Abstract
Background
Mortality in patients with COVID-19 pneumonia and systemic hyperinflammation is high. We aimed to examine whether mavrilimumab, an anti-granulocyte–macrophage colony-stimulating factor receptor-α monoclonal antibody, added to standard management, improves clinical outcomes in patients with COVID-19 pneumonia and systemic hyperinflammation.
Methods
This single-centre prospective cohort study included patients aged 18 years or older who were admitted to San Raffaele Hospital (Milan, Italy) with severe COVID-19 pneumonia, hypoxia, and systemic hyperinflammation. Patients received a single intravenous dose (6 mg/kg) of mavrilimumab added to standard care given by the hospital at the time. The control group consisted of contemporaneous patients with similar baseline characteristics who received standard care at the same hospital. The main outcome was time to clinical improvement (defined as improvement of two or more points on the seven-point ordinal scale of clinical status). Other outcomes included proportion of patients achieving clinical improvement, survival, mechanical ventilation-free survival, and time to fever resolution. Adverse events were monitored daily.
Findings
Between March 17 and April 15, 2020, 13 non-mechanically ventilated patients (median age 57 years [IQR 52–58], 12 [92%] men) received mavrilimumab and 26 patients (median age 60 [IQR 53–67], 17 [65%] men) in the control group received standard care. During the 28-day follow-up, no patients in the mavrilimumab group died, and seven (27%) patients in the control group died (p=0·086). At day 28, all patients in the mavrilimumab group and 17 (65%) patients in the control group showed clinical improvement (p=0·030), with earlier improvement in the mavrilimumab than in the control group (mean time to improvement 8 days [IQR 5 to 11] vs 19 days [11 to >28], p=0·0001). By day 28, one (8%) patient in the mavrilimumab group progressed to mechanical ventilation compared with nine (35%) patients in the control group who progressed to mechanical ventilation or died (p=0·14). By day 14, fever resolved in ten (91%) of 11 febrile patients in the mavrilimumab group, compared with 11 (61%) of 18 febrile patients in the control group (p=0·18); fever resolution was faster in mavrilimumab recipients versus controls (median time to resolution 1 day [IQR 1 to 2] vs 7 days [3 to >14], p=0·0093). Mavrilimumab was well tolerated, with no infusion reactions. Three (12%) patients in the control group developed infectious complications.
Interpretation
Mavrilimumab treatment was associated with improved clinical outcomes compared with standard care in non-mechanically ventilated patients with severe COVID-19 pneumonia and systemic hyperinflammation. Treatment was well tolerated. Confirmation of efficacy requires controlled testing.
Funding
IRCCS San Raffaele Scientific Institute.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly since its identification in patients with severe pneumonia in Wuhan, China.1 From December, 2019, COVID-19, which is caused by SARS-CoV-2, has caused an international outbreak of respiratory illness affecting more than 6·2 million people worldwide as of June 1, 2020. Current therapy for patients with COVID-19 is limited to non-specific, supportive care; global mortality among confirmed cases (6 284 065 cases with 375 902 deaths as of June 1, 2020), primarily due to respiratory failure, is approximately 6% (for data see Johns Hopkins University & Medicine [Baltimore, MD, USA] Coronavirus Resource Center).
The toll of the COVID-19 pandemic in Italy is severe (233 197 total cases as of June 1, amounting to 3855 cases per million population, and 33 475 deaths; for data see the Coronavirus Resource Center), and patients requiring hospital-based care often outnumber available resources. Effective treatments are urgently needed to reduce the individual and societal burden of the COVID-19 pandemic. Accumulating evidence suggests that a subgroup of patients with severe COVID-19 pneumonia develop a hyperinflammatory response, similar to the cytokine storm following chimeric antigen receptor (CAR) T-cell therapy or during macrophage activation syndrome,2 and resembling secondary haemophagocytic lymphohistiocytosis,3 which can contribute to mortality. Predictors of fatality from recent studies suggest that mortality might be due to virally triggered hyperinflammation.4
Research in context.
Evidence before this study
Patients with severe COVID-19 often develop respiratory failure that necessitates admission to the intensive care unit (ICU) or mechanical ventilation. Although no systematic literature search was done, we searched MEDLINE for research articles published in English between Jan 1 and March 17, 2020, and selected key evidence. In an initial report, about a third of patients with COVID-19 required admission to the ICU, and 15% of cases were fatal. In a subsequent report of 201 patients who were admitted to hospital, 42% developed acute respiratory distress syndrome, and 52% of these patients died. Effective treatments are needed to prevent disease escalation to a critical stage. Hyperinflammation, with its excessive cytokine production (known as a cytokine storm), has been identified as a key factor of poor prognosis in patients with COVID-19-related severe pneumonia, leading to high frequencies of respiratory failure and mortality. Several anti-inflammatory approaches targeting different cytokine pathways are among the potential treatments being evaluated currently. We hypothesised that blocking granulocyte–macrophage colony-stimulating factor (GM-CSF) signalling at the receptor would offer therapeutic benefit in addition to the standard of care.
Added value of this study
This study provides preliminary data that mavrilimumab treatment was associated with greater and faster improvement in a small population of non-mechanically ventilated patients with COVID-19-related severe pneumonia, hypoxia, and hyperinflammation, compared with a contemporaneous control cohort, with earlier discharge from the hospital and no progression to death with mavrilimumab treatment.
Implications of all the available evidence
These data represent the first evidence of a treatment effect of GM-CSF inhibition in COVID-19 and support further investigation of this biologic in controlled settings.
Granulocyte–macrophage colony-stimulating factor (GM-CSF) is a cytokine with a cardinal role in modulation of inflammation. Ligand binding to the GM-CSF receptor-α (GM-CSFRα) activates multiple pro-inflammatory pathways and, in macrophages and neutrophils, results in increased secretion of pro-inflammatory cytokines, including tumor necrosis factor, interleukin (IL)-1, IL-6, IL-23, and IL-12,5 as well as stimulation of multiple downstream signalling pathways, including Janus kinase 2 (JAK2)–signal transducer and activator of transcription 5 (STAT5), the mitogen-activated protein kinase (MAPK) pathway, and the phosphoinositide 3 kinase (PI3K) pathway, all of which influence activation and differentiation of myeloid cells.6, 7
Under physiological conditions, the concentration of circulating GM-CSF is low, but concentrations are elevated in inflammatory settings. Several cell types can serve as a source of GM-CSF, including fibroblasts, endothelial cells, macrophages, dendritic cells, T cells, neutrophils, eosinophils, and tumour cells, with most production occurring locally at the site of inflammation,6 thus functioning as a feedforward inflammatory amplifier.8
In hyperinflammation, immunomodulation is likely to be beneficial. Mavrilimumab is a monoclonal antibody (human isoform lgG4) that binds to GM-CSFRα and disrupts downstream signalling.9 Mavrilimumab has shown efficacy and safety across several phase 1 and phase 2 randomised trials in patients with rheumatoid arthritis.10 In addition, a phase 2 trial in giant cell arteritis is ongoing (NCT03827018). 32 patients with rheumatoid arthritis have received single intravenous doses of up to 10 mg/kg,11 and approximately 550 patients with rheumatoid arthritis have received repeat subcutaneous doses of up to 150 mg biweekly for up to 3 years.12 On the basis of clinical trial data, mavrilimumab shows low rates of serious infections compared with other immnomodulatory therapeutics used in rheumatoid arthritis.10, 11 We aimed to investigate whether mavrilimumab, added to standard management, improves clinical outcomes in patients admitted to hospital with COVID-19 pneumonia and systemic hyperinflammation.
Methods
Study design and patients
In this single-centre, prospective cohort study, clinical data from all patients who were admitted to San Raffaele Hospital (Milan, Italy) with COVID-19 were collected daily through a specifically designed, dedicated case report form according to an institutional protocol (COVID-BioB Study, ethical committee approval number 34/int/2020, registered with ClinicalTrials.gov, NCT04318366).13
We prospectively identified non-mechanically ventilated patients for treatment with mavrilimumab who fulfilled the following criteria: patients who were aged 18 years or older and diagnosed with COVID-19 pneumonia by detection of viral sequences at quantitative RT-PCR testing (nasopharyngeal swab) and radiological findings at chest x-ray or CT scan; had acute lung injury, defined as a ratio of the partial pressure of oxygen to the fraction of inspired oxygen (PaO2:FiO2) of 300 mm Hg or less, in the presence of bilateral pulmonary infiltrates by chest radiograph or CT scan, and no clinical evidence of left atrial hypertension;14 and had hyperinflammation, defined as elevation of serum inflammation markers C-reactive protein (CRP) to 100 mg/L or more (normal range <6 mg/L) or ferritin to 900 μg/L or more (normal range 30–400 μg/L), in the presence of any increase in lactate dehydrogenase (LDH; normal range 125–220 U/L). Exclusion criteria for this protocol were: management (including mechanical ventilation) in the intensive care unit (ICU); evidence of bacterial infection; and concomitant administration of other immunosuppressive biological agents or corticosteroids. A control cohort was also assembled during the active treatment period, consisting of consecutive contemporaneous patients who received local standard of care but were not treated with mavrilimumab for several reasons (no drug availability at the time of hospital admission [42% of cases], shortage of drug [50% of cases], absence of patient's consent [8% of cases]). Patients selected for the control group were comparable for age, sex, comorbidities (tobacco smoking, arterial hypertension, coronary artery disease, diabetes, chronic obstructive pulmonary disease, dyslipidaemia, and obesity), baseline inflammatory markers (serum CRP, ferritin, and LDH), and respiratory dysfunction (PaO2:FiO2, need for non-invasive ventilation, high-flow oxygen, and low-flow oxygen). The clinical outcomes of these patients were not known at the time of their selection for the control cohort.
The institutional review board approved the treatment protocol, administered under expanded access criteria, and all patients provided written informed consent.
Procedures
Patients received standard care given by the hospital at the time the protocol was conducted. All patients who were admitted to hospital with COVID-19 pneumonia received on admission treatment with oral hydroxychloroquine (200 mg twice a day), intravenous azithromycin (500 mg once daily until patient tested negative for urine antigen for Legionella pneumophila), oral lopinavir–ritonavir (400 mg and 100 mg, respectively, twice a day), and respiratory support with supplemental oxygen or non-invasive ventilation with continuous positive airway pressure (with a positive end expiratory pressure of 10 cm of water).
Mavrilimumab (provided by Kiniksa Pharmaceuticals, Lexington, MA, USA) was administered intravenously as a single dose of 6 mg/kg. The dose rationale, and by extension the intravenous route of administration for patients with COVID-19, was based on a combination of data from previous safety and efficacy evaluation of single and multiple doses in patients with rheumatoid arthritis, and the assessment of mavrilimumab lung distribution and pharmacodynamic effects in mice.15, 16 An extrapolation of these findings taken together with the known pathophysiology of COVID-19, in particular lung disease and hyperinflammation, led to the selected dose for this protocol.
Treatment with mavrilimumab (or follow-up for the control group) started when patients met the case definition for COVID-19 pneumonia with systemic hyperinflammation. The same medical team managed the patients in the mavrilimumab group and those in the control group.
Data on patients' clinical outcomes were evaluated from first fulfilment of eligibility criteria and for the following 28 days. Specifically, baseline was the day on which treatment with mavrilimumab was started for patients and the day of first fulfilment of eligibility criteria for controls. Clinical status, oxygen saturation (SaO2), PaO2, FiO2, PaO2:FiO2, axillary temperature, and CRP were assessed until discharge from hospital, day 28 of hospital stay, ICU admission, or death, whichever came first. Derived PaO2:FiO2 was calculated according to the formula: SaO2:FiO2=64 + 0·84 × (PaO2:FiO2).17 Serum CRP was evaluated on a near-daily basis, as indicated per clinical judgement. Repeated imaging of the chest (x-ray or CT scan) was performed in some patients as indicated as part of the monitoring of patients with pneumonia. Clinical status was assessed by a seven-point ordinal scale used in previous studies of patients admitted to hospital with severe influenza and COVID-19 and recommended by the WHO R&D Blueprint Group,18 which consists of the following numeric scale and scale descriptors: (1) patient discharged from the hospital; (2) hospitalisation, not requiring supplemental oxygen, no longer requiring ongoing medical care for COVID-19; (3) hospitalisation, not requiring supplemental oxygen, requiring ongoing medical care (COVID-19 related or otherwise); (4) hospitalisation, requiring supplemental low-flow oxygen therapy (FiO2 ≤35%); (5) hospitalisation, requiring nasal high-flow oxygen therapy (FiO2 ≥40%), non-invasive mechanical ventilation, or both; (6) hospitalisation, requiring invasive mechanical ventilation; and (7) death. The main outcome was time to clinical improvement (defined as improvement of two or more points on the seven-point ordinal scale for clinical assessment). Other clinical secondary outcomes were time to discharge from hospital, the proportion of patients reaching a score of one or two on the seven-point ordinal scale for clinical assessment, the proportion of patients without fever, time to resolution of fever without need for antipyretics for at least 48 h, overall survival, mechanical ventilation-free survival, serum CRP, and the PaO2:FiO2 ratio.
Monitoring of adverse events included daily clinical examination with vitals and blood tests; blood, sputum, and urine cultures were performed, as clinically indicated.
Statistical analysis
Continuous variables are reported as median (IQR) according to the distribution of the data, and categorical variables are reported as number and percentage. In the univariate analysis, we analysed categorical variables with Fisher's exact test (by doubling the exact one-tailed probability) or the Cochran-Mantel-Haenszel test. We assessed the time to main outcome after all patients had reached day 28, with failure to reach the outcome or mechanical ventilation or death before day 28 considered as right-censored at day 28 (right-censoring occurs when an event might have occurred after the last time a person was under observation, but the specific timing of the event is unknown). For time to fever resolution, we only analysed the first 14 days of data because fever resolution later than 14 days seemed to be clinically irrelevant in this setting; if present, fever was likely associated with another event, different from SARS-CoV-2 infection (ie, another superimposed infection). The time to main outcome was portrayed by Kaplan-Meier plots, and curves were compared with a log-rank test. Similarly, secondary endpoints were portrayed by Kaplan-Meier plots and compared with a log-rank test, with the exception of survival. Because there was no censoring before 28 days and no deaths in the mavrilimumab group, we used Fisher's exact test in preference to the log-rank test, which is based on asymptotic theory. We estimated median survivals from the Kaplan-Meier plots with accompanying 95% CIs. Statistical significance was defined as a p value of 0·05 or less. Data were analysed with SAS (version 9.4).
Role of the funding source
The funder of the study had no direct role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Between March 17 and April 15, 2020, 13 patients (median age 57 years [IQR 52–58], 12 [92%] men) with COVID-19 pneumonia and systemic hyperinflammation were treated with mavrilimumab and 26 contemporaneous control patients (median age 60 [IQR 53–67], 17 [65%] men; table 1 ) with COVID-19 pneumonia and systemic hyperinflammation were given standard care. Demographic and clinical characteristics of patients are summarised in table 1 and in the appendix 2 (p 1).
Table 1.
Demographic and baseline clinical characteristics
| Mavrilimumab group (n=13) | Control group (n=26) | p value* | ||
|---|---|---|---|---|
| Age, years | 57 (52–58) | 60 (53–67) | 0·19 | |
| Sex | .. | .. | 0·14 | |
| Male | 12 (92%) | 17 (65%) | .. | |
| Female | 1 (8%) | 9 (35%) | .. | |
| PaO2:FiO2 ratio, mm Hg | 196 (167–215) | 217 (138–258) | 0·43 | |
| PaO2:FiO2ratio | .. | .. | 0·48 | |
| PaO2:FiO2 200–300 mm Hg | 6 (46%) | 14 (54%) | ||
| PaO2:FiO2 100–199 mm Hg | 6 (46%) | 9 (35%) | .. | |
| PaO2:FiO2 <100 mm Hg | 1 (8%) | 3 (12%) | .. | |
| Respiratory support | .. | .. | 0·75 | |
| Low-flow oxygen† | 4 (31%) | 11 (42%) | .. | |
| High-flow oxygen‡ | 6 (46%) | 9 (35%) | .. | |
| Non-invasive ventilation with continuous positive airway pressure‡ | 3 (23%) | 6 (23%) | .. | |
| Patients with fever | 11 (85%) | 18 (69%) | 0·53 | |
| Fever duration, days | 11 (10–12) | 7 (4–10) | 0·0038 | |
| Duration of hospital stay before enrolment, days | 2 (1–2) | 1 (1–2) | 0·33 | |
| C-reactive protein, mg/L | 152 (100–177) | 123 (77–190) | 0·77 | |
| Lactate dehydrogenase, U/L | 420 (377–505) | 467 (354–522) | 0·72 | |
| Ferritin, μg/L | 2302 (1040–3217) | 1269 (854–3369) | 0·70 | |
| Interleukin-6, pg/L§ | 40 (28–60) | 47 (36–98) | 0·26 | |
| Lymphocyte count, cells per μL | 800 (700–1000) | 1050 (700–1300) | 0·16 | |
| Platelet count, cells per μL | 252 000 (190 000–285 000) | 222 500 (166 000–296 000) | 0·56 | |
Data are median (IQR) or n (%). PaO2:FiO2=partial pressure of oxygen:fraction of inspired oxygen.
Wilcoxon rank sum test was used for continuous variables. Fisher's exact test by doubling the one-sided p value was used for binary variables. Cochran-Mantel-Haenszel test with 1 degree of freedom was used to test the PaO2:FiO2 ratio, which has three ordinal categories. Cochran-Mantel-Haenszel test for testing general association was used to test respiratory function, which has three categories.
Corresponding to a score of four on the seven-point ordinal scale.
Corresponding to a score of five on the seven-point ordinal scale.
Baseline interleukin-6 concentrations were available for eight of 13 patients in the mavrilimumab group and 12 of 26 controls only.
11 (85%) patients in the mavrilimumab group and 18 (69%) patients in the control group were febrile. Considering patients who were dependent on supplemental oxygen at baseline, four (31%) patients in the mavrilimumab group and 11 (42%) patients in the control group were on supplemental low-flow oxygen (FiO2 ≤35%, corresponding to category four on the seven-point ordinal scale), six (46%) patients in the mavrilimumab group and nine (35%) patients in the control group were on high-flow oxygen (FiO2 ≥40% and not on non-invasive ventilation, corresponding to category five on the seven-point ordinal scale), and three (23%) patients in the mavrilimumab group and six (23%) patients in the control group were on non-invasive ventilation (corresponding to category five on the seven-point ordinal scale; p=0·75; table 1). The median PaO2:FiO2 ratio was comparably low across groups. No patients were on mechanical ventilation at baseline.
During the 28-day follow-up period, no patients in the mavrilimumab died, and seven (27%) patients in the control group died (Fisher's exact test: p=0·086; figure 1A ). All deaths occurred in patients with severe respiratory failure, defined as a score of higher than four on the seven-point ordinal scale (Cochran-Mantel-Haenszel test: p=0·0094 for the effect of baseline score on death, after stratification for treatment group); six (86%) of seven deaths occurred during the first week of follow-up, and the remaining death occurred on day 8.
Figure 1.
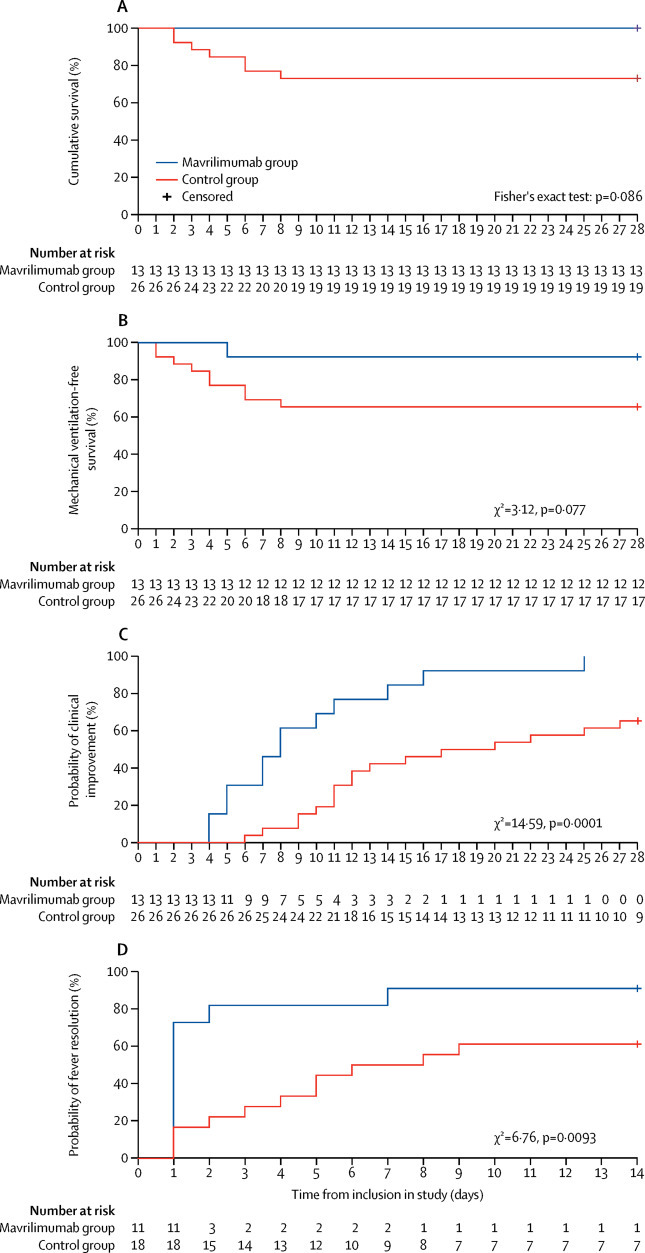
Clinical outcome measures in the mavrilimumab group versus the control group
(A) Cumulative survival estimated by a Kaplan-Meier curve at 28 days and compared with a Fisher's exact test. (B) Mechanical ventilation-free survival estimated by a Kaplan-Meier curve and compared with a log-rank test. (C) Time to clinical improvement estimated by a Kaplan-Meier curve and compared with a log-rank test. (D) Time to fever resolution estimated by a Kaplan-Meier curve and compared with a log-rank test.
At 28 days of follow-up, all patients in the mavrilimumab group and 17 (65%) patients in the control group had shown a clinical improvement of two or more points on the seven-point ordinal scale (p=0·030; table 2 ). One (8%) patient in the mavrilimumab group progressed to mechanical ventilation compared with nine (35%) patients in the control group who progressed to mechanical ventilation or died (p=0·14; table 2; figure 1B). The mavrilimumab-treated patient who progressed to mechanical ventilation achieved clinical improvement within the 28-day observation period. Mechanical ventilation-free survival was not significantly different between the mavrilimumab group and the control group (figure 1B).
Table 2.
Follow-up data of patients treated with mavrilimumab and the control group at day 28
| Mavrilimumab group (n=13) | Control group (n=26) | p value* | |
|---|---|---|---|
| Clinical improvement† | 13 (100%) | 17 (65%) | 0·030 |
| Days to clinical improvement‡ | 8 (5 to 11) | 19 (11 to >28) | 0·0001 |
| Days to discharge from hospital | 10 (9 to 12) | 20 (12 to >28) | 0.0030 |
| Days to resolution of fever in the first 2 weeks | 1 (1 to 2) | 7 (3 to >14) | 0·0093 |
| Fever resolution by day 14§ | 10 (91%) | 11 (61%) | 0·18 |
| Mechanical ventilation or death | 1 (8%) | 9 (35%) | 0·14 |
| Death | 0 (0%) | 7 (27%) | 0·086 |
| CRP reduction ≥75% | 11 (85%) | 11/25 (44%)¶ | 0·035 |
Data are n (%) or median (IQR).
Proportions were tested using Fisher's exact test by doubling the one-sided p value; time to event variables were analysed using the log-rank test.
Clinical improvement defined by live discharge from the hospital, improvement of at least two points from baseline on a modified seven-point ordinal scale (as recommended by the WHO R&D Blueprint Group), or both.
Patients who died are censored on day 28.
Fever resolution calculated on patients who were febrile at day 0—ie, 11 patients treated with mavrilimumab and 18 patients in the control group.
Only patients with post-baseline assessments were included in the analysis.
Notably, patients treated with mavrilimumab reached the clinical improvement endpoint in significantly fewer days than did the control group (median 8 days [IQR 5 to 11] days vs 19 days [11 to >28]), as demonstrated by the Kaplan-Meier plot (χ2=14·59, p=0·0001; figure 1C; table 2).
Accordingly, mavrilimumab treatment was associated with earlier discharge from hospital than was standard care (median 10 days [IQR 9 to 12] vs 20 days [12 to >28] days, p=0·0030; table 2). Changes in clinical status of individual patients are shown in figure 2 .
Figure 2.
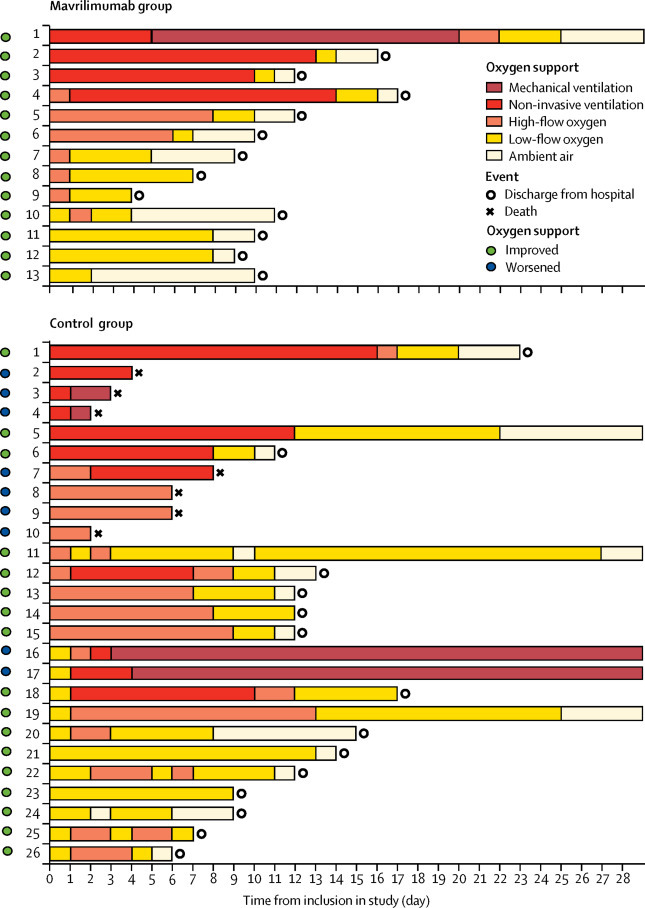
Changes in clinical status and oxygen support from baseline in individual patients
Baseline (day 0) was the day on which treatment with mavrilimumab was started for patients, and the day of first fulfilment of eligibility criteria for controls. A patient's status improved if the oxygen-support status improved by at least two points on a seven-point scale before day 28 or if the patient was discharged.
At day 28, the median increase in PaO2:FiO2 from baseline was higher in the mavrilimumab group than in the control group (275 mm Hg [IQR 202 to 313] vs 175 mm Hg [–63 to 287]), based on ANCOVA with baseline value as the covariate on log scale (p=0·026). All patients in the mavrilimumab group showed an improvement in PaO2:FiO2 by 25% or more, compared with 17 (65%) patients in the control group (p=0·030). All patients in the mavrilimumab group had a PaO2:FiO2 of 300 mm Hg or more at the last available follow-up, compared with 17 (65%) patients in the control group (p=0·030).
The improvement in respiratory function in patients treated with mavrilimumab was parallelled by reduction in serum CRP (last available median CRP 8 mg/L [6–28] vs 52 mg/L [14–141] in the control group; p=0·0068), corresponding to a CRP reduction of at least 75% in 11 (85%) patients in the mavrilimumab group and 11 (44%) of 25 patients with post-baseline assessments in the control group (p=0·035; table 2; see also appendix 2 p 4).
By day 14, fever resolved in ten (91%) of 11 febrile patients in the mavrilimumab group, compared with 11 (61%) of 18 febrile patients in the control group (p=0·18); time to resolution of fever was significantly shorter in mavrilimumab-treated patients than in the control group (median 1 day [IQR 1 to 2] vs 7 days [3 to >14]; χ2=6·76, p=0·0093; figure 1D).
As part of the monitoring of patients with pneumonia, CT scans were done on patients to assess radiological evolution of the disease. Imaging obtained at baseline and discharge for two representative patients treated with mavrilimumab showed significant improvement in lung opacification (figure 3 ), consistent with the overall improvement in their clinical status.
Figure 3.
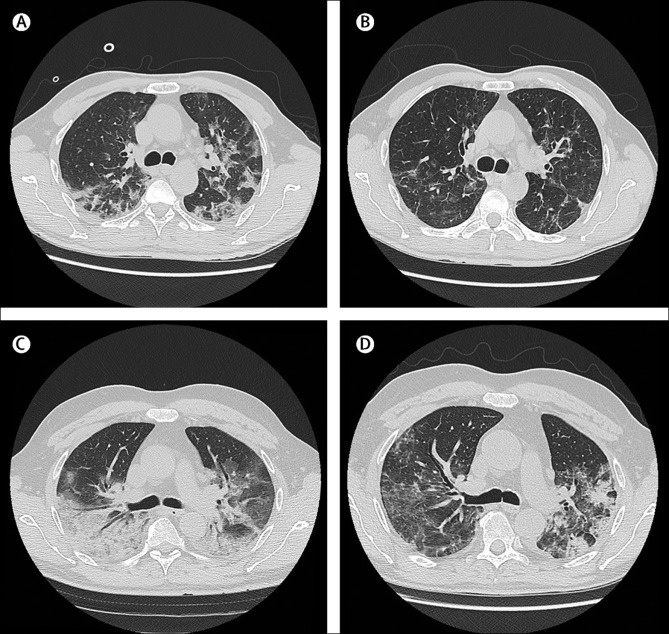
Radiographic findings in two patients in the mavrilimumab group
Lung CT scans of a man aged 58 years at day 0 (A) and at discharge from hospital on day 7 (B). At day 0, the patients was febrile, receiving oxygen through a face mask, with fraction of inspired oxygen (FiO2) of 0·4, partial pressure of oxygen (PaO2) of 86 mm Hg, lactate dehydrogenase (LDH) concentration of 374 U/L, and C-reactive protein (CRP) concentration of 100 mg/L. The day 0 lung CT scan shows presence of bilateral, blurred ground-glass opacities with crazy paving pattern and small dense consolidation areas. The CT scan at discharge (afebrile, on room air, oxygen saturation of 98%, LDH normalised, and CRP concentration of 12·5 mg/L), shows reduction and regression of these findings. Lung CT scans of a man aged 56 years at day 0 (C) and at discharge from hospital on day 14 (D). At day 0, the patient was febrile, receiving high-flow oxygen through a face mask with reservoir bag and continuous positive airway pressure 12 h per day, PaO2 of 176 mm Hg, LDH concentration of 944 U/L, and CRP concentration of 177 mg/L. The day 0 lung CT scan shows extensive involvement of the right lung with a posterior large consolidation area and aerial bronchogram; ground-glass opacities and crazy paving pattern are predominant on the left side. The CT scan at discharge (afebrile, on room air, oxygen saturation of 98%, LDH normalised, and CRP concentration of 28·2 mg/L), shows improvement in lung involvement.
Because it is currently under debate whether elevated D-dimer levels could be associated with a worse outcome in COVID-19,19, 20 we explored the available data in our patient population of 39 patients. However, since the D-dimer testing was not a part of our standard of care at the time of the treatment protocol, data were not available for 15 (38%) of the patients. Within the limited available data, the D-dimer values at baseline were median 0·8 μg/mL (IQR 0·4–1·5) in the mavrilimumab-treated patients and 1·7 μg/mL (1·4–3·9) in the control group. A further analysis showed that, in patients with available measurements, the level of D-dimer at baseline did not appear to affect the observed treatment effect (data not shown).
Mavrilimumab treatment was well tolerated in all patients, without infusion reactions. We did not observe any cases of neutropenia. An increase in CRP, white blood cells, and serum procalcitonin was observed in one patient treated with mavrilimumab, and this patient was admitted to the ICU 3 days after infusion. Empirical antibiotic treatment was started; however, microbiological cultures of blood and urine obtained before antibiotic treatment remained negative. Three (12%) patients in the control group developed infectious complications.
Discussion
These data suggest that administration of mavrilimumab in patients admitted to hospital with COVID-19 pneumonia and hyperinflammation improved clinical outcomes compared with local standard care only. Over the course of 28 days of follow-up, mavrilimumab treatment was associated with superior and earlier clinical improvements in respiratory parameters, faster resolution of inflammation, and fewer deaths compared with standard care. The improvement in respiratory outcomes with mavrilimumab resulted in earlier weaning from supplemental oxygen and in shorter hospital stays than with standard care alone. All mavrilimumab-treated patients attained clinical improvement, and none died during follow-up; conversely, 27% of control patients died. Mortality in the control group in our study is in line with that emerging from previous studies in similar clinical settings, considering patients with hyperinflammatory features.4, 21 This mortality rate is not surprisingly higher than that emerging from recent reports of patients admitted to hospital in the New York City area (NY, USA); this latest assessment was probably diluted by the inclusion of patients with COVID-19 without hyperinflammatory features.22 Patients in the control group died primarily during the first 8 days after enrolment (which was, in both groups, the first day that evidence for hyperinflammation was present). Considering a median disease duration of 6 days before baseline, the identified peak of death for patients in the control group is in line with the earliest peak of death initially described by authors from Wuhan.21 These data indirectly emphasise the cardinal role of rampant inflammation in early mortality and strengthen the rationale for immunomodulation in hyperinflammatory settings. Mavrilimumab was well tolerated in all patients.
The pathogenesis of COVID-19 pneumonia involves a maladaptive, detrimental inflammatory response in the lungs. Post-mortem studies of patients with COVID-19 revealed inflammatory exudates and rich infiltration of neutrophils and myeloid cells in air spaces.23 Detrimental inflammation in the lungs is parallelled by elevations in serum CRP and ferritin, which are markers of disease severity.4, 21
GM-CSF is a cytokine with a cardinal role in innate inflammation and is a potential mediator of the cytokine storm.7 The concentration of circulating GM-CSF is low under physiological conditions, and increases in inflammatory settings, being produced by several cell types at the site of inflammation and functioning as a feedforward inflammatory amplifier.6, 8 Moreover, GM-CSF regulates pulmonary surfactant homoeostasis and alveolar macrophage-mediated innate host defence.24
Mavrilimumab is an anti-GM-CSF-Rα monoclonal antibody, which inhibits the GM-CSF signalling axis in granulocytes and myeloid cells. In previous phase 2 studies in patients with rheumatoid arthritis, mavrilimumab dampened inflammation, improved clinical outcomes, and was well tolerated.9, 10 Notably, in clinical studies with mavrilimumab, there was no causal association apparent between administration of mavrilimumab and clinically significant respiratory disease. This finding is of great clinical importance considering the observation that high levels of autoantibodies against GM-CSF, as well as mutation of the α or β subunits of the GM-CSFR, have been associated with idiopathic and hereditary pulmonary alveolar proteinosis, respectively.25
Several anticytokine biological agents have the potential to dampen detrimental inflammation in COVID-19.26, 27 However, we theorised that inhibition of inflammatory cascades upstream could yield robust results. Mavrilimumab inhibits a cardinal pathway of granulocytes and myeloid cells upstream, thus quenching downstream production of myriad pro-inflammatory mediators involved in the pathogenesis of COVID-19. These preliminary results represent the first evidence of attenuation of hyperinflammation in COVID-19 pneumonia by inhibition of the GM-CSF pathway.
The findings reported in this study need to be considered in the light of several important limitations. The protocol design was the best possible under truly dramatic circumstances: quick access to a potentially life-saving medication was prioritised over investigational setup to quickly identify whether an efficacy signal could be perceived. As a consequence, patients could not be randomly assigned to receive mavrilimumab or the institutional standard of care; rather, patients who were contemporaneously admitted to hospital and who did not receive mavrilimumab due to several reasons (eg, no drug availability or shortage of drug and absence of patient consent) and with similar baseline characteristics were identified at time of hospital admission to serve as a contemporaneous control group, and their outcomes were compared prospectively to the clinical outcomes of patients receiving mavrilimumab. That being said, the absence of a pre-established randomisation process can nevertheless introduce risks for selection bias, treatment bias, or placebo effect. Furthermore, other clinical variables besides mavrilimumab treatment might have affected clinical outcomes, despite the matching of baseline demographics and clinical characteristics (appendix 2 pp 1–3). It should be noted that patients who received mavrilimumab had had a longer fever duration before enrolment than had control patients: this characteristic might have differentially affected clinical outcomes; however, any potential difference in disease stage would be small, especially considering the comparable number of days of hospital stay between groups. Additionally, the mavrilimumab group had a non-significant male predominance compared with the control group; however, women in general have better outcomes than men, providing a bias, if any, against mavrilimumab treatment.28
The relatively short follow-up of 28 days, although focused on outcome data that could be easily collected during the hospital stay, is inherently limited for longer-term efficacy and safety conclusions, even while acknowledging that near-term results with respect to the survival of patients treated with mavrilimumab were encouraging. This is the first evaluation of a novel therapeutic strategy in a setting overwhelmed by the COVID-19 pandemic, in order to tackle cogent and immediate clinical needs. Although these initial findings should be confirmed in subsequent placebo-controlled studies, dampening of hyperinflammation with mavrilimumab seems to have the potential to be beneficial for COVID-19.29
Understanding the limitations of these preliminary data, patients treated with mavrilimumab showed greater and faster improvement than did a control cohort receiving standard management. These encouraging preliminary results represent the first evidence of a treatment effect in COVID-19 with GM-CSF inhibition; further testing in controlled trials is warranted, and multicentre, double-blind, randomised, placebo-controlled studies are planned on the basis of the signal obtained here.
Data sharing
The individual anonymised data supporting the analyses contained in this manuscript are available to the editors for independent verification of the analyses as needed. Individual anonymised data supporting figure 2 can be made available to the public upon written request to the corresponding author, specifying the purpose of the request and planned analyses, if any.
Acknowledgments
Acknowledgments
This expanded access treatment protocol was funded by the San Raffaele Scientific Institute (Milan, Italy). Kiniksa Pharmaceuticals provided the drug and assisted by sharing pre-existing data on mavrilimumab, providing the Pharmacy Manual and the Investigator Brochure available for the ongoing phase 2 trial of mavrilimumab in Giant Cell Arteritis. We thank Kiniksa Pharmaceuticals for providing mavrilimumab and the following Kiniksa team members for their contributions: Fang Fang, Steven Chang, and Ben Hagberg for their advice in data analytics, Dave Nichols and Jeannie Celiberti for coordination of drug supply, Emmanuelle Hugentobler for assistance in manuscript preparation, and Randy Perrin, who facilitated the expanded access treatment protocol. We dedicate this work to the memory of health-care workers who have given their lives in the care of patients with COVID-19.
Contributors
GDL and LD conceptualised the study. AZ, MT, FC, and LD supervised the study. GDL, JFP, and LD were involved in preparing the protocol of the study. GDL, GC, CC, ED-T, and LD were involved in the clinical care of the patients. LD was responsible for funding acquisition. GDL, GC, CC, ED-T, PA, AT, NB, ST, FM, NF, PR-Q, AR, TD'A, PS, GL, FDC, and LD were involved in data curation. GDL, GC, JFP, BCT, and LD did formal analysis of data. GDL and LD were responsible for project administration. GDL, GC, and LD prepared the original draft of the manuscript. All authors were involved in writing, reviewing, and editing of the manuscript.
Declaration of interests
JFP reports compensation from Kiniksa Pharmaceuticals as an employee during the conduct of the protocol and outside the submitted work, and is an inventor on patent applications related to mavrilimumab. BCT has served as a consultant to Kiniksa Pharmaceuticals but declared no other competing interests. The other authors declare no competing interests.
Supplementary Materials
References
- 1.Chen N, Zhou M, Dong X. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burn TN, Weaver L, Rood JE. Genetic deficiency of interferon-γ reveals interferon-γ-independent manifestations of murine hemophagocytic lymphohistiocytosis. Arthritis Rheumatol. 2020;72:335–347. doi: 10.1002/art.41076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton JA. GM-CSF-dependent inflammatory pathways. Front Immunol. 2019;10 doi: 10.3389/fimmu.2019.02055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton JA. GM-CSF in inflammation and autoimmunity. Trends Immunol. 2002;23:403–408. doi: 10.1016/s1471-4906(02)02260-3. [DOI] [PubMed] [Google Scholar]
- 7.Shiomi A, Usui T. Pivotal roles of GM-CSF in autoimmunity and inflammation. Mediators Inflamm. 2015;2015 doi: 10.1155/2015/568543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu X, Han M, Li T. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinblatt ME, McInnes IB, Kremer JM. A randomized phase IIb study of mavrilimumab and golimumab in rheumatoid arthritis. Arthritis Rheumatol. 2018;70:49–59. doi: 10.1002/art.40323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burmester GR, Feist E, Sleeman MA, Wang B, White B, Magrini F. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor-α, in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann Rheum Dis. 2011;70:1542–1549. doi: 10.1136/ard.2010.146225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leon L, Penuelas M, Candel FJ. Indicator opportunistic infections after biological treatment in rheumatoid arthritis, 10 years follow-up in a real-world setting. Ther Adv Musculoskelet Dis. 2019;11 doi: 10.1177/1759720X19878004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crotti C, Biggioggero M, Becciolini A, Agape E, Favalli EG. Mavrilimumab: a unique insight and update on the current status in the treatment of rheumatoid arthritis. Expert Opin Investig Drugs. 2019;28:573–581. doi: 10.1080/13543784.2019.1631795. [DOI] [PubMed] [Google Scholar]
- 13.Zangrillo A, Beretta L, Silvani P. Fast reshaping of intensive care unit facilities in a large metropolitan hospital in Milan, Italy: facing the COVID-19 pandemic emergency. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov1. published online April 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 15.Campbell J, Nys J, Eghobamien L, Cohen ES, Robinson MJ, Sleeman MA. Pulmonary pharmacodynamics of an anti-GM-CSFRα antibody enables therapeutic dosing that limits exposure in the lung. MAbs. 2016;8:1398–1406. doi: 10.1080/19420862.2016.1215790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang B, Lau YY, Liang M. Mechanistic modeling of antigen sink effect for mavrilimumab following intravenous administration in patients with rheumatoid arthritis. J Clin Pharmacol. 2012;52:1150–1161. doi: 10.1177/0091270011412964. [DOI] [PubMed] [Google Scholar]
- 17.Rice TW, Wheeler AP, Bernard GR, Hayden DL, Schoenfeld DA, Ware LB. Comparison of the SpO2/FIO2 ratio and the PaO2/FIO2 ratio in patients with acute lung injury or ARDS. Chest. 2007;132:410–417. doi: 10.1378/chest.07-0617. [DOI] [PubMed] [Google Scholar]
- 18.WHO R&D Blueprint and COVID-19. 2020. https://www.who.int/teams/blueprint/covid-19
- 19.Gris JC, Quéré I, Pérez-Martin A, Lefrant JY, Sotto A. Uncertainties on the prognostic value of D-dimers in COVID-19 patients. J Thromb Haemost. 2020 doi: 10.1111/jth.14876. published online April 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Yan X, Fan Q. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020 doi: 10.1111/jth.14859. published online April 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goyal P, Choi JJ, Pinheiro LC. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020 doi: 10.1056/NEJMc2010419. published online April 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Shi L, Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapnell BC, Whitsett JA. GM-CSF regulates pulmonary surfactant homeostasis and alveolar macrophage-mediated innate host defense. Annu Rev Physiol. 2002;64:775–802. doi: 10.1146/annurev.physiol.64.090601.113847. [DOI] [PubMed] [Google Scholar]
- 25.Trapnell BC, Whitsett JA, Nakata K. Pulmonary alveolar proteinosis. N Engl J Med. 2003;349:2527–2539. doi: 10.1056/NEJMra023226. [DOI] [PubMed] [Google Scholar]
- 26.Campochiaro C, Della-Torre E, Cavalli G. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020 doi: 10.1016/j.ejim.2020.05.021. published online May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavalli G, De Luca G, Campochiaro C. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wenham C, Smith J, Morgan R. COVID-19: the gendered impacts of the outbreak. Lancet. 2020;395:846–848. doi: 10.1016/S0140-6736(20)30526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rouce RH. The earlier the better: timely mitigation of CRS. Blood. 2019;134:2119–2120. doi: 10.1182/blood.2019003618. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The individual anonymised data supporting the analyses contained in this manuscript are available to the editors for independent verification of the analyses as needed. Individual anonymised data supporting figure 2 can be made available to the public upon written request to the corresponding author, specifying the purpose of the request and planned analyses, if any.


