Abstract
Despite a sizeable body of research, the efficacy of therapeutic cancer vaccines remains limited when applied as sole agents. By using a prime:boost approach involving two viral cancer vaccines, we were able to generate large tumor-specific CD8+ T-cell responses in a murine model of disseminated pulmonary melanoma. Significant increases in the number and quality of circulating effector T-cells were documented when low-dose cyclophosphamide (CTX) was administered pre-vaccination to tumor-bearing but not tumor-free hosts. Interestingly, tumor-bearing mice receiving CTX and co-primed with a melanoma differentiation antigen together with an irrelevant control antigen exhibited significantly enhanced immunity against the tumor, but not the control antigen, in secondary lymphoid organs. This result highlighted an increased cancer-specific reactivity of vaccine-induced T-cell responses following CTX preconditioning. Additionally, an acute reduction of the frequency of peripheral regulatory T-cells (Tregs) was noticeable, particularly in the proliferating, presumably tumour-reactive, subset. Enhanced infiltration of lungs with multifunctional T-cells resulted in overt reduction in metastatic burden in mice pretreated with CTX. Despite doubling the median survival in comparison to untreated controls, most vaccinated mice ultimately succumbed to cancer progression. However, preconditioning of the virus-based vaccination with CTX resulted in a remarkable improvement of the therapeutic activity leading to complete remission in the majority of the animals. Collectively, these data reveal how CTX can potentiate specific cellular immunity in an antigen-restricted manner that is only observed in vaccinated tumor-bearing hosts while depleting replicating Tregs. A single low dose of CTX enhances antitumor immunity and the efficacy of this potent prime:boost platform by modulating the kinetics of the vaccine-specific responses. Clinical assessment of CTX combined with next-generation cancer vaccines is indicated.
Keywords: cyclophosphamide pre-conditioning, therapeutic cancer vaccine, oncolytic vesicular stomatitis virus, adenoviral vector, Tregs
Introduction
Induction of endogenous antitumor immune responses using vaccination has been investigated for decades and although progress has been gradual the full potential of this approach is yet to be realized.1 Using vaccines as sole therapeutic agents exerts limited clinical activity and major barriers to the success of vaccines include the inability of vaccines to overcome immunosuppressive cell populations, including regulatory T-cells (Tregs) and/or to mount robust responses against the targeted antigen(s).2 3 Combining cancer vaccines with other immunomodulants is being actively pursued by many investigators with the hope that such polypharmacy will help overcome these major obstacles.4
We have developed a heterologous prime:boost cancer vaccination.5–7 The strategy relies on (1) an immune prime mediated by a replication-incompetent adenoviral vaccine administered intramuscularly, followed by (2) a boost using an oncolytic replication-competent rhabdoviral vaccine infused systemically. Both viral vectors harbor the same transgene that encodes a tumor-associated antigen (TAA). We have shown that this approach generates tumor-specific CD8+ T-cell responses of particularly remarkable magnitudes owing to the ability of the boosting vector to directly engage splenic central memory T-cells (Tcm).8 In murine studies, such adaptive anticancer immunity results in prolonged survival and efficacy in various solid tumor models thus offering an ideal platform to study the kinetics of vaccine-induced antitumor immunity.5–7
Combining chemotherapeutic compounds with immunotherapy could be considered counterintuitive as numerous cytotoxic drugs demonstrate immunosuppressive functions.9 However, some agents have validated immunomodulatory functions particularly when administered at a low dose. Among these chemotherapeutics, cyclophosphamide (CTX) demonstrates pleiotropic effects that supports the induction of antitumor immunity. Briefly, CTX has been reported to (1) directly kill cancer cells in an immunogenic fashion, (2) influence dendritic cell (DC) homeostasis and promote tumor infiltration of DCs secreting more interleukin (IL)-12 and less IL-10, (3) polarize CD4+ T cells into type-1 and/or type-17 immune helpers, (4) stimulate the activation and proliferation of tumor-specific T lymphocytes and their infiltration inside the tumor bed and (5) reprogram and/or deplete immunosuppressive T regulatory cells (Tregs).10–12 However, the effect that CTX has on the dynamics of TAA-specific effector and Treg cells induced by anti-cancer vaccines has not been comprehensively characterized.
We hypothesized that combining our oncolytic vaccination approach with a single low dose of CTX would potentiate tumor-specific effector immune responses and deplete Tregs thereby favoring vaccine-mediated tumor control. This report shows that CTX preconditioning enhances the magnitude and quality of CD4+ and CD8+ T-cell responses induced by prime:boost oncolytic vaccination specifically in tumor-bearing hosts, alongside focusing their reactivity toward tumor-associated rather than tumor-irrelevant antigens. CTX preconditioning leads to increased infiltration of effector T-cells within the tumor microenvironment (TME) and reduced peripheral Tregs, particularly when proliferating. Remarkably, the combination therapy translated into complete and durable disease clearance in the majority of mice treated.
Materials & Methods
Mice
Female C57BL/6 mice (8–10 weeks old at study initiation) were purchased from Charles River Laboratory (Wilmington, Massachusetts, USA) and housed in a specific pathogen-free facility.
Recombinant viruses
Ad-empty, Ad-OVA and Ad-DCT are replication-deficient adenoviruses (E1/E3-deletion) based on the human serotype 5. VSV-DCT is a replication-competent oncolytic viral agent which derives from the wild-type Indiana strain of the vesicular stomatitis virus (VSV). Ad-empty has no transgene. Ad-DCT and VSV-DCT encode the human dopachrome tautomerase (DCT). The OVA transgene encodes the chicken ovalbumin and was used as a tumor-unrelated antigen control.
Cell culture
Murine melanoma B16-F10 cells (expressing the murine DCT antigen) were grown in F11-MEM containing 10% fetal bovine serum (FBS), 2 mM L-glutamine, 1 mM sodium pyruvate and vitamin solution, 0.01 mM non-essential amino acids, 50 mM β-mercaptoethanol, 100 U/mL penicillin, and 100 mg/mL streptomycin (all from Invitrogen, Grand Island, New York, USA).
In vivo treatments
For adenovirus injection, mice were anesthetized in a sealed chamber containing 5% inhalation isoflurane. Adenoviral vectors were administered intramuscularly at a total dose of 2×108 pfu (1×108 plaque forming units (pfu) in 50 µL phosphate-buffered saline (PBS) per thigh). VSV-DCT was injected intravenously in 200 µL PBS at a dose of 2×109 pfu. CTX was administered through intraperitoneal (i.p.) injection at 50 mg/kg in 200 µL of saline solution 1 day prior to Ad prime (other groups received 200 µL of saline solution as control).
Tumor challenge
For lung tumor engraftment, 2.5×105 B16-F10 melanoma cells were injected intravenously in 200 µL saline water. Mice were monitored daily and euthanized for tissue harvest or on signs of morbidity.
Tissue homogenates
Blood sampling was performed through retro-orbital sinus bleeding using a heparinized capillary tube. Blood was collected into a heparinized saline solution (30 U/mL). Peripheral white blood cells were cleared of erythrocytes in ammonium chloride potassium (ACK) buffer at room temperature for 5 min then washed in Hanks medium; the procedure was repeated twice. After harvest, lymph nodes and spleen were crushed between glass slides to release leukocytes. Splenic red blood cells were lysed in ACK buffer as described for blood. Lungs bearing melanoma metastases were dissociated enzymatically using a cocktail of 0.05% collagenase, 0.002% hyaluronidase and 0.02% DNAse I. Lung-infiltrating leukocytes were isolated using the EasySep Mouse CD90.2 Positive Selection Kit II (Stemcell Technologies, Vancouver, British Columbia, Canada) following manufacturer’s recommendations.
Peptides
Peptides corresponding to the immunodominant epitopes of DCT: (1) DCT180–188 SVYDFFVWL/“SVY” that binds to H-2Kb; 100% conserved between human and murine DCT and (2) DCT89–102 KFFHRTCKCTGNFA/“KFF” from human DCT that binds to I-Ab; differing from the murine epitope in position 92: His (human) → Asn (murine). Peptides corresponding to the immunodominant epitopes of OVA: (1) OVA257–264 SIINFEKL/“SIIN” that binds to H-2Kb and (2) OVA323–339 ISQAVHAAHAEINEAGR/“ISQ” that binds to I-Ab. Peptides were synthesized by Biomer Technologies (San Francisco, California, USA).
Antibodies
Monoclonal antibodies used in flow cytometry assays: anti-CD16/CD32 (clone 2.4G2) to block Fc receptors, anti-CD3 (clone 145-2 C11), anti-CD8α (clone 53-6.7), anti-CD4 (clone RM4-5), anti-CD25 (clone PC61) and anti-FoxP3 (clone FJK-16s) for detecting cell surface and intranuclear markers, and anti-interferon γ (IFNγ) (clone XMG1.2) and anti-tumor necrosis factor α (TNFα; clone MP6-XT22) for intracellular cytokine staining. All reagents were purchased from BD Pharmingen (Mississauga, Ontario, Canada) with the exception of anti-FoxP3 that was purchased from eBioscience/Thermo Fisher Scientific (Burlington, Ontario, Canada).
Antigen-specific T-cell responses
DCT-specific T-cell responses were measured 9 and 14 days post prime and 5 days post boost. Peripheral blood mononuclear cells (PBMCs) or splenocytes were incubated in complete Roswell Park Memorial Institute (RPMI) medium (ie supplemented with FBS 10%, penicillin–streptomycin 1% and L-glutamine 1%) with the SVY peptide (2 µg/mL) or KFF peptide (15 µg/mL) for DCT-specific CD8+ or CD4+ T-cell (re-)stimulation, respectively, or with the SIIN peptide (2 µg/mL) for OVA-specific CD8+ T-cell (re-)stimulation. Incubation was performed in an incubator (37°C, 5% CO2, 95% humidity) for 5 hours with brefeldin A (1 µg/mL, GolgiPlug BD Pharmingen) during the last 4 hours. Leukocytes were labeled with LIVE/DEAD fixable near-IR dye (Life Technologies/Thermo Fisher Scientific) following supplier’s recommendations to identify live lymphocytes. Cells were incubated with antibodies targeting CD16/CD32 before staining with fluorescent-labeled antibodies targeting T-cell markers. Then, cells were permeabilized and fixed with Cytofix/Cytoperm (BD Pharmingen) and stained for intracellular cytokines. Data were acquired using a FACSCanto flow cytometer with FACSDiva software (BD Pharmingen) and analyzed with FlowJo v10 for Mac (Tree Star, Oregon, USA).
In vivo BrdU labeling
5-Bromo-2'-deoxyuridine (BrdU) was first administered i.p. at 50 mg/kg immediately after tumor challenge (ie, 4 days prior to CTX and 5 days prior to Ad) and maintained over the time until tissue harvest in the drinking water at 0.8 mg/mL. BrdU-containing water was refreshed every day. Tregs that incorporated BrdU into their DNA during the S phase of the cell cycle were stained using the APC BrdU Flow Kit (BD Pharmagen) following supplier’s recommendations.
Statistical analyses
GraphPad Prism was used for graphing and statistical analyses. Immune response data were plotted used mean±SEM or SD. Unpaired Student’s two-tailed t-test was used to compare two groups, ordinary one-way analysis of variance was used for multiple groups. Survival curves were plotted using Kaplan-Meier method, and compared using the log-rank test. Differences considered significant when p≤0.05 (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001).
Results
CTX increases the magnitude and quality of circulating antigen-specific T-cells in tumor-bearing mice
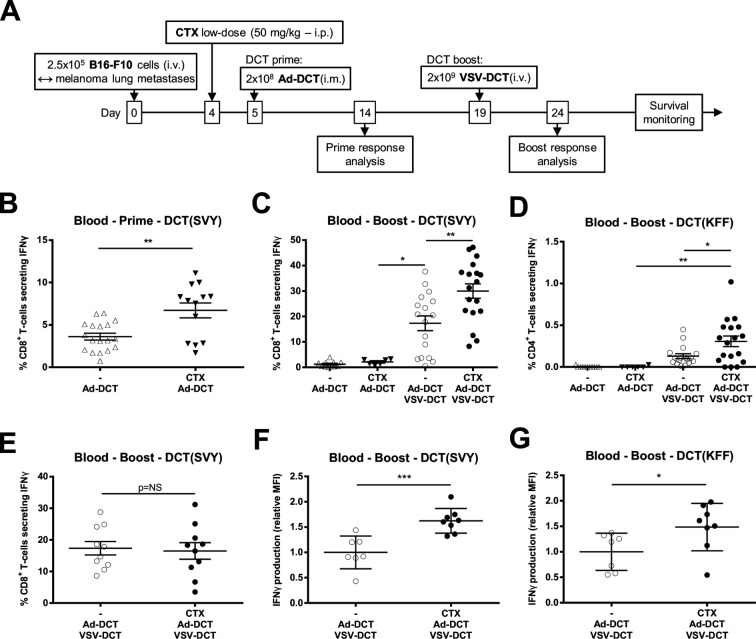
Mice bearing disseminated B16-F10 melanoma lung tumors were treated with varying combinations of vaccines encoding DCT, a well-characterized melanoma-associated antigen, with or without CTX preconditioning (figure 1A). Priming with Ad-DCT induced specific anti-DCT CD8+ T-cells and the magnitude of the circulating responses was significantly increased by a single low dose of CTX prior to priming (figure 1B, online supplementary figure S1). Boosting with systemic oncolytic VSV-DCT increased the magnitude of CD8+ T-cell responses compared with Ad alone and a further significant increase in both specific CD8+ Tc1 and CD4+ Th1 cell frequencies were seen when CTX was combined with this potent prime:boost platform (figure 1C, D, online supplementary figure S1) in a tumor-specific manner as no such effect was observed in tumor-free mice (figure 1E). CTX preconditioning also increased specific IFNγ production by both CD8+ and CD4+ T-cells when quantified using mean fluorescence intensities (figure 1F, G). Collectively, these data show that low-dose CTX improves the immunotherapeutic profile of this vaccine in a tumor-restricted manner.
Figure 1.
Cyclophosphamide (CTX) increases the magnitude and quality of virus-based vaccination in tumor-bearing mice. (A) Timeline for treatment of mice bearing B16-F10 lung tumors. To measure T-cell reactivity against the tumor-associated antigen dopachrome tautomerase (DCT), circulating lymphocytes have been restimulated ex vivo with peptides corresponding to the major histocompatibility complex (MHC)-I and MHC-II-restricted immunodominant epitopes DCT(SVY) and DCT(KFF), respectively. (B) Frequency of circulating CD8+ T-cells reacting against DCT(SVY) 9 days after priming with the adenovirus (Ad)-DCT or CTX + Ad-DCT. (C) Frequency of reactive CD8+ T-cells 5 days after boosting in melanoma lung tumor-bearing animals treated with Ad-DCT, CTX + Ad-DCT, Ad-DCT + vesicular stomatitis virus (VSV)-DCT, and CTX + Ad-DCT + VSV-DCT. (D) Frequency of circulating CD4+ T-cells reacting against DCT(KFF) 5 days after boosting in tumor-bearing mice that received Ad-DCT, CTX + Ad-DCT, Ad-DCT + VSV-DCT, and CTX + Ad-DCT + VSV-DCT. (E) Frequency of interferon γ (IFNγ)+ CD8+ T-cells 5 days after boosting in tumor-free animals treated with Ad-DCT + VSV-DCT and CTX + Ad-DCT + VSV-DCT. (F, G) Production of IFNγ illustrated as relative mean fluorescence intensity (MFI) in (F) DCT-specific CD4+ and (G) CD8+ T-cells following treatment of tumor-bearing mice with Ad-DCT + VSV-DCT and CTX + Ad-DCT + VSV-DCT. Data displayed in (B–E) consist of pools of at least two distinct experiments; dot plots indicate mean±SEM. Data displayed in (F) and (G) are results from one representative experiment; dot plots indicate mean±SD. i.m., intramuscular; i.p., intraperitoneal; i.v. intravenous.
jitc-2020-000981supp001.pdf (1.1MB, pdf)
CTX preconditioning focuses vaccine-induced immunity against the tumor
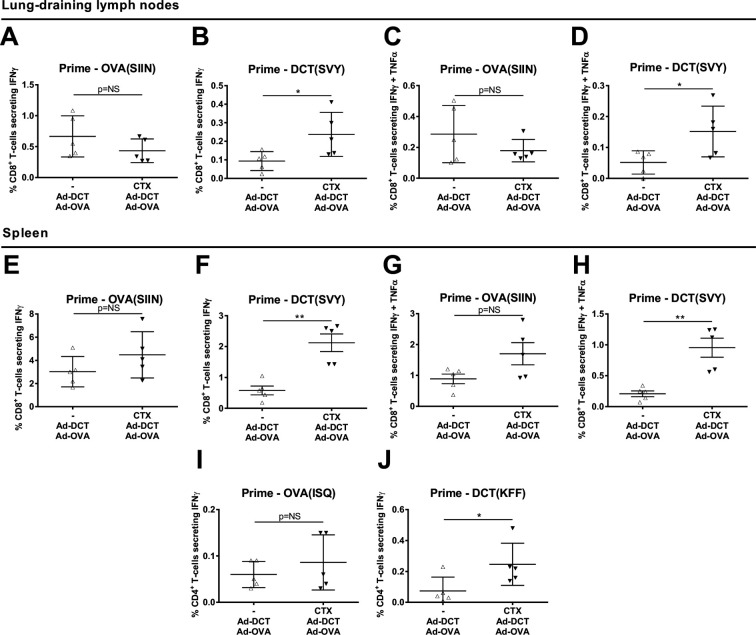
In order to determine the effect of CTX pretreatment with relation to tumor-antigen specificity, secondary lymphoid organs were harvested from tumor-bearing mice 9 days after co-priming with Ad-DCT plus an Ad vector expressing the tumor-unrelated xenoantigen ovalbumin (Ad-OVA). DCT-targeting and OVA-targeting immune responses were quantified using intracellular cytokine staining and flow cytometry. Within the tumor-draining lymph nodes, CTX pretreatment failed to significantly alter the magnitude of OVA-specific single IFNγ+ and dual IFNγ+ TNFα+ CD8+ T-cell responses whereas a significant expansion was noted in both DCT-specific populations (figure 2A–D). These findings were mirrored by OVA-specific and DCT-specific CD8+ splenocytes (figure 2E–H). No significant effect of CTX preconditioning on the magnitude of OVA-specific CD4+ Th1-cell frequencies was observed (figure 2I). However, CTX significantly increased the corresponding DCT-specific CD4+ T-cell population (figure 2J). Taken together, low-dose CTX significantly improves vaccine-induced immunity against a well-defined melanocytic TAA expressed in B16-F10 cells but has no effect on the immune response against a xenoantigen that is irrelevant to the tumor.
Figure 2.
Preconditioning with cyclophosphamide (CTX) focuses vaccine-induced immunity against the tumor. Frequency of (A) anti-ovalbumin (OVA) interferon γ (IFNγ)+, (B) anti-dopachrome tautomerase (DCT) IFNγ+, (C) anti-OVA IFNγ+ tumor necrosis factor α (TNFα)+ and (D) anti-DCT IFNγ+ TNFα+ CD8+ T-cells isolated from mediastinal lymph nodes draining melanoma lung metastases. Frequency of splenic (E) anti-OVA IFNγ+, (F) anti-DCT IFNγ+, (G) anti-OVA IFNγ+ TNFα+ and (H) anti-DCT IFNγ+ TNFα+ CD8+ T-cells. Frequency of splenic (I) anti-OVA and (J) anti-DCT IFNγ+ CD4+ T-cells. Samples harvested 9 days after co-vaccination with Ad-DCT and Ad-OVA with or without CTX preconditioning in mice bearing disseminated B16-F10 melanoma lung tumors. Data display mean±SD of one representative experiment.
Pre-vaccination CTX increases the CD8+ T-cells/Tregs ratio
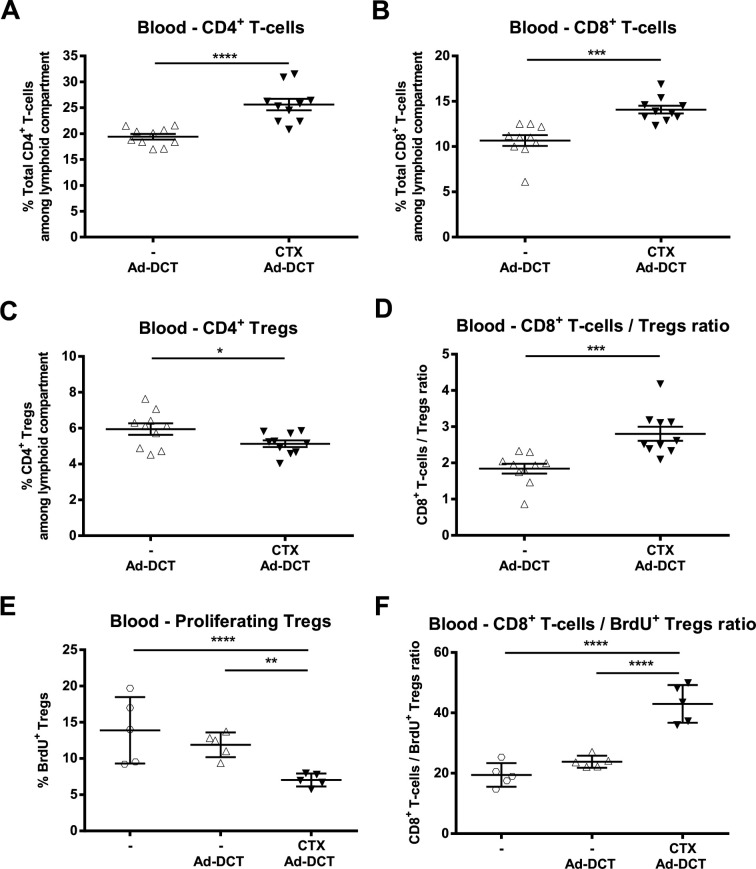
As Tregs have been shown to constrain the expansion of CD8+ T cells,13 we hypothesized that the increased magnitude of vaccine-induced TAA-specific T-cell responses observed after low-dose CTX would be accompanied by decreased Treg proliferation. We interrogated the dynamics of various short-term circulating T-cell subsets in tumor-bearing mice following Ad-DCT±CTX. At 24 hours post-Ad, CTX preconditioning did not result in generalized lymphodepletion when CD4+ and CD8+ T-cells were quantified (online supplementary figure S2). A relative expansion of CD4+ and CD8+ T-cell frequencies within the circulating lymphoid compartment was documented (figure 3A, B). Co-staining for CD4, CD25 and FoxP3 revealed a significant decrease in circulating Treg frequency in the CTX group (figure 3C) and ratios of CD8+ T-cells/CD4+ Tregs were significantly increased after preconditioning (figure 3D). BrdU was administered in the drinking water to tumor-bearing mice to quantify Treg proliferation, and blood samples were taken 72 hours post-Ad. A significant decrease in the frequency of proliferating Tregs (figure 3E) and increase in the ratio of total CD8+ T cells/BrdU+ Tregs (figure 3F) in the CTX group was noted. Thus, prevaccination treatment with CTX reduced the population of proliferating Tregs resulting in a favorable proportion of effector CD8+ T cells over immunosuppressive CD4+ Tregs.
Figure 3.
Pre-vaccination cyclophosphamide (CTX) increases the CD8+ T-cells/Tregs ratio and reduces proliferating Tregs in the periphery. Frequency of (A) total CD4+ T-cells, (B) total CD8+ T-cells and (C) CD4+ CD25+ FoxP3+ Tregs among the circulating lymphoid compartment of melanoma lung-metastatic mice. (D) Ratio of CD8+ T-cells/Tregs in peripheral blood samples taken 24 hours after adenovirus (Ad)-dopachrome tautomerase (DCT), either alone or after CTX preconditioning. Graphs display mean±SEM of two pooled experiments. Frequency of (E) proliferating 5-bromo-2'-deoxyuridine (BrdU)+ CD4+ CD25+ FoxP3+ Tregs and (F) ratio of CD8+ T cells/BrdU+ Tregs within the lymphoid compartment of blood samples collected 72 hours after treatment of melanoma lung-metastatic animals with Ad-DCT, administered either alone or after CTX preconditioning (untreated controls labeled “-"). Mean±SD displayed.
jitc-2020-000981supp002.pdf (68.4KB, pdf)
Chemotherapeutic preconditioning increases lung tumor infiltration by melanoma-specific effector T-cell populations and leads to durable tumor clearance
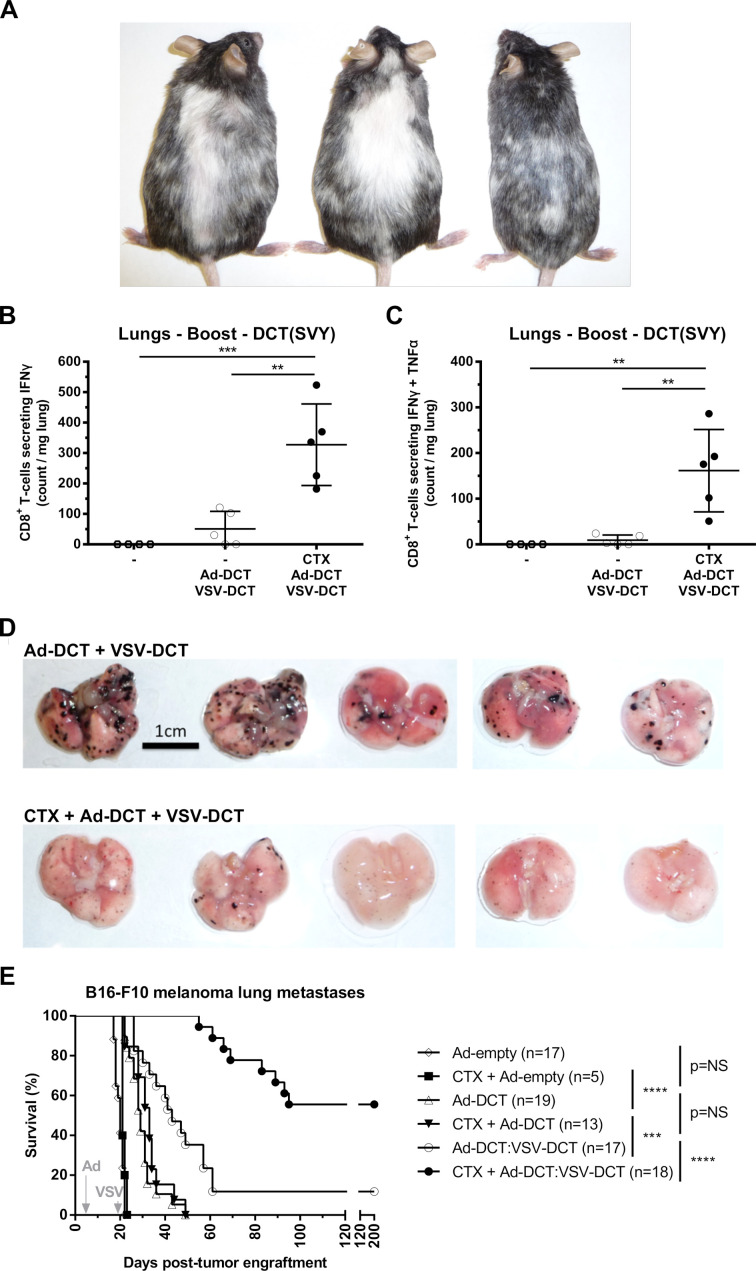
Further analyses were performed on mice bearing lung melanoma to ascertain the functional impact of CTX on the prime:boost vaccine. In long-term survivors, marked vitiligo was observed when animals were pretreated with CTX (figure 4A). As previously reported,7 this benign autoimmune manifestation resulted from a bystander elimination of melanocytes (which express the DCT antigen) by vaccine-induced CD8+ T-cells. Lungs were harvested from tumor-bearing mice 5 days after VSV-DCT boost, alongside untreated mice, and lymphocytes were isolated. Intracellular staining of CD8+ T-cells for IFNγ and TNFα revealed significant increases of DCT-specific, single and dual positive, populations within the TME in the CTX group, whereas no reactive T-cells were detected in untreated animals (figure 4B, C). At the same time point, lungs harvested were examined for gross lesions. Pretreatment with CTX resulted in an overt reduction in tumor burden (figure 4D). Ad-DCT:VSV-DCT resulted in long-term survival in a minority of mice. However, when this regimen was preceded by a single dose of CTX, the majority of mice were cured (figure 4E). Overall, low-dose CTX increases specific antitumor immunity resulting in a remarkable survival advantage in mice bearing aggressive lung disease. In conclusion, CTX preconditioning increased infiltration of the tissues harboring melanoma lesions by polyfunctional tumor-reactive effector T-cells which preceded durable tumor control.
Figure 4.
Chemotherapeutic preconditioning increases specific antimelanocytic effector activity. (A) Marked vitiligo observed in long-term surviving mice preconditioned with cyclophosphamide (CTX). Number of (B) interferon γ (IFNγ)+ and (C) IFNγ+ tumor necrosis factor α (TNFα)+ dopachrome tautomerase (DCT)-specific CD8+ tumor-infiltrating lymphocytes isolated from lungs with B16-F10 metastases after no treatment, adenovirus (Ad)-DCT + vesicular stomatitis virus (VSV)-DCT, and CTX + Ad-DCT + VSV-DCT. (D) Lungs with disseminated melanoma harvested 5 days post boost in mice treated with Ad-DCT + VSV-DCT and CTX + Ad-DCT + VSV-DCT. (E) Kaplan-Meier survival curves of mice with B16-F10 melanoma lung tumors following treatment with the different viral vaccines with or without CTX. Data displayed in (B) and (C) show mean±SD of one representative experiment. Data displayed in (E) consist of a pool of five distinct experiments. NS, not significant.
Discussion
By preconditioning mice with a single low dose of CTX, we observed improvement of both the functionality and magnitude of effector T-cell populations in tumor-bearing mice. Intriguingly, no benefit to the immunotherapeutic profile of the vaccine was noted in tumor-free mice, nor when vaccinating melanoma-bearing mice with a tumor-irrelevant antigen, uncovering an unexpected tumor/antigen-specific function of CTX preconditioning. This report also demonstrates that CTX pretreatment impairs Treg proliferation and improves the immune profile of a potent prime:boost vaccine. Ultimately, this approach resulted in durable tumor clearance in the majority of animals bearing an aggressive pulmonary melanoma model following oncolytic prime:boost vaccination.
CTX is one of the most widely used cytotoxics within the oncology clinic.14 At high doses, CTX is used as a primary antineoplastic agent for various malignancies as well as a preconditioning agent prior to bone marrow transplantation achieved by ablation of CTX-sensitive populations inclusive of effector lymphocytes.14 At lower doses, CTX selectively targets CD4+ Tregs due to their low intracellular concentrations of ATP, which in turn impairs their ability to synthesize glutathione, a key molecule in the metabolic inactivation of CTX.15 This dose-dependent effect is recapitulated in this model where we document reduced Treg proliferation as well as decreased total Treg frequencies in the absence of overt circulating CD4+ and CD8+ lymphodepletion.
The B16F10 model is remarkably aggressive, leading to endpoint of untreated animals within 20 days. At the time of CTX preconditioning (day 4), around 10 macroscopic foci of melanoma metastases can be observed in the lungs under a dissecting microscope.16 Its relevance with regards to the clinic are numerous.17 Indeed, 18% of patients with advanced melanoma develop lung metastases. In particular, approximately 60% of patients with pulmonary metastatic melanoma are diagnosed with >3 nodules; a criterion associated with ineligibility to surgical resection in 70% of the cases and with poor prognosis.18–20 This report builds on previous studies revealing Treg depletion and extended survival times when CTX is used in combination with vaccines in murine models.21 22 Expanding on these findings, we showed that CTX preconditioning enhances specific CD8+ T-cell responses against the TAA targeted by the vaccine and such effects are only observed in the setting of tumor-bearing animals. Intriguingly, we revealed that within secondary lymphoid organs, preconditioning selectively enhances CD4+ and CD8+ immunity against a TAA, but not against a tumor-irrelevant xenoantigen when tumor-bearing mice were co-vaccinated. Collectively, these results indicate that CTX modulates antitumor immunity by interfering with tumor-induced immunosuppression that relies on interactions between the host’s immune system and the immunoevasive neoplastic process. As such, preconditioning with CTX enhances immunity in an antigen-specific manner and future studies are required to determine whether the increases in quantity and quality of immune responses in the tumor-bearing animal can be solely ascribed to Treg depletion.
Regulatory T-cells have been shown to suppress antitumor CD8+ T-cells in vivo23 and an increased ratio of circulating CD8+ T-cells/Tregs is associated with clinical response to PD1 blockade in patients with advanced melanoma.24 Evidence for the positive effect of tumor-infiltrating lymphocyte (TIL) density on melanoma survival times is well established and therapeutic approaches to increase TIL density are therefore sought-after.25 The induction of poly-functional CD8+ T-cells is also beneficial, as such cells were enriched in long-term melanoma survivors following DC vaccination.26 In this report, CTX preconditioning favorably improved the ratio of CD8+ T-cells/Tregs and increased the magnitude of specific multifunctional T-cells within tumor-bearing lungs.
Low-dose CTX before therapeutic cancer vaccination appears well tolerated and has increased survival times in renal cell and biliary tract cancer.27 28 Based on this report and CTX’s favorable therapeutic profile, future clinical evaluation of single, low-dose CTX pretreatment should be considered for patients with melanoma in combination with the next generation of cancer therapeutics, including oncolytic vaccines.5–8 29 30
Acknowledgments
The authors want to thank Liang Zhang for her technical assistance with in vivo experimentations.
Footnotes
Contributors: JGP, MJA, KBS, BWB, STW, NK, and AJRM performed the experiments. JGP and MJA wrote the manuscript. BDL, GK, and YW supervised the study and edited the manuscript.
Funding: JGP was supported by the Seerave Foundation and the SIRIC Cancer Research and Personalized Medicine (CARPEM). BDL was supported by the Terry Fox Foundation, the Ontario Institute for Cancer Research, BioCanRx and Turnstone Biologics.
Competing interests: JGP, MJA, BWB, YW and BDL are named as inventors on patents for cancer vaccination involving an oncolytic rhabdovirus. These patents have been licensed to Turnstone Biologics of which JGP, BWB, YW and BDL are shareholders. KBS is an employee of Turnstone Biologics.
Patient consent for publication: Not required.
Ethics approval: All animal studies complied with Canadian Council on Animal Care guidelines and were approved by McMaster University’s Animal Research Ethics Board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information. Raw data remain available upon reasonable request.
References
- 1. Guo C, Manjili MH, Subjeck JR, et al. Therapeutic cancer vaccines: past, present, and future. Adv Cancer Res 2013;119:421–75. 10.1016/B978-0-12-407190-2.00007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. van der Burg SH, Arens R, Ossendorp F, et al. Vaccines for established cancer: overcoming the challenges posed by immune evasion. Nat Rev Cancer 2016;16:219–33. 10.1038/nrc.2016.16 [DOI] [PubMed] [Google Scholar]
- 3. Melief CJM, van Hall T, Arens R, et al. Therapeutic cancer vaccines. J Clin Invest 2015;125:3401–12. 10.1172/JCI80009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Branca MA. Rekindling cancer vaccines. Nat Biotechnol 2016;34:1019–24. 10.1038/nbt.3690 [DOI] [PubMed] [Google Scholar]
- 5. Atherton MJ, Stephenson KB, Pol J, et al. Customized viral immunotherapy for HPV-associated cancer. Cancer Immunol Res 2017;5:847–59. 10.1158/2326-6066.CIR-17-0102 [DOI] [PubMed] [Google Scholar]
- 6. Bridle BW, Stephenson KB, Boudreau JE, et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol Ther 2010;18:1430–9. 10.1038/mt.2010.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pol JG, Zhang L, Bridle BW, et al. Maraba virus as a potent oncolytic vaccine vector. Mol Ther 2014;22:420–9. 10.1038/mt.2013.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bridle BW, Nguyen A, Salem O, et al. Privileged antigen presentation in splenic B cell follicles maximizes T cell responses in prime-boost vaccination. J Immunol 2016;196:4587–95. 10.4049/jimmunol.1600106 [DOI] [PubMed] [Google Scholar]
- 9. Krisl JC, Doan VP, Chemotherapy DVP. Chemotherapy and transplantation: the role of immunosuppression in malignancy and a review of antineoplastic agents in solid organ transplant recipients. American Journal of Transplantation 2017;17:1974–91. 10.1111/ajt.14238 [DOI] [PubMed] [Google Scholar]
- 10. Abu Eid R, Razavi GSE, Mkrtichyan M, et al. Old-School chemotherapy in immunotherapeutic combination in cancer, a low-cost drug repurposed. Cancer Immunol Res 2016;4:377–82. 10.1158/2326-6066.CIR-16-0048 [DOI] [PubMed] [Google Scholar]
- 11. Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res 2012;72:3439–44. 10.1158/0008-5472.CAN-11-3912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sistigu A, Viaud S, Chaput N, et al. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol 2011;33:369–83. 10.1007/s00281-011-0245-0 [DOI] [PubMed] [Google Scholar]
- 13. McNally A, Hill GR, Sparwasser T, et al. Cd4+Cd25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci U S A 2011;108:7529–34. 10.1073/pnas.1103782108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Emadi A, Jones RJ, Brodsky RA. Cyclophosphamide and cancer: golden anniversary. Nat Rev Clin Oncol 2009;6:638–47. 10.1038/nrclinonc.2009.146 [DOI] [PubMed] [Google Scholar]
- 15. Zhao J, Cao Y, Lei Z, et al. Selective depletion of CD4+CD25+FoxP3+ regulatory T cells by low-dose cyclophosphamide is explained by reduced intracellular ATP levels. Cancer Res 2010;70:4850–8. 10.1158/0008-5472.CAN-10-0283 [DOI] [PubMed] [Google Scholar]
- 16. Sorensen MR, Pedersen SR, Lindkvist A, et al. Quantification of B16 melanoma cells in lungs using triplex Q-PCR--a new approach to evaluate melanoma cell metastasis and tumor control. PLoS One 2014;9:e87831. 10.1371/journal.pone.0087831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Overwijk WW, Restifo NP. B16 as a mouse model for human melanoma. Curr Protoc Immunol 2001;Chapter 20:Unit 20.1. 10.1002/0471142735.im2001s39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Soliman M, Petrella T, Tyrrell P, et al. The clinical significance of indeterminate pulmonary nodules in melanoma patients at baseline and during follow-up chest CT. Eur J Radiol Open 2019;6:85–90. 10.1016/j.ejro.2019.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petersen RP, Hanish SI, Haney JC, et al. Improved survival with pulmonary metastasectomy: an analysis of 1720 patients with pulmonary metastatic melanoma. J Thorac Cardiovasc Surg 2007;133:104–10. 10.1016/j.jtcvs.2006.08.065 [DOI] [PubMed] [Google Scholar]
- 20. Harpole DH, Johnson CM, Wolfe WG, et al. Analysis of 945 cases of pulmonary metastatic melanoma. J Thorac Cardiovasc Surg 1992;103:743–50. discussion 748-750. 10.1016/S0022-5223(19)34957-8 [DOI] [PubMed] [Google Scholar]
- 21. Liu J-Y, Wu Y, Zhang X-S, et al. Single administration of low dose cyclophosphamide augments the antitumor effect of dendritic cell vaccine. Cancer Immunol Immunother 2007;56:1597–604. 10.1007/s00262-007-0305-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peng S, Lyford-Pike S, Akpeng B, et al. Low-Dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer Immunol Immunother 2013;62:171–82. 10.1007/s00262-012-1322-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen M-L, Pittet MJ, Gorelik L, et al. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proc Natl Acad Sci U S A 2005;102:419–24. 10.1073/pnas.0408197102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacquelot N, Roberti MP, Enot DP, et al. Predictors of responses to immune checkpoint blockade in advanced melanoma. Nat Commun 2017;8:592. 19;8(1):592. 10.1038/s41467-017-00608-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee N, Zakka LR, Mihm MC, et al. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology 2016;48:177–87. 10.1016/j.pathol.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 26. Wimmers F, Aarntzen EHJG, Duiveman-deBoer T, et al. Long-lasting multifunctional CD8+ T cell responses in end-stage melanoma patients can be induced by dendritic cell vaccination. Oncoimmunology 2015;5:e1067745. 10.1080/2162402X.2015.1067745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shirahama T, Muroya D, Matsueda S, et al. A randomized phase II trial of personalized peptide vaccine with low dose cyclophosphamide in biliary tract cancer. Cancer Sci 2017;108:838–45. 10.1111/cas.13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012;18:1254–61. 10.1038/nm.2883 [DOI] [PubMed] [Google Scholar]
- 29. Atherton MJ, Stephenson KB, Tzelepis F, et al. Transforming the prostatic tumor microenvironment with oncolytic virotherapy. Oncoimmunology 2018;7:e1445459. 10.1080/2162402X.2018.1445459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pol JG, Acuna SA, Yadollahi B, et al. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic Maraba virus currently in first-in-human trials. Oncoimmunology 2019;8:e1512329. 10.1080/2162402X.2018.1512329 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-000981supp001.pdf (1.1MB, pdf)
jitc-2020-000981supp002.pdf (68.4KB, pdf)