Abstract
Background:
The goat is a multi-purpose animal producing meat, milk, hide, fiber and manure, and hence plays a significant role in providing supplementary income and livelihood to the huge number of asset-poor ranchers and landless workers of rural India. Aims: The present study is aimed to investigate the in vitro effects of prostaglandins E2 (PGE2) and F2α (PGF2α) on ovarian granulosa cells of a goat.
Methods:
The healthy, slightly atretic and atretic antral follicles of goats with diameter ranging from 3-8 mm were cultured with PGE2 and PGF2α (1.0 μg/ml) along with control for 24 h. Histomorphological analysis was done in order to study the prostaglandins induced changes in granulosa cells of all the three categories of follicles. The acridine orange (AO) and methylene blue (MB) staining were used to study the apoptotic index in all the three categories of follicles and the collected data were analyzed by ANOVA with Duncan post Hoc test.
Results:
Prostaglandins E2 revealed positive while PGF2α showed a negative effect on the rescue of apoptosis in granulosa cells. The PGE2 treated follicles revealed a reduction in the attributes of apoptosis in granulosa cells while PGF2α showed an increased in the apoptotic characteristics.
Conclusion:
The present study depicted that granulosa cell viability in vitro is dependent on the continuous supply of survival and growth factors and prostaglandin of E series synthesis is a crucial step in the suppression of granulosa cell apoptosis while that of F series induces apoptosis.
Key Words: Apoptosis, Granulosa cells, Prostaglandins, Ovaries
Introduction
Follicular atresia in the ovary is a degenerative process that involves apoptosis of granulosa cells and is regulated by gonadotropins, steroids, cytokines and growth factors (Manchanda et al., 2001 ▶). Most of the follicles (<5 mm in diameter) undergo atresia and regression during the follicular development and the follicles that attain >5 mm in diameter are destined to ovulate in goats and sheep. The regulation of follicular atresia by gonadotropins is well understood but little is known about its control by non-gonadotropin signals (Manchanda et al., 2001 ▶).
Prostaglandins are cyclic carboxylic acids, derived from arachidonic acid or closely related fatty acids which have potent and diverse physiological actions. The role of prostaglandins in the governance of follicular function of the ovary was first speculated based on sustaining that prohibition of prostaglandin synthesis by aspirin and indomethacin results in blocking of ovulation in rats (Armstrong and Grinwich, 1972 ▶; Orcyzk and Behrman, 1972 ▶). Similar findings were further observed in other species, including rabbits (Grinwich et al., 1972 ▶; O’Grady et al., 1972 ▶), mice (Lau et al., 1974 ▶), goldfish (Stacey and Pandey, 1975 ▶), rhesus and marmoset monkeys (Wallach et al., 1975 ▶; Maia et al., 1978 ▶), and pigs (Ainsworth et al., 1979 ▶). Another clue for the role of prostaglandins at the follicular level was supported by the findings that intra-follicular levels of prostaglandins of both the series i.e. E and F, elevated considerably in these species shortly before ovulation (Armstrong et al., 1974 ▶; Yang et al., 1974 ▶; Ainsworth et al., 1975 ▶; Bauminger and Lindner, 1975 ▶; Tsang et al., 1979 ▶). Triebwasser et al. (1983) ▶ already demonstrated that prostaglandins E2 (PGE2) can mimic the action of follicle stimulating hormone (FSH) on granulosa cells at the early stages of development. Prostaglandins E2 can trigger progesterone production in rat granulosa cells (Hiller et al., 1978 ▶). Thus prostaglandins are effective lipid compounds bearing distinct hormone-like effects, and are synthesized in many tissues and play various female reproductive functions such as steroidogenesis, tissue remodeling, neovascularization of the luteinizing follicle and modulation of the oocyte and ovulation. They are essential for follicle rupture and oocyte release in most of the mammals including bovine, ovine, and equine, etc. The reproductive physiology of goat concerning prostaglandins is less understood compared to cattle and pigs. Hence, it is inescapable to investigate practically the effects of prostaglandins on apoptosis in granulosa cells of goat ovarian tissue. Moreover, the intra-ovarian role of PGs in apoptosis has not been addressed so far in small ruminants.
Materials and Methods
Collection and culture of ovarian follicles in vitro
No animals were personally handled or sacrificed for our research purpose, only the reproductive tissues (ovaries only) of Jamnapari breed of goat (Capra hircus) were collected from government-approved abattoirs of Chandigarh (30.7333° N, 76.7794° E) in normal saline at 4°C.
Then the follicles were manually separated with fine forceps. The healthy antral follicles, slightly atretic follicles and atretic antral follicles having a diameter ranging from 3-8 mm were selected on a morphometric basis including color, the turbidity of follicular fluid and vascularity (Sharma and Bhardwaj, 2009). The follicles were then cultured with prostaglandins PGE2 product code number 14010 and Prostaglandins F2α (PGF2α) product code number 16010 (purchased from Cayman Chemical Co. 1180E, Ellsworth Road, Ann Arbor, Michigan 48108, USA) with the concentration of 1.0 μg/ml in DMEM (Dulbecco’s modified eagle medium), supplemented with antibiotics (HiMedia) 200-unit having concentration of penicillin 100IU/Ml and streptomycin 100 IU/Ml) in CO2 incubator (5% CO2, 95% humidity, 38°C) for 24 h as per experimental layout (Fig. 1).
Fig. 1.
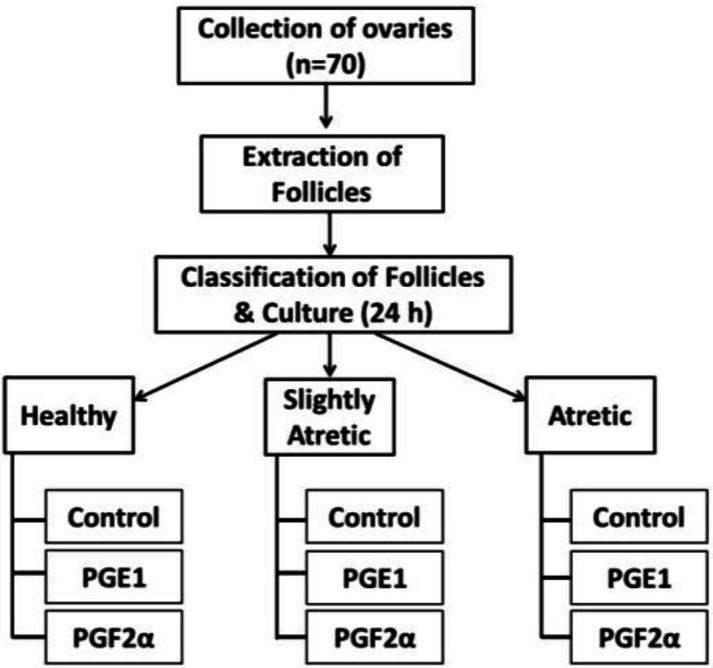
Experimental layout of the study
Histomorphological analysis
Histomorphological analysis was done according to the method of Pearse (1968). The cultured follicles after prostaglandin treatment were fixed in Bouin’s fixative for 24 h. After fixation washing of follicles under tap water was done for 2 h and then dehydration through a graded series of ethanol (30-100%) followed by embedding in paraffin wax at 60°C. Similar blocks were prepared, then trimmed and sectioned serially at 5 μm thickness. After this, the trimmed sections were stretched on albumin coated slides and air-dried for staining. Dewaxing of the sections was done by xylene followed by hydration and dehydration using a series of alcohol (30-100%) for staining with hematoxylin and eosin (H&E) to study the histomorphological characters of apoptosis in granulosa cells of ovarian follicles.
Preparation of granulosa cell suspension
The treated follicles were aspirated with the help of a 20-gauge needle on a 2 ml syringe containing phosphate buffer saline at pH 7.4. The cumulus-oocyte complexes were removed with the help of micropipettes using stereo-microscopes. The granulosa cell aggregates from the aspirates were allowed to centrifuge at 2000 rpm for 5 min. Then the supernatant was removed and the pellet formed was resuspended in phosphate buffer saline (PBS). This step was repeated thrice to remove the debris.
Apoptotic assay
Apoptotic analysis was done according to the method of Broaddus et al. (1996) ▶ and Dave et al. (2001) ▶. Granulosa cells apoptosis was evaluated by acridine orange (AO) staining and methylene blue (MB) staining, analyzed under the fluorescent and light microscope (Olympus, Japan). The cell suspension prepared from treated follicles was mixed with an equal quantity of AO and MB working solution which was prepared by dissolving 1 μL each of AO and MB in 1 ml PBS. Normal cells were identified by green fluorescence and unstained whereas apoptotic cells showed red fluorescence and blue stain. Quantification of healthy and apoptotic granulosa cells was done to measure percentage apoptosis.
Statistical analysis was done by applying ANOVA with Duncan Post Hoc test. Significance is implied at P<0.05.
Results
Histomorphological analysis
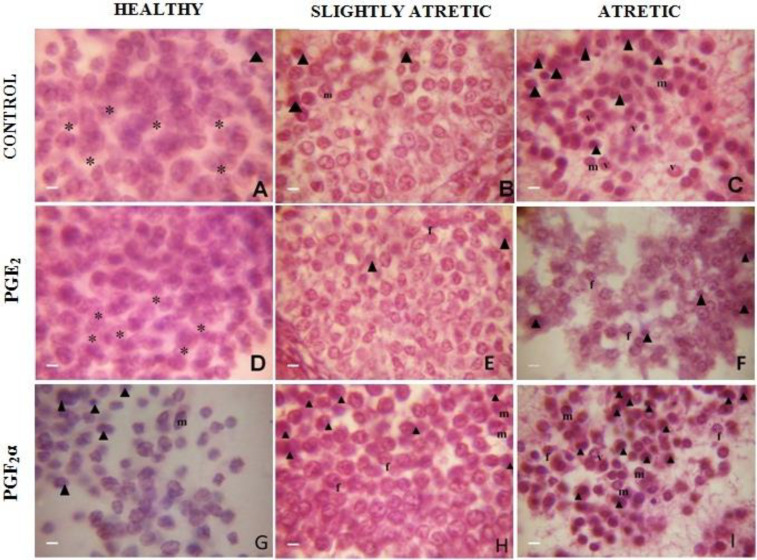
The healthy antral follicles of control group cultured for 24 h were composed of granulosa cells of normal contour having clear morphology. The cytoplasm was finely granulated and lightly stained; the nucleus was centrally located and spherical in outline. The cells reacted positively with eosin stain and are spherical (Fig. 2A). Slightly atretic antral follicles of the same group revealed the apoptotic granulosa cells lodged in between healthy cells. These apoptotic cells were characterized by their small size, pyknotic nucleus, dark staining and marginated chromatin (Fig. 2B). In atretic antral follicles of the same group cultured for the same duration, the apoptotic granulosa cells were more in number with densely stained chromatin material, fragmented nuclei, and hyalinized cytoplasm with varying degree of vacuolization and membrane blebbing was observed (Fig. 2C). In most of the degenerating cells, the cytoplasmic contents were condensed and clumped. Heterochromatization and chromatolysis of granulosa cells were observed. The cell membrane was loose, wrinkled and folded (Fig. 2C).
Fig. 2.
Histological appearance of granulosa cells in ovarian antral follicles showing morphological characteristics of apoptosis after PGE2 treatment at 1.0 μg/ml (D, E, F) and PGF2α treatment at 1.0 μg/ml (G, H, I) in comparison with control post haematoxylin and eosin staining (A, B, C) after 24 h at ×1000. The photograph reveals decreased and increased incidence of apoptotic characteristics. * Represents normal healthy cells with intact membrane, arrow head: Darkly stained pyknotic nuclei, m: Marginated chromatin, f: Fragmented chromatin, and v: Vacuolated cell
Prostaglandins E2 (1.0 μg/ml) treated healthy and slightly atretic antral follicles cultured for 24 h revealed chiefly healthy granulosa cells with intact cell membranes and maintained cellular integrity. The appearance of pyknotic nuclei, degenerative granulosa cells were minimum in number (Figs. 2D and E). Prostaglandins E2 treated atretic antral follicles cultured for 24 h demonstrated marked features of decreasing the attributes of apoptosis where most of the granulosa cells remained healthy with maintained cellular integrity. The appearance of pyknotic nuclei, vacuoles and condensed chromatin and degenerating granulosa cells were minimum (Fig. 2F). Prostaglandins E2 helped in the suppression of apoptosis by decreasing the incidence of the occurrence of apoptotic characters within granulosa cells. While the PGF2α (1.0 μg/ml) treated healthy and slightly atretic follicles cultured for 24 h reported an increasing incidence of characteristics of apoptosis within granulosa cells (Figs. 2G-I). Whereas the number of degenerative granulosa cells was more when atretic antral follicles were treated with PGF2α with the same dose for the same duration (Figs. 2G-I).
Fluorescence assay for detection and quantification of apoptosis
Moreover, the quantification of apoptosis revealed that PGE2 decreased the frequency of apoptosis in granulosa cells (Table 1). Scrutiny of apoptosis using fluorescent dye i.e. AO is based upon the differential
Table 1.
Apoptotic index in various experimental groups using AO and MB dye
| Stains | Treatments | Healthy (%) | Slightly atretic (%) | Atretic (%) |
|---|---|---|---|---|
| AO | Control (n=10) | 13.49 ± 1.89b | 18.79 ± 1.47b | 49.40 ± 3.87b |
| PGE1 (n=10) | 3.81 ± 2.34c | 8.29 ± 3.24c | 19.69 ± 3.32c | |
| PGF2α (n=10) | 25.17 ± 2.91a | 52.19 ± 2.27a | 89.21 ± 3.60a | |
| MB | Control (n=10) | 18.53 ± 3.25b | 25.08 ± 1.85b | 62.63 ± 2.64b |
| PGE1 (n=10) | 5.48 ± 1.70c | 16.56 ± 1.86b | 24.22 ± 2.37c | |
| PGF2α (n=10) | 31.50 ± 2.03a | 67.59 ± 4.14a | 92.69 ± 0.94a |
Values are expressed as mean±SEM. ANOVA with Duncan Post Hoc test was used for analysis; different letters indicate significant differences among means of treatment at each duration (P<0.05) after Duncan’s test. AO: Acridine orange, and MB: Methylene blue
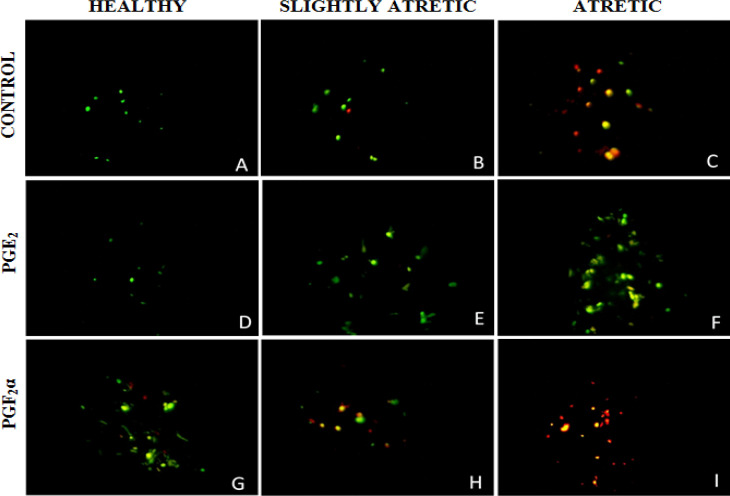
uptake of dye by live and apoptotic cells. Healthy cells with intact cell membranes and integrity appear green in color because of AO, whereas the apoptotic cells appear red due to the intercalation of AO dye. Prostaglandins E2 supplementation decreased the percentage of granulosa cells showing red fluorescence in healthy, slightly and atretic antral follicles. Whereas PGF2α increased the percentage of granulosa cells showing red color in healthy, slightly atretic and atretic antral follicles (Figs. 3A-I).
Fig. 3.
Fluorescent photographs of granulosa cells of ovarian antral follicles after PGE2 treatment at 1.0 μg/ml (D, E, F) and PGF2α treatment at 1.0 μg/ml (G, H, I) in comparison with control (A, B, C) stained with fluorescent dye AO depicting apoptotic granulosa cells with bright red fluorescence and normal live cells with green fluorescence after 24 h culture at ×400
Methylene blue staining
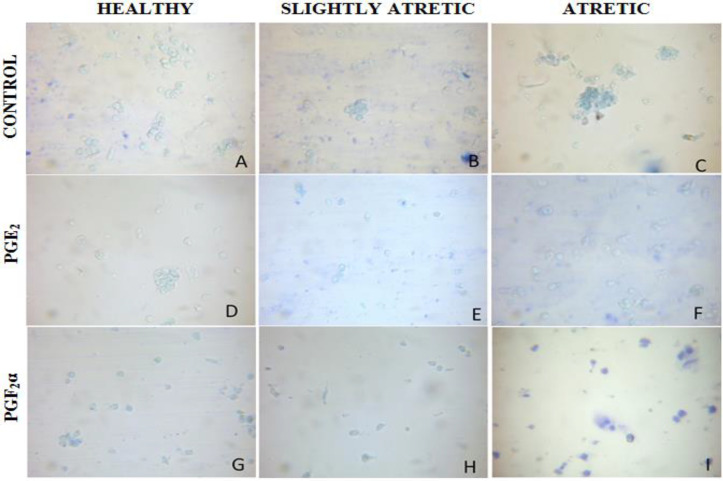
Furthermore, the MB staining of cells clearly showed that PGE2 has suppressed apoptosis in granulosa cells (Table 1). Analysis of granulosa cell apoptosis is based upon the differential staining of granulosa cells; the normal healthy cells appeared transparent whereas apoptotic cells appeared blue as dead cells took up the stain. Prostaglandins E2 treatment decreased the percentage of granulosa cells showing blue color whereas, in PGF2α treatment, most of the granulosa cells appeared blue (Figs. 4A-I).
Fig. 4.
Microphotographs showing apoptosis in granulosa cells of ovarian antral follicles using MB staining after PGE2 treatment at 1.0 μg/ml (D, E, F) and PGF2α treatment at 1.0 μg/ml (G, H, I) in comparison with control (A, B, C) depicting apoptotic granulosa cells with blue color and normal live cells with no color after 24 h culture at ×400
Discussion
Although PG synthesis in granulosa cells during ovarian follicular development is regulated by a complex of interactions of different factors, the physiological role of these eicosanoids in the control of ovarian cells function by these factors is unknown. The present results illustrate that notable change in the responsiveness of granulosa cells when cultured with PGs for 24 h. The results of the present study revealed that PGE2 suppressed the apoptosis in granulosa cells as already reported by Manchanda et al. (2001) ▶, who studied the role of prostaglandins in the suppression of apoptosis in hen granulosa cells and demonstrated that a prostaglandin synthesis is a necessary event in the suppression of granulosa cell apoptosis. Histomorphological analysis of PGE2 and PGF2α supplemented granulosa cells revealed that PGE2 reduced the frequency of apoptotic attributes within the granulosa cells of atretic antral follicles while PGF2α upregulated the process of apoptosis. The PGF2α activates the apoptotic signaling cascades have already been documented in corpus luteum (Yadav et al., 2005 ▶). Prostaglandins E2 suppressed the rate of apoptosis in healthy, slightly atretic and atretic follicles. Though the mechanism by which PGE2 mitigates the apoptotic action is not elucidated, some hypothetical roles of prostaglandin in ovarian follicular regulation have already been suggested by Armstrong (1981) ▶.
Growing theca cells release PGE2, which stimulates the production of cyclic adenosine monophosphate (cAMP) in theca and granulosa cells. The cAMP subsequently initiates the growth of follicles by exerting gonadotropin like action on granulosa cells, which do not yet possess receptors for pituitary gonadotropins. Prostaglandins F2α, of follicular origin, may initiate or participate in the processes of luteolysis and atresia, through interactions of specific receptors (Armstrong, 1981 ▶).
Our findings are consistent with the study of Li et al. (1995) ▶, who documented PGs as central elements in cell signaling for mitogenesis induced by growth factors, and in oncogenic transformation. An increased PG production is associated with many tumor cells and is believed to control tumor growth and metastasis. Further, he demonstrated, compared to PGs of the F series, PGE1 and PGE2 are more effective in persuading transforming growth factor-α (TGF-α) induced DNA synthesis in hen granulosa cells. Prostaglandins of I series were also shown to increase in the cAMP production which was the main indicator of cell division in cultured granulosa cells in vitro but as compared to E series PGs of I series are lower stimulator for cAMP production (Goff et al., 1978 ▶). The goat granulosa cells are possibly regulated by a similar mechanism for multiplication or regression.
Steroidogenesis in granulosa cells requires the continuous supply of stimulating factors, and PGE2 is one of them; granulosa cells become more sensitive to PGE2 in the presence of FSH (Goff et al., 1983 ▶). Bowolaksono et al. (2008) ▶ who observed the production of PGs and their receptors in bovine corpus luteum cells also suggested that PGs have anti-apoptotic roles in bovine luteal steroidogenic cells.
The use of AO differential staining undoubtedly interpreted the apoptosis and DNA damage in granulosa cells. Acridine orange is fluorescent compound which intercalates between the adjacent base pairs of DNA and RNA and enhances its fluorescent intensity (Nafisi et al., 2007 ▶). Acridine orange is permeable to cell membrane stain apoptotic cells as red and non-apoptotic cells as green. The AO differential staining techniques aptly assisted in the analysis of PGs suppression or induction of cascades of apoptosis within granulosa cells. In our study, PGE2 is found to be capable of suppressing apoptosis, which is revealed through fluorescent assay. In PGE2 administered group, green fluorescence suggestive of live cells is higher as compared to orange-reddish fluorescence (an indicator of apoptosis). These findings endorse the role of PG in follicular growth and development as already documented in rats (Goff and Armstrong, 1983 ▶).
In summary, the present study shows that prostaglandin of E series synthesis is a crucial step in the suppression of granulosa cell apoptosis while that of F series has an apoptotic role. The availability of such factors or the capacity of granulosa cells to reciprocate them may be essential determinants of the fate of goat ovarian follicles.
Acknowledgements
A. K. Sharma acknowledges the financial assistance provided in the form of JRF by UGC, New Delhi. The authors are also thankful to the Department of Zoology, Kurukshetra University, Kurukshetra for providing Laboratory facilities. The authors acknowledge the SAP-UGC for financial support.
Conflict of interest
There is no conflict of interest between the authors.
References
- Ainsworth L, Baker RD, Armstrong DT. Pre-ovulatory changes in follicular fluid prostaglandin F levels in swine. Prostaglandins. 1975;9:915–925. doi: 10.1016/0090-6980(75)90079-9. [DOI] [PubMed] [Google Scholar]
- Ainsworth L, Tsang BK, Downey BR, Baker RD, Marcus GJ, Armstrong DT. Effects of indomethacin on ovulation and luteal function in gilts. Bio. Reprodn. 1979;21:401–411. doi: 10.1095/biolreprod21.2.401. [DOI] [PubMed] [Google Scholar]
- Armstrong DT. Prostaglandins and follicular functions. Reproduction. 1981;62:283–291. doi: 10.1530/jrf.0.0620283. [DOI] [PubMed] [Google Scholar]
- Armstrong DT, Grinwich DL. Blockade of spontaneous and LH-induced ovulation in rats by indomethacin, an inhibitor of prostaglandin biosynthesis. Prostaglandins. 1972;1:21–28. doi: 10.1016/0090-6980(72)90062-7. [DOI] [PubMed] [Google Scholar]
- Armstrong DT, Moon YS, Zamecnik J. Evidence for a role of ovarian prostaglandins in ovulation. In: Mougdal, NR (Ed.), Gonadotropins and gonadal function. 1st Edn. Elsevier Inc; 1974. pp. 343–356. [Google Scholar]
- Bauminger S, Lindner HR. Periovulatory changes in ovarian prostaglandin formation and their hormonal control in the rat. Prostaglandins. 1975;9:737–751. doi: 10.1016/0090-6980(75)90111-2. [DOI] [PubMed] [Google Scholar]
- Bowolaksono A, Nishimura R, Hojo T, Sakumoto R, Acosta TJ, Okuda K. Anti-apoptotic roles of prostaglandin E2 and F2alpha in bovine luteal steroidogenic cells. Bio. Reprodn. 2008;79:310–317. doi: 10.1095/biolreprod.107.066084. [DOI] [PubMed] [Google Scholar]
- Broaddus VC, Yang L, Scavo LM, Ernst JD, Boylan AM. Asbestos induces apoptosis of human and rabbit pleural mesothelial cells via reactive oxygen species. J. Clinic. Investig. 1996;98:2050–2059. doi: 10.1172/JCI119010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave U, Shousha S, Westaby D. Methylene blue staining: is it really useful in Barrett’s esophagus? Gastro. Endosco. 2001;53:333–335. doi: 10.1016/s0016-5107(01)70408-7. [DOI] [PubMed] [Google Scholar]
- Goff AK, Armstrong DT. Changes in responsiveness of rat granulosa cells to prostaglandin E2 and follicle-stimulating hormone during culture. Cana. J. Physio. Pharmacol. 1983;61:608–613. doi: 10.1139/y83-093. [DOI] [PubMed] [Google Scholar]
- Goff AK, Zamecnik J, Ali M, Armstrong DT. Prostaglandin I2 stimulation of granulosa cell cyclic AMP production. Prostaglandins. 1978;15:875–879. doi: 10.1016/0090-6980(78)90154-5. [DOI] [PubMed] [Google Scholar]
- Grinwich DL, Kennedy TG, Armstrong DT. Dissociation of ovulatory and steroidogenic actions of luteinizing hormone in rabbits with indomethacin, an inhibitor of prostaglandin biosynthesis. Prostaglandins. 1972;1:89–96. doi: 10.1016/0090-6980(72)90071-8. [DOI] [PubMed] [Google Scholar]
- Hillier SG, Zeleznik AJ, Ross GT. Independence of steroidogenic capacity and luteinizing hormone receptor induction in developing granulosa cells. Endocrinology. 1978;102:937–946. doi: 10.1210/endo-102-3-937. [DOI] [PubMed] [Google Scholar]
- Lau IF, Saksena SK, Chang MC. Prostaglandins F and ovulation in mice. Reproduction. 1974;40:467–469. doi: 10.1530/jrf.0.0400467. [DOI] [PubMed] [Google Scholar]
- Li J, Tsang BK. Prostaglandins mediate the stimulation of deoxyribonucleic acid synthesis by transforming growth factor α in hen granulosa cells during ovarian follicular development. Bio. Reprodn. 1995;52:1050–1058. doi: 10.1095/biolreprod52.5.1050. [DOI] [PubMed] [Google Scholar]
- Maia JrH, Barbosa I, Coutinho EM. Inhibition of ovulation in marmoset monkeys by indomethacin. Fert. Ster. 1978;29:565–570. doi: 10.1016/s0015-0282(16)43287-5. [DOI] [PubMed] [Google Scholar]
- Manchanda R, Kim JM, Tsang BK. Role of prostaglandins in the suppression of apoptosis in hen granulosa cells by transforming growth factor alpha. Reproduction. 2001;122:91–101. [PubMed] [Google Scholar]
- Nafisi S, Saboury AA, Keramat N, Neault JF, Tajmir-Riahi HA. Stability and structural features of DNA intercalation with ethidium bromide, acridine orange and methylene blue. J. Mol. Struct. 2007;827:35–43. [Google Scholar]
- O’Grady JP, Caldwell BV, Auletta FJ, Speroff L. The effects of an inhibitor of prostaglandin synthesis (indomethacin) on ovulation, pregnancy, and pseudopregnancy in the rabbit. Prostaglandins. 1972;1:97–106. doi: 10.1016/0090-6980(72)90072-x. [DOI] [PubMed] [Google Scholar]
- Orczyk GP, Behrman HR. Ovulation blockade by aspirin or indomethacin-in vivo evidence for a role of prostaglandin in gonadotrophin secretion. Prostaglandins. 1972;1:3–20. doi: 10.1016/0090-6980(72)90061-5. [DOI] [PubMed] [Google Scholar]
- Pearse AGE. Histochemistry, theoretical and applied: theoretical and applied. 3rd Edn. Boston, USA: 1968. [Google Scholar]
- Sharma RK, Bhardwaj JK. Ultrastructural characterization of apoptotic granulosa cells in caprine ovary. J. Micro. 2009;236:236–242. doi: 10.1111/j.1365-2818.2009.03281.x. [DOI] [PubMed] [Google Scholar]
- Stacey NE, Pandey S. Effects of indomethacin and prostaglandins on ovulation of goldfish. Prostaglandins. 1975;9:597–607. doi: 10.1016/0090-6980(75)90065-9. [DOI] [PubMed] [Google Scholar]
- Triebwasser WF, Clark MR, LeMaire WJ, Marsh JM. Localization and in vitro synthesis of prostaglandins in components of rabbit preovulatory Graafian follicles. Prostaglandins. 1978;16:621–632. doi: 10.1016/0090-6980(78)90192-2. [DOI] [PubMed] [Google Scholar]
- Tsang BK, Ainsworth L, Downey BR, Armstrong DT. Pre-ovulatory changes in cyclic AMP and prostaglandin concentrations in follicular fluid of gilts. Prostaglandins. 1979;17:141–148. doi: 10.1016/0090-6980(79)90086-8. [DOI] [PubMed] [Google Scholar]
- Wallach EE, de la Cruz A, Hunt J, Wright KH, Stevens VC. The effect of indomethacin on HMG-HCG induced ovulation in the rhesus monkey. Prostaglandins. 1975;9:645–658. doi: 10.1016/0090-6980(75)90072-6. [DOI] [PubMed] [Google Scholar]
- Yadav VK, Lakshmi G, Medhamurthy R. Prostaglandin F2α-mediated activation of apoptotic signaling cascades in the corpus luteum during apoptosis involvement of caspase-activated DNase. J. Bio. Chem. 2005;280:10357–10367. doi: 10.1074/jbc.M409596200. [DOI] [PubMed] [Google Scholar]
- Yang NS, Marsh JM, LeMaire WJ. Post ovulatory changes in the concentration of prostaglandins in rabbit Graafian follicles. Prostaglandins. 1974;6:37–44. doi: 10.1016/s0090-6980(74)80038-9. [DOI] [PubMed] [Google Scholar]