Many transgender and gender diverse people want to have children, but cross-sex hormone treatments limit fertility and complete gender affirming genital surgeries preclude it (Auer et al., 2018; Charter, Ussher, Perz, & Robinson, 2018; Kyweluk, Sajwani, & Chen, 2018). Stopping hormones requires foregoing gender affirming treatment, but cannot guarantee recovery of mature gametes. Cryopreserving gametes for in vitro fertilization (IVF) is more reliable, but it is costly and requires forecasting future fertility goals which are inherently stochastic. More importantly, since it necessitates gestational surrogacy, it disallows patients the fundamental experience of carrying a child, and though a technically straightforward solution to uterine infertility, it is not without emotional, social, and legal complications. Uterus transplantation (UTx) is currently being investigated for patients with absolute uterine factor infertility (AUFI) desiring pregnancy (Brännström et al., 2015; Ejzenberg et al., 2018). The Montreal Criteria, which are the international ethical standards governing UTx, require recipients to be genetically female (Lefkowitz, Edwards, & Balayla, 2013). The purpose of this editorial is to use the case-reasoning method of applied medical ethics to suggest consideration of UTx for transgender women.
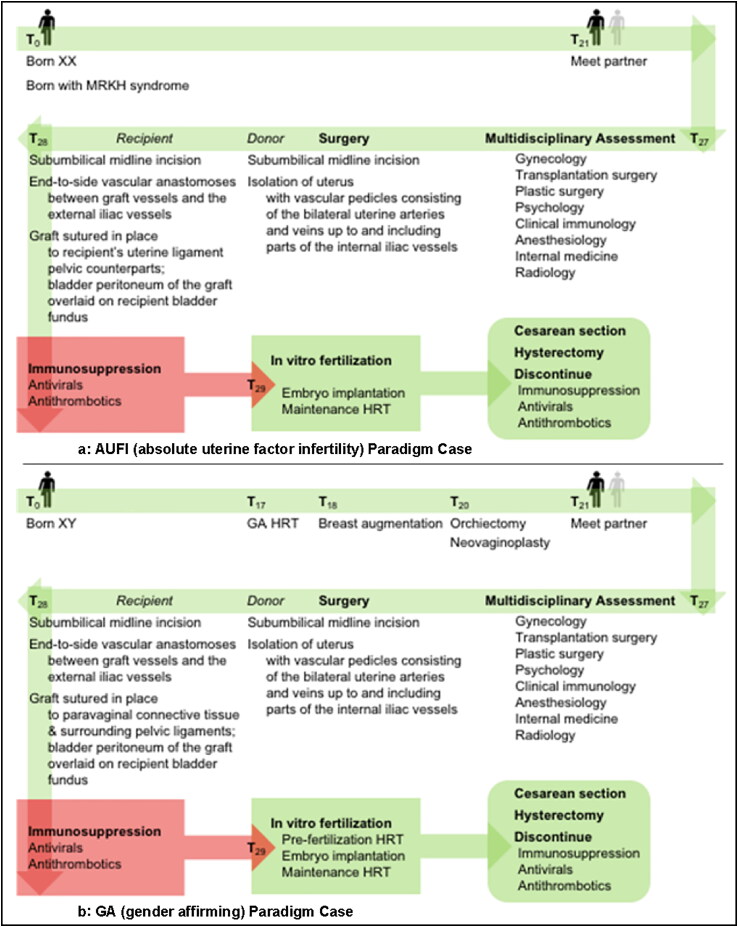
The 2013 updated Montreal Criteria were reviewed and all justifications for excluding transgender women were indexed. Components of each justification were separated and matched to their representative prima facie duties, and the relationship between competing prima facie duties was identified. For the purpose of this editorial two paradigm cases were constructed (See Figure 1a,b). “AUFI” (Absolute Uterine Factor Infertility) was modeled after the cisgender women with absolute uterine infertility factor assigned as patients in the Uterus Transplantation Trial at Baylor University Medical Center in Dallas, Texas, USA, as well as the described treatment techniques implemented in the trial (Testa et al., 2017). “GA” was modeled after a patient pursuing gender affirming care and eventually UTx. We analyzed the degree of matching between AUFI and GA to more accurately define the relationship between the prima facie duties. We defined “risk” as an aggregate of risks to donor, recipient, and fetus. Firstly, we identified all justification(s) in the Montreal Criteria for prohibiting UTx in transgender females. We identified one justification:
Figure 1.
(a) AUFI Paradigm Case: Timeline of events for genetic XX person with absent uterus due to MRKH syndrome pursuing uterine transplantation. AUFI = absolute uterine factor infertility, MRKH = Mayer-Rokitansky-Küster-Hauser syndrome, HRT = Hormone Replacement Therapy. (b) GA Paradigm Case: Timeline of events for genetic XY person undergoing gender affirming care and later pursuing uterine transplantation. GA = gender affirming, HRT = Hormone Replacement Therapy.
‘Uterine transplant offers the same promise of a solution for males or trans individuals wishing to gestate a child as it does for genetic females with UFI. Nevertheless, the Montreal Criteria require that the recipient be a genetic female. This warrants both justification and discussion. To date, only female recipients have been used in animal and human trials of uterine transplant. There are many interesting yet daunting theoretical medical issues concerning uterine transplant with a nongenetic female recipient, including the creation of adequate uterine vascularization de novo, the necessity for appropriate hormone replacement to sustain implantation and pregnancy, and the placement of the uterus in a nongynecoid pelvis.’
Next, we separated the justification into its component(s) and matched each component to its corresponding prima facie duty:
“There are many interesting yet daunting theoretical medical issues concerning uterine transplant with a nongenetic female recipient, including the creation of adequate uterine vascularization de novo, the necessity for appropriate hormone replacement to sustain implantation and pregnancy, and the placement of the uterus in a nongynecoid pelvis.” [Non-maleficence]
“However, it certainly bears mentioning that there does not seem to be a prima facie ethical reason to reject the idea of performing uterine transplant on a male or trans patient. A male or trans patient wishing to gestate a child does not have a lesser claim to that desire than their female counterparts. The principle of autonomy is not sex-specific. This right is not absolute, but it is not the business of medicine to decide what is unreasonable to request for a person of sound mind, except as it relates to medical and surgical risk, as well as to distribution of resources. A male who identifies as a woman, for example, arguably has UFI, no functionally different than a woman who is born female with UFI.” [Autonomy]
Since the first stipulation of the Montreal criteria explicitly excludes nongenetic females including transgender women as candidates for Utx, we identified the implied relationship between the two competing prima facie duties elaborated by the authors to be: Medical and surgical risk [Non-maleficence] > Right to gestate a child [Autonomy]. We aimed to interrogate the specific points corresponding to the prima facie duty of non-maleficence, which articulate a purported increase in medical and surgical risk of Utx in nongenetic females. In doing so, we sought to reevaluate the authors’ claimed relationship, in which non-maleficence is escalated to surpass a transgender woman’s autonomous right to gestate. We used the case-reasoning method of applied ethics to evaluate any increased risks in the GA paradigm case when compared to the AUFI paradigm case:
The method of uterine vascularization de novo (end-to-side vascular anastomoses between graft vessels and recipient external iliac vessels) did not differ between AUFI and GC. Suture sites for fixing the graft in its pelvic location differed minimally.
Post-transplant immunosuppression regimens were identical in both cases; this was the zone of highest risk and equal between both cases.
Hormone therapy to sustain GA implantation and pregnancy did not differ significantly from AUFI or established in vitro fertilization regimens and thus did not portend increased risk.
Nongynecoid pelvis (and its potential to cause cephalopelvic disproportion during vaginal delivery) was not identified as adding increased risk to GA, since the mode of delivery in both paradigm cases was by cesarean section.
In conclusion, we analyzed each theoretical medical and surgical issue concerning UTx in transgender women and examined to what extent they differed from cisgender females. We were unable to identify any increases in risk, from initial assessment to delivery and subsequent hysterectomy. We propose that transgender women that have pursued gender affirming care should not be excluded from the prima facie duty relationships recognized in cisgender females due to assigned male sex at birth. As the authors of the Montreal Criteria have stressed, we emphasize that these criteria must be dynamic and evolve to parallel the changing landscape of medicine, surgery, and transgender health.
Declaration of interest statement
The authors declare they have no conflicts of interest.
Vikram G. Mookerjee
Department of Plastic Surgery, Rhode Island Hospital, Brown University Alpert Medical School, Providence, RI, USA
mook@bu.edu
Daniel Kwan
Department of Plastic Surgery, Rhode Island Hospital, Brown University Alpert Medical School, Providence, RI, USA
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Auer, M. K., Fuss, J., Nieder, T. O., Briken, P., Biedermann, S. V., Stalla, G. K., … Hildebrandt, T. (2018). Desire to have children among transgender people in Germany: A cross-sectional multi-center study. Journal of Sexual Medicine, 15(5), 757–767. doi: 10.1016/j.jsxm.2018.03.083 [DOI] [PubMed] [Google Scholar]
- Brännström, M., Johannesson, L., Bokström, H., Kvarnström, N., Mölne, J., Dahm-Kähler, P., … Nilsson, L. (2015). Livebirth after uterus transplantation. Lancet, 385(9968), 607–616. doi: 10.1016/S0140-6736(14)61728-1 [DOI] [PubMed] [Google Scholar]
- Charter, R., Ussher, J. M., Perz, J., & Robinson, K. (2018). The transgender parent: experiences and constructions of pregnancy and parenthood for transgender men in Australia. International Journal of Transgenderism, 19(1), 64–77. doi: 10.1080/15532739.2017.1399496 [DOI] [Google Scholar]
- Ejzenberg, D., Andraus, W., Baratelli Carelli Mendes, L. R., Ducatti, L., Song, A., Tanigawa, R., … Chada Baracat, E. (2018). Livebirth after uterus transplantation from a deceased donor in a recipient with uterine infertility. Lancet, 392(10165), 2697–2704. doi: 10.1016/S0140-6736(18)31766-5 [DOI] [PubMed] [Google Scholar]
- Kyweluk, M. A., Sajwani, A., & Chen, D. (2018). Freezing for the future: transgender youth respond to medical fertility preservation. International Journal of Transgenderism, 19(4), 401–416. doi: 10.1080/15532739.2018.1505575 [DOI] [Google Scholar]
- Lefkowitz, A., Edwards, M., & Balayla, J. (2013). Ethical considerations in the era of the uterine transplant: An update of the Montreal Criteria for the ethical feasibility of uterine transplantation. Fertility and Sterility, 4, 924–926. [DOI] [PubMed] [Google Scholar]
- Testa, G., Koon, E. C., Johannesson, L., McKenna, G. J., Anthony, T., Klintmalm, G. B., … Olausson, M. (2017). Living donor uterus transplantation: A single center's observations and lessons learned from early setbacks to technical success. American Journal of Transplantation, 11, 2901–2910. [DOI] [PubMed] [Google Scholar]