Graphical abstract
Keywords: Propolis, SARS-CoV-2, COVID-19, Antiviral, Anti-inflammatory, PAK1 blocker
Abstract
Propolis, a resinous material produced by honey bees from plant exudates, has long been used in traditional herbal medicine and is widely consumed as a health aid and immune system booster. The COVID-19 pandemic has renewed interest in propolis products worldwide; fortunately, various aspects of the SARS-CoV-2 infection mechanism are potential targets for propolis compounds. SARS-CoV-2 entry into host cells is characterized by viral spike protein interaction with cellular angiotensin-converting enzyme 2 (ACE2) and serine protease TMPRSS2. This mechanism involves PAK1 overexpression, which is a kinase that mediates coronavirus-induced lung inflammation, fibrosis, and immune system suppression. Propolis components have inhibitory effects on the ACE2, TMPRSS2 and PAK1 signaling pathways; in addition, antiviral activity has been proven in vitro and in vivo. In pre-clinical studies, propolis promoted immunoregulation of pro-inflammatory cytokines, including reduction in IL-6, IL-1 beta and TNF-α. This immunoregulation involves monocytes and macrophages, as well as Jak2/STAT3, NF-kB, and inflammasome pathways, reducing the risk of cytokine storm syndrome, a major mortality factor in advanced COVID-19 disease. Propolis has also shown promise as an aid in the treatment of various of the comorbidities that are particularly dangerous in COVID-19 patients, including respiratory diseases, hypertension, diabetes, and cancer. Standardized propolis products with consistent bioactive properties are now available. Given the current emergency caused by the COVID-19 pandemic and limited therapeutic options, propolis is presented as a promising and relevant therapeutic option that is safe, easy to administrate orally and is readily available as a natural supplement and functional food.
1. Introduction
The COVID-19 pandemic is of grave concern due its impact on human health and on the economy. It is much more deadly than influenza and other types of diseases that recently have had worldwide impact [1], forcing countries to take unusual measures such as limiting travel, closing schools, businesses, and other locations where many people can come into contact with each other. Various public healthcare strategies have been adopted in an attempt to reduce the impact of the disease, but with limited effectiveness, as the virus continues to spread, often through asymptomatic patients [2]. Unfortunately, tests to determine if people are infectious or were previously infected are not widely available, often are costly, and frequently do not provide timely and accurate results. Various therapeutic alternatives have been proposed and tested; however, most require more robust data in clinical trials before they can be widely and safely used [3].
Isolation and stay-at-home measures do not effectively protect essential workers, especially health care personnel, who have become infected and are dying at alarming rates [4]. Economic and other necessities limit how well and how long these isolation measures can be maintained, especially in poor countries and in poor communities such as slums and favelas [5,6]. As populations gradually try to get back to normalcy, reducing social distancing, and people in “non-essential professions” return to the workplace, they become more at risk for infection. In this scenario, any options that could help ameliorate disease progression and its consequences, even marginally, would be useful. The world needs safe alternatives to help reduce the impact of this deadly disease.
Natural products, which have historically been widely used to help avoid and alleviate diseases [[7], [8], [9]], are among the options being considered as an adjuvant treatment for SARS-CoV-2 infection [10], because they are generally inexpensive, widely available, and rarely have undesirable side effects. Some have proven antiviral activity [[11], [12], [13]]. An important advantage of using natural remedies is that people who have other health problems or who have mild flu-related symptoms, but do not have the means or courage to visit an already overcrowded medical facility, could take simple and inexpensive measures to help reduce the impact of infection with SARS-CoV-2.
Considering the large number of deaths and other types of damage that the COVID-19 pandemic is causing, there is an urgent need to find treatments that have been approved as safe, and potentially able to inhibit the new coronavirus, reduce its infectivity, and/or alleviate the symptoms of infection [14,15]. Along this line, propolis and its components emerge as potential candidate materials that could help to reduce the pathophysiological consequences of SARS-CoV-2 infection [10].
Infection by SARS-CoV-2, the virus that causes COVID-19, is characterized by binding between viral spike proteins and angiotensin-converting enzyme 2 (ACE2) [16]. Activation of the spike protein is mediated through proteases, such as TMPRSS2, which play important roles in the viral infection [17]. After entry, followed by endocytosis, coronavirus infection causes PAK1 upregulation, a kinase that mediates lung inflammation, lung fibrosis and other critical mortality factors. Increased PAK1 levels also suppress the adaptive immune response, facilitating viral replication [10,18]. SARS-CoV-2 infection is associated with increased levels of chemokines and activated pro-inflammatory cytokines that lead to the development of atypical pneumonia, with rapid respiratory impairment and pulmonary failure [19]. Immunological/inflammatory phenomena (such as cytokine release syndrome) have been shown to be important in the spectrum of SARS-CoV-2 infection. These mechanisms are associated with organ dysfunction more than the viral load per se [20]. Along this line, a retrospective observational study found higher serum levels of pro-inflammatory cytokines such as IL-6, IL-1, and TNF-α, in patients with severe COVID-19, compared to individuals with mild disease [21].
There is considerable evidence that propolis can reduce and alleviate the symptoms of inflammatory diseases by affecting various metabolic cycles [[22], [23], [24]]. Recently, several studies have shown that propolis extract and some of its components act against several important targets in the pathophysiological context of the disease caused by SARS-CoV-2, such as reducing TMPRSS2 expression, and reducing ACE2 anchorage, which would otherwise facilitate entry of the virus into the cell; this is in addition to immunomodulation of monocytes / macrophages (reducing production of and eliminating IL-1 beta and IL-6), reduction of the transcription factors NF-KB and JAK2 / STAT3 and blocking PAK1, which determine inflammatory activities and fibrosis caused by COVID-19 [[25], [26], [27], [28]].
Various comorbidities have been associated with severe COVID19 symptoms and a greater chance of patients requiring intensive care; these include hypertension and diabetes. Also, mortality rates of COVID19 patients are much higher in those with cardiovascular disease, chronic respiratory disease, and diabetes [29,30]. There is considerable evidence that these conditions could be alleviated by treatment with propolis. This includes research in animal models of diabetes [31,32], hypertension [33,34], and cardiovascular disease [35,36]. Propolis has properties that are particularly relevant to SARS-CoV-2 infection, such as immune system fortification, reduced viral replication, and anti-inflammatory action [22,24,28,37,38].
2. Propolis and its properties
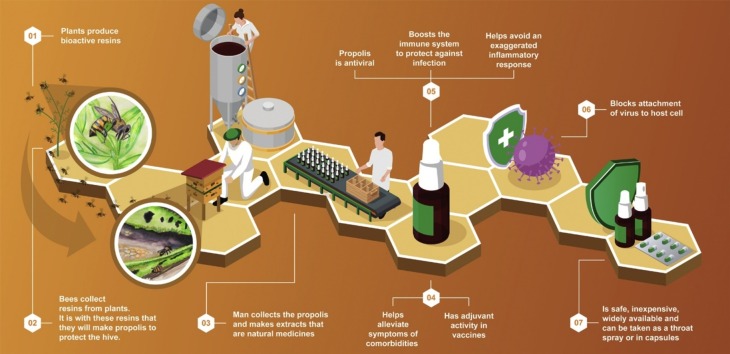
Propolis is a product derived from resins and plant exudates. Plants defend themselves from pathogens mainly by producing phytochemicals, many of which have been extracted and used in medicine [39]. Plant defense substances collected by bees include phenols and terpenoids [[40], [41], [42]]. Phytochemical compounds that show promise for the inhibition of coronavirus in humans include quercetin, myricetin, and caffeic acid, all components of propolis [43]. Honey bees and many other species of social bees recognize these antimicrobial properties and selectively collect and process these plant products to make propolis, which they use to protect the colony [44]. The production and use of propolis by honey bees evolved to the point that these social bees have considerably fewer immune genes than solitary insect species [45]. Bees in colonies that produce more propolis are healthier and live longer [46], and propolis consumption by the bees augments their immune response to bacterial challenge [47].
The composition of propolis varies according to the plant species available in each region [42,48,49]. As this variability can affect their medicinal properties, standardized propolis products have been developed to help meet the need for a product that does not vary in the main bioactive components and is safe, with minimal interaction with pharmaceutical drugs and proven efficacy in clinical trials [[50], [51], [52], [53]]. In recent decades, it has been shown to have antimicrobial (including antiviral), anti-inflammatory, immunomodulatory, antioxidant, and anticancer properties [54]. Propolis has historically been widely used to alleviate various diseases [[7], [8], [9],55]; it also has been considered, among other natural alternatives, as an adjuvant treatment for SARS-CoV-2 infection [10], because it is generally inexpensive, widely available and rarely causes undesirable side effects.
Some types of propolis that are highly valued for their medicinal properties, such as Brazilian green propolis, are mainly produced by bees from materials they collect from specific plants, in this case Baccharis dracunculifolia [56]. After the botanical origin of the propolis has been identified, extracts of the plant can be made to develop useful products, such as a medicinal mouthwash [57]. However, the medicinal properties of these plant extracts are often inferior compared to the propolis that the bees make from these plant materials [[58], [59], [60]].
3. Why propolis may be a good fit for dealing with COVID-19
Among natural medicine alternatives, propolis has been widely studied and is already extensively consumed in many countries [38,55,[61], [62], [63]]. For example, propolis products, such as throat sprays and extracts, are produced by hundreds of companies in Brazil and are sold as a health aid in nearly every pharmacy throughout the country, demonstrating on a practical basis that they can be safely consumed. These propolis products, and the raw material for their manufacture, are extensively exported by Brazilian companies, especially to Asian countries, including Japan, South Korea, and China [50,64]. The importance of propolis in Chinese, Japanese, Russian and Korean medicine is reflected in the number of patents for propolis containing products registered by 2013, including about 1200 by China and 300 – 400 each for Japan, Russia and Korea [42]. Since 2013, about 1400 new propolis-related patents were applied for in the US patent office. It is a key ingredient in traditional Chinese medicine [65]. Japanese scientists have isolated and patented various Brazilian propolis components for cancer treatment [66], demonstrating their usefulness. In fact, propolis has a wide spectrum of pharmacological properties and is a dietary supplement that is commonly consumed by both healthy and sick people as a preventative precaution and for treatment [[67], [68], [69]]. It is also used in veterinary medicine, due its antibacterial, antifungal, antiviral, antiparasitic, hepatoprotective, and immunomodulatory activities [70].
In the wake of the coronavirus outbreak, South Korea has seen a boon in the use of functional foods. According to their Ministry of Food and Drug Safety, “health functional foods” are nutrients that have been proven to be beneficial to health [71]. In March of this year, in response to the coronavirus pandemic, the ministry eased regulations for propolis, which is considered a functional food, and allowed new oral formulations [72]. However, despite considerable evidence that propolis can reduce and alleviate disease symptoms, its acceptance as a health-promoting supplement in human medicine has been limited in many countries such as the USA because of a relevant criticism that propolis products are not standardized and vary in their components and biological activity. In part, this is because propolis varies with the species of plants available in each region, from which the bees collect resins to produce it [42,48,49]. However, standardized propolis products have recently become available to help fill the need for a product that does not vary in the main bioactive components and effectiveness [50,52]. One such option, a standardized Brazilian propolis extract blend [54], has been tested for safety and effectiveness in clinical trials for treating kidney disease and diabetes [51], denture stomatitis [73], and burn patients [74]. Therefore, propolis as a nutraceutical or functional food should be considered as a resource that could help fight against the COVID-19 pandemic.
4. Some propolis compounds can potentially interact with SARS-CoV-2 MPRO
The research community has examined the genetic code of coronavirus and the mechanisms underlying the damages caused by SARS-CoV-2, to help search for drugs and/or potential targets in order to inactive the virus and reduce the damage that it causes. The main protease of coronavirus SARS-CoV-2, MPRO (3-chymotrypsin-like cysteine enzyme), is essential for coronavirus processing of polyproteins and for its life cycle, and therefore inhibition of the active site of this enzyme is a relevant target for drug discovery [75].
Along this line, Hashem evaluated various natural compounds with an in silico approach (molecular docking) to try to find useful options for treating SARS-CoV-2 infection. Curiously, caffeic acid phenethyl ester (CAPE), galangin, chrysin and caffeic acid, substances found in several different types of propolis around the world, appeared as potential drugs against this viral target (Table 1 ) [76]. Specifically, CAPE was predicted to interact with SARS-CoV-2 MPRO in a similar study [77]. Therefore, although it will be necessary to run in vitro assays to evaluate the potential anti-SARS-CoV-2 effects of propolis and/or its constituents, these in silico results are well boding.
Table 1.
Potential pathways through which propolis and its components could attenuate SARS-CoV-2 infection and its consequences.
| N | Targets | Aspect of SARS-CoV-2 infection | Propolis Components | Effect of the components and type of evidence |
|---|---|---|---|---|
| 1 | Viral RNA-dependent RNA polymerase (RdRp) and Spike glycoprotein (SGp) | Viral component that attaches to host cell | Limonin, Quercetin and Kaempferol | Inhibitory potential with high binding energy to viral components from -9 to -7.1 kcal/mol (in silico) [15] |
| 2 | 3a Channel Protein | Viral component that attaches to host cell | Kaempferol | Blocks the 3a channel that is encoded by ORF 3a of SARS-CoV (in vitro) [238] |
| 3 | ACE2 | Main receptor for viral entry | Myricetin, Caffeic Acid Phenethyl Ester, Hesperetin and Pinocembrin | Inhibitory potential with high binding energy to ACE2 (-8.97 kcal/mol) (in silico) [26] |
| Kaempferol | Inhibitory potential with high binding energy to ACE2 (-7.5 kcal/mol) (in silico) [239] | |||
| Quercetin | Inhibitory potential with high binding energy to ACE2 (-10.4 kcal/mol) (in silico) [25] | |||
| 3 | TMPRSS2 | Serine protease that mediates spike protein priming for viral entry | Kaempferol | Downregulates androgen receptors such as PSA and TMPRSS2 in a prostate cancer model (in vitro) [83] |
| 4 | PAK-1 | PAK-1 (RAC/CDC42-activated kinases) - Responsible for suppression of immune system in hosts | Caffeic Acid and Caffeic Acid Phenethyl Ester | Downregulates PAK-1 associated with Rac1 activation (in vitro) [18] |
| Inhibits PAK-1 directly or up-stream, blocking coronaviral infection (Review) [10] | ||||
| 5 | 3C-like protease | Mediates the proteolytic processing of replicase polypeptides 1a and 1ab into functional proteins in SARS-CoV-2 infection | Hesperetin | Inhibits cleavage activity of 3CLpro (in vitro) [240] |
| 6 | Inflammatory response | Response to viral infection that leads to organ injury | Propolis Extract | Inhibits NF-kB activation (in vitro) [241] |
| Induces Ca2+ signaling in dendritic cells in Peyer’s patches, improving the immune response (in vitro) [242] | ||||
| Attenuates the inflammatory response through intracellular ROS and NO levels with downregulation of IL‐1β and IL‐6 expression (in vitro) [27] | ||||
| Regulates IFN-γ, IL-6, and IL-10 cytokines in an experimental asthma model (in vivo) [243] | ||||
| Increases TGF-β and IL-10 levels, which contribute to the regulation of the inflammatory process in Acute Pulmonary Inflammation (in vivo) [24] | ||||
| Inhibits the production of ROS, RNS, NO, cytokines IL-1α, IL-1β, IL-4, IL-6, IL-12p40, IL-13, TNF-α, G-CSF, GM-CSF, MCP-1, MIP-1α, MIP-1β, and RANTES in stimulated J774A.1 macrophages (in vitro) [244] | ||||
| Kaempferol | Reduces TNF-α, IL-6, VEGF via the ERK-NFkB-cMyc-p21 pathway (in vitro) [83] | |||
| Caffeic Acid Phenethyl Ester | Inhibits NF-kB activation in HTLV-1 infection (in vitro) [245] | |||
| Modulates JAK/STAT signaling and attenuates oxidative stress and inflammation [87]. | ||||
| Immunomodulation | Adaptive immune response against viral infection | Propolis Extract | Increases humoral and cellular response in mice immunized with Suid herpesvirus type 1[106] | |
| Suppresses the differentiation of Th17 cells by inhibition of IL-6-induced phosphorylation of signal transducer and activator of transcription 3 (STAT3) (in vivo) [88] | ||||
| 7 | Thrombosis | Blood clotting dysregulation caused by viral infection | Quercetin | Inhibits thrombin in thrombotic manifestations (in vitro) [246] |
| 8 | Viral replication | Viral translation | Kaempferol and Hesperetin | Inhibits internal ribosomal entry site (IRES) activity required for viral protein translation (in vitro) [247] |
| Transcription | Kaempferol | Inhibits human immunodeficiency virus reverse transcriptase-associated DNA polymerase as well as RNAase H and RNase H activities (in vitro) [248] | ||
| Presents potent anti-HIV-1 reverse transcriptase activity (in vitro) [249] | ||||
| Endocytosis | Quercetin | Decreases Akt phosphorylation and viral endocytosis of Rhinovirus (in vivo) [250] | ||
| Replication and virion integrity | Prevents up-regulation of diacylglycerol acyltransferase (DGAT) required for hepatitis C virus replication (in vitro) [251] | |||
| Replication | Decreases heat shock proteins and Hepatitis B virus transcription levels (in vitro) [252] | |||
| Endocytosis | Caffeic Acid | Inhibits Hepatitis B virus-DNA replication (in vivo & in vitro) [253] | ||
| Endocytosis | Inhibits influenza A virus (IAV) replication (in vitro) [254] | |||
| Endocytosis | Inhibits influenza A virus (IAV) activity through neuraminidases (in vitro) [255] | |||
| Transcription | Caffeic Acid Phenethyl Ester | Inhibits HIV-1 integrase (Review) [256] |
5. Propolis can interact with ACE2 and TMPRSS2, potentially blocking or reducing SARS-CoV-2 invasion of the host cell
SARS-CoV-2 strongly binds to angiotensin-converting enzyme 2 (ACE2), using this enzyme as a receptor for invasion and replication in the host cell [17,78], causing damage and increasing interpersonal transmission [26,79]. Consequently, ACE inhibitors have been considered as useful drug alternatives. However, potential deleterious effects on users of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have emerged as a concern for treatment of COVID-19 patients. An observational study involving 8,910 patients did not confirm this suspicion, and therefore these classes of drugs remain an important tool against potential cardiovascular events [80].
Inhibition of ACE2 enzyme is an important target for treatment against SARS-CoV-2 infection [15,81]. Güler et al. [26] prepared an alcoholic extract of propolis and identified some hydroxycinnamic acids (caffeic acid, p-coumaric acid, t-cinnamic acid and CAPE), the flavanons rutin and myricetin, and the flavones hesperidin, chrysin and pinocembrin. Using molecular docking evaluations, they found that rutin had the highest binding energy to ACE2, followed by myricetin, caffeic acid phenethyl ester, hesperetin and pinocembrin. Rutin interacts with zinc fingers of the active sites of ACE2, a metalloprotease that presents the same zinc finger in ACE [26].
In addition to the in silico evidence, Osés et al. [82] evaluated several types of propolis for various characteristics, including inhibition of ACE. They found strong inhibition for most of the propolis types they studied, with higher than 90% ACE inhibition. The best results were found with the propolis components catechin and p-coumaric acid.
ACE2 and TMPRSS2 (transmembrane serine protease 2) on the surface of host cells are used by SARS-CoV-2 via interaction with spike glycoproteins in order to proceed with invasion and replication [15]. Vardhan & Sahoo [15] studied several molecules commonly found in medicinal herbs using molecular docking procedures with relevant targets, such as RNA-dependent RNA polymerase (RdRp), ACE2 and spike glycoproteins and compared the resulting scores with those of hydroxychloroquine [15]. Limonin was the most active compound; however, quercetin and kaempferol, also propolis compounds, gave high docking scores [15]. Kaempferol was studied in prostate cancer models, and the expression of TMPRSS2 was reduced, showing a potential mechanism of action for an antitumoral effect [83]. Kaempferol could be an important propolis component for use against COVID-19, since it is involved in the inhibition of TMPRSS2 [83], potentially interacting with ACE2, RdRp and spike glycoprotein (SGp) [15], besides its antiviral activity [84] (Table 1).
6. Propolis blocks PAK-1, potentially avoiding lung fibrosis and restoring a normal immune response
Among the possible targets for controlling COVID-19 damage, the major “pathogenic” kinase PAK1 is key. It is an essential component in malaria and viral infections, but it is also involved in a wide variety of other diseases and disease conditions, including cancer, inflammation, and immuno-suppression, when abnormally activated. Consequences of PAK1 activation include lung fibrosis [10], which is an aggravating factor in COVID-19. PAK1 is activated by RAC. Xu et al. [18] demonstrated that caffeic acid and its ester (CAPE), components of propolis, can inactivate RAC, consequently inhibiting PAK1. The inactivation of PAK1 directly, or up-stream, can potentially attenuate coronavirus pathogenesis [10]. B-cells and T-cells are lymphocytes that produce specific antibodies against viruses and other intruders, and PAK1 contributes to their suppression. PAK1 inhibitors can both help combat the virus and restore a normal immune response [10].
Propolis from Europe and temperate Asia, usually made by bees from resins collected from Poplar trees, has predominantly flavonoid compounds, while green propolis (from Baccharis dracunculifolia), a propolis exclusively found in Brazil, has various kinds of flavonoids and prenylated phenylpropanoids, such as artepellin C, baccharin and drupanin. These and all other types of propolis can inactivate PAK1 [10]. Artepillin C selectively inhibits PAK1 [85] (Table 1).
Some studies have shown that propolis can act as an immunostimulant, with the ability to improve the immune response. Its components increase neutralizing antibody titers, activate phagocytosis, and increase IFN-γ levels and the number of lymphocytes [86]. An increase in IFN-γ levels was also detected by Shimizu et al. [28], who evaluated the mechanisms involved in the effects of some types of propolis in a herpes simplex animal model.
CAPE (caffeic acid phenethyl ester) is a potent inhibitor of activation of NF-kB in myelo-monocytic cells. Ansorge et al. [37] demonstrated that propolis, CAPE, quercetin, hesperidin and some other propolis flavonoids can inhibit the cytokine production of Th1 and Th2 type T cells, while increasing TGF-beta 1, an important anti-inflammatory cytokine. Moreover, CAPE can attenuate oxidative stress and inflammation through down-regulation of JAK2/STAT3 signaling [87] as well as having an immunomodulatory effect, in which CAPE inhibits IL-6 phosphorylation and STAT3, which are important for pro-inflammatory Th17 development [88].
Besides the anti-inflammatory effect of CAPE and kaempferol, Paulino et al. [89] evaluated the anti-inflammatory effect of artepellin C in rat paw edema and in cell cultures, demonstrating that the activity was at least in part mediated by prostaglandin E2 and NO inhibition through NF-kB modulation. Artepillin C is an important biomarker of Brazilian green propolis (botanical source Baccharis dracunculifolia).
Immune modulation is desirable since coronavirus infection dysregulates the immune response in the initial phases of infection, which facilitates viral replication. However, in later stages of COVID-19, the body develops an exaggerated inflammatory response, which can greatly damage the lungs and other organs. Propolis, different from typical immunosuppressants, can help avoid immunosuppression during the initial phases of disease and, in later stages, reduce an exaggerated host inflammatory response, inhibiting excess IL-6, IL-2 and JAK signaling [90]. CAPE, a propolis component, is also known as an immune-modulating agent [91] and should be considered as an alternative to help reduce an exaggerated inflammatory response. In a mouse model, propolis had immunomodulatory action in vivo on Toll-like receptor expression and on pro-inflammatory cytokine production [92].
There is ample evidence for interference of propolis and/or its components with viral replication and infectivity, potentially decreasing lung inflammation due to anti-inflammatory properties, while promoting immune system fortification. These are useful properties that could help minimize the symptoms and deleterious effects of COVID-19 (Fig. 1 ).
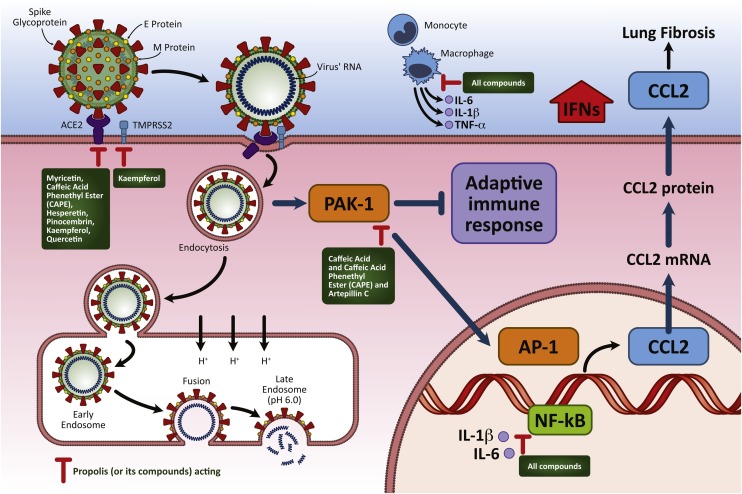
Fig. 1.
Major pathways through which propolis can interfere with SARS-CoV-2 attachment to the host cell, viral replication, and pathophysiological consequences. SARS-CoV-2 entry into target cells requires spike protein binding to ACE2 and activation by TMPRSS2. After binding, several signals are triggered, allowing viral endocytosis and PAK1 activation, which reduces the adaptive immune response and antibody production against the virus. PAK1 also stimulates CCL2 production, which generates a fibrotic response. Viral infection induces nuclear transition factor NF-KB activation, generating local pro-inflammatory cytokine production. Propolis-derived compounds downregulate the expression of TMPRSS2 and the anchoring ACE2, which limits entry of the virus. Furthermore, they promote NF-KB and monocyte/macrophage immunomodulation, reducing pro-inflammatory cytokine overproduction, and they reduce PAK1 activation, increasing the production of antibodies against SARS-CoV-2.
7. Propolis as an antiviral substance
Propolis has been tested against various viral disease organisms; initial successes have prompted research to determine the most useful components, which may be modified to produce more active and specific pharmaceuticals [93]. Viruses that were controlled by propolis in animal models with suggestion for control in humans include influenza [11,94], herpes simplex virus type 2 [95], and HIV [93,96]. Shimizu et al. [28] evaluated three different types of propolis in ethanol extracts, using a murine model of herpes simplex virus type 1. Despite the chemical differences due to the different plant origins of the resins the bees used to produce the propolis (Baccharis dracunculifolia, Baccharis eriodata and Myrceugenia euosma), all three propolis extracts not only had direct anti-HSV-1 effects, but also stimulated immunological activity against intradermal HSV-1 infection in mice.
Antiviral activity of propolis has been reported for DNA and RNA viruses (poliovirus, herpes simplex virus, and adenovirus) in an in vitro model (cultured cells). The best results were obtained against poliovirus and herpes virus, with 99.9% inhibition of the latter, at a propolis concentration of 30 ug/ml [97]. The propolis components chrysine and kaempferol caused a concentration-dependent reduction of intracellular replication of herpes-virus strains when host cell monolayers were infected and subsequently cultured in a drug-containing medium. Quercetin, another propolis component, had the same effect, but only at the highest concentrations tested (60 ug/mL) against various human herpes simplex virus strains, with a intracellular replication reduction of approximately 65%, while it reduced the infectivity of bovine herpes virus, human adenovirus, human coronavirus, and bovine coronavirus about 50%. The reduction was 70% in the case of rotavirus [84].
8. Anti-inflammatory and immunomodulatory properties of propolis
The most critical cases of COVID-19, which require ventilator-assisted intensive care and often result in prolonged ventilator dependency and death, are a result of an exaggerated inflammatory response to infection [98]. SARS-CoV-2 infection is associated with increased levels of chemokines and activated pro-inflammatory cytokines that lead to the development of atypical pneumonia, with rapid respiratory impairment and pulmonary failure [19]. Immunological/inflammatory phenomena (such as cytokine release syndrome) have been shown to be important mortality factors in SARS-CoV-2 infection. Higher serum levels of pro-inflammatory cytokines such as IL-6, IL-1, and TNF-α, are found in patients with severe COVID-19 compared to those of individuals with mild disease [21]. Molecular mechanisms involved in this immune process are the targets of various synthetic medicines being tested in patients, including ciclesonide, hydroxy chloroquine, ivermectin, and ketorolac, which are PAK1 blockers [10]. PAK1 (RAC/CDC42-activated kinase 1) is overexpressed in the lung in response to SARS-CoV-2 infection and is a critical mediator of the cytokine storm that frequently results in mortality in hospitalized patients [99]. Fortuitously, propolis components are effective PAK1 blockers (Table 1).
There is considerable evidence that propolis can reduce and alleviate the symptoms of inflammatory diseases [[22], [23], [24]] and has immunomodulatory properties [24,37]. However, these properties can vary according to the plant origin of the propolis, as well as the extraction process/solvent used and the inflammatory protocol (cell culture, animal models, induction by lipopolysaccharides) when the propolis extracts are tested [50]. Tests with animal models have shown that propolis can reduce the levels of IL-6 and TNF-alpha, which are key pro-inflammatory mediators, and increase the levels of the regulatory cytokine IL-10 [24]. Kaempferol, a propolis component, reduces IL-6, TNF-alpha, and VEGF (vascular endothelial growth factor) via the ERK-NFkB-cMyc-p21 pathway [83] (Table 1).
Tests on macrophage cell cultures also demonstrated that propolis inhibits the production of IL-1 beta, an important component of the inflammasome inflammatory pathway, in diseases such as rheumatoid arthritis, lupus and other autoimmune diseases [55]. Although the mechanisms of action are not well elucidated, these propolis components have potential as complementary supplements in the preventive treatment of chronic inflammatory diseases [100].
9. Propolis has potential as a vaccine adjuvant
Propolis is considered a safe immunostimulant and a potent vaccine adjuvant [101]. Propolis has been widely tested as a vaccine adjuvant, because it induces an earlier immune response and provides a longer protection period [102]. It is also included as an adjuvant ingredient in traditional Chinese medicine [103]. Propolis flavonoids have potential as adjuvants, enhancing IgG, IL-4, and IFN-γ in serum [104]. Fernandes et al. [86] found that propolis exerted a positive adjuvant effect on vaccines that were developed against canine coronavirus. They assayed IFN-γ, which is an effective way to measure the cellular response induced by a vaccine. In a mouse model, propolis, added as an adjuvant to inactivated swine herpesvirus type 1 vaccine, stimulated increased cellular and humoral responses, increasing IFN-γ [105,106]. Propolis enhanced the immune response to inactivated porcine parvovirus vaccine in guinea pigs [107]. When added to a Trichomonas vaginalis protein vaccine, propolis increased the IgG antibody response 4-10 times in mice, compared to the protein alone [108]. Propolis was also effective as an adjuvant in the immunization of cattle with bovine herpesvirus [105]. It improved the humoral and cellular responses in mice inoculated with inactivated virus vaccines [109]. Propolis as an adjuvant gave a similar immune response (increasing IFN-γ levels), to Alum and Freund’s adjuvant in mice vaccinated with an HIV-1 polytope vaccine candidate, with less risk of undesirable side effects [110].
10. Comorbidities and evidence of how propolis can help reduce their impact in COVID-19 patients
10.1. Cancer
Cancer is considered a relevant comorbidity factor for COVID-19. Cancer patients have a 3-4 times higher risk of progressing to severe COVID-19 disease than patients without comorbidities. Also, the hospital environment during the coronavirus pandemic can interfere with or delay the treatment that cancer patients should receive. Patients with symptoms may choose not to risk a visit to a clinic or hospital to determine if they have cancer [111]. Alternative therapies could help retard cancer or reduce the impact of cancer and cancer treatment in COVID-19 patients.
Propolis has potential as a complementary therapy for cancer. It has shown efficacy against various types, including bladder, blood, brain, breast, colon, head and neck, kidney, liver, pancreas, prostate, and skin cancers [112]. Propolis could help prevent cancer progression; in various parts of the world it is considered an alternative therapy for cancer treatment [113]. Propolis extracts have been found to inhibit tumor cell growth both in vitro and in vivo, including inhibition of angiogenesis, demonstrating potential for the development of new anticancer drugs [[114], [115], [116]]. Various components of propolis have been shown to inhibit cancer cell growth, including cinnamic acid [66], CAPE [[117], [118], [119]], quercetin [120], and chrysin [121]. Propolis and its components normally have little impact on normal cells, displaying differential cytotoxicity in liver cancer, melanoma and breast cell carcinoma cell lines [122,123]. Propolis enhances the activity of tumor necrosis factor related apoptosis inducing ligand (TRAIL) in cancer cells [124].
10.2. Hypertension and cardiovascular disease
Hypertension and cardiovascular disease are considered relevant comorbidities for COVID-19 [[125], [126], [127]]. Propolis has demonstrated anti-hypertensive effects in rat models [33,34,128,129]. In Cameroon, it is used in popular medicine to treat various ailments, including high blood pressure [130]. Propolis has been widely used as a dietary supplement for its health benefits, including cardiovascular protective effects [131,132]. In a human trial, consumption of propolis improved critical blood parameters, including HDL, GSH and TBARS levels, demonstrating that it could contribute to a reduced risk for cardiovascular disease [132].
10.3. Obesity
Obesity is a major comorbidity and predictor of increased mortality in COVD-19 patients. Obesity and SARS-CoV-2 both induce an inflammatory process, exacerbating SARS-CoV-2 infection in the obese [133]. Propolis reduced inflammation and prevented hyperlipidemia and metabolic syndromes in highly caloric diet induced obesity in mice. Body weight gain, visceral adipose tissue, liver and serum triglycerides, cholesterol, and non-esterified fatty acids were all reduced in the propolis fed mice [78,134]. Caffeic acid phenethyl ester, a propolis component, is a natural anti-obesity agent [135].
10.4. Thromboembolism, thrombosis and microthrombosis
Microthrombosis, disseminated intravascular coagulation, and consequent multiorgan failure are common in severely affected COVID19 patients, with associated high mortality rates [[136], [137], [138], [139]]. Anticoagulants are sometimes prescribed to such patients because they can reduce mortality (Tang et al. 2020). An elevated level of plasminogen activator inhibitor-1 (PAI-1) is a biomarker and risk factor for thrombosis and atherosclerosis [140,141]. Various types of evidence demonstrate that propolis can reduce platelet aggregation and other thrombosis-related parameters. Propolis decreased thrombotic tendencies in mice by suppressing lipopolysaccharide-induced increases in PAI-1 levels [142,143]. Propolis downregulated platelet-derived growth factor and platelet endothelial cell adhesion molecules in low-density lipoprotein knockout mice [144]. Platelet aggregation was reduced by propolis in tests on human blood in vitro [145] and in other in vitro tests [146]. Caffeic acid phenethyl ester (CAPE), a well-studied bioactive propolis component, inhibits collagen induced platelet activation [147].
10.5. Old age
The elderly are more often affected by chronic inflammation, characterized by systemically increased levels of proinflammatory cytokines, which can contribute to development of a cytokine storm, a major cause of COVID-19 mortality [148]. Propolis has antioxidant properties, which could help retard or reduce aging processes [149]. CAPE, a propolis component, increased the lifespan of Caenorhabditis elegans, a common model organism for aging studies [150]. Propolis consumption protected against cognitive decline in elderly subjects (humans) exposed to high altitudes [151]. Serum TGF-β1 and IL-10 levels were significantly higher in propolis-treated elderly subjects, helping reduce inflammation, which could be the mechanism of protection against cognitive decline. Activity of superoxide dismutase (SOD), a key antioxidant in men treated with propolis was increased, while malondialdehyde, a marker of oxidative stress, decreased [152]. The same tendencies were detected in a diabetic rat model [153]. Propolis has the potential to reduce neurodegenerative damage through antioxidant activity, which helps protect against cognitive impairment in Alzheimer’s disease as well as aging [154,155]. In a mouse model of Alzheimer’s disease, coniferaldehyde, an active ingredient in propolis, had neuroprotective effects. It reduced brain β-amyloid deposits and pathological changes in the brain, helping preserve learning and memory capacity [156]. The angiotensin system, which is key to SARS-CoV-2 invasion of host cells, is associated with senescence. One of the reasons that SARS-CoV-2 causes significantly higher mortality in older patients may be that they have a larger number of senescent lung cells, which are a vulnerable target for viral infection and can help promote viral replication. That would make senolytic drugs useful to help the elderly survive COVID-19. Quercetin, a propolis component, which has been proposed as a therapeutic for treatment of COVID-19, has senolytic activity [157].
10.6. Diabetes
Common comorbidities with high death rates in critically ill COVID19 patients include diabetes [30]. Given the relation between diabetes and inflammation, and that flavonoids, major bioactive components of propolis, protect against free radicals and other pro-oxidative compounds, it is plausible that propolis consumption can reduce the risk of diabetes [158]. Brazilian propolis has become popular as a healthy dietary supplement in various parts of the world because it can help prevent inflammation and diabetes [159]. Propolis was found to reduce blood glucose, blood lipids and free radicals in diabetic rats [31,160]. It also reduced glycemia [32,161] and insulin resistance [[162], [163], [164]] in diabetic rats. Experimental diabetic nephropathy was also prevented [165]. Diabetes symptoms were reduced in a diabetic mouse model [166], apparently by attenuating immune activation in adipose tissues.
Clinical trials with diabetic patients demonstrated that propolis consumption improved antioxidant parameters [167], glycemic control [168,169], and the lipid profile and renal function [170]. Propolis is also an antimicrobial agent with wound healing properties [171,172], which has proven especially useful for diabetic patients [173,174], who tend to develop difficult to heal wounds.
Caffeic acid phenethyl ester (CAPE) is considered an anti-obesity agent with beneficial effects on inflammation and diabetes [175]. CAPE reduced insulin resistance in diabetic mice and in hepatic cell culture [176]. Chrysin, another component of propolis, also has antidiabetic properties [177].
10.7. Kidney diseases
COVID-19 is an important threat for patients with comorbidities such as renal or hepatic impairment [178]. The kidney is a common target of SARS-CoV-2 [179]. COVID-19 patients are at increased risk of kidney impairment [79], and consequently many patients with COVID-19 present renal dysfunction [180]. Increased mortality is common in COVID-19 patients with chronic kidney disease and in those undergoing hemodialysis [181]. Propolis has shown protective effects against kidney diseases. Nephropathy was prevented by propolis treatment in animal models [165,182,183]. Brazilian red propolis attenuated hypertension and renal damage in a rat renal ablation model [184]. Anti-diabetic activity of propolis in a rat model reduced liver damage [160]. In a pioneering clinical trial, propolis reduced proteinuria in patients with chronic kidney disease [51].
10.8. Bacterial infection
Bacterial infection is a common complication in COVID-19 [79]. Propolis has a long history of use for its antibacterial properties and could help treat bacterial infections in COVID-19 patients. The healing properties of propolis are referred to throughout the Old Testament, and propolis was prescribed by Hippocrates in Ancient Greece for the treatment of sores and ulcers [185]. Propolis has been popular for centuries in Russia and other countries in Eastern Europe for its antibacterial properties [186]. The pharmacological value of propolis comes from a natural mixture of antibacterial substances, instead of only one or a few substances as in most medicines [187]. Propolis components galangin, pinocembrin, rutin, quercetin, and naringenin, as well as CAPE increase bacterial membrane permeability, which could explain their antimicrobial properties [188]. De Campos et al. [189] showed that the main mechanism of action of propolis is rupture and lysis of bacterial cells. Propolis has demonstrated antibacterial activity against Staphylococcus aureus, Staphylococcus epidermidis, and E. coli [190], including methicillin-resistant and methicillin-susceptible strains of Staphylococcus aureus [191]. Adding propolis extract to the antibiotics, ampicillin, gentamycin and streptomycin, vancomycin and oxacillin increased their antibacterial activity against Staphylococcus aureus [192,193]. The extract also reduced cell adhesion and consequent biofilm formation by this bacterium [193]. Propolis (sometimes known as bee glue) has antibacterial activity against human tubercle bacillus, but often has only limited activity against Gram-negative bacilli. These antimicrobial properties appear to be due to its high flavonoid content [186]. Propolis can help avoid bacterial tooth decay [194]. Propolis and some of its components inhibit bacterial motility [195]. Black poplar, Populus nigra, tree resin is the main source of the propolis used for medicinal purposes in Europe; it contains phenols and flavonoids that have well known antimicrobial properties [196]. Propolis can be bacteriostatic and or bactericidal, depending on the concentration [197]. An ethanol extract of propolis inhibited microbial growth and biofilm formation by Pseudomonasaeruginosa [198]. The antibacterial properties of propolis make it a useful ingredient in a wound healing biofilm [199].
11. Limitations: Lack of standardization
Man has used propolis as an herbal medicine for thousands of years. Various useful activities have been described for propolis, including, and not limited to antiviral, antibacterial, antifungal, anti-inflammatory, immunoregulatory, antioxidant, and wound healing properties [200,201]. However, plant geographical source, bee species, seasonality and climatic differences can dramatically affect chemical composition [48,202]. These details, along with variations in the processing and solvent extraction processes (which can selectivity extract some compounds according to their polarity) [203], can influence its biological properties. This can affect and limit the repeatability of tests and confuse the compilation of results used to determine appropriate dosages for human clinical trials, ultimately causing insecurity for prescribers.
Considerable work has gone into understanding the mechanisms involved in the biological properties of propolis [54,189,204,205]. Also, efforts have been made to improve technological and analytical processes to determine adequate extraction procedures that preserve its bioactive compounds and consistently provide the best pharmacological properties for each medical condition [50,200,201,203,[206], [207], [208]]. However, although propolis is a product that can be offered to the market in several presentations and with different classifications according to the type of product, possibly as a health supplement, food supplement, cosmetic and/or hygiene product, the various beneficial effects that appear in published research were not accepted by the European Commission as acceptable "claims", based on the argument that there are qualitative and quantitative variations in the bioactive flavonoids, which are dependent on the raw material provided by beekeepers. Those factors, and the lack of standardized extraction and preparation methods, are reasons that do not permit this type of approval [209], justifying the standardization proposed by Chan [38].
Although in the bee products field “standardization” is not yet a normal procedure, this reality already exists in the phytopharmaceutical industry. When working with herbal products, it is normal to find differences in the raw material received, since plants suffer a strong influence of the environment, including seasonality, soil treatment, other plant species nearby, and various other conditions, resulting in batches of plant materials that are often chemically different, qualitatively and/or quantitatively. It is not possible to have identical batches when working with this type of material; however, minimal standardization is needed in order to validate safety and efficacy studies and guarantee useful characteristics when a product is offered to the market [210].
The definition of “Standardization” by the American Herbal Product association is: “Standardization refers to the body of information and control necessary to produce material of reasonable consistency” [211]. The mechanisms and technologies available to meet this goal are available; however, challenges also exist. Nikam et al. [210] present useful guidelines for those who intend to develop such standardization.
11.1. A Standardized Propolis Product
In Brazil, 12 main types of propolis have already been described [201]. Due to this great variability and the limitations that these differences cause in the research, development and industrial fields, Berretta et al. [54] developed a Standardized Propolis Extract, named EPP-AF® (Patent Letter no. 0405483-0, approved by Industrial Property Magazine on July 23, 2019), which possesses reproducibility batch-to-batch for a group of phenolic and flavonoid compounds, in addition to a characteristic HPLC fingerprint and consistent biological effects (antimicrobial activity) [54]. Several studies have been conducted with this extract, including analytical development and validation [54,212,213], biological effects such as antimicrobial, antifungal and wound healing properties [54,205,[214], [215], [216], [217]], and anti-inflammatory and immunoregulatory activities [22,24].
12. Safety and Efficacy Studies
12.1. Non-Clinical Studies
Besides the long history of traditional use of propolis for treating diseases, various studies in animals have demonstrated the safety of propolis [[218], [219], [220]]. Safety studies for EPP-AF® have been conducted using an in vitro Ames Test, demonstrating a lack of abnormalities in the bacterial strains that were evaluated (unpublished data) and a lack of abnormalities in a micronucleus test (in vitro) [221]. Tavares et al. [222] also studied propolis using a micronucleus tool, with the mutagenicity agent doxorubicin as a positive control. They found that the propolis behaved as a “Janus” compound; it was genotoxic at higher concentrations and chemopreventive at lower ones. This demonstrates the importance of the appropriate dosage and model for testing, which are needed to correctly extrapolate to clinical trials. Additionally, acute and subchronic animal toxicity tests were performed; even at very high treatment levels, EPP-AF® propolis did not reach an LD50 dose (maximum tested 3000 mg/kg) [223]. The safety data from tests with Wistar rats (1000 mg/kg) and rabbits (300 mg/kg), and the conversion factor proposed by the US Food and Drug Agency were used to propose the dosages for human trials [50]
12.2. Clinical Trials
A clinical safety study was carried out at the Ribeirão Preto School of Medicine of the University of São Paulo (FMRP/USP) with healthy volunteers in order to assess the safety of ingesting 375 mg / day of Standardized Propolis Extract (EPP-AF®), for five days. No adverse events were observed. The study pointed to the absence of acute toxicity after the oral use of Standardized Propolis Extract (EPP-AF®) at a dose of 375 mg daily for five days. The significant positive variation observed in the parameter HDL cholesterol needs further studies with a larger number of patients to confirm this beneficial effect on the cardiovascular system (unpublished data).
In addition, and more important in this case, a study to evaluate drug interaction was performed using a cocktail approach to analyze the main hepatic metabolizing enzymes (cytochrome P450 enzymes - CYPs) and the transport enzyme Pgp. The results showed that this standardized propolis extract is safe and without risk of drug interaction, according to the criteria established by the WHO [53]. The propolis tested in the interaction study was provided in tablets; consequently, these results cannot necessarily be extrapolated to a propolis alcohol extract.
Using a propolis preparation, a clinical trial that was randomized, double-blind, and placebo-controlled was conducted with 430 children (1-5 years old) in Israel with a placebo elixir and Chizukit (a standard over-the counter drug containing Echinacea extract 50 mg/ml (Echinacea purpurea and E. angustifolia), 50 mg/ml of propolis extract and 10 mg/ml of vitamin C, for respiratory tract infection gave good results [224]. Another clinical study was done for asthma treatment in adults [204]. The study, which used a propolis water extract, demonstrated reduction in key pro-inflammatory cytokines, including tumor necrosis factor (TNF-a), ICAM-1, IL-6, IL-8 and a 3-fold increase in the “protective” cytokine IL-10; the levels of prostaglandins E2, F2a and leukotriene D4 were reduced significantly [204].
A randomized double-blind placebo controlled clinical study of 32 patients with Chronic Kidney Disease, demonstrated safety of the Standardized Propolis Extract (EPP-AF®) at an oral dose of 500 mg / day after administration during 12 months, with significant reduction in proteinuria and urinary MCP1 in the propolis group compared to the placebo [51], with no side effects. Another clinical study conducted with the Standardized Propolis Extract (EPP-AF®) in healthy volunteers aimed to assess the antioxidant activity. There was a reduction in cell damage induced by oxidative stress in healthy volunteers, due to an increase in the enzymatic antioxidant capacity, especially affecting superoxide dismutase (SOD), and decreasing lipid peroxidation and DNA oxidation (8-OHDG) (article submitted). These data indicate the important protective effect that propolis has on cells, tissues and on the human body, reducing the effects of aging, degenerative diseases and several other conditions that involve these oxidation processes.
Another relevant clinical trial was conducted by Soroy et al. [225] on dengue hemorrhagic fever patients. Their double-blind, randomized and placebo-controlled trial evaluated the propolis product PropoelixTM (two 200 mg capsules, three times a day), demonstrating an improvement in platelet counts and a decrease in TNF-alfa, promoting a reduction in the duration of hospitalization time of the patients.
The current COVID-19 pandemic has promoted strong interest in propolis as a therapeutic option. As a consequence, a clinical trial of Brazilian green propolis extract (EPP-AF) for treatment of COVID-19 patients was recently initiated in Brazil (https://clinicaltrials.gov/ct2/show/NCT04480593).
12.3. Dosages
Clinical trials with propolis have been conducted in various regions of the world, most of them with the limitation of a lack of standardization. Berretta et al. [50,51,53] evaluated many of them; the most common dosage used was 500 mg/day for adults. Considering the case of EPP-AF®, the clinical data until now support dosages of 375 - 500 mg of propolis/day; however, non-clinical trials indicate that much higher dosages can be tolerated and may be useful [211]. The dose of 500 mg/day would be equivalent to 30 drops of propolis extract (with 11% w/v of dry matter), 3 to 4 times a day, diluted in about 100 ml of water, or 3 to 4 units/day of capsules or tablets with the equivalent amount of extract. For preventive purposes, 30 drops/day or one capsule, are usually taken. However, considering the dosage safely used by Soroy et al. [225] of 1200 mg/day, in more severe cases of COVID-19, dosages higher than 500 mg/day could be useful.
13. Supplements, Food and Hygiene Products made with Propolis
Propolis extracts and sprays, often combined with medicinal herb extracts and honey, are now found in nearly every pharmacy in Brazil, attesting to their safety, popularity and usefulness in this country, where hundreds of companies currently produce these “natural medicines”. For each regulatory category and country, the technical rules to be followed for propolis products vary. Some countries such as Brazil, Canada, United States of America, China, South Korea, Japan, Australia and the European Community already possess regulation for propolis [50]; consequently, propolis can be easily found by consumers at a low cost and potentially can be useful for preventive and/or curative purposes in the early stages of disease.
14. Why consider using nutraceuticals or other natural alternatives instead of relying on modern pharmaceuticals?
Propolis is extensively used in foods and beverages because of its benefits for human health. It contains hundreds of natural compounds, including aldehydes, coumarins, polyphenols, steroids and inorganic compounds, with a broad spectrum of biological and pharmacological properties, including antimicrobial, antioxidant, anti-inflammatory, immunomodulatory, antitumor, anticancer, antiulcer, hepatoprotective, cardioprotective, and neuroprotective actions [226]. The health industry has always used natural products, including propolis, as an alternative source of drugs [62]. The complex mix of propolis components can provide greater health benefits than would be apparent by analyzing the individual effects of components, apparently due to synergistic effects [97].
Modern medicine relies on powerful drugs that have specific and strong impacts on disease organisms and on the body. This strategy and adequate sanitary measures have proven to be highly effective, resulting in an almost constant increase in human lifespan, which has more than doubled since 1900 to over 70 years [227]. However, the system in place for approving pharmaceuticals also has some disadvantages, including the long lead time and considerable funding needed to discover new options, test them for safety and effectiveness, and after 5-10 years, obtain approval for their use. Due to the high costs involved and the possibility that this extended process will not result in a product that will compensate the investment required, potentially useful materials may never become available. Another problem is that modern medicine can be quite expensive, with constantly increasing costs for individuals and countries. Consequently, adequate healthcare may not be available to everyone who needs it. Side effects of many of these powerful medicines are also a concern. Doctors and patients often need to weigh the risk of drug side effects against the consequences of the disease. Also, for some diseases no effective drug is available and patient care focuses on relieving symptoms and the consequences of infection.
Among alternatives to modern drugs, there has long been a traditional use of natural health products. However, such products normally cannot be registered as medicines; the considerable investment needed to qualify them as such often would not be compensated because they are difficult to obtain a patent for and people could easily purchase or collect them. Curiously, one of the strategies for developing modern drugs is to carefully dissect the components of natural products, determine which ones have desirable activity, patent and synthesize them and then go through the expensive process of getting them approved, though with some possibility that such products could give a return on the investment because of the patents. A case in point is Brazilian green propolis, for which there is considerable evidence of anticancer properties [228,229]. This propolis is not patented, but some if its components were isolated, and synthesized, and are now patented drugs for cancer treatment [230]. Brazil continues to produce and export large quantities of green propolis, especially to Asian countries, but various patented components are the property of companies in other countries.
In some parts of the world, the equivalent of the US Food and Drug Administration (FDA), officially classifies certain natural products as “Functional Foods” or some other similar category. As such, they can be produced and marketed and used by people who believe they will be good for their health. To be classified as functional foods, these agencies require proof that they are safe and that they have proven health benefits [71,231]. This option provides alternatives that are normally inexpensive and do not require prescriptions. Specifically, propolis has been suggested as a prophylactic treatment for high risk groups in the current COVID-19 pandemic [232].
Some investment is necessary to help qualify and register natural medicines, which may be provided by companies or by government programs that recognize the need for this type of investment, or both. In Brazil, the Sao Paulo state research agency (FAPESP), has a program called PIPE (http://www.fapesp.br/pipe/) that helps small companies finance this type of research for products that they recognize would not normally be developed without this type of support, including natural pest control alternatives for agriculture and “natural medicine” formulations. Various research projects on propolis products have been financed by this FAPESP PIPE program including wound healing and antifungal products [54,205,[214], [215], [216]], and the development of a standardized propolis formulation [54]; tests of this product were made for safety and effectiveness in patients with chronic kidney disease and diabetes [51]; both diseases are the subjects of projects supported by FINEP (Brazilian Study and Projects Financing Agency).
While modern medicines normally have only one or just a few active components, natural products can have many. Propolis, for example, has hundreds of components [226], many of which have properties that have the potential to help treat various types of disease or have various modes of action against a specific disease and its consequences [[233], [234], [235]]. Another consideration is that a strong specific effect, such as that of an anticoagulant used in an effort to prevent the microthromboses that have become a serious consequence of advanced COVID19 [236], requires specific dosing in order to avoid excess bleeding and other dangerous side effects [237], and such drugs are not a safe option for patients that have some types of blood disease, or various heart and vessel disorders. A natural anticoagulant could give some protection and at a level sufficient to reduce the risk of thrombosis without strong side effects. Propolis has demonstrated anticoagulant properties [147].
15. Conclusions
Considering the large number of deaths and other types of damage that the COVID-19 pandemic is causing, there is an urgent need to find therapies that can help avoid or reduce SARS-CoV-2 infection and its consequences. Propolis has proven anti-inflammatory and immunoregulatory effects, including PAK-1 inhibition. Also, attachment to ACE2, a major target of the SARS-CoV-2 virus for host cell invasion, is inhibited by propolis. Propolis components, including CAPE, rutin, quercetin, kaempferol and myricetin have demonstrated in silico a strong interaction with ACE2. Kaempferol reduced the expression of TMPRSS2. In addition to these activities, propolis does not interact with the main liver enzymes or with other key enzymes; according to criteria adopted by the World Health Organization, therefore propolis can be used concurrently with the main drugs without risk of potentiation or inactivation.
To determine if propolis specifically affects SARS-CoV-2 will require more research. But given that propolis is a risk-free product, except for those who may develop an allergy to it, the known biological activities of this natural bee product lead us to suggest its use for reducing the risk and impact of infection and as an adjunct to treatment.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgements
The present study received no funding from any source or any governing body. We thank Cristiane Melo for preparing the drawings of the bee collecting resin and the hive opening with propolis and Mr. Adriel Santos for kindly preparing the graphical abstract figure. Some of the research cited involving the authors of this paper was funded by the Brazilian research funding agencies FAPESP, CAPES, FINEP and CNPq, and by the health products company Apis Flora. The development and testing of the Brazilian standardized propolis product were partially funded by the São Paulo state research funding institution, FAPESP, as well as the federal agencies FINEP and CNPq.
References
- 1.Vardeny O., Madjid M., Solomon S.D. Applying the Lessons of Influenza to COVID-19 During a Time of Uncertainty. Circulation. 2020;141(21):1667–1669. doi: 10.1161/CIRCULATIONAHA.120.046837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Setti L., Kirienko M., Dalto S.C., Bonacina M., Bombardieri E. FDG-PET/CT findings highly suspicious for COVID-19 in an Italian case series of asymptomatic patients. Eur J Nucl Med Mol Imaging. 2020 doi: 10.1007/s00259-020-04819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 4.Lapolla P., Mingoli A., Lee R. Deaths from COVID-19 in healthcare workers in Italy—What can we learn? Infect Control Hosp Epidemiol. 2020:1–2. doi: 10.1017/ice.2020.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corburn J., Vlahov D., Mberu B., Riley L., Caiaffa W.T., Rashid S.F., Ko A., Patel S., Jukur S., Martínez-Herrera E., Jayasinghe S., Agarwal S., Nguendo-Yongsi B., Weru J., Ouma S., Edmundo K., Oni T., Ayad H., Health Slum. Arresting COVID-19 and Improving Well-Being in Urban Informal Settlements. J Urban Health. 2020;97:348–357. doi: 10.1007/s11524-020-00438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pereira R.J., Nascimento G.N.L.D., Gratão L.H.A., Pimenta R.S. The risk of COVID-19 transmission in favelas and slums in Brazil. Public Health. 2020;183:42–43. doi: 10.1016/j.puhe.2020.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowan M.M. Plant Products as Antimicrobial Agents. Clin Microbiol Rev. 1999;12(4):564–582. doi: 10.1128/CMR.12.4.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakkali F., Averbeck S., Averbeck D., Idaomar M. Biological effects of essential oils--a review. Food Chem Toxicol. 2008;46(2):446–475. doi: 10.1016/j.fct.2007.09.106. [DOI] [PubMed] [Google Scholar]
- 9.Saklani A., Kutty S.K. Plant-derived compounds in clinical trials. Drug Discov Today. 2008;13(3-4):161–171. doi: 10.1016/j.drudis.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Maruta H., He H. PAK1-blockers: Potential Therapeutics against COVID-19. Med Drug Discov. 2020 doi: 10.1016/j.medidd.2020.100039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serkedjieva J., Manolova N., Bankova V. Anti-influenza virus effect of some propolis constituents and their analogues (esters of substituted cinnamic acids) J Nat Prod. 1992;55(3):294–302. doi: 10.1021/np50081a003. [DOI] [PubMed] [Google Scholar]
- 12.Calixto J. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33(2):179–189. doi: 10.1590/S0100-879X2000000200004. [DOI] [PubMed] [Google Scholar]
- 13.Calixto J.B. Twenty-five years of research on medicinal plants in Latin America: a personal view. J Ethnopharmacol. 2005;100(1-2):131–134. doi: 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Uddin M M.F., Rizvi T.A., Loney T., Suwaidi H.A., Al-Marzouqi A.H.H., Eldin A.K., Alsabeeha N., Adrian T.E., Stefanini C., Nowotny N., Alsheikh-Ali A., Senok A.C. SARS-CoV-2/COVID-19: Viral Genomics, Epidemiology, Vaccines, and Therapeutic Interventions. Viruses. 2020;12:526. doi: 10.3390/v12050526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vardhan S., Sahoo S.K. Searching inhibitors for three important proteins of COVID-19 through molecular docking studies. arXiv. 2020 2004.08095. [Google Scholar]
- 16.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol. 2020;94(7):e00127–20. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu J.W., Ikeda K., Kobayakawa A., Ikami T., Kayano Y., Mitani T., Yamori Y. Downregulation of Rac1 activation by caffeic acid in aortic smooth muscle cells. Life Sci. 2005;76(24):2861–2872. doi: 10.1016/j.lfs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Ding Y., He L., Zhang Q., Huang Z., Che X., Hou J., Wang H., Shen H., Qiu L., Li Z., Geng J., Cai J., Han H., Li X., Kang W., Weng D., Liang P., Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol. 2004;203(2):622–630. doi: 10.1002/path.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stebbing J., Phelan A., Griffin I., Tucker C., Oechsle O., Smith D., Richardson P. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. 2020;71(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hori J.I., Zamboni D.S., Carrao D.B., Goldman G.H., Berretta A.A. The Inhibition of Inflammasome by Brazilian Propolis (EPP-AF) Evid Based Complement Alternat Med. 2013;2013:418508. doi: 10.1155/2013/418508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pineros A.R., de Lima M.H.F., Rodrigues T., Gembre A.F., Bertolini T.B., Fonseca M.D., Berretta A.A., Ramalho L.N.Z., Cunha F.Q., Hori J.I., Bonato V.L.D. Green propolis increases myeloid suppressor cells and CD4(+)Foxp3(+) cells and reduces Th2 inflammation in the lungs after allergen exposure. J Ethnopharmacol. 2020;252:112496. doi: 10.1016/j.jep.2019.112496. [DOI] [PubMed] [Google Scholar]
- 24.Machado J.L., Assuncao A.K., da Silva M.C., Dos Reis A.S., Costa G.C., Arruda Dde S., Rocha B.A., Vaz M.M., Paes A.M., Guerra R.N., Berretta A.A., do Nascimento F.R. Brazilian green propolis: anti-inflammatory property by an immunomodulatory activity. Evid Based Complement Alternat Med. 2012;2012:157652. doi: 10.1155/2012/157652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sekiou O., Omar I., Bouziane Z., Bouslama A. Djemel. In-silico identification of potent inhibitors of COVID-19 main protease (Mpro) and Angiotensin converting enzyme 2 (ACE2) from natural products. ChemRxiv. 2020 doi: 10.26434/chemrxiv.12181404. [DOI] [Google Scholar]
- 26.Güler H.I., Tatar G., Yildiz O., Belduz A.O., Kolayli S. Investigation of potential inhibitor properties of ethanolic propolis extracts against ACE-II receptors for COVID-19 treatment by Molecular Docking Study. ScienceOpen Preprints. 2020 doi: 10.14293/S2199-1006.1.SOR-.PP5BWN4.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asgharpour F., Moghadamnia A.A., Motallebnejad M., Nouri H.R. Propolis attenuates lipopolysaccharide-induced inflammatory responses through intracellular ROS and NO levels along with downregulation of IL-1beta and IL-6 expressions in murine RAW 264.7 macrophages. J Food Biochem. 2019;43(8) doi: 10.1111/jfbc.12926. e12926. [DOI] [PubMed] [Google Scholar]
- 28.Shimizu T., Takeshita Y., Takamori Y., Kai H., Sawamura R., Yoshida H., Watanabe W., Tsutsumi A., Park Y.K., Yasukawa K., Matsuno K., Shiraki K., Kurokawa M. Efficacy of Brazilian Propolis against Herpes Simplex Virus Type 1 Infection in Mice and Their Modes of Antiherpetic Efficacies. Evid Based Complement Alternat Med. 2011;2011:976196. doi: 10.1155/2011/976196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., Du B., Li L.J., Zeng G., Yuen K.Y., Chen R.C., Tang C.L., Wang T., Chen P.Y., Xiang J., Li S.Y., Wang J.L., Liang Z.J., Peng Y.X., Wei L., Liu Y., Hu Y.H., Peng P., Wang J.M., Liu J.Y., Chen Z., Li G., Zheng Z.J., Qiu S.Q., Luo J., Ye C.J., Zhu S.Y., Zhong N.S. China Medical Treatment Expert Group for, Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stein R.A. COVID-19: Risk. groups, mechanistic insights and challenges. Int J Clin Pract. 2020 doi: 10.1111/ijcp.13512. e13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuliang H.U., Hepburn H.R., Xuan H., Chen M., Daya S., Radloff S.E. Effects of propolis on blood glucose, blood lipid and free radicals in rats with diabetes mellitus. Pharmacol Res. 2005;51(2):147–152. doi: 10.1016/j.phrs.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 32.Al-Hariri M., Eldin T.G., Abu-Hozaifa B., Elnour A. Glycemic control and anti-osteopathic effect of propolis in diabetic rats. Diabetes Metab Syndr Obes. 2011;2011(4):377–384. doi: 10.2147/DMSO.S24159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishima S., Yoshida C., Akino S., Sakamoto T. Antihypertensive effects of Brazilian propolis: identification of caffeoylquinic acids as constituents involved in the hypotension in spontaneously hypertensive rats. Biol Pharm Bull. 2005;28(10):1909–1914. doi: 10.1248/bpb.28.1909. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama H., Sumitou Y., Sakamoto T., Araki Y., Hara H. Antihypertensive effects of flavonoids isolated from brazilian green propolis in spontaneously hypertensive rats. Biol Pharm Bull. 2009;32(7):1244–1250. doi: 10.1248/bpb.32.1244. [DOI] [PubMed] [Google Scholar]
- 35.Chopra S., Pillai K.K., Husain S.Z., Giri D.K. Propolis protects against doxorubicin-induced myocardiopathy in rats. Exp Mol Pathol. 1995;62(3):190–198. doi: 10.1006/exmp.1995.1021. [DOI] [PubMed] [Google Scholar]
- 36.Fang Y., Sang H., Yuan N., Sun H., Yao S., Wang J., Qin S. Ethanolic extract of propolis inhibits atherosclerosis in ApoE-knockout mice. Lipids Health Dis. 2013;12:123. doi: 10.1186/1476-511X-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ansorge R.D., Lendeckel S. Propolis and some of its constituents down-regulate DNA synthesis and inflammatory cytokine production but induce TGF-beta1 production of human immune cells. Z Naturforsch C J Biosci. 2003;58(7) doi: 10.1515/znc-2003-7-823. [DOI] [PubMed] [Google Scholar]
- 38.Chan G.C.-F., Cheung K.-W., Sze D.M.-Y. The Immunomodulatory and Anticancer Properties of Propolis. Clin Rev Allerg Immu. 2013;44(3):262–273. doi: 10.1007/s12016-012-8322-2. [DOI] [PubMed] [Google Scholar]
- 39.Balandrin M.F., Klocke J.A., Wurtele E.S., Bollinger W.H. Natural plant chemicals: sources of industrial and medicinal materials. Science. 1985;228(4704):1154–1160. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- 40.Langenheim J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J Chem Ecol. 1994;20(6):1223–1280. doi: 10.1007/BF02059809. [DOI] [PubMed] [Google Scholar]
- 41.Cheng A.-X., Lou Y.-G., Mao Y.-B., Lu S., Wang L.-J., Chen X.-Y. Plant Terpenoids: Biosynthesis and Ecological Functions. J Integr Plant Biol. 2007;49(2):179–186. doi: 10.1111/j.1744-7909.2007.00395.x. [DOI] [Google Scholar]
- 42.Toreti V.C., Sato H.H., Pastore G.M., Park Y.K. Recent progress of propolis for its biological and chemical compositions and its botanical origin. Evid Based Complement Alternat Med. 2013;2013:697390. doi: 10.1155/2013/697390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mani J.S., Johnson J.B., Steel J.C., Broszczak D.A., Neilsen P.M., Walsh K.B., Naiker M. Natural product-derived phytochemicals as potential agents against coronaviruses: A review. Virus Res. 2020;284:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simone-Finstrom M., Spivak M. Propolis and bee health: the natural history and significance of resin use by honey bees. Apidologie. 2010;41(3):295–311. doi: 10.1051/apido/2010016. [DOI] [Google Scholar]
- 45.Evans J.D., Spivak M. Socialized medicine: individual and communal disease barriers in honey bees. J Invertebr Pathol. 2010;103(Suppl 1):S62–S72. doi: 10.1016/j.jip.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 46.Nicodemo D., Malheiros E.B., De Jong D., Couto R.H.N. Increased brood viability and longer lifespan of honeybees selected for propolis production. Apidologie. 2014;45(2):269–275. doi: 10.1007/s13592-013-0249-y. [DOI] [Google Scholar]
- 47.Turcatto A.P., Lourenço A.P., De Jong D. Propolis consumption ramps up the immune response in honey bees infected with bacteria. Apidologie. 2018;49(3):287–296. doi: 10.1007/s13592-017-0553-z. [DOI] [Google Scholar]
- 48.Bankova V. Recent trends and important developments in propolis research. Evid Based Complement Alternat Med. 2005;2(1):29–32. doi: 10.1093/ecam/neh059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miguel M., Nunes S., Dandlen S.A., Cavaco A.M., Antunes M.D. Phenols, flavonoids and antioxidant activity of aqueous and methanolic extracts of propolis (Apis mellifera L.) from Algarve, South Portugal. Food Sci Technol. 2014;34(1) doi: 10.1590/S0101-20612014000100002. [DOI] [Google Scholar]
- 50.Berretta A.A., Arruda C., Miguel F., Baptista N., Nascimento A., Marquele- Oliveira F., Hori J., Barud H., Damaso B., Ramos C., Ferreira R., Bastos J. In: Superfood and Functional Food - An Overview of Their Processing and Utilization. Waisundara V., editor. Intech Open; London: 2017. Functional Properties of Brazilian Propolis: From Chemical Composition Until the Market; pp. 55–98. [DOI] [Google Scholar]
- 51.Silveira M.A.D., Teles F., Berretta A.A., Sanches T.R., Rodrigues C.E., Seguro A.C., Andrade L. Effects of Brazilian green propolis on proteinuria and renal function in patients with chronic kidney disease: a randomized, double-blind, placebo-controlled trial. BMC Nephrol. 2019;20(1):140. doi: 10.1186/s12882-019-1337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaccaria V., Garzarella E.U., Di Giovanni C., Galeotti F., Gisone L., Campoccia D., Volpi N., Arciola C.R., Daglia M. Multi Dynamic Extraction: An Innovative Method to Obtain a Standardized Chemically and Biologically Reproducible Polyphenol Extract from Poplar-Type Propolis to Be Used for Its Anti-Infective Properties. Materials. 2019;12(22):3746. doi: 10.3390/ma12223746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cusinato D.A.C., Martinez E.Z., Cintra M.T.C., Filgueira G.C.O., Berretta A.A., Lanchote V.L., Coelho E.B. Evaluation of potential herbal-drug interactions of a standardized propolis extract (EPP-AF(R)) using an in vivo cocktail approach. J Ethnopharmacol. 2019;245:112174. doi: 10.1016/j.jep.2019.112174. [DOI] [PubMed] [Google Scholar]
- 54.Berretta A.A., Nascimento A.P., Bueno P.C., Vaz M.M., Marchetti J.M. Propolis standardized extract (EPP-AF(R)), an innovative chemically and biologically reproducible pharmaceutical compound for treating wounds. Int J Biol Sci. 2012;8(4):512–521. doi: 10.7150/ijbs.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ramos A., Miranda J. Propolis: a review of its anti-inflammatory and healing actions. J Venom Anim Toxins Trop Dis. 2007;13(4):697–710. doi: 10.1590/S1678-91992007000400002. [DOI] [Google Scholar]
- 56.Barth O., Freitas A., Matsuda A., Almeida-Muradian L. Botanical origin and Artepillin-C content of Brazilian propolis samples. Grana. 2013;52(2):129–135. doi: 10.1080/00173134.2012.747561. [DOI] [Google Scholar]
- 57.Pedrazzi V., Leite M.F., Tavares R.C., Sato S., do Nascimento G.C., Issa J.P.M. Herbal mouthwash containing extracts of Baccharis dracunculifolia as agent for the control of biofilm: clinical evaluation in humans. ScientificWorldJournal. 2015;2015 doi: 10.1155/2015/712683. 712683-712683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park Y.K., Fukuda I., Ashida H., Nishiumi S., Yoshida K.-i., Daugsch A., Sato H.H., Pastore G.M. Suppressive Effects of Ethanolic Extracts from Propolis and Its Main Botanical Origin on Dioxin Toxicity. J Agr Food Chem. 2005;53(26):10306–10309. doi: 10.1021/jf058111a. [DOI] [PubMed] [Google Scholar]
- 59.da Silva Filho A.A., de Sousa J.P., Soares S., Furtado N.A., Andrade e Silva M.L., Cunha W.R., Gregório L.E., Nanayakkara N.P., Bastos J.K. Antimicrobial activity of the extract and isolated compounds from Baccharis dracunculifolia D. C. (Asteraceae) Z Naturforsch C J Biosci. 2008;63(1–2):40–46. doi: 10.1515/znc-2008-1-208. [DOI] [PubMed] [Google Scholar]
- 60.Búfalo M.C., Figueiredo A.S., de Sousa J.P., Candeias J.M., Bastos J.K., Sforcin J.M. Anti-poliovirus activity of Baccharis dracunculifolia and propolis by cell viability determination and real-time PCR. J Appl Microbiol. 2009;107(5):1669–1680. doi: 10.1111/j.1365-2672.2009.04354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Castaldo S., Capasso F. Propolis, an old remedy used in modern medicine. Fitoterapia. 2002;73(Suppl 1):S1–S6. doi: 10.1016/s0367-326x(02)00185-5. [DOI] [PubMed] [Google Scholar]
- 62.Silva-Carvalho R., Baltazar F., Almeida-Aguiar C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid Based Complement Alternat Med. 2015;2015:206439. doi: 10.1155/2015/206439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kuropatnicki A.K., Szliszka E., Krol W. Historical Aspects of Propolis Research in Modern Times. Evid Based Complement Alternat Med. 2013;2013:964149. doi: 10.1155/2013/964149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sun S., He J., Liu M., Yin G., Zhang X. A Great Concern Regarding the Authenticity Identification and Quality Control of Chinese Propolis and Brazilian Green Propolis. J Food Nutr Res. 2019;7(10):725–735. doi: 10.12691/jfnr-7-10-6. [DOI] [Google Scholar]
- 65.Sun J.L., Hu Y.L., Wang D.Y., Zhang B.K., Liu J.G. Immunologic enhancement of compound Chinese herbal medicinal ingredients and their efficacy comparison with compound Chinese herbal medicines. Vaccine. 2006;24(13):2343–2348. doi: 10.1016/j.vaccine.2005.11.053. [DOI] [PubMed] [Google Scholar]
- 66.Akao Y M.H., Matsumoto K., Ohguchi K., Nishizawa K., Sakamoto T., Araki Y., Mishima S., Nozawa Y. Cell growth inhibitory effect of cinnamic acid derivatives from propolis on human tumor cell lines. Biol Pharm Bull. 2003;26(7):1057. doi: 10.1248/bpb.26.1057. [DOI] [PubMed] [Google Scholar]
- 67.Banskota A.H., Tezuka Y., Kadota S. Recent progress in pharmacological research of propolis. Phytother Res. 2001;15(7):561–571. doi: 10.1002/ptr.1029. [DOI] [PubMed] [Google Scholar]
- 68.Furtado Júnior J.H.C., Valadas L.A.R., Fonseca S., Lobo P.L.D., Calixto L.H.M., Lima A.G.F., de Aguiar M.H.R., Arruda I.S., Lotif M.A.L., Rodrigues Neto E.M., Fonteles M.M.F. Clinical and Microbiological Evaluation of Brazilian Red Propolis Containing-Dentifrice in Orthodontic Patients: A Randomized Clinical Trial. Evid Based Complement Alternat Med. 2020;2020:8532701. doi: 10.1155/2020/8532701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gajek G., Marciniak B., Lewkowski J., Kontek R. Antagonistic Effects of CAPE (a Component of Propolis) on the Cytotoxicity and Genotoxicity of Irinotecan and SN38 in Human Gastrointestinal Cancer Cells In Vitro. Molecules. 2020;25(3):658. doi: 10.3390/molecules25030658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Santos L.M., Fonseca M.S., Sokolonski A.R., Deegan K.R., Araújo R.P., Umsza-Guez M.A., Barbosa J.D., Portela R.D., Machado B.A. Propolis: types, composition, biological activities, and veterinary product patent prospecting. J Sci Food Agric. 2020;100(4):1369–1382. doi: 10.1002/jsfa.10024. [DOI] [PubMed] [Google Scholar]
- 71.He-rim J. The Investor, The Korea Herald; Korea: 2020. ‘Health functional foods’ see boom in wake of virus outbreak.http://www.theinvestor.co.kr/view.php?ud=20200305000843 [Google Scholar]
- 72.Koe T. 2020. New rules: South Korea expands propolis oral formats, removes upper limit for functional ingredients.https://www.nutraingredients-asia.com/Article/2020/03/09/ [Google Scholar]
- 73.Pina G.d.M.S., Lia E.N., Berretta A.A., Nascimento A.P., Torres E.C., Buszinski A.F.M., de Campos T.A., Coelho E.B., Martins V.d.P. Efficacy of Propolis on the Denture Stomatitis Treatment in Older Adults: A Multicentric Randomized Trial. Evid Based Complement Alternat Med. 2017;2017:8971746. doi: 10.1155/2017/8971746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berretta A.A. Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo; Ribeirão Preto: 2007. Pesquisa pré-clínica e clínica de um gel termorreversível contendo extrato padronizado de própolis (EPP-AF) para a redução do tempo de cicatrização de lesões em pacientes queimados, Ph.D. thesis. [DOI] [Google Scholar]
- 75.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses, The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hashem H. IN Silico Approach of Some Selected Honey Constituents as SARS-CoV-2 Main Protease (COVID-19) Inhibitors. EJMO. 2020;4(3):196–200. doi: 10.26434/chemrxiv.12115359. [DOI] [Google Scholar]
- 77.Kumar V., Dhanjal J.K., Kaul S.C., Wadhwa R., Sundar D. Withanone and caffeic acid phenethyl ester are predicted to interact with main protease (M(pro)) of SARS-CoV-2 and inhibit its activity. J Biomol Struct Dyn. 2020:1–13. doi: 10.1080/07391102.2020.1772108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mehra M.R., Desai S.S., Kuy S., Henry T.D., Patel A.N. Cardiovascular Disease, Drug Therapy, and Mortality in Covid-19. N Engl J Med. 2020;382 doi: 10.1056/NEJMoa2007621. e102. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Sanchis-Gomar F., Lavie C.J., Perez-Quilis C., Henry B.M., Lippi G. Angiotensin-Converting Enzyme 2 and Antihypertensives (Angiotensin Receptor Blockers and Angiotensin-Converting Enzyme Inhibitors) in Coronavirus Disease 2019. Mayo Clin Proc. 2020;95(6):1222–1230. doi: 10.1016/j.mayocp.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oses S.M., Marcos P., Azofra P., de Pablo A., Fernandez-Muino M.A., Sancho M.T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Different Geographical Areas: Needs for Analytical Harmonization. Antioxidants. 2020;9(1):75. doi: 10.3390/antiox9010075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Da J., Xu M., Wang Y., Li W., Lu M., Wang Z. Kaempferol Promotes Apoptosis While Inhibiting Cell Proliferation via Androgen-Dependent Pathway and Suppressing Vasculogenic Mimicry and Invasion in Prostate Cancer. Anal Cell Pathol. 2019;2019:1907698. doi: 10.1155/2019/1907698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Debiaggi M., Tateo F., Pagani L., Luini M., Romero E. Effects of propolis flavonoids on virus infectivity and replication. Microbiologica. 1990;13(3):207–213. [PubMed] [Google Scholar]
- 85.Messerli S.M., Ahn M.-R., Kunimasa K., Yanagihara M., Tatefuji T., Hashimoto K., Mautner V., Uto Y., Hori H., Kumazawa S., Kaji K., Ohta T., Maruta H. Artepillin C (ARC) in Brazilian green propolis selectively blocks oncogenic PAK1 signaling and suppresses the growth of NF tumors in mice. Phytother Res. 2009;23(3):423–427. doi: 10.1002/ptr.2658. [DOI] [PubMed] [Google Scholar]
- 86.Fernandes M.H.V., Ferreira L.N., Vargas G.D.A., Fischer G., Hübner S.O. Effect of Water Extract from Brown Propolis on Production of IFN-ϒ After Immunization Against Canine Parvovirus (Cpv) and Canine Coronavirus (Ccov) Ciênc Anim Bras. 2015;16(2):235–242. doi: 10.1590/1089-6891v16i223458. [DOI] [Google Scholar]
- 87.Mahmoud A.M., Abd El-Twab S.M. Caffeic acid phenethyl ester protects the brain against hexavalent chromium toxicity by enhancing endogenous antioxidants and modulating the JAK/STAT signaling pathway. Biomed Pharmacother. 2017;91:303–311. doi: 10.1016/j.biopha.2017.04.073. [DOI] [PubMed] [Google Scholar]
- 88.Okamoto Y., Tanaka M., Fukui T., Masuzawa T. Brazilian propolis inhibits the differentiation of Th17 cells by inhibition of interleukin-6-induced phosphorylation of signal transducer and activator of transcription 3. Immunopharm Immunot. 2012;34(5):803–809. doi: 10.3109/08923973.2012.657304. [DOI] [PubMed] [Google Scholar]
- 89.Paulino N., Abreu S.R., Uto Y., Koyama D., Nagasawa H., Hori H., Dirsch V.M., Vollmar A.M., Scremin A., Bretz W.A. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur J Pharmacol. 2008;587(1–3):296–301. doi: 10.1016/j.ejphar.2008.02.067. [DOI] [PubMed] [Google Scholar]
- 90.Nile S.H., Nile A., Qiu J., Li L., Jia X., Kai G. COVID-19: Pathogenesis, cytokine storm and therapeutic potential of interferons. Cytokine Growth F R. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li C.C., Wang X.J., Wang H.R. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov Today. 2019;24(3):726–736. doi: 10.1016/j.drudis.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Orsatti C.L., Missima F., Pagliarone A.C., Bachiega T.F., Búfalo M.C., Araújo J.P., Jr., Sforcin J.M. Propolis immunomodulatory action in vivo on Toll-like receptors 2 and 4 expression and on pro-inflammatory cytokines production in mice. Phytother Res. 2010;24(8):1141–1146. doi: 10.1002/ptr.3086. [DOI] [PubMed] [Google Scholar]
- 93.Ito J., Chang F.R., Wang H.K., Park Y.K., Ikegaki M., Kilgore N., Lee K.H. Anti-AIDS agents. 48.(1) Anti-HIV activity of moronic acid derivatives and the new melliferone-related triterpenoid isolated from Brazilian propolis. J Nat Prod. 2001;64(10):1278–1281. doi: 10.1021/np010211x. [DOI] [PubMed] [Google Scholar]
- 94.Shimizu T., Hino A., Tsutsumi A., Park Y.K., Watanabe W., Kurokawa M. Anti-influenza virus activity of propolis in vitro and its efficacy against influenza infection in mice. Antivir Chem Chemother. 2008;19(1):7–13. doi: 10.1177/095632020801900102. [DOI] [PubMed] [Google Scholar]
- 95.Nolkemper S., Reichling J., Sensch K.H., Schnitzler P. Mechanism of herpes simplex virus type 2 suppression by propolis extracts. Phytomedicine. 2010;17(2):132–138. doi: 10.1016/j.phymed.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 96.Harish Z., Rubinstein A., Golodner M., Elmaliah M., Mizrachi Y. Suppression of HIV-1 replication by propolis and its immunoregulatory effect. Drugs Exp Clin Res. 1997;23(2):89–96. [PubMed] [Google Scholar]
- 97.Amoros M., Simões C.M., Girre L., Sauvager F., Cormier M. Synergistic effect of flavones and flavonols against herpes simplex virus type 1 in cell culture. Comparison with the antiviral activity of propolis. J Nat Prod. 1992;55(12):1732–1740. doi: 10.1021/np50090a003. [DOI] [PubMed] [Google Scholar]
- 98.England J.T., Abdulla A., Biggs C.M., Lee A.Y.Y., Hay K.A., Hoiland R.L., Wellington C.L., Sekhon M., Jamal S., Shojania K., Chen L.Y.C. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2020:100707. doi: 10.1016/j.blre.2020.100707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maruta H., Kittaka A. Chemical evolution for taming the ‘pathogenic kinase’ PAK1. Drug Discov Today. 2020;25(6):959–964. doi: 10.1016/j.drudis.2020.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tozser J., Benko S. Natural Compounds as Regulators of NLRP3 Inflammasome-Mediated IL-1beta Production. Mediators Inflamm. 2016;2016:5460302. doi: 10.1155/2016/5460302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ashry el S.H., Ahmad T.A. The use of propolis as vaccine’s adjuvant. Vaccine. 2012;31(1):31–39. doi: 10.1016/j.vaccine.2012.10.095. [DOI] [PubMed] [Google Scholar]
- 102.Fan Y., Guo L., Hou W., Guo C., Zhang W., Ma X., Ma L., Song X. The Adjuvant Activity of Epimedium Polysaccharide-Propolis Flavone Liposome on Enhancing Immune Responses to Inactivated Porcine Circovirus Vaccine in Mice. Evid Based Complement Alternat Med. 2015;2015:972083. doi: 10.1155/2015/972083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang L., Hu Y., Xue J., Wang F., Wang D., Kong X., Li P., Xu W. Compound Chinese herbal medicinal ingredients can enhance immune response and efficacy of RHD vaccine in rabbit. Vaccine. 2008;26(35):4451–4455. doi: 10.1016/j.vaccine.2008.06.075. [DOI] [PubMed] [Google Scholar]
- 104.Tao Y., Wang D., Hu Y., Huang Y., Yu Y., Wang D. The immunological enhancement activity of propolis flavonoids liposome in vitro and in vivo. Evid Based Complement Alternat Med. 2014;2014:483513. doi: 10.1155/2014/483513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fischer G., Cleff M.B., Dummer L.A., Paulino N., Paulino A.S., de Oliveira Vilela C., Campos F.S., Storch T., D’Avila Vargas G., de Oliveira Hübner S., Vidor T. Adjuvant effect of green propolis on humoral immune response of bovines immunized with bovine herpesvirus type 5. Vet Immunol Immunopathol. 2007;116(1–2):79–84. doi: 10.1016/j.vetimm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Fischer G., Conceicao F.R., Leite F.P., Dummer L.A., Vargas G.D., Hubner Sde O., Dellagostin O.A., Paulino N., Paulino A.S., Vidor T. Immunomodulation produced by a green propolis extract on humoral and cellular responses of mice immunized with SuHV-1. Vaccine. 2007;25(7):1250–1256. doi: 10.1016/j.vaccine.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 107.Ma X., Guo Z., Shen Z., Wang J., Hu Y., Wang D. The immune enhancement of propolis adjuvant on inactivated porcine parvovirus vaccine in guinea pig. Cell Immunol. 2011;270(1):13–18. doi: 10.1016/j.cellimm.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 108.Sena-Lopes Â., Bezerra F.S.B., das Neves R.N., de Pinho R.B., Silva M.T.O., Savegnago L., Collares T., Seixas F., Begnini K., Henriques J.A.P., Ely M.R., Rufatto L.C., Moura S., Barcellos T., Padilha F., Dellagostin O., Borsuk S. Chemical composition, immunostimulatory, cytotoxic and antiparasitic activities of the essential oil from Brazilian red propolis. PLoS One. 2018;13(2) doi: 10.1371/journal.pone.0191797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fischer G., Paulino N., Marcucci M.C., Siedler B.S., Munhoz L.S., Finger P.F., Vargas G.D., Hübner S.O., Vidor T., Roehe P.M. Green propolis phenolic compounds act as vaccine adjuvants, improving humoral and cellular responses in mice inoculated with inactivated vaccines. Mem Inst Oswaldo Cruz. 2010;105(7):908–913. doi: 10.1590/S0074-02762010000700012. [DOI] [PubMed] [Google Scholar]
- 110.Mojarab S., Shahbazzadeh D., Moghbeli M., Eshraghi Y., Bagheri K.P., Rahimi R., Savoji M.A., Mahdavi M. Immune responses to HIV-1 polytope vaccine candidate formulated in aqueous and alcoholic extracts of Propolis: Comparable immune responses to Alum and Freund adjuvants. Microb Pathog. 2020;140:103932. doi: 10.1016/j.micpath.2019.103932. [DOI] [PubMed] [Google Scholar]
- 111.Raymond E., Thieblemont C., Alran S., Faivre S. Impact of the COVID-19 Outbreak on the Management of Patients with Cancer. Targ Oncol. 2020;15:249–259. doi: 10.1007/s11523-020-00721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Patel S. Emerging Adjuvant Therapy for Cancer: Propolis and its Constituents. J Diet Suppl. 2016;13(3):245–268. doi: 10.3109/19390211.2015.1008614. [DOI] [PubMed] [Google Scholar]
- 113.Frión-Herrera Y., Gabbia D., Scaffidi M., Zagni L., Cuesta-Rubio O., De Martin S., Carrara M. The Cuban Propolis Component Nemorosone Inhibits Proliferation and Metastatic Properties of Human Colorectal Cancer Cells. Int J Mol Sci. 2020;21(5):1827. doi: 10.3390/ijms21051827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song Y.S., Park E.H., Jung K.J., Jin C. Inhibition of angiogenesis by propolis. Arch Pharm Res. 2002;25(4):500–504. doi: 10.1007/BF02976609. [DOI] [PubMed] [Google Scholar]
- 115.Orsolić N., Basić I. Antitumor, hematostimulative and radioprotective action of water-soluble derivative of propolis (WSDP) Biomed Pharmacother. 2005;59(10):561–570. doi: 10.1016/j.biopha.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 116.Watanabe M.A., Amarante M.K., Conti B.J., Sforcin J.M. Cytotoxic constituents of propolis inducing anticancer effects: a review. J Pharm Pharmacol. 2011;63(11):1378–1386. doi: 10.1111/j.2042-7158.2011.01331.x. [DOI] [PubMed] [Google Scholar]
- 117.Wu J., Omene C., Karkoszka J., Bosland M., Eckard J., Klein C.B., Frenkel K. Caffeic acid phenethyl ester (CAPE), derived from a honeybee product propolis, exhibits a diversity of anti-tumor effects in pre-clinical models of human breast cancer. Cancer Lett. 2011;308(1):43–53. doi: 10.1016/j.canlet.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Akyol S., Ozturk G., Ginis Z., Armutcu F., Yigitoglu M.R., Akyol O. In vivo and in vitro antıneoplastic actions of caffeic acid phenethyl ester (CAPE): therapeutic perspectives. Nutr Cancer. 2013;65(4):515–526. doi: 10.1080/01635581.2013.776693. [DOI] [PubMed] [Google Scholar]
- 119.Chang H., Wang Y., Yin X., Liu X., Xuan H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement Altern Med. 2017;17(1):471. doi: 10.1186/s12906-017-1984-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Orsolić N., Knezević A.H., Sver L., Terzić S., Basić I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J Ethnopharmacol. 2004;94(2–3):307–315. doi: 10.1016/j.jep.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 121.Sawicka D., Car H., Borawska M.H., Nikliński J. The anticancer activity of propolis. Folia Histochem Cytobiol. 2012;50(1):25–37. doi: 10.2478/18693. [DOI] [PubMed] [Google Scholar]
- 122.Grunberger D., Banerjee R., Eisinger K., Oltz E.M., Efros L., Caldwell M., Estevez V., Nakanishi K. Preferential cytotoxicity on tumor cells by caffeic acid phenethyl ester isolated from propolis. Experientia. 1988;44(3):230–232. doi: 10.1007/BF01941717. [DOI] [PubMed] [Google Scholar]
- 123.Nagaoka T., Banskota A.H., Tezuka Y., Saiki I., Kadota S. Selective antiproliferative activity of caffeic acid phenethyl ester analogues on highly liver-metastatic murine colon 26-L5 carcinoma cell line. Bioorg Med Chem. 2002;10(10):3351–3359. doi: 10.1016/s0968-0896(02)00138-4. [DOI] [PubMed] [Google Scholar]
- 124.Szliszka E., Czuba Z.P., Domino M., Mazur B., Zydowicz G., Krol W. Ethanolic extract of propolis (EEP) enhances the apoptosis- inducing potential of TRAIL in cancer cells. Molecules. 2009;14(2):738–754. doi: 10.3390/molecules14020738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cook T.M. The importance of hypertension as a risk factor for severe illness and mortality in COVID-19. Anaesthesia. 2020;75(7):976–977. doi: 10.1111/anae.15103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahajan K., Chandra K. Cardiovascular comorbidities and complications associated with coronavirus disease 2019. Med J Armed Forces India. 2020 doi: 10.1016/j.mjafi.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Emami A., Javanmardi F., Pirbonyeh N., Akbari A. Prevalence of Underlying Diseases in Hospitalized Patients with COVID-19: a Systematic Review and Meta-Analysis. Arch Acad Emerg Med. 2020;8(1) doi: 10.22037/aaem.v8i1.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kubota Y., Umegaki K., Kobayashi K., Tanaka N., Kagota S., Nakamura K., Kunitomo M., Shinozuka K. Anti-hypertensive effects of Brazilian propolis in spontaneously hypertensive rats. Clin Exp Pharmacol Physiol. 2004;31(Suppl 2):S29–S30. doi: 10.1111/j.1440-1681.2004.04113.x. [DOI] [PubMed] [Google Scholar]
- 129.Zhou H., Wang H., Shi N., Wu F. Potential Protective Effects of the Water-Soluble Chinese Propolis on Hypertension Induced by High-Salt Intake. Clin Transl Sci. 2020 doi: 10.1111/cts.12770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zingue S., Nde C.B.M., Michel T., Ndinteh D.T., Tchatchou J., Adamou M., Fernandez X., Fohouo F.T., Clyne C., Njamen D. Ethanol-extracted Cameroonian propolis exerts estrogenic effects and alleviates hot flushes in ovariectomized Wistar rats. BMC Complement Altern Med. 2017;17(1):65. doi: 10.1186/s12906-017-1568-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yuan W., Chang H., Liu X., Wang S., Liu H., Xuan H. Brazilian Green Propolis Inhibits Ox-LDL-Stimulated Oxidative Stress in Human Umbilical Vein Endothelial Cells Partly through PI3K/Akt/mTOR-Mediated Nrf2/HO-1 Pathway. Evid Based Complement Alternat Med. 2019;2019:5789574. doi: 10.1155/2019/5789574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Mujica V., Orrego R., Pérez J., Romero P., Ovalle P., Zúñiga-Hernández J., Arredondo M., Leiva E. The Role of Propolis in Oxidative Stress and Lipid Metabolism: A Randomized Controlled Trial. Evid Based Complement Alternat Med. 2017;2017:4272940. doi: 10.1155/2017/4272940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Michalakis K., Ilias I. SARS-CoV-2 infection and obesity: Common inflammatory and metabolic aspects. Diabetes Metab Syndr. 2020;14(4):469–471. doi: 10.1016/j.dsx.2020.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Koya-Miyata S., Arai N., Mizote A., Taniguchi Y., Ushio S., Iwaki K., Fukuda S. Propolis prevents diet-induced hyperlipidemia and mitigates weight gain in diet-induced obesity in mice. Biol Pharm Bull. 2009;32(12):2022–2028. doi: 10.1248/bpb.32.2022. [DOI] [PubMed] [Google Scholar]
- 135.Rayalam S., Mills D., Azhar Y., Miller E., Wang X. Caffeic Acid Phenethyl Ester and Its Fluorinated Derivative as Natural Anti-obesity Agents (P06-089-19) Curr Dev Nutr. 2019;3(Supplement_1) doi: 10.1093/cdn/nzz031.P06-089-19. [DOI] [Google Scholar]
- 136.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., Loda M., Looney M.R., McAllister F., Rayes R., Renaud S., Rousseau S., Salvatore S., Schwartz R.E., Spicer J.D., Yost C.C., Weber A., Zuo Y., Egeblad M. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bertoletti L., Couturaud F., Montani D., Parent F., Sanchez O. Venous thromboembolism and COVID-19. Respir Med Res. 2020;78:100759. doi: 10.1016/j.resmer.2020.100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jose R.J., Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. 2020 doi: 10.1016/S2213-2600(20)30216-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., Arbous M.S., Gommers D.A.M.P.J., Kant K.M., Kaptein F.H.J., van Paassen J., Stals M.A.M., Huisman M.V., Endeman H. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vaughan D.E. PAI-1 and atherothrombosis. J Thromb Haemost. 2005;3(8):1879–1883. doi: 10.1111/j.1538-7836.2005.01420.x. [DOI] [PubMed] [Google Scholar]
- 141.Cesari M., Pahor M., Incalzi R.A. Plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovasc Ther. 2010;28(5):e72–e91. doi: 10.1111/j.1755-5922.2010.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ohkura N., Oishi K., Kihara-Negishi F., Atsumi G.-I., Tatefuji T. Effects of a diet containing Brazilian propolis on lipopolysaccharide-induced increases in plasma plasminogen activator inhibitor-1 levels in mice. J Intercult Ethnopharmacol. 2016;5(4):439–443. doi: 10.5455/jice.20160814112735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kitamura H. Effects of Propolis Extract and Propolis-Derived Compounds on Obesity and Diabetes: Knowledge from Cellular and Animal Models. Molecules. 2019;24(23):4394. doi: 10.3390/molecules24234394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Daleprane J.B., Abdalla D.S. Emerging roles of propolis: antioxidant, cardioprotective, and antiangiogenic actions. Evid Based Complement Alternat Med. 2013;2013:175135. doi: 10.1155/2013/175135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bojić M., Antolić A., Tomičić M., Debeljak Ž., Maleš Ž. Propolis ethanolic extracts reduce adenosine diphosphate induced platelet aggregation determined on whole blood. Nutr J. 2018;17(1):52. doi: 10.1186/s12937-018-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang Y.-X., Yang T.-T., Xia L., Zhang W.-F., Wang J.-F., Wu Y.-P. Inhibitory Effect of Propolis on Platelet Aggregation In Vitro. J Healthc Eng. 2017;2017:3050895. doi: 10.1155/2017/3050895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hsiao G., Lee J.J., Lin K.H., Shen C.H., Fong T.H., Chou D.S., Sheu J.R. Characterization of a novel and potent collagen antagonist, caffeic acid phenethyl ester, in human platelets: in vitro and in vivo studies. Cardiovasc Res. 2007;75(4):782–792. doi: 10.1016/j.cardiores.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 148.Gubernatorova E.O., Gorshkova E.A., Polinova A.I., Drutskaya M.S. IL-6: Relevance for immunopathology of SARS-CoV-2. Cytokine Growth F R. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kocot J., Kiełczykowska M., Luchowska-Kocot D., Kurzepa J., Musik I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid Med Cell Longev. 2018;2018:7074209. doi: 10.1155/2018/7074209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Havermann S., Chovolou Y., Humpf H.U., Wätjen W. Caffeic acid phenethylester increases stress resistance and enhances lifespan in Caenorhabditis elegans by modulation of the insulin-like DAF-16 signalling pathway. PLoS One. 2014;9(6) doi: 10.1371/journal.pone.0100256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zhu A., Wu Z., Zhong X., Ni J., Li Y., Meng J., Du C., Zhao X., Nakanishi H., Wu S. Brazilian Green Propolis Prevents Cognitive Decline into Mild Cognitive Impairment in Elderly People Living at High Altitude. J Alzheimers Dis. 2018;63(2):551–560. doi: 10.3233/JAD-170630. [DOI] [PubMed] [Google Scholar]
- 152.Jasprica I., Mornar A., Debeljak Z., Smolcić-Bubalo A., Medić-Sarić M., Mayer L., Romić Z., Bućan K., Balog T., Sobocanec S., Sverko V. In vivo study of propolis supplementation effects on antioxidative status and red blood cells. J Ethnopharmacol. 2007;110(3):548–554. doi: 10.1016/j.jep.2006.10.023. [DOI] [PubMed] [Google Scholar]
- 153.Sameni H.R., Ramhormozi P., Bandegi A.R., Taherian A.A., Mirmohammadkhani M., Safari M. Effects of ethanol extract of propolis on histopathological changes and anti-oxidant defense of kidney in a rat model for type 1 diabetes mellitus. J Diabetes Investig. 2016;7(4):506–513. doi: 10.1111/jdi.12459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ni J., Wu Z., Meng J., Zhu A., Zhong X., Wu S., Nakanishi H. The Neuroprotective Effects of Brazilian Green Propolis on Neurodegenerative Damage in Human Neuronal SH-SY5Y Cells. Oxid Med Cell Longev. 2017;2017:7984327. doi: 10.1155/2017/7984327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ayaz M., Sadiq A., Junaid M., Ullah F., Ovais M., Ullah I., Ahmed J., Shahid M. Flavonoids as Prospective Neuroprotectants and Their Therapeutic Propensity in Aging Associated Neurological Disorders. Front Aging Neurosci. 2019;11:155. doi: 10.3389/fnagi.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dong Y., Stewart T., Bai L., Li X., Xu T., Iliff J., Shi M., Zheng D., Yuan L., Wei T., Yang X., Zhang J. Coniferaldehyde attenuates Alzheimer’s pathology via activation of Nrf2 and its targets. Theranostics. 2020;10(1):179–200. doi: 10.7150/thno.36722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sargiacomo C., Sotgia F., Lisanti M.P. COVID-19 and chronological aging: senolytics and other anti-aging drugs for the treatment or prevention of corona virus infection? Aging. 2020;12(8):6511–6517. doi: 10.18632/aging.103001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vinayagam R., Xu B. Antidiabetic properties of dietary flavonoids: a cellular mechanism review. Nutr Metab. 2015;12(1):60. doi: 10.1186/s12986-015-0057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Tiveron A.P., Rosalen P.L., Franchin M., Lacerda R.C., Bueno-Silva B., Benso B., Denny C., Ikegaki M., Alencar S.M. Chemical Characterization and Antioxidant, Antimicrobial, and Anti-Inflammatory Activities of South Brazilian Organic Propolis. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0165588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.El Adaouia Taleb R., Djebli N., Chenini H., Sahin H., Kolayli S. In vivo and in vitro anti-diabetic activity of ethanolic propolis extract. J Food Biochem. 2020;44 doi: 10.1111/jfbc.13267. [DOI] [PubMed] [Google Scholar]
- 161.Matsui T., Ebuchi S., Fujise T., Abesundara K.J., Doi S., Yamada H., Matsumoto K. Strong antihyperglycemic effects of water-soluble fraction of Brazilian propolis and its bioactive constituent, 3,4,5-tri-O-caffeoylquinic acid. Biol Pharm Bull. 2004;27(11):1797–1803. doi: 10.1248/bpb.27.1797. [DOI] [PubMed] [Google Scholar]
- 162.Zamami Y., Takatori S., Koyama T., Goda M., Iwatani Y., Doi S., Kawasaki H. [Effect of propolis on insulin resistance in fructose-drinking rats] Yakugaku Zasshi. 2007;127(12):2065–2073. doi: 10.1248/yakushi.127.2065. [DOI] [PubMed] [Google Scholar]
- 163.Li Y., Chen M., Xuan H., Hu F. Effects of Encapsulated Propolis on Blood Glycemic Control, Lipid Metabolism, and Insulin Resistance in Type 2 Diabetes Mellitus Rats. Evid Based Complement Alternat Med. 2012;2012:981896. doi: 10.1155/2012/981896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Aoi W., Hosogi S., Niisato N., Yokoyama N., Hayata H., Miyazaki H., Kusuzaki K., Fukuda T., Fukui M., Nakamura N., Marunaka Y. Improvement of insulin resistance, blood pressure and interstitial pH in early developmental stage of insulin resistance in OLETF rats by intake of propolis extracts. Biochem Biophys Res Commun. 2013;432(4):650–653. doi: 10.1016/j.bbrc.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 165.Abo-Salem O.M., El-Edel R.H., Harisa G.E., El-Halawany N., Ghonaim M.M. Experimental diabetic nephropathy can be prevented by propolis: Effect on metabolic disturbances and renal oxidative parameters. Pak J Pharm Sci. 2009;22(2):205–210. [PubMed] [Google Scholar]
- 166.Kitamura H., Naoe Y., Kimura S., Miyamoto T., Okamoto S., Toda C., Shimamoto Y., Iwanaga T., Miyoshi I. Beneficial effects of Brazilian propolis on type 2 diabetes in ob/ob mice: Possible involvement of immune cells in mesenteric adipose tissue. Adipocyte. 2013;2(4):227–236. doi: 10.4161/adip.25608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gao W., Pu L., Wei J., Yao Z., Wang Y., Shi T., Zhao L., Jiao C., Guo C. Serum Antioxidant Parameters are Significantly Increased in Patients with Type 2 Diabetes Mellitus after Consumption of Chinese Propolis: A Randomized Controlled Trial Based on Fasting Serum Glucose Level. Diabetes Ther. 2018;9(1):101–111. doi: 10.1007/s13300-017-0341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Samadi N., Mozaffari-Khosravi H., Rahmanian M., Askarishahi M. Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: a randomized, double-blind clinical trial. J Integr Med. 2017;15(2):124–134. doi: 10.1016/S2095-4964(17)60315-7. [DOI] [PubMed] [Google Scholar]
- 169.Hesami S., Hashemipour S., Shiri-Shahsavar M.R., Koushan Y., Khadem Haghighian H. Administration of Iranian Propolis attenuates oxidative stress and blood glucose in type II diabetic patients: a randomized, double-blind, placebo-controlled, clinical trial. Caspian J Intern Med. 2019;10(1):48–54. doi: 10.22088/cjim.10.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zakerkish M., Jenabi M., Zaeemzadeh N., Hemmati A.A., Neisi N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci Rep. 2019;9(1):7289. doi: 10.1038/s41598-019-43838-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Oryan A., Alemzadeh E., Moshiri A. Potential role of propolis in wound healing: Biological properties and therapeutic activities. Biomed Pharmacother. 2018;98:469–483. doi: 10.1016/j.biopha.2017.12.069. [DOI] [PubMed] [Google Scholar]
- 172.Picolotto A., Pergher D., Pereira G.P., Machado K.G., da Silva Barud H., Roesch-Ely M., Gonzalez M.H., Tasso L., Figueiredo J.G., Moura S. Bacterial cellulose membrane associated with red propolis as phytomodulator: Improved healing effects in experimental models of diabetes mellitus. Biomed Pharmacother. 2019;112:108640. doi: 10.1016/j.biopha.2019.108640. [DOI] [PubMed] [Google Scholar]
- 173.Afkhamizadeh M., Aboutorabi R., Ravari H., Fathi Najafi M., Ataei Azimi S., Javadian Langaroodi A., Yaghoubi M.A., Sahebkar A. Topical propolis improves wound healing in patients with diabetic foot ulcer: a randomized controlled trial. Nat Prod Res. 2018;32(17):2096–2099. doi: 10.1080/14786419.2017.1363755. [DOI] [PubMed] [Google Scholar]
- 174.Henshaw F.R., Bolton T., Nube V., Hood A., Veldhoen D., Pfrunder L., McKew G.L., Macleod C., McLennan S.V., Twigg S.M. Topical application of the bee hive protectant propolis is well tolerated and improves human diabetic foot ulcer healing in a prospective feasibility study. J Diabetes Complicat. 2014;28(6):850–857. doi: 10.1016/j.jdiacomp.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 175.Shin S.H., Seo S.G., Min S., Yang H., Lee E., Son J.E., Kwon J.Y., Yue S., Chung M.Y., Kim K.H., Cheng J.X., Lee H.J., Lee K.W. Caffeic acid phenethyl ester, a major component of propolis, suppresses high fat diet-induced obesity through inhibiting adipogenesis at the mitotic clonal expansion stage. J Agric Food Chem. 2014;62(19):4306–4312. doi: 10.1021/jf405088f. [DOI] [PubMed] [Google Scholar]
- 176.Nie J., Chang Y., Li Y., Zhou Y., Qin J., Sun Z., Li H. Caffeic Acid Phenethyl Ester (Propolis Extract) Ameliorates Insulin Resistance by Inhibiting JNK and NF-κB Inflammatory Pathways in Diabetic Mice and HepG2 Cell Models. J Agric Food Chem. 2017;65(41):9041–9053. doi: 10.1021/acs.jafc.7b02880. [DOI] [PubMed] [Google Scholar]
- 177.Ramírez-Espinosa J.J., Saldaña-Ríos J., García-Jiménez S., Villalobos-Molina R., Ávila-Villarreal G., Rodríguez-Ocampo A.N., Bernal-Fernández G., Estrada-Soto S. Chrysin Induces Antidiabetic, Antidyslipidemic and Anti-Inflammatory Effects in Athymic Nude Diabetic Mice. Molecules. 2017;23(1):67. doi: 10.3390/molecules23010067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.D’Marco L., Puchades M.J., Romero-Parra M., Gorriz J.L. Diabetic Kidney Disease and COVID-19: The Crash of Two Pandemics. Front Med. 2020;7:199. doi: 10.3389/fmed.2020.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Perico L., Benigni A., Remuzzi G. Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. Nephron. 2020;144(5):213–221. doi: 10.1159/000507305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020;181(4):905–913. doi: 10.1016/j.cell.2020.04.004. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Alberici F., Delbarba E., Manenti C., Econimo L., Valerio F., Pola A., Maffei C., Possenti S., Piva S., Latronico N., Focà E., Castelli F., Gaggia P., Movilli E., Bove S., Malberti F., Farina M., Bracchi M., Costantino E.M., Bossini N., Gaggiotti M., Scolari F. Management Of Patients On Dialysis And With Kidney Transplant During SARS-COV-2 (COVID-19) Pandemic In Brescia, Italy. Kidney Int Rep. 2020;5(5):580–585. doi: 10.1016/j.ekir.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bhadauria M. Propolis Prevents Hepatorenal Injury Induced by Chronic Exposure to Carbon Tetrachloride. Evid Based Complement Alternat Med. 2012;2012:235358. doi: 10.1155/2012/235358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Boutabet K., Kebsa W., Alyane M., Lahouel M. Polyphenolic fraction of Algerian propolis protects rat kidney against acute oxidative stress induced by doxorubicin. Indian J Nephrol. 2011;21(2):101–116. doi: 10.4103/0971-4065.82131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Teles F., da Silva T.M., da Cruz Júnior F.P., Honorato V.H., de Oliveira Costa H., Barbosa A.P.F., de Oliveira S.G., Porfírio Z., Libório A.B., Borges R.L., Fanelli C. Brazilian Red Propolis Attenuates Hypertension and Renal Damage in 5/6 Renal Ablation Model. PLoS ONE. 2015;10(1) doi: 10.1371/journal.pone.0116535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Cushnie T.P., Lamb A.J. Antimicrobial activity of flavonoids. Int J Antimicrob Agents. 2005;26(5):343–356. doi: 10.1016/j.ijantimicag.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Grange J.M., Davey R.W. Antibacterial properties of propolis (bee glue) J R Soc Med. 1990;83(3):159–160. doi: 10.1177/014107689008300310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kujumgiev A., Tsvetkova I., Serkedjieva Y., Bankova V., Christov R., Popov S. Antibacterial, antifungal and antiviral activity of propolis of different geographic origin. J Ethnopharmacol. 1999;64(3):235–240. doi: 10.1016/s0378-8741(98)00131-7. [DOI] [PubMed] [Google Scholar]
- 188.Cornara L., Biagi M., Xiao J., Burlando B. Therapeutic Properties of Bioactive Compounds from Different Honeybee Products. Front Pharmacol. 2017;8:412. doi: 10.3389/fphar.2017.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Campos J.V.d., Assis O.B.G., Bernardes-Filho R. Atomic force microscopy evidences of bacterial cell damage caused by propolis extracts on E. coli and S. aureus. Food Sci Technol. 2019;40:55–61. doi: 10.1590/fst.32018. [DOI] [Google Scholar]
- 190.Bankova V., Christov R., Kujumgiev A., Marcucci M.C., Popov S. Chemical composition and antibacterial activity of Brazilian propolis. Z Naturforsch C J Biosci. 1995;50(3-4):167–172. doi: 10.1515/znc-1995-3-402. [DOI] [PubMed] [Google Scholar]
- 191.Boisard S., Le Ray A.-M., Landreau A., Kempf M., Cassisa V., Flurin C., Richomme P. Antifungal and Antibacterial Metabolites from a French Poplar Type Propolis. Evid Based Complement Alternat Med. 2015;2015:319240. doi: 10.1155/2015/319240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Al-Ani I., Zimmermann S., Reichling J., Wink M. Antimicrobial Activities of European Propolis Collected from Various Geographic Origins Alone and in Combination with Antibiotics. Medicines. 2018;5(1):2. doi: 10.3390/medicines5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Scazzocchio F., D’Auria F.D., Alessandrini D., Pantanella F. Multifactorial aspects of antimicrobial activity of propolis. Microbiol Res. 2006;161(4):327–333. doi: 10.1016/j.micres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 194.Libério S.A., Pereira A.L., Araújo M.J., Dutra R.P., Nascimento F.R., Monteiro-Neto V., Ribeiro M.N., Gonçalves A.G., Guerra R.N. The potential use of propolis as a cariostatic agent and its actions on mutans group streptococci. J Ethnopharmacol. 2009;125(1):1–9. doi: 10.1016/j.jep.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 195.Mirzoeva O.K., Grishanin R.N., Calder P.C. Antimicrobial action of propolis and some of its components: the effects on growth, membrane potential and motility of bacteria. Microbiol Res. 1997;152(3):239–246. doi: 10.1016/S0944-5013(97)80034-1. [DOI] [PubMed] [Google Scholar]
- 196.Popova M., Silici S., Kaftanoglu O., Bankova V. Antibacterial activity of Turkish propolis and its qualitative and quantitative chemical composition. Phytomedicine. 2005;12(3):221–228. doi: 10.1016/j.phymed.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 197.Mazzarello V., Donadu M.G., Ferrari M., Piga G., Usai D., Zanetti S., Sotgiu M.A. Treatment of acne with a combination of propolis, tea tree oil, and Aloe vera compared to erythromycin cream: two double-blind investigations. Clin Pharmacol. 2018;2018(10):175–181. doi: 10.2147/CPAA.S180474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Meto A., Colombari B., Meto A., Boaretto G., Pinetti D., Marchetti L., Benvenuti S., Pellati F., Blasi E. Propolis Affects Pseudomonas aeruginosa Growth, Biofilm Formation, eDNA Release and Phenazine Production: Potential Involvement of Polyphenols. Microorganisms. 2020;8(2):243. doi: 10.3390/microorganisms8020243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Loureiro K.C., Barbosa T.C., Nery M., Chaud M.V., da Silva C.F., Andrade L.N., Corrêa C.B., Jaguer A., Padilha F.F., Cardoso J.C., Souto E., Severino P. Antibacterial activity of chitosan/collagen membranes containing red propolis extract. Pharmazie. 2020;75(2):75–81. doi: 10.1691/ph.2020.9050. [DOI] [PubMed] [Google Scholar]
- 200.Park Y.K., Koo M.H., Abreu J.A., Ikegaki M., Cury J.A., Rosalen P.L. Antimicrobial properties of propolis on oral microorganisms. Curr Microbiol. 1998;36:24–28. doi: 10.1007/s002849900274. [DOI] [PubMed] [Google Scholar]
- 201.Park Y.K., Alencar S.M., Aguiar C.L. Botanical origin and chemical composition of Brazilian propolis. J Agr Food Chem. 2002;50(9):2502–2506. doi: 10.1021/jf011432b. [DOI] [PubMed] [Google Scholar]
- 202.Sforcin J., Fernandes A.J., Lopes C., Bankova V., Funari S. Seasonal effect on Brazilian propolis antibacterial activity. J Ethnopharmacol. 2000;73(1-2):243–249. doi: 10.1016/s0378-8741(00)00320-2. [DOI] [PubMed] [Google Scholar]
- 203.Galeotti F., Maccari F., Fachini A., Volpi N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods. 2018;7(3):41. doi: 10.3390/foods7030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Khayyal M.T. A.S. el-Ghazaly, A.S. el-Khatib, Mechanisms involved in the antiinflammatory effect of propolis extract. Drugs Exp Clin Res. 1993;19(5):197–203. [PubMed] [Google Scholar]
- 205.de Castro P.A., Savoldi M., Bonatto D., Barros M.H., Goldman M.H., Berretta A.A., Goldman G.H. Molecular characterization of propolis-induced cell death in Saccharomyces cerevisiae. Eukaryot Cell. 2011;10(3):398–411. doi: 10.1128/EC.00256-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Pietta G.C., PG, Pietta A.M. Analytical methods for quality control of propolis. Fitoterapia. 2002;73:S7–S20. doi: 10.1016/s0367-326x(02)00186-7. [DOI] [PubMed] [Google Scholar]
- 207.Marquiafável F.S., Nascimento A.P., Barud H.d.S., Marquele-Oliveira F., de-Freitas L.A.P., Bastos J.K., Berretta A.A. Development and characterization of a novel standardized propolis dry extract obtained by factorial design with high artepillin C content. J Pharm Technol Drug Res. 2015;4:1. doi: 10.7243/2050-120X-4-1. [DOI] [Google Scholar]
- 208.Cunha I., SawayaI A., Caetano F., ShimizuI M.T., MarcucciIII M.C., DrezzaI F.T., PoviaI G.S., Carvalho P.d.O. Factors that influence the yield and composition of Brazilian propolis extracts. J Braz Chem Soc. 2004;15(6):964–970. doi: 10.1590/S0103-50532004000600026. [DOI] [Google Scholar]
- 209.European Food Safety Authority Panel on Dietetic Products, Scientific Opinion on the substantiation of health claims related to propolis (ID 1242, 1245, 1246, 1247, 1248, 3184) and flavonoids in propolis (ID 1244, 1644, 1645, 3526, 3527, 3798, 3799) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010;8(10):1810. doi: 10.2903/j.efsa.2010.1810. [DOI] [Google Scholar]
- 210.Nikam P.H., Kareparamban J., Aruna J., Kadam V. Future Trends in Standardization of Herbal Drugs. J Appl Pharm Sci. 2012;2(6):38–44. doi: 10.7324/JAPS.2012.2631. [DOI] [Google Scholar]
- 211.Waldesch F.G., Konigswinter B., Remagen H. Medpharm CRC Press; Boca Raton: 2003. Herbal Medicinal Products - Scientific and regulatory basis for development quality assurance and marketing authorisation. [Google Scholar]
- 212.Souza J., Tacon L., Correia C., Bastos J., Freitas L. Spray-dried propolis extract, II: prenylated components of green propolis. Pharmazie. 2007;62(7):488–492. [PubMed] [Google Scholar]
- 213.Rocha B.A., Bueno P.C.P., Vaz M.M.d.O.L.L., Nascimento A.P., Ferreira N.U., Moreno G.d.P., Rodrigues M.R., Costa-Machado A.R.d.M., Barizon E.A., Campos J.C.L., de Oliveira P.F., Acésio N.d.O., Martins S.d.P.L., Tavares D.C., Berretta A.A. Evaluation of a Propolis Water Extract Using a Reliable RP-HPLC Methodology and In Vitro and In Vivo Efficacy and Safety Characterisation. Evid Based Complement Alternat Med. 2013;2013:670451. doi: 10.1155/2013/670451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.de Castro P., Savoldi M., Bonatto D., Malavazi I., Goldman M., Berretta A., Goldman G. Transcriptional profiling of Saccharomyces cerevisiae exposed to propolis. BMC Complement Altern Med. 2012;12:194. doi: 10.1186/1472-6882-12-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Berretta A.A., de Castro P.A., Cavalheiro A.H., Fortes V.S., Bom V.P., Nascimento A.P., Marquele-Oliveira F., Pedrazzi V., Ramalho L.N., Goldman G.H. Evaluation of Mucoadhesive Gels with Propolis (EPP-AF) in Preclinical Treatment of Candidiasis Vulvovaginal Infection. Evid Based Complement Alternat Med. 2013;2013:641480. doi: 10.1155/2013/641480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Barud Hda S., de Araujo A.M., Junior, Saska S., Mestieri L.B., Campos J.A., de Freitas R.M., Ferreira N.U., Nascimento A.P., Miguel F.G., Vaz M.M., Barizon E.A., Marquele-Oliveira F., Gaspar A.M., Ribeiro S.J., Berretta A.A. Antimicrobial Brazilian Propolis (EPP-AF) Containing Biocellulose Membranes as Promising Biomaterial for Skin Wound Healing. Evid Based Complement Alternat Med. 2013;2013:703024. doi: 10.1155/2013/703024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Marquele F.D., Oliveira A.R., Bonato P.S., Lara M.G., Fonseca M.J. Propolis extract release evaluation from topical formulations by chemiluminescence and HPLC. J Pharm Biomed Anal. 2006;41(2):461–468. doi: 10.1016/j.jpba.2005.12.022. [DOI] [PubMed] [Google Scholar]
- 218.Mohammadzadeh S., Shariatpanahi M., Hamedi M., Ahmadkhaniha R., Samadi N., Ostad S.N. Chemical composition, oral toxicity and antimicrobial activity of Iranian propolis. Food Chemistry. 2007;103(4):1097–1103. doi: 10.1016/j.foodchem.2006.10.006. [DOI] [Google Scholar]
- 219.Dobrowolski J., Vohora S., Sharma K., Shah S., Naqvi S., Dandiya P. Antibacterial, Antifungal, Antiamoebic, Antiinflammatory and Antipyretic Studies on Propolis Bee Products. J Ethnopharmacol. 1991;35(1):77–82. doi: 10.1016/0378-8741(91)90135-Z. [DOI] [PubMed] [Google Scholar]
- 220.Mani F., Damasceno H.C., Novelli E.L., Martins E.A., Sforcin J.M. Propolis: Effect of different concentrations, extracts and intake period on seric biochemical variables. J Ethnopharmacol. 2006;105(1–2):95–98. doi: 10.1016/j.jep.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 221.Senedese J.M., Rodrigues A.R., Furtado M.A., Faustino V.D., Berretta A.A., Marchetti J.M., Tavares D.C. Assessment of the mutagenic activity of extracts of brazilian propolis in topical pharmaceutical formulations on Mammalian cells in vitro and in vivo. Evid Based Complement Alternat Med. 2011;2011:315701. doi: 10.1093/ecam/nen049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tavares D.C., Mazzaron Barcelos G.R., Silva L.F., Chacon Tonin C.C., Bastos J.K. Propolis-induced genotoxicity and antigenotoxicity in Chinese hamster ovary cells. Toxicol In Vitro. 2006;20(7):1154–1158. doi: 10.1016/j.tiv.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 223.Reis C.M.F., Carvalho J.C.T., Caputo L.R.G., Patricio K.C.M., Barbosa M.V.J., Chieff A.L., Bastos J.K. Atividade antiinflamatória, antiúlcera gástrica e toxicidade subcrônica do extrato etanólico de própolis. Rev Bras Farmacogn. 2000;9-10(1):43–52. doi: 10.1590/S0102-695X2000000100005. [DOI] [Google Scholar]
- 224.Cohen H.A., Varsano I., Kahan E., Sarrell E.M., Uziel Y. Effectiveness of an herbal preparation containing echinacea, propolis, and vitamin C in preventing respiratory tract infections in children: a randomized, double-blind, placebo-controlled, multicenter study. Arch Pediatr Adolesc Med. 2004;158(3):217–221. doi: 10.1001/archpedi.158.3.217. [DOI] [PubMed] [Google Scholar]
- 225.Soroy L., Bagus S., Yongkie I.P., Djoko W. The effect of a unique propolis compound (PropoelixTM) on clinical outcomes in patients with dengue hemorrhagic fever. Infect Drug Resist. 2014;7:323–329. doi: 10.2147/IDR.S71505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lotfy M. Biological activity of bee propolis in health and disease. Asian Pac J Cancer Prev. 2006;7(1):22–31. [PubMed] [Google Scholar]
- 227.Finch C.E. Evolution in health and medicine Sackler colloquium: Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc Natl Acad Sci U S A. 2010;107(Suppl 1):1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ahn M.R., Kunimasa K., Ohta T., Kumazawa S., Kamihira M., Kaji K., Uto Y., Hori H., Nagasawa H., Nakayama T. Suppression of tumor-induced angiogenesis by Brazilian propolis: major component artepillin C inhibits in vitro tube formation and endothelial cell proliferation. Cancer Lett. 2007;252(2):235–243. doi: 10.1016/j.canlet.2006.12.039. [DOI] [PubMed] [Google Scholar]
- 229.Szliszka E., Zydowicz G., Janoszka B., Dobosz C., Kowalczyk-Ziomek G., Krol W. Ethanolic extract of Brazilian green propolis sensitizes prostate cancer cells to TRAIL-induced apoptosis. Int J Oncol. 2011;38(4):941–953. doi: 10.3892/ijo.2011.930. [DOI] [PubMed] [Google Scholar]
- 230.Chuang M.-h., Peng C.-y., Chi C.-y., Chung H.-y., Liu C.-t., Kuo H.-c. U.-M.B. Technology; United States: 2016. Device method of making artepillin c in propolis for anti-cancer.https://patents.google.com/patent/US20170226042A1/en [Google Scholar]
- 231.Ferrari C.K. Functional foods, herbs and nutraceuticals: towards biochemical mechanisms of healthy aging. Biogerontology. 2004;5(5):275–289. doi: 10.1007/s10522-004-2566-z. [DOI] [PubMed] [Google Scholar]
- 232.Bachevski D., Damevska K., Simeonovski V., Dimova M. Back to the basics: Propolis and COVID-19. Dermatol Ther. 2020;2020 doi: 10.1111/dth.13780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Sforcin J.M. Propolis and the immune system: a review. J Ethnopharmacol. 2007;113(1):1–14. doi: 10.1016/j.jep.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 234.Salatino A., Fernandes-Silva C.C., Righi A.A., Salatino M.L. Propolis research and the chemistry of plant products. Nat Prod Rep. 2011;28(5):925–936. doi: 10.1039/c0np00072h. [DOI] [PubMed] [Google Scholar]
- 235.de Mendonça I.C.G., Porto I.C.C.d.M., do Nascimento T.G., de Souza N.S., Oliveira J.M.d.S., Arruda R.E.d.S., Mousinho K.C., dos Santos A.F., Basílio-Júnior I.D., Parolia A., Barreto F.S. Brazilian red propolis: phytochemical screening, antioxidant activity and effect against cancer cells. BMC Complem Altern Med. 2015;15:357. doi: 10.1186/s12906-015-0888-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Porfidia A., Pola R. Venous thromboembolism and heparin use in COVID-19 patients: juggling between pragmatic choices, suggestions of medical societies and the lack of guidelines. J Thromb Thrombolysis. 2020;50:68–71. doi: 10.1007/s11239-020-02125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Schwarz S., Sauter D., Wang K., Zhang R., Sun B., Karioti A., Bilia A.R., Efferth T., Schwarz W. Kaempferol derivatives as antiviral drugs against the 3a channel protein of coronavirus. Planta Med. 2014;80(2-3):177–182. doi: 10.1055/s-0033-1360277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Yang L., Li Y.-T., Miao J., Wang L., Fu H., Li Q., Wen W.-B., Zhang Z.-Y., Song R.-W., Liu X.-G., Wang H.-W., Cui H.-T. Network pharmacology studies on the effect of Chai-Ling decoction in coronavirus disease 2019. Traditional Medicine Research. 2020;5(3):145. doi: 10.12032/TMR20200324170. [DOI] [Google Scholar]
- 240.Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., Hsieh C.C., Chao P.D. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antiviral Res. 2005;68(1):36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wu Z., Liu Y., Zhu A., Wu S., Nakanishia H. Brazilian green propolis suppresses microglia-mediated neuroinflammation by inhibiting NF-kB activation. J Neurol Sci. 2017;381 doi: 10.1016/j.jns.2017.08.1910. [DOI] [Google Scholar]
- 242.Adachi T., Tezuka H., Tsuji N.M., Ohteki T., Karasuyama H., Kumazawa T. Propolis induces Ca2+ signaling in immune cells. Biosci Microbiota Food Health. 2019;38(4):141. doi: 10.12938/bmfh.19-011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sy L.B., Wu Y.L., Chiang B.L., Wang Y.H., Wu W.M. Propolis extracts exhibit an immunoregulatory activity in an OVA-sensitized airway inflammatory animal model. Int Immunopharmacol. 2006;6(7):1053–1060. doi: 10.1016/j.intimp.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 244.Szliszka E., Kucharska A.Z., Sokol-Letowska A., Mertas A., Czuba Z.P., Krol W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid Based Complement Alternat Med. 2013;2013:976415. doi: 10.1155/2013/976415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Shvarzbeyn J., Huleihel M. Effect of propolis and caffeic acid phenethyl ester (CAPE) on NFkappaB activation by HTLV-1 Tax. Antiviral Res. 2011;90(3):108–115. doi: 10.1016/j.antiviral.2011.03.177. [DOI] [PubMed] [Google Scholar]
- 246.Shi Z.H., Li N.G., Tang Y.P., Wei L., Lian Y., Yang J.P., Hao T., Duan J.A. Metabolism-based synthesis, biologic evaluation and SARs analysis of O-methylated analogs of quercetin as thrombin inhibitors. Eur J Med Chem. 2012;54:210–222. doi: 10.1016/j.ejmech.2012.04.044. [DOI] [PubMed] [Google Scholar]
- 247.Tsai F.J., Lin C.W., Lai C.C., Lan Y.C., Lai C.H., Hung C.H., Hsueh K.C., Lin T.H., Chang H.C., Wan L., Sheu J.J., Lin Y.J. Kaempferol inhibits enterovirus 71 replication and internal ribosome entry site (IRES) activity through FUBP and HNRP proteins. Food Chem. 2011;128(2):312–322. doi: 10.1016/j.foodchem.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 248.Min B.S., Tomiyama M., Ma C.M., Nakamura N., Hattori M. Kaempferol acetylrhamnosides from the rhizome of Dryopteris crassirhizoma and their inhibitory effects on three different activities of human immunodeficiency virus-1 reverse transcriptase. Chem Pharm Bull (Tokyo). 2001;49(5):546–550. doi: 10.1248/cpb.49.546. [DOI] [PubMed] [Google Scholar]
- 249.Behbahani M., Sayedipour S., Pourazar A., Shanehsazzadeh M. In vitro anti-HIV-1 activities of kaempferol and kaempferol-7-O-glucoside isolated from Securigera securidaca. Res Pharm Sci. 2014;9(6):463–469. [PMC free article] [PubMed] [Google Scholar]
- 250.Ganesan S., Faris A.N., Comstock A.T., Wang Q., Nanua S., Hershenson M.B., Sajjan U.S. Quercetin inhibits rhinovirus replication in vitro and in vivo. Antiviral Res. 2012;94(3):258–271. doi: 10.1016/j.antiviral.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Rojas A., Del Campo J.A., Clement S., Lemasson M., Garcia-Valdecasas M., Gil-Gomez A., Ranchal I., Bartosch B., Bautista J.D., Rosenberg A.R., Negro F., Romero-Gomez M. Effect of Quercetin on Hepatitis C Virus Life Cycle: From Viral to Host Targets. Sci Rep. 2016;6:31777. doi: 10.1038/srep31777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Cheng Z., Sun G., Guo W., Huang Y., Sun W., Zhao F., Hu K. Inhibition of hepatitis B virus replication by quercetin in human hepatoma cell lines. Virol Sin. 2015;30(4):261–268. doi: 10.1007/s12250-015-3584-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Wang G.F., Shi L.P., Ren Y.D., Liu Q.F., Liu H.F., Zhang R.J., Li Z., Zhu F.H., He P.L., Tang W., Tao P.Z., Li C., Zhao W.M., Zuo J.P. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Res. 2009;83(2):186–190. doi: 10.1016/j.antiviral.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 254.Utsunomiya H., Ichinose M., Ikeda K., Uozaki M., Morishita J., Kuwahara T., Koyama A.H., Yamasaki H. Inhibition by caffeic acid of the influenza A virus multiplication in vitro. Int J Mol Med. 2014;34(4):1020–1024. doi: 10.3892/ijmm.2014.1859. [DOI] [PubMed] [Google Scholar]
- 255.Xie Y., Huang B., Yu K., Shi F., Liu T., Xu W. Caffeic acid derivatives: a new type of influenza neuraminidase inhibitors. Bioorg Med Chem Lett. 2013;23(12):3556–3560. doi: 10.1016/j.bmcl.2013.04.033. [DOI] [PubMed] [Google Scholar]
- 256.Fesen M.R., Pommier Y., Leteurtre F., Hiroguchi S., Yung J., Kohn K.W. Inhibition of HIV-1 integrase by flavones, caffeic acid phenethyl ester (CAPE) and related compounds. Biochem Pharmacol. 1994;48(3):595–608. doi: 10.1016/0006-2952(94)90291-7. [DOI] [PubMed] [Google Scholar]