Abstract
The relationship between food environments and diabetes morbidity is vastly understudied, despite the well-recognized linkage between dietary quality and diabetes complications. Further, literature demonstrates that attributes of places can have nonlinear relationships with health outcomes. This study examines the extent to which “food swamps” are associated with greater rates of hospitalizations for complications among adults with diabetes over time as well as the linearity of this relationship. We conduct a longitudinal county-level analysis of 832 counties across 16 U.S. states in 2010, 2012, and 2014 using data from the USDA Food Environment Atlas and the AHRQ Health Care Cost and Utilization Project State Inpatient Databases. Food swamp severity is measured as the percentage of food outlets in a county that sell primarily unhealthy foods. Hierarchical linear mixed models with county random intercepts are estimated, controlling for area-level covariates and state and year fixed effects. Curvilinear relationships are explored by additively incorporating quadratic terms. We find that, over the study period, mean food swamp severity remained relatively stable. Mean hospitalization rates decreased from 296.72 to 262.82 hospitalizations per 1,000 diabetic adults (p<0.001). In adjusted models, greater food swamp severity was associated with higher hospitalization rates in a curvilinear manner (severity: β=2.181, p=0.02; severity2: β=−0.017, p=0.04), plateauing at approximately 64% unhealthy outlets, a saturation point observed in 17% of observations. Policies that limit saturation of the environment with unhealthy outlets may help in the prevention of diabetic complications, but more saturated counties will likely require more extensive intervention.
Keywords: Food environment, diabetes, hospitalizations, complications, morbidity
Introduction
Diabetes is one of the most prevalent chronic conditions in the United States; recent estimates suggest that it affects over 30 million or 12 percent of American adults (Centers for Disease Control and Prevention, 2017). Individuals with diabetes are at increased risk of developing a variety of serious complications, from acute issues like ketoacidosis to longer term complications such as cardiovascular disease, kidney disease, nerve damage, and problems of the eyes and feet (Centers for Disease Control and Prevention, 2018). Such complications are the source of diabetes-related morbidity and mortality, and they result in high volumes of hospitalizations. In the U.S., it is estimated that 7.2 million hospital discharges were related to diabetes in 2014 and that over 69 billion dollars were spent on diabetes-related inpatient hospitalizations in 2017 (American Diabetes Association, 2018; Centers for Disease Control and Prevention, 2017). Among adults with diabetes, the leading risk factors for developing complications include poor glycemic control, high blood pressure, and high cholesterol (Centers for Disease Control and Prevention, 2017; Deshpande, Harris-Hayes, & Schootman, 2008; Tziomalos & Athyros, 2015; Yau et al., 2012). These intermediate outcomes of diabetes can be influenced by many factors, such as physical inactivity, stress, and treatment adherence, but they are also greatly affected by unhealthy diet. As a result, individuals with diabetes are advised to adhere to diets that are low in processed carbohydrates, saturated and trans fats, cholesterol, and sodium (Bantle et al., 2008; Mayo Clinic, n.d.).
One’s ability to adhere to a recommended diet, however, may be prejudiced by contextual influences. A plethora of research on neighborhood characteristics has found that attributes of places may be determinants of health outcomes, independent of the attributes of the individuals who live within these places (Diez Roux, 2001; Kawachi & Berkman, 2003). With regard to diabetes management and diet, individuals certainly have varying preferences, abilities, and degrees of knowledge, but we are increasingly learning that dietary choices can also be influenced by the surrounding food environment, including the availability of both healthy and unhealthy foods. It is true that previous studies on the relationship between singular aspects of food availability, such as the number of or distance to grocery stores or fast food outlets, and dietary outcomes have yielded mixed results (Caspi, Sorensen, Subramanian, & Kawachi, 2012; Cobb et al., 2015). However, some studies have sought to capture the overall nature of the food environment by focusing on the relative rate of outlets selling mostly unhealthy foods to outlets selling mostly healthy foods, and have more consistently found significant associations with dietary measures such as fruit and vegetable and fast food intake and purchasing (Caspi et al., 2012; Clary et al., 2016; Colón-Ramos et al., 2017; Hager et al., 2017; Mason, Bentley, & Kavanagh, 2013; Thornton & Kavanagh, 2012) and obesity (Babey, Diamant, Hastert, & Harvey, 2008; Cobb et al., 2015; Cooksey-Stowers, Schwartz, & Brownell, 2017; Feng, Astell-Burt, Badland, Mavoa, & Giles-Corti, 2018; Spence, Cutumisu, Edwards, Raine, & Smoyer-Tomic, 2009; Truong, Fernandes, An, Shier, & Sturm, 2010) in the expected directions. Environments that are considered unhealthy by these relative measures, where outlets selling unhealthy goods predominate over outlets selling healthy goods, have been described as “food swamps” (Rose et al., 2009). If such environments encourage diets that are disproportionately lower in fruits and vegetables and higher in fast food and processed snacks, they may place adults with diabetes who live and work within them at higher risk of developing complications and exhibit higher complication rates as a result.
Further, it is possible that relationships between the food environment and diet and related outcomes are nonlinear. Previous work on a variety of subjects has shown that neighborhood characteristics, such as the severity of food swamps as well as community socioeconomic status, land use mix, and natural environment availability (Lei, 2017; Mezuk et al., 2016; Wu, Prina, Jones, Matthews, & Brayne, 2017), can have curvilinear associations with health outcomes. In the food environment context, the addition of a singular healthy or unhealthy outlet may have a dissimilar influence on food choice when more or less of these outlets already exist. For instance, in a relatively healthy food environment, a new fast food outlet would be highly notable, but in an environment overly saturated with unhealthy options, the overall change to the environment would be small and may not shift behavior. If true, such a pattern would be important to consider when designing intervention strategies.
While the relationship between food environment and diabetes prevalence (M. Ahern, Brown, & Dukas, 2011; Babey et al., 2008; Frankenfeld, Leslie, & Makara, 2015; Gebreab et al., 2017; Haynes-Maslow & Leone, 2017; Lee et al., 2018; Richardson et al., 2017; Salois, 2012), incidence (Auchincloss et al., 2009; Christine et al., 2015; Gebreab et al., 2017; Mezuk et al., 2016; Polsky, Moineddin, Glazier, Dunn, & Booth, 2016), and glycemic control among diabetic adults has been examined previously (Berkowitz et al., 2018; Tabaei et al., 2017; Zhang et al., 2017), the relationship between food environment and diabetes-related morbidity is almost entirely unstudied. This analysis builds on our previous work in which we examined the association of food swamp severity and hospitalization rates and found that counties with unhealthier food environments have higher all-cause hospitalization rates among adults with diabetes (Anonymous, 2019). However, the study was cross-sectional and did not explore the possibility of a nonlinear relationship between food swamp severity and hospitalization rates. It also used a limited measure of food environment, comprising only fast food outlets and grocery stores. As such, this current study incorporates additional data and aims to assess the extent to which county-level food swamp severity, measured more comprehensively, is associated with higher county-level hospitalization rates among adults with diabetes in the United States over time. Further, it will examine whether this association is constant across all levels of unhealthy outlet saturation.
Materials & Methods
Study Sample
Data on the food environment came from the U.S. Department of Agriculture Economic Research Service (USDA ERS) Food Environment Atlas, which provides statistics on a range of food environment indicators for U.S. counties, including counts of outlet types. The USDA classifies outlet types according to North American Industry Classification System (NAICS) codes. The most recent estimates that have been released for the relevant variables are from 2009, 2012, and 2014 (Economic Research Service (ERS), U.S. Department of Agriculture (USDA), n.d.). Data on the rate of hospitalizations among diabetic adults came from the Agency for Healthcare Research & Quality (AHRQ) Health Care Cost and Utilization Project (HCUP) state inpatient databases and the Centers for Disease Control and Prevention (CDC) for 16 states (AZ, AR, CO, FL, GA, IA, MA, MI, MN, NJ, NM, NY, OR, RI, VT and WA) for years 2010 through 2014. The HCUP state inpatient databases contain the universe of all-payer hospital inpatient records for each participating state (Agency for Healthcare Research and Quality, 2018). The Centers for Disease Control and Prevention uses Bayesian multilevel modeling on data from the Behavioral Risk Factor Surveillance System and the U.S. Census Bureau to calculate county and year-specific estimates of diagnosed diabetes (Centers for Disease Control and Prevention, 2013). Data on relevant county-level covariates were obtained from the U.S. Department of Health and Human Services Area Health Resources Files (AHRF). All data sources were linked using Federal Information Processing System (FIPS) county codes.
The final analytic sample included data for 832 counties across 16 states in years 2010, 2012, and 2014. Counties with populations under 5,000 (n=41) were dropped to ensure large enough denominators to reliably estimate hospitalization rates (J. Ahern, Matthay, Goin, Farkas, & Rudolph, 2018; Chauhan et al., 2011), as were four outlier observations from 2010 where hospitalization rates drastically differed from their 2012 and 2014 rates.
Measures
Food swamp severity was assessed using a relative measure that represented the percentage of food outlets in a county that sell primarily unhealthy foods. These outlets included fast food restaurants (NAICS code 722211) and convenience stores (NAICS codes 445120 and 447110). The total outlet count additionally included grocery stores (NAICS code 445110) and full-service restaurants (NAICS code 722110). There are multiple ways of quantifying food swamps, but percentage measures such as this have been utilized by several recent studies (Luan, Law, & Quick, 2015; Mezuk et al., 2016; Mui, Gittelsohn, & Jones-Smith, 2017; Mui, Jones-Smith, Thornton, Pollack Porter, & Gittelsohn, 2017; Truong et al., 2010). Using a percentage measure rather than a ratio of unhealthy to healthy outlets allows for the inclusion of counties with zero healthy outlets that would be dropped for having an invalid denominator with a ratio measure.
The main outcome variable was the inpatient hospitalization rate among adult county residents with diabetes. Individuals were linked to their home counties using the FIPS code of residence listed on the hospitalization record. Rates were calculated by dividing the total number of hospital admissions with any-listed diagnosis of Clinical Classification Software code 49 (“diabetes mellitus without complication”) or 50 (“diabetes mellitus with complications”) incurred by county residents over age 20 in each calendar year by the CDC’s estimated number of diagnosed adults with diabetes within the county in that year. Rates were presented as the number of hospitalizations per 1,000 adult county residents with diabetes.
These rates included admissions for all diagnoses among individuals with diabetes, excluding only admissions for pregnancy and patients transferred from other hospitals. We included all diagnoses because poor glycemic control, high blood pressure, and high cholesterol can result in an array of complications among adults with diabetes, many of which may initially seem unrelated to diabetes. For instance, diabetes affects the blood vessels and nerves that control the heart, and acute myocardial infarction and stroke are by far the most frequent reasons for hospitalization among adults with diabetes (Centers for Disease Control and Prevention, 2017; Gregg et al., 2014). These admissions may be missed by stricter coding definitions of diabetes-related complications (Gibbons, Soljak, Millett, Valabhji, & Majeed, 2014). Further, having diabetes can increase the cost and difficulty of treating one’s co-occurring conditions (American Diabetes Association, 2018). For example, diabetes can impact immune function, resulting in reduced resistance to influenza and pneumonia, and can damage blood vessels in the lungs, causing further exacerbations in individuals with chronic obstructive pulmonary disease (Deshpande et al., 2008; Rogliani, Lucà, & Lauro, 2015). We sought to capture complications that result from these interplays as well.
Several other variables were used to capture county-level health systems and sociodemographic characteristics relevant to hospitalization rates and food environment. The percentage of diabetic adult hospitalizations admitted through the emergency room, the percentage of hospitalized diabetic adults that were Medicaid beneficiaries, and the mean number of comorbidities per diabetic patient admitted were created using the HCUP analytic sample. The number of primary care physicians per 1,000 residents, median household income, population density (log transformed), and the percentage of the county population that is non-Hispanic black, Hispanic, female, and over age 65 were sourced from the AHRF. The number of recreational facilities per 1,000 residents was obtained from the USDA Food Environment Atlas.
Statistical analysis
A hierarchical linear mixed regression model was used to estimate the association of food swamp severity and hospitalization rates among adults with diabetes, accounting for the nesting of biennial observations within counties using county random intercepts. Standard errors were clustered at the county level to adjust for the non-independence of these observations. To allow for a curvilinear relationship, polynomial iterations of the food swamp severity variable were tested in an additive manner, ceasing when an iteration was no longer significant at the 0.05 level. The model further included indicators for the years 2012 and 2014 to account for time trends and for states to account for any clustering of counties within states, which may have their own policies, programs, etc. that affect hospitalization rates. The model also controlled for the time-varying health-system and sociodemographic covariates previously noted, and variance inflation factors (VIF) were examined for model covariates to assess collinearity.
Sensitivity analyses
Several sensitivity analyses were performed to examine the robustness of the main results to alternative measurement and modeling decisions. First, the regression model was estimated using a more restrictive definition of diabetes-related complications. This definition comprised only hospitalizations with a principal diagnosis that met the AHRQ Prevention Quality Indicator™ Version 6.0 specifications for diabetes with short-term complications, diabetes with long-term complications, uncontrolled diabetes, or lower extremity amputation among patients with diabetes (Agency for Healthcare Research and Quality, 2016). The ICD-9 codes for each qualified diagnosis are included in the appendix, and included such diagnoses as ketoacidosis, and renal, ophthalmic and peripheral circulatory manifestations. Diagnoses such as acute myocardial infarction and stroke were not included in this definition. Second, similar food environments can have different impacts on diet and related outcomes at varying levels of financial security (Allcott et al., 2017; Jones-Smith et al., 2013). To examine this possibility, an interaction between food swamp severity and financial resources, measured by median household income and, alternatively, by the percent living in poverty, was assessed. Third, to ensure that any association of food swamp severity and hospitalization rates was not driven by changes in diabetes prevalence estimates (the denominator), the model was run using the log transformed count of hospitalizations as the outcome and additionally controlling for the log transformed number of diabetic county residents. Finally, in an attempt to identify endogeneity from outlets differentially locating in areas for reasons that influence hospitalizations (i.e. demand for unhealthy foods), the change in food swamp severity between 2010 and 2014 was regressed on a variety of baseline county characteristics, including the diabetes prevalence rate, median household income, logged population density, and the percent of the population that is non-Hispanic black, Hispanic, over age 65, and live in urban areas, as well as state indicators.
Data were analyzed using Stata, version 14, College Station, TX, between June 2018 and June 2019.
Results
The mean food swamp severity remained relatively stable over the study period; it increased by less than one percentage point from 53.63 percent unhealthy outlets to 54.38 percent unhealthy outlets, but this increase was not statistically significant (p=0.157). The mean hospitalization rate decreased from 2010 to 2014, from 296.72 hospitalizations to 262.82 hospitalizations per 1,000 adults with diabetes (p<0.001) (Table 1). Common primary diagnoses among these hospitalizations included atrial fibrillation, subendocardial infarction, septicemia, pneumonia, obstructive chronic bronchitis, kidney failure, and ketoacidosis. Both variables exhibited far more variation between counties than they did between years for each county. The between-county standard deviation for food swamp severity was 10.56 while the between-year standard deviation was only 3.22. The between-county standard deviation for hospitalization rates was 80.80 while the between-year standard deviation was 34.36.
Table 1.
Health-Systems Related and Sociodemographic Characteristics of Counties, 2010-2014
| Variable | 2010 Mean (95% CI) n=828 |
2012 Mean (95% CI) n=832 |
2014 Mean (95% CI) n=832 |
|---|---|---|---|
| Hospitalization rate (per 1,000 diabetic residents) | 296.72 (290.48, 302.96) | 269.09 (263.17, 275.02) | 262.82 (257.35, 268.30) |
| Food swamp severity | 53.63 (52.92, 54.35) | 54.14 (53.37, 54.92) | 54.38 (53.63, 55.15) |
| Percentage admitted in ED | 53.08 (51.79, 54.38) | 45.83 (43.84, 47.82) | 50.09 (48.12, 53.07) |
| Percentage of patients with Medicaid | 18.19 (17.44, 18.94) | 18.80 (18.10, 19.51) | 21.37 (20.73, 22.01) |
| Mean comorbidity burden | 3.60 (3.57, 3.62) | 3.74 (3.71, 3.76) | 3.89 (3.87, 3.92) |
| Primary care physicians (per 1,000 residents) | 0.59 (0.57, 3.62) | 0.59 (0.57, 0.61) | 0.60 (0.58, 0.62) |
| Recreational facilities (per 1,000 residents) | 0.09 (0.08, 0.09) | 0.08 (0.7, 0.8) | 0.08 (0.07, 0.08) |
| Median household income (in thousands) | 44.45 (43.68, 45.22) | 45.91 (45.10, 46.73) | 48.08 (47.22, 48.94) |
| Population density (population/square miles) | 348.80 (200.28, 497.33) | 353.39 (202.48, 504.30) | 359.17 (205.94, 512.40) |
| Percentage of population non-Hispanic Black | 9.36 (8.40, 10.31) | 9.73 (8.77, 10.69) | 9.85 (8.89, 10.81) |
| Percentage of population Hispanic | 8.86 (8.04, 9.67) | 9.25 (8.43, 10.06) | 9.56 (8.73, 10.38) |
| Percentage of population female | 50.01 (49.85, 50.17) | 49.92 (49.76, 50.08) | 49.91 (49.74, 50.07) |
| Percentage of population over age 65 | 15.86 (15.56, 16.16) | 16.80 (16.49, 17.12) | 17.76 (17.44, 18.09) |
Note. Boldface indicates statistical significance (p<0.05) in t-test of means compared to 2010. a denotes estimate from 2009 rather than 2010.
Source. USDA Food Environment Atlas 2009-2014, AHRQ Health Care Cost and Utilization Project (HCUP) state inpatient files 2010-2014, HHS Area Health Resources File (AHRF) 2010-2014.
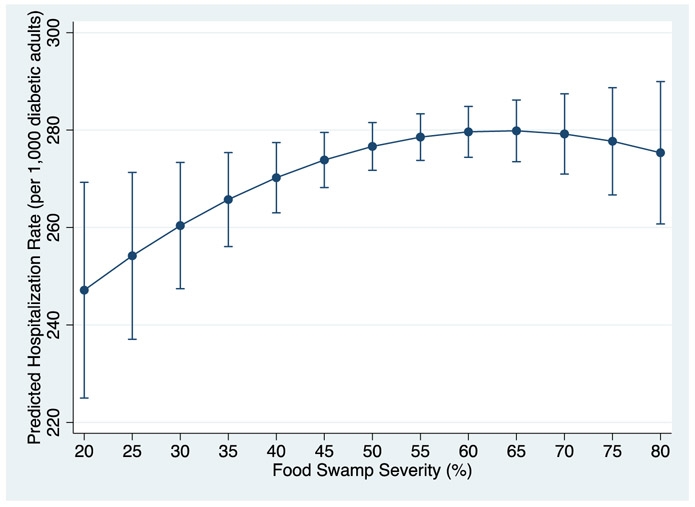
Results from the multivariate mixed models with varying quadratic terms indicated that food swamp severity had a significant positive and curvilinear association with hospitalization rates among adults with diabetes at the county level (Table 2). A squared food swamp severity term was significant (β=−0.017, p=0.038), indicating that the strength of the association attenuated as food swamp severity increased. In essence, the association was stronger in environments with lower relative rates of unhealthy food outlets, but the magnitude leveled off after a certain point of saturation by unhealthy outlets (Figure 1). This point of saturation was approximately 64 percent, a quantity achieved by only 17 percent of the county-year observations. The mean VIF was 1.58; all variables were below 2.45, with most below 2.00. Cubic and higher polynomial food swamp terms, when added, were not significant and were not included in the final model.
Table 2.
The Association between County Food Swamp Scores and Hospitalization Rates among Adults with Diabetes, 2010-2014 (n=2,490)
| Coefficient | 95% Confidence Interval |
|
|---|---|---|
| Food swamp severity | 2.181* | (0.390, 3.972) |
| Food swamp severity2 | −0.017* | (−0.033, −0.001) |
| Time trend | ||
| 2012 | −32.959*** | (−37.329, −28.590) |
| 2014 | −45.902*** | (−52.360, −39.444) |
| Percentage admitted in ED | 0.146 | (−0.026, 0.318) |
| Percentage of patients with Medicaid | 0.007 | (−0.408, 0.421) |
| Mean comorbidity burden | 53.009*** | (39.704, 66.314) |
| Primary care physicians (per 1,000 residents) | 16.843* | (0.786, 32.899) |
| Recreational facilities (per 1,000 residents) | −33.482 | (−94.619, 27.655) |
| Median household income (in thousands) | −1.673*** | (−2.132, −1.214) |
| Log-transformed population density | 8.848*** | (4.160, 13.536) |
| Percentage of population non-Hispanic Black | −0.195 | (−0.775, 0.385) |
| Percentage of population Hispanic | 1.502*** | (0.913, 2.091) |
| Percentage of population female | 2.996* | (0.480, 5.512) |
| Percentage of population over age 65 | 0.802 | (−0.450, 2.054) |
Note. Table presents estimates from hierarchical linear mixed model with county-level random intercepts and state indicator variables. Standard errors are clustered at county level. Boldface indicates statistical significance
p<0.05
p<0.01
p<0.001).
Figure 1:
Predicted hospitalization rates by food swamp severity (with 95% CI)
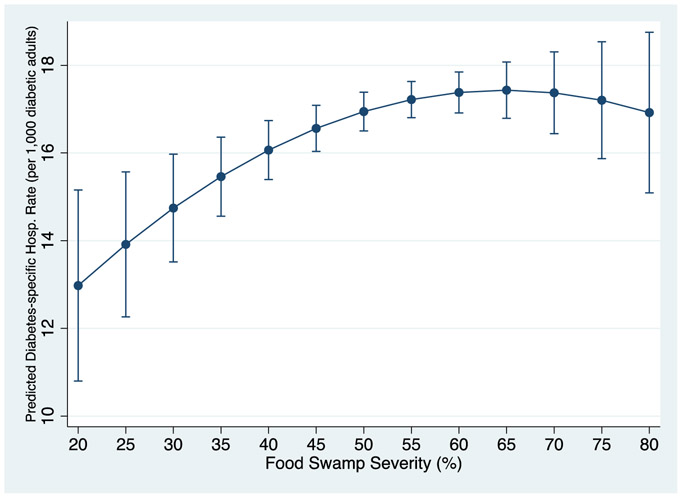
The model analyzing hospitalization rates for strictly diabetes-related hospitalizations yielded a consistent pattern of results (food swamp severity: β=0.284, p=0.005; food swamp severity2: β=−0.002, p=0.023). The coefficients were smaller in magnitude, as these particular hospitalizations were relatively rare (baseline mean=17.74 hospitalizations per 1,000 diabetic adults). However, the rate of change across the distribution of food swamp severity was similar to that of the original model, as shown in Figure 2. Terms for the interaction of food swamp severity with median household income and with the percent living in poverty were not significant when included in the model. The model decomposing hospitalization rates into hospitalization and prevalence counts was concordant with the original model. No baseline county characteristics were significantly associated with changes in food swamp severity over time, indicating that they were not related to outlet entry and exit.
Figure 2:
Predicted diabetes-specific hospitalization rates by food swamp severity (with 95% CI)
Discussion
The results of this study indicate that, in the U.S., food swamp severity is associated with higher rates of hospitalizations for complications among diabetic adults at the county level, even after adjusting for relevant covariates. The results are consistent with previous research that elucidates how the oversaturation of the environment with unhealthy outlets might influence eating behaviors among adults with diabetes. High prevalence of unhealthy foods diminishes the likelihood of resisting temptation to purchase them, as desire for a product can be prompted by visual cues. The more prevalent these products are, the more visually salient they are, meaning desires are more frequently triggered and the odds are higher that we will eventually succumb to them (Armel, Beaumel, & Rangel, 2008; Laibson, 2001; Milosavljevic, Navalpakkam, Koch, & Rangel, 2012). This is especially true considering willpower is a limited resource (Baumeister, Vohs, & Tice, 2007; Hagger, Wood, Stiff, & Chatzisarantis, 2010). Further, greater density of unhealthy outlets makes unhealthy foods more convenient to obtain than healthier foods, and we are more likely to purchase products when doing so does not require additional time and effort than when they are less readily available (Berry, Seiders, & Grewal, 2002; Meiselman, Hedderley, Staddon, Pierson, & Symonds, 1994; Meyers & Stunkard, 1980; Thorndike, Sonnenberg, Riis, Barraclough, & Levy, 2012). In addition, literature on tobacco suggests that when products are more available and visually salient, they may contribute to a social norm that the consumption of these products is ordinary and perhaps even more commonplace than is true (Albers, Siegel, Cheng, Biener, & Rigotti, 2004; Alesci, Forster, & Blaine, 2003; Charlesworth & Glantz, 2005).
Our results indicate that strategies that limit the oversaturation of counties with unhealthy outlets may help prevent diabetic complications. However, the finding that the relationship plateaus in the most extreme food swamps suggests that more extensive food environment changes may be needed to prevent complications in these counties, perhaps because these areas are so oversaturated with unhealthy outlets that small increases in healthy outlets or decreases in unhealthy outlets do little to impact the overall food environment. Such minor changes in outlet distribution might be imperceptible and thus unlikely to alter the influences that drive food purchasing and consumption decisions. This point of saturation may also help partially explain why studies on the entry of new grocery stores (Allcott et al., 2017; Cummins, Flint, & Matthews, 2014; Dubowitz et al., 2015; Elbel et al., 2015; Zhang et al., 2016) or fast food moratoriums (Sturm & Hattori, 2015) have found null or clinically small results for dietary quality and obesity. These policies are often implemented in the poorest quality food environments in which the introduction of one healthy outlet or the curtailment of further unhealthy outlets could have little impact on the existing degree of saturation. Certainly, these situations are complex and null results could stem from a range of factors (price and quality of foods sold, transportation resources, etc.), but a curvilinear relationship between outlets and outcomes should potentially be explored.
Our study is one of the first to assess the relationship between the food environment and diabetes-related morbidity. Our previous work similarly identified a positive association, but, as mentioned, was cross-sectional and did not consider nonlinearity (Anonymous, 2019). As such, these findings are consistent yet contribute additional insight into our limited knowledge about this relationship. Given the burden of diabetes, it is important that we understand the diversity of factors that may contribute to complications among diabetic adults, including neighborhood characteristics. Although recent studies have suggested that the rates of complications have decreased in recent years (Centers for Disease Control and Prevention, 2007; Gregg et al., 2014), these rates are still quite high. For instance, in 2010 it was estimated that in the U.S. 45.5 of every 10,000 adults with diabetes was hospitalized for acute myocardial infarction, compared to only 25.8 of every 10,000 adults without diabetes (Gregg et al., 2014). Furthermore, with the population aging and diabetes prevalence increasing, more individuals are at risk and the absolute number of complications may continue to rise (Gregg et al., 2014). By broadening our understanding of the factors that influence complications, we may be able to recognize and utilize additional avenues to prevent some of these complications.
It might be suggested that counties with higher percentages of unhealthy food outlets are simply the same counties that exhibit other qualities associated with increased rates of hospitalizations among diabetic adults, but this data suggest that food swamps, at least at the county level, represent a wholly separate concept. For instance, food swamp severity is at best moderately correlated with measures of socioeconomic status or deprivation, such as median household income, percent living in poverty, and unemployment rate (ρ=−0.27, 0.40, 0.16, respectively) and only weakly correlated with measures of access to preventive care, such as the number of primary care physicians and federally qualified health centers per population and the percent of adults without health insurance (ρ=−0.28, 0.02, 0.26, respectively). Thus, if this analysis’ findings can be corroborated, food swamp measures may prove to be additional indicators with which we can identify areas that warrant increased attention and intervention.
This study has important limitations to consider when interpreting the results. First, the observational methods used limit causal inference. However, this study builds upon previous analyses that have used causal methods such as instrumental variables to successfully link food environment with other related outcomes, such as obesity (Cooksey-Stowers et al., 2017; Dunn, 2010). Highway exits as an instrumental variable, which was used in these studies, was not appropriate for our analysis, as transit is related to health services access and hospital utilization, but these studies bolster confidence in our identified relationship despite our inability to make causal claims. Second, due to data availability, the food swamp severity variable did not include some outlets that may meaningfully contribute to the food environment, such as farmers’ markets and specialty stores. While the outlets included likely encompass a sizeable portion of food purchases, future studies may want to consider additional outlet types, when possible. Third, the time period analyzed was chosen based on data availability and may not accurately correspond to the etiologic processes under study. Longer periods in which more change can be observed should be analyzed in the future. Also, due to availability, the observations from 2010 include food environment data from 2009, but this practice of merging data from multiple years has been previously utilized (Rundle et al., 2008). As seen in the results, food environment changes vary marginally over short time periods and it is expected that the 2009 estimates are quite close to what would have been observed in 2010. Finally, the unit of analysis is the county level, which forfeits some precision that could have been obtained by using smaller units and masks existing within-county heterogeneity. However, larger units like counties are more likely to capture a greater share of individuals’ daily travel routes compared to smaller units. Food shopping often takes place further from home than would be observed with such units. For instance, a Los Angeles-based study found that only approximately 22 percent of those surveyed shopped for groceries within their home census tracts (Inagami, Cohen, Finch, & Asch, 2006). However, it remains that county boundaries are arbitrarily drawn and may not truly reflect the space in which people spend their time, risking spatial misclassification. Further, the use of the county unit also makes us unable to incorporate residential selection and other important individual potential confounders. Individual-level analyses would allow us to consider these aspects and explore the mechanisms behind this association, but unfortunately such analyses were not possible in this study. Hospitalization data does not provide information on adults with diabetes who did not experience hospitalization, rendering us without an appropriate comparison group at the individual level. Individual-level analyses using alternative outcome data and geographic information systems-based measures should be pursued when such data is available at the national level. However, the aggregate-level conclusions we can draw from this study may still be useful for policy discussions because they highlight the challenges faced by communities oversaturated by unhealthy outlets.
Conclusion
U.S. counties with greater percentages of unhealthy food outlets have higher rates of hospitalizations among adults with diabetes, but this relationship plateaus at a point of extreme saturation by unhealthy outlets. Understanding this food swamp saturation point may provide insight into geographic disparities in diabetes complication rates across the country as well as new ways in which policy makers and practitioners can prevent diabetic complications and the resulting morbidity and mortality.
Appendix
Table A1.
ICD-9 Codes & Diagnoses Included in AHRQ Prevention Quality Indicators
| Prevention Quality Indicator 01: Diabetes with Short-term Complications | |||
|---|---|---|---|
| ICD-9 Code |
Diagnosis | ICD-9 Code |
Diagnosis |
| 25010 | Diabetes with ketoacidosis, type II | 25022 | Diabetes with hyperosmolarity, type II, uncontrolled |
| 25011 | Diabetes with ketoacidosis, type I | 25023 | Diabetes with hyperosmolarity, type I, uncontrolled |
| 25012 | Diabetes with ketoacidosis, type II, uncontrolled | 25030 | Diabetes with other coma, type II |
| 25013 | Diabetes with ketoacidosis, type I, uncontrolled | 25031 | Diabetes with other coma, type II |
| 25020 | Diabetes with hyperosmolarity, type II | 25032 | Diabetes with other coma, type II, uncontrolled |
| 25021 | Diabetes with hyperosmolarity, type I | 25033 | Diabetes with other coma, type I, uncontrolled |
| Prevention Quality Indicator 03: Diabetes with Long-term Complications | |||
| ICD-9 Code |
Diagnosis | ICD-9 Code |
Diagnosis |
| 25040 | Diabetes with renal manifestations, type II | 25070 | Diabetes with peripheral circulatory disorders, type II |
| 25041 | Diabetes with renal manifestations, type II | 25071 | Diabetes with peripheral circulatory disorders, type I |
| 25042 | Diabetes with renal manifestations, type II, uncontrolled | 25072 | Diabetes with peripheral circulatory disorders, type II, uncontrolled |
| 25043 | Diabetes with renal manifestations, type I, uncontrolled | 25073 | Diabetes with peripheral circulatory disorders, type I, uncontrolled |
| 25050 | Diabetes with ophthalmic manifestations, type II | 25080 | Diabetes with other specified manifestations, type II |
| 25051 | Diabetes with ophthalmic manifestations, type I | 25081 | Diabetes with other specified manifestations, type I |
| 25052 | Diabetes with ophthalmic manifestations, type II, uncontrolled | 25082 | Diabetes with other specified manifestations, type II, uncontrolled |
| 25053 | Diabetes with ophthalmic manifestations, type I, uncontrolled | 25083 | Diabetes with other specified manifestations, type I, uncontrolled |
| 25060 | Diabetes with neurological manifestations, type II | 25090 | Diabetes with unspecified complication, type II |
| 25061 | Diabetes with neurological manifestations, type I | 25091 | Diabetes with unspecified complication, type I |
| 25062 | Diabetes with neurological manifestations, type II, uncontrolled | 25092 | Diabetes with unspecified complication, type II, uncontrolled |
| 25063 | Diabetes with neurological manifestations, type I, uncontrolled | 25093 | Diabetes with unspecified complication, type I, uncontrolled |
| Prevention Quality Indicator 14: Uncontrolled Diabetes | |||
| ICD-9 Code |
Diagnosis | ICD-9 Code |
Diagnosis |
| 25002 | Diabetes mellitus without mention of complication, type II, uncontrolled | 25003 | Diabetes mellitus without mention of complication, type I, uncontrolled |
| Prevention Quality Indicator 16: Lower Extremity Amputation among Patients with Diabetes | |||
| Lower Extremity Amputation | |||
| ICD-9 Code |
Diagnosis | ICD-9 Code |
Diagnosis |
| 8410 | Lower limb amputation, not otherwise specified | 8416 | Disarticulation of knee |
| 8412 | Amputation through foot | 8417 | Amputation above knee |
| 8414 | Amputation of ankle through malleoli of tibia and fibula | 8418 | Disarticulation of Hip |
| 8415 | Other amputation below knee | 8419 | Abdominopelvic amputation |
| Diabetes | |||
| ICD-9 Code |
Diagnosis | ICD-9 Code |
Diagnosis |
| 25000 | Diabetes mellitus without mention of complications, type II | 25050 | Diabetes with ophthalmic manifestations, type II |
| 25001 | Diabetes mellitus without mention of complications, type I | 25051 | Diabetes with ophthalmic manifestations, type I |
| 25002 | Diabetes mellitus without mention of complications, type II, uncontrolled | 25052 | Diabetes with ophthalmic manifestations, type II, uncontrolled |
| 25003 | Diabetes mellitus without mention of complications, type I, uncontrolled | 25053 | Diabetes with ophthalmic manifestations, type I, uncontrolled |
| 25010 | Diabetes with ketoacidosis, type II | 25060 | Diabetes with neurological manifestations, type II |
| 25011 | Diabetes with ketoacidosis, type I | 25061 | Diabetes with neurological manifestations, type I |
| 25012 | Diabetes with ketoacidosis, type II, uncontrolled | 25062 | Diabetes with neurological manifestations, type II, uncontrolled |
| 25013 | Diabetes with ketoacidosis, type I, uncontrolled | 25063 | Diabetes with neurological manifestations, type I, uncontrolled |
| 25020 | Diabetes with hyperosmolarity, type II | 25070 | Diabetes with peripheral circulatory disorders, type II |
| 25021 | Diabetes with hyperosmolarity, type I | 25071 | Diabetes with peripheral circulatory disorders, type I |
| 25022 | Diabetes with hyperosmolarity, type II, uncontrolled | 25072 | Diabetes with peripheral circulatory disorders, type II, uncontrolled |
| 25023 | Diabetes with hyperosmolarity, type I, uncontrolled | 25073 | Diabetes with peripheral circulatory disorders, type I, uncontrolled |
| 25030 | Diabetes with other coma, type II | 25080 | Diabetes with other specified manifestations, type II |
| 25031 | Diabetes with other coma, type II | 25081 | Diabetes with other specified manifestations, type I |
| 25032 | Diabetes with other coma, type II, uncontrolled | 25082 | Diabetes with other specified manifestations, type II, uncontrolled |
| 25033 | Diabetes with other coma, type I, uncontrolled | 25083 | Diabetes with other specified manifestations, type I, uncontrolled |
| 25040 | Diabetes with renal manifestations, type II | 25090 | Diabetes with unspecified complication, type II |
| 25041 | Diabetes with renal manifestations, type II | 25091 | Diabetes with unspecified complication, type I |
| 25042 | Diabetes with renal manifestations, type II, uncontrolled | 25092 | Diabetes with unspecified complication, type II, uncontrolled |
| 25043 | Diabetes with renal manifestations, type I, uncontrolled | 25093 | Diabetes with unspecified complication, type I, uncontrolled |
Source. Agency for Healthcare Research and Quality (AHRQ) Prevention Quality Indicators Technical Specifications, Version 6.0
References:
- Agency for Healthcare Research and Quality. (2016, October). Prevention Quality Indicators Technical Specifications. Retrieved from https://www.qualityindicators.ahrq.gov/Modules/PQI_TechSpec_ICD09_v60.aspx
- Agency for Healthcare Research and Quality. (2018). Introduction to the HCUP State Inpatient Databases (SID). Retrieved from https://www.hcup-us.ahrq.gov/db/state/siddist/Introduction_to_SID.pdf
- Ahern J, Matthay EC, Goin DE, Farkas K, & Rudolph KE (2018). Acute changes in community violence and increases in hospital visits and deaths from stress-responsive diseases. Epidemiology, 29(5), 684–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahern M, Brown C, & Dukas S (2011). A national study of the association between food environments and county-level health outcomes. The Journal of Rural Health, 27(4), 367–379. [DOI] [PubMed] [Google Scholar]
- Albers AB, Siegel M, Cheng DM, Biener L, & Rigotti NA (2004). Relation between local restaurant smoking regulations and attitudes towards the prevalence and social acceptability of smoking: A study of youths and adults who eat out predominantly at restaurants in their town. Tobacco Control, 13(4), 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alesci NL, Forster JL, & Blaine T (2003). Smoking visibility, perceived acceptability, and frequency in various locations among youth and adults☆. Preventive Medicine, 36(3), 272–281. [DOI] [PubMed] [Google Scholar]
- Allcott H, Diamond R, Dubé J-P, Handbury J, Rahkovsky I, & Schnell M (2017). Food Deserts and the Causes of Nutritional Inequality (Working Paper No. 24094). 10.3386/w24094 [DOI] [Google Scholar]
- American Diabetes Association. (2018). Economic Costs of Diabetes in the US in 2017. Diabetes Care, 41(5), 917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armel KC, Beaumel A, & Rangel A (2008). Biasing simple choices by manipulating relative visual attention. Judgment and Decision Making, 3(5), 396–403. [Google Scholar]
- Auchincloss AH, Roux AVD, Mujahid MS, Shen M, Bertoni AG, & Carnethon MR (2009). Neighborhood resources for physical activity and healthy foods and incidence of type 2 diabetes mellitus: The Multi-Ethnic study of Atherosclerosis. Archives of Internal Medicine, 169(18), 1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babey SH, Diamant AL, Hastert TA, & Harvey S (2008). Designed for disease: The link between local food environments and obesity and diabetes.
- Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, … Wheeler ML (2008). Nutrition recommendations and interventions for diabetes: A position statement of the American Diabetes Association. Diabetes Care, 31 Sup(10.2337/dc08-S061), S61–S78. Retrieved from http://care.diabetesjournals.org/content/31/Supplement_1/S61.full?ijkey=0139447811c08f073edda51cfc6585e745215d6b&keytype2=tf_ipsecsha [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Vohs KD, & Tice DM (2007). The strength model of self-control. Current Directions in Psychological Science, 16(6), 351–355. [Google Scholar]
- Berkowitz SA, Karter AJ, Corbie-Smith G, Seligman HK, Ackroyd SA, Barnard LS, … Wexler DJ (2018). Food Insecurity, Food “Deserts,” and Glycemic Control in Patients With Diabetes: A Longitudinal Analysis. Diabetes Care, dc171981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry LL, Seiders K, & Grewal D (2002). Understanding service convenience. Journal of Marketing, 66(3), 1–17. [Google Scholar]
- Caspi CE, Sorensen G, Subramanian S, & Kawachi I (2012). The local food environment and diet: A systematic review. Health & Place, 18(5), 1172–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2007). Prevalence of self-reported cardiovascular disease among persons aged> or= 35 years with diabetes–United States, 1997-2005. MMWR. Morbidity and Mortality Weekly Report, 56(43), 1129. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2013). Methods and References for County-Level Estimates and Ranks and State-Level Modeled Estimates. Retrieved from https://www.cdc.gov/diabetes/pdfs/data/calculating-methods-references-county-level-estimates-ranks.pdf
- Centers for Disease Control and Prevention. (2017). National diabetes statistics report, 2017. Retrieved from https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf
- Centers for Disease Control and Prevention. (2018, August 30). About Chronic Disease ∣ Chronic Disease Prevention and Health Promotion ∣ CDC. Retrieved September 12, 2018, from National Center for Chronic Disease Prevention and Health Promotion website: https://www.cdc.gov/chronicdisease/about/index.htm [Google Scholar]
- Charlesworth A, & Glantz SA (2005). Smoking in the movies increases adolescent smoking: A review. Pediatrics, 116(6), 1516–1528. [DOI] [PubMed] [Google Scholar]
- Chauhan P, Cerdá M, Messner SF, Tracy M, Tardiff K, & Galea S (2011). Race/ethnic-specific homicide rates in new york city: Evaluating the impact of broken windows policing and crack cocaine markets. Homicide Studies, 15(3), 268–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christine PJ, Auchincloss AH, Bertoni AG, Carnethon MR, Sánchez BN, Moore K, … Roux AVD (2015). Longitudinal associations between neighborhood physical and social environments and incident type 2 diabetes mellitus: The Multi-Ethnic Study of Atherosclerosis (MESA). JAMA Internal Medicine, 175(8), 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clary C, Lewis DJ, Flint E, Smith NR, Kestens Y, & Cummins S (2016). The local food environment and fruit and vegetable intake: A geographically weighted regression approach in the ORiEL Study. American Journal of Epidemiology, 184(11), 837–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb LK, Appel LJ, Franco M, Jones-Smith JC, Nur A, & Anderson CA (2015). The relationship of the local food environment with obesity: A systematic review of methods, study quality, and results. Obesity, 23(7), 1331–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colón-Ramos U, Monge-Rojas R, Cremm E, Rivera IM, Andrade EL, & Edberg MC (2017). How Latina mothers navigate a ‘food swamp’ to feed their children: A photovoice approach. Public Health Nutrition, 20(11), 1941–1952. 10.1017/S1368980017000738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey-Stowers K, Schwartz MB, & Brownell KD (2017). Food Swamps Predict Obesity Rates Better Than Food Deserts in the United States. International Journal of Environmental Research and Public Health, 14(11), 1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins S, Flint E, & Matthews SA (2014). New neighborhood grocery store increased awareness of food access but did not alter dietary habits or obesity. Health Affairs, 33(2), 283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande AD, Harris-Hayes M, & Schootman M (2008). Epidemiology of diabetes and diabetes-related complications. Physical Therapy, 88(11), 1254–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux AV (2001). Investigating neighborhood and area effects on health. American Journal of Public Health, 91(11), 1783–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubowitz T, Ghosh-Dastidar M, Cohen DA, Beckman R, Steiner ED, Hunter GP, … Sloan JC (2015). Diet and perceptions change with supermarket introduction in a food desert, but not because of supermarket use. Health Affairs, 34(11), 1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn RA (2010). The effect of fast-food availability on obesity: An analysis by gender, race, and residential location. American Journal of Agricultural Economics, 92(4), 1149–1164. [Google Scholar]
- Economic Research Service (ERS), U.S. Department of Agriculture (USDA). (n.d.). Food Environment Atlas. Retrieved from https://www.ers.usda.gov/data-products/food-environment-atlas/
- Elbel B, Moran A, Dixon LB, Kiszko K, Cantor J, Abrams C, & Mijanovich T (2015). Assessment of a government-subsidized supermarket in a high-need area on household food availability and children’s dietary intakes. Public Health Nutrition, 18(15), 2881–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Astell-Burt T, Badland H, Mavoa S, & Giles-Corti B (2018). Modest ratios of fast food outlets to supermarkets and green grocers are associated with higher body mass index: Longitudinal analysis of a sample of 15,229 Australians aged 45 years and older in the Australian National Liveability Study. Health & Place, 49, 101–110. [DOI] [PubMed] [Google Scholar]
- Frankenfeld CL, Leslie TF, & Makara MA (2015). Diabetes, obesity, and recommended fruit and vegetable consumption in relation to food environment sub-types: A cross-sectional analysis of Behavioral Risk Factor Surveillance System, United States Census, and food establishment data. BMC Public Health, 15(1), 491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebreab SY, Hickson DA, Sims M, Wyatt SB, Davis SK, Correa A, & Diez-Roux AV (2017). Neighborhood social and physical environments and type 2 diabetes mellitus in African Americans: The Jackson Heart Study. Health & Place, 43, 128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons DC, Soljak MA, Millett C, Valabhji J, & Majeed A (2014). Use of hospital admissions data to quantify the burden of emergency admissions in people with diabetes mellitus. Diabetic Medicine, 31(8), 971–975. [DOI] [PubMed] [Google Scholar]
- Gregg EW, Li Y, Wang J, Rios Burrows N, Ali MK, Rolka D, … Geiss L (2014). Changes in diabetes-related complications in the United States, 1990–2010. New England Journal of Medicine, 370(16), 1514–1523. [DOI] [PubMed] [Google Scholar]
- Hager ER, Cockerham A, O’Reilly N, Harrington D, Harding J, Hurley KM, & Black MM (2017). Food swamps and food deserts in Baltimore City, MD, USA: associations with dietary behaviours among urban adolescent girls. Public Health Nutrition, 20(14), 2598–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, & Chatzisarantis NL (2010). Ego depletion and the strength model of self-control: A meta-analysis. Psychological Bulletin, 136(4), 495. [DOI] [PubMed] [Google Scholar]
- Haynes-Maslow L, & Leone LA (2017). Examining the relationship between the food environment and adult diabetes prevalence by county economic and racial composition: An ecological study. BMC Public Health, 17(1), 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagami S, Cohen DA, Finch BK, & Asch SM (2006). You are where you shop: Grocery store locations, weight, and neighborhoods. American Journal of Preventive Medicine, 31(1), 10–17. [DOI] [PubMed] [Google Scholar]
- Jones-Smith JC, Karter AJ, Warton EM, Kelly M, Kersten E, Moffet HH, … Laraia BA (2013). Obesity and the food environment: Income and ethnicity differences among people with diabetes: the Diabetes Study of Northern California (DISTANCE). Diabetes Care, DC_122190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawachi I, & Berkman LF (2003). Neighborhoods and health. Oxford University Press. [Google Scholar]
- Laibson D (2001). A cue-theory of consumption. The Quarterly Journal of Economics, 116(1), 81–119. [Google Scholar]
- Lee DC, Gallagher MP, Gopalan A, Osorio M, Vinson AJ, Wall SP, … Elbel B (2018). Identifying Geographic Disparities in Diabetes Prevalence Among Adults and Children Using Emergency Claims Data. Journal of the Endocrine Society, 2(5), 460–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei L (2017). The impact of community context on children’s health and nutritional status in China. Social Science & Medicine, 179, 172–181. [DOI] [PubMed] [Google Scholar]
- Luan H, Law J, & Quick M (2015). Identifying food deserts and swamps based on relative healthy food access: A spatio-temporal Bayesian approach. International Journal of Health Geographics, 14(1), 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason KE, Bentley RJ, & Kavanagh AM (2013). Fruit and vegetable purchasing and the relative density of healthy and unhealthy food stores: Evidence from an Australian multilevel study. J Epidemiol Community Health, 67(3), 231–236. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic. (n.d.). Hyperglycemia in diabetes—Symptoms and causes. Retrieved January 12, 2018, from Mayo Clinic; website: http://www.mayoclinic.org/diseases-conditions/hyperglycemia/symptoms-causes/syc-20373631 [Google Scholar]
- Meiselman HL, Hedderley D, Staddon SL, Pierson BJ, & Symonds CR (1994). Effect of effort on meal selection and meal acceptability in a student cafeteria. Appetite, 23(1), 43–55. [DOI] [PubMed] [Google Scholar]
- Meyers AW, & Stunkard AJ (1980). Food accessibility and food choice: A test of Schachter’s externality hypothesis. Archives of General Psychiatry, 37(10), 1133–1135. [DOI] [PubMed] [Google Scholar]
- Mezuk B, Li X, Cederin K, Rice K, Sundquist J, & Sundquist K (2016). Beyond access: Characteristics of the food environment and risk of diabetes. American Journal of Epidemiology, 183(12), 1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosavljevic M, Navalpakkam V, Koch C, & Rangel A (2012). Relative visual saliency differences induce sizable bias in consumer choice. Journal of Consumer Psychology, 22(1), 67–74. [Google Scholar]
- Mui Y, Gittelsohn J, & Jones-Smith JC (2017). Longitudinal associations between change in neighborhood social disorder and change in food swamps in an urban setting. Journal of Urban Health, 94(1), 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mui Y, Jones-Smith JC, Thornton RL, Pollack Porter K, & Gittelsohn J (2017). Relationships between Vacant Homes and Food Swamps: A Longitudinal Study of an Urban Food Environment. International Journal of Environmental Research and Public Health, 14(11), 1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polsky JY, Moineddin R, Glazier RH, Dunn JR, & Booth GL (2016). Relative and Absolute Availability of Fast Food Restaurants in Relation to the Development of Diabetes: A Population-Based Cohort Study. Canadian Journal of Diabetes, 40(5), S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AS, Ghosh-Dastidar M, Beckman R, Flórez KR, DeSantis A, Collins RL, & Dubowitz T (2017). Can the introduction of a full-service supermarket in a food desert improve residents’ economic status and health? Annals of Epidemiology, 27(12), 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogliani P, Lucà G, & Lauro D (2015). Chronic obstructive pulmonary disease and diabetes. COPD Research and Practice, 1(1), 3. [Google Scholar]
- Rose D, Bodor JN, Swalm CM, Rice JC, Farley TA, & Hutchinson PL (2009). Deserts in New Orleans? Illustrations of urban food access and implications for policy. Ann Arbor, MI: University of Michigan National Poverty Center/USDA Economic Research Service Research. [Google Scholar]
- Rundle A, Neckerman KM, Freeman L, Lovasi GS, Purciel M, Quinn J, … Weiss C (2008). Neighborhood food environment and walkability predict obesity in New York City. Environmental Health Perspectives, 117(3), 442–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salois MJ (2012). Obesity and diabetes, the built environment, and the ‘local’food economy in the United States, 2007. Economics & Human Biology, 10(1), 35–42. [DOI] [PubMed] [Google Scholar]
- Spence JC, Cutumisu N, Edwards J, Raine KD, & Smoyer-Tomic K (2009). Relation between local food environments and obesity among adults. BMC Public Health, 9(1), 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R, & Hattori A (2015). Diet and obesity in Los Angeles County 2007–2012: Is there a measurable effect of the 2008 “Fast-Food Ban”? Social Science & Medicine, 133, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaei BP, Rundle AG, Wu WY, Horowitz CR, Mayer V, Sheehan DM, & Chamany S (2017). Associations of Residential Socioeconomic, Food, and Built Environments With Glycemic Control in Persons With Diabetes in New York City From 2007–2013. American Journal of Epidemiology, 187(4), 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorndike AN, Sonnenberg L, Riis J, Barraclough S, & Levy DE (2012). A 2-Phase Labeling and Choice Architecture Intervention to Improve Healthy Food and Beverage Choices. American Journal of Public Health, 102(3), 527–533. 10.2105/AJPH.2011.300391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LE, & Kavanagh AM (2012). Association between fast food purchasing and the local food environment. Nutrition & Diabetes, 2(12), e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong K, Fernandes M, An R, Shier V, & Sturm R (2010). Measuring the physical food environment and its relationship with obesity: Evidence from California. Public Health, 124(2), 115–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziomalos K, & Athyros VG (2015). Diabetic nephropathy: New risk factors and improvements in diagnosis. The Review of Diabetic Studies: RDS, 12(1–2), 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y-T, Prina AM, Jones A, Matthews FE, & Brayne C (2017). The built environment and cognitive disorders: Results from the Cognitive Function and Ageing Study II. American Journal of Preventive Medicine, 53(1), 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, … Grauslund J (2012). Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care, DC_111909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YT, Laraia BA, Mujahid MS, Blanchard SD, Warton EM, Moffet HH, & Karter AJ (2016). Is a reduction in distance to nearest supermarket associated with BMI change among type 2 diabetes patients? Health & Place, 40, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YT, Mujahid MS, Laraia BA, Warton EM, Blanchard SD, Moffet HH, … Karter AJ (2017). Association between neighborhood supermarket presence and glycated hemoglobin levels among patients with type 2 diabetes mellitus. American Journal of Epidemiology, 185(12), 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]