Abstract
Introduction
Statins, also known as 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase inhibitors, are lipid-lowering agents that are central in preventing or reducing the complications of atherosclerotic cardiovascular disease. Because statins have anti-inflammatory properties, there is considerable interest in their therapeutic potential in other chronic inflammatory conditions. We aim to identify the statin with the greatest ability to reduce systemic inflammation, independent of the underlying disease entity.
Methods and analysis
We aim to conduct a comprehensive search of published and peer-reviewed randomised controlled clinical trials, with at least one intervention arm of a Food & Drug Administration-licensed or European Medicines Agency-licensed statin and a minimum treatment duration of 12 weeks. Our objective is to investigate the effect of statins (atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin, simvastatin) on lipid profile, particularly, cholesterol low-density lipoprotein and inflammation markers such as high-sensitive C reactive protein (hsCRP), CRP, tumour necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-6, IL-8, soluble cluster of differentiation 14 (sCD14) or sCD16 in adults, published in the last 20 years (between January 1999 and December 2019). We aim to identify the most potent statin to reduce systemic inflammation and optimal dosing. The following databases will be searched: Medline, Scopus, Web of Science and Cochrane Library of Systematic Reviews. The risk of bias of included studies will be assessed by Cochrane Risk of Bias Tool and Quality Assessment Tool for Quantitative Studies. The quality of studies will be assessed, to show uncertainty, by the Jadad Score. If sufficient evidence is identified, a meta-analysis will be conducted with risk ratios or ORs with 95% CIs in addition to mean differences.
Ethics and dissemination
Ethics approval is not required as no primary data will be collected. Results will be presented at conferences and published in a peer-reviewed journal.
PROSPERO registration number
CRD42020169919
Keywords: clinical pharmacology, immunology, microbiology, infectious diseases, molecular biology
Strengths and limitations of this study.
This study will include randomised controlled clinical trials to determine the most effective statin on the combined reduction of lipid profile and inflammatory biomarkers.
High-quality clinical trials will be reviewed accurately to generate reliable evidence.
This study will be conducted following Preferred Reporting Items for Systematic Review and Meta-Analysis Protocol guidelines.
Variation of statin doses among included studies will likely produce heterogeneity that will subsequently reduce the sample size of the meta-analysis.
Introduction
Statins are US Food & Drug Administration (FDA) approved lipid-lowering drugs (table 1) that have been on the market for more than 30 years1 and are widely prescribed to patients who are at high risk of cardiovascular diseases.2 Statins exert their function via inhibiting 3-hydroxy-3-methylglutaryl coenzyme-A (HMG-CoA) reductase which converts HMG-CoA into L-mevalonate resulting in reduced cholesterol biosynthesis.3 The cholesterol biosynthesis inhibition via statins results in the upregulation of low-density lipoprotein (LDL) receptors on the cell surface which consequently leads to increased uptake and clearance of LDL in the circulating blood. This ultimately lowers LDL-cholesterol (LDL-C) and decreases the risk associated with lipoprotein deposition in the arterial wall and progression to atherosclerosis and vascular disease. In addition to statins’ LDL-C lowering ability, statins also inhibit protein prenylation,4 which is an important biological process that mediates protein-protein interaction and anchoring of cell membrane proteins.5 The ability of statins to inhibit isoprenoids, important metabolites in the protein prenylation pathway, accounts for their lipid-independent pleiotropic effects.6 7 Indeed statins have been reported to have anti-inflammatory, antioxidant antiproliferative and immunomodulatory effects independent of their cholesterol-lowering ability.8 The reported vascular effects of statins are wide-ranging and include improvement of endothelial functioning, decreasing oxidative stress and maintenance of coronary artery plaque stability.8 Statins may also lower the risk of liver cancer.9 The anti-inflammatory effects vary among the different types of currently licensed statins with various meta-analyses reporting differential efficacy in reducing inflammation in chronic obstructive pulmonary disease (COPD).10 Statins (table 1) are categorised into two main groups according to their solubility: (1) hydrophilic statins which include pravastatin and rosuvastatin and these display high hepato-selectivity with increased first-pass effect and (2) lipophilic statins which are characterised by passive diffusion into cells; these include atorvastatin, simvastatin, lovastatin, fluvastatin, pitavastatin and cerivastatin.11
Table 1.
List of statins as a single-ingredient product licensed by the Food & Drug Administration and European Medicines Agency
| Statin | Solubility | Type | Synthetic state | Pharmacokinetic parameters | |||||||||
| Cytochrome P450 subclass | Half-life (hours) | Clearance (L/hour) | Hepatic extraction | Excretion | |||||||||
| Systemic | Oral | Hepatic | Renal | Urine | Faecal | ||||||||
| Atorvastatin | Lipophilic | Fully synthetic | Active drug | CYP3A4 | Mean* | 17.8 | 157 | >70% | 1.20% | 70% | |||
| Range† | 13.8–20.7 | ||||||||||||
| Lipitor‡ | Dose§: 20, 40, 80 mg | REF: 58 | REF: 59 | REF: 58 | REF: 60 | ||||||||
| REF: 61 62 | |||||||||||||
| Cerivastatin | Lipophilic | Fully synthetic | Active drug | CYP2C8 and CYP3A4 | Mean* | 2.96 | 13 | 24%–30% | 70% | ||||
| Range† | 2.2–4.0 | ||||||||||||
| Lipobay‡¶ | Dose§: 0.2 to 0.3 mg | ||||||||||||
| REF: 63–65 | REF: 63 | REF: 66 67 | REF: 66 67 | ||||||||||
| Fluvastatin | Lipophilic | Natural statin | Active drug | CYP2C9 some CYP2C8 | Mean* | 1.9 | 68 | 120–180 | 69 | 73% | 6% | 93% | |
| Range† | 1.5–2.4 | ||||||||||||
| Lescol‡ | Dose§: 40 to 80 mg | REF: 68 | REF: 69 70 | REF: 71 | REF: 72 | REF: 68 | REF: 68 | ||||||
| REF: 73–76 | |||||||||||||
| Lovastatin | Lipophilic | Natural statin | Prodrug | CYP3A4 | Mean* | 2.7 | 18–75 | 175–351 | 69% | 9.60% | 83.20% | ||
| Range† | 2.6–2.8 | ||||||||||||
| Mevacor‡** | Dose§: 20 to 40 mg | REF: 71 | REF: 69 77 78 78 | REF: 79 | REF: 71 | REF: 71 | |||||||
| REF: 76 78 80 | |||||||||||||
| Pitavastatin | Lipophilic | Fully synthetic | Active drug | Partially: CYP2C8 and CYP2C9 | Mean* | 10.7 | 16–26 | 15% | 79% | ||||
| Range† | 6.9–13.1 | ||||||||||||
| Livalo‡ | Dose§: 1, 2, 4 mg | REF: 81–84 | REF: 85 | REF: 85 | |||||||||
| REF: 81–84 86 87 | |||||||||||||
| Rosuvastatin | Hydrophilic | Fully synthetic | Active drug | Partially: CYP2CP and CYPC19 | Mean* | 14.2 | 49 | 273–281 | 82 | 12 | 63% | 5%–10% | 90% |
| Range† | 10.1–24.4 | ||||||||||||
| Crestor‡ | Dose§: 5, 10, 20, 40 mg | ||||||||||||
| REF: 88–93 | REF: 94 | REF: 88 | REF: 95 | REF: 94 | REF: 94 | REF: 94 96 | REF: 96 | ||||||
| Pravastatin | Hydrophilic | Semisynthetic | Active drug | None | Mean* | 2.17 | 57 | 24–27 | 46%–66% | 20% | 71% | ||
| Range† | 1.6–2.6 | ||||||||||||
| Pravachol‡ | Dose§: 10, 20, 40 mg | REF: 97 | REF: 97–99 | REF: 59 | REF: 97 | REF: 97 | |||||||
| REF: 80 97 100–103 | |||||||||||||
| Simvastatin | Lipophilic | Semisynthetic | Prodrug | CYP3A4 | Mean* | 4.6 | 32 | 2000–3100 | >79% | 13% | 58% | ||
| Range† | 1.6–7.9 | ||||||||||||
| Zocor‡ | Dose§: 20, 40, 60 mg | REF: 104 | REF:105–107 | REF:79 | REF: 104 108 | REF:104 108 | |||||||
| REF: 92 105 106 109–115 | |||||||||||||
Cerivastatin is withdrawn from the market and lovastatin is not licensed in Great Briatin and Switzerland
*Mean calculated as the average of the means of the cited references.
†Range of the means from the cited references.
‡Common brand name.
§Half-life reported from indicated doses from the cited references.
¶Withdrawn from the market due to rhabdomyolysis in 2001.
**Not commonly prescribed anymore and not licensed in Great Britain and Switzerland.
Statins and inflammation
Inflammatory responses to various clinical conditions result in elevated secretion and activity of acute inflammatory proteins such as Creactive protein (CRP). In the liver, CRP is mainly secreted by hepatocytes in response to interleukin-6 (IL-6).12 Increased secretion of IL-6 and CRP further exacerbate the inflammatory milieu through secretion of pro-inflammatory cytokines such as tumour necrosis factor (TNF), activation of the complement pathway, apoptosis, phagocytosis and nitric oxide release.13 Previous clinical trials have reported statin therapy to reduce CRP levels through an LDL-C independent mechanism,14 15 resulting in better clinical outcomes in patients with reduced CRP.16 In addition, atorvastatin therapy was shown to reduce inflammatory biomarkers such as high-sensitive CRP (hsCRP) and IL-6 in patients with unstable angina who received the percutaneous coronary intervention and furthermore reduced cardiac troponin I and creatine kinase muscle brain suggesting a reduction in cardiac myocyte necrosis.17 Additionally, the PRINCE randomised controlled trial (RCT) reported pravastatin (40 mg/day) therapy to have a significant reduction in CRP levels following 12 and 24 weeks of treatment.14 Statin therapy further resulted in the downregulation of other inflammatory biomarkers, such as IL-8 and sCD14, in patients with coronary artery inflammation.18 19 Currently it is not fully elicited on how different types of statins (hydrophilic or lipophilic, table 1) or the treatment duration differentially affect immune responses.
Mechanisms to reduce inflammation
Statins are selectively taken up by hepatocytes and decrease inflammatory responses by regulating the expression of various cell surface molecules/receptors, transcription factors, cytokines, chemokines and other soluble inflammatory mediators.20 Furthermore, their ability to be taken up by other cell types, including immune cells, depending on the expression of cell membrane transport proteins and their chemical properties.11 21 Statins can enter their target cells either through passive diffusion11 or active transport which involves transmembrane proteins within the organic anionic-transporting polypeptide 21 22 and Na+taurocholate cotransporting polypeptides groups.23
Effects on cell surface receptor
Even though statins were shown to have no effect on peripheral frequencies of circulating CD14++CD16−, CD14++CD16+ and CD14+CD16++ monocyte subsets, statins were shown to reduce expression of cell surface receptors such as vascular endothelial growth factor receptor-2, toll-like receptor (TLR)-4 and tyrosine kinase receptor Tie2 which are involved in proliferation, migration and pathogen recognition within all monocyte populations.24 Furthermore, statins downregulate the expression of TLR-2, human leukocyte antigen-DR and CC-chemokine receptor-2 on monocytes, while increasing peroxisome proliferator activated receptor-γ activity, which enhances their anti-inflammatory properties.17 25 The ability of statins to reduce chemokine and chemokine receptor expression on human vascular endothelial cells and human primary macrophages is achieved via inhibition of the isoprenoid geranylgeranyl pyrophosphate pathway.26
Effect on cell signalling
Statins are documented to affect cellular functionality of both monocytes and T cells through altering activation of lymphocyte function-associated antigen (LFA)-1 integrin molecules that are involved in lymphocyte adhesion, migration and transduction of co-stimulatory signals to T cells during antigen presentation.27 Activation of LFA-1 integrin molecules leads to conformational changes in their structures, thus increasing their binding affinity for their respective substrates, which further enhances pro-inflammatory responses.28 However, cellular uptake of statins is reported to inhibit these conformational changes in LFA-1 molecules and further enhance their anti-inflammatory properties.27 Statins also modulate immune responses through alteration of cell-to-cell interaction. Here statins suppress monocyte-derived dendritic cells resulting in reduced T cell activation, proliferation and T helper differentiation.25
Downstream effects on soluble biomarkers
Statins inhibit monocyte chemoattractant protein-1 secretion, resulting in decreased leucocyte recruitment during inflammation.29 Statins suppress the production of pro-inflammatory cytokines such as IL-6 and IL-8 in IL-1β-stimulated synoviocytes from rheumatoid arthritis patients via interference in protein prenylation and nuclear factor κB (NF-κB) pathway.30
Classification of statins
Statins are classified based on several different factors.
Source of origin: They are classified as natural, semisynthetic or fully synthetic (table 1). Natural statins are acquired from fungal fermentation and these include lovastatin. Simvastatin and pravastatin are classified as semisynthetic statins because they are produced through direct alkylation of lovastatin and hydroxylation of mevastatin, respectively. Fully synthetic statins are produced from different substrates and these include pitavastatin, rosuvastatin, fluvastatin, atorvastatin and cerivastatin.11
Pharmacological properties: Two pharmacological properties differentiate statins; they are either prodrugs or active drugs (table 1). Prodrug statins include lovastatin and simvastatin; they are administered in an inactive state and are activated through hydrolysis by liver enzymes. Atorvastatin, cerivastatin, fluvastatin and pravastatin are administered as active drugs.11
Physiochemical properties: Statins are classified as lipophilic or hydrophilic (table 1). Atorvastatin, simvastatin, lovastatin, fluvastatin, cerivastatin and pitavastatin are relatively lipophilic statins as they dissolve efficiently in lipid/fat solution. Cytochrome P450 enzymes metabolise most lipophilic statins except pitavastatin, which is only partially metabolised by this pathway. Hydrophilic statins such as rosuvastatin and pravastatin are not significantly metabolised by the cytochrome P450 system.11 Pravastatin and rosuvastatin are classified as hydrophilic statins as they dissolve efficiently in water. Pravastatin and rosuvastatin are excreted largely as the parent compound into faeces, urine and bile.31 32
Liver selectivity: The hepato-selective processing of statins is defined by their solubility profile; therefore, lipophilic statins diffused passively through hepatocyte cell membranes, whereas hydrophilic statins’ uptake occurs through carrier transmembrane proteins.11
Statins in clinical conditions other than cardiovascular disease
Inflammation
Statin therapy has been reported to have a wide range of potentially beneficial effects. These include the improved clinical outcome of chronic kidney disease in patients presenting with acute coronary syndrome.33 Statins also reduced mortality in patients with cirrhosis with bacteraemia and pneumonia.34 Additionally, a 2-year treatment period with atorvastatin was associated with milder disease progression in patients with relapsing-remitting multiple sclerosis.35 However, a study by Birnbaum et al reported that disease progression was exacerbated by atorvastatin combined with beta interferon in patients with multiple sclerosis.36 Moreover, statin users developed significantly less uveitis.37 Atorvastatin and rosuvastatin also inhibited the micro-inflammatory state and improved the nutritional status in patients who had maintenance haemodialysis.38 In a retrospective observational study, pitavastatin usage significantly decreased the mortality risk in Japanese patients who had haemodialysis.39 However, Palmer et al published a systemic review of RCTs and reported statins to be associated with uncertain adverse events in adults treated with dialysis regardless of serum cholesterol levels; furthermore, statin treatment showed no beneficial effects on mortality and cardiovascular events for patients who had dialysis.40 Rosuvastatin therapy was shown to reduce the levels of inflammatory markers, such as IL-6 and hsCRP, leading to resolved systemic inflammation and improved endothelial-dependent vascular function in patients with COPD.41 Furthermore, a 6-month atorvastatin (80 mg) therapy improved cough on a quality-of-life scale in patients with bronchiectasis.42
Cancer
The Reduction by Dutasteride of Prostate Cancer Events (REDUCE) RCT reported the effect of statins; specifically simvastatin, lovastatin, atorvastatin and fluvastatin in the reduction of inflammatory responses in both acute and chronic prostate inflammation.43 Furthermore, in a retrospective cohort study, patients with COPD had a lower risk of prostate cancer following simvastatin, atorvastatin, pravastatin, fluvastatin and lovastatin therapy.44 Inversely, an observational study by Emilsson et al that used observational data from the Surveillance, Epidemiology and End Results (SEER)-Medicare Databases on 17 372 patients with cancer, reported that treatment with statins within 6 months after cancer diagnosis did not improve patients’ survival rates when followed up for 3 years.45
Central nervous system
Statins have a major effect on the central nervous system, particularly on cognition and neurological disorders and may decrease the risk of Alzheimer’s disease (AD) and Parkinson’s disease through direct impact on neurodegeneration and microglia, respectively.46 However, the Lipitor’s effect in Alzheimer’s dementia (LEADc) RCT showed that even though atorvastatin (80 mg/day) treatment was well tolerated without unexpected adverse events in patients with AD, this treatment did not have significant beneficial effects on AD over a 72-week period.47 Additionally, Sano et al further showed in an RCT that despite a significant reduction in cholesterol, simvastatin (20 mg/day) treatment did not prevent the progression of symptoms in individuals with mild to moderate AD.48
Infection
Statins are reported to have a great effect on vaginal microbiome via reduced proportions of Gardnerella vaginalis and increased proportions of beneficial lactobacilli.49 In addition, statins diminished the risk of infections in patients with type 2 diabetes.50 Inversely, in patients with dementia statin, therapy was associated with increased risk of infection.51 However, it was reported that statin use in patients with asthma chronic pulmonary disease overlap syndrome was associated with lower tuberculosis (TB) and pneumonia risks after adjustment for multiple confounding factors.52 Statin use was also associated with a lower risk of active TB.53 54 Statin therapy also reduced the mycobacterial growth in human macrophages and mice by induction of autophagy and phagosome maturation.55 Furthermore, many studies have stated the potential use of statins as host-directed therapy against infectious diseases caused by viruses, protozoa, fungi and bacteria.56
Most of the data on statins as therapeutic agents originate from observational studies. This further highlights the need to perform RCTs to evaluate statins’ immunomodulatory effects independent of their cholesterol-lowering ability. This protocol describes the investigation of commonly available statins (table 1) atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin and simvastatin focusing on their effect to reduce systemic inflammation in humans. Here, cerivastatin and lovastatin will be excluded due to decommissioned status from the market and the lack of license in Great Britain and Switzerland, respectively. This systematic review will address the hypothesis that pravastatin and rosuvastatin are inferior to other statins in reducing systemic inflammation due to increased first-pass effect.
Objectives
Primary objective
To identify the type of statin with the best potential to reduce systemic inflammation (statin type stratification).
Secondary objective
To identify the optimal dose for each statin to reduce systemic inflammation (statin dose stratification).
Methods and design
Population
The systematic review will include high-quality RCTs on adults of at least 18 years of age who have been treated with either atorvastatin, fluvastatin, pitavastatin, pravastatin, rosuvastatin or simvastatin, and in whom LDL-C, and at least one of the following markers of systemic inflammation: hsCRP, CRP, TNF-α, IL-1β, IL-6, IL-8, sCD14 or sCD16 are measured before and after statin treatment.
Patient and public involvement
This is a systemic review and meta-analysis protocol which will address the anti-inflammatory effects of statins. This study does not involve patients and/or the public at any stage as primary data will not be collected.
Study design
This systematic review will consider published and peer-reviewed randomised controlled clinical trials with at least one intervention arm of an FDA-licensed or European Medicines Agency (EMA)-licensed statin and a minimum treatment duration of 12 weeks.
Search strategy
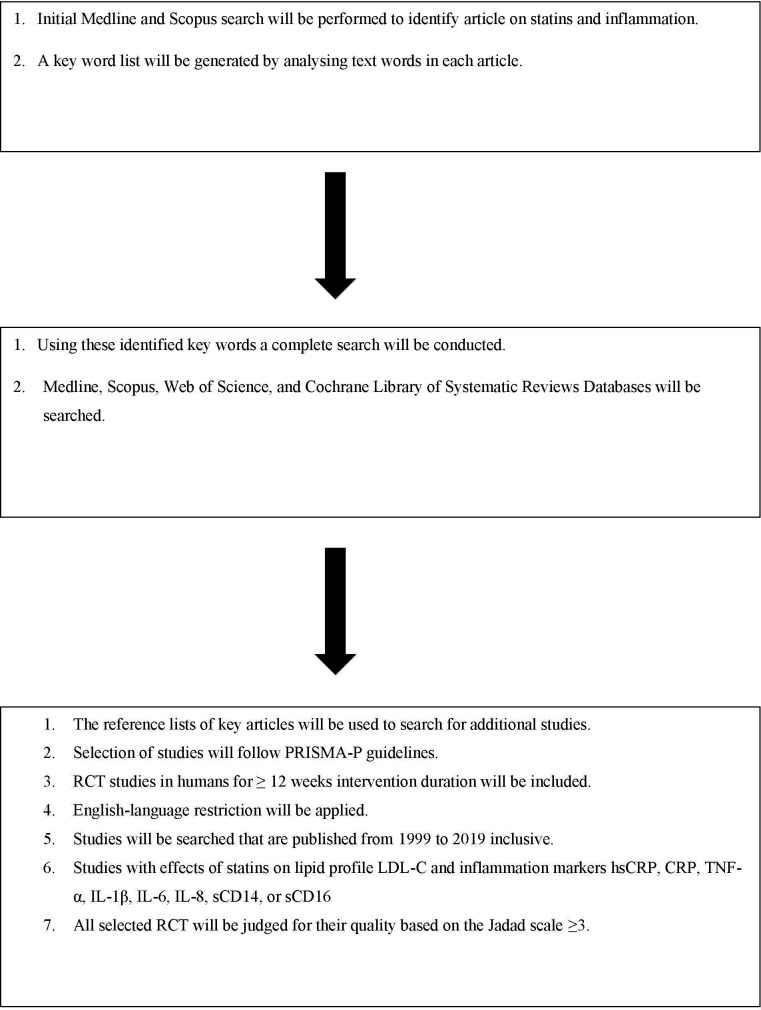
The search strategy (see online supplementary file 1) aims to identify published and peer-reviewed articles with available full text. A stepwise approach will identify the selected articles. As indicated in figure 1, an initial limited search of Medline and Scopus will be undertaken; this will be followed by the analysis of the text words contained in the titles and abstracts, and of the index terms used to describe each article. A second search, using all identified keywords and index terms, will then be undertaken across all included databases. In the third step, the reference lists of key articles will be searched for additional studies. Studies will be restricted to the English language and to those published from 1999 to 2019, inclusive. The databases that will be searched are Medline, Scopus, Web of Science and Cochrane Library of Systematic Reviews.
Figure 1.
A schematic process of the systemic review. CRP, C reactive protein; hsCRP, high-sensitive C reactive protein;IL, interleukin; PRISMA-P, Preferred Reporting Items for Systematic Review and Meta-Analysis Protocol; RCT, randomised controlled clinical trial; TNF-α, tumour necrosis factor alpha.
bmjopen-2020-039034supp001.pdf (78.8KB, pdf)
Eligibility criteria
Inclusion criteria
RCTs in humans.
Adults of at least 18 years of age.
At least one intervention arm including an FDA-licensed or EMA-licensed statin.
Minimum treatment duration of 12 weeks.
Studies that report the effects of statins on lipid profile LDL-C and inflammation markers hsCRP, CRP, TNF-α, IL-1β, IL-6, IL-8, sCD14 or sCD16.
Publication year: January 1999 to December 2019.
Exclusion criteria
RCT including participants with malignancies.
RCT including participants with autoimmune diseases.
RCT with cerivastatin (decommissioned from the market) or lovastatin (not commonly prescribed anymore and its usage is associated with more risks than beneficial effects) as intervention therapy.
Genetic studies.
Study selection
The primary selection of publications will depend on the information contained in their titles and abstracts and will be conducted by two independent investigators and reported using Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols guidelines (see online supplementary file 2). When the reviewers disagree, the article will be re-assessed by a third reviewer.
Quality assessment
Two reviewers will independently verify selected articles to reduce the source of bias. All selected RCTs will be graded for their quality based on the Jadad Scale (see online supplementary file 3), the Oxford quality scoring system which is a widely used checklist for classification of quality of evidence.57
Risk of bias assessment
Two reviewers will assess the risk of bias, based on the Cochrane Risk of Bias Tool for RCTs (see online supplementary file 4). The source of bias will be judged as high, low or unclear for the following domains: random sequence generation, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting of outcome and other sources of bias.
Data extraction
Quantitative and qualitative data will be extracted from selected papers: higher scores in grading, low risk in the evaluation and depending on publication bias tool used. The data extracted will include three domains: (1) identification of the study (year publication, first author’s name, PubMed identification number, title, journal name and impact factor); (2) methodology (study type, co-medication with statin intervention, target population (median/mean age, gender distribution, race), target condition, comorbidities, statin, type, dose, duration) and (3) outcomes (change (or relevant data to estimate change) in lipid profile LDL-C and inflammation markers hsCRP, CRP, TNF-α, IL-1β, IL-6, IL-8, sCD14 or sCD16). For data extraction, two independent Microsoft Excel spreadsheets will be compiled by two reviewers to summarise the data from the included studies. The spreadsheets will then be combined into one. The overall agreement rate between the two investigators will be calculated using Cohen’s κ statistic. Disagreements will be resolved by a third investigator.
Management of missing data
The investigator will be contacted via email in the case that key study specifics or outcome data are missing. If a response is not received within 2 weeks, a reminder email will be sent. A further 2 weeks waiting period will be allowed for responses; if no response or connection is established with the investigator, these studies will be excluded from the analysis.
Data management
Data management will be the responsibility of investigators. A Google Drive folder with shared access among the investigators will be provided for the systematic review which will encompass the protocol, manuscripts and supplementary files from included and excluded studies, as well as documentation of steps in data extraction and analysis, risk of bias and quality assessment. A back-up of the records will be stored on a second hard drive. EndNote V.X9 reference management software will be used in the study.
Outcomes
The primary outcome is the mean difference in systemic inflammatory markers and the secondary outcome is the change in lipid profile between study arms at the end of the statin intervention. The outcomes of the systemic review will be classified into primary and secondary outcomes as follows:
Systemic inflammatory markers: Data will be provided as a change in percent over time for hsCRP, CRP, TNF-α, IL-1β, IL-6, IL-8, sCD14 or sCD16.
Lipid profile: Data will be provided as a change in percent over time for total cholesterol, LDL, high-density lipoprotein and triglycerides.
Analysis
Descriptive analysis
Studies will be categorised by each type of statin intervention and comparison, with data tabulated in narrative form to illustrate the study populations, interventions, durations and outcomes. The outcomes from included studies will provide the following:
Type of intervention (statin) and sample size.
Intervention outcomes will include the change in lipid profile and other inflammatory biomarkers such as hsCRP, CRP, TNF-α, IL-1β, IL-6, IL-8, sCD14 or sCD16.
The outcomes will be analysed together using the Cochrane Review Manager V.5.3 software, according to the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions. If statistical heterogeneity is detected, the random-effects model will be adopted. In terms of considerable statistical heterogeneity, a qualitative summary will be provided by a table, as described above. This will be done by the lead investigator in liaison with a second investigator for accuracy.
Statistical analysis
We will analyse dichotomous data as risk ratios or ORs with 95% CIs and continuous data as mean differences or standardised mean differences. We will perform meta-analyses only if the treatment participants (age group), the underlying clinical question (disease type) and outcomes (assessed inflammatory markers) are similar enough. If an RCT consists of multiple arms, we will include only the relevant arms. A meta-analysis on LDL-C will be performed to assess the potency of statins; for each study, this will be reported as standardised mean differences with its 95% CI. A scatter plot of the percentage change in LDL-C against percentage change in inflammatory biomarkers over a specific time period will be performed to assess the correlation between lipid profile and inflammation. Heterogeneity and potential sources of heterogeneity will be assessed and quantified using I2 and Q statistics. Funnel plot and Egger’s test will be used to assess publication and small sample size bias. Subgroup analysis of identified studies will be stratified based on statin type, concentration and intervention period. Univariable and multivariable meta-regression analysis will be used to investigate the potential sources of heterogeneities. Potential outliers will be investigated in a sensitivity analysis by dropping each study at a time. The Duval and Tweedie trim-and-fill will be used to adjust estimates for the effects of publication bias, if any.
This systematic review will provide further insight into the effectiveness of statins to reduce systemic inflammation in various stages of chronic disease conditions, inform on the most potent statin to reduce systemic inflammation and optimal dosing. In addition, this study will add and improve the existing knowledge of the effects of statins on inflammatory markers and may further provide a basis for future clinical trials in specific diseases.
Supplementary Material
Footnotes
Twitter: @solima
Contributors: RG and FT conceived and planned the idea. BM, SS and MO designed the study protocol. SS and BM designed the figure and wrote the first draft. RG, FT and MO revised the protocol. APK and DB provided valuable insight into data acquisition and statistical analysis. DB and RW revised and designed the reporting of literature. SM, KS, EN, GG and CS critically reviewed the protocol. All authors have approved and contributed to the final written manuscript.
Funding: This publication was produced by StatinTB which is part of the EDCTP2 programme supported by the European Union (grant number RIA2017T-2004-StatinTB). RJW is supported by the Francis Crick Institute which is funded by Wellcome (FC0010218); Medical Research Council (FC0010218) and Cancer Research UK (FC0010218). RJW also receives support from Wellcome (104803, 203135). The work was supported by the Wellcome Trust CIDRI-Africa 203135Z/16/Z fund.
Disclaimer: The views and opinions of authors expressed herein do not necessarily state or reflect those of EDCTP and Wellcome Trust.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Endo A. A historical perspective on the discovery of statins. Proc Jpn Acad Ser B Phys Biol Sci 2010;86:484–93. 10.2183/pjab.86.484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366:1267–78. 10.1016/S0140-6736(05)67394-1 [DOI] [PubMed] [Google Scholar]
- 3.Istvan ES, Deisenhofer J. Structural mechanism for statin inhibition of HMG-CoA reductase. Science 2001;292:1160–4. 10.1126/science.1059344 [DOI] [PubMed] [Google Scholar]
- 4.Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med 2001;5:378–87. 10.1111/j.1582-4934.2001.tb00172.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem 1996;65:241–69. 10.1146/annurev.bi.65.070196.001325 [DOI] [PubMed] [Google Scholar]
- 6.Kavalipati N, Shah J, Ramakrishan A, et al. Pleiotropic effects of statins. Indian J Endocrinol Metab 2015;19:554–62. 10.4103/2230-8210.163106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol 2005;45:89–118. 10.1146/annurev.pharmtox.45.120403.095748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanco-Colio LM, Tuñón J, Martín-Ventura JL, et al. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int 2003;63:12–23. 10.1046/j.1523-1755.2003.00744.x [DOI] [PubMed] [Google Scholar]
- 9.Tran KT, McMenamin Úna C, Coleman HG, et al. Statin use and risk of liver cancer: evidence from two population-based studies. Int J Cancer 2020;146:1250-1260. 10.1002/ijc.32426 [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Chang R, Yao J, et al. Effectiveness of long-term using statins in COPD - a network meta-analysis. Respir Res 2019;20:17. 10.1186/s12931-019-0984-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schachter M, Chemical SM. Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 2005;19:117–25. 10.1111/j.1472-8206.2004.00299.x [DOI] [PubMed] [Google Scholar]
- 12.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J 1990;265:621–36. 10.1042/bj2650621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sproston NR, Ashworth JJ. Role of C-reactive protein at sites of inflammation and infection. Front Immunol 2018;9:754. 10.3389/fimmu.2018.00754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Albert MA, Danielson E, Rifai N, et al. Effect of statin therapy on C-reactive protein levels: the pravastatin inflammation/CRP evaluation (Prince): a randomized trial and cohort study. JAMA 2001;286:64–70. 10.1001/jama.286.1.64 [DOI] [PubMed] [Google Scholar]
- 15.Schaefer EJ, McNamara JR, Asztalos BF, et al. Effects of atorvastatin versus other statins on fasting and postprandial C-reactive protein and lipoprotein-associated phospholipase A2 in patients with coronary heart disease versus control subjects. Am J Cardiol 2005;95:1025–32. 10.1016/j.amjcard.2005.01.023 [DOI] [PubMed] [Google Scholar]
- 16.Ridker PM, Cannon CP, Morrow D, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005;352:20–8. 10.1056/NEJMoa042378 [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Liu C, Zhang L, et al. Intensive atorvastatin therapy attenuates the inflammatory responses in monocytes of patients with unstable angina undergoing percutaneous coronary intervention via peroxisome proliferator-activated receptor γ activation. Inflammation 2015;38:1415–23. 10.1007/s10753-015-0116-2 [DOI] [PubMed] [Google Scholar]
- 18.Toribio M, Fitch KV, Sanchez L, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS 2017;31:797–806. 10.1097/QAD.0000000000001427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waehre T, Damås JK, Gullestad L, et al. Hydroxymethylglutaryl coenzyme a reductase inhibitors down-regulate chemokines and chemokine receptors in patients with coronary artery disease. J Am Coll Cardiol 2003;41:1460–7. 10.1016/S0735-1097(03)00263-8 [DOI] [PubMed] [Google Scholar]
- 20.McKenney JM. Pharmacologic characteristics of statins. Clin Cardiol 2003;26:32–8. 10.1002/clc.4960261507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalliokoski A, Niemi M. Impact of OATP transporters on pharmacokinetics. Br J Pharmacol 2009;158:693–705. 10.1111/j.1476-5381.2009.00430.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grube M, Köck K, Oswald S, et al. Organic anion transporting polypeptide 2B1 is a high-affinity transporter for atorvastatin and is expressed in the human heart. Clin Pharmacol Ther 2006;80:607–20. 10.1016/j.clpt.2006.09.010 [DOI] [PubMed] [Google Scholar]
- 23.Greupink R, Dillen L, Monshouwer M, et al. Interaction of fluvastatin with the liver-specific Na+ -dependent taurocholate cotransporting polypeptide (NTCP). Eur J Pharm Sci 2011;44:487–96. 10.1016/j.ejps.2011.09.009 [DOI] [PubMed] [Google Scholar]
- 24.Jaipersad AS, Shantsila E, Blann A, et al. The effect of statin therapy withdrawal on monocyte subsets. Eur J Clin Invest 2013;43:1307–13. 10.1111/eci.12183 [DOI] [PubMed] [Google Scholar]
- 25.Yilmaz A, Reiss C, Weng A, et al. Differential effects of statins on relevant functions of human monocyte-derived dendritic cells. J Leukoc Biol 2006;79:529–38. 10.1189/jlb.0205064 [DOI] [PubMed] [Google Scholar]
- 26.Veillard NR, Braunersreuther V, Arnaud C, et al. Simvastatin modulates chemokine and chemokine receptor expression by geranylgeranyl isoprenoid pathway in human endothelial cells and macrophages. Atherosclerosis 2006;188:51–8. 10.1016/j.atherosclerosis.2005.10.015 [DOI] [PubMed] [Google Scholar]
- 27.Schramm R, Menger MD, Harder Y, et al. Statins inhibit lymphocyte homing to peripheral lymph nodes. Immunology 2007;120:315–24. 10.1111/j.1365-2567.2006.02505.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraemohs L, Koenen RR, Ostermann G, et al. The functional interaction of the beta 2 integrin lymphocyte function-associated antigen-1 with junctional adhesion molecule-A is mediated by the I domain. J Immunol 2004;173:6259–64. 10.4049/jimmunol.173.10.6259 [DOI] [PubMed] [Google Scholar]
- 29.Romano M, Diomede L, Sironi M, et al. Inhibition of monocyte chemotactic protein-1 synthesis by statins. Lab Invest 2000;80:1095–100. 10.1038/labinvest.3780115 [DOI] [PubMed] [Google Scholar]
- 30.Lazzerini PE, Lorenzini S, Selvi E, et al. Simvastatin inhibits cytokine production and nuclear factor-kB activation in interleukin 1beta-stimulated synoviocytes from rheumatoid arthritis patients. Clin Exp Rheumatol 2007;25:696–700. [PubMed] [Google Scholar]
- 31.Hatanaka T. Clinical pharmacokinetics of pravastatin: mechanisms of pharmacokinetic events. Clin Pharmacokinet 2000;39:397–412. 10.2165/00003088-200039060-00002 [DOI] [PubMed] [Google Scholar]
- 32.Martin PD, Warwick MJ, Dane AL, et al. Metabolism, excretion, and pharmacokinetics of rosuvastatin in healthy adult male volunteers. Clin Ther 2003;25:2822–35. 10.1016/S0149-2918(03)80336-3 [DOI] [PubMed] [Google Scholar]
- 33.Natanzon SS, Matetzky S, Beigel R, et al. Statin therapy among chronic kidney disease patients presenting with acute coronary syndrome. Atherosclerosis 2019;286:14–19. 10.1016/j.atherosclerosis.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 34.Hung T-H, Tsai C-C, Lee H-F. Statin use in cirrhotic patients with infectious diseases: a population-based study. PLoS One 2019;14:e0215839. 10.1371/journal.pone.0215839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lanzillo R, Moccia M, Russo CV, et al. Therapeutic lag in reducing disability progression in relapsing-remitting multiple sclerosis: 8-year follow-up of two randomized add-on trials with atorvastatin. Mult Scler Relat Disord 2019;28:193–6. 10.1016/j.msard.2018.12.042 [DOI] [PubMed] [Google Scholar]
- 36.Birnbaum G, Cree B, Altafullah I, et al. Combining beta interferon and atorvastatin may increase disease activity in multiple sclerosis. Neurology 2008;71:1390–5. 10.1212/01.wnl.0000319698.40024.1c [DOI] [PubMed] [Google Scholar]
- 37.Borkar DS, Tham VM, Shen E, et al. Association between statin use and uveitis: results from the Pacific ocular inflammation study. Am J Ophthalmol 2015;159:707–13. 10.1016/j.ajo.2015.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian J, Hou X, Hu L, et al. Efficacy comparison of atorvastatin versus rosuvastatin on blood lipid and microinflammatory state in maintenance hemodialysis patients. Ren Fail 2017;39:153–8. 10.1080/0886022X.2016.1256309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ota Y, Kitamura M, Muta K, et al. Effect of statin on life prognosis in Japanese patients undergoing hemodialysis. PLoS One 2019;14:e0224111. 10.1371/journal.pone.0224111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer SC, Navaneethan SD, Craig JC, et al. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst Rev 2013;9:CD004289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neukamm A, Høiseth AD, Einvik G, et al. Rosuvastatin treatment in stable chronic obstructive pulmonary disease (RODEO): a randomized controlled trial. J Intern Med 2015;278:59–67. 10.1111/joim.12337 [DOI] [PubMed] [Google Scholar]
- 42.Mandal P, Chalmers JD, Graham C, et al. Atorvastatin as a stable treatment in bronchiectasis: a randomised controlled trial. Lancet Respir Med 2014;2:455–63. 10.1016/S2213-2600(14)70050-5 [DOI] [PubMed] [Google Scholar]
- 43.Allott EH, Howard LE, Vidal AC, et al. Statin use, serum lipids, and prostate inflammation in men with a negative prostate biopsy: results from the reduce trial. Cancer Prev Res 2017;10:319–26. 10.1158/1940-6207.CAPR-17-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin H-W, Lin L-F, Chen H-C, et al. Chronic obstructive pulmonary disease with short-acting inhaled pharmacotherapy increases the risk of prostate cancer: a two-stage database approach. PLoS One 2018;13:e0203377. 10.1371/journal.pone.0203377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Emilsson L, García-Albéniz X, Logan RW, et al. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol 2018;4:63–70. 10.1001/jamaoncol.2017.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willey JZ, Elkind MSV. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors in the treatment of central nervous system diseases. Arch Neurol 2010;67:1062–7. 10.1001/archneurol.2010.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010;74:956–64. 10.1212/WNL.0b013e3181d6476a [DOI] [PubMed] [Google Scholar]
- 48.Sano M, Bell KL, Galasko D, et al. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology 2011;77:556–63. 10.1212/WNL.0b013e318228bf11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abdelmaksoud AA, Girerd PH, Garcia EM, et al. Association between statin use, the vaginal microbiome, and Gardnerella vaginalis vaginolysin-mediated cytotoxicity. PLoS One 2017;12:e0183765. 10.1371/journal.pone.0183765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pouwels KB, Widyakusuma NN, Bos JHJ, et al. Association between statins and infections among patients with diabetes: a cohort and prescription sequence symmetry analysis. Pharmacoepidemiol Drug Saf 2016;25:1124–30. 10.1002/pds.4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yeh L-T, Tang C-Y, Yang S-F, et al. Association between statin use and sepsis risk in patients with dementia: a retrospective cohort study. Int J Environ Res Public Health 2019;16:1626 10.3390/ijerph16091626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yeh J-J, Lin C-L, Hsu C-Y, et al. Statin for tuberculosis and pneumonia in patients with Asthma⁻Chronic pulmonary disease overlap syndrome: a time-dependent population-based cohort study. J Clin Med 2018;7:381–5. 10.3390/jcm7110381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su VY-F, Su W-J, Yen Y-F, et al. Statin use is associated with a lower risk of TB. Chest 2017;152:598–606. 10.1016/j.chest.2017.04.170 [DOI] [PubMed] [Google Scholar]
- 54.Lai C-C, Lee M-TG, Lee S-H, et al. Statin treatment is associated with a decreased risk of active tuberculosis: an analysis of a nationally representative cohort. Thorax 2016;71:646–51. 10.1136/thoraxjnl-2015-207052 [DOI] [PubMed] [Google Scholar]
- 55.Parihar SP, Guler R, Khutlang R, et al. Statin therapy reduces the Mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J Infect Dis 2014;209:754–63. 10.1093/infdis/jit550 [DOI] [PubMed] [Google Scholar]
- 56.Parihar SP, Guler R, Brombacher F. Statins: a viable candidate for host-directed therapy against infectious diseases. Nat Rev Immunol 2019;19:104–17. 10.1038/s41577-018-0094-3 [DOI] [PubMed] [Google Scholar]
- 57.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12. 10.1016/0197-2456(95)00134-4 [DOI] [PubMed] [Google Scholar]
- 58.Stern RH, Yang BB, Horton M, et al. Renal dysfunction does not alter the pharmacokinetics or LDL-cholesterol reduction of atorvastatin. J Clin Pharmacol 1997;37:816–9. 10.1002/j.1552-4604.1997.tb05629.x [DOI] [PubMed] [Google Scholar]
- 59.Corsini A, Bellosta S, Baetta R, et al. New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther 1999;84:413–28. [DOI] [PubMed] [Google Scholar]
- 60.Davidson MH. Controversy surrounding the safety of cerivastatin. Expert Opin Drug Saf 2002;1:207–12. 10.1517/14740338.1.3.207 [DOI] [PubMed] [Google Scholar]
- 61.Gibson DM, Bron NJ, Richens A, et al. Effect of age and gender on pharmacokinetics of atorvastatin in humans. J Clin Pharmacol 1996;36:242–6. 10.1002/j.1552-4604.1996.tb04194.x [DOI] [PubMed] [Google Scholar]
- 62.Cilla DD, Whitfield LR, Gibson DM, et al. Multiple-Dose pharmacokinetics, pharmacodynamics, and safety of atorvastatin, an inhibitor of HMG-CoA reductase, in healthy subjects. Clin Pharmacol Ther 1996;60:687–95. 10.1016/S0009-9236(96)90218-0 [DOI] [PubMed] [Google Scholar]
- 63.Mück W, Ritter W, Ochmann K, et al. Absolute and relative bioavailability of the HMG-CoA reductase inhibitor cerivastatin. Int J Clin Pharmacol Ther 1997;35:255–60. [PubMed] [Google Scholar]
- 64.Schall R, Müller FO, Hundt HK, et al. No pharmacokinetic or pharmacodynamic interaction between rivastatin and warfarin. J Clin Pharmacol 1995;35:306–13. 10.1002/j.1552-4604.1995.tb04065.x [DOI] [PubMed] [Google Scholar]
- 65.Kantola T, Kivistö KT, Neuvonen PJ. Effect of itraconazole on cerivastatin pharmacokinetics. Eur J Clin Pharmacol 1999;54:851–5. 10.1007/s002280050566 [DOI] [PubMed] [Google Scholar]
- 66.Bayer BAYCOL (cerivastatin sodium tablets), 1997. [Google Scholar]
- 67.Mück W, Unger S, Kawano K, et al. Inter-ethnic comparisons of the pharmacokinetics of the HMG-CoA reductase inhibitor cerivastatin. Br J Clin Pharmacol 1998;45:583–90. 10.1046/j.1365-2125.1998.00717.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tse FL, Jaffe JM, Troendle A. Pharmacokinetics of fluvastatin after single and multiple doses in normal volunteers. J Clin Pharmacol 1992;32:630–8. 10.1002/j.1552-4604.1992.tb05773.x [DOI] [PubMed] [Google Scholar]
- 69.Desager JP, Horsmans Y. Clinical pharmacokinetics of 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors. Clin Pharmacokinet 1996;31:348–71. 10.2165/00003088-199631050-00003 [DOI] [PubMed] [Google Scholar]
- 70.Smith HT, Jokubaitis LA, Troendle AJ, et al. Pharmacokinetics of fluvastatin and specific drug interactions. Am J Hypertens 1993;6:375S–82. 10.1093/ajh/6.11.375S [DOI] [PubMed] [Google Scholar]
- 71.Lennernäs H, Fager G. Pharmacodynamics and pharmacokinetics of the HMG-CoA reductase inhibitors. similarities and differences. Clin Pharmacokinet 1997;32:403–25. 10.2165/00003088-199732050-00005 [DOI] [PubMed] [Google Scholar]
- 72.Lindahl A, Sandström R, Ungell AL, et al. Jejunal permeability and hepatic extraction of fluvastatin in humans. Clin Pharmacol Ther 1996;60:493–503. 10.1016/S0009-9236(96)90145-9 [DOI] [PubMed] [Google Scholar]
- 73.Kantola T, Backman JT, Niemi M, et al. Effect of fluconazole on plasma fluvastatin and pravastatin concentrations. Eur J Clin Pharmacol 2000;56:225–9. 10.1007/s002280000127 [DOI] [PubMed] [Google Scholar]
- 74.Siekmeier R, Lattke P, Mix C, et al. Dose dependency of fluvastatin pharmacokinetics in serum determined by reversed phase HPLC. J Cardiovasc Pharmacol Ther 2001;6:137–45. 10.1177/107424840100600205 [DOI] [PubMed] [Google Scholar]
- 75.Smit JW, Wijnne HJ, Schobben F, et al. Effects of alcohol consumption on pharmacokinetics, efficacy, and safety of fluvastatin. Am J Cardiol 1995;76:89A–96. 10.1016/S0002-9149(05)80026-8 [DOI] [PubMed] [Google Scholar]
- 76.Kivistö KT, Kantola T, Neuvonen PJ. Different effects of itraconazole on the pharmacokinetics of fluvastatin and lovastatin. Br J Clin Pharmacol 1998;46:49–53. 10.1046/j.1365-2125.1998.00034.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pentikainen PJ, Saraheimo M, Schwartz JI, et al. Comparative pharmacokinetics of lovastatin, simvastatin and pravastatin in humans. J Clin Pharmacol 1992;32:136–40. 10.1002/j.1552-4604.1992.tb03818.x [DOI] [PubMed] [Google Scholar]
- 78.Pan HY, Triscari J, DeVault AR, et al. Pharmacokinetic interaction between propranolol and the HMG-CoA reductase inhibitors pravastatin and lovastatin. Br J Clin Pharmacol 1991;31:665–70. 10.1111/j.1365-2125.1991.tb05590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Blum CB. Comparison of properties of four inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Am J Cardiol 1994;73:D3–11. 10.1016/0002-9149(94)90626-2 [DOI] [PubMed] [Google Scholar]
- 80.Pan HY, DeVault AR, Wang-Iverson D, et al. Comparative pharmacokinetics and pharmacodynamics of pravastatin and lovastatin. J Clin Pharmacol 1990;30:1128–35. 10.1002/j.1552-4604.1990.tb01856.x [DOI] [PubMed] [Google Scholar]
- 81.Qi X, Ding L, Wen A, et al. Simple LC-MS/MS methods for simultaneous determination of pitavastatin and its lactone metabolite in human plasma and urine involving a procedure for inhibiting the conversion of pitavastatin lactone to pitavastatin in plasma and its application to a pharmacokinetic study. J Pharm Biomed Anal 2013;72:8–15. 10.1016/j.jpba.2012.09.026 [DOI] [PubMed] [Google Scholar]
- 82.Wen J, Xiong Y. OATP1B1 388A>G polymorphism and pharmacokinetics of pitavastatin in Chinese healthy volunteers. J Clin Pharm Ther 2010;35:99–104. 10.1111/j.1365-2710.2009.01071.x [DOI] [PubMed] [Google Scholar]
- 83.Chen Y, Zhang W, Huang W-hua, et al. Effect of a single-dose rifampin on the pharmacokinetics of pitavastatin in healthy volunteers. Eur J Clin Pharmacol 2013;69:1933–8. 10.1007/s00228-013-1554-0 [DOI] [PubMed] [Google Scholar]
- 84.Shang D, Deng S, Yao Z, et al. The effect of food on the pharmacokinetic properties and bioequivalence of two formulations of pitavastatin calcium in healthy Chinese male subjects. Xenobiotica 2016;46:34–9. 10.3109/00498254.2015.1046153 [DOI] [PubMed] [Google Scholar]
- 85.Kowa LIVALO® (pitavastatin) tablet, 2016. [Google Scholar]
- 86.Luo Z, Zhang Y, Gu J, et al. Pharmacokinetic properties of single- and multiple-dose pitavastatin calcium tablets in healthy Chinese volunteers. Curr Ther Res Clin Exp 2015;77:52–7. 10.1016/j.curtheres.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ando H, Tsuruoka S, Yanagihara H, et al. Effects of grapefruit juice on the pharmacokinetics of pitavastatin and atorvastatin. Br J Clin Pharmacol 2005;60:494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu H-F, Hristeva N, Chang J, et al. Rosuvastatin pharmacokinetics in Asian and white subjects wild type for both OATP1B1 and BCRP under control and inhibited conditions. J Pharm Sci 2017;106:2751–7. 10.1016/j.xphs.2017.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee E, Ryan S, Birmingham B, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in white and Asian subjects residing in the same environment. Clin Pharmacol Ther 2005;78:330–41. 10.1016/j.clpt.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 90.Zhang W, Yu B-N, He Y-J, et al. Role of BCRP 421C>A polymorphism on rosuvastatin pharmacokinetics in healthy Chinese males. Clin Chim Acta 2006;373:99–103. 10.1016/j.cca.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 91.Li Y, Jiang X, Lan K, et al. Pharmacokinetic properties of rosuvastatin after single-dose, oral administration in Chinese volunteers: a randomized, open-label, three-way crossover study. Clin Ther 2007;29:2194–203. 10.1016/j.clinthera.2007.10.005 [DOI] [PubMed] [Google Scholar]
- 92.Birmingham BK, Bujac SR, Elsby R, et al. Impact of ABCG2 and SLCO1B1 polymorphisms on pharmacokinetics of rosuvastatin, atorvastatin and simvastatin acid in Caucasian and Asian subjects: a class effect? Eur J Clin Pharmacol 2015;71:341–55. 10.1007/s00228-014-1801-z [DOI] [PubMed] [Google Scholar]
- 93.Birmingham BK, Bujac SR, Elsby R, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol 2015;71:329–40. 10.1007/s00228-014-1800-0 [DOI] [PubMed] [Google Scholar]
- 94.Martin PD, Warwick MJ, Dane AL, et al. Absolute oral bioavailability of rosuvastatin in healthy white adult male volunteers. Clin Ther 2003;25:2553–63. 10.1016/S0149-2918(03)80316-8 [DOI] [PubMed] [Google Scholar]
- 95.Bergman E, Forsell P, Tevell A, et al. Biliary secretion of rosuvastatin and bile acids in humans during the absorption phase. Eur J Pharm Sci 2006;29:205–14. 10.1016/j.ejps.2006.04.015 [DOI] [PubMed] [Google Scholar]
- 96.McTaggart F, Buckett L, Davidson R, et al. Preclinical and clinical pharmacology of rosuvastatin, a new 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor. Am J Cardiol 2001;87:28B–32. 10.1016/s0002-9149(01)01454-0 [DOI] [PubMed] [Google Scholar]
- 97.Singhvi SM, Pan HY, Morrison RA, et al. Disposition of pravastatin sodium, a tissue-selective HMG-CoA reductase inhibitor, in healthy subjects. Br J Clin Pharmacol 1990;29:239–43. 10.1111/j.1365-2125.1990.tb03626.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kyrklund C, Backman JT, Neuvonen M, et al. Gemfibrozil increases plasma pravastatin concentrations and reduces pravastatin renal clearance. Clin Pharmacol Ther 2003;73:538–44. 10.1016/S0009-9236(03)00052-3 [DOI] [PubMed] [Google Scholar]
- 99.Kyrklund C, Backman JT, Neuvonen M, et al. Effect of rifampicin on pravastatin pharmacokinetics in healthy subjects. Br J Clin Pharmacol 2004;57:181–7. 10.1046/j.1365-2125.2003.01972.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Almeida S, Filipe A, Almeida A, et al. Comparative study on the bioequivalence of two formulations of pravastatin. data from a crossover, randomised, open-label bioequivalence study in healthy volunteers. Arzneimittelforschung 2006;56:70–5. 10.1055/s-0031-1296704 [DOI] [PubMed] [Google Scholar]
- 101.Ogawa K, Hasegawa S, Udaka Y, et al. Individual difference in the pharmacokinetics of a drug, pravastatin, in healthy subjects. J Clin Pharmacol 2003;43:1268–73. 10.1177/0091270003257232 [DOI] [PubMed] [Google Scholar]
- 102.Pan WJ, Gustavson LE, Achari R, et al. Lack of a clinically significant pharmacokinetic interaction between fenofibrate and pravastatin in healthy volunteers. J Clin Pharmacol 2000;40:316–23. 10.1177/00912700022008874 [DOI] [PubMed] [Google Scholar]
- 103.Escobar Y, Venturelli CR, Hoyo-Vadillo C. Pharmacokinetic properties of pravastatin in Mexicans: an open-label study in healthy adult volunteers. Curr Ther Res Clin Exp 2005;66:238–46. 10.1016/j.curtheres.2005.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mauro VF. Clinical pharmacokinetics and practical applications of simvastatin. Clin Pharmacokinet 1993;24:195–202. 10.2165/00003088-199324030-00002 [DOI] [PubMed] [Google Scholar]
- 105.Sunkara G, Reynolds CV, Pommier F, et al. Evaluation of a pharmacokinetic interaction between valsartan and simvastatin in healthy subjects. Curr Med Res Opin 2007;23:631–40. 10.1185/030079906X167471 [DOI] [PubMed] [Google Scholar]
- 106.Park S-J, Yeo C-W, Shim E-J, et al. Pomegranate juice does not affect the disposition of simvastatin in healthy subjects. Eur J Drug Metab Pharmacokinet 2016;41:339–44. 10.1007/s13318-015-0263-8 [DOI] [PubMed] [Google Scholar]
- 107.O’Brien SG, Meinhardt P, Bond E, et al. Effects of imatinib mesylate (STI571, Glivec) on the pharmacokinetics of simvastatin, a cytochrome P450 3A4 substrate, in patients with chronic myeloid leukaemia. Br J Cancer 2003;89:1855–9. 10.1038/sj.bjc.6601152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merck ZOCOR (simvastatin) tablets, 1991. [Google Scholar]
- 109.Kyrklund C, Backman JT, Kivistö KT, et al. Rifampin greatly reduces plasma simvastatin and simvastatin acid concentrations. Clin Pharmacol Ther 2000;68:592–7. 10.1067/mcp.2000.111414 [DOI] [PubMed] [Google Scholar]
- 110.Ucar M, Neuvonen M, Luurila H, et al. Carbamazepine markedly reduces serum concentrations of simvastatin and simvastatin acid. Eur J Clin Pharmacol 2004;59:879–82. 10.1007/s00228-003-0700-5 [DOI] [PubMed] [Google Scholar]
- 111.Neuvonen PJ, Kantola T, Kivistö KT. Simvastatin but not pravastatin is very susceptible to interaction with the CYP3A4 inhibitor itraconazole. Clin Pharmacol Ther 1998;63:332–41. 10.1016/S0009-9236(98)90165-5 [DOI] [PubMed] [Google Scholar]
- 112.Mousa O, Brater DC, Sunblad KJ, et al. The interaction of diltiazem with simvastatin. Clin Pharmacol Ther 2000;67:267–74. 10.1067/mcp.2000.104609 [DOI] [PubMed] [Google Scholar]
- 113.Lilja JJ, Kivistö KT, Neuvonen PJ. Grapefruit juice-simvastatin interaction: effect on serum concentrations of simvastatin, simvastatin acid, and HMG-CoA reductase inhibitors. Clin Pharmacol Ther 1998;64:477–83. 10.1016/S0009-9236(98)90130-8 [DOI] [PubMed] [Google Scholar]
- 114.Backman JT, Kyrklund C, Kivistö KT, et al. Plasma concentrations of active simvastatin acid are increased by gemfibrozil. Clin Pharmacol Ther 2000;68:122–9. 10.1067/mcp.2000.108507 [DOI] [PubMed] [Google Scholar]
- 115.Tubic-Grozdanis M, Hilfinger JM, Amidon GL, et al. Pharmacokinetics of the CYP 3A substrate simvastatin following administration of delayed versus immediate release oral dosage forms. Pharm Res 2008;25:1591–600. 10.1007/s11095-007-9519-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-039034supp001.pdf (78.8KB, pdf)