Abstract
Introduction
“What effective ways of motivation, support and technologies help people with cystic fibrosis improve and sustain adherence to treatment?” was identified as one of the James Lind Alliance Priority Setting Partnership’s top 10 research questions in cystic fibrosis (CF). Using electronic questionnaires, we aimed to gain a deeper understanding of this research priority.
Method
The work was led by the steering group representative of the UK CF community consisting of patients, carers and healthcare professionals (HCPs). Electronic questionnaires were completed over a 4-week period and promoted via online forums such as Twitter, the UK CF Trust and US CF Foundation websites and via professional networks. Analysis of the closed questions was completed using Microsoft Excel, with keyword analysis and the final thematic analysis completed using NVivo software.
Results
There were 313 respondents; 176/313 (56%) were from people with CF and their families. HCPs comprised of 10 professional groups accounting for 137/313 (44%) of respondents, with global involvement of participants with the majority from the UK. Common themes identified as impacting on adherence included: having no time, treatment burden, competing life demands, fatigue and the patient’s general health. Having a routine was identified as the most frequently used motivational strategy, valued by both the patient and professional community. However, some strategies were valued more by HCPs than used in practice by patients; these included the use of short-term goal setting and technology use.
Conclusion
Adherence to treatment is crucial, however it is often suboptimal and strategies valued by HCPs to promote adherence are not always shared by patients. To promote adherence clinicians and researchers should be mindful that in a condition where treatment burden and time pressures are considerable, any interventions should focus on simplifying care and reducing treatment burden.
Keywords: cystic fibrosis
Key messages.
This study further explored one of the top 10 James Lind Alliance Priority Setting Partnership questions “What effective ways of motivation, support and technologies help people with cystic fibrosis improve and sustain adherence to treatment?”
Treatment burden in cystic fibrosis (CF) is high and this affects adherence. We have highlighted the common themes impacting on adherence and the strategies used by both the patient and clinical CF community to improve this, including the role of digital technology.
Through our work we have also demonstrated areas for future research which will be of benefit to the patient, clinical and research CF community.
Introduction
Cystic fibrosis (CF) is an autosomal recessive multisystem disorder requiring a demanding daily regimen which may include airway clearance, inhaled therapies, pancreatic enzyme replacement therapies (PERT) and nutritional supplements.1 In the UK there are 10 000 people living with the condition, of which 40% are under the age of 16 years, with a predicted life expectancy of 47 years for those born in 2017.2 In the USA this number is 34 000.3 The formation of specialised CF centres in conjunction with improvements in respiratory treatments, better nutrition and the development of cystic fibrosis transmembrane conductance regulator (CFTR) modulator therapies have improved clinical outcomes for patients. The associated treatment burden is high with patients typically spending two to two and a half hours a day on their treatment regimens.4 5 Treatment adherence is crucial, with low adherence associated with increased pulmonary exacerbations, hospitalisations and lower respiratory function.6 However, adherence rates in CF are suboptimal.6
In a recent global James Lind Alliance (JLA) Priority Setting Partnership exercise, the CF patient and clinical community jointly identified the top 10 clinical research questions important to the CF community.7 The following question was included in the top 10; “What effective ways of motivation, support and technologies help people with cystic fibrosis improve and sustain adherence to treatment?”. To gain a deeper understanding of this research priority question we undertook an online survey to gain views from the CF community.
We aimed to first understand the challenges people with CF (pwCF) face with regards to adherence and second to summarise the motivation and support strategies used in promoting adherence. We aimed to better understand the views of the patient CF community and their families and to determine if these views differed from those of healthcare professionals (HCPs). In addition, technology is having an increasing role within healthcare and its importance has been recognised by the World Health Organisation (WHO).8 Our final aim through exploration of this question was to explore the role of technologies in promoting treatment adherence in CF.
Methods
Data collection
This work was led by a steering group representative of the UK CF community consisting of pwCF, parents of pwCF and multidisciplinary HCPs. An electronic questionnaire was co-produced with contributions from steering group representatives using SurveyMonkey (http://www.surveymonkey.com) and comprised of both closed questions and narrative free-text responses (online supplementary file 1). This was broadly separated within the survey into the three main areas of interest with regards to adherence: motivation, support and technology. The survey was open to pwCF, their friends and families and HCPs for a period of 4 weeks (March to April 2019) and was inclusive of all ages. There were separate streams for lay and professional respondents to complete. Participants were asked to agree to the privacy policy prior to taking part and General Data Protection Regulation (GDPR) guidance was used for the management of any personally identifiable data. The survey was promoted on Twitter using the twitter handle @questionCF as well as by the group @CFAware, the UK CF Trust, the US CF Foundation and via professional networks. This work was supported by the UK CF Trust under the title ‘James Lind CF2’. The University of Nottingham Research Ethics Committee deemed the original JLA work to not require ethical approval and this was an extension of this work.
bmjresp-2020-000601supp001.pdf (3.4MB, pdf)
Data analysis
Data were captured automatically by SurveyMonkey and downloaded to Microsoft Excel for quantitative data and NVivo qualitative data software package (QSR International) for the free-text comments. Informed by an analytical approach previously developed by the group9 a structured, iterative approach to data analysis was used which incorporated descriptive statistics, qualitative content analysis and qualitative thematic analysis.
Descriptive statistics were generated for the closed response questions and the word frequency function of the NVivo package was used to perform a content analysis10 of those questions which offered a free-text response. Semantically linked words (ie, tired, tiring) were combined in the counts and words such as ‘adherence’ and ‘motivation’ were removed (their presence in the question artificially increasing their count). These quantitative data were merged and reviewed for each area of interest (motivation, support, technology). This was particularly useful with regards to identifying the types of technology used.
Synthesis of the data identified simple trends within the focus of answers and constituted an initial phase of thematic analysis.11 From the thematic analysis we identified areas of interest (termed codes) and overarching ideas which might explain them (termed themes). Following this, all free-text responses were reviewed and mapped to these codes/themes. Given the variations in the quality and quantity of the free-text responses, qualitative data could be mapped to multiple codes/themes. Additional codes/themes were also created in response to novel comments. To ensure consistency, the coding was validated by two authors to ensure appropriateness of interpretation. Throughout, attention was paid to both frequent codes and key themes as well as for minority opinions and novel perspectives on the research question. Data generated by professionals and non-professionals (pwCF, parents of pwCF, and so on) were considered separately to allow comparison across these groups.
Results
Demographics
In total, 313 responses were received; of which 176/313 (56%) were from pwCF and their families and 137/313 (44%) were from HCPs. Among the lay community 103/176 (58%) responses were from pwCF, 70/176 (40%) from a parent of a child or children with CF and 3/176 (2%) from another relative or friend. HCP responses comprised 10 professional groups (table 1), of which dieticians and physiotherapists both accounted for 20% of responses. Not all respondents answered every question and so we have included the denominator where we present raw numbers. The majority of respondents (159/222, 72%) were from the UK with 52/222 (23%) from the USA and the remainder from Europe, Canada, Australia and New Zealand. The median age of pwCF was 22 years (1 to 74 years).
Table 1.
Professional demographics of healthcare professionals completing the survey. Healthcare professionals represented 10 professional groups
| Professional group | Number responses | Percentage |
| Physiotherapist | 28 | 20.4% |
| Dietitian | 28 | 20.4% |
| Psychologist | 22 | 16.1% |
| Researcher | 14 | 10.2% |
| Social worker | 11 | 8.0% |
| Nurse | 10 | 7.3% |
| Respiratory paediatrician | 7 | 5.1% |
| Respiratory physician | 7 | 5.1% |
| Pharmacist | 3 | 2.2% |
| Junior doctor | 2 | 1.5% |
| Unknown demographic | 5 | 3.6% |
| Total | 137 |
Support
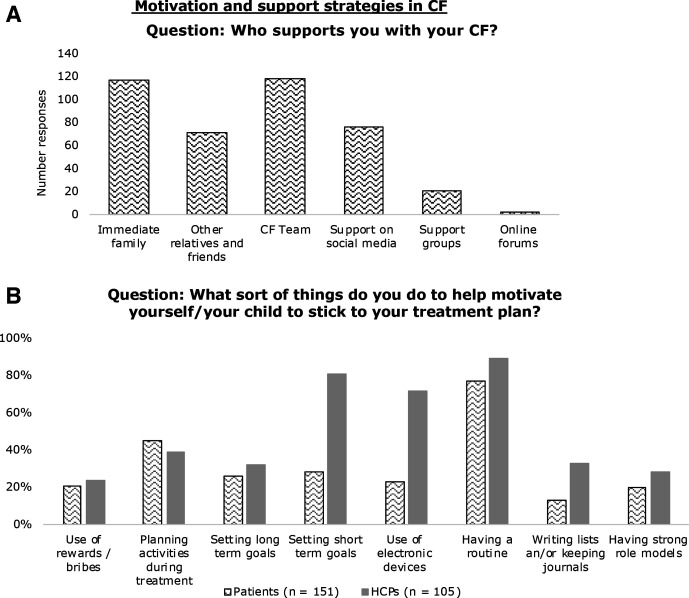
Both groups recognised the importance of support being available from a variety of sources (figure 1A). Immediate family and the CF team were identified as key support networks for pwCF in both quantitative and thematic analysis. These were also identified as positive support networks by HCPs, in addition to a minority also identifying mental health services as a key area of support.
Figure 1.
(A) “Who supports you with your CF?” was answered by 147 people from the patient and carer community to help build a picture of support networks in CF. (B) Lay community (151 respondents) and HCPs (105 respondents) responses into motivation strategies used to promote adherence. CF, cystic fibrosis; HCPs, healthcare professionals.
When asked if they were able to talk honestly with their CF team about adherence, 127/149 (85%) of pwCF and their families agreed. Most (98/151, 65%) felt they were well supported and their team understood their treatment demands. However, the thematic analysis for this question revealed that there was greater expression of negative comments compared with positive responses relating to their CF team. Included within this were the feelings of letting their team down or feeling judged about their treatment adherence. In addition, others described that although their CF team were supportive, they did not fully understand the associated treatment burden or complexities of the disease (Quotes 1 and 2).
Quote 1, pwCF: I have absolute confidence in our team's academic knowledge of the disease and what it requires, but since none of them live it, I'm not entirely sure they fully grasp what the burden of care can truly be for patients and families.
Quote 2, pwCF: I feel great support when I'm doing it. I don't always feel that support when I'm not doing it. There's a tendency to think that I don't understand why this is important or that I am not trying hard enough. I have to explain it's a process of changing a whole system and that's incredibly difficult… It's not lack of motivation or lack of education/knowledge. I get it. I just can't get it done.
Motivation
Among pwCF and their families, 65/158 people (41%) reported full weekly treatment adherence, while 18% reported adherence to treatment less than half of the week. Within the lay community the most common theme identified impacting on adherence was ‘having no time’ which encompassed competing work and school commitments. Free-text comments relating to the node ‘No Time’ was used 111 times, with an additional 40 coded to ‘Competing Commitments’. Other factors were associated treatment burden including equipment cleaning and preparation time, working outside their usual routine, the patient’s general health and tiredness (Quote 3). In addition, a small number discussed mental health; although this was to a lesser extent than HCPs.
Quote 3, Family member with CF: Sometimes we're just tired—tired of setting up and breaking down equipment; tired of soaking and sterilising pieces; tired of figuring out how to fit treatments into our social activities as a family.
Our survey showed that 102/104 (98%) of HCPs incorporated asking about barriers to adherence into their consultations, however time constraints were identified as a factor preventing this occurring at every interaction. A minority felt that this wasn’t always appropriate to do at every consultation, for example, if focussing on alternative goals or instead choosing to focus on encouragement and what a patient had done well.
Figure 1B illustrates motivating techniques either used by pwCF and their families or promoted by HCPs. Both groups acknowledged that having a routine was important and was the most commonly identified strategy for promoting adherence; used by 116/151 (77%) patients and 93/105 (89%) of HCPs. It was also the most commonly coded strategy relating to adherence in the free-text comments. However, there were also discrepancies between the two groups with some motivational strategies valued more highly by HCPs than by pwCF and their families. This was most apparent with short-term goal setting 85/105 (81%) versus 42/151 (28%), respectively, and using electronic devices 76/105 (72%) versus 34/151 (23%), respectively. A negative view of technology by a pwCF (Quote 4) included:
Quote 4, pwCF: I don't use health / med trackers as it just feels like another thing to have to manage (input data, more alarms etc).
Positive influential factors on adherence identified by patients through thematic analysis were: shortening preparation and treatment times such as time sterilising equipment; making treatments more portable or disposable; and having more physical support with their treatments at home. Comments relating to cleaning and sterilising equipment were coded 29 times. Free-text comments from HCPs highlighted that they valued patient-led goal setting; identifying health beliefs and patient-specific barriers; and motivational interviewing in promoting adherence.
The majority of pwCF and their families (144/151, 95%) said they understood exactly what their treatments were for; 131/151 (87%) felt that this made adherence easier. Also, a number of patients described that education made them more ‘motivated’ to take their treatments and valued the importance of being informed. Nevertheless, it was suggested that treatment knowledge does not always reduce the treatment burden (Quote 5), while others felt that they didn’t receive adequate education. HCPs also valued highly the use of education to increase adherence through understanding.
Quote 5, pwCF: I know the importance but often doesn't make it easier or less exhausting to do.
Technology
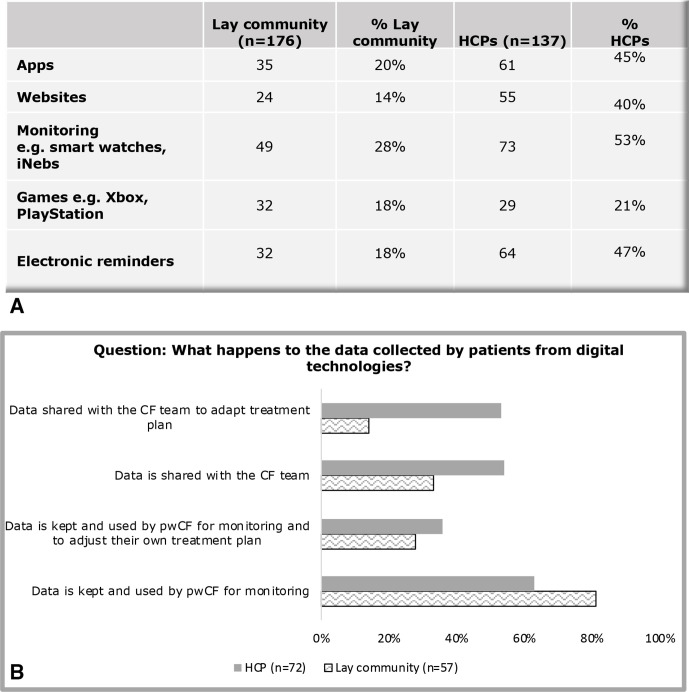
In addition to the frequency of electronic device use varying between the two groups, the types of technologies preferred also differed (figure 2A). HCPs promoted the use of devices for monitoring (73/137, 53%), electronic reminders (64/137, 47%) and Apps (applications) (61/137, 45%), however only 35/176 (20%) of pwCF reported using Apps and 32/176 (18%) using electronic devices in the closed question responses. Instead most used technology for monitoring of their condition through the use of data tracking nebulisers and smart watches (49/176, 28% of lay respondents).
Figure 2.
The use of digital technologies in CF. (A) Technologies used by the lay community (176 people) to help manage their treatment regimen versus what HCPs believe their patient’s to be using (137 people). (B) Views of how patients share their data that they collect from using digital technology versus what HCPs believe happen to these data. Respondents were able to select multiple responses. CF, cystic fibrosis; HCPs, healthcare professionals; pwCF, people with CF.
Among patients who did use digital technology, ‘Reminders’ and its synonyms were the most frequently used words by pwCF and their families (used 38 times), with 59 responses coded to the node ‘Technology – Reminders’. For example, using timers or Apps to remind themselves to take medications or complete treatments, with some also linking these data to smart watches. Tracking of their condition was also discussed including tracking of their physical parameters, medications, trends and activity levels. This was enabled through the use of fitness watches such as Fitbit and Apple watches which were also used to promote exercise.
However, in contrast with the closed questions, for those who used technology, thematic analysis showed a greater emphasis on the use of Apps. The words App or Apps were used 69 times in responses. Apps was the most frequently used word relating to technology. A small number of pwCF used technology to order repeat prescriptions.
In keeping with the descriptive analysis of the closed responses, thematic analysis was largely positive from HCPs in regard to technology, who felt remote monitoring of a condition, including real-time feedback in the case of data tracking nebulisers was beneficial. Conversely, some professionals felt this level of adherence monitoring was too invasive, describing it as ‘punitive and exposing’ while others reflected on the lack of evidence base behind technology in CF (Quote 6).
Quote 6, HCP: I don't specifically recommend any (besides using phone for reminders) because none have proven efficacy. In absence of efficacy, it falls on patient's personal experience
Figure 2B highlights the variations in data sharing practices from technology reported between pwCF and their CF team. Participants were able to select several responses. Our data show that there is a discrepancy over the perceived amount of data shared between pwCF and HCPs. There are also differences in perception over how these data are used to amend treatment plans. A higher proportion (45/72, 63% HCPs and 46/57, 81% pwCF) felt that pwCF kept their data to themselves for their own health monitoring while 39/72 (54%) HCPs versus 19/57 (33%) pwCF felt that pwCF shared their data with their CF team. However, a larger percentage of HCPs believe that their patients shared their data with their CF team allowing them to adapt their treatment plan to what was done in practice by pwCF (38/72, 53% vs 8/57, 14%, respectively). The consensus among HCPs was that data are owned by the patients to be shared as they wished but was beneficial for providing an objective measure of adherence and to adjust treatment plans.
Perhaps unsurprisingly, HCPs were more aware of trials relating to technology than their patients. One in 10 patients were aware of trials relating to technology. The most commonly identified trials by both groups were CFHealthHub12 and Project Fizzyo (ISRCTN51624752).13 Both groups identified CF-specific Apps able to combine multiple aspects of CF care in one place, for example, calorie counting, medication reminders and monitoring as an area of future research interest relating to technology, with some HCPs expanding that these should be developed in conjunction with pwCF. The lay community also suggested other trials including home monitoring of pulmonary function testing and physiological observations (heart rate/saturations), virtual clinics and trials involving physiotherapy. HCPs also expressed that current technology had limited evidence for its use in CF (Quote 7).
Quote 7, HCP: I think there is an amazing opportunity to use technology to promote adherence. However, this has to be well thought through and evidence based. Merely providing adherence data won’t improve adherence unless it is backed by evidence-based adherence support.
Discussion
This study had global involvement of pwCF, their families and HCPs and through the use of a web-based survey collected their views on which motivation strategies, support networks and technologies help people with cystic fibrosis improve and sustain adherence to treatment. The current study confirms our previous work which showed that treatment burden in CF is high and patients, their families and HCPs recognise this as a barrier to adherence.5 Patients described the relentlessness of the condition and identified that additional competing demands, such as from school and work, were barriers to adherence. Related themes were having no time (including time for preparation and cleaning of equipment) and tiredness. The high associated treatment burden in CF has been well described, with treatments typically taking two to two and a half hours to complete,4 5 needing adaptation and adjustment as the nature of the condition changes.
In this study and in our previous work we highlight that the most burdensome treatments identified by patients were the most likely to be omitted.5 However, other research has suggested that there is not always a clear relationship between objective treatment burden and adherence. This was demonstrated by Ball et al who found that teenagers with CF were most adherent to treatment during school-term weekdays, despite this being a time when there were many competing demands.14 Thus, the authors concluded there was value in integrating treatments into a daily routine.
Routine has been shown to be advantageous in adherence in CF. Hoo et al15 found that patients with higher levels of adherence had the highest habit scores, lower median intravenous antibiotic days and a trend towards higher forced expiratory volume in one second compared with those with low adherence. Habit formation is advantageous as it reduces the need for conscious effort. Within our study both groups recognised the importance of having a routine and acknowledged that adherence was reduced when routine was disrupted.
In this study treatment burden is frequently given by participants as a reason for non-adherence. However, it is also possible that some respondents may have been reluctant to give a full explanation for their reasons for non-adherence. Drabble et al16 demonstrated that for patients who gave ‘forgot’ as a reason for non-adherence the underlying reasons for forgetting differed between high and low adherers. With regard to treatment burden, this may be a more acceptable rationalisation for some pwCF to disclose than the underlying reason for their non-adherence. We hope that through the anonymity of the survey this was minimised. In addition, perception of treatment burden can be influenced by coping strategies used. Work in type 1 diabetes17 found that patients with a maladaptive coping style to treatments (ie, avoidant coping) had greater levels of diabetes-specific distress. This resulted in amplifying the perception of their treatment burden followed by a deterioration in their self-management and glycaemic control. This may also be relevant for some pwCF with a similar coping style, thus amplifying an already significant treatment burden. Furthermore, where ‘burden’ is given as an explanation of suboptimal adherence, a behavioural change approach might interpret this as indicating the challenge faced by pwCF in establishing a sustainable routine in the face of a complex system.18
Our study found that CF education was valued and patients recognised the importance of being well informed, helping them to stay motivated when the immediate benefits were not seen. However, it was felt that education did not always relieve treatment burden. Education to improve self-management and adherence is used in a number of chronic conditions, with a number of self-management education strategies used in CF since the 1990s.19 Evidence of self-management education in CF however is still poor. A Cochrane review19 found that there was insufficient quantity or quality of evidence based on current trials to draw any firm conclusions around its role in CF, with further trials needed. It did acknowledge that there was some limited evidence that improved knowledge may result in a positive change to a small number of behaviours in CF.
The use of digital technology is a rapidly growing area within healthcare and its importance recognised by the WHO.8 Its use in CF has increased over the last 20 years yet there is currently limited evidence of its efficacy in improving long-term adherence in CF.20 This study has highlighted that although technology is being used by a group within the CF community to aid treatment management; it is promoted more by HCPs than used in practice by the majority of pwCF at present.21 In addition, the types of technologies preferred by pwCF can be different to what is recommended by their HCPs. In particular the use of Apps provided interesting data. Although closed text responses reported relatively low use of Apps, thematic analysis provided more of an insight into their use than was captured by the closed question alone. Apps was the most frequently used word in relation to types of technologies used, with its use mainly linked to providing reminders. In addition, CF-specific Apps capable of combining multiple aspects of care was identified as an area of research interest. Studies such as MyCyFapp22 for patients with CF-related pancreatic insufficiency goes some way to addressing this research area. Its App has multiple functions including allowing the user to calculate their optimal PERT dosing, complete symptom scoring and providing nutritional education. However, to our knowledge, there is no CF-specific App supported by trial data which allows patients to self-manage all aspects of their CF care in one place. Home monitoring, trials involving physiotherapy and virtual clinics were also identified, although these have previously been used in trials in CF.20
Ongoing studies including CFHealthHub12 and Project Fizzyo13 aim to address adherence in CF through the use of digital technology. Project Fizzyo combines the use of Fitbit exercise trackers with chipped airway clearance devices with feedback given on technique via an App. Computer games were also developed to be used at different intervals within the study to assess their impact on adherence. Their aim is to improve the quality of airway clearance and reduce treatment burden by making physiotherapy more enjoyable. CFHealthHub collects data through data tracking nebulisers, accessible to both clinicians and pwCF to provide an objective measure of adherence. This, if handled sensitively, aims to enable clinicians to support patients more appropriately and improve adherence. Additional online supportive tools such as education, problem-solving and goal setting are available for patients.
The patient’s view is paramount in guiding future research and a strength of this study was that it addressed an area considered a priority by the CF community. Perspectives of both the patient and clinical CF community were sought to give a deeper understanding of the issues affecting adherence. We had global reach with a large number of responses with an almost equal split between the HCP and patient community. Thematic analysis was beneficial as it allowed for a more in-depth analysis of this research question. Third, public and patient involvement was maintained throughout the study and were part of the steering group as well as through manuscript preparation. In addition, the survey was anonymous which may have encouraged openness and honesty in the participants’ responses.
We have identified several limitations to this study. First, we acknowledge that self-reporting of adherence is subject to both recall and social desirability bias. Also, the demographics and opinions of people who did not take part are unknown. This may be of particular relevance as the surveys were largely promoted on digital platforms. Groups which either chose not to use these platforms or do not have access may not share the same views as those who chose to engage in the survey, especially in relation to the use of digital technologies. However, due to the relatively young demographic of pwCF this group is more likely to be familiar with using these platforms in their everyday lives. In addition, although a structured approach was taken for data analysis the nature of the data means that the data will always require a degree of subjective interpretation. Finally, the majority of respondents were from the UK where healthcare is universal and free at the point of use. However, we feel though that the results are still generalisable to all countries where digital technologies are accessible.
Conclusion
Adherence to treatment is crucial but it is often suboptimal and difficult for pwCF to achieve. Through this exercise, we have shared the opinions of the CF community in relation to adherence, the challenges faced and what role digital technology has in promoting adherence. We hope that this research will provide first a deeper insight into the current challenges, as well as demonstrate areas for future research. This study would recommend further trials looking at methods of improving CF patient care through the three key themes identified in our analysis: support, motivation and technology. In particular, trials looking at reducing treatment burden or the role of CF specific App-based technology to manage their CF care. However, researchers should be mindful that in a condition where treatment burden and time pressures are considerable, any interventions should not add additional demands and instead focus on simplifying care and reducing treatment burden.
Acknowledgments
We would like to thank all the people with cystic fibrosis (CF) and their families and the multidisciplinary CF healthcare professionals for taking part in this survey. Members of the James Lind Alliance CF2 steering group include: Brownlee K, Collins S, Daniels T, Davies G, Duff AJA, Elliot ZC, Gathercole K, Hurley MN, Leighton PA, Rayner OC, Rowbotham NJ, Smith SJ, Chandran S, Nash EF, Smyth AR (Chair) and Wilson P.
Footnotes
Contributors: All authors were involved in the study design process and contributed in the preparation of this manuscript.
Funding: This work was supported by the UK Cystic Fibrosis Trust. NJR is an NIHR Academic Clinical Fellow at University of Nottingham. GD is an NIHR Clinical Trials Fellow and was previously supported by a NIHR Clinical Lectureship at UCL.
Competing interests: NR has given lectures at meetings sponsored by Teva and received non-financial support from Teva and Vertex. AS has provided consultancy for Vertex and holds a current unrestricted research grant from Vertex. He has taken part in clinical trials sponsored by Vertex, Raptor and Insmed. He has given lectures at meetings sponsored by Teva and Vertex. GD has given lectures at meetings sponsored by Chiesi. GD is a collaborator on Project Fizzyo. TD has completed consultancy work for Zambon who supply INeb nebuliser systems which have adherence monitoring capacity.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Data are available on request from Professor Alan Smyth, Division of Child Health, Obstetrics & Gynaecology, Evidence Based Child Health Group, Obstetrics & Gynaecology, Queens Medical Centre, University of Nottingham, E FLoor East Block, Nottingham, NG7 2UH, UK
References
- 1.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: challenges to disease self-management. J Cyst Fibros 2009;8:91–6. 10.1016/j.jcf.2008.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cystic Fibrosis Trust Uk cystic fibrosis registry annual data report 2017. London: Cystic Fibrosis Trust, 2018. [Google Scholar]
- 3.Knapp EA, Fink AK, Goss CH, et al. . The cystic fibrosis Foundation patient registry. design and methods of a national observational disease registry. Ann Am Thorac Soc 2016;13:1173–9. 10.1513/AnnalsATS.201511-781OC [DOI] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Trust Cystic fibrosis insight survey – report on the 2017 and 2018 surveys. London: Cystic Fibrosis Trust, 2018. [Google Scholar]
- 5.Davies G, Rowbotham NJ, Smith S, et al. . Characterising burden of treatment in cystic fibrosis to identify priority areas for clinical trials. J Cyst Fibros 2020;19:499–502. 10.1016/j.jcf.2019.10.025 [DOI] [PubMed] [Google Scholar]
- 6.Eakin MN, Riekert KA. The impact of medication adherence on lung health outcomes in cystic fibrosis. Curr Opin Pulm Med 2013;19:687–91. 10.1097/MCP.0b013e3283659f45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowbotham NJ, Smith S, Leighton PA, et al. . The top 10 research priorities in cystic fibrosis developed by a partnership between people with CF and healthcare providers. Thorax 2018;73:388–90. 10.1136/thoraxjnl-2017-210473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation Recommendations on digital interventions for health system strengthening. Geneva, Switzerland: World Health Organisation, 2019: p. 1–150. [Google Scholar]
- 9.Palser SC, Rayner OC, Leighton PA, et al. . Perception of first respiratory infection with Pseudomonas aeruginosa by people with cystic fibrosis and those close to them: an online qualitative study. BMJ Open 2016;6:e012303. 10.1136/bmjopen-2016-012303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res 2005;15:1277–88. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- 11.Howitt D, Cramer D. Introduction to research methods in psychology. England: Pearson, 2014. [Google Scholar]
- 12.ISRCTN55504164 A randomised controlled trial and parallel process evaluation to determine whether CFHealthHub, an intervention to help CF patients build better treatment habits, offers any benefit over usual care to adults with CF, 2017. Available: http://www.isrctn.com/ISRCTN55504164
- 13.ISRCTN51624752 Project Fizzyo: remote monitoring and gaming technology for children with cystic fibrosis 2018.
- 14.Ball R, Southern KW, McCormack P, et al. . Adherence to nebulised therapies in adolescents with cystic fibrosis is best on week-days during school term-time. J Cyst Fibros 2013;12:440–4. 10.1016/j.jcf.2012.12.012 [DOI] [PubMed] [Google Scholar]
- 15.Hoo ZH, Gardner B, Arden MA, et al. . Role of habit in treatment adherence among adults with cystic fibrosis. Thorax 2019;74:197–9. 10.1136/thoraxjnl-2017-211453 [DOI] [PubMed] [Google Scholar]
- 16.Drabble SJ, O'Cathain A, Arden MA, et al. . When is forgetting not forgetting? A Discursive analysis of differences in forgetting talk between adults with cystic fibrosis with different levels of adherence to nebulizer treatments. Qual Health Res 2019;29:2119–31. 10.1177/1049732319856580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iturralde E, Weissberg-Benchell J, Hood KK. Avoidant coping and diabetes-related distress: pathways to adolescents' type 1 diabetes outcomes. Health Psychol 2017;36:236–44. 10.1037/hea0000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arden MA, Drabble S, O'Cathain A, et al. . Adherence to medication in adults with cystic fibrosis: an investigation using objective adherence data and the theoretical domains framework. Br J Health Psychol 2019;24:357–80. 10.1111/bjhp.12357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savage E, Beirne PV, Ni Chroinin M, et al. . Self-Management education for cystic fibrosis. Cochrane Database Syst Rev 2014:CD007641. 10.1002/14651858.CD007641.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calthorpe RJ, Smith S, Gathercole K, et al. . Using digital technology for home monitoring, adherence and self-management in cystic fibrosis: a state-of-the-art review. Thorax 2020;75:72–7. 10.1136/thoraxjnl-2019-213233 [DOI] [PubMed] [Google Scholar]
- 21.Rowbotham NJ, Smith SJ, Davies G, et al. . Can exercise replace airway clearance techniques in cystic fibrosis? A survey of patients and healthcare professionals. J Cyst Fibros 2020;19:e19–24. 10.1016/j.jcf.2019.10.026 [DOI] [PubMed] [Google Scholar]
- 22.Boon M, Calvo-Lerma J, Claes I, et al. . Use of a mobile application for self-management of pancreatic enzyme replacement therapy is associated with improved gastro-intestinal related quality of life in children with cystic fibrosis. J Cyst Fibros 2020;19:562–8. 10.1016/j.jcf.2020.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjresp-2020-000601supp001.pdf (3.4MB, pdf)