack of data regarding the utility of percutaneous coronary intervention (PCI) following return of spontaneous circulation (ROSC). The American College of Cardiology (ACC) states that fibrinolysis can be considered as an option for the relatively stable ST elevation myocardial infarction (STEMI) patient with active COVID-19, but that PCI remains the treatment of choice [85]. Regarding unstable patients, including those who suffered cardiac arrest, the ACC also recommends patients be intubated prior to arriving to the catherization lab to decrease the risk of viral aerosolization and staff exposure [85].
3.6.2. Targeted temperature management
There is currently no evidence on the impact of targeted temperature management (TTM) in post-cardiac arrest patients with COVID-19. Given the neurologic benefit of TTM in non-COVID-19 patients, it should still be considered following ROSC and, if applicable, part of the patient's immediate post cardiac arrest treatment [86,87].
3.6.3. Extracorporeal membranous oxygenation
Use of extracorporeal membranous oxygenation (ECMO) can be a viable rescue strategy in carefully selected patients. However, ECMO is a resource-intensive technique restricted to highly specialized centers, limiting its widespread use. Although there are insufficient data to support ECMO during CPR (E-CPR), several major organizations recommend against it, given the potential for cross-contamination of staff, the significant use of PPE, and questionable risk-to-benefit ratio in patients with multiple co-morbidities or multiple organ failure [48,88,89]. A study by Yang et al. examined critically ill patients with COVID-19 and found that five of the six (83.3%) patients who were placed on ECMO did not survive [60].
3.6.4. Transportation
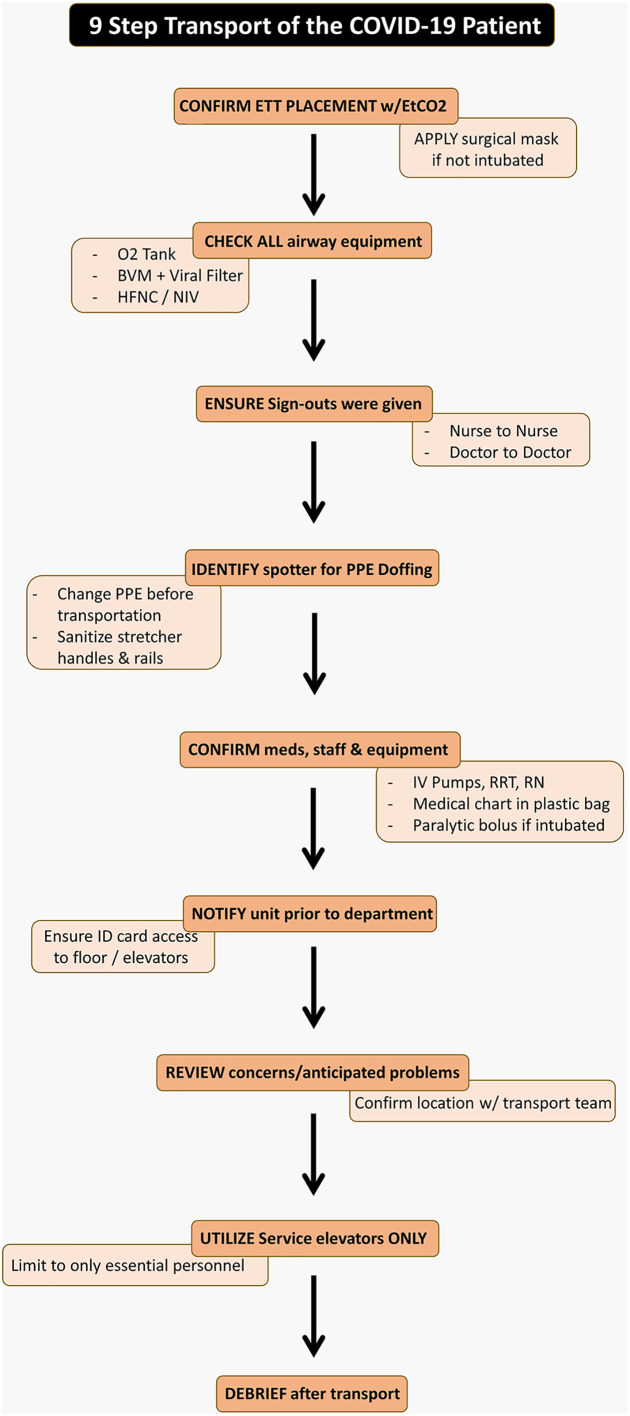
The transportation of the COVID-19 patient requires a team effort. The process should be conducted in a thorough and meticulous fashion with close attention to detail to protect staff and other patients in the hospital. Following patient stabilization, the 9 step process outlined in Fig. 5 should occur after nurse to nurse and physician to physician sign out has occurred.
Fig. 5.
Nine step algorithm to safely transport a COVID-19 patient.
3.6.5. Debriefing
Following the cardiac arrest, both the IPR and OPR teams should convene for a debrief. The debrief should be a reflective conversation about performance in a specified clinical setting or scenario [90,91]. Performance refers to a combination of taskwork and teamwork; the former represents the team's overall ability to adhere to a resuscitation algorithm or in this case, the team composition mentioned previously [92]. Taskwork also refers to specific psychomotor skills such as performing CPR. Given the novelty of this team composition when resuscitating COVID-19 patients, this dedicated debrief and feedback time is of utmost importance. It should be utilized to identify areas for improvement in duties, responsibilities, and communication. Both regular debriefing and feedback sessions are associated with improved ROSC, neurologic outcome, and time delay to first compression [[93], [94], [95], [96]]. Regardless of patient outcomes, team members need to recognize that treating COVID-19 infected patients requires a different approach and assist each another with the challenges that arise in adapting to this new method.
3.6.6. Ethical considerations and controversies
Although this review focuses primarily on in-hospital cardiac arrest, obtaining a thorough history from EMS personnel regarding the events that led up to the cardiac arrest continues to remain vital and may serve to guide further treatment. Regarding prehospital resuscitation, patients who meet ALL of the following criteria may not benefit from continued resuscitation:
-
-
Cardiac arrest not witnessed by EMS personnel
-
-
No ROSC at any point during prehospital resuscitation
-
-
Absence of shockable rhythm at any point during prehospital resuscitation [28]
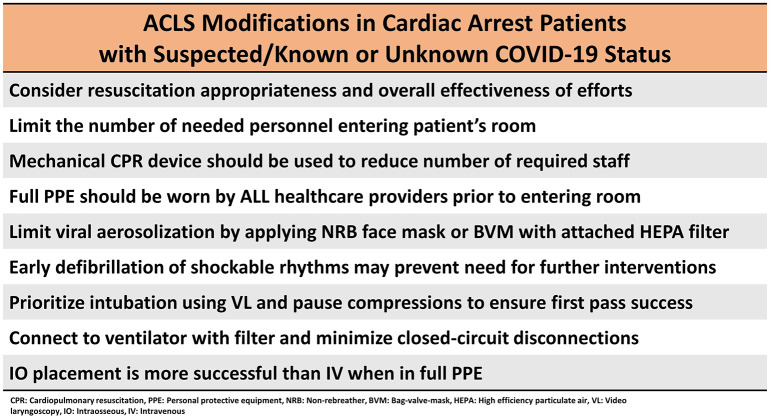
The difficulty in performing CPR as well as other life-saving interventions and procedures while wearing full PPE must be highlighted. While vital to staff safety, the use of appropriate PPE has been shown to decrease the effectiveness of compressions and impair healthcare provider's skills when performing advanced airway procedures [45,59,97,98]. Furthermore, resuscitation teams need to be aware of PPE's reduction in healthcare provider field of vision and interference with team communication, as these are vital when managing any patient in cardiac arrest [99,100]. A summary of the changes and modifications to advanced life support can be found in Fig. 6 .
Fig. 6.
Summary of ACLS modifications in cardiac arrest patients with suspected/known or unknown COVID-19 status.
3.6.7. Do not attempt resuscitation / Do not resuscitate considerations
Many clinicians have suggested a universal do-not-attempt-resuscitate (DNAR) / do-not-resuscitate (DNR) policy for all patients infected with COVID-19 infection, regardless of the patient's desires or family's wishes. The ethics of such DNAR/DNR decisions are complex and require thoughtful consideration by clinicians, ethicists, and legal representatives, among many others involved parties. Given the high mortality and poor overall outcome, the risks and benefits of CPR should be assessed for each patient on a case-by-case basis. In addition to the extremely poor prognosis of a COVID-19 patient in cardiorespiratory arrest, several other factors must be considered, including the exposure risk to resuscitation team members, extensive use of PPE required for all team members, and the delay in response to resuscitation mandated by appropriate donning of PPE prior to entry into the patient room. These decisions should be made at an institutional level.
4. Conclusion
COVID-19 is highly communicable and poses significant risk to healthcare providers. Avoiding aerosol-generating procedures and only performing such interventions when necessary with the appropriate PPE are vital when treating critically ill patients with COVID-19. Resuscitation status must be considered early so that patient prognosis, provider safety, and equipment/supply use are all optimized; the prior “do it all” approach to resuscitation likely no longer applies to the patient with severe COVID-19 illness. The most appropriate approach involves a realistic appraisal of the situation with patient and family awareness of the poor prognosis of severely ill COVID-19 patients; the clinical team must consider each patient on a case-by-case basis.
While the main tenants of ACLS have not drastically changed, this review helps identify important modifications to managing cardiac arrest patients. Among these are utilizing a mechanical compression device to limit needed personnel and prioritizing the placement of an SGA or intubation to prevent further aerosolization of this virus. Healthcare institutions should utilize a structured and organized team, consisting of the minimal number of team members needed inside and outside the patient's room, to further guarantee the safety of healthcare providers. In addition to early goals of care discussions with COVID-19 patients and/or their proxy, healthcare providers must identify the underlying cause of the patient's cardiac arrest and determine the risk/benefit of aggressive interventions in these critically ill patients.
Declaration of Competing Interests
None.
Acknowledgements
BL, TM, MR, WB, MG, and MS conceived the idea for this manuscript and contributed substantially to the writing and editing of the review. This manuscript did not utilize any grants, and it has not been presented in abstract form. This clinical review has not been published, it is not under consideration for publication elsewhere, its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright-holder. This review does not reflect the views or opinions of the U.S. government, Department of Defense, U.S. Army, U.S. Air Force, or SAUSHEC EM Residency Program.
References
- 1.Wuhan Coronavirus (2019-nCoV) Global Cases (by Johns Hopkins CSSE). Accessed March 26, 2020. https://gisanddata.maps.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
- 2.Guan W., Ni Z., Hu Y. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. Published online February 28:NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bansal M. Cardiovascular disease and COVID-19. Diabetes Metab Syndr. 2020;14(3):247–250. doi: 10.1016/j.dsx.2020.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y.-Y., Ma Y.-T., Zhang J.-Y., Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020:1–2. doi: 10.1038/s41569-020-0360-5. Published online March 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guo T., Fan Y., Chen M. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. Published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi S., Qin M., Shen B. Association of Cardiac Injury with Mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. Published online March 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tong Z.-D., Tang A., Li K.-F. Potential Presymptomatic transmission of SARS-CoV-2, Zhejiang Province, China, 2020. Emerg Infect Dis. 2020;26(5):1052–1054. doi: 10.3201/eid2605.200198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bai Y., Yao L., Wei T. Presumed asymptomatic carrier transmission of COVID-19. JAMA. 2020 doi: 10.1001/jama.2020.2565. Published online February 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rothe C., Schunk M., Sothmann P. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghinai I., McPherson T.D., Hunter J.C. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet Lond Engl. 2020;395(10230):1137–1144. doi: 10.1016/S0140-6736(20)30607-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng K., Poon B.H., Kiat Puar T.H. COVID-19 and the risk to health care workers: a case report. Ann Intern Med. 2020 doi: 10.7326/L20-0175. Published online March 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cascella M., Rajnik M., Cuomo A., Dulebohn S.C., Di Napoli R. StatPearls Publishing; StatPearls: 2020. Features, evaluation and treatment coronavirus (COVID-19)http://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- 15.Edelson Dana P., Sasson Comilla, Chan Paul S., et al. Interim Guidance for Basic and Advanced Life Support in Adults, Children, and Neonates With Suspected or Confirmed COVID-19: From the Emergency Cardiovascular Care Committee and Get With the Guidelines®-Resuscitation Adult and Pediatric Task Forces of the American Heart Association in Collaboration with the American Academy of Pediatrics, American Association for Respiratory Care, American College of Emergency Physicians, The Society of Critical Care Anesthesiologists, and American Society of Anesthesiologists: Supporting Organizations: American Association of Critical Care Nurses and National EMS Physicians. Circulation. 0(0). 10.1161/CIRCULATIONAHA.120.047463 [DOI]
- 16.CDC Recommendations for Preventing the Spread of Vancomycin Resistance Recommendations of the Hospital Infection Control Practices Advisory Committee (HICPAC) 2020. https://www.cdc.gov/mmwr/preview/mmwrhtml/00039349.htm Accessed April 20. [DOI] [PubMed]
- 17.Centers for Disease Control and Prevention (CDC) Update: management of patients with suspected viral hemorrhagic fever--United States. MMWR Morb Mortal Wkly Rep. 1995;44(25):475–479. [PubMed] [Google Scholar]
- 18.Christian M.D., Loutfy M., McDonald L.C. Possible SARS coronavirus transmission during cardiopulmonary resuscitation. Emerg Infect Dis. 2004;10(2):287–293. doi: 10.3201/eid1002.030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Advanced Cardiac Life Support Recommendations for ALL Patients During the COVID-19 Pandemic - Alfred Health Guideline. INTENSIVE. 2020. https://intensiveblog.com/advanced-cardiac-life-support-recommendations-for-all-patients-during-the-covid-19-pandemic-alfred-health-guideline/ Published March 26, 2020. Accessed March 27.
- 20.Resuscitation Council UK Statement on COVID-19 in relation to CPR and resuscitation in healthcare settings. https://www.resus.org.uk/media/statements/resuscitation-council-uk-statements-on-covid-19-coronavirus-cpr-and-resuscitation/covid-healthcare/
- 21.Chan J.F.-W., Yuan S., Kok K.-H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. The Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seto W.H., Tsang D., Yung R.W.H. Effectiveness of precautions against droplets and contact in prevention of nosocomial transmission of severe acute respiratory syndrome (SARS) Lancet Lond Engl. 2003;361(9368):1519–1520. doi: 10.1016/s0140-6736(03)13168-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wax R.S., Christian M.D. Practical recommendations for critical care and anesthesiology teams caring for novel coronavirus (2019-nCoV) patients. Can J Anaesth J Can Anesth. 2020 doi: 10.1007/s12630-020-01591-x. Published online February 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yam L.Y.C., Chen R.C., Zhong N.S. SARS: ventilatory and intensive care. Respirol Carlton Vic. 2003;8(Suppl):S31–S35. doi: 10.1046/j.1440-1843.2003.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cabrini L., Landoni G., Zangrillo A. Minimise nosocomial spread of 2019-nCoV when treating acute respiratory failure. The Lancet. 2020;395(10225):685. doi: 10.1016/S0140-6736(20)30359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Twu S.-J., Chen T.-J., Chen C.-J. Control measures for severe acute respiratory syndrome (SARS) in Taiwan. Emerg Infect Dis. 2003;9(6):718–720. doi: 10.3201/eid0906.030283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chow C.B. Post-SARS infection control in the hospital and clinic. Paediatr Respir Rev. 2004;5(4):289–295. doi: 10.1016/j.prrv.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.COVID Critical care: protected code blue (confirmed or presumptive COVID-19 case). COVID critical care - Ontario, Canada. https://covidcriticalcare.ca/cardiac-arrest/
- 29.Verbeek J.H., Ijaz S., Mischke C. Personal protective equipment for preventing highly infectious diseases due to exposure to contaminated body fluids in healthcare staff. Cochrane Database Syst Rev. 2016;4 doi: 10.1002/14651858.CD011621.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.CDC CDC Recommendations on Interim Infection Prevention and Control Recommendations for Patients with Suspected or Confirmed Coronavirus Disease 2019 (COVID-19) in Healthcare Settings. Centers for Disease Control and Prevention. 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/infection-control-recommendations.html Published February 11, 2020. Accessed April 11.
- 31.Müller M., Jürgens J., Redaèlli M., Klingberg K., Hautz W.E., Stock S. BMJ Open. 2018;8(8) doi: 10.1136/bmjopen-2018-022202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reader T.W., Flin R., Mearns K., Cuthbertson B.H. Interdisciplinary communication in the intensive care unit. Br J Anaesth. 2007;98(3):347–352. doi: 10.1093/bja/ael372. [DOI] [PubMed] [Google Scholar]
- 33.Burley D. Better communication in the emergency department. Emerg Nurse J RCN Accid Emerg Nurs Assoc. 2011;19(2):32–36. doi: 10.7748/en2011.05.19.2.32.c8509. [DOI] [PubMed] [Google Scholar]
- 34.Dayton E., Henriksen K. Communication failure: basic components, contributing factors, and the call for structure. Jt Comm J Qual Patient Saf. 2007;33(1):34–47. doi: 10.1016/s1553-7250(07)33005-5. [DOI] [PubMed] [Google Scholar]
- 35.Tool S.B.A.R. Situation-Background-Assessment-Recommendation | IHI - Institute for Healthcare Improvement. http://www.ihi.org:80/resources/Pages/Tools/SBARToolkit.aspx
- 36.Dunsford J. Structured communication: improving patient safety with SBAR. Nurs Womens Health. 2009;13(5):384–390. doi: 10.1111/j.1751-486X.2009.01456.x. [DOI] [PubMed] [Google Scholar]
- 37.De Meester K., Verspuy M., Monsieurs K.G., Van Bogaert P. SBAR improves nurse–physician communication and reduces unexpected death: a pre and post intervention study. Resuscitation. 2013;84(9):1192–1196. doi: 10.1016/j.resuscitation.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Lee S.Y., Dong L., Lim Y.H., Poh C.L., Lim W.S. SBAR: towards a common interprofessional team-based communication tool. Med Educ. 2016;50(11):1167–1168. doi: 10.1111/medu.13171. [DOI] [PubMed] [Google Scholar]
- 39.Shao F., Xu S., Ma X. In-hospital cardiac arrest outcomes among patients with COVID-19 pneumonia in Wuhan, China. Resuscitation. 2020 doi: 10.1016/j.resuscitation.2020.04.005. Published online April 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Atkinson P, French J, Lang E, McColl T, Mazurik L. Just the Facts: Protecting frontline clinicians during the COVID-19 pandemic. Cjem.:1–5. doi: 10.1017/cem.2020.359 [DOI] [PMC free article] [PubMed]
- 41.Morgenstern J. First10EM: COVID Resuscitation Principles. First10EM. 2020. https://first10em.com/covid-resuscitation-principles/ Published March 22.
- 42.Farkas J. Internet book of critical care: COVID-19. EMCrit Project. 2020 https://emcrit.org/ibcc/covid19/ [Google Scholar]
- 43.Brewster D.J., Chrimes N.C., Do T.B. Consensus statement: safe airway society principles of airway management and tracheal intubation specific to the COVID-19 adult patient group. Med J Aust. 2020;212(10):1. doi: 10.5694/mja2.50598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rezaie S. REBEL EM: COVID-19: airway management. REBEL EM - Emergency Medicine Blog. 2020 https://rebelem.com/covid-19-airway-management/ Published March 1. [Google Scholar]
- 45.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: guidelines from the difficult airway society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020 doi: 10.1111/anae.15054. Published online March 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brown C., Moiser J., Law A., Carlson J., Gibbs M. COVID-19 a 3-step approach to intubation • LITFL • Coronavirus Update. Life in the Fast Lane • LITFL • Medical Blog. 2020. https://litfl.com/covid-3-step-approach-to-intubation/ Published March 19.
- 47.Peng PWH, Ho P-L, Hota SS. Outbreak of a new coronavirus: what anaesthetists should know. Br J AnaesthPublished online February 2020:S0007091220300982. doi: 10.1016/j.bja.2020.02.008. [DOI] [PMC free article] [PubMed]
- 48.Alhazzani W., Møller M.H., Arabi Y.M. Surviving Sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020 doi: 10.1007/s00134-020-06022-5. Published online March 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Song W., Liu Y., Ouyang Y. Recommendations on cardiopulmonary resuscitation strategy and procedure for novel coronavirus pneumonia. Resuscitation. 2020 doi: 10.1016/j.resuscitation.2020.03.023. Published online April 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Canelli R., Connor C.W., Gonzalez M., Nozari A., Ortega R. Barrier Enclosure during Endotracheal Intubation. N Engl J Med. 2020 doi: 10.1056/NEJMc2007589. Published online April 3. NEJMc2007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Begley JL, Lavery KE, Nickson CP, Brewster DJ. The aerosol box for intubation in COVID-19 patients: an in-situ simulation crossover study. Anaesthesia. n/a(n/a). doi: 10.1111/anae.15115 [DOI] [PMC free article] [PubMed]
- 52.Bianco F., Incollingo P., Grossi U., Gallo G. Preventing transmission among operating room staff during COVID-19 pandemic: the role of the aerosol box and other personal protective equipment. Updates Surg. 2020:1–4. doi: 10.1007/s13304-020-00818-2. Published online May 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jazuli F., Bilic M., Hanel E., Ha M., Hassall K., Trotter B.G. Endotracheal intubation with barrier protection. Emerg Med J EMJ. 2020 doi: 10.1136/emermed-2020-209785. Published online June 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mechanical Ventilation of SARS Patients: Lessons from the 2003 SARS outbreak. ECRI. 2020. https://www.ecri.org/components/HDJournal/Pages/Mechanical-Ventilation-of-SARS-Patients-2003-SARS-Outbreak.aspx Accessed April 21.
- 55.Caputo N.D., Strayer R.J., Levitan R. Early Self-Proning in Awake, Non-intubated Patients in the Emergency Department: A Single ED’s Experience during the COVID-19 Pandemic. Acad Emerg Med. 2020 doi: 10.1111/acem.13994. Published online April 22: acem.13994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeFilippis Ersilia M., Ranard Lauren S., Berg David D. Cardiopulmonary Resuscitation During the COVID-19 Pandemic: A View from Trainees on the Frontline. Circulation. doi: 10.1161/CIRCULATIONAHA.120.047260 [DOI] [PMC free article] [PubMed]
- 57.Adler M.D., Krug S., Eiger C. Impact of Personal Protective Equipment on the Performance of Emergency Pediatric Tasks. Pediatr Emerg Care. 2020;24 doi: 10.1097/PEC.0000000000002028. Published online February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen J., Lu K.-Z., Yi B., Chen Y. Chest compression with personal protective equipment during cardiopulmonary resuscitation: a randomized crossover simulation study. Medicine (Baltimore) 2016;95(14) doi: 10.1097/MD.0000000000003262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim T.H., Kim C.H., Shin S.D., Haam S. Influence of personal protective equipment on the performance of life-saving interventions by emergency medical service personnel. SIMULATION. 2016;92(10):893–898. doi: 10.1177/0037549716662322. [DOI] [Google Scholar]
- 60.Yang X., Yu Y., Xu J. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30079-5. Published online February. S2213260020300795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang J, Hajizadeh N, Moore EE, et al. Tissue Plasminogen Activator (tPA) Treatment for COVID-19 Associated Acute Respiratory Distress Syndrome (ARDS): A Case Series. J Thromb Haemost. doi: 10.1111/jth.14828 [DOI] [PMC free article] [PubMed]
- 63.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0188. Published online March 16. [DOI] [PubMed] [Google Scholar]
- 64.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Middeldorp S., Coppens M., van Haaps T.F. Incidence of venous thromboembolism in hospitalized patients with COVID-19. J Thromb Haemost JTH. 2020 doi: 10.1111/jth.14888. Published online May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Garcia-Olivé I., Sintes H., Radua J., Abad Capa J., Rosell A. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med. 2020;169:106023. doi: 10.1016/j.rmed.2020.106023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Suyama J., Knutsen C.C., Northington W.E., Hahn M., Hostler D. IO versus IV access while wearing personal protective equipment in a HazMat scenario. Prehospital Emerg Care Off J Natl Assoc EMS Physicians Natl Assoc State EMS Dir. 2007;11(4):467–472. doi: 10.1080/10903120701536982. [DOI] [PubMed] [Google Scholar]
- 68.Lamhaut L., Dagron C., Apriotesei R. Comparison of intravenous and intraosseous access by pre-hospital medical emergency personnel with and without CBRN protective equipment. Resuscitation. 2010;81(1):65–68. doi: 10.1016/j.resuscitation.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 69.Castle N., Owen R., Hann M., Clark S., Reeves D., Gurney I. Impact of chemical, biological, radiation, and nuclear personal protective equipment on the performance of low- and high-dexterity airway and vascular access skills. Resuscitation. 2009;80(11):1290–1295. doi: 10.1016/j.resuscitation.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 70.Smereka J., Szarpak L., Filipiak K.J., Jaguszewski M., Ladny J.R. Which intravascular access should we use in patients with suspected/confirmed COVID-19? Resuscitation. 2020 doi: 10.1016/j.resuscitation.2020.04.014. Published online April 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Loeb M., McGeer A., Henry B. SARS among critical care nurses, Toronto. Emerg Infect Dis. 2004;10(2):251–255. doi: 10.3201/eid1002.030838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7(4) doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Raboud J., Shigayeva A., McGeer A. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One. 2010;5(5) doi: 10.1371/journal.pone.0010717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Couper K., Taylor-Phillips S., Grove A. International Liason Committee on Resuscitation. 2020. COVID-19 infection risk to rescuers from patients in cardiac arrest.https://costr.ilcor.org/document/covid-19-infection-risk-to-rescuers-from-patients-in-cardiac-arrest [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fritz Z., Perkins G.D. Cardiopulmonary resuscitation after hospital admission with covid-19. BMJ. 2020;369:m1387. doi: 10.1136/bmj.m1387. [DOI] [PubMed] [Google Scholar]
- 76.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. Published online March 27. [DOI] [PubMed] [Google Scholar]
- 77.Gottlieb M., Alerhand S. Ultrasonography: a useful adjunct in cardiac arrest. Ann Emerg Med. 2020;75(4):514–515. doi: 10.1016/j.annemergmed.2019.07.025. [DOI] [PubMed] [Google Scholar]
- 78.Guideline for Ultrasound Transducer Cleaning and Disinfection. acep.org. Published June 2018. Accessed April 23, 2020. https://www.acep.org/globalassets/new-pdfs/policy-statements/guideline-for-ultrasound-transducer-cleaning-and-disinfection.pdf
- 79.Luo W., Yu H., Gou J. Clinical Pathology of Critical Patient with Novel Coronavirus Pneumonia (COVID-19) 2020. https://www.preprints.org/manuscript/202002.0407/v1 Published online February 27.
- 80.Li T., Lu H., Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–690. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu J., Wang L., Zhao L. 2020. Risk Assessment of Venous Thromboembolism and Bleeding in COVID-19 Patients. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Edler C., Schröder A.S., Aepfelbacher M. Dying with SARS-CoV-2 infection—an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020 doi: 10.1007/s00414-020-02317-w. Published online June 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin Immunol Orlando Fla. 2020 doi: 10.1016/j.clim.2020.108427. Published online April 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teran F., Burns K.M., Narasimhan M. Critical care transesophageal echocardiography in patients during the COVID-19 pandemic. J Am Soc Echocardiogr. 2020 doi: 10.1016/j.echo.2020.05.022. Published online May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Welt F.G.P., Shah P.B., Aronow H.D. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: from ACC’s interventional council and SCAI. J Am Coll Cardiol. 2020 doi: 10.1016/j.jacc.2020.03.021. Published online March 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hypothermia after Cardiac Arrest Study Group Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 87.Writing Group, Nolan J.P., Morley P.T. Therapeutic hypothermia after cardiac arrest: an advisory statement by the advanced life support task force of the international liaison committee on resuscitation. Circulation. 2003;108(1):118–121. doi: 10.1161/01.CIR.0000079019.02601.90. [DOI] [PubMed] [Google Scholar]
- 88.Ramanathan K., Antognini D., Combes A. Planning and provision of ECMO services for severe ARDS during the COVID-19 pandemic and other outbreaks of emerging infectious diseases. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30121-1. Published online March. S2213260020301211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Extracorporeal Life Support Organization Guidance Document on ECMO in COVID-19. 2020. https://www.elso.org/COVID19.aspx Accessed March 28.
- 90.Cheng A., Eppich W., Grant V., Sherbino J., Zendejas B., Cook D.A. Debriefing for technology-enhanced simulation: a systematic review and meta-analysis. Med Educ. 2014;48(7):657–666. doi: 10.1111/medu.12432. [DOI] [PubMed] [Google Scholar]
- 91.Raemer D., Anderson M., Cheng A., Fanning R., Nadkarni V., Savoldelli G. Research Regarding Debriefing as Part of the Learning Process. Simul Healthc J Soc Simul Healthc. 2011;6:S52–S57. doi: 10.1097/SIH.0b013e31822724d0. [DOI] [PubMed] [Google Scholar]
- 92.Cheng Adam, Nadkarni Vinay M., Beth Mancini Mary. Resuscitation education science: educational strategies to improve outcomes from cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2018;138(6):e82–e122. doi: 10.1161/CIR.0000000000000583. [DOI] [PubMed] [Google Scholar]
- 93.Jiang C., Zhao Y., Chen Z., Chen S., Yang X. Improving cardiopulmonary resuscitation in the emergency department by real-time video recording and regular feedback learning. Resuscitation. 2010;81(12):1664–1669. doi: 10.1016/j.resuscitation.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 94.Tannenbaum S.I., Cerasoli C.P. Do team and individual debriefs enhance performance? A Meta-Analysis. Hum Factors. 2012 doi: 10.1177/0018720812448394. Published online June 4. [DOI] [PubMed] [Google Scholar]
- 95.Edelson D.P., Litzinger B., Arora V. Improving in-hospital cardiac arrest process and outcomes with performance debriefing. Arch Intern Med. 2008;168(10):1063–1069. doi: 10.1001/archinte.168.10.1063. [DOI] [PubMed] [Google Scholar]
- 96.Boet S., Sharma B., Pigford A.-A., Hladkowicz E., Rittenhouse N., Grantcharov T. Debriefing decreases mental workload in surgical crisis: a randomized controlled trial. Surgery. 2017;161(5):1215–1220. doi: 10.1016/j.surg.2016.11.031. [DOI] [PubMed] [Google Scholar]
- 97.Coates M.J., Jundi A.S., James M.R. Chemical protective clothing; a study into the ability of staff to perform lifesaving procedures. J Accid Emerg Med. 2000;17(2):115–118. doi: 10.1136/emj.17.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Del Rossi G., Bodkin D., Dhanani A., Courson R.W., Konin J.G. Protective athletic equipment slows initiation of CPR in simulated cardiac arrest. Resuscitation. 2011;82(7):908–912. doi: 10.1016/j.resuscitation.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 99.Visentin L.M., Bondy S.J., Schwartz B., Morrison L.J. Use of personal protective equipment during infectious disease outbreak and nonoutbreak conditions: a survey of emergency medical technicians. CJEM. 2009;11(1):44–56. doi: 10.1017/s1481803500010915. [DOI] [PubMed] [Google Scholar]
- 100.Daugherty E.L., Perl T.M., Needham D.M., Rubinson L., Bilderback A., Rand C.S. The use of personal protective equipment for control of influenza among critical care clinicians: a survey study. Crit Care Med. 2009;37(4):1210–1216. doi: 10.1097/CCM.0b013e31819d67b5. [DOI] [PubMed] [Google Scholar]