Abstract
Coronavirus disease 2019 (COVID-19) may have a metabolic origin given strong links with risk factors such as lipids and glucose and co-morbidities such as obesity and type 2 diabetes mellitus. The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein mediates viral cellular entry via the ACE2 receptor. The cytoplasmic tail of this spike protein is heavily palmitoylated. Emerging studies suggest that SARS-CoV-2 alters lipid metabolism in the lung epithelial cells by modulating peroxisome proliferator-activated receptor alpha (PPARα), possibly contributing to lipotoxicity, inflammation and untoward respiratory effects. Disruption of this process may affect palmitoylation of SARS-CoV spike protein and thus infectivity and viral assembly. COVID-19 is also increasingly being recognized as a vascular disease, with several studies noting prominent systemic endothelial dysfunction. The pathogenesis of endothelial dysfunction may also be linked to COVID-19-mediated metabolic and inflammatory effects. Herein, exercise will be compared to fenofibrate as a possible therapeutic strategy to bolster resilience against (and help manage recovery from) COVID-19. This paper will explore the hypothesis that exercise may be a useful adjuvant in a setting of COVID-19 management/rehabilitation due to its effects on PPARα and vascular endothelial function.
Keywords: Exercise, COVID-19, Vascular, Endothelium, PPARα
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) currently shows no sign of disappearing on its own. Globally, Coronavirus disease 2019 (COVID-19) cases have surpassed 21 million, contributing to over 775,000 deaths. In the United States, the CDC projects that COVID-19 will be a top 10 leading cause of death for the year 2020. While we all eagerly await the development of a vaccine, scientists and clinicians have begun exploring “off-label” use of various drugs with that hope that strategic repurposing may help manage and treat COVID-19 [1]. Fenofibrate (a peroxisome proliferator-activated receptor alpha agonist) is one such medication that holds promise given its favorable effects on inflammation and endothelial function [1]. Herein, exercise will be compared to fenofibrate as a possible therapeutic strategy to bolster resilience against (and help manage recovery from) COVID-19. This paper will explore the hypothesis that exercise may be a useful adjuvant in a setting of COVID-19 management/rehabilitation due to its effects on PPARα and vascular endothelial function.
COVID-19 progression has been suggested to have a metabolic origin given that elevated glucose and lipid levels are risk factors. The SARS-CoV-2 spike protein mediates viral cellular entry via the ACE2 receptor (please see our previous paper on the possible role of exercise as a mediator of ACE2) [2]. The cytoplasmic tail of this spike protein is heavily palmitoylated (i.e. a 16 carbon fatty acid chain is added to palmitate), a common post-translational modification that increases the hydrophobic nature of a protein [3]. Emerging studies suggest that SARS-CoV-2 alters lipid metabolism in the lung epithelial cells by modulating PPARα, possibly contributing to lipotoxicity and untoward respiratory effects [4]. PPARα belongs to the nuclear receptor (NR) family and is considered a key transcriptional factor that regulates lipid metabolism. PPARα is constitutively expressed in the lung. Not surprisingly, alveolar epithelial cells have been shown to conduct fatty acid oxidation, a function that serves a critical role in maintaining optimal lung function [5]. Disruption of this process may affect palmitoylation of SARS-CoV spike protein and thus infectivity and viral assembly [3], [4]. In response to pulmonary inflammation induced by lipopolysaccharide (LPS) or TNFα, PPARα mRNA in the lung can be reduced by 50–60% [6]. Subsequently, there may be substantial impairment of fatty acid oxidation in alveolar epithelial cells contributing to diminished bioenergetics, epithelial cell apoptosis and acute lung injury [5]. Moreover, PPARα-deficient mice have an exaggerated pulmonary inflammatory response to LPS-induced inflammation [7]. Thus reductions in PPARα from COVID-19 may be an important effector of pulmonary inflammation and mechanistically involved in the pathogenesis of acute lung injury [5].
Alveolar epithelial cells are not the only cell line important for gas exchange and pulmonary inflammatory status. Pulmonary microvascular endothelial cells also play a role in maintaining homeostasis. In its quiescent state, the endothelium is anti-inflammatory and anti-thrombotic. Emerging studies suggest that COVID-19 may be a vascular disease, causing systemic endothelial activation and dysfunction [8]. Patients presenting with COVID-19 demonstrate elevated levels of von Willebrand Factor and P-selectin with levels of thrombomodulin correlating with mortality [8]. Endothelial cells express ACE2 receptors. SARS-CoV-2 may cause endothelial cell infection, endothelialitis (i.e. inflammation of the endothelium), apoptosis/pyroptosis and ultimately microvascular dysfunction [9]. Unlike influenza, patients who die from COVID-19 associated respiratory failure present with a distinct vascular phenotype. Histologic analysis of pulmonary microvessels reveal diffuse endothelial injury and disrupted endothelial cell membranes [10]. Thus, endotheliopathy may be a consequence of and contributor to the pathogenesis of COVID-19 [8], [11]. Like alveolar epithelial cells, pulmonary endothelial cells express PPARα [12] and conduct fatty acid oxidation which parenthetically is required for endothelial cell proliferation [13]. Indeed, PPARα is considered an endogenous regulator of endothelial colony-forming cells and circulating endothelial progenitor cell fate [14]. Disruption of this process in the endothelial cell (as described above) likely leads to inflammation and cytokine production (i.e. cytokine storm syndrome), reduction of nitric oxide and impaired vascular reactivity. Alterations in pulmonary vascular reactivity may affect gas exchange (i.e. alveolar-capillary barrier disruption) and be partially responsible for ventilation-perfusion mismatches and hypoxemia seen with COVID-19 [11].
As alluded to previously, PPARα-activation has anti-inflammatory effects mainly achieved via transrepression, a process whereby pro-inflammatory genes are downregulated. As such, use of the PPARα agonists may serve a useful therapeutic role by helping to reverse the inflammatory and metabolic changes induces by SARS-CoV-2. A recent study by Ehrlich et al found that the PPARα agonist fenofibrate prevented phospholipid accumulation within SARS-CoV-2 infected cells, blocking viral replication [4] Authors concluded that disrupting the SARS-CoV-2 lifecycle with fenofibrate could prove an effective therapeutic target in the ongoing battle against COVID-19. Fenofibrate has been shown to suppress the downregulation of PPARα activation caused by inflammation, attenuate cytokine production triggered by LPS or TNFα [6], [7], and improve fatty acid oxidation, preventing acute lung injury [5]. Indeed, Fenofibrate itself has anti-inflammatory properties [15]. Fenofibrate may also have a favorable effect on vascular endothelial function. Fibrates inhibit endothelin-1 production and increase nitric oxide production [16]. Specifically, fenofibrate has been shown to suppress microvascular inflammation and apoptosis through inhibition of nuclear factor-κB and activation of adenosine monophosphate (AMP)-activated protein kinase leading to endothelial nitric oxide synthase phosphorylation and NO production [17], [18], [19], [20], [21]. It is interesting to note that although AMPK is not a canonical NR co-regulator, it interacts with NRs and is highly involved in their regulation of energy metabolism. Fenofibrate may also increase tetrahydrobiopterin levels (BH4), an essential cofactor for eNOS and ultimately NO production [22], [23]. With increases in NO bioavailability, comes improved vascular reactivity in vivo [24], [25]. Although studies examining changes in endothelial function with fenofibrate in humans have been relegated to the brachial artery, changes in brachial endothelial-dependent flow-mediated dilation correlate with changes in coronary [26] and pulmonary vascular endothelial reactivity [27], suggesting the fenofibrate may have favorable systemic endothelial effects, particularly in vascular beds impacted by COVID-19 [28], [29], [30], [31].
As can be seen, Fenofibrate holds promise as a therapeutic agent to mitigate the detrimental cardio-pulmonary damage associated with COVID-19. And with this revelation comes an important hypothesis generating question. What else can be done to possibly disrupt SARS-CoV-2 mediated lipid metabolism derangement and inflammation? In one word – exercise.
Much of the research to date on PPARs and exercise has focused on modulation of other isoforms (namely PPARγ but also PPARδ/β) or key co-regulator/co-activators (e.g. peroxisome proliferator-activated receptor γ 1α, PGC-1α) in skeletal muscle. PPARα is expressed in cardiac myocytes, hepatocytes, enterocytes, lymphocytes, monocytes, adipocytes, smooth muscle cells, and as alluded to previously endothelial cells and epithelial cells. As such, PPARα plays an important role for systemic metabolic processes (heart, kidney, central nervous system, bone, intestines, pancreas, liver, lung). While PPARγ is responsible for synthesis and storage (adipogenesis and lipid synthesis), PPARα is involved with catabolism and oxidation. Along these lines, Iemitsu et al. demonstrated that exercise training was able to improve the age-associated decrease in PPARα mRNA and protein expression in the heart while also enhancing PPARα DNA binding to PPRE (response element). In turn, there were commensurate and favorable changes in PPARα target genes related to fatty acid metabolism (β-oxidation) such as carnitine palmitoyl transferase-I (CAT) and acyl-CoA synthase, 3-hydroxyacyl CoA dehydrogenase (HAD). Similarly, Zhang et al. reported that exercise training increased PPARα mRNA expression in liver with subsequent favorable changes in target genes related to fatty acid metabolism including carnitine palmitoyl transferase 1 (CPT-1), catalase (CAT) and ATP binding cassette transporter A1 (ABCA1). Horowitz et al. studied the effect of 12 weeks of endurance exercise training on PPARα skeletal muscle protein content in the vastus lateralis of young women [32]. Results revealed that exercise training doubled levels of muscle PPARα as well as PPARα target proteins (medium-chain and very long chain acyl-CoA dehydrogenase). Schmitt et al. examined PPARα mRNA expression in the tibialis anterior of habitually endurance exercise trained and untrained young men [33]. There was a trend (p = 0.1) for PPARα mRNA concentration to be higher in exercise trained compared to sedentary muscle. There were strong correlations noted between PPARα mRNA concentration and the expression of other genes involved in oxidative metabolism (hormone sensitive lipase, fatty acid binding protein and cytochrome c oxidase I). Taken together there is a limited but provocative literature supporting a role for exercise as a modulator of PPARα in various organs/tissues.
Exercise is also well established to have ubiquitous effects on systemic endothelial function [34], [35] and inflammation [36]. For an excellent review on the anti-inflammatory effects of exercise as they relate to immunovigilance against COVID-19, please see da Silveira et al. [37]. Regular/habitual exercise increases eNOS expression/activation and BH4 bioactivity while reducing expression and/or activity of ET-1, nuclear factor-κB, and NADHP oxidase resulting in increased NO bioavailability and increased vascular reactivity [38], [39]. Exercise also increases the number of circulating endothelial progenitor cells, suggesting a milieu that favors regeneration and re-endothelialization of injured endothelium [40]. Such an environment would prove valuable in a setting of COVID-19 mediated endothelial apoptosis and endothelial cell membrane disruption. Interestingly, PPARs may be required for exercise to attenuate endothelial dysfunction [41]. Research will be needed to explicitly explore PPARα as a mediator of exercise-induced improvements in endothelial function in specific vascular beds including the pulmonary circuit.
Although studies have yet to explore the effect of exercise on PPARα in the lung, it is reasonable to speculate that mechanisms responsible for transcriptional changes in the heart and skeletal muscle would be similar in the lung. That is, the lung as a target organ is essential for mounting an optimal exercise response and delivering oxygen rich blood to the working skeletal muscle (i.e. cardio-respiratory fitness). The classic Karlman Wasserman “gear wheel model” describes the integrated exercise response as linking mitochondria, skeletal muscle, heart-blood (circulatory system) and lungs as inter-connected cogs. Increases in mechanical and metabolic factors that govern changes in PPARα in the heart and skeletal muscle may spill over to the respiratory system to ensure a concerted effort to match metabolic demand with cardio-respiratory supply. That habitual exercise training can modulate PPARα in the lung remains, at this time, a hypothesis. Conversely, there may be some redundancy between PPARα and PPARδ/β such that PPARδ/β can compensate for reductions PPARα and this may be target organ specific and differentially affected by exercise [42]. Empirical data will be needed to support (or refute) our hypothesis. Given the known effect of COVID-19 on the heart as an incendiary for cardiac damage [43], [44], aforementioned findings of changes in cardiac PPARα with exercise training may still have important implications for overall cardiovascular function and cardiovascular disease risk [45].
As alluded to previously, the cytokine storm associated with COVID-19 may lead to reductions in PPARα. These reductions may have important implications for exercise capacity. Emerging studies suggest a role for PPARα in glucose and amino acid metabolism [46]. Genes involved in gluconeogenesis have been identified as targets of PPARα and these include phosphoenolpyruvate carboxykinase (Pck1), pyruvate carboxylase (Pcx), and lactate dehydrogenase A [42]. PPARα-knock out mice exhibit hypoglycemia and lower serum lactate levels, suggesting increased reliance on anaerobic metabolic pathways to generate gluconeogenic precursors [42]. Synthesis of glycogen is also affected in PPARα-knock out mice. Not surprisingly, these PPARα knock out mice demonstrate very low aerobic exercise tolerance compared to wild-type mice [42]. It is interesting to note that survivors of SARS and MERS present with reduced exercise capacity [47]. PPARα-knock out mice also gain more weight and adipose mass compared to wild-type animals, thus reductions in PPARα α- also have implications for obesity [48]. In PPARα-knock out mice chronically fed a high-fat diet, expression of inflammatory genes in adipose tissue is more pronounced compared to wild-type mice [49]. Parenthetically, an anti-obesity role for PPARα is supported by studies in which obese rodents were administered synthetic PPARα agonists such as fenofibrate and demonstrated marked weight loss [50]. Overall, reductions in PPARα from COVID-19 may cause a downward spiral whereby altered metabolism and inflammation contributes to diminished exercise capacity, which further begets unfavorable changes in metabolism and inflammation [51]. Changes in PPARα from COVID-19 may prime the body for fatigue, inactivity and obesity. Exercise and increases in cardiorespiratory fitness may thus be needed for secondary prevention to mitigate the possibility of further disuse, chronic disease and disability [52].
Studies are beginning to emerge suggesting that COVID-19 survivors may have reduced cardiopulmonary function, with even non-hospitalized patients presenting with notable dysfunction [53], [54]. Cardiopulmonary rehabilitation may be needed to help individuals regain functional quality of life [55]. Thus, exercise may be a useful adjuvant for the management and treatment of COVID-19 survivors. Compared to emerging drugs that are being repurposed for the possible treatment of COVID-19 and its related cardiopulmonary and metabolic sequela, exercise may be on PPAR.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
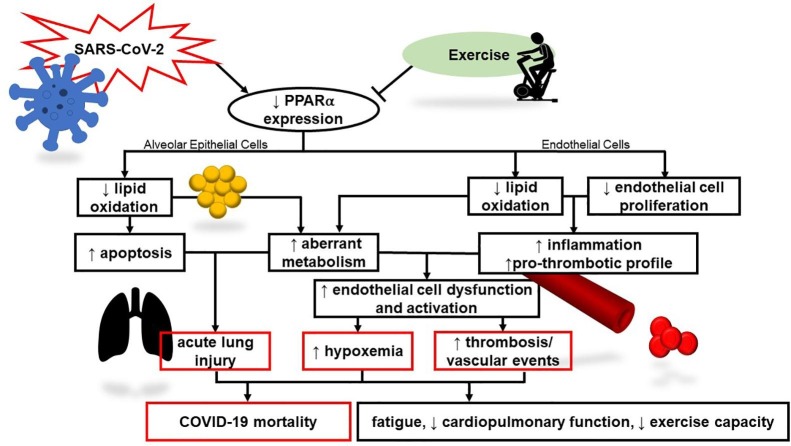
We wish to thank Sara Mascone for her assistance creating Fig. 1.
Fig. 1.
Theoretical model linking SARS-CoV-2 to COVID-19-mediated morbidity and mortality via PPARα. Viral infection may alter lipid metabolism and endothelial function contributing to inflammation and untoward systemic consequences. Exercise may have a favorable effect PPARα and is known to be anti-inflammatory and improve endothelial function.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mehy.2020.110197.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Rogosnitzky M., Berkowitz E., Jadad A.R. Delivering benefits at speed through real-world repurposing of off-patent drugs: the COVID-19 pandemic as a case in point. JMIR Public Health Surveill. 2020;6:e19199. doi: 10.2196/19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heffernan K.S., Jae S.Y. Exercise as medicine for COVID-19: An ACE in the hole? Med Hypotheses. 2020;142 doi: 10.1016/j.mehy.2020.109835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McBride C.E., Machamer C.E. Palmitoylation of SARS-CoV S protein is necessary for partitioning into detergent-resistant membranes and cell-cell fusion but not interaction with M protein. Virology. 2010;405:139–148. doi: 10.1016/j.virol.2010.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ehrlich A, Uhl S, Ioannidis K, Hofree M, tenOever BR, Nahmias Y. The SARS-CoV-2 Transcriptional Metabolic Signature in Lung Epithelium. SSRN Cell Metabolism. Available at SSRN: 10.2139/ssrn.3650499. [DOI]
- 5.Cui H., Xie N., Banerjee S., Ge J., Guo S., Liu G. Impairment of Fatty Acid Oxidation in Alveolar Epithelial Cells Mediates Acute Lung Injury. Am J Respir Cell Mol Biol. 2019;60:167–178. doi: 10.1165/rcmb.2018-0152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker J., Delayre-Orthez C., Frossard N., Pons F. Regulation of peroxisome proliferator-activated receptor-α expression during lung inflammation. Pulm Pharmacol Ther. 2008;21:324–330. doi: 10.1016/j.pupt.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Delayre-Orthez C., Becker J., Guenon I. PPARalpha downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respir Res. 2005;6:91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goshua G., Pine A.B., Meizlish M.L. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020 doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Varga Z., Flammer A.J., Steiger P. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England) 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ackermann M., Verleden S.E., Kuehnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. New Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huertas A., Montani D., Savale L. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J. 2020:2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue I., Shino K., Noji S., Awata T., Katayama S. Expression of peroxisome proliferator-activated receptor alpha (PPAR alpha) in primary cultures of human vascular endothelial cells. Biochem Biophys Res Commun. 1998;246:370–374. doi: 10.1006/bbrc.1998.8622. [DOI] [PubMed] [Google Scholar]
- 13.Schoors S., Bruning U., Missiaen R. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao Y., Chen J., Dong L.J. A Protective Effect of PPARα in Endothelial Progenitor Cells Through Regulating Metabolism. Diabetes. 2019;68:2131–2142. doi: 10.2337/db18-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu X., Otsuki M., Saito H. PPARalpha and GR differentially down-regulate the expression of nuclear factor-kappaB-responsive genes in vascular endothelial cells. Endocrinology. 2001;142:3332–3339. doi: 10.1210/endo.142.8.8340. [DOI] [PubMed] [Google Scholar]
- 16.Newaz M., Blanton A., Fidelis P., Oyekan A. NAD(P)H oxidase/nitric oxide interactions in peroxisome proliferator activated receptor (PPAR)alpha-mediated cardiovascular effects. Mutat Res. 2005;579:163–171. doi: 10.1016/j.mrfmmm.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 17.Tomizawa A., Hattori Y., Inoue T., Hattori S., Kasai K. Fenofibrate suppresses microvascular inflammation and apoptosis through adenosine monophosphate-activated protein kinase activation. Metab Clin Exp. 2011;60:513–522. doi: 10.1016/j.metabol.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 18.Murakami H., Murakami R., Kambe F. Fenofibrate activates AMPK and increases eNOS phosphorylation in HUVEC. Biochem Biophys Res Commun. 2006;341:973–978. doi: 10.1016/j.bbrc.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 19.Becker J., Delayre-Orthez C., Frossard N., Pons F. The peroxisome proliferator-activated receptor α agonist fenofibrate decreases airway reactivity to methacholine and increases endothelial nitric oxide synthase phosphorylation in mouse lung. Fundam Clin Pharmacol. 2012;26:340–346. doi: 10.1111/j.1472-8206.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- 20.Okayasu T., Tomizawa A., Suzuki K., Manaka K., Hattori Y. PPARalpha activators upregulate eNOS activity and inhibit cytokine-induced NF-kappaB activation through AMP-activated protein kinase activation. Life Sci. 2008;82:884–891. doi: 10.1016/j.lfs.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 21.Goya K., Sumitani S., Xu X. Peroxisome Proliferator-Activated Receptor α Agonists Increase Nitric Oxide Synthase Expression in Vascular Endothelial Cells. Arterioscler Thromb Vasc Biol. 2004;24:658–663. doi: 10.1161/01.ATV.0000118682.58708.78. [DOI] [PubMed] [Google Scholar]
- 22.Liu J., Lu C., Li F. PPAR-α agonist fenofibrate upregulates tetrahydrobiopterin level through increasing the expression of guanosine 5'-triphosphate cyclohydrolase-I in human umbilical vein endothelial cells. PPAR Res. 2011;2011:523520. doi: 10.1155/2011/523520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Esenboga K., Çiçek Ö F., Oktay A.A., Ayral P.A., Gürlek A. Advances in clinical and experimental medicine : official organ Wroclaw. Medical University; 2019. Effect of fenofibrate on serum nitric oxide levels in patients with hypertriglyceridemia; pp. 931–936. [DOI] [PubMed] [Google Scholar]
- 24.Walker A.E., Kaplon R.E., Lucking S.M., Russell-Nowlan M.J., Eckel R.H., Seals D.R. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension (Dallas, Tex. : 1979) 1979;60(2012):1517–1523. doi: 10.1161/HYPERTENSIONAHA.112.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sahebkar A., Giua R., Pedone C., Ray K.K., Vallejo-Vaz A.J., Costanzo L. Fibrate therapy and flow-mediated dilation: A systematic review and meta-analysis of randomized placebo-controlled trials. Pharmacol Res. 2016;111:163–179. doi: 10.1016/j.phrs.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Broxterman Ryan M., Witman Melissa A., Trinity Joel D. Strong Relationship Between Vascular Function in the Coronary and Brachial Arteries. Hypertension (Dallas, Tex. : 1979) 1979;74(2019):208–215. doi: 10.1161/HYPERTENSIONAHA.119.12881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts K.E., Heffernan K.S., Kuvin J.T., Karas R., Preston I.R., Hill N.S. Pulmonary Hypertension: Biomarkers and Translational Science. American Thoracic Society; 2011. Peripheral Vascular Endothelial Function and The Pulmonary Blood Pressure Response To Exercise In Pulmonary Hypertension, A62; p. A1995. [Google Scholar]
- 28.Wang G., He L., Liu J. Coronary flow velocity reserve is improved by PPAR-α agonist fenofibrate in patients with hypertriglyceridemia. Cardiovasc Ther. 2013;31:161–167. doi: 10.1111/j.1755-5922.2011.00307.x. [DOI] [PubMed] [Google Scholar]
- 29.Guo Q., Wang G., Namura S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab: Offi J Int Soc Cereb Blood Flow Metab. 2010;30:70–78. doi: 10.1038/jcbfm.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Vilarrupla A., Laviña B., García-Calderó H. PPARα activation improves endothelial dysfunction and reduces fibrosis and portal pressure in cirrhotic rats. J Hepatol. 2012;56:1033–1039. doi: 10.1016/j.jhep.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 31.Ouk T., Gautier S., Pétrault M. Effects of the PPAR-α agonist fenofibrate on acute and short-term consequences of brain ischemia. J Cereb Blood Flow Metab: Offi J Int Soc Cereb Blood Flow Metab. 2014;34:542–551. doi: 10.1038/jcbfm.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horowitz J.F., Leone T.C., Feng W., Kelly D.P., Klein S. Effect of endurance training on lipid metabolism in women: a potential role for PPARalpha in the metabolic response to training. Am J Physiol Endocrinol Metab. 2000;279:E348–355. doi: 10.1152/ajpendo.2000.279.2.E348. [DOI] [PubMed] [Google Scholar]
- 33.Schmitt B., Flück M., Décombaz J. Transcriptional adaptations of lipid metabolism in tibialis anterior muscle of endurance-trained athletes. Physiol Genomics. 2003;15:148–157. doi: 10.1152/physiolgenomics.00089.2003. [DOI] [PubMed] [Google Scholar]
- 34.Padilla J., Simmons G.H., Bender S.B., Arce-Esquivel A.A., Whyte J.J., Laughlin M.H. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 2011;26:132–145. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashor A.W., Lara J., Siervo M. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med (Auckland N.Z.) 2015;45:279–296. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- 36.Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 37.da Silveira M.P., da Silva Fagundes K.K., Bizuti M.R., Starck É., Rossi R.C., de Resende E.S.D.T. Physical exercise as a tool to help the immune system against COVID-19: an integrative review of the current literature. Clin Exp Med. 2020:1–14. doi: 10.1007/s10238-020-00650-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker A.E., Kaplon R.E., Pierce G.L., Nowlan M.J., Seals D.R. Prevention of age-related endothelial dysfunction by habitual aerobic exercise in healthy humans: possible role of nuclear factor κB. Clin Sci (Lond) 2014;127:645–654. doi: 10.1042/CS20140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seals D.R., Edward F. Adolph Distinguished Lecture: The remarkable anti-aging effects of aerobic exercise on systemic arteries. J Appl Physiol (Bethesda, Md. ;1985) 1985;117(2014):425–439. doi: 10.1152/japplphysiol.00362.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ribeiro F., Ribeiro I.P., Alves A.J. Effects of exercise training on endothelial progenitor cells in cardiovascular disease: a systematic review. Am J Phys Med Rehabil. 2013;92:1020–1030. doi: 10.1097/PHM.0b013e31829b4c4f. [DOI] [PubMed] [Google Scholar]
- 41.Cheang W.S., Wong W.T., Zhao L. PPARδ Is Required for Exercise to Attenuate Endoplasmic Reticulum Stress and Endothelial Dysfunction in Diabetic Mice. Diabetes. 2017;66:519–528. doi: 10.2337/db15-1657. [DOI] [PubMed] [Google Scholar]
- 42.Muoio D.M., MacLean P.S., Lang D.B. Fatty acid homeostasis and induction of lipid regulatory genes in skeletal muscles of peroxisome proliferator-activated receptor (PPAR) alpha knock-out mice. Evidence for compensatory regulation by PPAR delta. J Biol Chem. 2002;277:26089–26097. doi: 10.1074/jbc.M203997200. [DOI] [PubMed] [Google Scholar]
- 43.Heffernan K.S., Michos E.D., Gump B.B. Coronavirus Disease 2019 (COVID-19) and Cardiac Injury. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.2450. [DOI] [PubMed] [Google Scholar]
- 44.Gupta A., Madhavan M.V., Sehgal K. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Han L., Shen W.-J., Bittner S., Kraemer F.B., Azhar S. PPARs: regulators of metabolism and as therapeutic targets in cardiovascular disease. Part I: PPAR-α. Future Cardiol. 2017;13:259–278. doi: 10.2217/fca-2016-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kersten S., Mandard S., Escher P. The peroxisome proliferator-activated receptor alpha regulates amino acid metabolism. FASEB J : Off Publ Feder Am Soc Exp Biol. 2001;15:1971–1978. doi: 10.1096/fj.01-0147com. [DOI] [PubMed] [Google Scholar]
- 47.Ahmed H., Patel K., Greenwood D.C. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020;52 doi: 10.2340/16501977-2694. jrm00063. [DOI] [PubMed] [Google Scholar]
- 48.Sanchis-Gomar F., Lavie C.J., Mehra M.R., Henry B.M., Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95:1445–1453. doi: 10.1016/j.mayocp.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stec D.E., Gordon D.M., Hipp J.A. Loss of hepatic PPARα promotes inflammation and serum hyperlipidemia in diet-induced obesity. Am J Physiol Regul Integr Compar Physiol. 2019;317:R733–r745. doi: 10.1152/ajpregu.00153.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rachid T.L., Penna-de-Carvalho A., Bringhenti I., Aguila M.B., Mandarim-de-Lacerda C.A., Souza-Mello V. PPAR-α agonist elicits metabolically active brown adipocytes and weight loss in diet-induced obese mice. Cell Biochem Funct. 2015;33:249–256. doi: 10.1002/cbf.3111. [DOI] [PubMed] [Google Scholar]
- 51.Stienstra R., Duval C., Müller M., Kersten S. PPARs, obesity, and inflammation. PPAR Res. 2007;2007 doi: 10.1155/2007/95974. 95974-95974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohamed A.A., Alawna M. Role of increasing the aerobic capacity on improving the function of immune and respiratory systems in patients with coronavirus (COVID-19): A review. Diabetes Metab Syndrome. 2020;14:489–496. doi: 10.1016/j.dsx.2020.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Puntmann V.O., Carerj M.L., Wieters I. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao YM, Shang YM, Song WB et al., Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery, EClinicalMedicine. [DOI] [PMC free article] [PubMed]
- 55.Liu K., Zhang W., Yang Y., Zhang J., Li Y., Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complementary Ther Clin Pract. 2020;39 doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.